Abstract
Background
Urolithiasis is a common and often recurrent problem in dogs.
Objective
To evaluate trends in urolith composition in dogs and to assess risk factors for urolithiasis, including age, breed, sex, neuter status, urolith location, and bacterial urolith cultures.
Sample Population
A total of 10 444 uroliths and the dogs from which they were obtained.
Methods
The laboratory database at the UC Davis Gerald V. Ling Urinary Stone Analysis Laboratory was searched for all urolith submissions from dogs between January 2006 and December 2018. Mineral type, age, breed, sex, neuter status, urolith location, and urolith culture were recorded. Trends were evaluated and variables compared to evaluate risk factors.
Results
Calcium oxalate (CaOx) and struvite‐containing uroliths comprised the majority of all submissions from dogs, representing 47.0% and 43.6%, respectively. The proportion of CaOx‐containing uroliths significantly decreased from 49.5% in 2006 to 41.8% in 2018 (P = .006), with no change in the proportion of struvite‐containing urolith submissions. Cystine‐containing uroliths comprised 2.7% of all submissions between 2006 and 2018 and a significant nonlinear increase in this mineral type occurred over time (1.4% of all submissions in 2006 to 8.7% in 2018; P < .001). Of all cystine‐containing uroliths, 70.3% were from intact male dogs. Age, breed, and sex predispositions for uroliths were similar to those previously identified.
Conclusions and Clinical Importance
Although calcium oxalate‐ and struvite‐containing uroliths continue to be the most common uroliths submitted from dogs, a decrease in the proportion of CaOx‐containing uroliths and an increase in the proportion of cystine‐containing uroliths occurred during the time period evaluated.
Keywords: calcium oxalate, canine, cystine, struvite, urate, urolithiasis
Abbreviations
- 95% CI
95% confidence interval
- CaOx
calcium oxalate
- MUC
Minnesota Urolith Center
- OR
odds ratio
- UTI
urinary tract infection
1. INTRODUCTION
Urolithiasis is a common and often recurrent problem in dogs. Changing urolith trends in dogs have been recognized over the last several decades, with increases in the proportion of calcium oxalate (CaOx)‐containing uroliths and decreases in the proportion of struvite‐containing uroliths reported from various laboratories offering quantitative urolith analysis. 1 , 2 , 3 , 4 , 5 , 6 However, data from the Minnesota Urolith Center (MUC) in 2019 showed a higher prevalence of struvite‐containing uroliths compared with CaOx‐containing uroliths. 7 Urate‐containing uroliths are typically the third most common type reported in dogs. 1 , 4 , 6 Cystine and silica‐containing uroliths continue to be submitted to laboratories much less frequently, 1 , 2 , 3 , 6 and geographical differences in cystine submissions have been reported. 2 The Canadian Veterinary Urolith Centre identified significantly increased proportions of cystine uroliths from 1998 to 2014 4 and cystine was the third most common urolith type submitted to the MUC in 2019 in dogs. 7 Submissions of silica‐containing uroliths were the third most common at a laboratory in Mexico City, representing 13.3% of submissions, and suggesting a potential environmental role in silica urolith formation. 8 Ongoing evaluation of trends from different institutions can detect shifts in the proportions of various uroliths in dogs, identify potential risk factors, and allow evaluation of effectiveness of preventative strategies.
Risk factors for urolithiasis in dogs vary based on urolith composition, and include breed, age, sex, neuter status and in some cases, presence of urinary tract infection (UTI), especially with struvite‐containing uroliths and urease‐producing bacteria. 1 , 2 , 3 , 4 Calcium oxalate, urate, and cystine‐containing uroliths are more common in male dogs, whereas female dogs are predisposed to struvite‐containing uroliths because of their increased risk of UTI. 1 , 2 , 3 , 4 , 9
The purpose of our study was to determine the mineral composition of uroliths submitted from dogs to the UC Davis Gerald V. Ling Urinary Stone Analysis Laboratory between 2006 and 2018. Trends for mineral composition of uroliths and risk factors for development of uroliths in dogs during the past 12 years, including age, breed, sex, neuter status, urolith location, and urolith culture results, were evaluated.
2. MATERIALS AND METHODS
2.1. Study population
A computer‐assisted search of the urolith submissions at the Gerald V. Ling Urinary Stone Analysis Laboratory at the School of Veterinary Medicine, University of California, Davis was performed to identify all uroliths from dogs sent to the laboratory between January 1, 2006 and December 31, 2018. Information in the database was obtained from a standard form submitted with each urolith. Year of urolith submission, age, breed, sex, neuter status, mineral composition of the urolith, and location within the urinary tract and bacterial culture results of the urolith were recorded, if available. Individual dogs may have had >1 urolith submission during this time period and each submission was considered separately.
2.2. Mineral analysis
To determine the mineral composition of uroliths, every visibly distinct layer of each urolith was initially analyzed by use of the oil‐immersion method of optical crystallography with polarizing light microscopy, as previously described. 6 Infrared spectroscopy, x‐ray diffractometry, high pressure liquid chromatography, scanning electron microscopy using energy‐dispersive x‐rays, and electron probe microanalysis were used as adjunctive analytic methods when needed to definitively identify minerals or elements in the urolith. For each urolith, each mineral present in any amount was recorded and the percentage of each mineral within each layer was estimated by calculating a mean value for the crystal counts obtained by microscopic examination of 5 to 10 microscopic slide preparations using the oil immersion method of optical crystallography. The amount of specific minerals in individual uroliths varied from 1% to 100%. For uroliths with distinct layers and variations in the thickness of each distinct layer, it was not possible to accurately designate a single mineral as being predominant. For the purpose of consistency in reporting technique for the laboratory, uroliths composed of a mixture of ≥2 minerals, whether the multiple mineral components were contained in distinct layers or uniformly dispersed throughout the urolith, were reported as containing that mineral component. 6 , 10 In our study, they were counted once for each mineral detected in the urolith and, as a result, totals of minerals detected in uroliths was >100%. Calcium oxalate included CaOx monohydrate, dihydrate, or both and urate included uric acid, salts of uric acid (eg, ammonium urate), or both. With regard to location, the upper urinary tract was considered to include the kidney and ureter and the lower urinary tract included the bladder and urethra. Voided uroliths were considered separately. Because uroliths were retrieved from >1 location in some dogs, totals of location are higher than total number of uroliths containing each mineral type.
Urolith culture was performed as described previously. 11 Briefly, each urolith was washed 4 times with 50 mL of sterile saline for 10 seconds. Using sterile rongeurs, the urolith was cracked and the core scraped into a sterile mortar. The core then was ground after addition of 1 mL of sterile saline and a 0.1 mL aliquot of this mixture was used for aerobic bacterial culture.
2.3. Statistical analysis
Descriptive statistics were used with individual urolith types for age, breed, sex, neuter status, location, and urolith culture results. Because totals of minerals detected in uroliths was higher than the numbers of uroliths submitted, the totals for age, sex, and breed add to >100% when all mineral types are considered. The χ2 test was used to compare association between categorical variables (age group, breed, sex, neuter status, urolith location, and urolith bacterial culture results) for each urolith type. The χ2 tests for trend additionally were used to evaluate trends in urolith submissions over time and trends in age categories for individual urolith types. Logistic regression analysis was used to evaluate breed predilection for each mineral type by comparing breed distributions in dogs with individual urolith types to mixed breed dogs with that same mineral type (reference category). Results were reported as odds ratios (ORs) and 95% confidence intervals (95% CIs). For breed analysis, breeds with an OR > 3.5 and P < .001 or an OR < 0.29 and P < .001 were considered to be at clinically relevant higher or lower risk of developing particular mineral types compared to mixed breeds. Furthermore, OR for breeds previously reported to have an increased or decreased risk also were included in the results to provide meaningful past comparisons from the database. Breeds with a clinically relevant higher or lower risk of developing certain mineral types were not reported in results if there were <10 dogs of that breed in the Gerald V. Ling Urinary Stone Analysis Laboratory database between January 1, 2006 and December 31, 2018. P values <.05 were considered significant.
3. RESULTS
A total of 10 444 urolith submissions from dogs were analyzed at the Gerald V. Ling Urinary Stone Analysis Laboratory between January 1, 2006 and December 31, 2018. Of 10 444 submissions, 5747 (55.0%) contained >1 mineral. Data regarding sex of the dogs was missing for 40 urolith submissions, data on the age of the dog was missing for 222 urolith submissions, and data regarding breed was missing for 5 urolith submissions.
3.1. Calcium oxalate‐ and struvite‐containing uroliths
Of the 10 444 urolith submissions, 4912 (47.0%) were CaOx‐containing uroliths. A significant nonlinear decrease in the proportion of CaOx‐containing urolith submissions occurred over time, from 49.5% of all urolith submissions in 2006 to 41.8% in 2018 (P = .006). A total of 4552 struvite‐containing uroliths were submitted, representing 43.6% of all submissions from dogs. No significant change in the proportion of struvite‐containing uroliths occurred over time (P = .6), with struvite‐containing uroliths being 42.5% (468/1100) of total submissions in 2006 and 42.7% (241/565) in 2018 (Figure 1).
FIGURE 1.
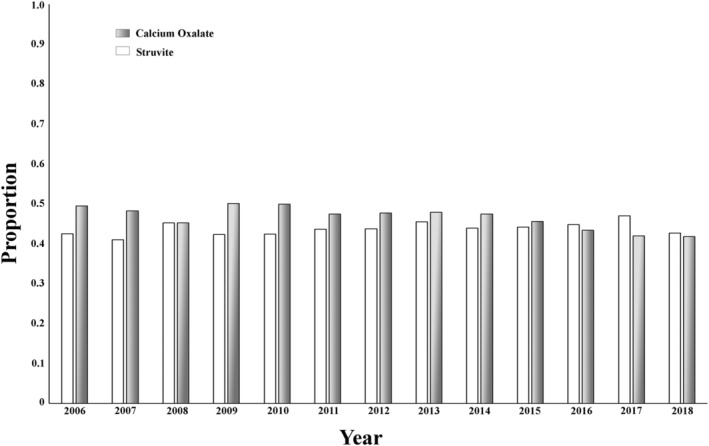
Proportion of all urolith submissions that were calcium oxalate‐containing (gray bars) and struvite‐containing (white bars) uroliths obtained from dogs and submitted for analysis from 2006 through 2018. There was a significant nonlinear decrease in the proportion of CaOx‐containing urolith submissions, (P = .006) while no significant change in the proportion of struvite‐containing uroliths was noted (P = .6). CaOx, calcium oxalate
Of all CaOx‐containing uroliths, 79.5% were from dogs ≥7 years of age. The proportion of CaOx‐containing uroliths among dogs ≥7 years of age (3818/6356; 60.0%) was significantly higher compared to the proportion of Ca‐Ox uroliths among dogs <7 years of age (986/3866; 25.5%; OR, 4.4; 95% CI, 4.0‐4.8; P < .001). Conversely, of all struvite‐containing uroliths submitted, 49.8% were from dogs <7 years of age. The proportion of struvite‐containing uroliths was significantly higher among dogs <7 years of age (2226/3866; 57.6%) compared to the proportion of struvite‐containing uroliths among dogs ≥7 years of age (2243/6356; 35.3%; OR, 2.5; 95% CI, 2.3‐2.7; P < .001; Figure 2). Almost 2/3 (62.0%) of all struvite‐containing uroliths occurred in dogs <4 years of age.
FIGURE 2.
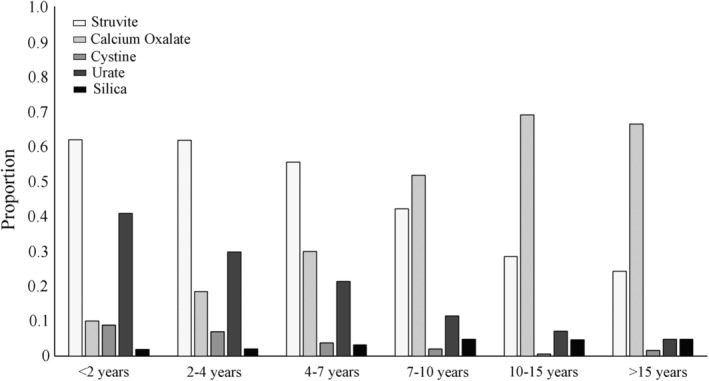
Proportion of the most common canine urolith types (CaOx, struvite, urate, cystine, and silica) categorized by age groups. CaOx, calcium oxalate
Associations between mineral types and sex of dogs are presented in Table 1. Calcium oxalate‐, urate‐, and cystine‐containing uroliths were more common in male dogs whereas female dogs had higher risk for struvite‐containing urolithiasis. The proportion of CaOx‐containing uroliths was significantly higher in neutered male dogs (3517/4737; 74.2%) compared to the proportion of CaOx‐containing uroliths in intact male dogs (410/863; 47.5%; OR, 3.2; 95% CI, 2.7‐3.7, P < .001). No significant difference was found for female dogs for neuter status for CaOx‐containing uroliths (P = .2).
TABLE 1.
Distribution of sex within each urolith mineral type for 10 444 uroliths
| Female | Male | Odds ratio (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| CaOx | 1296 | 24.8 | 3927 | 75.2 | 6.4 (5.8‐6.9) | <.001 |
| Struvite | 3682 | 81.1 | 856 | 18.9 | 18.2 (16.5‐20.1) | <.001 |
| Urate | 859 | 55.0 | 702 | 45.0 | 1.5 (1.4‐1.7) | <.001 |
| Cystine | 5 | 1.8 | 273 | 98.2 | 49.2 (19.6‐134.9) | <.001 |
| Silica | 54 | 13.0 | 360 | 87.0 | 6.0 (4.5‐8.2) | <.001 |
| Apatite | 2901 | 77.6 | 839 | 22.4 | 8.7 (7.9‐9.5) | <.001 |
| Brushite | 74 | 52.1 | 68 | 47.9 | — | .15 |
| Xanthine | 6 | 18.8 | 26 | 81.2 | 3.7 (1.5‐10.0) | .003 |
Note: Odds ratios and P values relate to the proportion of the mineral (compared to all other minerals) within each sex. Odds ratios are presented based on the sex with higher risk.
Abbreviations: 95% CI, 95% confidence interval; CaOx, calcium oxalate.
Breeds considered at clinically relevant higher risk (OR, > 3.5; P < .001) of developing CaOx‐containing uroliths compared to mixed breed dogs were the Standard Poodle (OR, 6.8; 95% CI, 2.6‐17.7), Pomeranian (OR, 6.1; 95% CI, 4.4‐8.4), Brussels Griffon (OR, 5.8; 95% CI, 2.2‐15.4), Miniature Pinscher (OR, 4.6; 95% CI, 2.4‐8.7), Lhasa Apso (OR, 3.9; 95% CI, 2.9‐5.4), and Maltese Terrier (OR, 3.7; 95% CI, 2.7‐5.1). The Miniature Schnauzer (OR, 2.5; 95% CI, 2.0‐3.0; P < .001) and Bichon Frise (OR, 1.4; 95% CI, 1.2‐1.7; P < .001) also had increased risk of developing CaOx‐containing uroliths. The Dalmatian (OR, 0.07; 95% CI, 0.03‐0.1) was the only breed at clinically relevant lower risk (OR, < 0.29; P < .001) of developing CaOx‐containing uroliths. No breeds were at clinically relevant higher risk of developing struvite‐containing uroliths compared to mixed breed dogs. However, 2 breeds had a clinically relevant lower risk of developing struvite‐containing uroliths: Dalmatian (OR, 0.08; 95% CI, 0.04‐0.2) and Miniature Pinscher (OR, 0.2; 95% CI, 0.09‐0.4).
3.2. Urate
The number of urate‐containing uroliths was 1560 (14.9% of all submissions). No significant change in the proportion of urate‐containing uroliths occurred between 2006 and 2018 (P = .08; Figure 3). The proportion of urate‐containing uroliths among dogs <7 years of age (961/3866; 24.9%) was significantly higher compared to the proportion of urate‐containing uroliths among dogs ≥7 years of age (588/6356; 9.3%; OR, 3.3; 95% CI, 2.9‐3.6; P < .001; Figure 2). Of urate‐containing uroliths, 62.0% were from dogs <7 years of age (961/1549; 62.0%) and urate‐containing uroliths most often were submitted from dogs that were between 4 and 7 years of age (554/1549, representing 35.7% of dogs with urate‐containing uroliths).
FIGURE 3.
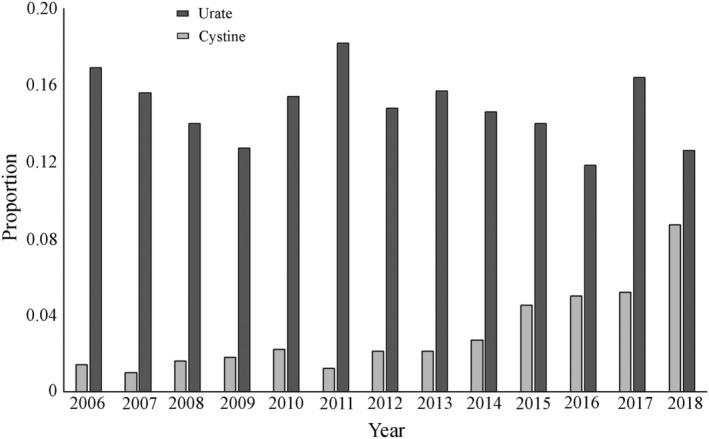
Proportion of urate‐ and cystine‐containing uroliths submitted from dogs between 2006 and 2018. A significant increase in the proportion of cystine‐containing uroliths was noted over time (P < .001)
The proportion of urate‐containing uroliths was significantly higher in male intact dogs (136/863; 15.7%) compared to the proportion of urate‐containing uroliths in neutered male dogs (566/4737; 11.9%; OR, 1.4; 95% CI, 1.1‐1.7; P = .002). No significant difference was found for female dogs based on neuter status (P = .2).
Breeds at a clinically relevant higher risk (OR, > 3.5; P < .001) for developing urate‐containing uroliths compared with mixed breed dogs were: Dalmatian (OR, 85.5; 95% CI, 44.6‐163.7), Bulldog (breed not further specified; OR, 7.9; 95% CI, 4.2‐14.7), English Bulldog (OR, 5.6; 95% CI, 3.9‐8.0), and Pitbull Terrier (OR, 3.7; 95% CI, 2.1‐6.5).
3.3. Cystine
Cystine‐containing uroliths represented 279 of 10 444 uroliths submissions from dogs (2.7%) over the time period studied. A significant nonlinear increase in the proportion of cystine‐containing uroliths submitted over time (P < .001) occurred, from 1.4% (15/1100) in 2006 to 8.7% (49/565) in 2018 (Figure 3).
Cystine‐containing uroliths were submitted more often from male dogs than female dogs (Table 1) and, of the 273 male dogs with cystine‐containing uroliths, 192 (70.3%) were reported as intact males (OR, 16.7; 95% CI, 12.5‐20; P < .001). A significant nonlinear trend for change in the proportion of male intact dogs with cystine‐containing uroliths occurred over time (P = .02; Figure 4). No significant difference in the proportion of cystine‐containing uroliths between female spayed and female intact dogs was observed (P = .5). The breeds of 5 female dogs with cystine‐containing uroliths were Pitbull Terrier (2), mixed breed (2), and Newfoundland (1).
FIGURE 4.
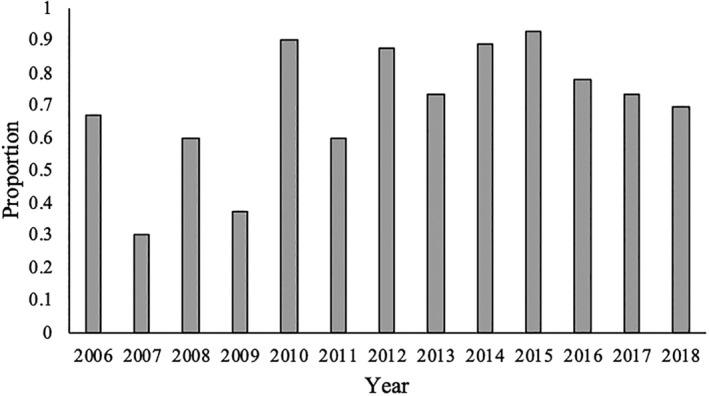
Proportion of male dogs with cystine‐containing uroliths that were male intact between 2006 and 2018. A significant nonlinear trend for changes in the proportion of male intact dogs with cystine‐containing uroliths was noted during this time (P = .02)
Of cystine‐containing uroliths, the proportion of cystine‐containing uroliths was significantly higher among dogs <7 years (190/3866; 4.9%) compared to the proportion of cystine‐containing uroliths among dogs ≥7 years of age (83/6356; 1.3%; OR, 3.9; 95% CI, 3.0‐5.1; P < .001). Of dogs with cystine‐containing uroliths, 60.8% (166/273) were between 2 and 7 years of age. A significant decrease in the proportion of cystine‐containing uroliths compared to other urolith types was observed with increasing age (P < .001; Figure 2).
Eight breeds were at clinically relevant higher risk of developing cystine‐containing uroliths compared to the risk of mixed breeds: Mastiff (OR, 52.7; 95% CI, 16.4‐169.5), Australian Cattle Dog (OR, 30.8; 95% CI, 11.4‐82.7), Pitbull Terrier (OR, 12.9; 95% CI, 6.6‐25.1), French Bulldog (OR, 13.8; 95% CI, 6.7‐28.5), Rottweiler (OR, 11.9; 95% CI, 4.6‐30.5), English Bulldog (OR, 10.1; 95% CI, 6.2‐16.2), and Bulldog (breed not further specified; OR, 9.1; 95% CI, 4.1‐20.4).
3.4. Silica
Of 10 444 uroliths submitted, 405 (3.9%) contained silica. The proportion of dogs with silica‐containing uroliths was significantly higher among dogs ≥7 years of age (299/6356; 4.7%) compared to the proportion of silica‐containing uroliths among dogs <7 years of age (104/3866; 2.7%; OR, 1.8; 95% CI, 1.4‐2.3; P < .001; Figure 2). Of dogs with silica‐containing uroliths, 74.2% (299/403) were ≥7 years of age.
The proportion of silica‐containing uroliths was significantly higher in neutered male dogs (323/4737; 6.8%) compared to the proportion of silica‐containing uroliths in intact male dogs (37/863; 4.3%) (OR, 1.6; 95% CI, 1.2‐2.4; P = .005). The proportion of silica‐containing uroliths was significantly higher in intact female (13/340, 3.8%) as compared with spayed female dogs (41/4464; 0.9%; OR, 4.3; 95% CI, 2.2‐8.3; P < .001).
Six breeds had clinically relevant higher risk of having silica‐containing uroliths compared to the risk in mixed breed dogs: German Shepherd (OR, 13.1; 95% CI, 7.2‐23.8), Border Collie (OR, 10.8; 95% CI, 5.2‐22.3), Pug (OR, 9.6; 95% CI, 7.3‐12.7), Australian Shepherd (OR, 9.5; 95% CI, 5.2‐17.3), Golden Retriever (OR, 5.9; 95% CI, 3.2‐10.6), and Samoyed (OR, 7.9; 95% CI, 2.9‐22.0).
3.5. Apatite
Apatite‐containing uroliths accounted for 3752/10 444 (35.9%) of all uroliths submitted from dogs. A significant nonlinear increase in the proportion of apatite‐containing uroliths occurred over time (P < .001), from 32.4% (356/1100) in 2006 to 37.7% (213/565) in 2018. Of apatite‐containing uroliths, 20/3752 (0.5%) were composed solely of apatite. In mixed uroliths, the most common coprecipitates were struvite (3175/3752; 84.6%), CaOx (935/3752; 24.9%), urate (509/3752; 13.6%), brushite (76/3752; 2.0%), silica (30/3752; 0.8%), and cystine (6/3752; 0.2%).
The proportion of apatite‐containing uroliths among dogs <7 years of age (1594/3866; 41.2%) was significantly higher compared to the proportion of apatite‐containing uroliths among dogs ≥7 years of age (2078/6356; 32.7%; OR, 1.4; 95% CI, 1.3‐1.6; P < .001). The overall proportion of dogs with apatite‐containing uroliths <7 years of age was 43.4% (1594/3672).
The proportion of apatite‐containing uroliths was significantly higher in spayed female dogs (2715/4737; 57.3%) compared to the proportion of apatite‐containing uroliths in intact female dogs (186/340; 54.7%; OR, 1.3; 95% CI, 1.02‐1.6; P = .03). No difference for male dogs based on neuter status was observed (P = .7).
3.6. Brushite
Of 10 444 urolith submissions from dogs, 141 contained brushite (1.4%). Age was known for dogs with 139 brushite‐containing uroliths and no significant difference (P = .5) in the proportion of brushite‐containing uroliths among dogs ≥7 (90/6356; 1.4%) and <7 (49/3866; 1.3%) years of age was identified. The majority (129/139; 92.1%) of brushite‐containing uroliths were submitted from dogs ≥4 years of age. No breeds were at substantially increased or decreased risk of having brushite‐containing uroliths compared with mixed breed dogs.
3.7. Xanthine
Of 10 444 uroliths, 33 (0.3%) contained xanthine. These uroliths were most often from dogs 4 to 7 years of age (11/33; 33.3%) and dogs 10 to 15 years of age (9/33; 27.3%). No significant difference in the proportion xanthine‐containing uroliths among dogs <7 years of age (18/3866; 0.5%) compared to the proportion of xanthine‐containing uroliths among dogs ≥7 years of age (17/6356; 0.3%; P = .1) was observed. Breed predispositions were not determined because of the small numbers of dogs with xanthine‐containing uroliths. Of dogs with xanthine‐containing uroliths where breed was reported, 9/25 (36.0%) were Dalmatians, 5/25 (20.0%) were mixed breed dogs and 3/25 (12.0%) were Cavalier King Charles Spaniels.
3.8. Urolith location
The most common location for CaOx‐ and struvite‐containing uroliths was the bladder (5909/6041; 97.8% and 4843/4996; 96.9%, respectively). Small numbers of each were removed from the upper urinary tract (75/6041; 1.2% for CaOx‐containing and 51/4996; 1.0% for struvite‐containing uroliths) or were voided (57/6041; 0.9% for CaOx‐containing and 102/4996; 2.0% for struvite‐containing uroliths).
Of 1750 locations recorded for urate‐containing uroliths, 96.9% (1695) were removed from the lower urinary tract, 1.4% (25) from the upper urinary tract, and 1.7% (30) were voided. The majority of cystine‐containing uroliths were removed from the lower urinary tract (371/372; 97.8%); only 2.2% (1/372) were removed from the upper urinary tract (1/372; 2.2%). Silica‐containing uroliths were removed from the upper urinary tract (5/449, 1.1%), lower urinary tract (431/449; 96.0%) or were voided (13/449; 2.9%). Of apatite‐containing uroliths where location was reported, 97.1% (4009/4127) were removed from the lower urinary tract, 1.2% (48/4127) from the upper urinary tract, and 1.7% (70/4127) were voided. Brushite‐containing uroliths most often were located in the lower urinary tract (94.4%; 170/180), with fewer removed from the upper urinary tract (2.8%; 5/180) or voided (2.8%; 5/180). Location was available for 32 xanthine‐containing uroliths, with 15.6% (5/32) submitted from the upper urinary tract and 84.4% (27/32) from the lower urinary tract (none were voided). The proportion of xanthine‐containing uroliths removed from the upper urinary tract was significantly higher than for other urolith types (OR, 16.6; 95% CI, 4.9‐44.6; P < .001).
3.9. Urolith bacterial culture results
Of 10 444 uroliths submitted, 3102 (29.7%) had aerobic bacterial urolith culture performed and of these, 784 (25.2%) were positive for growth. The most common bacteria isolated were Staphylococcus spp. (n = 587; 74.9%), Enterococcus spp. (n = 91; 11.6%), Escherichia coli (n = 54; 6.9%), Proteus spp. (n = 22; 2.8%), Pseudomonas spp. (n = 19; 2.4%), and Klebsiella spp. (n = 10; 1.3%).
Of 1435 cultured struvite‐containing uroliths, 600 were positive for growth (41.8%). Apatite‐containing uroliths were cultured 1188 times and of these, 476 (40.1%) were positive for bacterial growth. Compared with other mineral types, the proportion of positive bacterial cultures was higher in struvite‐containing (OR, 5.8; 95% CI, 4.8‐7.0; P < .001) and apatite‐containing (OR, 3.5; 95% CI, 2.9‐4.1; P < .001) uroliths.
4. DISCUSSION
Evaluation of trends in urolith submissions and risk factors for formation of different mineral types identified several important findings. Although CaOx‐ and struvite‐containing uroliths continue to be the most common mineral types submitted from dogs, the proportion of CaOx‐containing uroliths has decreased in the last 12 years. The absolute number and proportion of cystine‐containing uroliths have increased. Age, breed, and sex are similar to previously reported results for the various mineral types. 2 , 3 , 4 , 6
The small decrease in the proportion of CaOx‐containing uroliths during the time period of our study differs from previous increases reported almost worldwide, with significant increases in CaOx submissions reported in Asia and North America in 1999 vs 2010. It also contrasts with the previous trend at our laboratory from 1985 to 2006. 6 However, at the MUC, CaOx was the second most common mineral identified in 2019. 7 Several risk factors coexist for CaOx urolith formation in dogs and include diet (including moisture content, treats, and trace elements), breed, genetics, age, sex, body condition score, voiding behavior, metabolic derangements, alterations in intestinal flora, and environmental factors. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Urine of dogs with CaOx uroliths often has higher relative supersaturation of these minerals 22 and the urine calcium‐to‐creatinine ratio is significantly higher in CaOx urolith‐forming dogs compared to controls of the same breed, 14 , 23 suggesting calciuresis is an important factor. Diet is a key strategy used for prevention of CaOx urolithiasis, and a decrease in the proportion of CaOx‐containing uroliths could indicate that diets marketed for CaOx prevention, diets with increased moisture content, or both could be effective in decreasing recurrence. 24 , 25 However, dietary histories were not routinely available and were not analyzed in our study. Also, we were not able to reliably report first or recurrent episodes of CaOx urolithiasis. As a result, prospective studies to analyze the effects of various diets on CaOx recurrence would be valuable.
Given that specific dog breeds are predisposed to CaOx urolithiasis, it is possible that smaller numbers of such breeds were represented in our population, thereby decreasing the proportion of CaOx‐containing uroliths. However, this possibility seems unlikely because breed predispositions in our study were similar to those reported by others. 4 , 6 , 26 The Standard Poodle, the breed with the highest risk for CaOx‐containing uroliths in our study, has been reported as having a predisposition to CaOx urolithiasis in only 2 other studies. 4 , 5 The Miniature Schnauzer and Bichon Frise have a high risk of CaOx urolithiasis. 3 , 4 , 6 , 26 In our study, both breeds had higher risk compared to mixed breeds, but this risk was lower than that previously identified at our laboratory. 6 This difference could indicate increased awareness of CaOx urolithiasis resulting in improved breeding practices, more proactive prevention strategies, or a combination of these factors.
Similar to previous studies, middle‐aged to older dogs most often were affected by CaOx urolithiasis and male dogs were predisposed. 3 , 4 , 6 , 26 Female dogs were underrepresented, which could be because of anatomical differences allowing voiding of uroliths. Increased risk of CaOx‐containing uroliths was observed in neutered male compared with male intact dogs. This difference has been reported previously. It has been hypothesized that CaOx formation probably is not associated with hormonal differences in dogs. 26
A significant decrease in the proportion of struvite‐containing uroliths was observed from 1985 to 2006. 6 We found no significant change in the proportion of struvite‐containing uroliths from 2006 to 2018, with struvite‐containing uroliths remaining approximately 43% of total urolith submissions. The relative frequency of struvite urolith submissions to the MUC from dogs in the United Kingdom remained stable from 1997 to 2006. 3 When evaluating all struvite urolith submissions to the MUC in 2019, they exceeded those of CaOx. 7 Because dissolution of nonobstructive struvite uroliths should be attempted as the standard of care, 27 it was expected that submission of these uroliths would continue to decrease. However, large urolith burdens can be difficult to dissolve, and dissolution rates of struvite uroliths in dogs are variable, even with concurrent antimicrobial administration. 27 , 28 , 29 Furthermore, apatite, a common coprecipitate with struvite, 10 can hinder dissolution. It is unknown if submissions to our laboratory were from dogs in which dissolution had failed or if the veterinarian had not attempted medical dissolution. Female dogs continue to be overrepresented, likely because of increased risk of UTI by urease‐producing bacteria. 1 , 2 , 3 , 4 , 9 Therefore, the observation that struvite‐containing uroliths had a higher likelihood of positive bacterial cultures compared to other mineral types is not surprising.
Although apatite was the third most common mineral detected in our study and in past reports from our laboratory, pure apatite uroliths are uncommon. 6 , 30 Almost 85% of apatite was noted as coprecipitating with struvite, and less often with CaOx and urate. These data suggest management strategies specific for apatite prevention likely are not warranted.
Similar to previous studies, most dogs with struvite‐containing uroliths were young to middle‐aged. 2 , 3 , 6 No breed predispositions for struvite‐containing uroliths were identified, although other studies have identified breeds such as Bichon Frise, Miniature Schnauzer, Pekingese, Pug, and Shih‐tzu to be at increased risk. 2 , 4 , 6 Awareness of predisposed breeds by veterinarians could have resulted in medical dissolution being more likely to be prescribed when uroliths suspected to be struvite were found in these breeds. Risk factors for apatite‐containing uroliths were similar to those for struvite because of its common coprecipitation with this mineral. 6
Although cystine‐containing uroliths were only the sixth most common urolith type submitted to our laboratory, apatite‐containing uroliths are only higher because of coprecipitation with other minerals. The increase in both the proportion and absolute number of cystine‐containing submissions is interesting. This observation is in agreement with the numbers of cystine‐containing uroliths submitted to another urolith laboratory, 4 although the time periods differed. In that study, numbers of cystine‐containing uroliths increased over time—they were the sixth most common urolith noted and comprised 0.6% of all submissions between 1998 and 2014. 4 In 2019, cystine uroliths were the third most common urolith submitted to the MUC, representing 6% of total submissions, 7 whereas cystine‐containing uroliths were 8.7% of total submissions at our laboratory in 2018. Genetic mutations resulting in type I or type II cystinuria have been identified and predispose dogs to cystine urolithiasis; a type III (androgen dependent) form also has been described. 31 The breeds reported in our study, including the Mastiff, Pitbull Terrier, Bulldog breeds, and Rottweiler, are similar to those reported as predisposed in other studies. 2 , 3 , 4 , 6 Increased popularity of these breeds, decreased genetic screening in breeding programs, decreased rates of neutering, changes in diets, or a combination of these factors could account for the increase in cystine‐containing uroliths submitted. Intact males are considered at risk because of androgen‐dependent cystinuria 31 and indeed just over 70% of males with cystine‐containing uroliths were intact, slightly lower than reported in other studies. 2 , 32 , 33 The lower percentage of male intact dogs in our study could be a result of dogs having been recently neutered but with persistent cystine urolithiasis or having other underlying reasons for these uroliths being present. The young age of affected dogs in our study is consistent with cystine urolithiasis resulting from an inborn error of metabolism. 2 , 3 , 31 , 32
Similar to cystine‐containing uroliths, urate‐containing uroliths most often were noted in dogs <7 years of age with approximately 1/3 submitted from dogs between 4 and 7 years of age, in agreement with other studies. 2 , 3 , 6 In our population, urate‐containing uroliths were more common in female than in male dogs, and this observation contrasts with a male predilection reported in several other studies. 3 , 4 Dalmatians, Bulldogs, and English Bulldogs continue to have a higher risk of having urate‐containing uroliths related to the SLC2A9 mutation leading to hyperuricosuria. 2 , 3 , 4 , 6 , 34 , 35 No predisposition for urate‐containing uroliths was observed in Labrador Retrievers and Jack Russell terriers, although they have been identified as being at risk for urate urolithiasis based on genetic studies. 36 Pitbull Terriers only have been reported as having increased risk for urate‐containing uroliths in 1 other study despite being carriers of the hyperuricosuria mutation. 4 , 36 No trend for a change in the proportion of urate‐containing uroliths was observed, suggesting that strategies for prevention based on known risk factors such as genetic hyperuricosuria or presence of a portosystemic shunt are not effectively decreasing submissions to laboratories. Few xanthine‐containing uroliths were submitted, most often from the Dalmatian and Cavalier King Charles Spaniel breeds. The frequency of submissions from Dalmatians likely is a consequence of allopurinol administration for management of urate urolithiasis, but history of allopurinol administration was unknown in these dogs. The Cavalier King Charles Spaniel is recognized as having familial xanthinuria. 37
Silica‐containing uroliths continue to be uncommon, but geographic differences are recognized, and silica‐containing uroliths were the third most common urolith type from Mexico City. 8 Similar to other studies, most dogs with silica‐containing uroliths were male, 2 , 6 , 8 , 38 , 39 with this possibly reflecting increased likelihood of small silica‐containing uroliths being voided in females. Intact female dogs having higher risk of silica‐containing uroliths than spayed female dogs has not been described previously; neuter status has not been reported previously. 6 , 38 , 39 This finding could reflect the lifestyle of these dogs as working breeds, but no difference in risk was observed for intact male and neutered male dogs. Dogs with silica‐containing uroliths typically were older dogs, consistent with previous reports. 38 , 39 , 40 To our knowledge, predisposition for silica‐containing uroliths has not been described for the Pug, Australian Shepherd, or Border Collie, although some of these breeds previously have been recorded as having silica‐containing uroliths. 6 , 38 , 39 It is hypothesized that silica urolithiasis occurs in part in dogs because increased ingestion of silica, including in well water, soil, corn gluten feed, rice hulls, soybean hulls, or other plant sources. 39 These dog breeds could have higher exposure to dietary sources, particularly the Border Collie and Australian Shepherd as working dog breeds. Differences in breed predisposition also could be a consequence of the reporting method at our laboratory, where some uroliths classified as having silica only had a very small proportion of silica within the urolith. Pugs, Australian Shepherds, and Border Collies have been predisposed to struvite urolithiasis in some studies 3 , 4 , 5 and their predisposition to silica‐containing uroliths could reflect coprecipitation of silica with other mineral types such as struvite or an inherent predisposition to urolithiasis. 38 , 39
Trends have been reported for decreasing proportions of both CaOx‐ and struvite‐containing uroliths from the upper urinary tract. 6 Removal of upper urinary tract uroliths has decreased because of advancement of minimally invasive procedures for managing ureteral obstruction such as ureteral stents and subcutaneous ureteral bypass systems rather than ureterotomy with subsequent urolith removal. 41 , 42 Almost all mineral types were removed from the upper urinary tract with similar frequency, except for xanthine. Further investigation regarding this finding is warranted.
Staphylococcus spp. were the most common bacteria cultured from uroliths. Positive aerobic bacterial culture findings were similar to our previous data, 10 despite struvite‐containing uroliths in dogs rarely forming in the absence of infection. 27 These results could be associated with prior antimicrobial administration or lack of viable bacteria within the urolith layers. Based on these findings and new guidelines published for antimicrobial use for treatment of UTI, 43 bacterial cultures from uroliths other than struvite are not warranted because they do not alter management in most cases. Interpretation of struvite‐containing urolith culture results should be tailored to the dog and antimicrobials administered only if clinically indicated.
Data were from the UC Davis Gerald V. Ling Urinary Stone Analysis Laboratory and as a consequence, might not be representative of all urolith types, ages, breeds, and sex across the United States, although submissions were from various academic and private veterinary hospitals. We reported urolith submissions and it is possible multiple submissions could have been received from the same dog. Breeds were reported by the primary care veterinarian and it is possible mixed breed dogs were listed as purebreds or vice versa. There were limited missing data for dog age, breed, and sex, which could minimally affect results. In addition, because of the laboratory's reporting methods, the proportion of each mineral within uroliths varied and in some cases, represented <50% of the overall urolith composition. The advantage of this approach is that each mineral component in calculi, with or without distinct layering, is included. However, it could also result in possible overlap of different mineral components in a urolith and inclusion of components present in lower percentages.
5. CONCLUSIONS
Although CaOx‐ and struvite‐containing uroliths continue to be most common, the decrease in the proportion of CaOx‐containing uroliths contrasts with previous studies. Further evaluation is recommended to determine the cause, which is likely to be multifactorial. The increase in the proportion of cystine‐containing uroliths warrants further evaluation of risk factors. Ongoing evaluation of trends in mineral composition of uroliths submitted from dogs will be beneficial to evaluate emerging risk factors and effectiveness of preventative strategies.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Kopecny L, Palm CA, Segev G, Westropp JL. Urolithiasis in dogs: Evaluation of trends in urolith composition and risk factors (2006‐2018). J Vet Intern Med. 2021;35:1406–1415. 10.1111/jvim.16114
REFERENCES
- 1. Osborne CA, Lulich JP, Kruger JM, Ulrich LK, Koehler LA. Analysis of 451,891 canine uroliths, feline uroliths, and feline urethral plugs from 1981 to 2007: perspectives from the Minnesota Urolith Center. Vet Clin North Am Small Anim Pract. 2009;39:183‐197. [DOI] [PubMed] [Google Scholar]
- 2. Lulich JP, Osborne CA, Albasan H, Koehler LA, Ulrich LM, Lekcharoensuk C. Recent shifts in the global proportions of canine uroliths. Vet Rec. 2013;172:363‐369. [DOI] [PubMed] [Google Scholar]
- 3. Roe K, Pratt A, Lulich J, Osborne C, Syme HM. Analysis of 14,008 uroliths from dogs in the UK over a 10‐year period. J Small Anim Pract. 2012;53:634‐640. [DOI] [PubMed] [Google Scholar]
- 4. Houston DM, Weese HE, Vanstone NP, Moore AE, Weese JS. Analysis of canine urolith submissions to the Canadian Veterinary Urolith Centre, 1998‐2014. Can Vet J. 2017;58:45‐50. [PMC free article] [PubMed] [Google Scholar]
- 5. Ling GV, Thurmond MC, Choi YK, Franti CE, Ruby AL, Johnson DL. Changes in proportion of canine urinary calculi composed of calcium oxalate or struvite in specimens analyzed from 1981 through 2001. J Vet Intern Med. 2003;17:817‐823. [DOI] [PubMed] [Google Scholar]
- 6. Low WW, Uhl JM, Kass PH, Ruby AL, Westropp JL. Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985‐2006). J Am Vet Med Assoc. 2010;236:193‐200. [DOI] [PubMed] [Google Scholar]
- 7. Minnesota Urolith Center, University of Minnesota . 2019 Global Urolith Data. https://www.vetmed.umn.edu/sites/vetmed.umn.edu/files/globaldata.pdf. Accessed December 3, 2020.
- 8. Del Angel‐Caraza J, Diez‐Prieto I, Pérez‐García CC, et al. Composition of lower urinary tract stones in canines in Mexico City. Urol Res. 2010;38(3):201‐204. [DOI] [PubMed] [Google Scholar]
- 9. Ling GV, Franti CE, Johnson DL, Ruby AL. Urolithiasis in dogs III: prevalence of urinary tract infection and interrelations of infection, age, sex, and mineral composition. Am J Vet Res. 1998;59:643‐649. [PubMed] [Google Scholar]
- 10. Ling GV, Franti CE, Ruby AL, Johnson DL, Thurmond M. Urolithiasis in dogs I: mineral prevalence and interrelations of mineral composition, age, and sex. Am J Vet Res. 1998;59:624‐629. [PubMed] [Google Scholar]
- 11. Perry LA, Kass PH, Johnson DL, Ruby AL, Shiraki R, Westropp JL. Evaluation of culture techniques and bacterial cultures from uroliths. J Vet Diagn Invest. 2013;25:199‐202. [DOI] [PubMed] [Google Scholar]
- 12. Stevenson AE, Blackburn JM, Markwell PJ, Robertson WG. Nutrient intake and urine composition in calcium oxalate stone‐forming dogs: comparison with healthy dogs and impact of dietary modification. Vet Ther. 2004;5:218‐231. [PubMed] [Google Scholar]
- 13. Lulich JP, Osborne CA, Nagode LA, Polzin DJ, Parke ML. Evaluation of urine and serum analytes in Miniature Schnauzers with calcium oxalate urolithiasis. Am J Vet Res. 1991;52:1583‐1590. [PubMed] [Google Scholar]
- 14. Furrow E, Pattersen EE, Armstrong JP, et al. Fasting urinary calcium‐to‐creatinine and oxalate‐to creatinine ratios in dogs with calcium oxalate urolithiasis and breed‐matched controls. J Vet Intern Med. 2015;29:113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dijcker JC, Hagen‐Plantinga EA, Everts H, Bosch G, Kema IP, Hendriks WH. Dietary and animal‐related factors associated with the rate of urinary oxalate and calcium excretion in dogs and cats. Vet Rec. 2012;171:46‐52. [DOI] [PubMed] [Google Scholar]
- 16. Gnanandarajah JS, Abrahante JE, Lulich JP, Murtaugh MP. Presence of Oxalobacter formigenes in the intestinal tract is associated with the absence of calcium oxalate urolith formation in dogs. Urol Res. 2012;40(5):467‐473. [DOI] [PubMed] [Google Scholar]
- 17. Lekcharoensuk C, Osborne CA, Lulich JP, et al. Associations between dry dietary factors and canine calcium oxalate uroliths. Am J Vet Res. 2002;63:330‐337. [DOI] [PubMed] [Google Scholar]
- 18. Lekcharoensuk C, Lulich JP, Osborne CA, et al. Patient and environmental factors associated with calcium oxalate urolithiasis in dogs. J Am Vet Med Assoc. 2000;217:515‐519. [DOI] [PubMed] [Google Scholar]
- 19. Kennedy SM, Lulich JP, Ritt MG, Furrow E. Comparison of body condition score and urinalysis variables between dogs with and without calcium oxalate uroliths. J Am Vet Med Assoc. 2016;249:1274‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Killilea DW, Westropp JL, Shiraki R, et al. Elemental content of calcium oxalate stones from a canine model of urinary stone disease. PLOS One. 2015;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Furrow E, McCue ME, Lulich JP. Urinary metals in a spontaneous canine model of calcium oxalate urolithiasis. PLoS One. 2017;12:e0176595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevenson AE, Robertson WG, Markwell P. Risk factor analysis and relative supersaturation as tools for identifying calcium oxalate stone‐forming dogs. J Small Anim Pract. 2003;44:491‐496. [DOI] [PubMed] [Google Scholar]
- 23. Carr SV, Grant DC, DeMonaco SM, et al. Measurement of preprandial and postprandial urine calcium to creatinine ratios in male Miniature Schnauzers with and without urolithiasis. J Vet Intern Med. 2020;34:754‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allen HS, Swecker WS, Becvarova I, Weeth LP, Werre SR. Associations of diet and breed with recurrence of calcium oxalate cystic calculi in dogs. J Am Vet Med Assoc. 2015;246:1098‐1103. [DOI] [PubMed] [Google Scholar]
- 25. Queau Y. Nutritional management of urolithiasis. Vet Clin North Am Small Anim Pract. 2019;49:175‐186. [DOI] [PubMed] [Google Scholar]
- 26. Hunprasit V, Schreiner PJ, Bender JB, Lulich JP. Epidemiologic evaluation of calcium oxalate urolithiasis in dogs in the United States: 2010‐2015. J Vet Intern Med. 2019;33:2090‐2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lulich JP, Berent AC, Adams LG, Westropp JL, Bartges JW, Osborne CA. ACVIM small animal consensus recommendations on the treatment and prevention of uroliths in dogs and cats. J Vet Intern Med. 2016;30:1564‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abdullahi SU, Osborne CA, Leininger JR, Fletcher TF, Griffith DP. Evaluation of a calculolytic diet in female dogs with induced struvite urolithiasis. Am J Vet Res. 1984;45:1508‐1519. [PubMed] [Google Scholar]
- 29. Dear JD, Larsen JA, Bannasch M, et al. Evaluation of a dry therapeutic urinary diet and concurrent administration of antimicrobials for struvite cystolith dissolution in dogs. BMC Vet Res. 2019;15:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ling GV, Ruby AL. Canine uroliths: analysis of data derived from 813 speciments. Vet Clin North Am Small Anim Pract. 1986;16:303‐316. [DOI] [PubMed] [Google Scholar]
- 31. Brons AK, Henthorn PS, Raj K, et al. SLC3A1 and SLC7A9 mutations in autosomal recessive or dominant canine cystinuria: a new classification system. J Vet Intern Med. 2013;27:1400‐1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hesse A, Hoffmann J, Orzekowsky H, Neiger R. Canine cystine urolithiasis: a review of 1760 submissions over 35 years (1979‐2013). Can Vet J. 2016;57:277‐281. [PMC free article] [PubMed] [Google Scholar]
- 33. Florey J, Ewen V, Syme H. Association between cystine urolithiasis and neuter status of dogs within the UK. J Small Anim Pract. 2017;58:531‐535. [DOI] [PubMed] [Google Scholar]
- 34. Bannasch D, Safra N, Young A, et al. Mutations in the SLC2A9 gene cause hyperuricosuria and hyperuricemia in the dog. PLoS Genet. 2008;4:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karmi N, Safra N, Young A, Bannasch DL. Validation of a urine test and characterization of the putative genetic mutation for hyperuricosuria in Bulldogs and Black Russian Terriers. Am J Vet Res. 2010;71:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karmi N, Brown EA, Hughes SS, et al. Estimated frequency of the canine hyperuricosuria mutation in different dog breeds. J Vet Intern Med. 2010;24:1337‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Zuilen CD, Nickel RF, van Dijk TH, et al. Xanthinuria in a family of Cavalier King Charles spaniels. Vet Q. 1997;19:172‐174. [DOI] [PubMed] [Google Scholar]
- 38. Aldrich J, Ling GV, Ruby AL, Johnson DL, Franti CE. Silica‐containing urinary calculi in dogs (1981‐1993). J Vet Intern Med. 1997;11:288‐295. [DOI] [PubMed] [Google Scholar]
- 39. Osborne CA, Hammer RF, Klausner JS. Canine silica urolithiasis. J Am Vet Med Assoc. 1981;178:809‐813. [PubMed] [Google Scholar]
- 40. Osborne CA, Clinton CW, Kim KM, Mansfield CF. Etiopathogenesis, clinical manifestations, and management of canine silica urolithiasis. Vet Clin North Am Small Anim Pract. 1986;16:185‐207. [DOI] [PubMed] [Google Scholar]
- 41. Palm CA, Culp WTN. Nephroureteral obstructions: the use of stents and ureteral bypass systems for renal decompression. Vet Clin North Am Small Anim Pract. 2016;46:1183‐1192. [DOI] [PubMed] [Google Scholar]
- 42. Pavia PR, Berent AC, Weisse CW, Neiman D, Lamb K, Bagley D. Outcome of ureteral stent placement for treatment of benign ureteral obstruction in dogs: 44 cases (2010‐2013). J Am Vet Med Assoc. 2018;252:721‐731. [DOI] [PubMed] [Google Scholar]
- 43. Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J. 2019;247:8‐25. [DOI] [PubMed] [Google Scholar]


