Abstract
Advanced glycation end-products (AGEs) are a heterogeneous group of compounds formed by the non-enzymatic reaction between amino acids and reducing sugars, or dicarbonyls as intermediate compounds. Experimental studies suggest that AGEs may promote colorectal cancer, but prospective epidemiologic studies are inconclusive. We conducted a case–control study nested within a large European cohort. Plasma concentrations of three protein-bound AGEs—Nε-(carboxy-methyl)lysine (CML), Nε-(carboxy-ethyl)lysine (CEL) and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1)—were measured by ultra-performance liquid chromatography–tandem mass spectrometry in baseline samples collected from 1378 incident primary colorectal cancer cases and 1378 matched controls. Multivariable-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were computed using conditional logistic regression for colorectal cancer risk associated with CML, CEL, MG-H1, total AGEs, and [CEL+MG-H1: CML] and [CEL:MG-H1] ratios. Inverse colorectal cancer risk associations were observed for CML (OR comparing highest to lowest quintile, ORQ5 versus Q1 = 0.40, 95% CI: 0.27–0.59), MG-H1 (ORQ5 versus Q1 = 0.73, 95% CI: 0.53–1.00) and total AGEs (OR Q5 versus Q1 = 0.52, 95% CI: 0.37–0.73), whereas no association was observed for CEL. A higher [CEL+MG-H1: CML] ratio was associated with colorectal cancer risk (ORQ5 versus Q1 = 1.91, 95% CI: 1.31–2.79). The associations observed did not differ by sex, or by tumour anatomical sub-site. Although individual AGEs concentrations appear to be inversely associated with colorectal cancer risk, a higher ratio of methylglyoxal-derived AGEs versus those derived from glyoxal (calculated by [CEL+MG-H1: CML] ratio) showed a strong positive risk association. Further insight on the metabolism of AGEs and their dicarbonyls precursors, and their roles in colorectal cancer development is needed.
We evaluated colorectal cancer risk associated with blood levels of major advanced glycation end-products (AGEs). The AGEs examined were mostly associated with lower colorectal cancer risk. The ratio of methylglyoxal-derived versus glyoxal-derived AGEs was positively associated with colorectal cancer.
Introduction
Colorectal cancer (CRC) is the third most common incident cancer and the second leading cause of cancer death globally (1). A substantial body of epidemiologic evidence, particularly from large-scale prospective cohort studies, apportions a considerable contribution of modifiable dietary and lifestyle risk factors to CRC development (2–4). Western-type diets tend to promote the formation of advanced glycation end-products (AGEs), a heterogeneous class of pro-inflammatory and pro-oxidative compounds formed irreversibly by the non-enzymatic combination of amino acids and reducing sugars (5–8). AGEs can also be formed when proteins are glycated by highly reactive dicarbonyls such as glyoxal (GO) and methylglyoxal (MGO) absorbed from the diet, and/or smoking or produced as sugar and lipid metabolism by-products (8,9). GO and MGO have been reported to be over 20 000 times more potent in glycating amino acids, compared to sugars (10,11). As a consequence, most abundant AGEs in the body are derived from GO [(Nε-(carboxymethyl)lysine (CML)] or MGO [Nε-(carboxyethyllysine) (CEL) and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine, (MG-H1)] (12–14).
AGEs are thought to affect CRC development by promoting a pro-inflammatory and oxidative environment, primarily via binding to the receptor for AGEs (RAGE), a transmembrane protein that belongs to the immunoglobulin superfamily (15). Immunohistochemical expression of AGEs is higher in colon cancer tumours compared to adjacent normal tissues and AGEs have been shown to enhance and promote colon cancer growth in in vitro models (16–18). Animal studies show that AGEs can induce sustained inflammation in the colon and promote colon cancer development (19,20). However, two case–control studies nested within prospective studies have reported inconclusive findings for the association between circulating AGEs levels and CRC. In the Women’s Health Initiative (WHI) study, Chen et al. (21) found an inverse association between serum CML and CRC in women [odds ratio (OR) = 0.85, 95% confidence interval (CI): 0.49–1.47], while Jiao et al. (22) reported a positive association between circulating CML and CRC risk in male smokers (OR = 1.20, 95% CI: 0.64–2.26) in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study. These previous investigations focused on CML only and did not include other major AGEs or assess possible differences by sex or tumour anatomical sub-site. Furthermore, they detected AGEs by ELISA kits, which have low specificity and reproducibility (23).
The aim of the present study was to examine the associations between pre-diagnostic protein-bound circulating levels of CML, CEL and MG-H1 measured using ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) and CRC risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. The rationale for the selection of these three AGEs is threefold: they are considered as the most abundant in the body, they have very well-characterized chemical structures and they are derived from specific pathways of formation with CML being mainly derived from GO, whereas CEL and MG-H1 are mainly derived from MGO. For CML and CEL, lysine is the amino acid of the glycation site, whereas it is arginine for MG-H1. At the cellular level, the MGO-lysine adduct CEL is predominantly formed in the cytosol through the glycation of cytosol proteins, whereas the MGO-arginine adduct MG-H1 is equally found in cytosol, histone and mitochondria proteins (24). We hypothesized that protein-bound concentrations of these AGEs would be associated with a higher CRC risk. We also examined CRC risk associated with the ratios of AGEs from specific dicarbonyls similar to the [CEL:MG-H1] ratio assessed in previous studies (25,26), as a potential index of the chemical origin of the AGEs. Although MGO glycates amino acids to CEL and MG-H1, both these MGO-derived AGEs have different promoting factors as they are produced from lysine and arginine, respectively. Thus, we applied the ratio of [CEL:MG-H1] as a proxy of the potential differential glycating activities of MGO in the body.
Materials and methods
Study population and data collection
We conducted a nested case–control study within the EPIC cohort, an ongoing multicentre prospective study with participants recruited from 23 centres constellated in 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden and the UK) (27). A total of 521 324 participants were recruited into EPIC between 1992 and 2000. Detailed data on lifestyle, dietary and socio-demographic factors were collected at baseline from all the participants. Body weight and standing height were measured by trained health professionals using standardized protocols. Lifestyle variables such as smoking, physical activity and the level of education were collected using a validated, standardized questionnaire. Information on the highest attained educational level was categorized as none, primary, technical and professional, secondary or higher (college or university). Smoking was collected as status (current, past, never), by type of products (cigarettes, cigars, pipe), intensity (number of cigarettes) and duration (in years of smoking). Information on past smoking habits and the years since quitting smoking was collected in former smokers. Physical activity was defined according to the Cambridge physical activity definitions: inactive (sedentary job plus no recreational activity), moderately inactive (sedentary job with <0.5 h recreational activity daily/or standing job with no recreational activity), moderately active (sedentary job with 0.5–1 h recreational activity daily/or standing job with 0.5 h recreational activity daily/or physical job with no recreational activity) or active (sedentary job with >1 h recreational activity daily/or standing job with >0.5 h recreational activity daily/or physical job with at least some recreational activity/or heavy manual job) (28). Blood samples were collected and are stored in liquid nitrogen (−196°C) in biobank facilities located at the International Agency for Research on Cancer (IARC), or in local biobanks in Denmark (−150°C) and Sweden (−80°C at Malmö and Umeå) until analysis. Informed consent was obtained from all the participants. The EPIC study was approved by the IARC Ethical Committee and the local ethics committees pertaining to each participating centre.
Follow-up for CRC incidence and vital status
Vital status (98.4% complete) was ascertained on a regular basis using record linkage with centralized regional cancer registries (Denmark, Italy, the Netherlands, Norway, Spain, Sweden and UK) or via a combination of methods including use of health insurance records, connection with cancer and pathology registries and active follow-up through participants and their close relatives (France, Germany and Greece). Incident CRC cases were ascertained according to the classification by the International Classification of Diseases for Oncology (ICD-O, codes C18-C20). Colon cancer included tumours in the proximal site (C18.0-C18.5: from cecum to splenic flexure) or the distal segment (C18.6-C18.7: from descending colon down to sigmoid colon), while rectal cancer included tumours that occurred from the recto-sigmoid junction (C19) down to the rectum (C20). Tumours that arose in the anus and in the anal canal (C21) were not included in this analysis.
Nested case–control design study
A total of 1416 incident CRC cases were identified and matched on 1:1 ratio to controls by incidence density sampling from all cohort members alive and free of cancer at the time of diagnosis of the index case. Cases were selected sequentially in the order of date of diagnosis and based on sufficient biological sample availability. The following matching criteria were applied: age at blood collection (±1 year), sex, recruiting centre, time of the day at blood collection (±3 h), fasting status at blood collection (<3, 3–6 and >6 hours), and, additionally, among women by menopausal status (pre-menopause, peri-menopause and post-menopause) and hormone replacement therapy use at time of blood collection (yes/no). We excluded subjects within incomplete matched case sets (i.e. a case without a control or vice versa, n = 12), and 26 cases and their matched controls from Greece due to unforeseen data restriction issues. Thus, the final data analysis included 1378 CRC cases and their matched controls.
Laboratory analyses of AGEs
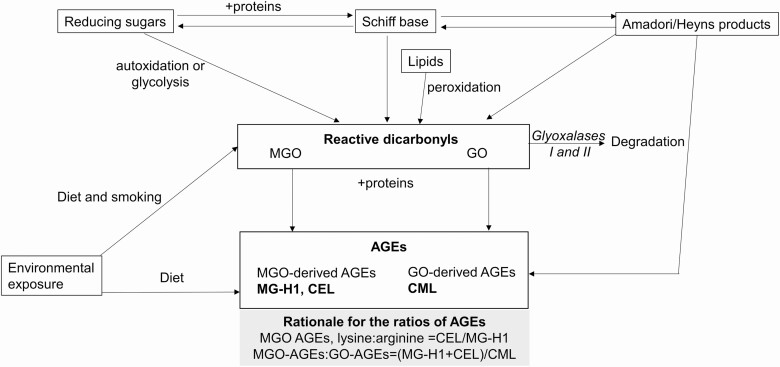
Plasma concentrations of protein-bound AGEs were determined with UPLC-MS/MS as previously described (29,30). In brief, protein-bound CML, CEL and MG-H1 were extracted from plasma using butanolic hydrochloric acid. The individual AGEs were quantified by calculating the area ratio of each unlabelled peak area to the corresponding internal standard. The sum of AGEs (ΣAGEs, in nmol/L) was calculated by summing up the circulating concentrations of CML, CEL and MG-H1 for each subject. We further calculated the ratios of the AGEs considering their dicarbonyl intermediates: MGO-derived:GO derived (i.e. CEL+MG-H1 divided by CML) (Figure 1). We also calculated the ratio of [CEL:MG-H1] to assess the influence of the relative abundance of lysine-sourced MGO-derived AGEs (CEL) versus arginine-sourced MGO-derived AGEs (MG-H1).
Figure 1.
Schematic representation of the formation of the AGEs and the rationale for the calculation of the ratios. AGE, advanced glycation end-product; CML, Nε-carboxy-methyllysine; CEL, Nε-carboxy-ethyllysine; GO, glyoxal; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; MGO, methylglyoxal. AGEs are absorbed from the diet or formed during the Maillard reactions from the Amadori or Heyns products and from the glycating actions of dicarbonyls such as MGO and GO. CML is derived from GO, whereas MG-H1 and CEL are derived from MGO.
Statistical analysis
Means, standard deviations or frequencies were calculated for all variables. Multivariable conditional logistic regression was used to estimate ORs and 95% CIs for CRC risk associated with circulating levels of protein-bound CML, CEL, MG-H1, ΣAGEs as well as [(CEL+MG-H1):CML] (i.e. MGO:GO AGEs) and [CEL:MG-H1]. For each main outcome variable (measured biomarker or calculated ratio), quintile cut points were determined based on the distribution in controls. We ran two models: Model 1 was conditioned on the matching factors and Model 2 was further adjusted for body mass index (BMI, continuous), height (continuous), highest attained education level (none, primary, technical and professional, secondary, higher), physical activity (inactive, moderately inactive, moderately active, active), smoking status/duration/intensity (never, current smokers 1 – ≤15, 16 – ≤25, >26 cigarettes/day; former smokers ≤10, 11 – ≤20, >20 years, occasional), and baseline intake levels of energy (continuous, kcal/day), alcohol, red and processed meats, dietary fibre and dairy products (all as continuous variables and as g/day). Tests for trend were run by using the median value of each quintile included in the model as continuous variables. Separate sub-group analyses were run by sex and anatomical sub-sites of CRC site (colon, rectal). The heterogeneity of the associations by sex, across anatomical sub-sites and in various sub-groups was assessed using the likelihood ratio test. We assessed the AGE-CRC association by sub-groups of type 2 diabetes (yes/no; self-reported at baseline) and obesity (defined as BMI ≥ 30 kg/m2). The potential bias of reverse causality in the AGE-CRC association was assessed by excluding cases diagnosed within the first 2 years. All the analyses were conducted using Stata 14.0 (StataCorp, College Station, TX, USA). Two-sided P-values <0.05 were statistically significant.
Results
Selected baseline characteristics of the study participants are shown in Table 1. Compared to controls, cases had higher BMI, higher intakes of alcohol and red and processed meats, and lower intakes of fruits, vegetables and dairy products. In addition, cases tended to be less physically active compared to controls.
Table 1.
Selected baseline demographic and lifestyle characteristics of study participants by colorectal cancer status, EPIC study 1992–2012
| Cases (n = 1378) | Controls (n = 1378) | |
|---|---|---|
| Women, % | 51.7 | 51.5 |
| Anthropometry, mean (SD) | ||
| BMI, kg/m2 | 26.7 ± 4.25 | 26.2 ± 3.74 |
| Waist circumference, cm | 90.4 ± 13.0 | 88.3 ± 12.1 |
| Waist-to-hip ratio | 0.88 ± 0.10 | 0.87 ± 0.10 |
| Lifestyle variables, n (%) | ||
| Smoking status frequency and intensity | ||
| Never | 542 (39.8) | 514 (37.9) |
| Current, 1–15 cigarettes/day | 139 (10.2) | 129 (9.51) |
| Current, 16–25 cigarettes/day | 94 (6.91) | 87 (6.40) |
| Current, 26+ cigarettes/day | 23 (1.69) | 20 (1.47) |
| Former, quit ≤ 10 years | 129 (9.48) | 139 (10.3) |
| Former, quit 11–20 years | 123 (9.04) | 144 (10.6) |
| Former, quit 20+ years | 177 (13.0) | 166 (12.2) |
| Current, pipe/cigar/occasional | 102 (7.49) | 125 (9.22) |
| Physical activity | ||
| Inactive | 361 (25.9) | 327 (23.3) |
| Moderately inactive | 448 (32.1) | 457 (32.6) |
| Moderately active | 311 (22.3) | 284 (20.3) |
| Active | 263 (18.9) | 314 (22.4) |
| Highest education level attained | ||
| None | 66 (4.85) | 68 (5.01) |
| Primary school completed | 490 (36.0) | 453 (33.4) |
| Technical/professional school | 343 (25.2) | 324 (23.9) |
| Secondary school | 184 (13.5) | 217 (16.0) |
| Higher education | 244 (17.9) | 247 (18.2) |
| Dietary intake, mean (SD) | ||
| Energy, kcal/day | 2127 ± 609 | 2124 ± 620 |
| Alcohol, g/day | 17.0 ± 22.1 | 15.4 ± 19.7 |
| Red and processed meats, g/day | 87.6 ± 53.1 | 85.1 ± 52.0 |
| Fruits and vegetables, g/day | 396 ± 233 | 421 ± 248 |
| Cereals, g/day | 216 ± 121 | 216 ± 119 |
| Dairy products, g/day | 331 ± 251 | 351 ± 244 |
| Fish and products, g/day | 28.2 ± 28.8 | 29.6 ± 30.6 |
| Sugar, cakes and confectionaries, g/day | 48.7 ± 66.6 | 48.7 ± 68.9 |
| Fats, g/day | 28.3 ± 15.6 | 27.9 ± 16.0 |
| Protein, g/day | 89.3 ± 27.9 | 90.3 ± 27.5 |
| AGEs biomarkers, mean (SD) | ||
| CML, nmol/l | 2719 ± 1046 | 2855 ± 1075 |
| CEL, nmol/l | 1475 ± 772 | 1475 ± 740 |
| MG-H1, nmol/l | 1056 ± 259 | 1079 ± 262 |
| ΣAGEs, nmol/l | 5250 ± 1488 | 5411 ± 1470 |
| CEL:MG-H1 | 1.45 ± 0.79 | 1.43 ± 0.75 |
| (CEL+MG-H1): CML | 1.01 ± 0.39 | 0.98 ± 0.38 |
Frequencies may not add up to 100% due to missing data. AGE, advanced glycation end products; BMI, body mass index; CML, Nε-carboxymethyllysine; CEL, Nε-carboxyethyllysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl) ornithine.
The associations of individual and combined AGEs, and their various ratios calculated based on pathway of AGEs derivation, are shown in Table 2. No statistically significant association was observed between CEL and CRC risk (OR comparing highest to lowest quintile, ORQ5 versus Q1 = 0.88, 95% CI: 0.64–1.19, Ptrend = 0.580), whereas inverse associations for CRC were observed for both CML (ORQ5 versus Q1 = 0.40, 95% CI: 0.27–0.59, Ptrend < 0.001) and MG-H1 (ORQ5 versus Q1 = 0.73, 95% CI: 0.53–1.00, Ptrend = 0.016). A near 50% lower odds for developing CRC was observed for ΣAGEs (ORQ5 versus Q1 = 0.52, 95% CI: 0.37–0.73, Ptrend < 0.001), mostly driven by CML and MG-H1. The ratio of [(CEL+MG-H1): CML] was associated with an increased risk or CRC (ORQ5 versus Q1 = 1.91, 95% CI: 1.31–2.79, Ptrend = 0.004).
Table 2.
ORs and 95% CI for colorectal cancer risk associated with circulating AGEs and their ratios, EPIC study 1992–2012
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P trend | |
|---|---|---|---|---|---|---|
| CML | ||||||
| Range, nmol/L | <2014 | 2014 – <2401 | 2401 – <2805 | 2805 – <3505 | ≥3505 | |
| Cases/controls | 302/276 | 338/276 | 280/275 | 247/276 | 211/275 | |
| Model 1a | 1.00 (ref.) | 0.96 (0.75–1.23) | 0.74 (0.57–0.97) | 0.58 (0.44–0.78) | 0.37 (0.26–0.52) | <0.001 |
| Model 2b | 1.00 (ref.) | 0.97 (0.75–1.26) | 0.79 (0.59–1.04) | 0.63 (0.46–0.86) | 0.40 (0.27–0.59) | <0.001 |
| CEL | ||||||
| Range, nmol/L | <986 | 986 – <1234 | 1234 – <1478 | 1478 – <1807 | ≥1807 | |
| Cases/controls | 272/276 | 270/276 | 286/276 | 290/275 | 260/275 | |
| Model 1a | 1.00 (ref.) | 0.96 (0.74–1.23) | 1.02 (0.78–1.33) | 1.01 (0.76–1.33) | 0.89 (0.66–1.19) | 0.605 |
| Model 2b | 1.00 (ref.) | 0.98 (0.76–1.27) | 1.04 (0.79–1.37) | 1.04 (0.78–1.39) | 0.88 (0.64–1.19) | 0.580 |
| MG-H1 | ||||||
| Range, nmol/L | <872 | 872 – <974 | 974 – <1082 | 1082 – <1248 | ≥1248 | |
| Cases/controls | 309/276 | 308/275 | 260/276 | 256/274 | 244/276 | |
| Model 1a | 1.00 (ref.) | 0.94 (0.73–1.20) | 0.75 (0.58–0.97) | 0.71 (0.54–0.93) | 0.68 (0.50–0.91) | 0.002 |
| Model 2b | 1.00 (ref.) | 0.97 (0.75–1.25) | 0.79 (0.61–1.03) | 0.77 (0.58–1.02) | 0.73 (0.53–1.00) | 0.016 |
| ΣAGEs, nmol/L | ||||||
| Range, nmol/L | <4284 | 4284 – <4848 | 4848 – <5414 | 5414 – <6306 | ≥6306 | |
| Cases/controls | 334/276 | 315/276 | 275/276 | 219/276 | 235/274 | |
| Model 1a | 1.00 (ref.) | 0.89 (0.70–1.14) | 0.73 (0.56–0.95) | 0.52 (0.39–0.68) | 0.48 (0.35–0.65) | <0.001 |
| Model 2b | 1.00 (ref.) | 0.93 (0.72–1.19) | 0.76 (0.58–1.00) | 0.54 (0.41–0.73) | 0.52 (0.37–0.73) | <0.001 |
| CEL:MG-H1 | ||||||
| Range | <0.89 | 0.89 – <1.15 | 1.15 – <1.43 | 1.43 – <1.81 | ≥1.81 | |
| Cases/controls | 247/276 | 274/276 | 263/275 | 295/275 | 298/275 | |
| Model 1a | 1.00 (ref.) | 1.09 (0.85–1.40) | 1.09 (0.83–1.42) | 1.27 (0.96–1.68) | 1.33 (0.98–1.80) | 0.047 |
| Model 2b | 1.00 (ref.) | 1.13 (0.87–1.47) | 1.08 (0.82–1.42) | 1.27 (0.95–1.70) | 1.26 (0.91–1.73) | 0.139 |
| (CEL+MG-H1): CML | ||||||
| Range | <0.66 | 0.66 – <0.86 | 0.86 – <1.02 | 1.02 – <1.24 | ≥1.24 | |
| Cases/controls | 233/276 | 279/276 | 263/275 | 280/275 | 322/275 | |
| Model 1a | 1.00 (ref.) | 1.49 (1.12–1.99) | 1.64 (1.19–2.27) | 1.70 (1.21–2.39) | 2.14 (1.50–3.05) | <0.001 |
| Model 2b | 1.00 (ref.) | 1.42 (1.05–1.90) | 1.54 (1.10–2.16) | 1.54 (1.08–2.19) | 1.91 (1.31–2.79) | 0.004 |
Quintiles were created based on the distribution in the control group. MG-H1 has one missing data, hence MG-H1, CEL:MG-H1 and (CEL+MG-H1): CML have 1377 cases and 1377 matched controls. AGE, advanced glycation end-product; CI, confidence interval; CML, Nε-carboxy-methyllysine; CEL, Nε-carboxy-ethyllysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; OR, odds ratio.
aModel 1 was conditioned on matching factors: age at blood collection (±1 year), sex, recruiting centre, time of the day at blood collection (±3 h), fasting status at blood collection (<3, 3–6 and >6 h), and, additionally, among women by menopausal status (pre-menopause, peri-menopause, and post-menopause) and hormone replacement therapy use at time of blood collection (yes/no).
bModel 2 model was Model 1 adjusted for BMI (continuous), height (continuous), education (none, primary, technical and professional, secondary, higher education), physical activity (inactive, moderately inactive, moderately active, active), smoking status, duration and intensity (never, 1–15 cigarettes/day, 16–25 cigarettes/day, over 26 cigarettes/day, former smokers who quit<10 years, former smokers who quit 11–20 years, former smokers who quit >20 years, current pipe-cigar and occasional smokers), energy intake (continuous), alcohol intake (continuous), processed meat intake (continuous), fibre intake (continuous) and dairy products intake (continuous).
We did not observe significant heterogeneity by sex or by tumour anatomical sub-sites for individual AGEs, ΣAGEs and [(CEL+MG-H1):CML] (Table 3). Analyses stratified by baseline diabetes status and by obesity as indicated by BMI ≥ 30 kg/m2 showed that [(CEL+MG-H1):CML] was associated with higher CRC risk in diabetic versus non-diabetic subjects, and in obese versus non-obese subjects (Table 4). The inverse associations observed with individual AGEs and for ΣAGEs were more prominent in obese individuals compared to non-obese ones.
Table 3.
ORs and 95% CI for colorectal cancer risk associated with 1 SD increase in circulating AGEs and their ratios, by sex and by tumour anatomical sub-site, EPIC study 1992–2012
| Colorectal cancer | Colon cancer | Rectal cancer | |||||
|---|---|---|---|---|---|---|---|
| Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | P heterogeneity by tumour sub-site | |
| CML, nmol/L | |||||||
| All | 1378/1378 | 0.75 (0.66–0.85) | 871/871 | 0.69 (0.58–0.83) | 503/503 | 0.81 (0.66–1.00) | 0.073 |
| Men | 679/679 | 0.69 (0.57–0.83) | 404/404 | 0.66 (0.51–0.86) | 272/272 | 0.67 (0.49–0.92) | 0.162 |
| Women | 699/699 | 0.81 (0.67–0.97) | 467/467 | 0.75 (0.59–0.96) | 231/231 | 0.90 (0.65–1.26) | 0.323 |
| Pheterogeneity by sex | 0.197 | 0.223 | 0.622 | ||||
| CEL, nmol/L | |||||||
| All | 1378/1378 | 0.98 (0.88–1.08) | 871/871 | 0.98 (0.86–1.11) | 503/503 | 1.00 (0.83–1.20) | 0.986 |
| Men | 679/679 | 1.01 (0.85–1.20) | 404/404 | 0.99 (0.77–1.25) | 272/272 | 1.10 (0.82–1.46) | 0.291 |
| Women | 699/699 | 0.97 (0.85–1.11) | 467/467 | 1.00 (0.85–1.17) | 231/231 | 0.83 (0.61–1.12) | 0.298 |
| Pheterogeneity by sex | 0.839 | 0.596 | 0.129 | ||||
| MG-H1, nmol/L | |||||||
| All | 1377/1377 | 0.88 (0.79–0.98) | 871/871 | 0.81 (0.71–0.93) | 503/503 | 0.99 (0.83–1.17) | 0.056 |
| Men | 678/678 | 0.83 (0.71–0.97) | 404/404 | 0.77 (0.62–0.95) | 272/272 | 0.87 (0.68–1.13) | 0.173 |
| Women | 699/699 | 0.92 (0.80–1.07) | 467/467 | 0.87 (0.72–1.05) | 231/231 | 1.01 (0.78–1.31) | 0.346 |
| Pheterogeneity by sex | 0.313 | 0.332 | 0.701 | ||||
| ΣAGEs, nmol/L | |||||||
| All | 1377/1377 | 0.81 (0.72–0.91) | 871/871 | 0.76 (0.65–0.89) | 503/503 | 0.76 (0.65–0.89) | 0.144 |
| Men | 678/678 | 0.76 (0.64–0.91) | 404/404 | 0.71 (0.55–0.91) | 272/272 | 0.81 (0.61–1.07) | 0.066 |
| Women | 699/699 | 0.85 (0.73–1.01) | 467/467 | 0.84 (0.68–1.03) | 231/231 | 0.85 (0.62–1.15) | 0.913 |
| Pheterogeneity by sex | 0.233 | 0.083 | 0.747 | ||||
| CEL:MG-H1 | |||||||
| All | 1377/1377 | 1.03 (0.93–1.14) | 871/871 | 1.05 (0.93–1.19) | 503/503 | 0.99 (0.82–1.21) | 0.517 |
| Men | 678/678 | 1.10 (0.91–1.32) | 404/404 | 1.07 (0.83–1.37) | 272/272 | 1.22 (0.87–1.70) | 0.572 |
| Women | 699/699 | 1.03 (0.90–1.17) | 467/467 | 1.08 (0.93–1.26) | 231/231 | 0.81 (0.60–1.09) | 0.175 |
| Pheterogeneity for sex | 0.528 | 0.967 | 0.110 | ||||
| (CEL+MG-H1):CML | |||||||
| All | 1377/1377 | 1.15 (1.03–1.29) | 871/871 | 1.15 (1.00–1.32) | 503/503 | 1.17 (0.94–1.46) | 0.630 |
| Men | 678/678 | 1.34 (1.10–1.64) | 404/404 | 1.27 (0.99–1.64) | 272/272 | 1.65 (1.12–2.44) | 0.852 |
| Women | 699/699 | 1.10 (0.95–1.27) | 467/467 | 1.15 (0.97–1.37) | 231/231 | 0.89 (0.65–1.23) | 0.292 |
| Pheterogeneity by sex | 0.084 | 0.292 | 0.065 | ||||
MG-H1 has one missing data, hence MG-H1, CEL:MG-H1 and (CEL+MG-H1): CML have 1377 cases and 1377 matched controls. Four cases of overlapping tumours were considered as colorectal cancer cases, but not classified as colon malignant tumour or rectal one. AGE, advanced glycation end-product; CI, confidence interval; CML, Nε-carboxy-methyllysine; CEL, Nε-carboxy-ethyllysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; OR, odds ratio.
Models were conditioned on matching factors: age at blood collection (±1 year), sex, recruiting centre, time of the day at blood collection (±3 h), fasting status at blood collection (<3, 3–6 and >6 h), and, additionally, among women by menopausal status (pre-menopause, peri-menopause and post-menopause) and hormone replacement therapy use at time of blood collection (yes/no) and adjusted for body mass index (continuous), height (continuous), education (none, primary, technical and professional, secondary, higher education), physical activity (inactive, moderately inactive, moderately active, active), smoking status, duration and intensity (never, 1–15 cigarettes/day, 16–25 cigarettes/day, over 26 cigarettes/day, former smokers who quit <10 years, former smokers who quit 11–20 years, former smokers who quit >20 years, current pipe-cigar and occasional smokers), energy intake (continuous), alcohol intake (continuous), processed meat intake (continuous), fibre intake (continuous) and dairy products intake (continuous).
Table 4.
ORs and 95% CI for colorectal cancer risk associated with 1 SD increase in circulating AGEs and their ratios, stratified by obesity and diabetes status, EPIC study 1992–2012
| Diabetesa | Obeseb | |||||
|---|---|---|---|---|---|---|
| Yes (n = 61) | No (n = 1099) | P for heterogeneity | Yes (n = 247) | No (n = 1131) | P for heterogeneity | |
| CML | 0.56 (0.25–1.25) | 0.74 (0.64–0.85) | 0.486 | 0.30 (0.19–0.47) | 0.85 (0.74–0.97) | <0.001 |
| CEL | 2.07 (0.89–4.82) | 0.99 (0.88–1.10) | 0.088 | 1.01 (0.68–1.50) | 1.01 (0.90–1.12) | 0.999 |
| MG-H1 | 1.20 (0.55–2.61) | 0.87 (0.78–0.98) | 0.443 | 0.46 (0.32–0.67) | 0.96 (0.86–1.07) | <0.001 |
| ΣAGEs | 1.07 (0.48–2.40) | 0.81 (0.72–0.92) | 0.499 | 0.38 (0.25–0.57) | 0.90 (0.80–1.02) | <0.001 |
| CEL:MG-H1 | 1.73 (0.78–3.84) | 1.05 (0.94–1.17) | 0.228 | 1.59 (1.11–2.27) | 1.02 (0.92–1.14) | 0.019 |
| (CEL+MG-H1):CML | 2.94 (1.21–7.15) | 1.19 (1.05–1.35) | 0.046 | 2.36 (1.60–3.47) | 1.10 (0.98–1.24) | <0.001 |
AGE, advanced glycation end-product; CI, confidence interval; CML, Nε-carboxy-methyllysine; CEL, Nε-carboxy-ethyllysine; MG-H1, Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine; OR, odds ratio.
Models were conditioned on matching factors: age at blood collection (±1 year), sex, recruiting centre, time of the day at blood collection (±3 h), fasting status at blood collection (<3, 3–6 and >6 hours), and, additionally, among women by menopausal status (pre-menopause, peri-menopause and post-menopause), and hormone replacement therapy use at time of blood collection (yes/no) and adjusted for height (continuous), education (none, primary, technical and professional, secondary, higher education), physical activity (inactive, moderately inactive, moderately active, active), smoking status, duration and intensity (never, 1–15 cigarettes/day, 16–25 cigarettes/day, over 26 cigarettes/day, former smokers who quit <10 years, former smokers who quit 11–20 years, former smokers who quit >20 years, current pipe-cigar and occasional smokers), energy intake (continuous), alcohol intake (continuous), processed meat intake (continuous), fibre intake (continuous) and dairy products intake (continuous).
aSelf-reported history of diabetes at baseline (n missing = 218).
bObesity was defined depending on body mass index (BMI). Obese: BMI ≥ 30 kg/m2 and non-obese: BMI < 30 kg/m2.
Discussion
In this study, we found that higher circulating levels of protein-bound CML, MG-H1 and ΣAGEs, but not CEL, were associated with a lower risk of CRC. We also observed that higher concentrations of MGO-derived AGEs relative to GO-derived AGEs were associated with higher CRC risk.
The inverse associations observed between the AGEs concentrations evaluated in our study and CRC risk contrast with our hypothesis that these AGEs contribute to colorectal carcinogenesis. Specifically, for CML, it is noteworthy that Jiao et al. (22) also reported similar inverse associations as observed in our study. However, their study was based on a sub-population of male, Finnish smokers and applied an ELISA-based methodology to assess relative differences of CML between cancer cases and controls. Unfortunately, ELISA methods have limited reproducibility and do not differentiate between protein-bound and free fractions of circulating AGEs. Our initial hypothesis for positive AGEs–CRC risk association was based on evidence from experimental studies indicating that AGEs are DNA-damaging and can directly induce sustained inflammation in colon tissues through binding with RAGE (17,19,31). We chose to analyze protein-bound AGEs because these are specifically recognized by RAGE (32–34).
We assessed CML, CEL and MG-H1 because they are thought to be the most abundant AGEs in the body and although there is evidence that they have harmful pro-inflammatory and pro-oxidative effects, their relationship with the colonic mucosa may be very complicated. The colonic mucosa may be exposed to a variety of other AGEs, both exogenously from the diet and those produced endogenously within the body and colonic milieu. Similar to endogenous AGEs, dietary AGEs may increase the body AGEs pool, interact directly with the colon mucosal and increase the risk for obesity (35). Some researchers have classified AGEs into two categories of ‘non-toxic’ and ‘toxic’ (36–38). This categorization still requires much further evidence, but it is noteworthy that we have previously shown an increased risk of rectal cancer with higher circulating levels of glyceraldehyde-derived AGEs (39), that have been categorized as ‘toxic’ (6,36). Another speculative explanation of our observations is that circulating AGE concentration may not be reflective of their levels in colorectal tissues where they may accumulate. There is a paucity of supportive data for this assertion, and little is known about whether the concentration and actions of specific AGEs may differ between tissues. Van Heijst et al. (40) observed varying AGE levels from an immunohistochemical expression of CML and the MGO-derived AGE argpyrimidine in various human tumours (muscle, colon, breast and larynx), suggesting that separate AGEs impact tissues differently. Therefore, relevant studies focusing on the functions, effects and the interactions of CML, CEL and MG-H1 and additional AGEs within normal and cancerous colonic tissues are warranted.
The positive association observed with the ratio of MGO-derived AGEs:GO-derived AGEs and CRC risk suggests that the conditions that may lead to more MGO-AGEs versus GO-AGEs may be important in CRC development. This result may implicate that higher circulating MGO versus GO may be of greater importance in CRC development than AGEs. Both MGO and GO are mainly detoxified through the glyoxalase system and by other enzymes such as aldo-keto reductases and dicarbonyl and L-xylulose reductase (41,42). Compared to GO, MGO is more reactive (43) but is rapidly and efficiently detoxified, mainly in the liver (42). This may possibly explain the higher CRC risk observed with MGO–AGE–GO–AGEs in obese individuals. Obesity is often associated with a degree of liver steatosis and decreased liver function and could possibly explain lower clearance of MGO with spillover into the circulation. It can be speculated that in the presence of a ‘competition’ between the production and the detoxification of dicarbonyls, MGO and its derived AGEs may be harmful to the colon tissue, and sustain systemic inflammation, compared to GO and derived AGEs—but this requires further investigation. Diabetes has been associated with a higher risk of CRC (44). Because diabetes is associated with poor glycaemic control, hyperglycaemia and enhanced production of AGEs, one would expect that the AGE–CRC association is higher in subjects with diabetes compared to those without. Additional studies should explore whether CRC risk associated with diabetes could be partially mediated through AGEs. Likewise, future studies may also explore to which extent treatment for diabetes may mitigate endogenous AGEs production and possibly CRC risk.
It is also noteworthy that dicarbonyls and some AGEs derived from them display hormetic properties, where lower levels are associated with beneficial health outcomes while higher levels are deleterious. Hormetic effects have been reported for lower levels of MGO which have been showed to prevent tumour growth, whereas higher levels promote tumour expansion (45). Surprisingly, it has been reported that another MGO-derived AGE, MG-H3, has anti-oxidative properties comparable with those of ascorbic acid (46). There is substantial evidence showing that the deleterious effects of AGEs are dependent upon the level of RAGE activity. In RAGE knockout mice, cancer development is greatly reduced, suggesting that the cancer-promotive and pro-inflammatory effects of AGEs are necessarily expressed in the presence of RAGE (47,48). Interestingly, there is mounting evidence showing that soluble RAGE, a free circulating isoform of RAGE, is inversely associated with CRC (21,49). The knowledge of AGEs metabolism and CRC need to be expanded, and additional studies are needed to better understand the role of dicarbonyls, and derived AGEs in the aetiology of CRC.
This study has several strengths, including the quantitative measurement of CEL, CML and MG-H1 by a state-of-the-art UPLC-MS/MS instrumental method. UPLC-MS/MS could be considered the gold standard method for the analysis of AGEs in plasma. UPLC-MS/MS could be used to accurately and precisely measure specific AGEs in both free and protein-bound forms. The major known drawback of using UPLC-MS/MS is its relatively higher cost and the necessity for trained personnel (23,50). Additional strengths include the prospective design, the large sample size and the ability to conduct analyses stratified by sex, and by anatomical sub-site (colon versus rectum). A limitation to our study is that we lack information on other AGEs produced from MGO including other MGO-derived hydroimidazolone (MG-Hs) such as MG-H2, MG-H3 and MG-H4, Nd-(4-carboxy-4,6-dimethyl5,6-dihydroxy-1,4,5,6-tetrahydropyrimidin-2-yl)-ornithine, argpyrimidine and cross-linking dimer methylglyoxal-lysine dimer (8), which may all have roles in CRC development. Another limitation is the use of plasma AGEs levels which are dependent on kidney and liver functions; hence they may not represent tissue levels. Further research is required to determine how circulating AGE measures in the same individual may relate to levels in colon tumour and normal colon tissues. Therefore, our assessment of AGEs in CRC development is far from complete, even though we analysed three major AGEs compounds. Also, our findings show that circulating measures of AGEs are likely to have differential associations with CRC, indicating that their posited detrimental properties may not be equivalent or that they vary in their pro-inflammatory capacity. More study is required on the individual and interactive roles of AGEs in the development of cancers and other chronic diseases. A deeper assessment of the qualitative pathways of AGEs production and their cumulative roles in cancer development may shed more insight into this fascinating topic. An additional limitation of this nested case–control study is the fact that blood samples and lifestyle factors were collected at baseline and may not necessarily reflect changes over time.
In conclusion, in this large, comprehensive prospective study, CML and MG-H1 are inversely associated with CRC risk, contrary to our initial hypothesis. However, we observed a significantly higher CRC risk with higher ratio of MGO-derived:GO-dervied AGEs. Our observations highlight the complexity of the proposed roles of AGEs in CRC development and suggest that AGEs levels may not be interpreted alone, but in consideration of their chemical origins. Additional studies examining toxic dicarbonyl AGE precursor compounds in CRC development and assessing the role of AGEs in the colonic milieu and within normal and tumorigenic colonic tissues are required. In addition, the development of laboratory instrumental methodologies for the assessment of a larger number of AGEs would aid greatly in better defining the roles of this diverse family of compounds in health and disease.
Acknowledgements
The authors would like to thank the EPIC study participants and staff for their valuable contribution to this research. The authors would also like to thank Mr Bertrand Hemon for the preparation of the databases. The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (the Netherlands); Health Research Fund (FIS)—Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology—ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford) (UK); and the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands. The EPIC-Norfolk study (doi: 10.22025/2019.10.105.00004) has received funding from the Medical Research Council (MR/N003284/1, MC-PC_13048 and MC-UU_12015/1). We are grateful to all the participants who have been part of the project and to the many members of the study teams at the University of Cambridge who have enabled this research. We are grateful to and acknowledge participants and collaborators from EPIC centres in France, Utrecht, Asturias, and Umea who also contributed data in this study. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php. Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Conflict of interest statement: None declared.
Glossary
Abbreviations
- AGE
advanced glycation end-product
- BMI
body mass index
- CEL
Nε-(carboxyethyl)lysine
- CI
confidence interval
- CML
Nε-(carboxymethyl)lysine
- CRC
colorectal cancer
- EPIC
European Prospective Investigation into Cancer and Nutrition
- GO
glyoxal
- IARC
International Agency for Research on Cancer
- ICD
International Classification of Diseases
- MG-H1
Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine
- MGO
methylglyoxal
- RAGE
receptor for AGE
- SD
standard deviation
- UPLC-MS/MS
ultra-performance liquid chromatography–tandem mass spectrometry
Funding
Funding (WCRF 2015/1391, PI: M.J.) was obtained from Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme.
References
- 1. Bray, F., et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Safiri, S., et al. (2019) The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol., 4, 913–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy, N., et al. (2019) Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Aspects Med, 69, 2–9. [DOI] [PubMed] [Google Scholar]
- 4. Mehta, R.S., et al. (2017) Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology, 152, 1944–1953.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cerami, C., et al. (1997) Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl Acad. Sci. USA, 94, 13915–13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takeuchi, M., et al. (2000) Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med., 6, 114–125. [PMC free article] [PubMed] [Google Scholar]
- 7. Scheijen, J.L.J.M., et al. (2016) Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: presentation of a dietary AGE database. Food Chem., 190, 1145–1150. [DOI] [PubMed] [Google Scholar]
- 8. Sharma, C., et al. (2015) Advanced glycation end-products (AGEs): an emerging concern for processed food industries. J. Food Sci. Technol., 52, 7561–7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schalkwijk, C.G., et al. (2020) Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev., 100, 407–461. [DOI] [PubMed] [Google Scholar]
- 10. Turk, Z. (2010) Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol. Res., 59, 147–156. [DOI] [PubMed] [Google Scholar]
- 11. Thornalley, P.J. (2005) Dicarbonyl intermediates in the Maillard reaction. Ann. NY Acad. Sci., 1043, 111–117. [DOI] [PubMed] [Google Scholar]
- 12. Nowotny, K., et al. (2018) Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev., 47, 55–66. [DOI] [PubMed] [Google Scholar]
- 13. Arribas-Lorenzo, G., et al. (2010) Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem., 58, 2966–2972. [DOI] [PubMed] [Google Scholar]
- 14. Raupbach, J., et al. (2020) Proteasomal degradation of glycated proteins depends on substrate unfolding: preferred degradation of moderately modified myoglobin. Free Radic. Biol. Med., 152, 516–524. [DOI] [PubMed] [Google Scholar]
- 15. Palanissami, G., et al. (2018) RAGE and its ligands: molecular interplay between glycation, inflammation, and hallmarks of cancer—a review. Horm. Cancer, 9, 295–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakellariou, S., et al. (2016) Clinical significance of AGE-RAGE axis in colorectal cancer: associations with glyoxalase-I, adiponectin receptor expression and prognosis. BMC Cancer, 16, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuniyasu, H., et al. (2003) Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int. J. Cancer, 104, 722–727. [DOI] [PubMed] [Google Scholar]
- 18. Chen, H., et al. (2014) Advanced glycation end products increase carbohydrate responsive element binding protein expression and promote cancer cell proliferation. Mol. Cell. Endocrinol., 395, 69–78. [DOI] [PubMed] [Google Scholar]
- 19. Shimomoto, T., et al. (2012) Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J. Gastroenterol., 47, 1073–1083. [DOI] [PubMed] [Google Scholar]
- 20. Wang, P., et al. (2018) Advanced glycation end products increase MDM2 expression via transcription factor KLF5. J. Diabetes Res., 2018, 3274084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen, L., et al. (2016) A prospective study of soluble receptor for advanced glycation end-products and colorectal cancer risk in postmenopausal women. Cancer Epidemiol., 42, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiao, L., et al. (2011) Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev., 20, 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Vos, L.C., et al. (2016) Advanced glycation end products: an emerging biomarker for adverse outcome in patients with peripheral artery disease. Atherosclerosis, 254, 291–299. [DOI] [PubMed] [Google Scholar]
- 24. Baldensperger, T., et al. (2020) Comprehensive analysis of posttranslational protein modifications in aging of subcellular compartments. Sci. Rep., 10, 7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmed, M.U., et al. (1997) N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J., 324 (Pt 2), 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verzijl, N., et al. (2000) Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J., 350(Pt 2), 381–387. [PMC free article] [PubMed] [Google Scholar]
- 27. Riboli, E., et al. (2002) European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr., 5(6B), 1113–1124. [DOI] [PubMed] [Google Scholar]
- 28. Wareham, N.J., et al. (2002) Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int. J. Epidemiol., 31, 168–174. [DOI] [PubMed] [Google Scholar]
- 29. Scheijen, J.L.J.M., et al. (2018) Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: the CODAM study. Clin. Nutr., 37, 919–925. [DOI] [PubMed] [Google Scholar]
- 30. Maasen, K., et al. (2019) High dietary glycemic load is associated with higher concentrations of urinary advanced glycation endproducts: the Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Am. J. Clin. Nutr., 110, 358–366. [DOI] [PubMed] [Google Scholar]
- 31. Stopper, H., et al. (2003) Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett., 190, 151–156. [DOI] [PubMed] [Google Scholar]
- 32. Xue, J., et al. (2014) The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry, 53, 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue, J., et al. (2011) Advanced glycation end product recognition by the receptor for AGEs. Structure, 19, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xie, J., et al. (2008) Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J. Biol. Chem., 283, 27255–27269. [DOI] [PubMed] [Google Scholar]
- 35. Cordova, R., et al. (2020) Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur. J. Nutr., 59, 2893–2904. [DOI] [PubMed] [Google Scholar]
- 36. Takata, T., et al. (2019) Intracellular toxic advanced glycation end-products in cardiomyocytes may cause cardiovascular disease. Sci. Rep., 9, 2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeuchi, M., et al. (2000) Neurotoxicity of advanced glycation end-products for cultured cortical neurons. J. Neuropathol. Exp. Neurol., 59, 1094–1105. [DOI] [PubMed] [Google Scholar]
- 38. Sato, T., et al. (2006) Toxic advanced glycation end products (TAGE) theory in Alzheimer’s disease. Am. J. Alzheimers. Dis. Other Demen., 21, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kong, S.Y., et al. (2015) The association between glyceraldehyde-derived advanced glycation end-products and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev., 24, 1855–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Heijst, J.W., et al. (2005) Advanced glycation end products in human cancer tissues: detection of N-epsilon-(carboxymethyl)lysine and argpyrimidine. Ann. NY Acad. Sci., 1043, 725–733. [DOI] [PubMed] [Google Scholar]
- 41. Kalousová, M., et al. (2010) Genetic predisposition to advanced glycation end products toxicity is related to prognosis of chronic hemodialysis patients. Kidney Blood Press. Res., 33, 30–36. [DOI] [PubMed] [Google Scholar]
- 42. Yang, K., et al. (2011) Differences in glyoxal and methylglyoxal metabolism determine cellular susceptibility to protein carbonylation and cytotoxicity. Chem. Biol. Interact., 191, 322–329. [DOI] [PubMed] [Google Scholar]
- 43. Maessen, D.E., et al. (2016) Energy restriction and Roux-en-Y gastric bypass reduce postprandial α-dicarbonyl stress in obese women with type 2 diabetes. Diabetologia, 59, 2013–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ma, Y., et al. (2018) Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br. J. Cancer, 119, 1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morgenstern, J., et al. (2020) The glyoxalase system—new insights into an ancient metabolism. Antioxidants (Basel), 9, 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang, T., et al. (2012) Exploring post-translational arginine modification using chemically synthesized methylglyoxal hydroimidazolones. J. Am. Chem. Soc., 134, 8958–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kwak, T., et al. (2017) Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene, 36, 1559–1572. [DOI] [PubMed] [Google Scholar]
- 48. Gebhardt, C., et al. (2008) RAGE signaling sustains inflammation and promotes tumor development. J. Exp. Med., 205, 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aglago, E.K., et al. (2021) Soluble receptor for advanced glycation end-products (sRAGE) and colorectal cancer risk: a case–control study nested within a European prospective cohort. Cancer Epidemiol. Biomarkers Prev., 30, 182–192. [DOI] [PubMed] [Google Scholar]
- 50. Perrone, A., et al. (2020) Advanced glycation end products (AGEs): biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell. Longev., 2020, 3818196. [DOI] [PMC free article] [PubMed] [Google Scholar]