Abstract
Background
Canine blood donors can be infected by various vector‐borne or other pathogens that could be an important cause of morbidity and death in transfusion recipients.
Hypothesis/Objectives
To estimate and predict positivity to transmittable blood‐borne pathogens in blood units collected from blood donor dogs in Canada.
Animals
Six thousand one hundred and fifty blood units from 1914 active blood donors registered to the Canadian Animal Blood Bank (CABB) between March 2010 and December 2016.
Methods
A registry‐based retrospective study. Blood units were screened by SNAP 4Dx/4Dx Plus and PCR panel tests. Information on blood donors and test results were extracted from multiple databases and collated. Logistic regressions were used to predict blood unit positivity.
Results
Of 1779 blood units, 0.56% were antibody‐positive for Anaplasma phagocytophilum/platys and 0% for Ehrlichia canis/ewingii. After exclusion of antibody‐positive units to Anaplasma spp., 1.1% of 6140 blood units were PCR‐positive to Anaplasma phagocytophilum, Bartonella spp., Brucella canis, “Candidatus Mycoplasma haematoparvum,” Mycoplasma haemocanis, or a combination of these pathogens. Babesia spp., Ehrlichia spp., and Leishmania spp. were not detected. Units from the first blood collection from a dog had higher odds of testing PCR‐positive (P < .001) for at least 1 pathogen than units from subsequent collections.
Conclusions and Clinical Importance
Although our study indicates a low probability of detecting blood‐borne pathogen in blood units collected by this Canadian blood bank, the presence of positive units highlights the importance of the preemptive identification and screening of blood units from healthy blood donors for safe blood banking, especially in first‐time donors.
Keywords: blood banking, canine vector‐borne diseases, infectious diseases, transfusion medicine
Abbreviations
- CABB
Canadian Animal Blood Bank
- CI
confidence intervals
- SOP
standard operating procedure
1. INTRODUCTION
Canine blood transfusions have become an integral part of veterinary medicine, providing a potentially life‐saving therapy for conditions where severe anemia or tissue hypoxia are present. 1 , 2 , 3 However, blood transfusion also carries risk for the recipient, among which is the transmission of pathogens from an infected donor. 4
In Canada, the Canadian Animal Blood Bank (CABB) is a nonprofit organization providing a centralized service to supply high quality blood products to field veterinarians. To mitigate the risk of pathogen transmission, only healthy dogs with negative SNAP 4Dx/4Dx Plus for Anaplasma spp. and Ehrlichia spp. are eligible for blood collection. Collected blood units are then submitted to PCR testing for Anaplasma spp., Ehrlichia spp., Bartonella spp., Babesia spp., Leishmania spp., “Candidatus Mycoplasma haematoparvum,” Mycoplasma haemocanis, and Brucella canis. A PCR‐positive blood unit for any of these pathogens is rejected, and the dog is deferred for life from the program.
Anaplasma and Ehrlichia are 2 rickettsial bacteria transmitted by ticks and other arthropods that can cause acute to subclinical syndromes in dogs 4 , 5 ; infected blood is a pathway for transmission. 6 , 7 Bartonella, an intraerythrocytic bacteria, is another vector‐borne pathogen that can cause clinical disease in dogs. 4 , 5 Transmission via transfusion has not been reported in dogs, but is possible in mice. 8 Babesia is a protozoan transmitted by ticks that cause clinical or subclinical infection in dogs. 9 Babesia transmission via transfusion is well documented in dogs, 10 , 11 and infected animals are unable to clear the infection. 9 Also caused by a protozoan and usually transmitted by sandflies, leishmaniasis can take many forms 4 ; its transmission via blood transfusion can result in a clinically severe visceral form. 12 Hemoplasmas, which encompasses Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum,” 13 are tick‐borne red cell parasites transmittable via blood transfusion that usually cause subclinical disease in dogs, 7 except in immunocompromised or splenectomized dogs where they might induce hemolytic anemia. 14 Another pathogen of importance is Brucella canis 15 ; although transfusion‐transmitted infection has not been documented in dogs, the potential exists due to the prolonged bacteremia associated with the infection. 7
All these pathogens occur in Canada, 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 except “Candidatus Mycoplasma haematoparvum.” Screening reduces the probability of transfusing a contaminated blood product, yet it cannot guarantee that all blood units are pathogen‐free considering the imperfect sensitivity of tests. 4 , 24 Moreover, for cost‐saving reasons, PCR testing is allowed by the CABB to be conducted in pools of at most 3 units, which could impact test sensitivity. To better appraise and mitigate the risk of transmission of these pathogens by blood transfusion in Canada, a better understanding of the presence of blood‐borne pathogens in blood units collected from canine donors is needed. The objectives of this study were to estimate the percentage of blood units testing antibody or PCR‐positive for blood‐borne pathogens in a Canadian sample of healthy canine blood donors, and generate predictive models to estimate these probabilities.
2. MATERIAL AND METHODS
A retrospective registry‐based study was conducted on data from canine blood donors of the CABB and their blood units collected between March 2010 and December 2016. The study was approved by the CABB management committee.
2.1. Animals
Donors were recruited on a voluntary basis by partner clinics, which form collection sites. The CABB Standard Operating Procedure (SOP) stated the following eligibility criteria, in addition to a signed owner consent form (http://www.canadiananimalbloodbank.ca/donor-enrollment-form/): (a) healthy based on annual physical examination performed by its regular veterinarian and even tempered; (b) ≥23 kg, but not overweight; (c) between 1 and 8 years of age; (d) current on their vaccinations (http://canadianbloodbank.ca/blood-donor-requirements/). Retirement of an active donor was generally fixed at 10 years of age but could be extended with veterinary approval.
Other recommended criteria included that the donors have no severe oral lesions, be spayed or neutered and received seasonal heartworm prevention. As determined in clinic to ensure the animal's safety, a packed cell volume above 40% and a total protein within reference intervals (6‐8 g/dL) were required before any blood collection. 25 Deferral was permanent if the donor had particular health conditions (eg, bleeding disorders, heart and liver diseases, diabetes, seizures, or epilepsy) and temporary if the donor was on certain prescribed medications or recently vaccinated.
2.2. Data collection on blood donors and blood units
For each recruited canine blood donor, a paper‐based registration form was filled out by its primary veterinarian. The information was then captured into the Global Office electronic web‐based health record system—Juvonno (https://www.globalofficesoftware.com/), and included data on the dog (name, date of birth, breed, sex), the owner (full name, address, phone number), and the participating clinic (name). When a dog was temporarily or permanently deferred, the previously recorded information was kept in the database and the reasons for exclusion could be noted (albeit not systematically) in a free‐text field. At the time of blood collection, each blood unit was uniquely identified with a unit record (UR) identification number provided by the CABB, and a corresponding donor summary sheet was completed. Only 1 blood unit per dog was collected at each blood collection event. Each UR number was captured in the Unit Record database along with the collection date, dog name and owner last name. In some cases, the registration form and donor summary sheet were scanned and added to the donor's record in the Global Office system.
2.3. SNAP 4Dx/4Dx Plus testing
Based on CABB protocol, all dogs at their first blood collection had to be tested using SNAP 4Dx or SNAP 4Dx Plus (IDEXX Laboratories, Inc) rapid tests, which are commercially available enzyme‐linked immunosorbent assays. These tests were performed using EDTA whole blood samples to detect antibodies against Anaplasma spp. (A. phagocytophilum and A. platys, p44/MSP2 region of the outer membrane), Ehrlichia spp. (E. canis and E. ewingii, p30/p30‐1 region of the outer membrane), and Borrelia burgdorferi (C6 peptide, derived from the VlsE outer surface protein), as well as Dirofilaria immitis antigen. 26 , 27 Annual SNAP 4Dx/4Dx Plus screening of all regular donors was also required, usually performed from April to August.
From March 22, 2010 to December 24, 2013, inclusively, SNAP 4Dx (before December 10, 2012) or 4Dx Plus (thereafter) tests were systematically performed postcollection at IDEXX Canada Reference Laboratories. If antibodies against Anaplasma spp. or Ehrlichia spp. were detected, the blood unit had to be discarded. After this period, SNAP 4Dx Plus tests were usually performed precollection at each veterinary clinic. As these latter data were not centralized and readily available, they were not included in the current project. Dogs with detected antibodies against Anaplasma spp. or Ehrlichia spp., as well as those with detected heartworm antigen (for dog's health reasons), were deferred precollection. Thus, blood units collected since 2014 were from a population of dogs prescreened using the SNAP 4Dx Plus within the year.
2.4. PCR testing
For the entire study period, each blood unit was systematically screened by a PCR at IDEXX Reference Laboratories (Blood Donor RealPCR Panel—Canine; IDEXX Laboratories, Inc) for 8 pathogens: Anaplasma spp., Babesia spp., Bartonella spp., Brucella canis, “Candidatus Mycoplasma haematoparvum,” Ehrlichia spp., Leishmania spp., and Mycoplasma haemocanis. Speciation for Anaplasma spp. and Ehrlichia spp. was provided for PCR‐positive tests. According to the CABB SOP, all blood units from the first collection of a dog had to be tested individually, but subsequent blood units could be tested in pools comprising 2 or 3 blood units for cost saving purposes. A negative status was attributed to each blood unit forming a negative pool. If the pool was positive, each unit was subsequently tested individually. PCR‐positive blood units to any of the 8 pathogens had to be discarded and the dog permanently excluded from the donor program.
2.5. Data extraction and management
Electronic files including all available information on blood donors, blood units and test results were extracted from the Global Office, Unit record and IDEXX databases. All information belonging to the same blood unit or dog were linked using the UR number or the combination of the blood collection date, dog name and owner last name, depending on the available information. Manual editing of name spelling was performed to enable matching when the additional available information such as the breed of dog, sex of dog and first name of the owner did support the match. The age of dogs at time of collection was calculated using the birth date and date of collection; however, due to a potential confusion between the birth date of the owner and of the dog in this field, all calculated dog age ≥13 years old were set as missing value. For each dog, the first registered blood unit collected was categorized as the first lifelong blood collection of the dog if it was tested individually in PCR, as this is a requirement of the CABB for first collections, and if no information indicative of a prior blood collection dated before March 2010 was found in a systematic search of the online databases. All second and following registered blood collections were considered as being at least the second collection. For dogs having their first lifelong collection identified in the database, a sequential number was attributed to each subsequent blood collection, from their first to their last collection registered. The time and reasons of dog retirement as a blood donor were extracted when available. All data manipulation and analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary).
2.6. Estimation and prediction of blood unit positivity
The percentage of blood units positive to SNAP 4Dx/4Dx Plus with 95% confidence intervals (CI) was estimated for each pathogen using the Surveyfreq procedure of SAS, with variance adjusted for potential clustering of blood units within dogs. However, in the absence of antibody‐positive blood units, Clopper‐Pearson exact estimates without clustering adjustment were used. A logistic regression model was then used to predict antibody positivity of blood units, defined as a blood unit in which antibodies against Anaplasma spp., Ehrlichia spp. or both were detected. One blood unit was randomly selected per dog to control for the potential correlation of multiple blood units per dog, as multilevel models could not be used due to the small number of positive blood units. A full model including the province, season of collection, age of dog at time of collection, sex, sequential order of blood collection, and detection of antibodies against B. burgdorferi (SNAP 4Dx/4Dx Plus) as categorical predictors (Table S1) was built. The breed was not included due to its sparse distribution, and the year of collection was excluded as it was not considered relevant in a clinical context. From this full model, a stepwise selection was used to keep only predictors with P < .05. Odds ratios were used to present the results. A ROC curve was estimated to assess the overall predictive ability of the model by evaluating the area under the curve (AUC), as well the model sensitivity and specificity at the available cut‐offs.
For the evaluation of the PCR‐positivity of blood units, antibody‐positive blood units for Anaplasma spp. or Ehrlichia spp. were excluded, because their rejection is required by CABB protocol. The percentage of blood units positive to PCR with 95% CI was estimated as described previously, for each pathogen separately and for all pathogens combined (ie, PCR positivity to at least 1 pathogen). Logistic regression models were then used to predict PCR‐positivity in blood units, defined as DNA detection for any of the 8 pathogens. The same predictors as previously described were used (Table S2), with the exception that the antibody detection against B. burgdorferi was not considered due to the large number of missing data. Two datasets were created for the selection of predictors, each one including a random selection of half of the dogs with 1 blood unit randomly selected by dog. The first dataset was used as a training sample to select a set of predictors with P < .05 with a stepwise approach from a full model. The second dataset was used to validate the selected predictors by estimating a logistic model only including these predictors. Finally, to improve the accuracy of the prediction, a final model was built using the predictors demonstrating a consistent association with the outcome in both the training and the validation dataset. For this final model, 1 blood unit per dog was again randomly selected. Odds ratios, ROC curve and predictive values were estimated as previously described.
2.7. Spatial statistics
Each blood unit was geocoded at the census subdivision level of the dog's owner, which is equivalent to a municipality, using all information available on the address of residency. The distribution of blood units according to their PCR status was mapped in ArcGIS version 10.5, with data aggregated at centroids of the census consolidated subdivisions as defined by Statistics Canada (2016) to improve visualization. Boundary files were obtained from Commission for Environmental Cooperation and from Statistics Canada. Spatial clusters of PCR‐positive blood units (for any of the pathogen) were investigated using the Kulldorff spatial scan test. 28 A Bernoulli model scanning for high‐risk areas was used. Statistical significance was determined using 9999 permutations.
3. RESULTS
3.1. Data description
We obtained information on 6602 blood units collected from March 22, 2010 to December 21, 2016. From these blood units, 452 were excluded due to: the absence of a valid blood UR number (n = 18), merging of multiple blood units collected from the same dog within a 2‐week period (n = 34), missing information on the dog in the Global Office database (n = 135), or missing PCR test results (n = 265). This resulted in 6150 blood units from 1914 dogs belonging to 1587 different owners and collected at 56 veterinary clinics.
Between 1 and 27 blood units (median: 2 units) were collected on each dog over the study period. A time lag from 35 to 1925 days (median: 126 days) was observed between consecutive blood collections from the same dog. Dogs were on median 4.9 years old at time of collection, ranging from 5 months to 12 years. A total of 1056 dogs were male and 843 were females, with missing information for 15 dogs. The number of active dogs (ie, with at least 1 collection) increased from 422 dogs in 2011 (the first year with complete data) to 834 dogs in 2016. Information was available on 198 dogs that retired from the blood bank during the study period. The reasons evoked were mostly retirement (n = 70), positive screening for vector‐borne diseases excluding B. burgdorferi seropositivity (n = 45), positive screening for B. burgdorferi (n = 5), inactive status (n = 47), health issues (n = 17), and death (n = 19). Each owner had between 1 and 8 active blood donor dogs, with 83% of them owning only 1. The number of veterinary clinics from which dogs were collected steadily increased over time from 29 clinics in 2011 to 52 clinics in 2016. Each clinic was associated to 1 to 1226 blood units (median = 46) over the study period.
3.2. Blood unit antibody positivity via SNAP 4Dx/4Dx Plus testing
During the study period, 1779/6150 blood units were tested by SNAP 4Dx/4Dx Plus at an IDEXX Reference Laboratory. All tests were performed within 6 days of blood collection, with 89% of them done within 2 days. Antibodies against B. burgdorferi and Anaplasma spp. were detected from 2.87% and 0.56% of the blood units, respectively. No blood units were positive for either Ehrlichia spp. or Dirofilaria immitis (Table 1).
TABLE 1.
Percentage of positive SNAP 4Dx/4Dx Plus tests with 95% confidence intervals for infectious agents in 1779 blood units from 982 canine blood donors, Canada, 2010‐2014
| Pathogens antibodies or antigens | Number positive | Percentage of positivity (%) | |
|---|---|---|---|
| Estimate | 95% CI | ||
| Dirofilaria immitis antigens a | 0 | 0.00 | 0.00‐0.21 |
| Anaplasma phagocytophilum/platys antibodies | 10 | 0.56 | 0.12‐1.00 |
| Borrelia burgdorferi antibodies a | 51 | 2.87 | 1.48‐4.26 |
| Ehrlichia canis/ewingii antibodies a | 0 | 0.00 | 0.00‐0.21 |
Abbreviation: CI, confidence intervals.
Confidence intervals were computed using the exact method, with no adjustment for clustering.
The distribution of Anaplasma spp. antibody‐positive blood units is presented in Supporting Information Table S1. From the full model predicting antibody positivity to Anaplasma spp. (because all SNAP 4Dx/4Dx Plus tests were negative to Ehrlichia spp.), only 1 predictor was kept. The odds of antibody positivity to Anaplasma spp. were higher when antibodies against B. burgdorferi were detected (OR = 17.3, 95% CI 3.19‐94.2, P = .001). The predictive ability of the model was low (AUC = 0.63, 95% CI 0.45‐0.81). When using the detection of antibodies against B. burgdorferi to predict antibody positivity to Anaplasma spp., the sensitivity of the prediction was 28.6% (2/7) and the specificity was 97.7% (953/975).
Among the 10 blood units (from 7 dogs) with a positive SNAP 4Dx/4Dx Plus for Anaplasma spp., all were submitted to PCR. Two blood units (from 2 dogs) were PCR‐positive for Anaplasma spp.; all others were PCR‐negative for all agents. Of the 7 dogs, 4 dogs were permanently deferred as blood donors, including the 2 with PCR‐positive results. Of the 3 remaining dogs, dog 1 had Anaplasma spp. antibodies detected on its first 2 collected blood units, was antibody‐negative on the third unit, and no SNAP 4Dx/4Dx Plus result was available for the 5 subsequent units. Similarly, dog 2 was antibody‐positive on its first 2 collected blood units and then stopped being a blood donor. Dog 3 had 3 blood units collected; the first and third units were antibody‐positive, but no result was available for the second. The PCR results from the 10 antibody‐positive blood units for Anaplasma spp. from these 7 dogs were excluded from PCR‐positivity analyses, as they should have been rejected (information not available).
3.3. Blood unit positivity via PCR assays
The PCR assays were completed at a maximum of 14 days postcollection (median: 2 days), with 99.7% of the tests done within a 6‐day delay. Of the 6140 blood units tested, 2087 were exclusively tested by individual PCR, 4019 were tested in pools only, and 34 were tested in pools and then individually. From the 4019 blood units only tested in pools, 58 were actually first collection, therefore derogating from CABB protocol. All blood units composing a PCR‐positive pooled sample were retested by individual PCR (Supporting Information Table S3).
Less than 0.8% of blood units were PCR‐positive for each pathogen (Table 2). Overall, 1.1% (95% CI 0.79‐1.33) of blood units were PCR‐positive to at least 1 pathogen. Anaplasma phagocytophilum was identified in all Anaplasma‐positive blood units. Babesia spp., Ehrlichia spp., and Leishmania spp. were not detected. In 4 blood units, Mycoplasma haemocanis and Candidatus Mycoplasma haematoparvum were both detected, whereas Mycoplasma haemocanis and Bartonella spp. were both detected in 1 unit.
TABLE 2.
Percentage of positive PCR test with 95% confidence intervals for various infectious agents in 6140 blood units a from 1911 canine blood donors, Canada, 2010‐2016
| Pathogens DNA | Number positive | Percentage of positivity (%) | |
|---|---|---|---|
| Estimate | 95% CI | ||
| Anaplasma phagocytophilum | 4 | 0.065 | 0.001‐0.13 |
| Babesia spp. b | 0 | 0.00 | 0.00‐0.06 |
| Bartonella spp. | 5 | 0.081 | 0.01‐0.15 |
| Brucella canis | 2 | 0.033 | 0.00‐0.078 |
| “Candidatus Mycoplasma haematoparvum” | 18 | 0.29 | 0.15‐0.44 |
| Ehrlichia spp. b | 0 | 0.00 | 0.00‐0.06 |
| Leishmania spp. b | 0 | 0.00 | 0.00‐0.06 |
| Mycoplasma haemocanis | 47 | 0.77 | 0.54‐1.00 |
Abbreviation: CI, confidence intervals.
SNAP 4Dx/4Dx Plus‐positive blood units were excluded (n = 10 blood units and 3 dogs).
Confidence intervals were computed using the exact method, with no adjustment for clustering.
All but 3 of the 69 dogs with a PCR‐positive blood unit for at least 1 pathogen were permanently deferred. For the first dog, which was PCR‐positive to Brucella canis, the blood unit was discarded, but the dog had 5 additional blood units collected during the study period (all were PCR‐negative). Another dog had Mycoplasma haemocanis detected on its first collected blood unit, but the blood unit was mislabeled, and another healthy dog was excluded from the donor program instead. The first blood unit was sold; it is not known if the product was transfused. On its second collection, 218 days later, the former dog tested once more PCR‐positive and was hence permanently deferred. A third dog tested PCR‐positive for “Candidatus Mycoplasma haematoparvum” on its first blood collection; the donor was permanently deferred, and the blood unit discarded. Its owner had 2 additional dogs; 1 also tested positive and the other was clear. When presenting at the clinic for a second donation, 133 days later, the owner brought the wrong dog and consequently the PCR panel was again “Candidatus Mycoplasma haematoparvum”‐positive.
For the model predicting PCR‐positivity in blood units, the only variable selected from the training dataset was the sequential order of blood collections. The odds of PCR‐positivity were higher at first collection compared to subsequent collections (OR = 7.98, 95% CI 2.79‐22.9, P < .001). This variable remained statistically significant in the validation dataset and had a similar strength of association (OR = 10.5, 95% CI 3.17‐34.5, P < .001). According to the final model, including 1 randomly selected blood unit from 1908 dogs, the odds ratio estimates was 10.8 (95% CI 3.9‐30.0). The predictive ability of the final model was limited (AUC = 0.68, 95% CI 0.65‐0.72). When using the sequential order of the collection (first vs others) to predict PCR‐positive blood units, the sensitivity of the prediction was 93.3% (56/60) and the specificity was 43.6% (807/1849). The distribution of blood units according to their sequential order of collection and PCR results is presented in Figure 1 for the 6116 blood units from the 1899 dogs for which the information was available for each blood unit.
FIGURE 2.
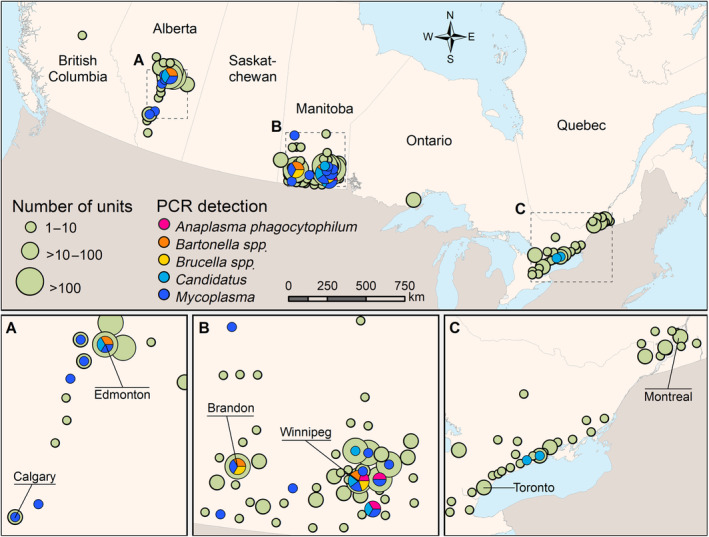
Geographical distribution of canine blood units from active blood donors in the Canadian Animal Blood Bank between March 2010 and December 2016, and location of positive blood unit(s) for each pathogen according to PCR testing. Each pathogen dot represents a location where at least 1 positive blood unit was detected. Blood units were geolocated at the centroid of the census consolidated subdivisions of the dog owner, as defined by Statistics Canada, 2016. The location and names of large cities and cities with large number of donations are indicated
3.4. Spatial statistics
Of the 6140 blood units tested in PCR, 6107 could be geolocated. They were distributed in 109 consolidated census divisions. The geographical distribution of blood donor dogs is presented in Figure 2. No spatial cluster of PCR‐positive blood units was detected (P = .4).
FIGURE 1.
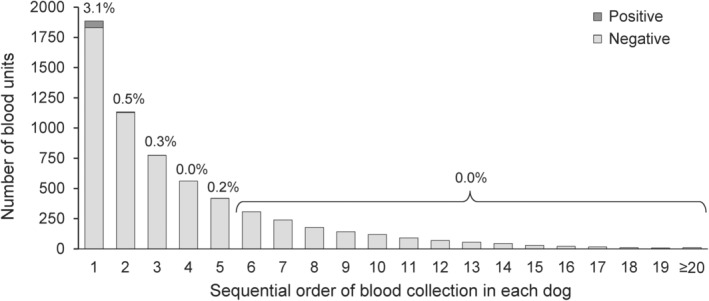
Distribution of 6116 blood units collected by the Canadian Animal Blood Bank between March 2010 and December 2016 according to their PCR‐positivity to transmittable blood‐borne pathogens in dogs, by sequential order of blood collection in each dog
4. DISCUSSION
This study reports the risks of exposure to blood‐borne pathogens during blood transfusion in Canadian dogs. It is recommended that pathogens of importance in transfusion medicine are systematically screened in blood donors; they are identifiable, transmittable by blood, capable of causing subclinical infection, and the cause of an important disease in recipients. 7 The pathogens systematically screened by the CABB are in accordance with the most recent evidence‐based recommendations included in the 2016 Consensus Statement of the ACVIM and best practices. 7 , 25
The estimated percentages of positivity we report among blood units collected from healthy dogs is low for all pathogens, a finding consistent with a similar, but smaller, study conducted on blood donor dogs in the United Kingdom in 2007‐2012. 29 It is also coherent with the low seroprevalences for these agents in Canadian dogs. 16 , 18 , 19 , 20 , 22 , 23 The highest percentage of PCR positivity in blood units was observed for Mycoplasma haemocanis, at 0.77%. This finding is in accordance with those of a 2012 study from the United States with prevalences of 0.6% and 0.7% for Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum,” respectively. 30 To our knowledge, this is the first report of “Candidatus Mycoplasma haematoparvum” in Canadian dogs. Even though clinical importance of hemoplasma infection in dogs is debatable, the potential risk for immunocompromised recipient warrants permanent deferral of PCR‐positive donors, notably that antimicrobial therapy does not reliably eliminate these organisms. 7 We did not detect Babesia in any blood unit, despite the inclusion in our database of blood units from 54 dogs of breeds that have a higher prevalence of infection in the United States, namely Greyhounds, 31 American Pit Bull Terriers and American Staffordshire Terriers. 32 , 33 Similarly, no Leishmania infection was detected. In Canada, leishmaniasis occurs mainly in dogs imported from countries where it is endemic. 34 However, a seroprevalence survey of Foxhounds conducted in 2000‐2003 found that canine visceral leishmaniasis is enzootic in Ontario and Nova Scotia, as well as in Eastern United States 35 ; no Foxhounds were included in our study.
The use of SNAP 4Dx/4Dx Plus screening allowed for the detection of Anaplasma spp. antibodies in dogs, while PCR testing enabled the additional detection of antigen‐positive blood units. This strategy of combining serological and PCR assays in parallel can increase sensitivity of detection. 36 Since 2014, most SNAP 4Dx Plus were performed precollection directly at the veterinary clinics. Although this results in a positive impact on dog welfare as it prevents the collection of blood units that would then be rejected due to a positive in‐house test, it has the drawback of making the information about antibody testing less available for monitoring purposes. For these reasons, it would be interesting to centralize and make available the SNAP 4Dx Plus results performed in‐house in the future. The likely low prevalence of Anaplasma spp. infection in canine blood donors in Canada allows CABB to be highly selective and systematically defer antibody‐positive dogs to minimize pathogen transmission risks, even when they are PCR‐negative; however, the inclusion of antibody‐positive but PCR‐negative dogs can be justified in endemic areas to have a sufficient donor pool. 7
Our results highlight a correlation between antibody positivity to Anaplasma spp. and to B. burgdorferi. Coinfections with Anaplasma spp. and B. burgdorferi are well documented in both dogs and Ixodes scapularis ticks, including in Canada. 23 , 37 , 38 Extra care, such as avoiding the use of pooled testing, might be warranted in testing blood units from B. burgdorferi‐positive dogs, as they might be more at risk for Anaplasma spp. infection. Seropositivity for B. burgdorferi per se is not considered a valid reason for deferral, because it is not transmittable via transfusion, 7 which aligns with the current CABB practice.
We observed higher odds of a positive PCR result at time of first collection. Two elements could explain this finding. First, a higher sensitivity of detection is likely at time of first collection because the protocol for pathogen screening only allows individual testing, whereas pooling of samples for PCR testing is permitted for subsequent collections. Second, as only dogs with PCR‐negative blood units are eligible for subsequent collection, it is likely that the most at‐risk dogs were already deferred. Interestingly, our descriptive results suggests that this higher probability of PCR‐positivity in first donation is consistent for most pathogens (Table S2). Although the predictive ability was limited, this advocates in favor of blood donors that could contribute to the blood bank on a regular (albeit safe for the dog's health) and long‐term basis. In that perspective, a better understanding of the reasons underlying withdrawal of a dog as a blood donor is warranted, for which only fragmental information was available in this study.
No spatial variation was observed in blood unit positivity, despite previous reports of spatiotemporal variations in the risk of exposure to vector ticks for many of these pathogens in Canada. 23 However, blood collection was not equally distributed across Canada, with few units originating from Eastern provinces, limiting the ability to detect potential high‐risk areas. This might be partially explained by the fact that the activities of CABB were first implemented in the Western provinces before expanding to other areas as observed in the increased number of participating clinics over time.
Apart from Anaplasma phagocytophilum, all pathogens detected by PCR were either only detected, or more frequently detected, in blood units tested individually compared to pooled samples, despite the fact that 2 times more blood units were tested in pools. This raises the question about the potential negative impact of pooling on test sensitivity, which could have led to false‐negative tests. To our knowledge, the impact of pooling on PCR test sensitivity in this context has not yet been determined. This would be important to investigate as the cost‐benefit of pooling might not warrant the potential negative impact on detection ability. Overall, the specificity of PCR tests was likely high given the absence or low percentages of PCR‐positive dogs for most pathogens.
Our results outline various noncompliance issues with the SOP that can happen when coordinating a multicenter blood donor program. The first situation refers to the absence of permanent dog deferral after the detection of a PCR‐positive blood unit, which was observed for 3 of the 69 dogs with a PCR‐positive unit. Some of these deviations from the protocol were explained by technical errors. In another case, involving a Brucella canis‐positive test, the clinical team suspected a false‐positive test result, which was supported by negative test results on subsequent collections. The specificity of PCR for Brucella is not perfect and false positives can be attributed to many factors, including contamination. 15 , 39 , 40 Another observation was the report of a permanent deferral for at least 5 dogs due to the detection of antibodies against B. burgdorferi, whereas the current CABB protocol, supported by the ACVIM Consensus Statement, 7 is not to exclude healthy seropositive dogs because there is no evidence that B. burgdorferi can be transmitted via transfusion. Finally, 58 blood units were tested in pooled PCR only at time of first collection, despite the CABB protocol clearly stating that first‐time donors are to be tested individually. Operating a blood donor program at the national level has its challenges, as many different actors need to comply with centralized protocols, whether it is registered veterinary technicians, participating veterinarians or students. The large number and expected high turnover of participants, along with the very infrequent use of procedures associated with positive tests, increases the risk of errors and underlines the need for continuous education for all people involved.
The absence of a unique key identifier between all merged databases not only led to the necessity of using a combination of complex and time‐consuming merging strategies, but also increases the likelihood that some incorrect matches were done, especially when considering the inconsistent spelling of names, missing values and common sharing of owner or dog names. For instance, ≥14 dogs were named “Charlie,” “Bella,” or “Jake,” which was one of the information available for matching. Moreover, UR numbers, another important key, were sometimes misread, incorrectly entered or reused after some time. Nevertheless, we are confident that the meticulous validation of the merged datasets proved successful. Another limitation was the fact the age of the owner was sometimes recorded instead of the age of the dog, leading to a potential misclassification or missing value for dog age. Also, the dog owner most recent address of residency was attributed to all blood units from this dog, because the historical records were overwritten at each database update. Thus, we strongly recommend the use of a unique identification number for each canine blood donor in addition to the UR number, the use of validation rules during data entry and the preservation of all historical records, which would strongly increase the feasibility, timeliness and informativeness of using these data for monitoring the infectious status of blood units. Finally, the databases did not systematically capture the information on blood unit rejection; a complete traceability system from the donor to the recipient would be useful to document compliance with procedures and potential negative outcomes after a transfusion.
Overall, the percentage of blood units with detectable blood‐borne pathogens of clinical importance was low among units collected by CABB over the study period, suggesting a low risk of exposure for dogs receiving blood products from this source. However, their detection in some units support the importance of screening systematically and rigorously for these pathogens, especially at time of first blood collection and for B. burgdorferi antibody‐positive dogs. More research is needed on the impact of blood pooling on PCR test sensitivity, which could have led to an underestimation of the risk. This database represents a valuable source of information for monitoring blood‐borne pathogens in Canadian dog blood donors. This is particularly relevant in the current context of climate change leading to a northern expansion of tick populations and an increase in the seasonal risk period, 41 which is likely to increase the risk of exposure to pathogens of importance in transfusion medicine in Canadian dogs.
CONFLICT OF INTEREST DECLARATION
A collaborative agreement between the Small Animal Blood Bank of the Centre Hospitalier Universitaire Vétérinaire (CHUV) of the Université de Montréal and the Canadian Animal Blood Bank exists since March 2018 to facilitate access to canine blood products to veterinary clinics throughout Canada; as such Dr. Blais (director of the CHUV Blood Bank) receives no financial compensation, but sits on the scientific and executive boards of CABB. All other authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Table S1: Distribution of 1779 blood units from 982 canine blood donors tested by SNAP 4Dx/4Dx Plus according to their antibody positivity to Anaplasma spp. in a population of canine blood donors, Canada.
Table S2: Distribution of 6140 blood units from 1914 dogs according to their PCR positivity to 5 pathogens in a population of canine blood donors, Canada.
Table S3: Distribution of 6140a blood units from 1914 caning blood donors according to their pooled and individual PCR test results, Canada, 2010‐2016.
ACKNOWLEDGMENT
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant attributed to M.‐C. Blais (RGPIN‐2019‐04979). The publication fees were covered by the Canadian Animal Blood Bank, which had no role in the study design, data analysis, and interpretation of the results. The authors thank Ali Ramiche, Magali Decôme and Sandra Botelho for their contribution to this work, and IDEXX Laboratories and the Canadian Animal Blood Bank for facilitating data extraction.
Nury C, Blais M‐C, Arsenault J. Risk of transmittable blood‐borne pathogens in blood units from blood donor dogs in Canada. J Vet Intern Med. 2021;35:1316–1324. 10.1111/jvim.16139
Funding information Canadian Animal Blood Bank; Natural Sciences and Engineering Research Council of Canada Discovery Grant, Grant/Award Number: RGPIN‐2019‐04979
REFERENCES
- 1. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care. 2009;19:66‐73. [DOI] [PubMed] [Google Scholar]
- 2. Rozanski E, de Laforcade AM. Transfusion medicine in veterinary emergency and critical care medicine. Clin Tech Small Anim Pract. 2004;19:83‐87. [DOI] [PubMed] [Google Scholar]
- 3. Davidow B. Transfusion medicine in small animals. Vet Clin North Am Small Anim Pract. 2013;43:735‐756. [DOI] [PubMed] [Google Scholar]
- 4. Wardrop KJ, Reine N, Birkenheuer A, et al. Canine and feline blood donor screening for infectious disease. J Vet Intern Med. 2005;19:135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reine NJ. Infection and blood transfusion: a guide to donor screening. Clin Tech Small Anim Pract. 2004;19:68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egenvall A, Bjöersdorff A, Lilliehöök I, et al. Early manifestations of granulocytic ehrlichiosis in dogs inoculated experimentally with a Swedish Ehrlichia species isolate. Vet Rec. 1998;143:412‐417. [DOI] [PubMed] [Google Scholar]
- 7. Wardrop KJ, Birkenheuer A, Blais MC, et al. Update on canine and feline blood donor screening for blood‐borne pathogens. J Vet Intern Med. 2016;30:15‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silva MN, Vieira‐Damiani G, Ericson ME, et al. Bartonella henselae transmission by blood transfusion in mice. Transfusion. 2016;56:1556‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Irwin PJ. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2010;40:1141‐1156. [DOI] [PubMed] [Google Scholar]
- 10. Freeman MJ, Kirby BM, Panciera DL, Henik RA, Rosin E, Sullivan LJ. Hypotensive shock syndrome associated with acute Babesia canis infection in a dog. J Am Vet Med Assoc. 1994;204:94‐96. [PubMed] [Google Scholar]
- 11. Stegeman JR, Birkenheuer AJ, Kruger JM, Breitschwerdt EB. Transfusion‐associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc. 2003;222:959‐963. 952. [DOI] [PubMed] [Google Scholar]
- 12. Owens SD, Oakley DA, Marryott K, et al. Transmission of visceral leishmaniasis through blood transfusions from infected English foxhounds to anemic dogs. J Am Vet Med Assoc. 2001;219:1076‐1083. [DOI] [PubMed] [Google Scholar]
- 13. Sykes JE, Ball LM, Bailiff NL, Fry MM. ‘Candidatus Mycoplasma haematoparvum’, a novel small haemotropic mycoplasma from a dog. Int J Syst Evol Microbiol. 2005;55:27‐30. [DOI] [PubMed] [Google Scholar]
- 14. Lester SJ, Hume JB, Phipps B. Haemobartonella canis infection following splenectomy and transfusion. Can Vet J. 1995;36:444‐445. [PMC free article] [PubMed] [Google Scholar]
- 15. Cosford KL. Brucella canis: an update on research and clinical management. Can Vet J. 2018;59:74‐81. [PMC free article] [PubMed] [Google Scholar]
- 16. Herrin BH, Peregrine AS, Goring J, Beall MJ, Little SE. Canine infection with Borrelia burgdorferi, Dirofilaria immitis, Anaplasma spp. and Ehrlichia spp. in Canada, 2013‐2014. Parasit Vectors. 2017;10:244‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gary AT, Webb JA, Hegarty BC, Breitschwerdt EB. The low seroprevalence of tick‐transmitted agents of disease in dogs from southern Ontario and Quebec. Can Vet J. 2006;47:1194‐1200. [PMC free article] [PubMed] [Google Scholar]
- 18. Bosu WT, Prescott JF. A serological survey of dogs for Brucella canis in southwestern Ontario. Can Vet J. 1980;21:198‐200. [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins R, Hoquet F, Bourque R, Gosselin Y. A serological survey for Brucella canis in dogs in the province of Quebec. Can Vet J. 1979;20:315‐317. [PMC free article] [PubMed] [Google Scholar]
- 20. Qurollo BA, Chandrashekar R, Hegarty BC, et al. A serological survey of tick‐borne pathogens in dogs in North America and the Caribbean as assessed by Anaplasma phagocytophilum, A. platys, Ehrlichia canis, E. chaffeensis, E. ewingii, and Borrelia burgdorferi species‐specific peptides. Infect Ecol Epidemiol. 2014;4:10. 10.3402/iee.v3404.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaunt MC, Carr AP, Taylor SM. Serological survey of canine vector‐borne diseases in Saskatchewan, Canada. Can Vet J. 2018;59:1109‐1111. [PMC free article] [PubMed] [Google Scholar]
- 22. Villeneuve A, Goring J, Marcotte L, Overvelde S. Seroprevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, Ehrlichia canis, and Dirofilaria immitis among dogs in Canada. Can Vet J. 2011;52:527‐530. [PMC free article] [PubMed] [Google Scholar]
- 23. Evason M, Stull JW, Pearl DL, et al. Prevalence of Borrelia burgdorferi, Anaplasma spp., Ehrlichia spp. and Dirofilaria immitis in Canadian dogs, 2008 to 2015: a repeat cross‐sectional study. Parasit Vectors. 2019;12:64‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Drexel J, Andrews B, Eberts M, Breitschwerdt E, Chandrashekar R. Comparative evaluation of 2 in‐clinic assays for vector‐borne disease testing in dogs. Top Companion Anim Med. 2018;33:114‐118. [DOI] [PubMed] [Google Scholar]
- 25. Yagi K, Bean BL. Canine donor selection. In: Yagi K, Holowaychuk MK, eds. Manual of Veterinary Transfusion Medicine and Blood Banking. 1st ed. Ames, Iowa: John Wiley & Sons; 2016:187‐198. [Google Scholar]
- 26. Stillman BA, Monn M, Liu J, et al. Performance of a commercially available in‐clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J Am Vet Med Assoc. 2014;245:80‐86. [DOI] [PubMed] [Google Scholar]
- 27. Chandrashekar R, Mainville CA, Beall MJ, et al. Performance of a commercially available in‐clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res. 2010;71:1443‐1450. [DOI] [PubMed] [Google Scholar]
- 28. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26:1481‐1496. [Google Scholar]
- 29. Crawford K, Walton J, Lewis D, Tasker S, Warman SM. Infectious agent screening in canine blood donors in the United Kingdom. J Small Anim Pract. 2013;54:414‐417. [DOI] [PubMed] [Google Scholar]
- 30. Compton SM, Maggi RG, Breitschwerdt EB. Candidatus Mycoplasma haematoparvum and Mycoplasma haemocanis infections in dogs from the United States. Comp Immunol Microbiol Infect Dis. 2012;35:557‐562. [DOI] [PubMed] [Google Scholar]
- 31. Taboada J, Harvey JW, Levy MG, et al. Seroprevalence of babesiosis in Greyhounds in Florida. J Am Vet Med Assoc. 1992;200:47‐50. [PubMed] [Google Scholar]
- 32. Macintire DK, Boudreaux MK, West GD, Bourne C, Wright JC, Conrad PA. Babesia gibsoni infection among dogs in the southeastern United States. J Am Vet Med Assoc. 2002;220:325‐329. [DOI] [PubMed] [Google Scholar]
- 33. Birkenheuer AJ, Levy MG, Stebbins M, Poore M, Breitschwerdt E. Serosurvey of antiBabesia antibodies in stray dogs and American pit bull terriers and American staffordshire terriers from North Carolina. J Am Anim Hosp Assoc. 2003;39:551‐557. [DOI] [PubMed] [Google Scholar]
- 34. Lennox WJ, Smart ME, Little PB. Canine leishmaniasis in Canada. Can Vet J. 1972;13:188‐190. [PMC free article] [PubMed] [Google Scholar]
- 35. Duprey ZH, Steurer FJ, Rooney JA, et al. Canine visceral leishmaniasis, United States and Canada, 2000‐2003. Emerg Infect Dis. 2006;12:440‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, Levy MG, Breitschwerdt EB. Comparison of serological and molecular panels for diagnosis of vector‐borne diseases in dogs. Parasit Vectors. 2014;7:127‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez‐Vicente S, Tagliafierro T, Coleman JL, et al. Polymicrobial nature of tick‐borne diseases. mBio. 2019;10(5):e02055‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chilton NB, Curry PS, Lindsay LR, et al. Passive and active surveillance for Ixodes scapularis (Acari: Ixodidae) in Saskatchewan, Canada. J Med Entomol. 2019;57:156‐163. [DOI] [PubMed] [Google Scholar]
- 39. Keid LB, Soares RM, Vasconcellos SA, Salgado VR, Megid J, Richtzenhain LJ. Comparison of a PCR assay in whole blood and serum specimens for canine brucellosis diagnosis. Vet Rec. 2010;167:96‐99. [DOI] [PubMed] [Google Scholar]
- 40. Kauffman LK, Bjork JK, Gallup JM, Boggiatto PM, Bellaire BH, Petersen CA. Early detection of Brucella canis via quantitative polymerase chain reaction analysis. Zoonoses Public Health. 2014;61:48‐54. [DOI] [PubMed] [Google Scholar]
- 41. Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. Increased risk of tick‐borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019;45:83‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Distribution of 1779 blood units from 982 canine blood donors tested by SNAP 4Dx/4Dx Plus according to their antibody positivity to Anaplasma spp. in a population of canine blood donors, Canada.
Table S2: Distribution of 6140 blood units from 1914 dogs according to their PCR positivity to 5 pathogens in a population of canine blood donors, Canada.
Table S3: Distribution of 6140a blood units from 1914 caning blood donors according to their pooled and individual PCR test results, Canada, 2010‐2016.


