Abstract
PURPOSE
The incidence of symptomatic brain metastasis at diagnosis in non–small-cell lung cancer (NSCLC) is 5%-10%, and up to 40% develop during the disease course. There is a paucity of data supporting the role of brain imaging at diagnosis in asymptomatic cases particularly from resource-constraint settings. Here, we present our experience of mandatory baseline brain imaging with contrast-enhanced computed tomography (CECT) scans of all patients with NSCLC.
MATERIALS AND METHODS
This was a prospective observation study of patients with NSCLC with mandatory baseline brain CECT and a CNS examination. All histology proven patients with NSCLC diagnosed between January 2018 and October 2019 were included irrespective of stage.
RESULTS
A total of 496 patients were enrolled. The median age was 57 years (range, 23-84) with majority being males (75%) and smokers (66%). The prevalence of epidermal growth factor receptor mutations and anaplastic lymphoma kinase fusions was 33.4% and 12%, respectively. Brain imaging leads to upstaging in 7% cases. The prevalence of brain metastases was 21% (n = 104), with half being asymptomatic (51%). Factors associated with higher proportion of brain metastasis were young age (≤ 40 years), adenocarcinoma histology, poor Eastern Cooperative Oncology Group performance status (3 and 4), and high neutrophil-lymphocyte ratio (NLR) (> 2.5). After a median follow-up of 10.8 months (95% CI, 7.33 to 12.73), the median overall survival was 7.46 versus 12.76 months (hazard ratio 0.67; 95% CI, 0.46 to 0.96; P = .03) in patients with and without brain metastases, respectively. On multivariate analyses, high NLR and molecular graded prognostic assessment affected the overall survival significantly.
CONCLUSION
In our study, 21% of patients had brain metastasis at diagnosis detected with a mandatory baseline brain imaging with CECT. NLR and molecular graded prognostic assessment are significant predictors of survival in patients with brain metastasis.
INTRODUCTION
Lung cancer has been one of the most common cancers in the world for the last several decades. As per GLOBOCAN 2018, it is the most commonly diagnosed cancer (11.6%, 2.1 million new cases) and the leading cause of cancer death (18.4%, 1.8 million deaths).1 Non–small-cell lung cancer (NSCLC) constitutes about 85% of all lung cancers. About 60%-70% of patients with NSCLC have either stage III B or stage IV disease at presentation.2 The prevalence of brain metastasis at presentation is 15%-20%,3,4 and up to 40% eventually develop during its disease course.5 In about 25% of cases, brain is the first site of disease recurrence.6 The established risk factors for developing brain metastases are adenocarcinoma histology, positive driver mutation status, advanced nodal status, advanced tumor stage, and younger age.6,7 Brain metastases are associated with significant morbidity, mortality, and impaired quality of life. The available therapeutic approaches include whole brain radiotherapy (WBRT), surgery, stereotactic radiosurgery (SRS), chemotherapy/tyrosine kinase inhibitors (TKIs), and symptomatic or supportive treatment.5,8
CONTEXT
Key Objective
In this study, we evaluated the role of mandatory brain imaging using contrast-enhanced computed tomography (CECT) in patients with non-small cell lung cancer.
Knowledge Generated
With mandatory CECT brain at baseline, we could detect brain metastasis in 21% of patients, and half of them were asymptomatic. It resulted in over staging in 7% of patients. Neutrophil-lymphocyte ratio and molecular GPA (graded prognostic assessment) could significantly predict survival in patients with brain metastasis.
Relevance
Our study is particularly relevant in resource constraint settings and support that CECT based brain imaging can help in diagnosis and appropriate stratification of patients with brain metastasis.
The advancement in the diagnosis and better systemic control of extracranial disease have resulted in increased incidence of brain metastases in recent times.9 The baseline use of brain imaging in asymptomatic patients is a topic of conflict.10 A significant fraction of asymptomatic brain metastasis may be missed by neurologic examination alone in patients with lung cancer.4 Although NCCN recommends brain imaging, a contrast magnetic resonance imaging (MRI) is preferred, from stage II onward. However, recommendations for baseline brain imaging particularly in patients with advanced NSCLC who are asymptomatic for brain metastases are weak and controversial.11–13 There is a paucity of data regarding the exact frequency, risk factors, and clinicopathologic and molecular correlation of asymptomatic and symptomatic brain metastasis at presentation. Performing MRI for all the patients may not be feasible in resource-constraint settings like ours. Although contrast-enhanced computed tomography (CECT) is considered optional imaging modality for this purpose, data on its use as a screening modality to detect brain metastasis are sparse. In this prospective observational study, we aimed to assess the frequency of brain metastases detected by mandatory CECT in patients with NSCLC at the time of diagnosis and their associated clinicopathologic and molecular variables, and effect on survival outcome.
MATERIALS AND METHODS
It was a single-center prospective observational study conducted between January 2018 and July 2019, and patients were followed for a minimum of 6 months at an interval of 2 months. We included treatment-naive patients of age 18-75 years with a histopathologic diagnosis of NSCLC. Patients who have been treated outside before referral to our center or with renal failure defined as creatinine clearance < 30 mL/min were excluded. All patients underwent complete physical examination and comprehensive neurologic assessment. At baseline, a CECT head was performed to evaluate brain metastases and leptomeningeal involvement along with the extracranial staging evaluation. In view of financial constraints and logistics at our center, computed tomography (CT) was included as an imaging modality rather than MRI. Patients who had MRI or a positron emission tomography (PET), which included a contrast CT of brain already done before referral, were included in the study, and CECT was not repeated. All the images were reviewed by our radiology team. All patients were staged according to AJCC (8th ed). Extracranial staging was done either by PETCT or CECT. Epidermal growth factor receptor (EGFR) testing was done by polymerase chain reaction, or anaplastic lymphoma kinase (ALK) testing was done by IHC D5F3 on Ventana staining platform (Immunohistochemistry). Molecular graded prognostic assessment (molGPA) (20) was done, which is a composite score of variables such as age, number of brain metastases, extracranial metastases, Karnofsky performance scale, EGFR, or ALK gene status, and the score ranges from 0 to 4. Neutrophil lymphocyte ratio (NLR) was calculated by manual peripheral blood smear examination, and the cutoff level for high NLR was taken as ≥ 2.5 (16). The clinicopathologic, treatment, and molecular status details were collected. Patients were followed from the date of enrollment until last date of follow-up or death, whichever was earlier and those who stopped attending clinic were contacted on telephone. The data were censored on December 31, 2019. Overall survival was calculated from date of enrollment until the date of death or last contact when patient was known to be alive. Study protocol was approved by Institute Ethics Committee.
Study protocol was approved by Institute Ethics Committee with effect from January 31, 2018, Ref No. IECPG-570/20.12.12.2017,RT—42/31.1.2018, and all subjects have given their written informed consent before their inclusion. The research was conducted in accordance with the World Medical Association Declaration of Helsinki. Consent has been taken by the patients for the publication.
Statistical Analysis
The descriptive statistics were used to assess the baseline parameters. Overall survival was calculated by Kaplan-Meier method. Chi-square test was used to assess the correlation of variables with brain metastases. Logistics univariate and multivariate analyses were done by Cox proportion hazard model for evaluating factors affecting the survival. STATA version 13 (Stata Statistical Software: Release 13; StataCorp LP, College Station, TX) was used for all the statistical analysis.
RESULTS
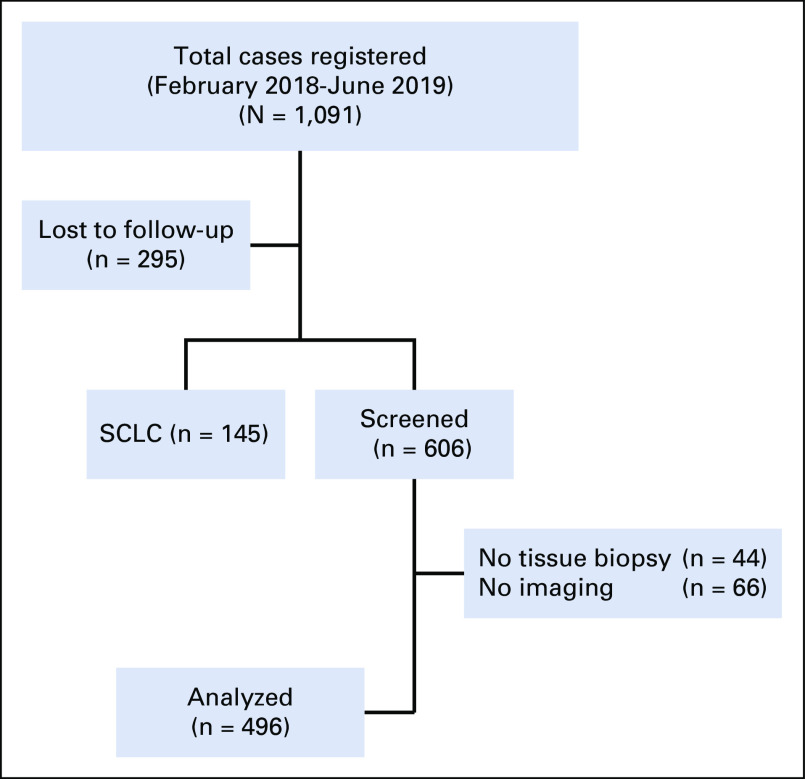
A total of 1,091 patients were registered at our lung cancer clinic during January 2018 to October 2019. Small-cell lung cancer was diagnosed in 145 patients, and 295 were lost to follow-up before complete evaluation. Eventually, 606 patients were screened of which 44 patients were excluded because of lack of a confirmed tissue diagnosis, and brain imaging could not be performed in 66 patients because of various reasons (20 had lost to follow-up, 24 did not underwent imaging despite advising, and 22 did not give consent). Finally, 496 patients were eligible for analysis as depicted in Figure 1.
FIG 1.
Flowchart depicting screening and final inclusion of the study patients. SCLC, small-cell lung cancer.
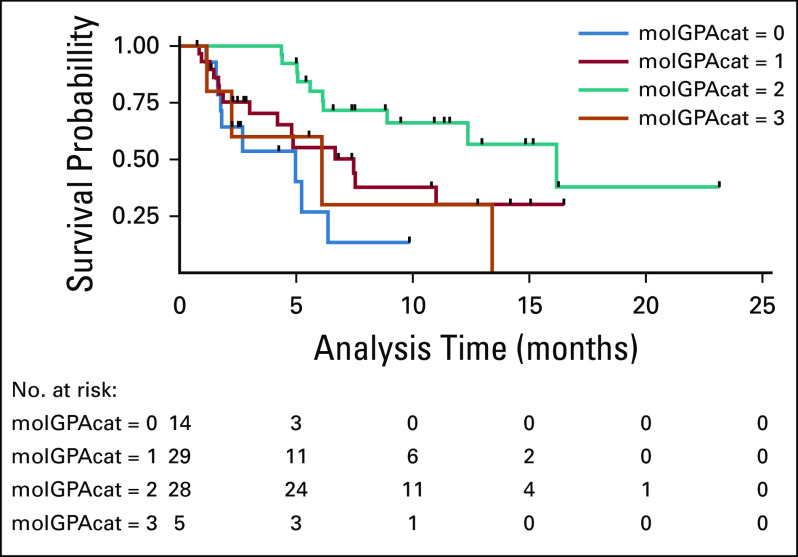
The median age was 57 years (range, 23-84) with the male predominance (75%, n = 374). Smokers constituted 66% (n = 326). Most patients (79%, n = 396) had good Eastern Cooperative Oncology Group performance status (ECOG PS) 1-2. The most common histopathology was adenocarcinoma (64%, n = 315) followed by squamous (27%, n = 134). EGFR mutation status was done in 81% (n = 283) of adenocarcinoma including NSCLC not otherwise specified, and a mutation was detected in 98 (33.4%) cases. The most common mutations were exon 19 deletion (62%, n = 61) and L858R (exon 21) (30%, n = 30). The ALK gene rearrangement status was available in 73% (n = 265) of adenocarcinoma and NSCLC not otherwise specified, with a positivity rate of 12% (n = 33). Molecular testing was not available in all the patients because of various reasons like tissue inadequacy and other logistics. Staging workup was done predominantly by CECT, and only 8.3% (n = 45) had staging by PETCT. On extracranial staging, patients with stage I were II (0.4%), stages IIA and IIB in 8 (1.6%) and 10 cases (2%) respectively, stages IIIA, IIIB, and IIIC in 37 (7%), 66 (13%), and 41 cases (8%), respectively, and stage IV disease in 332 cases (64%). Brain imaging leads to upstaging in 7% (n = 36) of cases, mostly from stage III (6%, n = 32) followed by stage II (1%, n = 4). Of patients who were upstaged, about 16 were asymptomatic for brain metastases. Baseline characteristics are described in Table 1. The imaging modality used for brain imaging was CECT (83%, n = 412) and MRI (17%, n = 84) as depicted in Table 1.
TABLE 1.
Baseline Characteristics of Patients (N = 496)
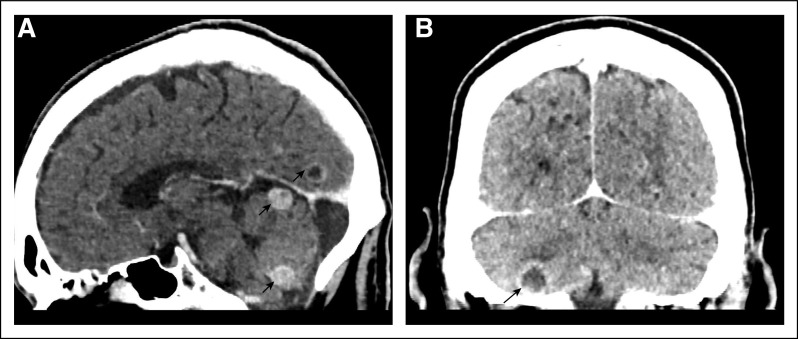
Brain metastases were detected in 104 cases (21%), and half of them (51%, n = 53) were asymptomatic. CECT was the modality in 51 (49%) patients, and MRI in 53 patients (51%). The CNS lesions were solitary in 41% (n = 43), 2-4 in number in 19% (n = 20), and > 4 in 38% (n = 40) and leptomeningeal disease in 1% (n = 1). Nearly half (52%, n = 54) of the lesions were supratentorial. Imaging characteristics were perilesional edema, enhancement, midline shift, and hemorrhage in 82% (n = 86), 68% (n = 71), 18% (n = 19), and 2% (n = 2) of cases, respectively. Few representative CECT images are shown in Figures 2A and 2B. Among the various strategies used, the CNS-directed and systemic therapy was used in 45% (n = 45), only systemic therapy in 32% (n = 33), and 27% (n = 28) did not receive any therapy. WBRT was given in 41% (n = 43) of patients, of which 53% (n = 23) received before starting systemic therapy. Among patients with brain metastases, 12 of 26 EGFR-positive (46%), 8 of 13 ALK-positive (61%), and 22 of 66 mutation-negative or unknown (33%) received WBRT. Four patients (one ALK-positive and three mutation-negative) underwent surgical resection of brain metastasis.
FIG 2.
Contrast enhanced computed tomography brain imaging showing (A) a well-defined round enhancing lesions in cerebellum and ring enhancing lesion in occipital lobe and (B) a well-defined ring enhancing lesion in the right inferior cerebellar region (indicated by arrows).
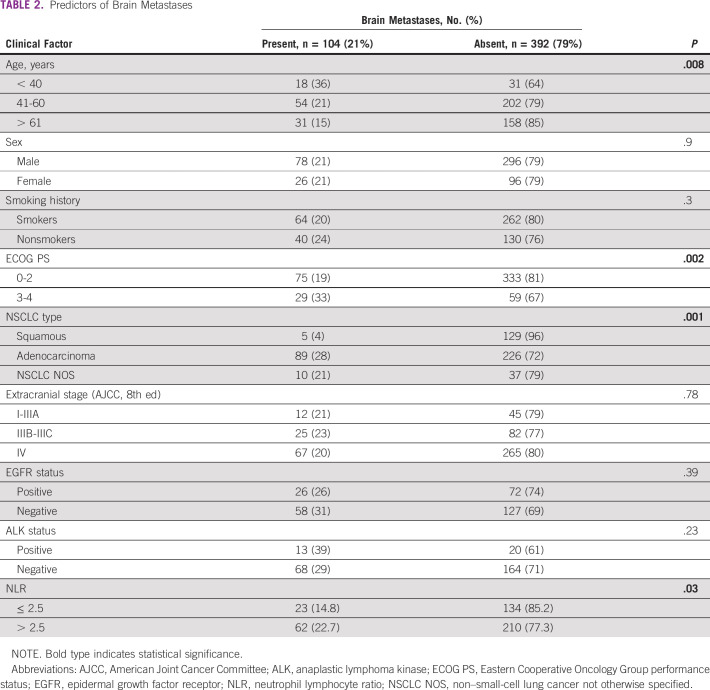
The factors predictive for brain metastases were younger age (≤ 40 years) (P = .008), adenocarcinoma histology (P = .001), ECOG PS 3-4 (P = .002), and high neutrophil lymphocyte ratio (NLR > 2.5) (P = .03) as depicted in Table 2. Although EGFR- and ALK-mutated patients had numerically higher frequency of brain metastasis as compared with wild type, the differences were not statistically significant.
TABLE 2.
Predictors of Brain Metastases
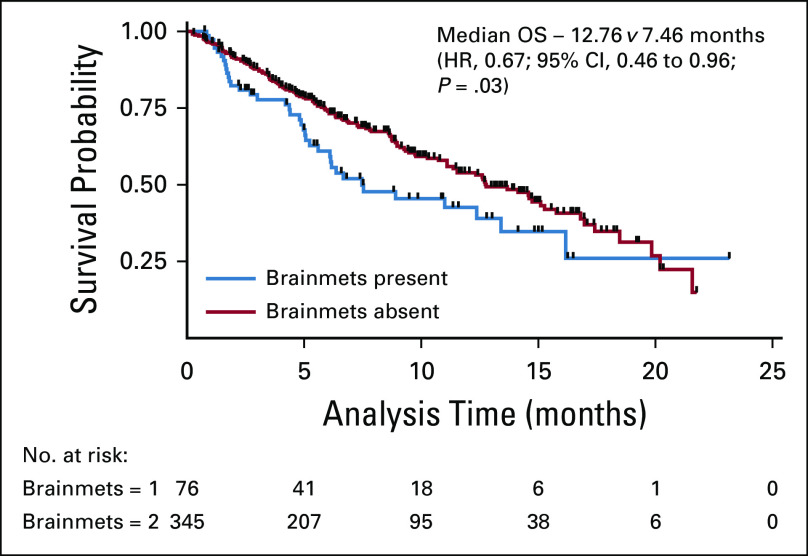
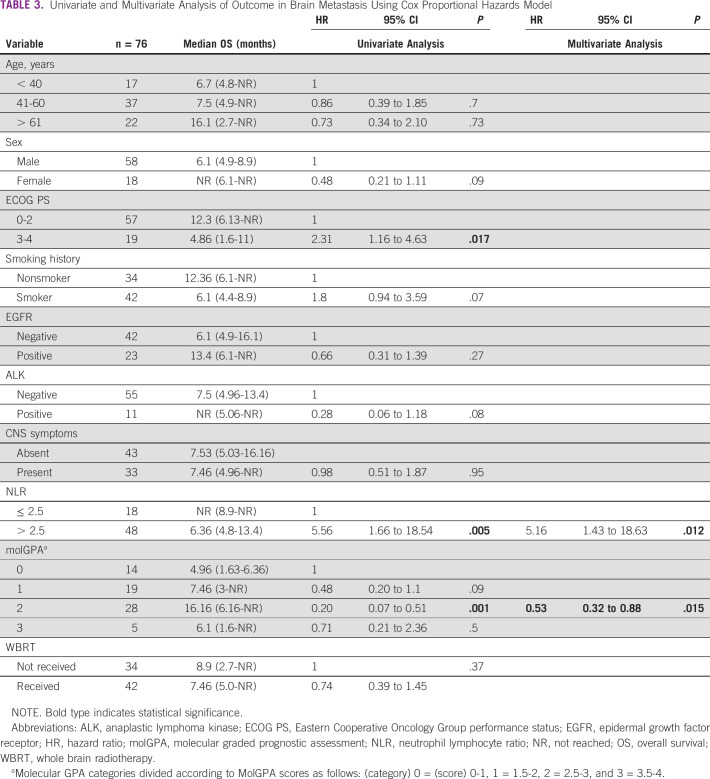
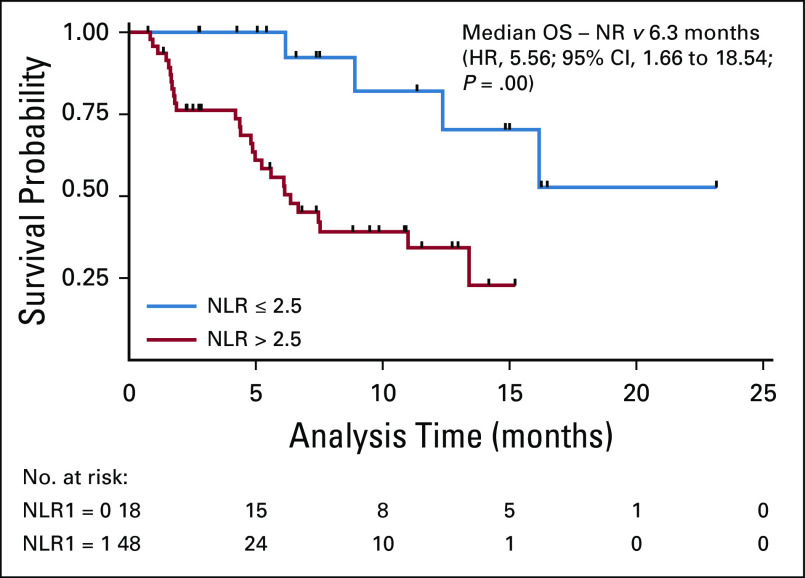
Patients who had not received any treatment were excluded from survival analysis. After a median follow-up of 10.8 months (95% CI, 7.33 to 12.73), the median overall survival was 7.46 versus 12.76 months (hazard ratio [HR] 0.67; 95% CI, 0.46 to 0.96; P = .03) in patients with and without brain metastases, respectively (Fig 3). Univariate analyses using Cox proportional hazards model for factors predicting survival in patient with brain metastases revealed that ECOG PS 3-4 (HR 2.31; 95% CI, 1.16 to 4.63; P = .017), NLR (> 2.5) (HR 5.56; 95% CI, 1.66 to 18.54; P = .005), and molGPA (HR 0.2; 95% CI, 0.07 to 0.51; P = .001) significantly affected the overall survival, as depicted in Table 3. On multivariate analyses, high NLR (HR 5.16; 95% CI, 1.43 to 18.63; P = .03) and molGPA (HR 0.53; 95% CI, 0.32 to 0.88; P = .012) remained significant as depicted in Table 3. There was no difference in survival of patients presenting with or without CNS symptoms. Figures 3 and 4 show the overall survival difference according to NLR and molGPA.
FIG 3.
OS with and without brain metastases. HR, hazard ratio; OS, overall survival.
TABLE 3.
Univariate and Multivariate Analysis of Outcome in Brain Metastasis Using Cox Proportional Hazards Model
FIG 4.
OS in brain metastasis stratified by NLR. HR, hazard ratio; NLR, neutrophil-lymphocyte ratio; NR, not reached; OS, overall survival.
DISCUSSION
The incidence of brain metastasis at the time of diagnosis detected predominantly with CECT in our study was 21%, with half of the cases being asymptomatic. Similar to our study, Kim et al3 in a prospective cohort of 183 patients with lung cancer using limited MRI brain reported brain metastases in 20.8% cases of which 81% were asymptomatic. In a retrospective analysis, Jena et al14 have reported a higher rate of brain metastasis (35%) using MRI and 46% of these patients were asymptomatic. Mandatory brain imaging at the time of diagnosis in NSCLC tends to pick up more asymptomatic metastasis, and the frequency varies as per the sensitivity of the modality used. The screening modality used in our study was CECT with the limited use of MRI, which might have affected the sensitivity in detection of brain metastases. Schoenmaekers et al11 have previously reported additional detection of brain metastasis in 4.7% of cases by MRI where CECT was negative. This would probably imply that CT scan is a good option as an initial screening modality as part of baseline staging where MRI could not be done in view of logistics and MRI can be preferred in highly suspicious CT-negative patients. It would have practical implications in resource-limited settings where MRI is not routinely available or affordable. European Society for Medical Oncology clinical practice guidelines do recognize CECT as an optional strategy for detection of brain metastasis in advanced disease but prefer MRI for patients undergoing curative intent treatment.12,13
Asymptomatic detection eventually results in upstaging of disease, which happened in 7% of patients in our study. Almost similar rates of upstaging have been reported by others.3,4 Early detection of brain metastases may result in timely initiation of locoregional therapies, which may affect the survival.
The majority of brain lesions in our study were solitary (41%), which was expected by routine imaging. The probability of offering local ablative-like SRS or surgery is higher in solitary brain lesion, which eventually may result in better survival outcome.15 However, in our cohort, none of these patients could undergo SRS or surgery because of various logistic reasons.
The predictive factors for brain metastases in our study were younger age (< 40 years), adenocarcinoma histology, poor ECOG PS, and high NLR (≥ 2.5). The adenocarcinoma histology is an established risk factor for brain metastases.7 It has been previously reported that the younger age group have an increased propensity for brain metastases similar to our study7 and timely management in this cohort might improve survival. In our study, poor ECOG PS has been associated with increased risk for brain metastases, which might indicate a more extensive stage or could be vice versa as the patients with brain metastases have a poor general condition overall. NLR is marker of systemic inflammatory response. Meta-analyses have shown adverse effect on overall survival in various solid malignancies with high NLR.16 It could also reflect higher disease burden or aggressive disease biology.17 In our study, it was found to be associated with brain metastases as reported earlier in another study.18 Higher nodal burden has also been reported to have a higher incidence of brain metastases in early and locally advanced disease. However, we could not demonstrate this association possibly because of smaller numbers of early and locally advanced cases in our cohort.
The frequency of brain metastases in ALK-positive patients was higher (39%) as compared with ALK-negative patients (29%); however, it was not significant statistically. We did not observe any difference in frequency of brain metastases in EGFR-mutated patients, perhaps owing to smaller numbers. This is in contrary to the studies, which have demonstrated higher incidence of brain metastases in EGFR- and ALK-mutated patients.19,20 Another study, which included majority patients from India, reported approximately two-fold higher incidence of brain metastasis in patients harboring EGFR mutations.21
The survival of patients with brain metastasis remains dismal. The median overall survival of 7.46 and 12.76 months in patients with and without brain metastases in our study is lower compared with that in other studies.3,21 It could be possibly due to the fact that it was not an interventional study and the treatment arms were not supervened. Even WBRT could be received by only 41% of the patients with brain metastasis, and the use of CNS effective newer TKIs was very limited.
The stratification of the patients with brain metastases using validated molGPA score can help the clinician for the prognosis and for therapeutic decisions. We have used this score in our Indian population, which helps in better categorization and intensification of treatment from the very beginning. Sperduto et al22 stratified patients with brain metastases using molGPA score, but the median survival was remarkable (nearly 4 years) in those with adenocarcinoma histology and a molGPA score of 3.5-4 compared with ours, which might be due to the lower use of targeted therapy, and only half received combined systemic and CNS-directed therapy because of the logistics and patient preferences. The patients who had an NLR of ≤ 2.5 had significantly better median survival in our study (Fig 5). A similar study had shown a better overall survival in low NLR cohort.23
FIG 5.
OS in brain metastases stratified by molGPA. molGPA categories divided according to molGPA scores as follows: (category) 0 = (score) 0-1, 1 = 1.5-2, 2 = 2.5-3, and 3 = 3.5-4. molGPA, molecular graded prognostic assessment; OS, overall survival.
The strengths of our study are being the largest prospective study from resource-limited setting and demonstrating feasibility of CECT-based screening for brain metastasis. The limitations include the limited use of MRI in CECT-negative patients. As it was an observational study, treatment interventions like SRS and WBRT were not controlled based on the symptoms or number of brain metastases. WBRT could be delivered only in 41% of patients because of logistics, which might have an impact on survival. It was a single-center study. There was limited use of CNS-effective TKIs because of high cost.
In conclusion, mandatory baseline brain imaging with CECT leads to detection of brain metastases in up to 21%, with half being asymptomatic. The disease is upstaged in 7% after brain imaging. molGPA helps in appropriate risk stratification for a better patient care and management.
Prabhat Singh Malik
Research Funding: BMS India Pvt Ltd
Deepam Pushpam
Stock and Other Ownership Interests: Caplin Point Laboratories
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part as an abstract at European Lung Cancer Congress (ELCC), April 10-13, 2019, Geneva, Switzerland.
DATA SHARING STATEMENT
The authors declare that the data are available in Microsoft Office Sheets format and can be verified by the authors when needed. Patient personal details have been deidentified.
AUTHOR CONTRIBUTIONS
Conception and design: Prabhat Singh Malik, Sushmita Pathy
Provision of study material or patients: Gundu Naresh, Prabhat Singh Malik, Deepali Jain
Collection and assembly of data: Gundu Naresh, Prabhat Singh Malik, Sachin Khurana, Deepam Pushpam, Mukesh Yadav
Data analysis and interpretation: Gundu Naresh, Prabhat Singh Malik, Deepam Pushpam, Vinod Sharma, Deepali Jain, Sushmita Pathy
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Prabhat Singh Malik
Research Funding: BMS India Pvt Ltd
Deepam Pushpam
Stock and Other Ownership Interests: Caplin Point Laboratories
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship Mayo Clin Proc 83584–5942008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SY, Kim JS, Park HS, et al. Screening of brain metastasis with limited magnetic resonance imaging (MRI): Clinical implications of using limited brain MRI during initial staging for non-small cell lung cancer patients J Korean Med Sci 20121–1262005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochstenbag MMH, Twijnstra A, Hofman P, et al. MR-imaging of the brain of neurologic asymptomatic patients with large cell or adenocarcinoma of the lung. Does it influence prognosis and treatment? Lung Cancer Amst Neth 42189–1932003 [DOI] [PubMed] [Google Scholar]
- 5.Moro-Sibilot D, Smit E, de Castro Carpeño J, et al. Non-small cell lung cancer patients with brain metastases treated with first-line platinum-doublet chemotherapy: Analysis from the European FRAME study Lung Cancer 90427–4322015 [DOI] [PubMed] [Google Scholar]
- 6.Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: Risk factors analysis Cancer 95605–6122002 [DOI] [PubMed] [Google Scholar]
- 7.Gaspar LE, Chansky K, Albain KS, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: A retrospective review by the Southwest Oncology Group J Clin Oncol 232955–29612005 [DOI] [PubMed] [Google Scholar]
- 8.Elaimy AL, Mackay AR, Lamoreaux WT, et al. Multimodality treatment of brain metastases: An institutional survival analysis of 275 patients. World J Surg Oncol. 2011;9:69. doi: 10.1186/1477-7819-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciardi S, de Marinis F.Multimodality management of non-small cell lung cancer patients with brain metastases Curr Opin Oncol 2286–932010 [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman O.Is routine baseline brain imaging needed for all newly diagnosed non-small-cell lung cancer patients? J Comp Eff Res 8569–5752019 [DOI] [PubMed] [Google Scholar]
- 11.Schoenmaekers JJAO, Dingemans A-MC, Hendriks LEL.Brain imaging in early stage non-small cell lung cancer: Still a controversial topic? J Thorac Dis 10S2168–S21712018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 29iv192–iv2372018suppl 4 [DOI] [PubMed] [Google Scholar]
- 13.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up Ann Oncol 28iv1–iv212017suppl 4 [DOI] [PubMed] [Google Scholar]
- 14.Jena A, Taneja S, Talwar V, et al. Magnetic resonance (MR) patterns of brain metastasis in lung cancer patients: Correlation of imaging findings with symptom J Thorac Oncol 3140–1442008 [DOI] [PubMed] [Google Scholar]
- 15.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial Lancet Oncol 101037–10442009 [DOI] [PubMed] [Google Scholar]
- 16.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Okita R, Saisho S, et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J Surg Oncol. 2015;13:291. doi: 10.1186/s12957-015-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh YW, Choi J-H, Ahn MS, et al. Baseline neutrophil–lymphocyte ratio is associated with baseline and subsequent presence of brain metastases in advanced non-small-cell lung cancer. Sci Rep. 2016;6:38585. doi: 10.1038/srep38585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino M, Soejima K, Mitsudomi T.Brain metastases in oncogene-driven non-small cell lung cancer Transl Lung Cancer Res 8S298–S3072019suppl 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin D-Y, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas J Thorac Oncol 9195–1992014 [DOI] [PubMed] [Google Scholar]
- 21.Bhatt VR, D'Souza SP, Smith LM, et al. Epidermal growth factor receptor mutational status and brain metastases in non–small-cell lung cancer J Glob Oncol 3208–2172017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol 3827–8312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedrés S, Torrejon D, Martínez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer Clin Transl Oncol 14864–8692012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data are available in Microsoft Office Sheets format and can be verified by the authors when needed. Patient personal details have been deidentified.