Abstract
Objective.
Cardiovascular disease (CVD) is accelerated in patients with systemic lupus erythematosus and lupus nephritis (LN). Despite the literature suggesting that renal arteriosclerosis predicts CVD in other glomerulonephritis diseases, arteriosclerosis grading and reporting might be particularly overlooked in LN biopsies. Our objective was to examine the burden of renal arteriosclerosis in LN and to assess whether arteriosclerosis is underreported in LN biopsies.
Methods.
We identified all patients with LN undergoing kidney biopsy between 1994 and 2017 at an academic center. We interpreted LN biopsy reports to classify the Banff categories of absent, mild, moderate, or severe renal arteriosclerosis. The prevalence of renal arteriosclerosis was compared with the prevalence published for age-matched healthy peers, and predictors of arteriosclerosis were examined. We overread biopsies for Banff renal arteriosclerosis grading and compared to pathology reports.
Results.
Among 189 incident patients with LN, renal arteriosclerosis prevalence was 2 decades earlier compared to their healthy peers, affecting 40% of patients ages 31–39 years with LN compared to 44% of healthy peers ages 50–59 years. A multivariable analysis showed a 3-fold higher odds of renal arteriosclerosis in patients ages ≥30 years with LN. LN chronicity on biopsy results predicted a 4-fold higher odds of renal arteriosclerosis. The overreads determined that 50% of standard LN biopsy reports missed reporting the presence or absence of renal arteriosclerosis.
Conclusion.
Renal arteriosclerosis is accelerated by 2 decades in patients with LN compared to their healthy peers and is overlooked by pathologists in half of the routine biopsy reports. We propose incorporating Banff renal arteriosclerosis grading in all LN biopsy reports.
INTRODUCTION
Premature cardiovascular disease (CVD) in patients with systemic lupus erythematosus (SLE) has been recently attributed to the interplay between inflammatory and immune mechanisms of atherosclerosis (1,2). Further, lupus nephritis (LN) is an independent risk factor for CVD, conferring a 9-fold higher risk of CVD events compared to healthy peers (3) and a 6-fold higher risk compared to patients with SLE without LN (4).
Methods to identify and prevent CVD early in patients with LN have not been determined, aside from managing CVD risk factors such as hypertension, tobacco use, and hyperlipidemia. There is limited information on early indicators of CVD in patients with LN. Hence, health care teams cannot implement timely preventive strategies to reduce the CVD burden in patients with LN (1,3,4). Clinicians urgently need early predictors of CVD in patients with LN to prevent related morbidity and mortality.
The classification system of the International Society of Nephrology/Renal Pathology Society (ISN/RPS) for LN primarily focuses on glomerular pathology and places no emphasis on standard or systematic grading of vascular lesions (5,6). Other renal pathology classification systems, such as the Banff classification system used in renal transplantation, apply quantitative assessment across all renal structures, including the vasculature (7). Therefore, nonglomerular biopsy findings, including renal arterial changes, may have been overlooked as a method to identify CVD risk in patients with LN (8). A few contradicting studies have evaluated the burden of renal arterial changes in LN (8–13), but only 2 studies examined the association between CVD events and renal arterial changes (9,10). Both studies found a poor association between graded renal arterial changes and CVD events (9,10). However, none of these LN studies used standard systematic grading for renal arterial changes, such as Banff criteria that are universally used to grade renal arteriosclerosis in all transplant and donor biopsy results (8–13).
Among patients with IgA nephropathy and renal transplant patients and donors, researchers use Banff scoring to grade renal arterial changes (14–16). They report that severe renal arteriosclerosis is an early predictor of CVD in both IgA nephropathy and transplant patients (14,16). Other studies report a similar correlation between renal arteriosclerosis and coronary atherosclerosis, suggesting that renal arteriosclerosis is an initial step associated with accelerated atherosclerosis and CVD (14,16,17).
We hypothesized that a similar correlation exists in patients with LN but is often missed due to the absence of systematic reporting of renal arterial changes. The underreporting of renal arteriosclerosis may explain contradicting results in prior LN studies, and systematic reporting could offer new methods to target CVD prevention.
In our current study, our objective was to examine the burden of renal arteriosclerosis in kidney biopsy reports of patients with LN and to compare the prevalence rates of renal arteriosclerosis in patients with LN to the prevalence in healthy kidney donors by age group. We also aimed to use systematic Banff criteria on a subsample of LN biopsy reports to assess whether arteriosclerosis and its severity are underreported in pathology reports. We hypothesized that renal arteriosclerosis burden would occur at a younger age in patients with LN, compared to healthy donors. We also hypothesized that arteriosclerosis is underreported on routine LN pathology reports, indicating a need for standard use of systematic Banff criteria to grade arteriosclerosis in all LN biopsy reports.
PATIENTS AND METHODS
Cohort.
We identified all consecutive patients with LN who underwent native renal biopsy between 1994 and 2017 at the University of Wisconsin Hospital and Clinics. We abstracted data on patient and disease characteristics from a comprehensive renal biopsy database and electronic health records. We used the 1997 updated American College of Rheumatology and Systemic Lupus International Collaborating Clinics 2012 criteria to validate the SLE diagnoses (18,19). We included the first native LN biopsy reports for all validated patients with SLE in our cohort. We excluded subsequent pathology reports after incident LN diagnosis, patients with transplant kidneys, and those who did not meet SLE diagnostic criteria and the ISN/RPS 2004 classification for LN (6). The study was approved by the University of Wisconsin Human Subjects Committee with a waiver of informed consent (number 2016-1260).
Covariates: sociodemographics and comorbidity.
Using electronic health record and database information, we recorded sociodemographic and comorbidity information at the time of biopsy. Patient and disease characteristics included age, sex, race, smoking status, and comorbidities. Hypertension, hyperlipidemia, and diabetes mellitus were assessed using International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes, a problem list diagnosis, or medication use. CVD events before LN diagnosis were assessed according to American Heart Association guidelines (20–22). Chronic kidney disease stage was assessed using the glomerular filtration rate at the time of biopsy (23). A modified CVD risk count was calculated by summing 7 risk factors used in arteriosclerotic cardiovascular disease (ASCVD) scoring: age, sex, race, smoking history, hyperlipidemia (high total cholesterol or low high-density lipoprotein cholesterol), hypertension, and diabetes mellitus, plus chronic kidney disease stage ≥3 and previous CVD events (24). We categorized the modified ASCVD risk to be present for patients with >1 risk factor based on studies reporting CVD events in patients with >1 traditional CVD risk factor (25). Patients with 0 or 1 risk factor on a modified ASCVD count were categorized as having a negative ASCVD score.
Renal histopathology.
Renal biopsy was performed for clinical indication (increase in serum creatinine, hematuria, and/or proteinuria). Fixed sections of kidney tissue were stained with hematoxylin and eosin, periodic acid–Schiff, and Masson’s tri-chrome stain for pathologic analysis. Immunofluorescent staining was performed on frozen sections. Electron microscopic analysis was performed on ultrathin sections. Pathologic assessment of all kidney biopsy reports was performed by clinical renal pathologists according to ISN/RPS guidelines (6). Using a comprehensive database, we abstracted the following data from renal pathology reports: class of LN, the presence or absence of chronic lesions on biopsy, and the degree of arteriosclerosis. We abstracted the LN class (I–VI), categorized as proliferative or nonproliferative, and LN chronicity (present/absent) according to ISN/RPS guidelines (6). Per the 2003 ISN/RPS guidelines, renal pathologists are required to report the number of glomeruli with active or chronic lesions, but reporting the chronicity index (scores) in all LN pathology reports is not mandatory (6). The LN chronicity index (scores) was not uniformly reported in all LN biopsy reports. Hence, consistent with the 2003 ISN/RPS guidelines, we defined LN chronicity as the presence of any chronic lesions on pathology reports, which were uniformly reported in all LN biopsy reports. The primary outcome of renal arteriosclerosis was graded using pathology reports, in which details were classified into Banff categories. Consistent with Banff, the renal arteriosclerosis biopsy report findings were categorized as none (0% luminal narrowing or not reported or no reporting on arteriosclerosis present/absent), mild (≤25% narrowing or reported as mild), moderate (26–50% narrowing or reported as moderate), and severe (>50% narrowing or reported as severe) (Table 1) (7).
Table 1.
Interpretations of biopsy-reported renal arteriosclerosis using the Banff scoring system
| Grade | Banff criteria for renal arteriosclerosis scoring | Biopsy interpretation using the Banff categories |
|---|---|---|
| None | 0% luminal narrowing | 0% narrowing or reported as not present or not reported |
| Mild | <25% luminal narrowing | <25% narrowing or reported as mild |
| Moderate | 26–50% luminal narrowing | 26–50% narrowing or reported as moderate |
| Severe | >50% luminal narrowing | >50% narrowing or reported as severe |
Overread using Banff criteria.
Next, a blinded study pathologist (YH) overread a 25% convenience sample (n = 43 biopsy reports). This sample was randomly selected for overread, including approximately 50% with and without reported renal arteriosclerosis with oversampling of recent biopsy reports (2014–2017), which could have improved with new standards in transplant biopsy grading. Light microscopy biopsy slides were analyzed to grade renal arteriosclerosis and other renal arterial changes, using the standard Banff criteria for grading renal arterial changes. According to the Banff criteria, renal arteriosclerosis was directly interpreted from the slides as none, mild (<25%), moderate (26–50%), and severe (>50%) luminal narrowing (7). For control comparison, we used the published rates of renal arteriosclerosis in kidney donors, graded using the standard Banff criteria and reported by age group (26).
Statistical analysis.
Descriptive data were expressed as the mean ± SD (for normally distributed data) or median and range (for data that were not normally distributed). We compared the prevalence of arteriosclerosis present and moderate or severe renal arteriosclerosis between our cohort and published controls by age group using a chi-square test.
We used a chi-square test and univariable logistic regression models to examine the association between the presence of renal arteriosclerosis, testing 10-year and 15-year age groups, modified ASCVD count, LN proliferative class, LN chronicity, and 1-year, 2-year, and 5-year SLE duration before LN diagnosis periods. Based on the findings of the univariable analy sis, we found a significant association between patients ages ≥30 years with LN and the presence of renal arteriosclerosis. Further, we noted an accelerated risk of arteriosclerosis in patients ages ≥30 years with LN in comparison with age-matched healthy donors. Therefore, for multivariable logistic regression models, we categorized patients with LN by age into 2 groups: age <30 years and ≥30 years. Further, SLE duration at the time of LN diagnosis was categorized in 2 periods: LN diagnosis within 2 years of SLE and LN diagnosis after 2 years of SLE for multivariable analysis. This decision was based on the recent studies emphasizing the accelerated risk of CVD or related arterial changes earlier, within the first 2 years of the SLE disease course (27,28). Variables with a P value less than 0.1 in univariable models and LN proliferative class were included in multivariable analyses. For patients with reports lacking information on the presence or absence of arteriosclerosis, we supplemented with available overread Banff grade classification. We used univariable and multivariable logistic regression to analyze associations between supplemented renal arteriosclerosis and covariables. We calculated kappa agreement and predictive values for establishing the diagnostic accuracy of biopsy reports in comparison with the overread Banff arteriosclerosis grade. Statistical software R, version 3.4.1, was used for the analysis (29).
RESULTS
Patient and disease characteristics.
Patient and disease characteristics of the cohort are summarized in Table 2. A total of 189 patients with incident LN met the inclusion criteria for the study. The median patient age at the time of kidney biopsy was 25 years (range 2–79 years). Of the 189 patients, 78% were female, 73% were White, 17% were from other minority races, and 10% had missing race data. At the time of biopsy, 23% were ever smokers, and 34% had >1 risk factor of the modified ASCVD count. Regarding LN disease characteristics, 41% of patients were classified as proliferative, LN chronicity was present in 38%, and 49% of patients were diagnosed with LN within 2 years of SLE diagnosis. In biopsy reports, we found that 41% of patients with LN had renal arterial changes. In total, 31% had renal arteriosclerosis and 12% had hyaline arteriolosclerosis.
Table 2.
Lupus nephritis cohort characteristics (n = 189)*
| Characteristic | Value |
|---|---|
| Age, median (range) years | 25 (2–79) |
| Female | 148 (78) |
| Race | |
| White | 138 (73) |
| African American | 17 (9) |
| Asian/other | 15 (8) |
| Missing | 19 (10) |
| Smoker, ever | 44 (23) |
| Modified ASCVD count, >1 risk factor | 64 (34) |
| Lupus duration | |
| <2 years | 93 (49) |
| ≥2 years | 44 (23) |
| Missing | 52 (28) |
| LN class | |
| Proliferative | 78 (41) |
| Nonproliferative | 91 (48) |
| Missing LN class | 20 (11) |
| LN chronicity present | 72 (38) |
| Renal arteriosclerosis | |
| Mild | 43 (24) |
| Moderate | 13 (6) |
| Severe | 2 (1) |
| Arteriolar hyalinosis present | 23 (12) |
Values are the number (%) unless indicated otherwise. ASCVD = arteriosclerotic cardiovascular disease; LN = lupus nephritis.
Burden of renal arteriosclerosis by age group and published comparisons.
We found that 40% of our patients with LN age ≥30 years had renal arteriosclerosis and >10% of patients had moderate-to-severe arteriosclerosis on biopsy reports. More strikingly, >50% of patients with LN age ≥30 years had renal arteriosclerosis when biopsy reports were overread using the standard Banff criteria to grade renal arteriosclerosis. We found that the prevalence of renal arteriosclerosis increased with age. Among the age group 60–69 years, the burden of moderate-to-severe arteriosclerosis (by routine pathology reports or supplemented with overread renal arteriosclerosis grade) was 1 in 3.
The onset of any arteriosclerosis in patients with LN was 2 decades earlier compared to the published prevalence in healthy kidney donors (Figure 1). Our LN cohort’s prevalence of 41% of any renal arteriosclerosis at ages 30– 39 years was comparable to healthy kidney donors ages 50–59 years (41% versus 44%; P = 0.9). Likewise, patients with LN ages 40–49 years had a comparable prevalence to controls age 60–69 years (52% versus 51%; P = 0.95). Moreover, the burden of moderately severe arteriosclerosis in patients with LN for the age group 60–69 years was 5-fold higher than reported in age-matched healthy donors (33% versus 6%) (Figure 2).
Figure 1.

Prevalence of any renal arteriosclerosis by age group in patients with lupus nephritis (LN) on biopsy reports (black bars) compared to the published prevalence of any arteriosclerosis in healthy donors by age groups. NR = not reported; * = the comparator group started at age 18–29 years (yo) (26).
Figure 2.
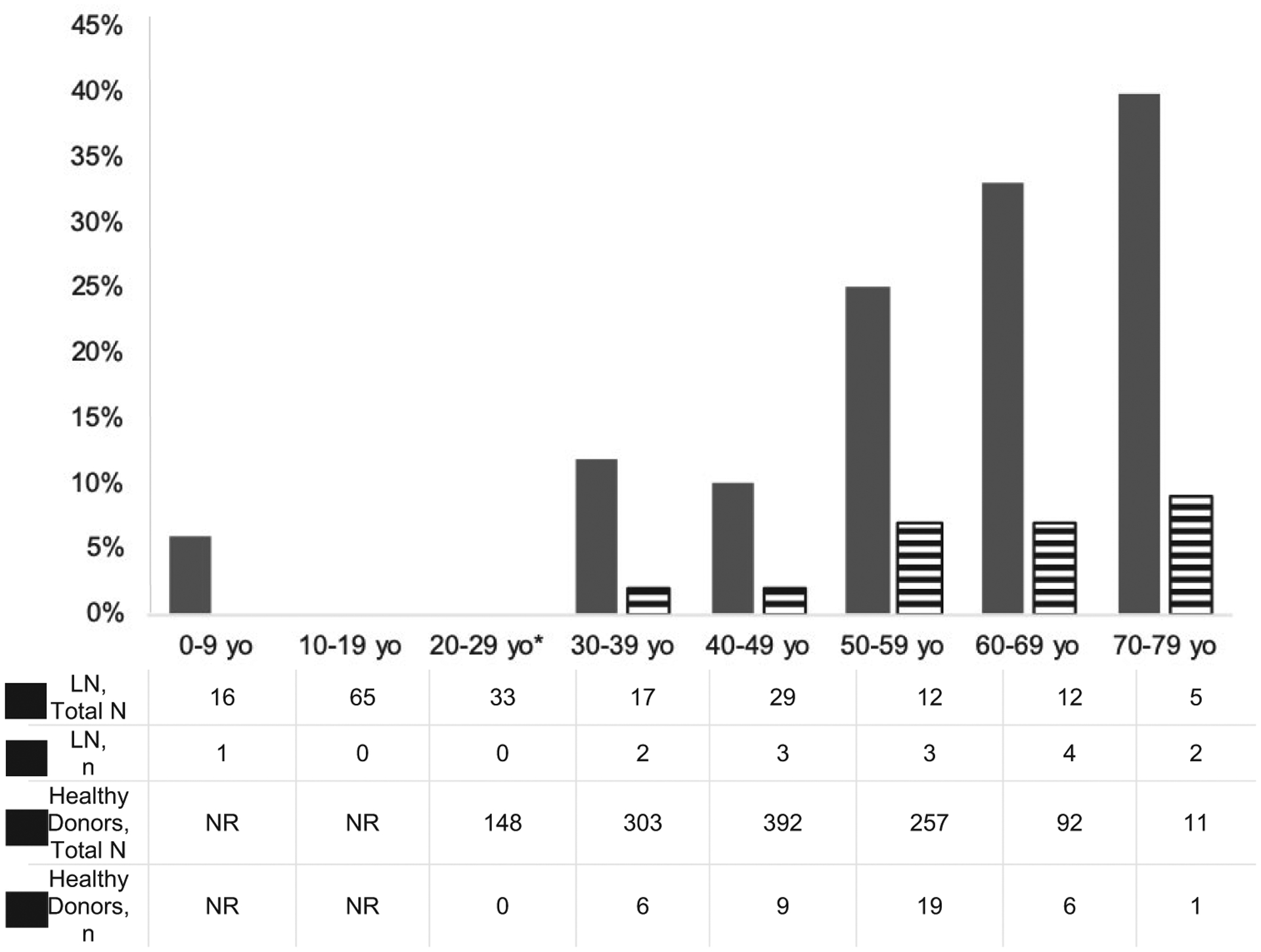
Prevalence of moderate-to-severe renal arteriosclerosis in patients with lupus nephritis (LN) on biopsy reports (black bars) compared to the published prevalence of moderate-to-severe arteriosclerosis in healthy donors by age group. NR = not reported; * = the comparator group started at age 18–29 years (yo) (26).
Predictors of renal arteriosclerosis.
Using the full LN cohort, we found that patients with LN age ≥30 years (OR 6.9 [95% confidence interval (95% CI) 3.5–14]), and LN chronicity (OR 3.0 [95% CI 1.5–5.7]) were predictors of renal arteriosclerosis on univariable analysis. Modified ASCVD count and LN diagnosis within 2 years of SLE were kept in the model as possible predictors of renal arteriosclerosis (P < 0.1). In multivariable analyses, we found a 3-fold higher odds of renal arteriosclerosis in patients with LN ages ≥30 years versus patients with LN ages <30 years (odds ratio [OR] 3.3 [95% CI 1.3–9.1], P = 0.02). Further, LN chronicity predicted a 4-fold greater odds of renal arteriosclerosis (OR 4.0 [95% CI 1.5– 11.6], P = 0.01) (Table 3). LN proliferative class, modified ASCVD count, and SLE disease duration were not associated with the presence of renal arteriosclerosis on the multivariable analysis. Using supplemented arteriosclerosis grades that added available information from the overread Banff analysis, we found a similar odds of an increase in the age group ≥30 years compared with patients with LN age <30 years (Table 3).
Table 3.
Predictors of reported and supplemented renal arteriosclerosis in lupus nephritis patients*
| Reported renal arteriosclerosis | Supplemented renal arteriosclerosis† | |||
|---|---|---|---|---|
| Variable | Unadjusted | Adjusted | Unadjusted | Adjusted |
| Age <30 years | Ref. | Ref. | Ref. | Ref. |
| Age ≥30 years | 6.9 (3.5–14)‡ | 3.3 (1.3–9.1)‡ | 5.3 (2.8–10)‡ | 3.2 (1.3–8.1)‡ |
| ASCVD count ≤1 | Ref. | Ref. | Ref. | Ref. |
| ASCVD count >1 | 1.8 (0.9–3.7) | 1.3 (0.5–3.4) | 2.5 (1.3–4.7) | 2.0 (0.8–4.9) |
| SLE duration<2 years | Ref. | Ref. | Ref. | Ref. |
| SLE duration ≥2 years | 2.0 (0.9–4.5) | 1.3 (0.5–3.7) | 1.8 (0.8–3.6) | 0.8 (0.3–2.1) |
| LN chronicity absent | Ref. | Ref. | Ref. | Ref. |
| LN chronicity present | 3.0 (1.5–5.7)‡ | 4.0 (1.5–11.6)‡ | 2.7 (1.5–5)‡ | 2.9 (1.1–7.7)‡ |
| LN nonproliferative | Ref. | Ref. | Ref. | Ref. |
| LN proliferative | 1.2 (0.6–2.5) | 0.9 (0.3–2.5) | 1.0 (0.6–1.9) | 0.8 (0.3–1.9) |
Values are the odds ratio (95% confidence interval). ASCVD = arteriosclerotic cardiovascular disease; LN = lupus nephritis; Ref. = reference; SLE = systemic lupus erythematosus.
Supplemented renal arteriosclerosis: reported renal arteriosclerosis with none or not reported was supplemented with overread grades.
Statistically significant.
Diagnostic accuracy of reports compared to Banff renal arteriosclerosis overread.
For a 25% convenience sample (n = 43 patients), we overread renal biopsy slides using the Banff criteria to grade renal arteriosclerosis. The sociodemographic and disease characteristics of the patients in our convenience sample included a mean age of 31 years at the time of LN diagnosis, 25% African American patients, and 72% female patients, and 49% of patients had LN chronicity on their pathology reports (all P > 0.05). We found a poor agreement between overread grades using the Banff criteria for renal arteriosclerosis and the original pathology reports (κ = 0.25). More than 50% of the original pathology reports missed renal arteriosclerosis (negative predictive value 49%), and nearly 40% of all reports lacked details on arterial changes or arteriosclerosis, whereas the positive predictive value of reported renal arteriosclerosis was 80%. The specificity of the biopsy reports for reported renal arteriosclerosis in comparison to overread arteriosclerosis, using the Banff criteria, was 84%.
DISCUSSION
To our knowledge, this is one of the first studies examining the burden of renal arteriosclerosis in incident patients with LN using the standard Banff interpretation and comparing the prevalence of renal arteriosclerosis to healthy donors. Our study found that renal arteriosclerosis is common and accelerated in patients with LN compared to age-matched healthy controls. Specifically, we found that renal arteriosclerosis appeared 2 decades earlier in LN in comparison to healthy donors (26). We found that patients with LN who were ages ≥30 years and LN chronicity predicted the presence of renal arteriosclerosis. We also found that renal arteriosclerosis reporting and grading in LN biopsy reports were missed or overlooked in more than one-half of the routine pathology reports, because of the lack of standard guidelines on reporting renal arterial changes, although when the presence of renal arteriosclerosis was reported on routine pathology reports, it was an accurate report. Our study highlights the prevalence of renal arteriosclerosis in patients with LN along with gaps in current pathology reports, suggesting a need to standardize reporting and grading of renal arteriosclerosis in all LN biopsies by using universal Banff arteriosclerosis grading.
Historically, renal arteriosclerosis has been overlooked in patients with LN. The first report on renal involvement in SLE by Appel et al in 1978 (8) graded renal arteriosclerosis in all kidney biopsy reports, among many other factors, but few comments on this finding were mentioned. In their report, approximately 60% of the cohort ages 10–57 years who had any renal arteriosclerosis was similar to our rate of 51%. A few other studies have globally reviewed renal arterial changes. Two studies reported a <20% burden of arteriosclerosis, suggesting risk in “older hypertensive adults” with LN (11,12). Barber et al (9) and Huang et al (10) reported a 57% prevalence of any renal arterial changes in patients with LN at younger ages. None of these studies used systematic grading for renal arteriosclerosis, such as Banff criteria to report renal arteriosclerosis, which could explain such contradictory findings (8–13). Unlike prior reports, we found no correlation between renal arteriosclerosis and traditional risk factors, including hypertension, other ASCVD risk factors, or proliferative LN (8,9,11,13).
Renal arteriosclerosis is now established as an early predictor of incident CVD events in IgA nephropathy (14,15). In IgA nephropathy, Myllamäki et al (14,15) scored renal arteriosclerosis using the standard Banff criteria and found severe arteriosclerosis to be significantly associated with CVD events (37% severe arteriosclerosis with CVD events versus 17% severe arteriosclerosis without CVD events; P < 0.05). Similarly, in renal transplant recipients, antibody-mediated severe arteriosclerosis was a strong predictor of major cardiovascular events (OR 4.1 [95% CI 2.4–7.1], P < 0.0001) (16). These studies have emphasized a common mechanistic pathway leading to both renal arteriosclerosis and atherosclerosis. But a similar relationship in patients with LN, who are at a 9-fold higher risk of CVD compared to their healthy peers, is yet to be elucidated. Therefore, we plan to perform future studies to grade renal arteriosclerosis in all LN biopsy reports, using a systematic renal arteriosclerosis grading criterion, such as Banff, and examine its role as an early predictor of CVD events in patients with LN.
Despite the solid positive predictive value of routine pathology reporting on the presence of arteriosclerosis (80%), overall renal arteriosclerosis was underreported in the routine LN pathology reports. Unlike in transplant biopsy reports that routinely use the Banff criteria to grade renal arteriosclerosis, in LN biopsies, >50% of the pathology reports did not comment on the presence of renal arteriosclerosis. The current ISN/RPS guidelines for LN biopsies do not provide recommendations on the standard use of systematic criteria to grade renal arteriosclerosis, such as the Banff criteria, in all LN biopsy reports. Recently, the ISN/RPS committee acknowledged the importance of renal arterial changes in LN, yet a standard systematic grading system to grade renal arteriosclerosis in all LN biopsies is still lacking (5,6). The presence of renal arteriosclerosis could be used as an early predictor of CVD events that will help clinicians to implement timely CVD preventive strategies and reduce CVD-related morbidity and mortality in patients with LN. Therefore, our study underscores the need for a universal standard to systematically report renal arteriosclerosis as an actionable precursor of CVD in patients with LN. This study supports the standard use of systematic Banff criteria to grade renal arteriosclerosis in all LN biopsies and calls for future prospective studies to explore the role of arteriosclerosis as an early predictor of CVD in patients with LN.
Despite the strengths of this study, including the inclusion of a validated incident LN cohort and using the systematic Banff criteria for renal arteriosclerosis grading, we also acknowledge a few limitations. First, our midwestern center had 73% White patients and may not fully represent the LN population in the US. Likewise, renal biopsies were not a standard procedure in all patients with LN. For example, our cohort may be missing patients with LN who received empiric treatment for LN without a biopsy. However, we believe that this limitation reflects real-life practice. Unlike prior reports, we found no correlation between renal arteriosclerosis and hypertension, >1 ASCVD risk factor, or proliferative LN, likely due to sample size limitations. Moreover, in the absence of standard guidelines for reporting arteriosclerosis in LN biopsies, nearly 50% of biopsy reports did not comment on the presence or absence of arteriosclerosis. Therefore, our prevalence estimates for renal arteriosclerosis are conservative and might underreport the true burden of renal arteriosclerosis in patients with LN. We also acknowledge that the published data on the prevalence of renal arteriosclerosis in healthy donors could underrepresent the true prevalence of renal arteriosclerosis in the healthy population. Further, because renal arteriosclerosis grading in LN biopsies and healthy donors was performed by different pathologists, there could have been an interobserver bias in renal arteriosclerosis grading. We attempted to overcome the limitation that renal arteriosclerosis is underreported in LN biopsy reports by overreading 25% of the LN biopsy reports, and applying overread arteriosclerosis grades, using standard Banff criteria, when routine pathology reports lacked details on the presence or absence of renal arteriosclerosis. In supplemented regression models, results were unchanged, and we again reported a good association between patients ages ≥30 years with LN and the presence of LN chronicity, and the presence of renal arteriosclerosis (Table 3). While overreading was not feasible in this study, in future studies we plan to overread all biopsy reports to grade renal arteriosclerosis, using the standard Banff criteria, and to collaborate with other diverse LN centers to examine the association between Banff renal arteriosclerosis grades and CVD events in patients with LN.
To conclude, we found that renal arteriosclerosis appeared in patients with LN 2 decades before their healthy peers. Despite the high specificity of renal arteriosclerosis in current biopsy reports, we found significant sensitivity gaps (>50%) in routine pathology reporting on renal arteriosclerosis in LN biopsy reports. Hence, our study underscores a need for universal use of systematic Banff renal arteriosclerosis grading criteria in all LN biopsies, similar to transplant pathology reporting standards.
SIGNIFICANCE & INNOVATIONS.
Renal arteriosclerosis is accelerated and premature in patients with lupus nephritis (LN) by 2 decades in comparison with their healthy peers.
Despite the high specificity of renal arteriosclerosis reporting in current biopsy reports, we found significant sensitivity gaps (>50%) in routine pathology reporting on renal arteriosclerosis in LN biopsy results.
Our study underscores a need for universal use of systematic Banff renal arteriosclerosis grading criteria in all LN biopsy results, similar to transplant pathology reporting standards.
Clinicians urgently need early predictors of cardiovascular disease (CVD) in patients with LN to prevent related morbidity and mortality, and renal arteriosclerosis on pathology reports could be an early predictor of CVD in patients with LN.
Footnotes
No potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Salmon JE, Roman MJ. Accelerated atherosclerosis in systemic lupus erythematosus: implications for patient management. Curr Opin Rheumatol 2001;13:341–4. [DOI] [PubMed] [Google Scholar]
- 2.Abusamieh M, Ash J. Atherosclerosis and systemic lupus erythematosus. Cardiol Rev 2004;12:267–75. [DOI] [PubMed] [Google Scholar]
- 3.Hermansen ML, Lindhardsen J, Torp-Pedersen C, Faurschou M, Jacobsen S. The risk of cardiovascular morbidity and cardiovascular mortality in systemic lupus erythematosus and lupus nephritis: a Danish nationwide population-based cohort study. Rheumatology (Oxford) 2017;56:709–15. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Bao G, Niyibizi M, Drenkard C, Lim SS. Differences between early and late cardiovascular disease in a population-based cohort of systemic lupus erythematosus patients [abstract]. Arthritis Rheumatol 2016;68 Suppl 10. [Google Scholar]
- 5.Bajema IM, Wilhelmus S, Alpers CE, Bruijn JA, Colvin RB, Cook HT, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93:789–96. [DOI] [PubMed] [Google Scholar]
- 6.Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004;65:521–30. [DOI] [PubMed] [Google Scholar]
- 7.Liapis H, Gaut JP, Klein C, Bagnasco S, Kraus E, Farris AB III, et al. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant 2017;17:140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appel GB, Silva FG, Pirani CL, Meltzer JI, Estes D. Renal involvement in systemic lupus erythematosus (SLE): a study of 56 patients emphasizing histologic classification. Medicine (Baltimore) 1978;57:371–410. [DOI] [PubMed] [Google Scholar]
- 9.Barber C, Herzenberg A, Aghdassi E, Su J, Lou W, Qian G, et al. Evaluation of clinical outcomes and renal vascular pathology among patients with lupus. Clin J Am Soc Nephrol 2012;7:757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Han SS, Qin DD, Wu LH, Song Y, Yu F, et al. Renal interstitial arteriosclerotic lesions in lupus nephritis patients: a cohort study from China. PLoS One 2015;10:e0141547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banfi G, Bertani T, Boeri V, Faraggiana T, Mazzucco G, Monga G, et al. Renal vascular lesions as a marker of poor prognosis in patients with lupus nephritis. Gruppo Italiano per lo Studio della Nefrite Lupica (GISNEL). Am J Kidney Dis 1991;18:240–8. [DOI] [PubMed] [Google Scholar]
- 12.Descombes E, Droz D, Drouet L, Grunfeld JP, Lesavre P. Renal vascular lesions in lupus nephritis. Medicine (Baltimore) 1997;76: 355–68. [DOI] [PubMed] [Google Scholar]
- 13.Grishman E, Venkataseshan VS. Vascular lesions in lupus nephritis. Mod Pathol 1988;1:235–41. [PubMed] [Google Scholar]
- 14.Myllamäki J, Syrjanen J, Helin H, Pasternack A, Kattainen A, Mustonen J. Vascular diseases and their risk factors in IgA nephropathy. Nephrol Dial Transplant 2006;21:1876–82. [DOI] [PubMed] [Google Scholar]
- 15.Myllamäki J, Honkanen T, Syrjanen J, Helin H, Rantala I, Pasternack A, et al. Uric acid correlates with the severity of histopathological parameters in IgA nephropathy. Nephrol Dial Transplant 2005;20:89–95. [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Vernerey D, Viglietti D, Aubert O, Duong Van Huyen JP, Empana JP, et al. Determinants and outcomes of accelerated arteriosclerosis: major impact of circulating antibodies. Circ Res 2015;117:470–82. [DOI] [PubMed] [Google Scholar]
- 17.Tracy RE. Histologic characteristics of coronary artery in relation to the renovasculopathies of hypertension. Ann Diagn Pathol 1998;2:159–66. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College of Rheumatology. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 1999;99:2829–48. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med 2017;22:NP1–43. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–350. [DOI] [PubMed] [Google Scholar]
- 25.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, Garcia FA, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 26.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010;152:561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels CM, Buhr KA, Goldberg JW, Bell CL, Visekruna M, Nekkanti S, et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol 2014;41:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urowitz MB, Gladman DD, Anderson NM, Su J, Romero-Diaz J, Bae SC, et al. Cardiovascular events prior to or early after diagnosis of systemic lupus erythematosus in the systemic lupus international collaborating clinics cohort. Lupus Sci Med 2016;3:e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2017. [Google Scholar]


