Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, corticosteroids, high-flow nasal oxygen, intensive care, mechanical ventilation, remdesivir
Abstract
Objectives:
To compare patient management and outcome during the first and second waves of the coronavirus 2019 pandemic.
Design:
Single-center prospective cohort study.
Setting:
Tertiary-care University Hospital.
Patients:
All adult patients admitted in either the first (from March 15 to May 15, 2020) or second (from October 1 to November 30, 2020) wave of coronavirus disease 2019.
Interventions:
None.
Measurements and Main Results:
Primary outcome was 30-day mortality. During the second wave of the coronavirus disease 2019 pandemic, 33 patients (4.8%) were transferred due to overcrowding and excluded from analysis. There were 341 (first wave of the coronavirus disease 2019 pandemic) and 695 (second wave of the coronavirus disease 2019 pandemic) coronavirus disease 2019 patients admitted to the hospital, with median age first wave of the coronavirus disease 2019 pandemic as 68 (57–80) and second wave of the coronavirus disease 2019 pandemic as 71 (60–80) (p = 0.15), and similar admission severity. For the first wave of the coronavirus disease 2019 pandemic versus second wave of the coronavirus disease 2019 pandemic, 30-day mortality was 74/341 (22%) and 98/662 (15%) (p = 0.007). In the ward, 11/341 (3.2%) and 404/662 (61%) received dexamethasone (p < 0.001); 6/341 (2%) and 79/662 (12%) received high-flow nasal oxygen (p < 0.0001); 2/341 (0.6%) and 88/662 (13.3%) received remdesivir (p < 0.0001); 249/341 (73%) and 0/662 (0%) received hydroxychloroquine (p < 0.0001); and 87/341 (26%) and 128/662 (19%) (p = 0.024) patients were transferred to ICU. On ICU admission, median Sequential Organ Failure Assessment was 6 (3–7) and 4 (3–6) (p = 0.02). High-flow nasal oxygen was given to 16/87 (18%) and 102/128 (80%) (p < 0.001); 69/87 (79%) and 56/128 (44%) received mechanical ventilation (p < 0.001) with durations 17 days (10–26 d) and 10 days (5–17 d) (p = 0.01). Median ICU length of stay was 14 days (5–27 d) and 6 days (3–11 d) (p < 0.001). Finally, 16/87 (18%) and 8/128 (6%) received renal replacement therapy (p = 0.0055); and 64/87 (74%) and 51/128 (40%) needed vasopressor support (p < 0.001).
Conclusions:
The main therapeutic changes between the first wave of the coronavirus disease 2019 pandemic and the second wave of the coronavirus disease 2019 pandemic were use of steroids, unrestrictive use of high-flow nasal oxygen for hypoxemic patients, and transfer of patients to other geographic areas in the case of ICU overcrowding. These changes were associated with a decrease in 30-day mortality, ICU admission, and organ support.
During the coronavirus disease 2019 (COVID-2019) pandemic, several questions on patient management and therapeutic options arose concerning care of moderate and severe COVID-19 patients requiring oxygen support or mechanical ventilation (MV) (1).
Initially, steroids were not recommended, because it could decrease virus clearance, as suggested in other viral infections (2, 3). However, single-center studies, later confirmed by the Recovery study, demonstrated a significant decrease in mortality in patients requiring oxygen support or MV when treated by dexamethasone (4). A further meta-analysis confirmed these results (5). In addition, remdesivir was also recommended in patients with oxygen support in the first and subsequent second waves of the COVID-19 pandemic (6). In contrast, hydroxychloroquine was not recommended by the time of the second wave due to the lack of evidence (7). Finally, high-flow nasal oxygen (HFNO) was initially suspected to aerosolize severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in room air (8), and its use was limited to flows under 30 L/min. However, later studies in the first wave showed the risk of aerosolization was limited even when using HFNO at flow as high as 60 L/min (9). All these results show substantial, progressive, and evidence-driven modification in the management of COVID-19 in our hospital, and internationally (7, 10).
This study assesses the impact of these changes in patient management on patient outcomes.
MATERIALS AND METHODS
Study Design and Participants
This prospective single-center cohort study was conducted at the University Hospital of Liege. The Ethics Committee of the University Hospital of Liege (Comité d’éthique hospitalo-universitaire de Liège [707]) reviewed the study and approved it (Reference: 2021/032). Informed consent was not required, because the study did not modify patients’ management and the data were anonymously collected.
All adult patients admitted to the University Hospital of Liege for acute respiratory failure due to SARS-CoV-2 pneumonia diagnosed with a positive polymerase chain reaction (PCR) for SARS-CoV-2 in nasal swab or other respiratory samples during the 5 days of their admission in the hospital or in the 14 days before admission were included. The detection of SARS-CoV-2 was performed by reverse transcription polymerase chain reaction using the Cobas SARS-CoV-2 assay (Roche, Switzerland) for the detection of the ORF1ab and E genes. The results were reported as cycle thresholds in order to give an approximation of the viral load.
Patients were admitted in either the first wave of COVID-19 from March 15 to May 15, 2020 (W1), or the second wave from October 1 to November 30, 2020 (W2) (11). Patients with positive SARS-CoV-2 PCR and primarily hospitalized for scheduled or urgent surgery were excluded. The hospital expanded its total ICU capacity from 58 to 68 beds during W1 and 71 beds during W2, with 10 to 12 beds dedicated to non-COVID-19 critically ill patients. In the ward, 196 beds were dedicated to COVID-19 patients during the two waves.
Procedure
During W1, Belgian public health services did not recommend the use of steroids because of the risk of decreasing the clearance of the SARS-CoV-2, and recommend hydroxychloroquine treatment in COVID-19 patients. Antibiotic treatment was started in all COVID-19 patients and stopped after 48 hours if PCR multiplex on respiratory samples revealed no pulmonary infection. Due to the high potential risk of aerosolization in room air and monitoring needs, patients did not receive HFNO in the ward. All patients requiring oxygen support over 15 L/min with reservoir mask and with respiratory rate above 30/min were admitted to ICU. Once in ICU, patients received HFNO with a maximum flow of 30 L/min, because it was believed the risk of aerosolization was too high above this value. ICU discharge was allowed when patients were weaned from HFNO and required oxygen supply only by normal flow.
During W2, dexamethasone in all COVID-19 patients under oxygen support or MV was started early at 6 mg/d for 10 days. Patients requiring oxygen support, but not MV, and in whom COVID-19 symptoms started less than 5 days previously received IV remdesivir with a starting dose of 200 mg and then a daily dose of 100 mg a day for 5–10 days. Antibiotics were started for suspected bacterial infections, based on the presence of parenchymatous condensation on computerized tomography scanner of the lung, leucocytes and positive culture in the endotracheal aspirate, or in the sputum in nonintubated patients, or evidence of septic shock. HFNO was used in the ward under appropriate monitoring and in the ICU at flows up to 60 L/min. Patients with refractory hypoxemia (Pao2 < 60 mm H2O) under HFNO or mild hypoxemia with a respiratory rate above 30/min were admitted to ICU. Once in ICU, patients could receive noninvasive ventilation and could benefit from prone position while in spontaneous ventilation. Patients were discharged from ICU when weaned from MV, regardless they were still on HFNO or on normal-flow oxygen supply.
During W2, to minimize risk of severe ICU overcrowding, patients were regularly transferred to other Belgian areas or to Germany, provided they had been in a stable condition for more than 48 hours, preferably under MV, to ensure safe transport.
During both W1 and W2, all personnel protection measures against aerosolization risk remained in force, as well as COVID-19 patient isolation measures. Enoxaparin was given at a dosage of 1 mg/kg once a day, except in the case where a full anticoagulation was clearly requested by another pathology.
Major differences in the clinical management of COVID-19 patients in the first and second wave are depicted in Figure 1.
Figure 1.
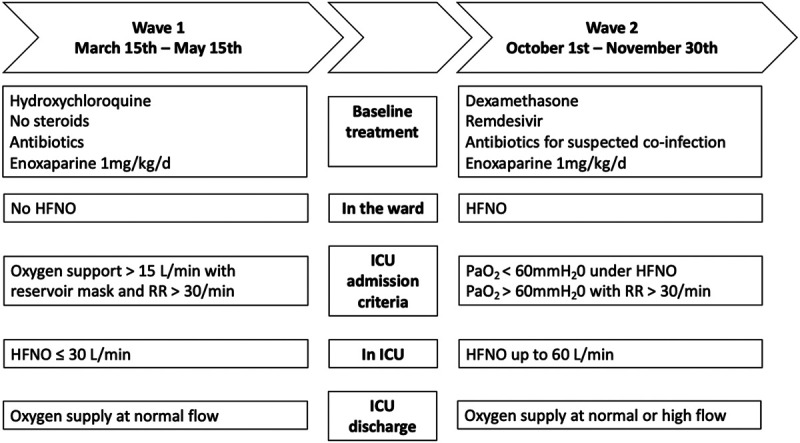
Clinical management of coronavirus disease 2019 patients during the first and second waves of the pandemic. HFNO = high-flow nasal oxygen, RR = respiratory rate.
Data Collection
We prospectively collected data on hospital admission, during hospitalization, on ICU admission, and during the ICU stay. On hospital admission, demographic data, comorbidities, SARS-CoV-2 viral load, physiologic, and biological values were collected. During hospitalization, the use of drugs oriented against COVID-19 (hydroxychloroquine, remdesivir, and steroids), use of HFNO, and date of ICU admission were collected. On ICU admission, patients’ characteristics, the same physiologic and biological values as on hospital admission, and the Sequential Organ Failure Assessment (SOFA) score were collected. Characteristics of patients transferred to other hospitals were also collected on ICU admission.
During the first week of ICU stay, the highest creatinine, bilirubin, C-reactive protein (CRP), and d-dimer values, along with the lowest platelet count, were collected. Over the entire ICU stay, data on the use of enoxaparin, antibiotic, vasopressors, renal replacement therapy (RRT), extracorporeal membrane oxygenation, the delay between ICU admission and intubation, duration of HFNO and MV, the length of ICU stay, and 28-day ICU-free and ventilator-free days were collected.
Outcome
The primary outcome was 30-day mortality. Secondary outcomes were: 30-day mortality of ICU patients, admission to ICU, length of ICU stay, delay between ICU admission and intubation, use and duration of MV, 28-day ICU-free days and ventilator-free days; use and duration of HFNO, vasopressor support, RRT, and risk factors of mortality.
Statistical Methods
Quantitative variables were reported as median (Q1–Q3) and categorical variables as number (%). Kruskal-Wallis and Fisher exact tests were used as appropriate. Cox regression models were used to assess risk factors of mortality. Hazard ratio and 95% CIs were reported. All the variables with p < 0.1 were selected for the multivariate model. A value of p < 0.05 was considered significant. Overall survival is presented by Kaplan-Meier curves. Missing data were not replaced. Calculations were performed using SAS (version 9.4, SAS institute, Cary, NC) and R (version 3.6.2, Foundation for Statistical Computing, Vienna, Austria). All statistical analyses were done by the Biostatistics and Medico-Economic Information Department of the University Hospital of Liege (Liege, Belgium).
RESULTS
During W1 and W2, respectively, 341 and 695 patients fulfilling all the inclusion criteria were hospitalized for COVID-19. Thirty-three W2 patients (4.8%) were transferred to other hospitals due to local overcrowding and excluded from analysis (Fig. 1, Supplement Digital Content, http://links.lww.com/CCX/A643). Their severity on ICU admission was the same as patients who remained (Table 1, Supplement Digital Content, http://links.lww.com/CCX/A642).
TABLE 1.
Patients Characteristics on Hospital Admission, Evolution and Treatment During the First and the Second Waves of Coronavirus Disease 2019 Pandemic
| Variables | First Wave of the COVID-19 Pandemic | Number of Patients | Second Wave of the COVID-19 Pandemic | Number of Patients | p |
|---|---|---|---|---|---|
| Age (yr) | 68 (57–80) | 341 | 71 (60–80) | 662 | 0.15 |
| Body mass index (kg/m2) | 27.2 (24–31) | 257 | 27.0 (24–31) | 509 | 0.054 |
| Sex (male) | 200 (59%) | 341 | 386 (58%) | 662 | 0.92 |
| Chronic kidney disease | 37 (10.9%) | 341 | 66 (10%) | 662 | 0.66 |
| Diabetes | 125 (37%) | 341 | 283 (43%) | 662 | 0.063 |
| Hypertension | 181 (53%) | 341 | 322 (49%) | 662 | 0.18 |
| Bilirubin (mg/dL) | 0.68 (0.5–0.97) | 336 | 0.64 (0.48–0.9) | 640 | 0.14 |
| C-reactive protein (mg/L) | 81.8 (33.6–166) | 339 | 78.2 (29.0–146) | 645 | 0.15 |
| Creatinine (mg/dL) | 0.99 (0.81–1.35) | 338 | 0.99 (0.77–1.38) | 644 | 0.68 |
| d-dimer (ng/mL) | 924 (540–1,782) | 297 | 1,020 (576–2,004) | 552 | 0.13 |
| Ferritin (µg/L) | 1,003 (531–2,329) | 77 | 743 (327–2,017) | 189 | 0.041 |
| Lymphocyte count (103/mm3) | 0.94 (0.66–1.23) | 337 | 0.90 (0.63–1.31) | 644 | 0.51 |
| Platelet count (103/mm3) | 195 (155–263) | 337 | 209 (161–274) | 644 | 0.15 |
| Spo2 (%) | 94 (90–96) | 340 | 93 (90–96) | 658 | 0.53 |
| Respiratory rate (/min) | 22 (19–29) | 325 | 20 (18–25) | 575 | 0.0003 |
| Heart rate (/min) | 90 (79–103) | 341 | 88 (76–101) | 662 | 0.15 |
| Mean arterial pressure (mm Hg) | 94.0 (86.0–103) | 341 | 94.3 (83.6–105) | 662 | 0.48 |
| Viral load (cycle threshold) | 27.5 (23–31) | 279 | 26.5 (21–32) | 454 | 0.91 |
| Hyrdoxychloroquine | 249 (73%) | 341 | 0 (0%) | 662 | <0.0001 |
| Remdesivir | 2 (0.6%) | 341 | 88 (13.3%) | 662 | <0.0001 |
| Dexamethasone | 11 (3.2%) | 341 | 404 (61%) | 662 | <0.0001 |
| Transfer in ICU | 87 (26%) | 341 | 128 (19%) | 662 | 0.024 |
| 30-d mortality | 74 (22%) | 341 | 99 (15%) | 662 | 0.007 |
| Age of deceased patients (yr) | 79.5 (71–86) | 74 | 82 (74–88) | 99 | 0.069 |
COVID-19 = coronavirus disease 2019.
Quantitative variables are reported as median (Q1–Q3) and categorical variables as number (%). Kruskal-Wallis and Fisher exact tests are used as appropriate.
On hospital admission, demographic data, comorbidities, physiologic and biological data, and viral load were the same in W1 and W2, except ferritin and respiratory rate, which were slightly but significantly higher in W1 patients (Table 1). Missing viral load data in Table 1 is due to some tests being performed outside the hospital or not reported in the patient’s medical file by the laboratory. During W1, 11/341 patients (3.2%) were treated with dexamethasone growing to 404/662 patients (61%) in W2 (p < 0.0001). Dexamethasone was started 14 days (13–22 d) and 1 day (0–1 d) after hospital admission in W1 and W2, respectively (p < 0.0001). Remdesivir was given in 2/341 W1 patients (0.6%) and 88/662 W2 patients (13.3%) (p < 0.0001), starting at 16 days (11–21 d) and 1 days (0–1 d) after admission (p = 0.009). Hydroxychloroquine was given in 249/341 W1 patients (73%) and 0/662 W2 patients (0%) (p < 0.0001). During W1, 87/341 patients (26%) were transferred from the ward to ICU, whereas 128/662 (19%) during W2 (p = 0.024) (Table 1).
In the ward, 6/341 W1 patients (2%) and 79/662 W2 patients (12%) received HFNO (p < 0.0001) for 4 days (2—6 d) and 3 days (2–7 d) (p = 0.97). Two of six W1 patients (33%) and 34/79 W2 patients (43%) receiving HFNO in the ward were transferred to ICU (p = 0.64). The two W1 patients who were transferred to the ICU required immediate intubation and MV.
During W1 and W2, 87/341 (26%) and 128/662 (19%) (p = 0.024) patients were admitted to ICU (Fig. 1, Supplement Digital Content, http://links.lww.com/CCX/A643). On ICU admission, median SOFA score was 6 (3–7) in W1 and 4 (3–6) in W2 (p = 0.02), but Pao2/Fio2 ratio was 108 (84–134) and 91 (71–116), respectively (p = 0.027) (Table 2). On ICU admission, lymphocyte count, CRP, d-dimer, and bilirubin values were statistically significantly higher in W1 than in W2 patients (Table 2).
TABLE 2.
Patients Characteristics on ICU Admission During the First and second Waves of Coronavirus Disease 2019 Pandemic
| Variables | First Wave of the COVID-19 Pandemic | Number of Patients | Second Wave of the COVID-19 Pandemic | Number of Patients | p |
|---|---|---|---|---|---|
| Age (yr) | 65 (54–71) | 87 | 67.0 (59.5–74.0) | 128 | 0.078 |
| Body mass index (kg/m2) | 30.8 (26.3–34.6) | 80 | 28.1 (25.1–32.2) | 117 | 0.0056 |
| Chronic kidney disease | 19 (21.8%) | 87 | 25 (19.5%) | 128 | 0.68 |
| Diabetes | 43 (49.4%) | 87 | 59 (46%) | 128 | 0.63 |
| Hypertension | 40 (46%) | 87 | 88 (72.1%) | 122 | 0.0008 |
| Sequential Organ Failure Assessment score | 6 (3–7) | 85 | 4 (3–6) | 120 | 0.021 |
| C-reactive protein (mg/L) | 173 (113–244) | 86 | 111 (63.9–197) | 127 | 0.0005 |
| Bilirubin (mg/dL) | 0.75 (0.54–1.13) | 86 | 0.65 (0.48–0.89) | 126 | 0.036 |
| Creatinine (mg/dL) | 0.97 (0.78–1.36) | 86 | 0.87 (0.70–1.23) | 127 | 0.11 |
| d-dimer (ng/mL) | 1,895 (922–4,717) | 67 | 1,280 (603–2,043) | 118 | 0.0018 |
| Ferritin (µg/L) | 1,968 (746–4,461) | 40 | 1,327 (612–2,725) | 83 | 0.21 |
| Lymphocyte count (103/mm3) | 0.80 (0.56–1.11) | 86 | 0.66 (0.42–0.96) | 127 | 0.010 |
| Platelet count (103/mm3) | 205 (163-268) | 86 | 233 (159–305) | 127 | 0.14 |
| Pao2/Fio2 (mm Hg) | 108 (84.4–134) | 56 | 91.2 (70.5–116) | 80 | 0.028 |
| Mean arterial pressure (mm Hg) | 66.3 (59.7–73.7) | 87 | 71.0 (64.5–77.8) | 128 | 0.027 |
COVID-19 = coronavirus disease 2019.
Quantitative variables are reported as median (Q1–Q3) and categorical variables as number (%). Kruskal-Wallis and Fisher exact tests are used as appropriate.
During the first week of ICU stay, CRP, creatinine, and bilirubin reached higher maximum values in W1 than W2 patients (Table 3). During ICU stay, 16/87 (18%) and 102/128 (80%) received HFNO (p < 0.0001) with a flow of 36 L/min (30–50 L/min) and 50 L/min (46–60 L/min) (p < 0.001) and 69/87 (79%) and 56/128 (44%) were mechanically ventilated (p < 0.001), respectively. The delay between ICU admission and intubation was longer in W2 than in W1. Median length of ICU stay, median 28-day ICU-free days, and median MV duration were shorter in W2 than W1 (Table 3). Finally, fewer W2 patients received RRT, antibiotic, and vasopressors (Table 3).
TABLE 3.
Characteristics of the ICU Stay During the First and Second Waves of the Coronavirus Disease 2019 Pandemic
| Variables | First Wave of the COVID-19 Pandemic | Number of Patients | Second Wave of the COVID-19 Pandemic | Number of Patients | p |
|---|---|---|---|---|---|
| Hospitalization LOS (d) | 8 (5–15) | 341 | 8 (5–13) | 662 | 0.030 |
| Hospitalization LOS, ICU patients (d) | 22 (11–39) | 87 | 13 (9–23) | 128 | 0.0028 |
| 30-d ICU mortality | 25 (29%) | 87 | 31 (24%) | 128 | 0.61 |
| LOS in ICU (d) | 13.8 (5.1–26.8) | 87 | 6.3 (2.8–11.0) | 128 | <0.0001 |
| ICU-free days on day 28 | 0 (0–14) | 87 | 17.5 (0.0–23.0) | 128 | <0.0001 |
| MV | 69 (79%) | 87 | 56 (44%) | 128 | <0.001 |
| MV (d) | 17 (10–26) | 69 | 10.0 (5.0–17.5) | 56 | 0.010 |
| MV-free days on day 28 | 1 (0–12) | 69 | 0.5 (0.0–19.0) | 56 | 0.32 |
| Prone position (number of patients) | 55 (63%) | 87 | 57 (45%) | 128 | 0.007 |
| Prone position (d) | 3 (0–6) | 87 | 0 (0–3) | 128 | 0.0018 |
| HFNO in ICU | 16 (18%) | 87 | 102 (80%) | 128 | <0.0001 |
| HFNO in ICU (d) | 2 (1–2) | 5 | 5 (3–7) | 59 | 0.0027 |
| Delay between MV and ICU admission (d) | 0.08 (0.02–0.78) | 69 | 1.12 (0.04–3.05) | 56 | 0.0019 |
| Renal replacement therapy | 16 (18.4%) | 87 | 8 (6.3%) | 128 | 0.0055 |
| Norepinephrine use | 64 (73.6%) | 87 | 51 (39.8%) | 128 | <0.0001 |
| Enoxaparin use | 83 (95.4%) | 87 | 117 (91.4%) | 128 | 0.26 |
| Enoxaparin daily dose (mg) | 70 (51–87) | 87 | 76 (60–96) | 128 | 0.082 |
| Extracorporeal membrane oxygenation | 2 (2.3%) | 87 | 3 (2.3) | 125 | 0.98 |
| Antibiotic use | 78 (89.7%) | 87 | 85 (66.4%) | 128 | <0.0001 |
| C-reactive protein (mg/L) (max) | 284.8 (193–354) | 87 | 183.2 (109–269) | 128 | <0.0001 |
| d-dimer (ng/mL) (max) | 1,768 (967–4,262) | 78 | 1,916.0 (1,172–4,689) | 124 | 0.24 |
| Platelet count (103/mm3) (minimum values observed during the first 7 d in ICU) | 167 (131–208) | 87 | 185 (136–238) | 128 | 0.16 |
| Creatinine (mg/dL) (max) | 1.46 (1.01–2.36) | 87 | 1.14 (0.89–1.75) | 128 | 0.0053 |
| Bilirubine (mg/dL) (max) | 1.16 (0.75–1.90) | 87 | 0.85 (0.68–1.20) | 128 | 0.0007 |
COVID-19 = coronavirus disease 2019, HFNO = high-flow nasal oxygen, LOS = length of stay, max = maximum values observed during the first 7 d in ICU, MV = mechanical ventilation.
Quantitative variables are reported as median (Q1–Q3) and categorical variables as number (%). Kruskal-Wallis and Fisher exact tests are used as appropriate.
During W1 and W2, 1/57 (2%) and 46/90 (51%) patients were discharged from ICU while on HFNO (p < 0.001).
During W1 and W2, 30-day mortality was 74/341 (22%) and 99/662 (15%) (p = 0.007) and 30-day ICU mortality was 25/87 (29%) and 31/128 (24%) (p = 0.61), respectively. The overall survival is presented by Kaplan-Meier curves (Fig. 2, Supplement Digital Content, http://links.lww.com/CCX/A644, and Fig. 3, Supplement Digital Content, http://links.lww.com/CCX/A645). In ICU, 18/25 (72%) and 24/31 (77%) patients died while a withholding or a withdrawing therapy has been previously decided, respectively, during W1 and W2 (p = 0.76). Cox regression models showed that independent risk factors for 30-day mortality of hospitalized patients were: W1, higher age, higher CRP and serum creatinine values, and lower platelet count and Spo2 values on hospital admission (Table 4). Cox regression models showed that independent risk factors for 30-day mortality of ICU patients were: higher age, SOFA score on ICU admission, and lower lymphocyte count on ICU admission (Table 5).
TABLE 4.
Risk Factors for 30-d Mortality of Hospitalized Coronavirus Disease 2019 Patients During the First and Second Waves of the Coronavirus Disease 2019 Pandemic
| Parameter | n | Univariate | Multivariate (n = 960) | ||
|---|---|---|---|---|---|
| HR (95% CI) | pa | HR (95% CI) | p | ||
| Wave (s) | 1,003 | 0.75 (0.56–1.02) | 0.066 | 0.69 (0.51–0.94) | 0.019 |
| Age (yr) | 1,003 | 1.06 (1.05–1.08) | <0.0001 | 1.08 (1.06–1.09) | <0.0001 |
| Body mass index (kg/m2) | 766 | 0.99 (0.96–1.02) | 0.43 | ||
| Gender (male) | 1,003 | 1.28 (0.93–1.75) | 0.13 | ||
| Chronic kidney disease (yes) | 1,003 | 1.67 (1.15–2.43) | 0.0069 | ||
| Diabetes (yes) | 1,003 | 0.81 (0.60–1.103) | 0.18 | ||
| Hypertension (yes) | 1,003 | 1.33 (0.98–1.81) | 0.070 | ||
| Total bilirubin on hospital admission (mg/dL, Ln) | 976 | 1.06 (0.82–1.36) | 0.68 | ||
| C-reactive protein on hospital admission (mg/L) | 984 | 1.002 (1.0001–1.003) | 0.053 | 1.002 (1.001–1.004) | 0.0064 |
| Creatinine on hospital admission (mg/dL, Ln) | 982 | 1.68 (1.32–2.13) | <0.0001 | 1.87 (1.39–2.50) | <0.0001 |
| d-dimers on hospital admission (µg/L, Ln) | 849 | 1.20 (1.04–1.38) | 0.012 | ||
| Procalcitonin on hospital admission (µg/L, Ln) | 847 | 1.32 (1.20–1.45) | <0.0001 | ||
| Ferritin on hospital admission (µg/L, Ln) | 266 | 1.30 (0.99–1.69) | 0.053 | ||
| Fibrinogen on hospital admission (g/L) | 965 | 0.93 (0.85–1.01) | 0.098 | ||
| Lactate dehydrogenase on hospital admission (U/L, Ln) | 927 | 1.95 (1.40–2.71) | <0.0001 | ||
| Lymphocyte count on hospital admission (103/mm3, Ln) | 981 | 0.75 (0.58–0.97) | 0.027 | ||
| Platelet count on hospital admission (103/mm3, Ln) | 981 | 0.71 (0.50–0.99) | 0.044 | 0.68 (0.48–0.97) | 0.033 |
| Oxygen saturation on hospital admission (%) | 998 | 0.98 (0.96–0.99) | 0.0010 | 0.97 (0.95–0.98) | <0.0001 |
| Respiratory frequency on hospital admission (beats/min, Ln) | 900 | 1.82 (1.25–2.66) | 0.0018 | ||
| Heart rate on hospital admission (beats/min, Ln) | 1,003 | 1.002(0.99–1.01) | 0.70 | ||
| Mean arterial pressure on hospital admission (mm Hg, Ln) | 1,003 | 0.99 (0.98–0.995) | 0.0031 | ||
| Temperature on hospital admission (°C, Ln) | 991 | 0.89 (0.74–1.08) | 0.24 | ||
HR = hazard ratio, Ln = Neperian logarithm.
Cox regression models are used to assess risk factors of mortality. All the variables with p < 0.1 were selected for the multivariate model.
aAdjusted for wave.
TABLE 5.
Risk Factors for 30-d Mortality of ICU Cornavirus Disease 2019 Patients During the First and second Waves of the Cornavirus Disease 2019 pandemic
| Variables | n | Univariate | Multivariate (n = 199) | ||
|---|---|---|---|---|---|
| HR (95% CI) | pa | HR (95% CI) | p | ||
| Wave (s) | 215 | 1.03 (0.61–1.75) | 0.91 | 0.68 (0.37–1.24) | 0.21 |
| Age (yr) | 215 | 1.06 (1.03–1.10) | 0.0001 | 1.06 (1.03–1.10) | 0.0004 |
| Body mass index (kg/m2) | 197 | 0.99 (0.95–1.05) | 0.97 | ||
| Gender (male) | 215 | 1.37 (0.73–2.55) | 0.33 | ||
| Chronic kidney disease (yes) | 215 | 1.80 (1.03–3.16) | 0.040 | ||
| Diabetes (yes) | 215 | 0.79 (0.46–1.33) | 0.37 | ||
| Hypertension (yes) | 215 | 1.03 (0.59–1.80) | 0.91 | ||
| Sequential Organ Failure Assessment on ICU admission | 205 | 1.13 (1.05–1.21) | 0.0007 | 1.13 (1.03–1.25) | 0.0083 |
| Pao2/Fio2 on ICU admission (mm Hg, Ln) | 133 | 0.64 (0.27–1.53) | 0.32 | ||
| Mean arterial pressure on ICU admission (mm Hg) | 215 | 0.96 (0.94–0.98) | 0.0007 | ||
| Lymphocytes on ICU admission (103/mm3, Ln) | 212 | 0.57 (0.40–0.83) | 0.0033 | 0.57 (0.38–0.85) | 0.0057 |
| C-reactive protein on ICU admission (mg/L) | 213 | 1.00 (0.99–1.003) | 0.73 | ||
| d-dimers on ICU admission (µg/L, Ln) | 185 | 1.05 (0.80–1.37) | 0.73 | ||
| Creatinine on ICU admission (mg/dL, Ln) | 213 | 1.40 (0.96–2.04) | 0.085 | ||
| Total bilirubin on ICU admission (mg/dL, Ln) | 212 | 0.90 (0.58–1.42) | 0.66 | ||
| Platelet count on ICU admission (103/mm3, Ln) | 213 | 0.60 (0.36–1.004) | 0.052 | ||
| Ferritin on ICU admission (µg/L, Ln) | 123 | 1.12 (0.82–1.55) | 0.47 | ||
| Troponin on ICU admission (ng/L, Ln) | 209 | 1.26 (1.12–1.43) | 0.0003 | ||
HR = hazard ratio, Ln = Neperian logarithm.
Cox regression models are used to assess risk factors of mortality. All the variables with p < 0.1 were selected for the multivariate model.
aAdjusted for wave.
DISCUSSION
In our single tertiary-care hospital, we could compare two cohorts of consecutive patients during the first two waves of the COVID-19 pandemic in Belgium. Our results showed W2 patient management drastically differed from W1. W2 patients were more frequently treated with dexamethasone and remdesivir, and received HFNO more frequently and for longer, in both the ward and ICU. No patients received hydroxychloroquine during W2. Due to the local risk of ICU overcrowding, during W2, 33 patients were transferred to other hospitals. All these modifications in patient management were associated with a decrease in: the number of patients admitted to ICU from the ward, ICU length of stay, the number of patients under MV, the length of MV, RRT, vasopressor use, antibiotic use, and biological inflammatory syndrome on ICU admission and during ICU stay. This global change in patient management during W2 was also associated with a decrease in 30-day mortality.
Changes in patient management strategy from W1 to W2 were justified by several studies during W1 (7). In particular, during W1, Belgian and international recommendations limited HFNO to the ICU and a rate of 30 L/min. Similarly, Belgian and international recommendations restricted steroid use in W1, and hydroxychloroquine was given to almost all COVID-19 patients (2, 10, 12). In contrast, the Recovery study and subsequent meta-analysis led to early steroid use in W2 (4, 5). Furthermore, remdesivir was also used for selected patients based on the results of Beigel et al (6), and hydroxychloroquine use was discontinued due to lack of evidence (7, 13, 14). HFNO use was expanded to the wards and a 60 L/min limit authorized (9), where personal protection equipment and COVID-19 patient isolation measures were used to reduce aerosol contamination risk (15). Finally, to avoid severe ICU overcrowding, regular patient transfers to other hospitals were introduced, where overcrowding and strained ICU capacity is associated with increased ICU mortality (16).
These “bundles” of major patient management modifications were associated with a series of cascading changes. Patients were admitted to the ICU later or less often, and had a shorter stay. Consequently, W2 patients transferred to the ICU had more severe respiratory dysfunction on ICU admission, whereas their inflammatory syndrome was less severe. Table 1 shows biological values were the same on hospital admission during W1 and W2. However, Table 2 shows on ICU admission, lymphocytes, CRP, d-dimers, and bilirubin were lower during W2 than W1. Table 3 shows during the first week of ICU stay, CRP, bilirubin, and creatinine values increased less during W2 than W1. Once in the ICU, HFNO duration was longer and MV was less frequent and shorter. Finally, patients were anticipatively transferred to other hospitals to mitigate critical care burden.
In this study, most of the W2 patients were already treated by dexamethasone on ICU admission. Steroid use can explain the lower CRP values during ICU stay and the lower d-dimer values on ICU admission. This latter biomarker has been linked to severity of acute respiratory distress syndrome (ARDS) (17). Another marker of severity, SOFA score, was also lower in W2 patients, because they were frequently intubated later than the first day of ICU despite a lower Pao2/Fio2 ratio due to wider use of HFNO. The use of organ supports was less frequent in W2 patients, but this change did not translate into a lower mortality rate during W2 in the multivariate analysis.
There are few studies comparing management strategy during COVID-19 pandemic (18). Contou et al (19) compared patient management and outcome in ICU patients during W1 and W2, where early steroid use featured in W2 was minimal in W1. In addition, they gave intermediate or full dose of thromboprophylaxis in half of the patients during W1 and all patients during W2. Unlike this study, they did not use antiviral treatment. Their main changes between W1 and W2 were associated with an increase in the delay between ICU admission and intubation, a decrease in the number of patients requiring MV, a decrease in the number of thrombotic events, and a decrease in d-dimer values. However, they did not observe a decrease in the ICU length of stay, duration of MV, RRT, antibiotic use, or vasopressor support. Furthermore, although SOFA score and patient age were similar to our present study, their proportion of mechanically ventilated and RRT patients was higher, which may explain their higher mortality rate. However, in both studies, ICU mortality did not decrease between the two periods, despite a decrease in d-dimer values.
Dennis et al (20) measured temporal survival trends in COVID-19 patients requiring critical care. From March 1 to June 27, 2020, they observed that 30-day ICU survival increased markedly over the study from 58% to 84%, attributing changes to the introduction of effective treatments as part of the Recovery study, improved physician understanding of the disease process, and falling critical care burden. Similarly, Bravata et al (21) examined whether COVID-19 mortality among patients in the general ward was associated with ICU strain. Hospital mortality varied over time from 25% to 12.5% according to strains on critical care capacity. COVID-19 ICU demand was associated with increased mortality for patients with critical COVID-19, supporting the idea strained critical care capacity was associated with increased COVID-19 ICU mortality.
Evidence-driven modification of our global patient management strategy between W1 and W2 decreased hospital, but not ICU, mortality. This result is explained, in part, by preventing degradation of COVID-19 patients, thus reducing the need for ICU. Indeed, the ICU mortality rate of W1 and W2 patients was the same as patients with non-COVID-19–induced ARDS (22). However, mortality is not the only outcome to be considered. In this pandemic, the use of critical care resources and long-term physical and functional outcomes of survivors must also be considered. Any decrease in ICU admission, MV duration, and length of stay reduces local overcrowding and may, therefore, improve the quality of care (16). It is anticipated that less RRT, shorter ICU length of stay, and shorter MV duration decrease the need of post ICU rehabilitation, the occurrence of post ICU syndrome, and therefore facilitate fast recovery of the patients.
The main limitation of this study is its single-center design. However, the sample size was large, and this single-center design was able to ensure good homogeneity in patient management during the two periods of the study. The single-center prospective design of the study also resulted in few missing data thanks to the electronic medical file used in our hospital. Finally, while only a single center, the changes in patient care were evidence-driven and relatively binary in the significant increase or decrease in use of a treatment approach (e.g., steroid use) between the waves. Thus, the present results would be very likely to generalize and transfer to other centers. Another limitation is the lack of data about the number of patients who were not admitted in ICU during both waves due to poor prognosis. However, our triage decisions did not change with time, so we could expect this did not influence the present results.
CONCLUSIONS
In a single tertiary hospital, the main therapeutic changes between W1 and W2 of the COVID-19 pandemic were eliminating use of hydroxychloroquine, use of remdesivir for patients under oxygen support and sick for less than 5 days, early use of steroids for hypoxemic patients including patients under MV, unrestrictive use of HFNO for hypoxemic patients, and transfer of patients to other geographical areas in case of overcrowding. These changes were associated with a decrease in 30-day mortality, ICU admission, length of ICU stay, and organ support.
ACKNOWLEDGMENTS
We thank our colleagues who have worked so hard to provide excellent clinical care during this global pandemic: Jean-Luc Canivet, Grace Kisoka, Nathalie Layios, Didier Ledoux, Paul Massion, Philippe Morimont, Gilles Parzibut, Sonia Piret, Sebastien Robinet, Patricia Wiesen, and all the medical teams, the nurses, physiotherapists, and psychologists of the ICU and of the ward.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
All authors contributed to the study conception and design. Data collection was performed by Dr. Lambermont, Dr. Cavalleri, Dr. Gillet, Ms. Seidel, and Ms. Thys. Analysis was performed by Dr. Lambermont, Ms. Seidel, Dr. Chase, Dr. Misset, and Dr. Delanaye. The first draft of the article was written by Dr. Lambermont, and all authors commented on previous versions of the article. All authors read and approved the final article.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Jamil S, Mark N, Carlos G, et al. : Diagnosis and management of COVID-19 disease. Am J Respir Crit Care Med. 2020; 201:P19–P20 [DOI] [PubMed] [Google Scholar]
- 2.Sanders JM, Monogue ML, Jodlowski TZ, et al. : Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020; 323:1824–1836 [DOI] [PubMed] [Google Scholar]
- 3.Arabi YM, Fowler R, Hayden FG: Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020; 46:315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19. N Eng J Med. 2021; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sterne JAC, Murthy S, Diaz JV, et al. ; WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020; 324:1330–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020; 383:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhazzani W, Evans L, Alshamsi F, et al. : Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First update. Crit Care Med. 2021; 49:e219–e234 [DOI] [PubMed] [Google Scholar]
- 8.Murthy S, Gomersall CD, Fowler RA: Care for critically ill patients with COVID-19. JAMA. 2020; 323:1499–1500 [DOI] [PubMed] [Google Scholar]
- 9.Gaeckle NT, Lee J, Park Y, et al. : Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020; 202:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhazzani W, Møller MH, Arabi YM, et al. : Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandromme M, De Pauw R, Serrien B, et al. ; Sciensano: Covid-19 Clinical Hospital Surveillance Report. Available at: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Hospital_epidemiology_Part_1.pdf. Accessed February 1, 2021
- 12.Russell CD, Millar JE, Baillie JK: Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020; 395:473–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalcanti AB, Zampieri FG, Rosa RG, et al. : Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020; 383:2041–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horby P, Mafham M, Linsell L, et al. : Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020; 383:2030–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haymet A, Bassi GL, Fraser JF: Airborne spread of SARS-CoV-2 while using high-flow nasal cannula oxygen therapy: Myth or reality? Intensive Care Med. 2020; 46:2248–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagshaw SM, Wang X, Zygun DA, et al. : Association between strained capacity and mortality among patients admitted to intensive care: A path-analysis modeling strategy. J Crit Care. 2018; 43:81–87 [DOI] [PubMed] [Google Scholar]
- 17.Grasselli G, Tonetti T, Protti A, et al. ; Collaborators. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: A multicentre prospective observational study. Lancet Respir Med. 2020; 8:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prescott HC, Levy MM: Survival from severe coronavirus disease 2019: Is it changing? Crit Care Med. 2021; 49:351–353 [DOI] [PubMed] [Google Scholar]
- 19.Contou D, Fraissé M, Pajot O, et al. : Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021; 25:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dennis JM, McGovern AP, Vollmer SJ, et al. : Improving survival of critical care patients with coronavirus disease 2019 in England: A national cohort study, March to June 2020. Crit Care Med. 2021; 49:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs Hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellani G, Laffey JG, Pham T, et al. ; LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


