Abstract
PURPOSE
COVID-19 has affected cancer care worldwide. Clinical trials are an important alternative for the treatment of oncologic patients, especially in Latin America, where trials can be the only opportunity for some of them to access novel and, sometimes, standard treatments.
METHODS
This was a cross-sectional study, in which a 22-question survey regarding the impact of the COVID-19 pandemic on oncology clinical trials was sent to 350 representatives of research programs in selected Latin American institutions, members of the Latin American Cooperative Oncology Group.
RESULTS
There were 90 research centers participating in the survey, with 70 of them from Brazil. The majority were partly private or fully private (n = 77; 85.6%) and had confirmed COVID-19 cases at the institution (n = 57; 63.3%). Accruals were suspended at least for some studies in 80% (n = 72) of the responses, mostly because of sponsors' decision. Clinical trials' routine was affected by medical visits cancelation, reduction of patients' attendance, reduction of other specialties' availability, and/or alterations on follow-up processes. Formal COVID-19 mitigation policies were adopted in 96.7% of the centers, including remote monitoring and remote site initiation visits, telemedicine visits, reduction of research team workdays or home office, special consent procedures, shipment of oral drugs directly to patients' home, and increase in outpatient diagnostic studies. Importantly, some of these changes were suggested to be part of future oncology clinical trials' routine, particularly the ones regarding remote methods, such as telemedicine.
CONCLUSION
To our knowledge, this was the first survey to evaluate the impact of COVID-19 on Latin American oncology clinical trials. The results are consistent with surveys from other world regions. These findings may endorse improvements in clinical trials' processes and management in the postpandemic period.
INTRODUCTION
In December 2019, a novel coronavirus named SARS-CoV-2 emerged as a cause of pneumonia in a rising number of patients in China.1,2 By March 11, 2020, COVID-19, the illness caused by this virus, was declared a pandemic.3 There have been approximately 108 million cases and more than 2.3 million deaths reported because of COVID-19 worldwide. Latin America has been heavily affected, accounting for more than 20 million cases and approximately 643,000 deaths reported by February 16.4 Health care systems have been coping with a rising number of severely affected patients demanding hospitalizations, including in intensive care units.5 As a consequence, elective visits, procedures, and treatments have been postponed because of COVID-19, with a significant impact on patients with other illnesses.6
CONTEXT
Key Objective
Was there an impact on oncology clinical trials in Latin America during the COVID-19 pandemic?
Knowledge Generated
A survey performed in several research centers in Latin America has shown that clinical trials have been deeply affected during the COVID-19 pandemic, with suspension of accruals, reduction of patients' attendance, and other alterations on clinical trials' procedures. Of note, remote methods, such as telemedicine, remote site initiation visits, and remote monitoring, have been widely used.
Relevance
The findings may endorse future changes in conduction of oncology clinical trials, especially the incorporation of remote technologies to the research centers' routine.
Patients with cancer may have an increased risk of developing severe events.7-9 Active cancer is an independent factor for COVID-19 mortality, and thus, patients who are potential candidates for enrollment in clinical trials could have a higher risk of dying from COVID-19.10 Clinical trials represent an important opportunity to access novel treatments for patients with cancer in all stages of the disease, but especially to those who have exhausted standard-of-care treatments and still carry a good performance status.11 In Latin American countries, clinical trials are sometimes the only possibility for patients in the public health system to have access to optimal standard-of-care treatments and/or innovative targeted therapies or immunotherapy.12-14 Clinical research may be one of the most affected oncology areas during the pandemic, mostly because of the need for frequent in-person visits to the research sites and consequently, more exposure to COVID-19.15 At the same time, staff who face patients directly, including research staff in institutions participating in clinical trials, are at higher risk of SARS-CoV-2 infection.16 Other services such as study operations and logistics may even face delays that jeopardize the conformity to planned activities at a site level.
To preserve patients' safety, along with compliance with Good Clinical Practice standards, several regulatory agencies published guidance for sponsors and study sites on how to conduct clinical research during the COVID-19 pandemic, which includes evaluation of continuing enrollment of patients onto ongoing trials, remote visits, remote monitoring, and alternative methods of investigational products delivery, among other measures.17-19 However, there are local characteristics that should be taken into account when adopting these measures.20
Formal policies were adopted in 64% of US research centers, based on a survey recently conducted by ASCO. The Dana-Farber Cancer institute has classified their policies regarding research during the COVID-19 pandemic into levels, which varied from minimal restriction on level 1 (only reduction of on-site staff work) to several restrictions on level 4, including suspension of nontherapeutic research and direct interaction with the patients only if strictly necessary.21
There is very limited information on how COVID-19 has been affecting cancer clinical research worldwide, particularly in low- and middle-income countries. To understand the impact of the pandemic on oncology clinical trials, we performed a survey addressed to investigators and research centers across Latin America.
METHODS
Study Design and Participants
This was a cross-sectional study that involved a survey sent to investigators and research coordinators of cancer research centers in several countries in Latin American members of the Latin American Cooperative Oncology Group (LACOG). The research protocol was approved by the Ethics committee from Hospital Sírio-Libanês, São Paulo, Brazil, on June 8, 2020. A protocol amendment adding questions to the survey was approved on June 11, 2020. Informed consent was obtained electronically from the person who answered the survey.
Procedures
The survey consisted of 22 questions regarding COVID-19 and cancer research centers institutional activities during the pandemic. The first questions were about the institution or research center characteristics, such as country of origin, type of health insurance coverage (public v private), and COVID-19 cases confirmed at local level. Specific questions regarding research center activities were number of active cancer clinical trials, if formal policies on the research field were developed and which policies were implemented (remote visits, home office, remote monitoring, remote site initiation visits (SIVs), changes on delivery of investigational products, suspension of feasibilities, or others), and if there was a temporary suspension on clinical trials recruitment and the reason for these suspensions in each institution. Questions about the selection procedures, consent, protocol deviations, ethics committee activities, and availability of other teams (such as radiology, surgery, and pathology) in clinical trials were also included in the survey. The last question inquired about the changes implemented during the COVID-19 pandemic that could be continued in the postpandemic scenario. The survey consisted mostly of multiple-choice questions, except for the number of open clinical trials in each institution, which required an exact number as a response. An English version of the survey is available in the Data Supplement.
The survey was hosted on Google Forms and was sent by e-mail and social media (WhatsApp Messenger) for members of LACOG in 350 institutions (250 located in Brazil and 100 in other Latin American countries). Brazilian institutions received the e-mail and survey in Portuguese, and the other Latin American institutions in Spanish. The survey was open to answers from June 26 to September 11, 2020, which included the period of the higher incidence of COVID-19 cases in Latin America so far. To improve the response rate, reminders were sent by e-mail and social media during the period that the survey was open. Initially, the survey was sent to only one representative of each institution. However, because of the low rates of responses, even after sending reminders, the survey was sent to more than one representative of each institution.
The survey results were submitted anonymously, and hence, specific data from each institution or individual cannot be identified.
Statistical Analysis
Data were gathered and analyzed using the SAS statistical software (version 9.4; SAS Institute, Inc, Cary, NC). The obtained data were submitted to descriptive and analytical statistics. To characterize the epidemiologic profile of the participants, absolute and relative frequencies and measures of central tendency (mean) were applied in the descriptive analysis.
RESULTS
Of the 350 questionnaires, 90 responses were obtained for the survey (25%). The majority of responders were from Brazilian research sites (77.8%; Fig 1A). Most of the institutions were partly private or fully private (85.6%; Fig 1B), had confirmed COVID-19 cases (63.3%), and were located in geographic regions with stable or increasing COVID-19 cases (83.4%; Fig 1C) at the time that the survey was conducted. The median number of open oncology or hematology-oncology clinical trials was 10 (1-50) per institution.
FIG 1.
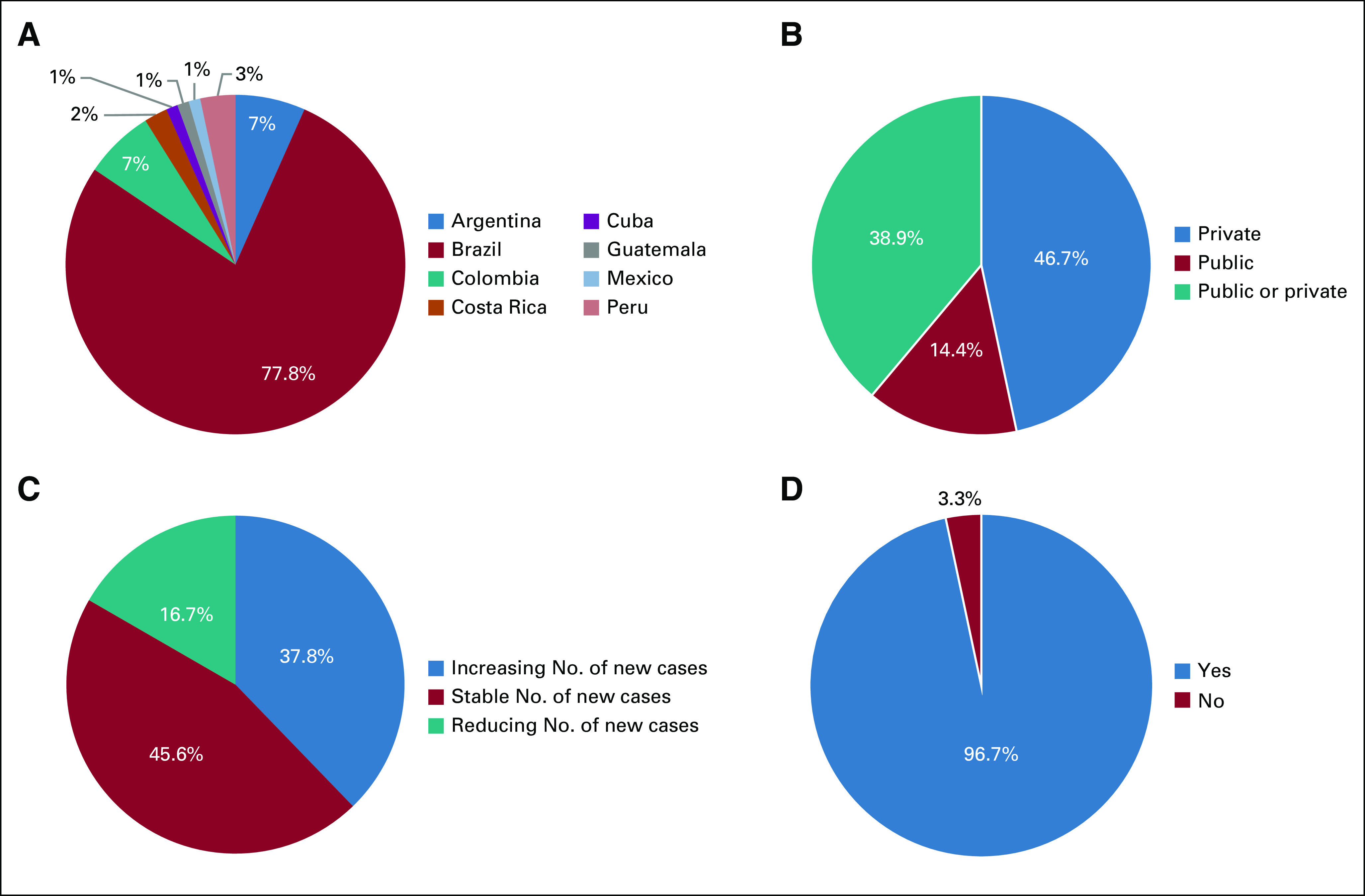
Epidemiology data gathered from LACOG survey responses. (A) Geographic distribution of participating centers. (B) Nature of the participant institutions. (C) Local COVID-19 situation. (D) Existence of policies directed to clinical trials during the COVID-19 pandemic. LACOG, Latin American Cooperative Oncology Group.
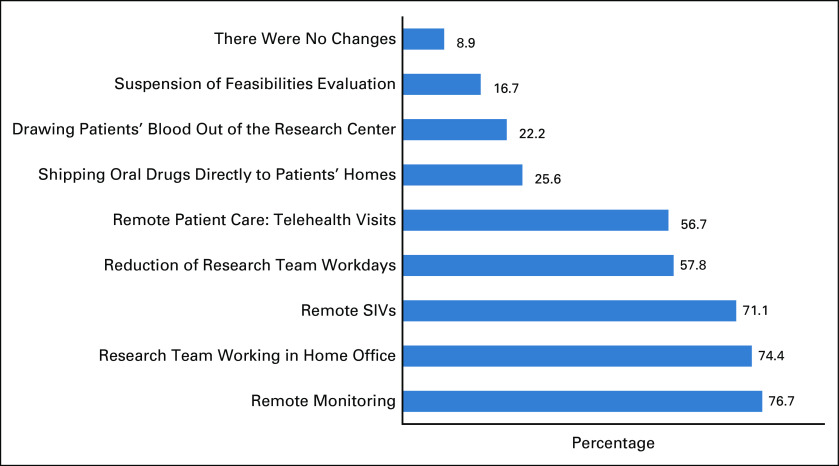
There were specific institutional policies directed to clinical trials during the COVID-19 pandemic in 96.7% of the participant institutions (Fig 1D). Only 8.9% of research centers did not have any change in their processes, 56.7% adopted telemedicine for patient evaluation, 74.4% adopted home office procedures for the research team, and 76.7% had remote monitoring (Fig 2).
FIG 2.
Institutional policies directed to clinical trials during the COVID-19 pandemic. SIV, site initiation visit.
Temporary interruption of clinical trial accrual occurred in 80% of the participant centers (Fig 3A), mainly because of the sponsors' decision (48.8%). All clinical trial accruals were stopped in 14.4% of the institutions. In 27.8%, < 50% of trials had an interruption, and in 18.9% of the centers, there were no interruptions (Fig 3B). Changes to the screening processes such as prioritizing patients with the largest potential to benefit from therapies, considering the severity of the disease, protocol's safety, protocol efficacy expectation, and other factors were implemented in 27.7% of the participant institutions. Nevertheless, 56.5% continued including all patients fitting the eligibility criteria, regardless of the factors mentioned above. Participants reported that patients treated in their institutions did not show any resistance (unwillingness) (66.6%) to participate in clinical trials during the pandemic, despite the required procedures and number of in-person visits for triage.
FIG 3.
Interruption of oncology clinical trials accrual during the COVID-19 pandemic. (A) Proportion of interruption of accrual in oncology clinical trials in the participant institutions (percentage of trials with interrupted accrual compared with the total of open studies at the research site). (B) Accrual interruption by clinical trial sponsor type.
Different procedures for consent were adopted only in 15.6% of the institutions, which included electronic consent and investigator's and patients' signatures on different dates (Fig 4B). Frequency of Ethics Committees meetings was not affected in 64.4% (Fig 4A) of the institutions.
FIG 4.
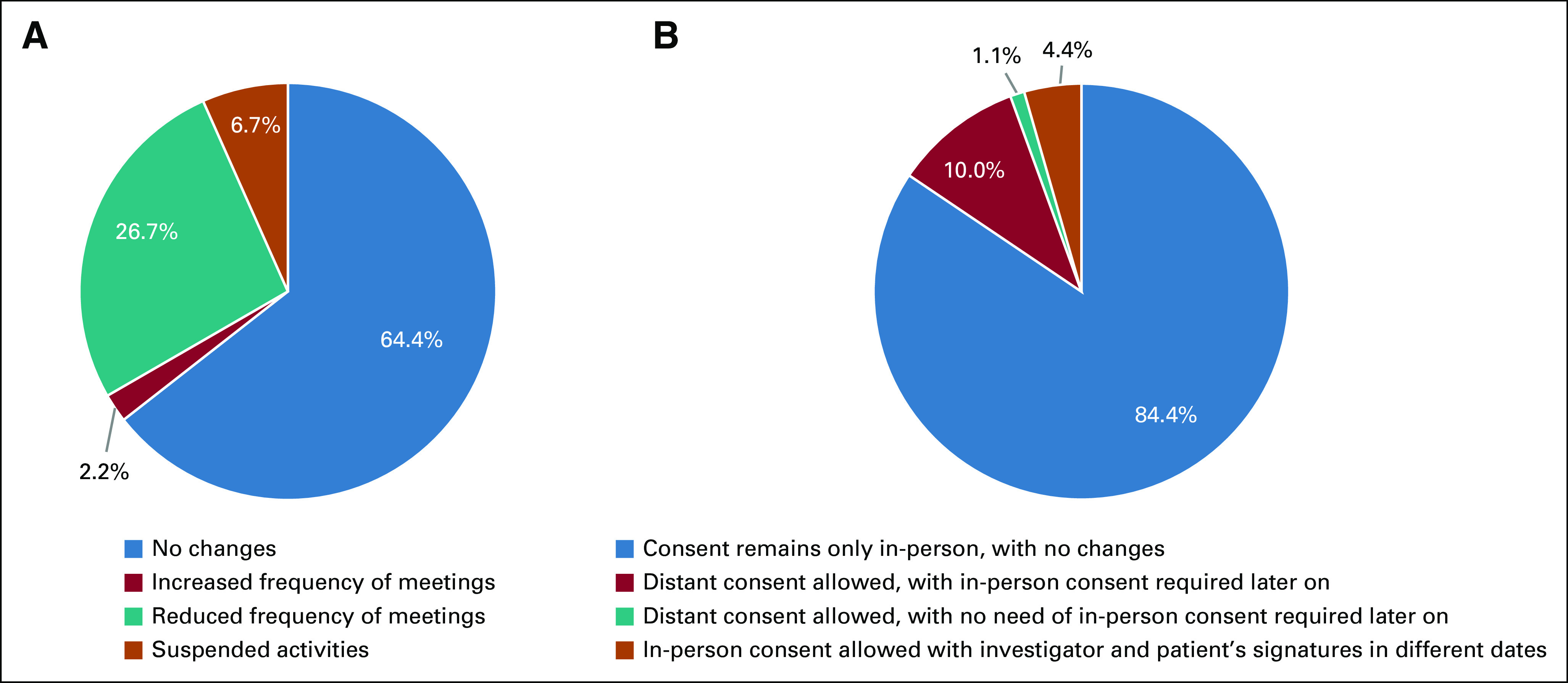
Adjustments in Ethics Committees meetings and Informed Consent procedures in oncology clinical trials during the COVID-19 pandemic. (A) Periodicity of Ethics Committees meetings. (B) Special consent procedures adopted during the COVID-19 pandemic.
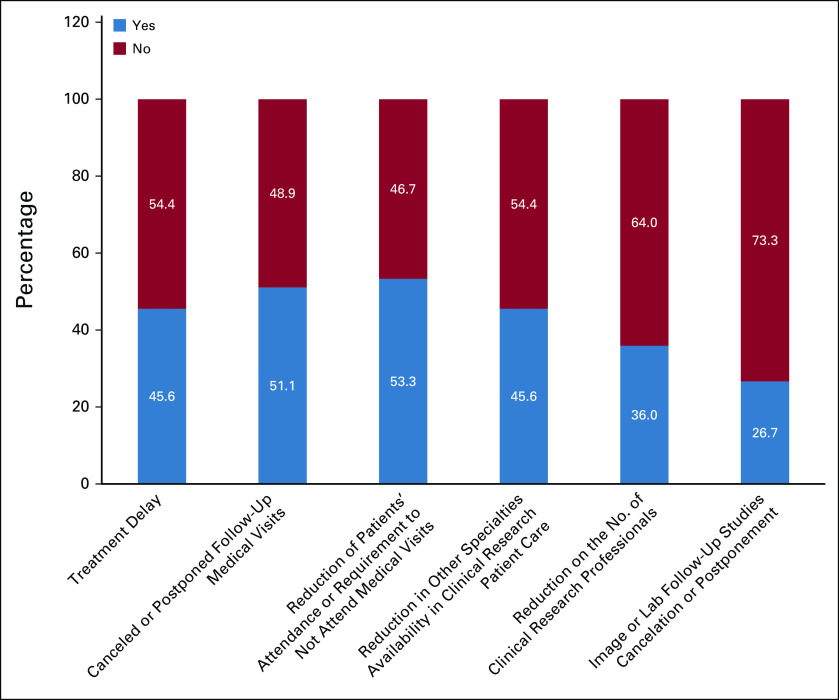
Changes in oncology clinical trials' procedures during the COVID-19 pandemic are described in Figure 5. Treatment delays related to COVID-19 issues were experienced in 45.6% of research sites, which implied in protocol deviations. Image and laboratory facilities were operating normally in most of the research sites (73.3%). Postponement of follow-up image studies, while maintaining a normal laboratory routine, occurred in 20%, and delays of both modalities in 6.6%. Cancelation and/or postponement of medical visits occurred in 51.1%. There was a decrease in patients' attendance or requirement not to attend medical visits in 53.3% of sites. About half (54.4%) of the centers had other medical specialties (radiology, surgery, or pathology) on a normal working schedule. A reduction in the number of research staff during the pandemic was reported by 36% of sites.
FIG 5.
Changes in oncology clinical trials' procedures during the COVID-19 pandemic.
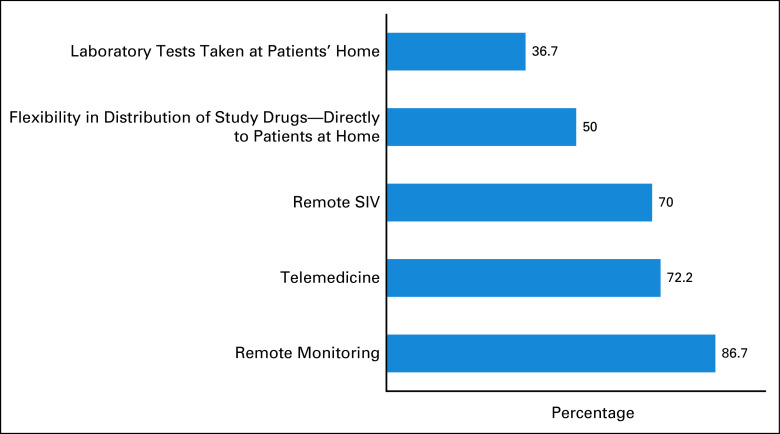
Suggested practices to be adopted on oncology clinical trials in the post-COVID-19 pandemic are described in Figure 6. Most respondents recommend remote monitoring (86.7%), implementation of telemedicine (72.2%), and remote SIVs (70%) as possible improvements.
FIG 6.
Suggested measures to be adopted on oncology clinical trials in the post-COVID-19 era. SIV, site initiation visit.
DISCUSSION
COVID-19 has challenged cancer care.22 A pandemic is a rare event that leads to reflections in many aspects of medical care. Patients, caregivers, and health care workers have experienced extreme psychologic distress.23,24 COVID-19 has challenged the medical community to implement a much needed, urgent, and immediate response, while dealing with overcrowded hospitals often with not enough supplies to offer the best care to affected patients.25,26
Oncology clinical trials continuity has been a subject of great concern during this period. Although trials represent a significant component of oncologic treatment, they come along with a strict routine and very specific procedures,15,27 which could be largely affected during the pandemic. ASCO performed a survey among US-based research programs, showing important changes in clinical trials during the pandemic.28 To our knowledge, this is the first survey to address the issue in Latin America.
Current results have shown, indeed, a significant impact on the conduct of oncology clinical trials in Latin America. There were specific institutional policies directed to clinical trials during the COVID-19 pandemic in 96.7% of the participant institutions. Of note, there was a difference in the percentage of nonexistence of specific institutional policies compared with percentage of changes in the institutional research processes (3.3% v 8.9%). The authors believe that despite having formal policies regarding clinical trials during the COVID-19 pandemic, some institutions did not apply these policies into their clinical trial routine for unknown reasons, justifying this difference between the two responses. The authors speculate that the reasons might be related to local pandemic status (eg, low number of cases), staff conditions (eg, small research site), and number of patients included in ongoing clinical trials (eg, few patients).
Accruals of at least one modality of clinical trials (sponsored or academic) have been suspended according to 80% of the responses. An analysis of a combination of global surveys and interviews of oncology clinical trials investigators and pooled data from IQVIA and clinicaltrials.gov has also demonstrated a negative impact on trials' accruals.29 In Europe and the United States, enrollment of new patients remained as usual in only 20% and 14% of institutions, respectively. Asia was less affected, with 60% of their trials recruiting normally.29 At Dana-Farber Cancer Institute, there was a statistically significant (P = .007) decrease in clinical trials' enrollment as of March 2020 (median = 86 patients), compared with the period between January 2018 and February 2020 (median = 205 patients per month). Moreover, 8.6% of enrolling trials were temporarily or permanently closed and 27.1% were put on hold, mainly because of restrictions on biospecimen collection.21 A recent cohort has also examined enrollments, particularly in studies conducted by the SWOG Cancer Research Network, a National Cancer Institute–sponsored National Clinical Trials Network Group. This analysis showed a significant decrease in oncology clinical trials' enrollment over the period that coincided with an increase in COVID-19 cases.30 During the LACOG survey period, cases of COVID-19 were stable or increasing in the region of the majority (80%) of sites, which could explain the high rate of accrual interruption. On the other hand, when the survey was performed, COVID-19 has been declared a pandemic for over 3 months. Therefore, almost all institutions had prepared and developed formal policies directed to the management of oncology clinical trials during this period, and accruals' suspension supposedly could be one of these policies.
Modifications on clinical trials' procedures and routines have affected a large proportion (40%) of sites in Latin America. US research centers have also suffered the same problems, with delays on routine services in 38%, decrease in patient's ability or willingness to come to the site, and limited services from other specialties in more than 50% of responses.28 During a unique situation as a pandemic, it is hard to control treatment delays and other specialties' availability. Ensuring protocol flexibility to external stressors and having contingency plans while maintaining patients' safety as a priority are some of the strategies that have been adopted.27
In a clinical trial routine, it is important to consider patients' requirements and concerns not to attend medical visits. Consequently, we observed an important trend toward the use of remote methods to avoid in-person visits, for both patients and sponsors. More than 50% of sites implemented telemedicine visits, and more than 70% organized remote SIVs and remote monitoring. US-based research programs had a higher percentage of telemedicine visits (87.5%), with similar rates of remote SIVs and remote monitoring.28 The lower rates in Latin America might be attributed to limitations such as the access of the population to a good quality internet connection that allows the telemedicine visits, to the absence of legislation regarding telemedicine in some countries, and also to the paucity of telehealth training available across the Latin American countries.31 Despite being more than 50%, there was an unexpectedly low rate of patient unattendance or requirement not to attend medical visits, which could also be explained by the patients' limitations associated with the lower rates of telemedicine visits, especially the access to a good quality internet connection, hence the preference for the on-site visits.
The low rates of remote consent could be due to the absence of regulation on this topic by regulatory authorities, and ethics committees be explained by the heterogeneity of ethics committees' procedures across Latin American countries. In addition, some countries demand a national approval to clinical trials' procedures, whereas others allow local evaluation.32 During the pandemic, more than 25% of the Ethics Committees had their frequency of meetings reduced, which could lead to a longer time of wait for a remote consent approval. These differences and requirements may difficult the approval of remote consent, added to the difficulty of application, since there is an already mentioned lack of internet access by the general population.
When asked about future suggestions in clinical trials, the use of these tools (telemedicine visits, remote SIVs, and remote monitoring) was the most frequent answers (more than 70% of the responders). The discussion regarding remote methods for patient care was strongly raised during the pandemic,33 and there were several suggestions for their adoption.27,34-36 In ASCO's survey, telemedicine visits were suggested as an opportunity to improve clinical trials by more than 90% of the respondents, along with remote SIVs and remote monitoring by 70.9% and 64.5%, respectively.28 These methods were carried out or planned by the majority of respondents in other survey, endorsing these methods as potential improvements for future clinical trials.29 This information could lead to a reassessment of clinical trials' procedures and incorporation of these processes as part of the trials' management. Finally, the incorporation of computer-based tools to help the informed consent process can also be discussed.37
Reducing in-person assessments with telehealth visits would allow a higher number of patients to be managed within the research centers' capacity. Moreover, the recruitment of patients who live far away from the research site could be facilitated.27 Although telemedicine allows for an adequate patient evaluation in a good proportion of cases, it is important to consider its limitations and recognize as an example, the need for invaluable in-person interactions particularly related to important information collected through physical examination. In Latin America, there might be additional barriers to patients' access to digital communication tools, especially in rural areas. Nevertheless, there have been efforts toward expanding telehealth in Latin-American countries.38,39
Changing data monitoring from in-person to remote methods is certainly a challenge since frequently there is an excess of documents to be checked and a few institutions use electronic medical records in Latin America.40 However, both remote monitoring and SIVs have been successfully implemented in many research sites during the pandemic, opening a possibility for these procedures to be mostly remote in the future and increasing clinical trials' cost effectiveness.34,41
This study has some limitations. The low response rate (25%) should be recognized. As the responses were anonymous, it was not possible to identify multiple responses from the same institution. Despite the fact that the authors believe this is an unlikely scenario, they recognize that this is an important limitation of the study. Also, most of the responders were from Brazilian research sites, which are an important epicenter of the pandemic, possibly prompting an overestimation of the COVID-19 impact in clinical trials.4 Nonetheless, Brazil is the leading country in the number of ongoing clinical trials in Latin America.4,42 However, the findings of the survey are consistent with data from other reports,28,29 showing that this pandemic has largely affected oncology research across world regions.
In conclusion, our survey showed that oncology clinical trials have been significantly affected during the pandemic in Latin America. The impact was greater in clinical trials' accrual, protocol compliance, and monitoring. Incorporation of technology to allow remote protocol procedures has been consistently and largely applied as a risk reducing strategy to protect both patients and site personnel. The results of our survey are consistent with other reports in the literature. Altogether, these results indicate that some of the changes implemented during these unusual COVID-19 times could represent an opportunity for sponsors, investigators, and regulatory agencies to reassess procedures and improve both the patient and staff experience while participating in clinical research.
ACKNOWLEDGMENT
We would like to thank all the investigators who took the time to answer this LACOG survey and helped us to delineate conclusions regarding clinical trials during the COVID-19 pandemic in Latin America. We would like to thank SAS software for providing a free license to LACOG.
Aline B. Lara Gongora
Honoraria: MSD Oncology, Amgen
Denis L. Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, BMS Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, BMS Brazil, Janssen-Cilag
Angelica Nogueira-Rodrigues
Honoraria: Roche, MSD, AstraZeneca
Consulting or Advisory Role: Roche, AstraZeneca, MSD, Eisai
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, Biomarin, Bristol Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Clinica Atlantis, INC Research, Halozyme, Covance, Celgene, inVentiv Health, Merck KGaA, Shanghai Henlius Biotech, Polyphor, PharmaMar
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Fernando Maluf
Honoraria: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Sanofi/Aventis, Merck Serono, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Merck Serono, AstraZeneca
Research Funding: Janssen-Cilag, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer Schering Pharma
Rachel Riechelmann
Honoraria: Novartis, Amgen, MSD Oncology, Servier, Roche/Genentech
Consulting or Advisory Role: Bayer, Roche/Genentech, Ipsen, Astellas Pharma
Research Funding: Bayer, Roche/Foundation Medicine, Amgen
Henry Gomes
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol Myers Squibb, Lilly
Research Funding: MSD Oncology
William N. William
Honoraria: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck, Lilly, Pfizer
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, Bristol Myers Squibb, Bayer, Roche
Speakers' Bureau: Boehringer Ingelheim
Research Funding: OSI Pharmaceuticals, Boehringer Ingelheim, Merck, Lilly, Bristol Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Merck, Bristol Myers Squibb, Roche/Genentech
Camilla A. F. Yamada
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Bayer, Janssen
Gilberto de Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb
Daniela D. Rosa
Consulting or Advisory Role: Roche, Novartis, AstraZeneca, Lilly, GlaxoSmithKline, Sanofi, Libbs, Pfizer, Amgen, Zodiac Pharma
Speakers' Bureau: Novartis, Lilly, Pfizer
Travel, Accommodations, Expenses: Roche
Andreia C. de Melo
Honoraria: MSD Oncology, Novartis, BMS Brazil
Speakers' Bureau: BMS Brazil, MSD Oncology
Research Funding: Roche, MSD Oncology, BMS Brazil, Novartis, Clovis Oncology, AstraZeneca
Travel, Accommodations, Expenses: MSD Oncology
Denisse Bretel
Consulting or Advisory Role: Merck
Oscar Arrieta
Honoraria: AstraZeneca, Merck Sharp & Dohme, Roche
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Pfizer
Research Funding: AstraZeneca, Roche, Merck Sharp & Dohme
Andrés F. Cardona
Honoraria: Bristol Myers Squibb, Roche, Boehringer Ingelheim, AbbVie, Merck Sharp & Dohme, Celldex
Consulting or Advisory Role: Roche, Merck Sharp & Dohme, Novartis, AstraZeneca, Bristol Myers Squibb, Foundation Medicine, Boehringer Ingelheim, Foundation for Clinical and Applied Cancer Research (FICMAC)
Speakers' Bureau: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Novartis, Foundation for Clinical and Applied Cancer Research (FICMAC), Foundation Medicine
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Foundation Medicine
Diogo A. Bastos
Honoraria: MSD, Roche, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, AstraZeneca, Bayer
Consulting or Advisory Role: Roche, Bayer, Janssen-Cilag, MSD Oncology
Research Funding: Janssen-Cilag, Pfizer, Astellas Pharma
Travel, Accommodations, Expenses: Janssen-Cilag, Bayer
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Aline B. Lara Gongora, Gustavo Werutsky, Denis L. Jardim, Carlos H. Barrios, Clarissa Mathias, Rachel Riechelmann, William N. William, Andreia C. de Melo, Eva Bustamante, Oscar Arrieta, Andrés F. Cardona, Diogo A. Bastos
Provision of study materials or patients: Gustavo Werutsky, Clarissa Mathias, Henry Gomes, Camilla A. F. Yamada, Denisse Bretel
Collection and assembly of data: Aline B. Lara Gongora, Gustavo Werutsky, Carlos H. Barrios, Clarissa Mathias, Henry Gomes, Daniela D. Rosa, Andreia C. de Melo, Raul Sala, Denisse Bretel, Andrés F. Cardona, Diogo A. Bastos
Data analysis and interpretation: Aline B. Lara Gongora, Gustavo Werutsky, Denis L. Jardim, Angelica Nogueira-Rodrigues, Carlos H. Barrios, Clarissa Mathias, Fernando Maluf, Rachel Riechelmann, Maurício Fraga, Henry Gomes, William N. William, Camilla A. F. Yamada, Gilberto de Castro Junior, Daniela D. Rosa, Andreia C. de Melo, Raul Sala, Oscar Arrieta, Andrés F. Cardona, Diogo A. Bastos
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Aline B. Lara Gongora
Honoraria: MSD Oncology, Amgen
Denis L. Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, BMS Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, BMS Brazil, Janssen-Cilag
Angelica Nogueira-Rodrigues
Honoraria: Roche, MSD, AstraZeneca
Consulting or Advisory Role: Roche, AstraZeneca, MSD, Eisai
Carlos H. Barrios
Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, Biomarin, Bristol Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Clinica Atlantis, INC Research, Halozyme, Covance, Celgene, inVentiv Health, Merck KGaA, Shanghai Henlius Biotech, Polyphor, PharmaMar
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Fernando Maluf
Honoraria: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Sanofi/Aventis, Merck Serono, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Merck Serono, AstraZeneca
Research Funding: Janssen-Cilag, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer Schering Pharma
Rachel Riechelmann
Honoraria: Novartis, Amgen, MSD Oncology, Servier, Roche/Genentech
Consulting or Advisory Role: Bayer, Roche/Genentech, Ipsen, Astellas Pharma
Research Funding: Bayer, Roche/Foundation Medicine, Amgen
Henry Gomes
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol Myers Squibb, Lilly
Research Funding: MSD Oncology
William N. William
Honoraria: Roche/Genentech, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck, Lilly, Pfizer
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, Bristol Myers Squibb, Bayer, Roche
Speakers' Bureau: Boehringer Ingelheim
Research Funding: OSI Pharmaceuticals, Boehringer Ingelheim, Merck, Lilly, Bristol Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Merck, Bristol Myers Squibb, Roche/Genentech
Camilla A. F. Yamada
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Bayer, Janssen
Gilberto de Castro Junior
Honoraria: AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Roche
Consulting or Advisory Role: Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol Myers Squibb, AstraZeneca, Yuhan, Merck Serono
Speakers' Bureau: AstraZeneca, Bayer, Novartis, Roche, Merck Serono, Bristol Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novartis, Pfizer, Roche, AstraZeneca, Boehringer Ingelheim, Bayer, Bristol Myers Squibb
Daniela D. Rosa
Consulting or Advisory Role: Roche, Novartis, AstraZeneca, Lilly, GlaxoSmithKline, Sanofi, Libbs, Pfizer, Amgen, Zodiac Pharma
Speakers' Bureau: Novartis, Lilly, Pfizer
Travel, Accommodations, Expenses: Roche
Andreia C. de Melo
Honoraria: MSD Oncology, Novartis, BMS Brazil
Speakers' Bureau: BMS Brazil, MSD Oncology
Research Funding: Roche, MSD Oncology, BMS Brazil, Novartis, Clovis Oncology, AstraZeneca
Travel, Accommodations, Expenses: MSD Oncology
Denisse Bretel
Consulting or Advisory Role: Merck
Oscar Arrieta
Honoraria: AstraZeneca, Merck Sharp & Dohme, Roche
Consulting or Advisory Role: Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Pfizer
Research Funding: AstraZeneca, Roche, Merck Sharp & Dohme
Andrés F. Cardona
Honoraria: Bristol Myers Squibb, Roche, Boehringer Ingelheim, AbbVie, Merck Sharp & Dohme, Celldex
Consulting or Advisory Role: Roche, Merck Sharp & Dohme, Novartis, AstraZeneca, Bristol Myers Squibb, Foundation Medicine, Boehringer Ingelheim, Foundation for Clinical and Applied Cancer Research (FICMAC)
Speakers' Bureau: Merck Sharp & Dohme, Roche, Bristol Myers Squibb, Novartis, Foundation for Clinical and Applied Cancer Research (FICMAC), Foundation Medicine
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb, Roche, Boehringer Ingelheim, Foundation Medicine
Diogo A. Bastos
Honoraria: MSD, Roche, Bristol Myers Squibb, Janssen-Cilag, Astellas Pharma, AstraZeneca, Bayer
Consulting or Advisory Role: Roche, Bayer, Janssen-Cilag, MSD Oncology
Research Funding: Janssen-Cilag, Pfizer, Astellas Pharma
Travel, Accommodations, Expenses: Janssen-Cilag, Bayer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019 N Engl J Med 382727–7332020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China N Engl J Med 3821708–17202020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cucinotta D, Vanelli M.WHO declares COVID-19 a pandemic Acta Biomed 91157–1602020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Coronavirus Disease (COVID-19) Dashboard. 2020. https://covid19.who.int [Google Scholar]
- 5.Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations Lancet Respir Med 8506–5172020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum L.The untold toll—The pandemic's effects on patients without Covid-19 N Engl J Med 3822368–23712020 [DOI] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China Lancet Oncol 21335–3372020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a Tertiary Care Hospital in Wuhan, China JAMA Oncol 61108–11102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang F, Shi S, Zhu J, et al. Clinical characteristics and outcomes of cancer patients with COVID-19 J Med Virol 922067–20732020 [DOI] [PubMed] [Google Scholar]
- 10.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study Lancet 3951907–19182020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vignot S, Martin M, Albin N, et al. Facilitating access to new therapeutic options through clinical trials: The vision of a regulator to reconcile innovation and safety Ann Oncol 301694–16962019 [DOI] [PubMed] [Google Scholar]
- 12.Arai RJ, Guindalini RSC, Llera AS, et al. Personalizing precision oncology clinical trials in Latin America: An expert panel on challenges and opportunities Oncologist 24e709–e7192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raez LE, Cardona AF, Santos ES, et al. The burden of lung cancer in Latin-America and challenges in the access to genomic profiling, immunotherapy and targeted treatments Lung Cancer 1197–132018 [DOI] [PubMed] [Google Scholar]
- 14.Calderon-Aparicio A, Orue A. Precision oncology in Latin America: Current situation, challenges and perspectives. Ecancermedicalscience. 2019;13:920. doi: 10.3332/ecancer.2019.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borno HT, Small EJ. Does the COVID-19 outbreak identify a broader need for an urgent transformation of cancer clinical trials research? Contemp Clin Trials. 2020;92:105997. doi: 10.1016/j.cct.2020.105997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah ASV, Wood R, Gribben C, et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: Nationwide linkage cohort study. BMJ. 2020;371:m3582. doi: 10.1136/bmj.m3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Paula BHR, Araújo I, Bandeira L, et al. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic Lancet Oncol 21624–6272020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA Guidance on Conduct of Clinical Trials of Medical Products During COVID-19 Public Health Emergency. Guidance for Industry, Investigators, and Institutional Review Boards. 2020. [Google Scholar]
- 19.Guidance on the Management of Clinical Trials During the COVID-19 (Coronavirus) Pandemic. European Medicines Agency; 2020. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf [Google Scholar]
- 20.Bobato Lara Gongora A, Jardim DL, Bastos DA.Oncology clinical trials during the COVID-19 pandemic Oncology (Williston Park) 34265–2692020 [PubMed] [Google Scholar]
- 21.Tolaney SM, Lydon CA, Li T, et al. The Impact of COVID-19 on clinical trial execution at the Dana-Farber Cancer Institute. J Natl Cancer Inst. doi: 10.1093/jnci/djaa144. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saini KS, de las Heras B, de Castro J, et al. Effect of the COVID-19 pandemic on cancer treatment and research Lancet Haematol 7e432–e4352020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng KYY, Zhou S, Tan SH, et al. Understanding the psychological impact of COVID-19 pandemic on patients with cancer, their caregivers, and health care workers in Singapore JCO Glob Oncol 61494–15092020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Datta SS, Mukherjee A, Ghose S, et al. Addressing the mental health challenges of cancer care workers in LMICs during the time of the COVID-19 pandemic JCO Glob Oncol 61490–14932020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willan J, King AJ, Jeffery K, et al. Challenges for NHS hospitals during covid-19 epidemic. BMJ. 2020;368:m1117. doi: 10.1136/bmj.m1117. [DOI] [PubMed] [Google Scholar]
- 26.Ranney ML, Griffeth V, Jha AK. Critical supply shortages—The need for ventilators and personal protective equipment during the Covid-19 pandemic. N Engl J Med. 2020;382:e41. doi: 10.1056/NEJMp2006141. [DOI] [PubMed] [Google Scholar]
- 27.Doherty GJ, Goksu M, de Paula BHR.Rethinking cancer clinical trials for COVID-19 and beyond Nat Cancer 1568–5722020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterhouse DM, Harvey RD, Hurley P, et al. Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: Findings from an American Society of Clinical Oncology survey JCO Oncol Pract 16417–4212020 [DOI] [PubMed] [Google Scholar]
- 29.Upadhaya S, Yu JX, Oliva C, et al. Impact of COVID-19 on oncology clinical trials Nat Rev Drug Discov 19376–3772020 [DOI] [PubMed] [Google Scholar]
- 30.Unger JM, Blanke CD, LeBlanc M, et al. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open. 2020;3:e2010651. doi: 10.1001/jamanetworkopen.2020.10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce W, Schroeder D, Suchecki R. Telehealth in Latin America: Progress, challenges, and opportunities in the face of COVID-19. Telehealth Med Today. 2021;6((1)) doi: 10.30953/tmt.v6.238. [DOI] [Google Scholar]
- 32.Bergamasco A, Arredondo Bisono T, Castillon G, et al. Drug utilization studies in Latin America: A scoping review and survey of ethical requirements Value Health Reg Issues 17189–1932018 [DOI] [PubMed] [Google Scholar]
- 33.Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care J Am Med Inform Assoc 27957–9622020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabhan C, Choueiri TK, Mato AR. Rethinking clinical trials reform during the COVID-19 pandemic. JAMA Oncol. 2020;6:1327. doi: 10.1001/jamaoncol.2020.3142. [DOI] [PubMed] [Google Scholar]
- 35.Araujo DV, Watson GA, Siu LL.The day after COVID-19—Time to rethink oncology clinical research JAMA Oncol 723–242021 [DOI] [PubMed] [Google Scholar]
- 36.Fontana E, Arkenau H-T. Oncology clinical trials during the COVID-19 outbreak: Lessons learnt during the crisis and future opportunities. Cancer Treat Rev. 2020;88:102047. doi: 10.1016/j.ctrv.2020.102047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shelton AK, Freeman BD, Fish AF, et al. A computer-based education intervention to enhance surrogates' informed consent for genomics research Am J Crit Care 24148–1552015 [DOI] [PubMed] [Google Scholar]
- 38.Prieto-Egido I, Simó-Reigadas J, Liñán-Benítez L, et al. Telemedicine networks of EHAS Foundation in Latin America. Front Public Health. 2014;2:188. doi: 10.3389/fpubh.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos A, D'Agostino M, Bouskela M, et al. Uma visão panorâmica das ações de telessaúde na América Latina Rev Panam Salud Pública 35465–4702014 [PubMed] [Google Scholar]
- 40.Electronic Medical Records in Latin America and the Caribbean: An Analysis of the Current Situation and Recommendations for the Region. Washington DC: Pan American Health Organization; 2016. https://iris.paho.org/bitstream/handle/10665.2/28210/9789275118825_eng.pdf?sequence=1&isAllowed=y [Google Scholar]
- 41.Tan AC, Ashley DM, Khasraw M.Adapting to a pandemic—Conducting oncology trials during the SARS-CoV-2 pandemic Clin Cancer Res 263100–31032020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coronel E, Halstead D, Fregni F.Clinical research in Latin America: Obstacles and opportunities Clin Investig 1911–9132011 [Google Scholar]