Abstract
Objective:
MicroRNAs (miRNAs) regulate gene expression and are disease biomarkers. Rheumatoid arthritis (RA) patients have accelerated atherosclerosis leading to excess cardiovascular morbidity and mortality, but traditional risk factors for cardiovascular risk stratification are inadequate. In the general population miRNAs improve cardiovascular risk estimation beyond traditional risk factors. Our objective was to develop a miRNA panel that predicts coronary atherosclerosis in RA patients.
Methods:
Plasma small RNA Next Generation Sequencing (NGS) was performed on 161 RA patients whose Agatston scores for coronary artery calcium were previously measured. Random forest analysis of plasma NGS miRNA expression was used to determine which miRNAs best differentiated between those patients with and without coronary artery calcium. Top predictive miRNAs were assayed by quantitative PCR (qPCR). Elastic net regression was used to develop the most parsimonious models with qPCR-measured miRNA concentrations and clinical variables (age, sex, ACC/AHA 10-year risk score, DAS28 score, and diabetes) separately to predict presence of coronary artery calcium and high coronary artery calcium. C-statistics were used to assess performance model performance.
Results:
The top miRNAs which differentiated those with and without coronary atherosclerosis based on random forest analysis included let-7c-5p, miR-30e-5p, miR-30c-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-126–3p, miR-30a-5p, and miR-125a-5p. For coronary artery calcium prediction, addition of all miRNAs except miR-126–3p to clinical factors improved the c-statistic modestly from 0.86 to 0.87. For high coronary artery calcium prediction, addition of all miRNAs except miR-30c-5p to clinical factors improved the c-statistic from 0.75 to 0.80.
Conclusion:
A plasma miRNA panel improved prediction of high coronary artery calcium beyond traditional risk factors and RA disease activity. Further evaluation of the miRNA panel for prediction of coronary events in RA is necessary.
Introduction
MicroRNAs (miRNAs) are short RNA sequences approximately 22 nucleotides in length. They fine-tune gene expression, typically by binding to the 3’ untranslated region of an mRNA at a complementary region located approximately at bases 2–8 from the 5’ end, termed the seed region. Such binding of a miRNA to its target mRNA can lead to cleavage of the mRNA or translational repression [1]. miRNAs are under specific transcriptional pressures with differing patterns of expression depending on cell type and stressors [1, 2]. Some miRNAs are selectively exported out of cells [3] and transported via carriers such as extracellular vesicles, lipoproteins, and protein complexes to other cells to modify their gene expression [4–6]. Plasma miRNAs are remarkably stable, even after freeze-thaw cycles [7], making them excellent potential markers of disease.
Currently, markers of cardiovascular disease are lacking for patients with rheumatoid arthritis (RA). Traditional risk factors do not adequately assess risk of coronary atherosclerosis or coronary events among patients with RA [8, 9]. Even inclusion of RA-specific factors such as disease activity and inflammatory markers do not provide estimates that predict cardiovascular risk adequately [9]. Plasma miRNAs have been helpful biomarkers in many diseases, including RA [10, 11] and cardiovascular disease [12, 13]. Currently, there are several approved and available miRNA-based diagnostic tests including miRNA panel tests for thyroid and pancreatic cancer, identifying tumor of unknown origin, osteoporosis, and also for platelet function assessment which may be helpful for cardiovascular disease prediction and monitoring [14]. However, there has been little work identifying miRNAs which may be predictors of cardiovascular disease in RA.
Most studies assessing the utility of circulating miRNAs as biomarkers of disease have used a candidate approach based on known miRNA functions. Indeed, we previously used such a candidate approach to try to identify miRNAs which might be associated with coronary atherosclerosis in RA but were unsuccessful [15]. Because the mechanism of accelerated atherosclerosis in RA is unclear, we postulated that an unbiased approach to select candidate miRNA biomarkers might be more helpful than a candidate approach. Moreover, a panel of multiple miRNAs may be more helpful than a single miRNA. Thus, the goal of the study was to use an unbiased approach to develop a model which incorporated plasma miRNAs and clinical factors to predict the presence of 1) coronary artery calcium and 2) high coronary artery calcium, markers of coronary artery atherosclerosis.
Methods
Study population
This study included 161 patients with RA from a prior cross-sectional study examining atherosclerosis in patients with RA [8] who had coronary artery calcium measurements and plasma small RNA sequencing performed. Patients were at least 18 years of age and met classification criteria for RA [16]. Recruitment and study procedures were described previously [8]. The study was approved by the Vanderbilt Institutional Review Board and all patients gave written informed consent.
Clinical assessments and general laboratory measures
We collected demographic and clinical information by patient interviews and medical record review and calculated RA disease activity (DAS28 score) based on 28 joints and erythrocyte sedimentation rate (ESR) [17]. High-sensitivity C-reactive protein (hsCRP) concentrations, ESR, and fasting total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein (LDL), and triglyceride concentrations were measured by the Vanderbilt University Medical Center Clinical laboratory. Larsen score was measured as previously described [18] to assess damage to the hands, wrists, and feet was performed on 94 of the RA patients for whom radiographs of hands and feet were available.
The 2013 American College of Cardiology/American Heart Association (ACC/AHA) 10-year risk score, which predicts 10-year risk of nonfatal myocardial infarction or coronary heart disease death or stroke, was calculated for each subject based on age, race, total cholesterol, HDL cholesterol, blood pressure, treatment for hypertension, diabetes, and smoking status.
Coronary artery calcium measurement
Coronary artery calcium was measured by electron beam computed tomography and quantified by Agatston score, as previously described [19,20]. Briefly, scans were performed with an Imatron C-150 (GE/Imatron, South San Francisco, CA) with a protocol using a 100-msec scanning time and a single-slice thickness of 3 mm. Calcification within a coronary artery with a minimal attenuation of 130 Hounsfield units (HU) were identified and calcified plaque was defined present if 3 or more contiguous pixels were detected. All scans were read by one blinded investigator (PR), who is an expert at coronary artery calcium scoring. High coronary artery calcium was defined as a score ≥300 Agatston units or ≥75th percentile for age, sex, and ethnicity [21].
Small RNA Sequencing and Bioinformatics
NGS of small RNAs was performed and miRNAs quantified as previously described [10]. In brief, total RNA was extracted from stored plasma (EDTA) using Norgen Biotek Total RNA Purification kits (Thorold, Ontario, CA). Complementary DNA libraries were constructed using TruSeq Small RNA Library Preparation Kits (San Diego, California, USA). Quality assessment and size selection was performed at the Vanderbilt Technologies for Advanced Genomics (VANTAGE) core facility. NGS was performed using Illumina NextSeq500 in the VANTAGE core facility. TIGER (“Tools for Integrative Genome analysis of Extracellular sRNAs”), an in-house small RNA sequencing analysis pipeline, was used to quantify miRNAs [10, 22, 23]. Random forest analysis was used on to analyze the sequencing results and select those miRNAs that best differentiated RA patients with and without coronary artery calcium. To select the top-performing miRNAs for validation by qPCR, miRNAs which were low in abundance on NGS (baseMean <30) were excluded.
Quantitative PCR
Top-performing miRNAs selected by random forest analysis from the NGS results were validated by qPCR (Figure 1). Total RNA was extracted from the plasma as described above. After the initial lysis step, a spike-in control cocktail was added as previously described [10] to normalize for variability in RNA extraction efficiency. qScript microRNA cDNA synthesis kits (Quantabio, Beverly, MA, USA) were used to make cDNA and individual miRNA qPCR primers and PerfeCTa SYBR green supermix (Quantabio, Beverly, MA, USA) were used for qPCR. The qPCR was performed in a 384-well format with samples measured in triplicate on a CFX384 Real-Time PCR detection system (Bio-Rad, Hercules, CA, USA). The miRNA concentrations were determined from standard dilution curves of a DNA mimic of known concentration and normalized to the spike-in cocktail. A threshold of Ct ≥ 40 for ≥ half of samples was used to exclude miRNAs from further analysis; however, the lowest abundance miRNA used in model development (miR-4446–3p) had raw Ct median = 35 [interquartile range 34, 38].
Figure 1.
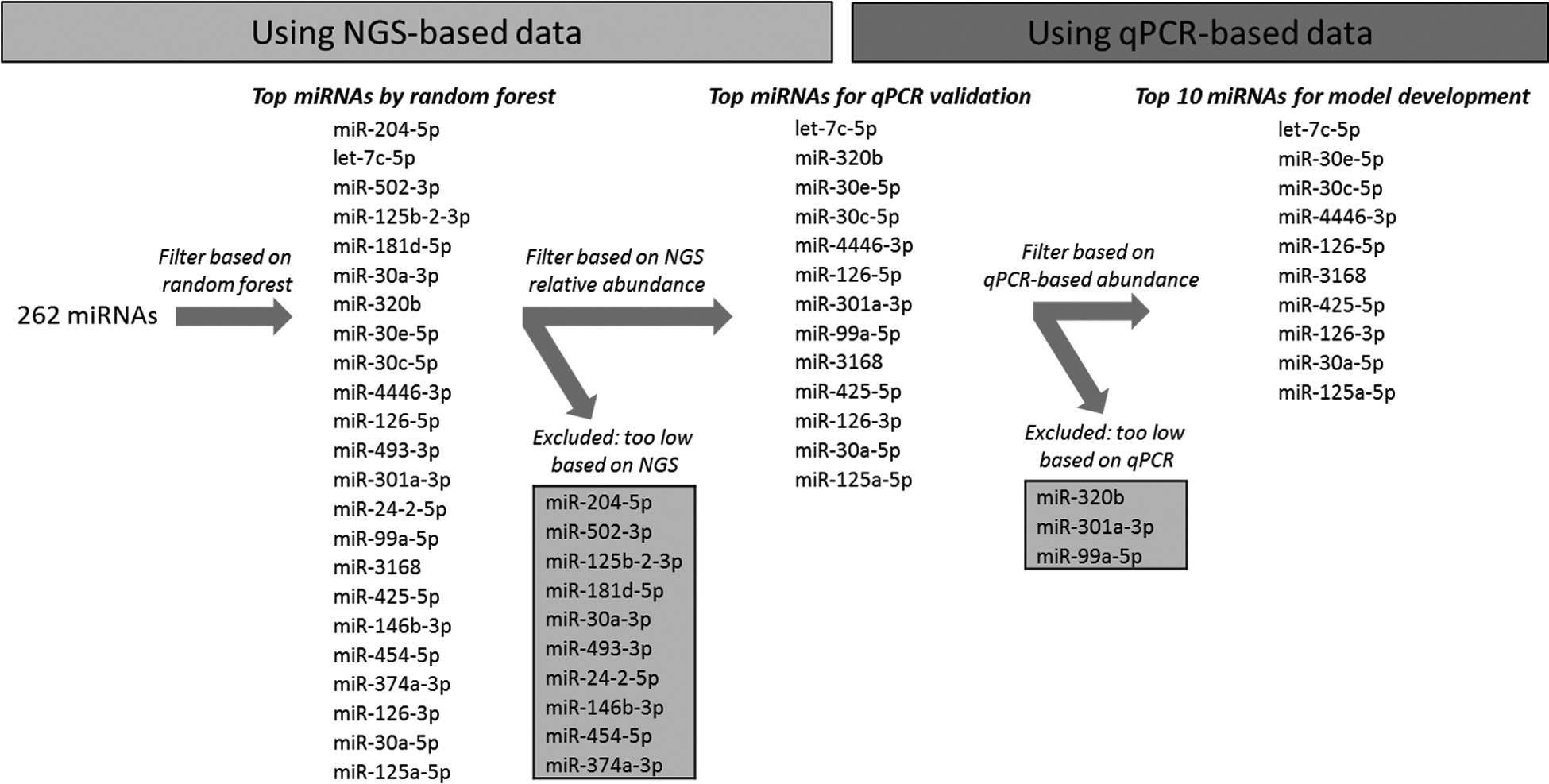
Schematic of study design. The relative abundance of plasma miRNAs based on next generation sequencing (NGS) was assessed for ability to predict the presence of coronary atherosclerosis using random forest analysis and ranked based on performance. Those which were too low in abundance were excluded. Top performing miRNAs were assayed by qPCR and those which were too low in abundance were excluded until a total of ten miRNAs were available for further model development.
Model development
Penalized logistic regression via elastic net regularization was used to develop a parsimonious model to predict 1) the presence of coronary artery calcium and 2) high coronary artery calcium. Clinical variables including age, sex, ACC/AHA 10-year risk score, DAS28 score, and diabetes, and plasma qPCR concentrations of the top 10 candidate miRNAs were included in the full model for variable selection. Clinical variables were forced into the final model and variable selection was permitted only among the miRNAs to develop the smallest miRNA panel. The elastic net regularization parameters were optimized to minimize the cross-validation error. Internal validation was performed using the .632 bootstrap method with 500 replications. The c-statistics were reported to measure model performance. We then compared models with clinical variables and miRNAs to models containing clinical variables only.
Pathway analysis
We performed pathway analysis on panel miRNAs using the Ingenuity Pathway Analysis (IPA) program (version 49932394, Qiagen). Upstream regulators and downstream targets of the panel miRNAs were filtered to include only experimentally observed or highly predicted targets in humans. Figures built using IPA were edited for clarity.
General statistics
Categorical data are presented as number (percent) and continuous data as median [interquartile range]. Univariable analyses were used to measure the association between each of the miRNA plasma concentrations and cardiovascular risk factors using linear regression for continuous and logistic regression for binary data. Triglycerides and hsCRP were analyzed on a natural log scale and effect size back transformed to original units. Data were analyzed using R (version 3.6.2) and SPSS (version 26).
Results
Subject Characteristics
The 161 patients with RA had a median age of 54 years, 69% were female, median DAS28 disease activity was 3.78, and 71% were rheumatoid factor positive. Coronary artery calcium was present in 82 subjects (51%) and 62 (39%) had high coronary artery calcium. Overall study design and clinical characteristics for all patients are presented in Figure 1 and Table 1, respectively. Details for subgroups based on the presence of high coronary artery calcium are presented in Supplemental Table 1.
Table 1.
Clinical characteristics of patients with RA
| All RA (N=161) | Without coronary calcium (n=79) | With coronary calcium (n=82) | P value | |
|---|---|---|---|---|
| Age, years | 54 [45, 63] | 48 [40, 54] | 60 [54, 68] | <0.001 |
| Sex, female | 111 (69) | 64 (81) | 47 (57) | 0.002 |
| Race, Caucasian | 143 (89) | 68 (86) | 75 (91) | 0.40 |
| DAS28, units | 3.83 [2.62, 4.75] | 3.46 [2.39, 4.40] | 4.05 [2.84, 5.01] | 0.04 |
| RF, positive | 110 (71) | 52 (69) | 58 (72) | 0.80 |
| hsCRP, mg/L | 4 [1, 11] | 3 [1, 10] | 5 [2, 12] | 0.09 |
| Disease duration, years | 4 [2, 18] | 3 [2,12] | 11 [2,20] | 0.003 |
| Larsen score, points | 2 [0, 16] | 0 [0, 13] | 2 [0, 19] | 0.38 |
| Coronary artery disease, # | 17 (11) | 0 (0) | 17 (21) | <0.001 |
| Hypertension, presence | 83 (52) | 29 (37) | 54 (66) | <0.001 |
| Systolic BP, mmHg | 134 [119, 146] | 129 [116, 138] | 137 [121, 151] | 0.004 |
| Diastolic BP, mmHg | 75 [68, 82] | 72 [67, 80] | 76 [69, 85] | 0.13 |
| Waist-hip ratio, units | 0.88 [0.81, 0.95] | 0.84 [0.78, 0.92] | 0.91 [0.83, 0.99] | <0.001 |
| BMI, kg/m2 | 28.3 [24.0, 33.0] | 28.3 [24.0, 33.5] | 28.3 [23.7, 31.5] | 0.34 |
| Total cholesterol, mg/dL | 185 [155, 211] | 183 [156, 204] | 188 [153, 214] | 0.70 |
| HDL cholesterol, mg/dL | 43 [37, 54] | 44 [38, 55] | 43 [36, 52] | 0.31 |
| LDL cholesterol, mg/dL | 112 [89, 135] | 105 [88, 134] | 114 [90, 136] | 0.44 |
| Triglycerides, mg/dL | 110 [80, 158] | 108 [81, 149] | 112 [80, 164] | 0.58 |
| DMII, presence | 18 (11) | 4 (5) | 14 (17) | 0.03 |
| Anti-hypertensive, use | 60 (37) | 16 (20) | 44 (54) | <0.001 |
| Corticosteroid, use | 87 (54) | 42 (53) | 45 (55) | 0.95 |
| Methotrexate, use | 115 (71) | 61 (77) | 54 (66) | 0.16 |
| Anti-TNF, use | 31 (19) | 17 (22) | 14 (17) | 0.61 |
Data are presented as median [interquartile range] for continuous variables and number (percent) for categorical variables. Abbreviations: RA= rheumatoid arthritis, DAS28=disease activity score based on 28 joints, RF=rheumatoid factor, hsCRP=high sensitivity C-reactive protein, ESR=erythrocyte sedimentation rate, BP=blood pressure, BMI=body mass index, DMII= diabetes mellitus type 2, TNF=tumor necrosis factor alpha. Larsen score is available for 93 subjects (40 without coronary artery calcium and 53 with coronary artery calcium).
Initial selection of candidate miRNAs based on NGS and qPCR
Random forest analysis was performed using the relative abundance of 262 miRNAs based on NGS to predict the presence of coronary artery calcium. We excluded the miRNAs which were low in abundance from further analysis, as discussed in methods section (Figure 1), and the remaining top performing miRNAs were assayed by qPCR. These included, in order of priority based on random forest analysis, let-7c-5p, miR-320b, miR-30e-5p, miR-30c-5p, miR-4446–3p, miR-126–5p, miR-301a-3p, miR-99a-5p, miR-3168, miR-425–5p, miR-126–3p, miR-30a-5p, and miR-125a-5p. Among these, miR-320b, miR-301a-3p, and miR-99a-5p were too low in abundance on qPCR as discussed in methods section and were excluded (Figure 1).
Data reduction and model development for presence of coronary calcium
Using plasma concentrations of the ten miRNAs (by qPCR) and clinical variables, we performed elastic net regression to reduce the number of miRNA predictors for the presence of coronary artery calcium. This method selected let-7c-5p, miR-30e-5p, miR-30c-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-30a-5p, and miR-125a-5p as predictors for the presence of coronary artery calcium. Thus, only miR-126–3p was removed due to lack of additional contribution to information in the model. Clinical predictors alone gave a c-statistic = 0.86 (95%CI: 0.80, 0.91) and the addition of the miRNA panel improved the prediction modestly to a c-statistic = 0.87 (95%CI: 0.82, 0.93) (Figure 2). Model characteristics are presented in Supplemental Table 2.
Figure 2.
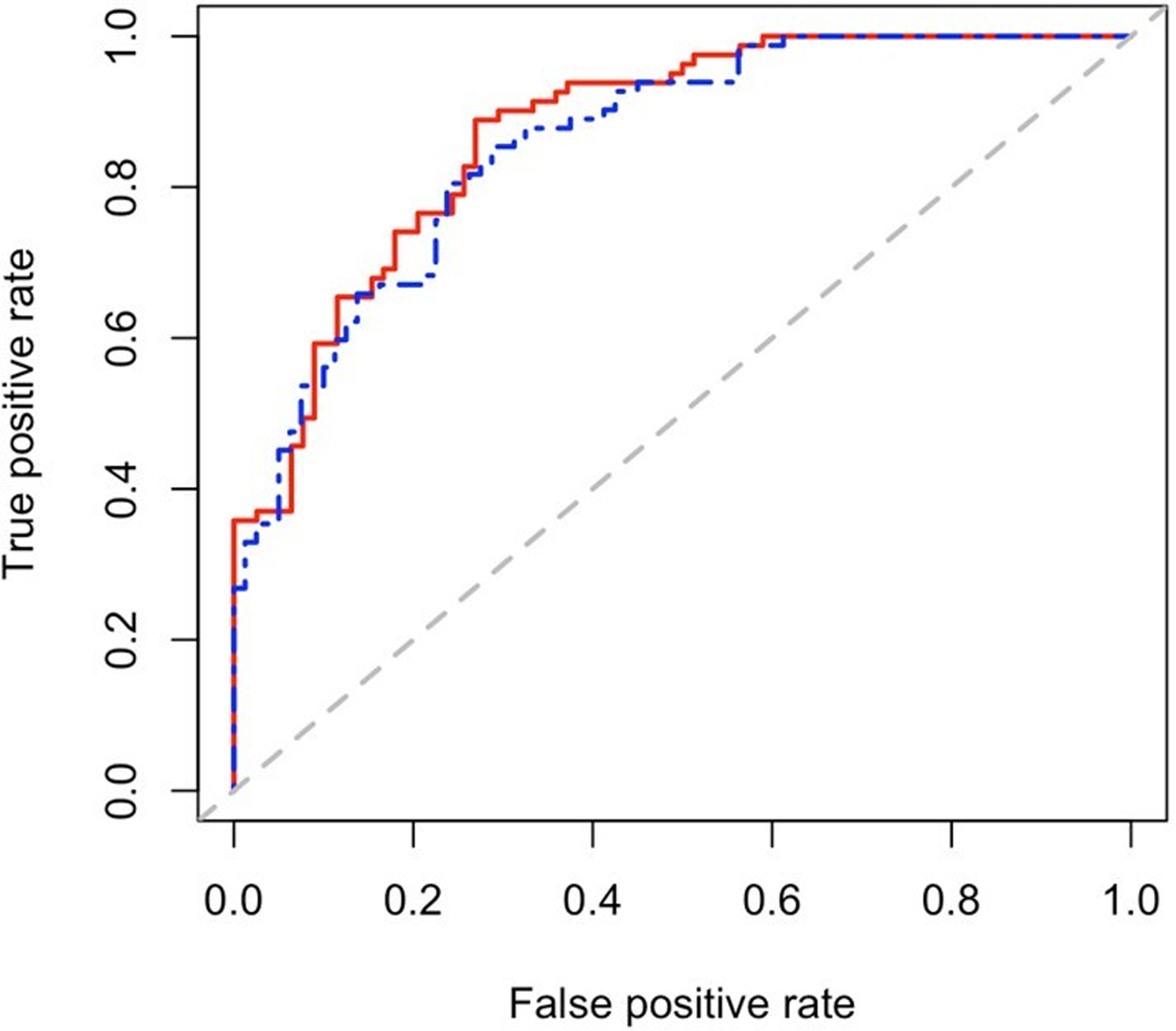
Receiver operating characteristic curve for prediction of presence of coronary calcium among patients with rheumatoid arthritis. Blue line represents the curve for clinical predicators alone (c-statistic = 0.86 (95% CI: 0.80, 0.91)) and red line represents the curve for clinical predictors plus let-7c-5p, miR-30e-5p, miR-30c-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-30a-5p, and miR-125a-5p (c-statistic = 0.87 (95% CI: 0.82, 0.93)).
Data reduction and model development for presence of high-risk coronary calcium
We also used qPCR-based plasma concentrations of the ten miRNAs and clinical variables in elastic net regression to reduce the number of predictors for prediction of high coronary artery calcium. This method selected let-7c-5p, miR-30e-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-126–3p, miR-30a-5p, and miR-125a-5p as predictors for high coronary artery calcium. Thus, only miR-30c-5p was removed due to lack of additional contribution to the model. Using the clinical predictors alone gave a c-statistic = 0.75 (95% CI 0.68, 0.83). The addition of the ten microRNAs improved the prediction for high coronary artery calcium to a c-statistic of 0.80 (95%CI 0.73, 0.86) (Figure 3, Supplemental Table 3).
Figure 3.
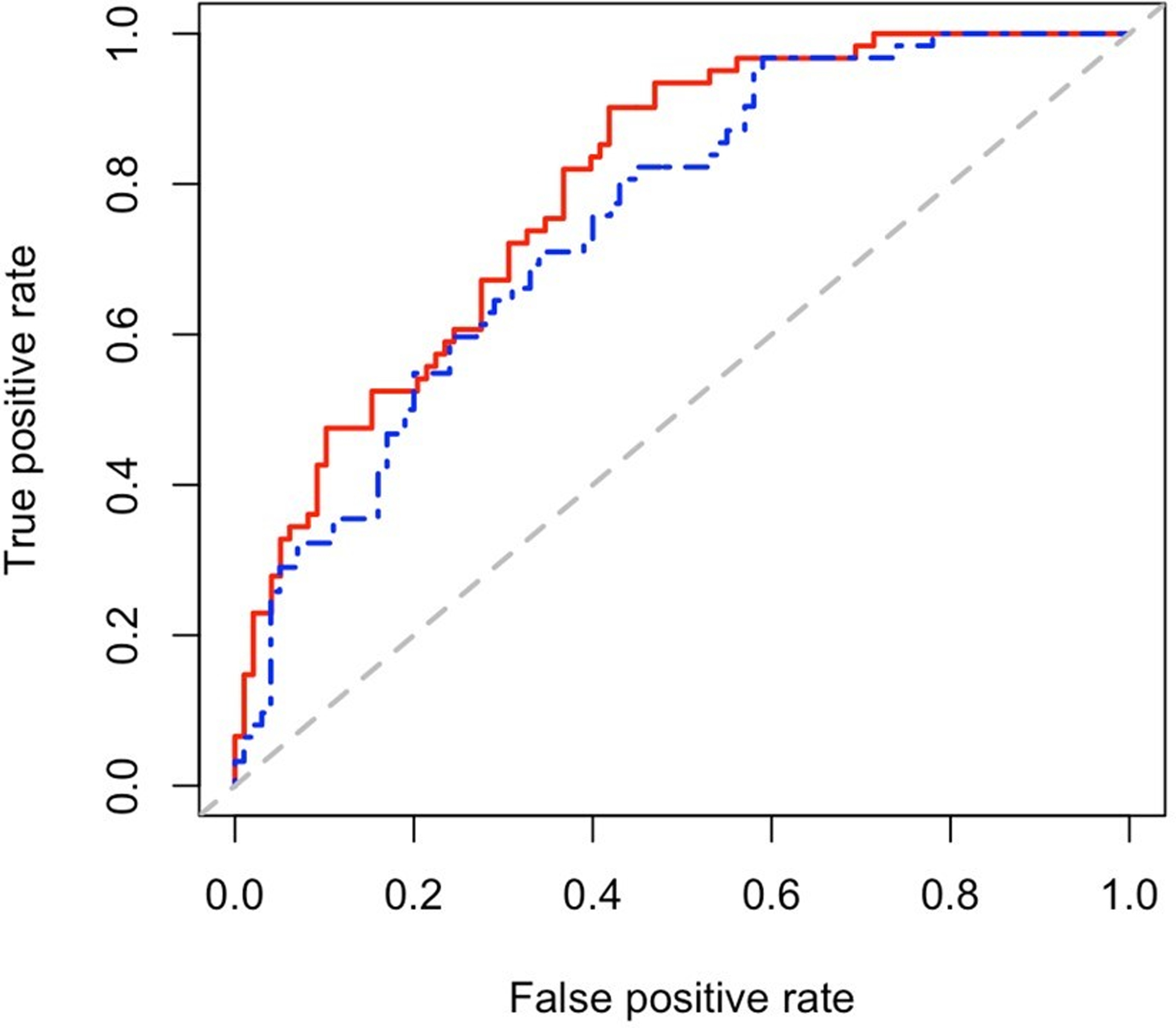
Receiver operating characteristic curve for prediction of presence of high-risk coronary calcium among patients with rheumatoid arthritis. Blue line represents curve for clinical predicators alone (c-statistic = 0.75 (95% CI: 0.68, 0.83)) and red line represents curve for clinical predictors plus miRNA panel let-7c-5p, miR-30e-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-126–3p, miR-30a-5p, and miR-125a-5p (c-statistic = 0.80 (95% CI: 0.73, 0.86)).
Analysis among RA patients without diabetes
Among those with presence of coronary calcium or high coronary calcium, there were significantly more patients with type 2 diabetes mellitus. Although the analyses above adjusted for diabetes both by presence of diabetes as a separate clinical predictor and by the ACC/AHA score, which includes diabetes, we performed an additional analysis excluding those with diabetes from analysis. Among 143 RA subjects without diabetes, the clinical predictors alone had an AUC=0.85 (95%CI: 0.79, 0.91), and clinical predictors plus the miRNA panel had an AUC=0.87 (95%CI: 0.81, 0.93), only modestly improving for prediction of coronary artery calcium. For prediction of high coronary artery calcium, the clinical predictors alone had an AUC=0.74 (95%CI: 0.66, 0.82), and clinical predictors plus the miRNA panel had an AUC=0.80 (95%CI: 0.73, 0.88), which is similar to analyses with the entire cohort.
Exploratory analysis: association of individual miRNAs with cardiovascular risk factors
We performed an exploratory analysis of the relationship between the 10 individual miRNAs and cardiovascular risk factors. In univariate analyses, individual miRNAs were not significantly associated with the presence of coronary artery calcium or high coronary artery calcium (Supplemental Table 4), or traditional risk factors such as age, hypertension, or cholesterol concentrations (Supplemental Table 5). miR-126–3p was weakly associated with serum triglycerides. None of the miRNAs were associated with CRP concentrations. Several miRNAs including miR-125a-5p, miR-30e-5p, and miR-3168 were significantly positively associated serum creatinine concentration (Supplemental Table 5).
Pathway analysis of miRNA panel- upstream regulators
Some of the top canonical pathways involved in the upstream regulation of panel of miRNAs included: the long noncoding RNA “HOX antisense intergenic (HOTAIR) regulatory pathway”, “Eukaryotic initiation factor 2 (EIF2) Signaling”, “Senescence pathway”, “Glucocorticoid receptor signaling” and “Role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis” (Supplementary Table 6, and Supplementary Figure 1). The most significant functional categories of the upstream regulators of the atherosclerosis panel miRNAs were mainly related to expression of RNA and protein, but also included fibrosis and apoptosis (Supplemental Table 7).
Pathway analysis of miRNA panel- downstream effects
The top canonical pathways which the panel miRNAs are predicted to affect include “Colorectal cancer metastasis signaling”, Molecular mechanisms of cancer”, “Systemic lupus erythematosus in B cell signaling pathway” (Supplemental Table 8). The most significant functional categories of miRNA targets were related to invasion of cells and cell movement. However, atherosclerosis was also a significant functional category (Supplemental Table 9) and the majority of miRNAs with known functions (let-7c-5p, miR-30a-5p, miR-30c-5p, miR-30e-5p, miR-125a-5p, miR126a-3p, miR-126a-5p) have direct or indirect effects on genes related to atherosclerosis (Supplemental Figure 2).
Discussion
The major finding of this study is that a panel of plasma miRNAs including let-7c-5p, miR-30e-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-126–3p, miR-30a-5p, and miR-125a-5p improved prediction of high coronary artery calcium among patients with RA.
The initial random forest analysis was designed to select miRNAs and clinical variables that predicted the presence of coronary calcium. This outcome leveraged the most statistical power because of the proportion of subjects in the groups (51% with coronary calcium and 49% without) was balanced. While improvement of the model with the addition of let-7c-5p, miR-30e-5p, miR-30c-5p, miR-4446–3p, miR-126–5p, miR-3168, miR-425–5p, miR-30a-5p, and miR-125a-5p was modest, the panel could still be an important biomarker. For example, in the Framingham Heart Study, the addition of CRP to the Framingham risk score increased the c-statistic from 0.863 to 0.865 for hard coronary heart disease outcomes, and increased the c-statistic from 0.795 to 0.799 for total cardiovascular disease [24].
Although the initial analysis to prioritize miRNAs for model development was based on the prediction of coronary calcium, there was only a small improvement in the model with the addition of miRNAs. However, there was a greater improvement in the model for prediction of high coronary calcium. One possibility for this difference in predictive capacity is that age is a major determinant of the presence versus absence of coronary calcium [25, 26]; low levels of coronary artery calcium are more strongly related to aging whereas high levels are more strongly related to accelerated atherosclerosis and cardiovascular events. Also, high coronary artery calcium was more difficult to predict using clinical measures alone, as evidenced by the baseline c-statistic for clinical predictors of 0.75, thus there was more opportunity for improvement. It is possible that the model is overfit, however, this is less likely because 1) initial selection of miRNAs was based on presence or absence of coronary artery calcium rather than high coronary artery calcium, 2) initial selection of miRNAs was based on NGS data, but the final model was developed using qPCR-based measurement, and 3) we used bootstrapping with cross validation to help reduce optimism of the models.
Individually the miRNAs that contributed to the model for high coronary artery calcium were not significantly independently associated high coronary artery calcium, yet as a panel they improved the AUC for prediction. A likely explanation for this finding is that a single miRNA species by itself often has modest effects on target expression [27], but the sum of the effect of multiple miRNAs can be amplified, given that miRNAs often act in regulatory networks, with one miRNA affecting multiple targets and multiple microRNAs affecting one gene [1].
Both miR-126–3p and miR-126–5p have established relevance for cardiovascular disease and were captured in the miRNA panel for high coronary calcium. miR-126 has been cited among the top nine most frequently reported miRNAs in atherosclerosis and hypertension [28], and biologically, miR-126–3p and miR-126–5p have critical roles in atherosclerosis. miR-126–3p downregulates vascular cell adhesion molecule 1 (VCAM-1) decreasing white blood cell adhesion to the endothelium [29], and decreases plaque formation. miR-126–5p, which is increased in endothelial cells after laminal shear stress, increases endothelial cell proliferation and limits atherosclerosis by downregulating Notch1 inhibitor delta-like 1 homolog (DLK1) [30, 31]. This parallels findings that miRNA alterations in peripheral blood monocyte subsets of patients with RA and cardiovascular disease may play a role in endothelial dysfunction [32].
Several members of the miRNA panel have been associated with vascular calcification. For example, decreases in miR-30c-5p cause increase in Runx2 leading increased vascular calcification in human coronary artery smooth muscle cells [33]. Additionally, miRNAs miR-30e and miR-125a are increased in atherosclerotic plaque compared to healthy vessel [34].
There are very few studies of miR-4446–3p in literature and we could find none in the context of atherosclerosis. However, most studies seeking cardiovascular biomarkers have used literature-based candidates for selection of miRNAs, so this miRNA may have been previously overlooked due to a paucity of information. Given lack of evidence associating miR-4446–3p with atherosclerosis or cardiovascular risk factors, the mechanism underlying its contribution to the predictive panels is uncertain. In cancer literature, miR-4446–3p is increased dramatically due to compressive forces in cancer-associated fibroblasts [35]. It would be interesting to know if miR-4446–3p is increased in the setting of arterial shear forces, because areas of shear stress are a major site for development of atherosclerosis [36]. Future work will be necessary to assess significance and function of miR-4446–3p.
We used a systems-based analysis to determine what potential functions the miRNAs as a group might have by assessing observed and predicted targets of the panel miRNAs. Most of the canonical pathways and functions identified were related to cancer. This might, in part, be because there is more information regarding miRNAs and cancer compared to other diseases but could also implicate mechanisms involving the immune system. Concordant with this possibility, pathways related to autoimmunity including SLE B cell and T cell exhaustion [37] were among the top targets, suggesting potential overlapping pathways in atherosclerosis and autoimmune disease. Although we found that atherosclerosis was a less significant pathway, it was interesting that even though we restricted our analysis to human data for known and highly predicted targets of the miRNAs, all but one of the miRNAs with known or highly predicted targets had a target related to atherosclerosis.
Similarly, we examined what could be driving expression of the panel miRNAs by assessing the top canonical pathways involved in their upstream regulation. The top two included the “HOTAIR regulatory pathway” and “EIF2 Signaling”. HOTAIR is a long non-coding RNA that increases in THP-1 macrophages treated with oxidized LDL and is instrumental in inducing oxidative stress and inflammation and apoptosis in the cells [38]. It is increased in monocytes and peripheral blood mononuclear cells from patients with coronary artery disease compared to control subjects in several small studies [39, 40]. The phosphorylation of eIF2 is induced by cellular stress, including lipid influx [41], and the eIF2 signaling pathway’s purpose is to globally block protein translation [38]. Thus, both of these identified pathways are involved in promoting or resolving cellular stresses important in the development of atherosclerosis and are potential targets for novel therapeutics [38, 41]. Lastly, components of an RA-specific pathway (“Role of macrophages, fibroblasts and endothelial cells in rheumatoid arthritis”) are upstream regulators of the miRNA panel, suggesting that RA-specific processes contribute to the composition of the miRNA atherosclerosis panel.
This study had some limitations. Basing initial selection of the miRNAs on NGS offered a broad and unbiased evaluation, but because NGS and qPCR do not have a 1:1 correlation and qPCR-based concentrations are used for the final model, it is possible that other miRNAs selected on qPCR-based concentrations could also predict coronary calcium. The study population was nearly 90% Caucasian, so it is unclear how this panel would perform in non-Caucasian populations. Although we preformed bootstrapping to improve the generalizability of the results, for development of potential biomarkers, replication in other cohorts will be necessary.
In conclusion, we determined that a panel of plasma miRNAs improves prediction of high coronary calcium in patients with RA. Replication of these findings in external cohorts and with hard cardiovascular outcomes will be helpful to fully evaluate the utility of the panel.
Supplementary Material
Key Point.
A plasma microRNA panel including let-7c-5p, miR-30a-5p, miR-30e-5p, miR-125a-5p, miR-126–3p, miR-126–5p, miR-425–5p, miR-3168 and miR-4446–3p improved prediction of high coronary artery calcium beyond clinical factors in patients with rheumatoid arthritis.
Funding:
Veterans Health Administration CDA IK2CX001269, Arthritis Foundation Delivering on Discovery grant, Alpha Omicron Pi, NIH Grants: P01HL116263, and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1.Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–97. [DOI] [PubMed] [Google Scholar]
- 2.Chen CZ, Li L, Lodish HF, Bartel DP. (2004) MicroRNAs modulate hematopoietic lineage differentiation. Science 303:83–6. [DOI] [PubMed] [Google Scholar]
- 3.Guduric-Fuchs J, O’Connor A, Camp B, O’Neill CL, Medina RJ, Simpson DA (2012) Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC genomics 13:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickman CT, Lawson J, Jabalee J, MacLellan SA, LePard NE, Bennewith KL, et al. (2017) Selective extracellular vesicle exclusion of miR-142–3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget 8:15252–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 108:5003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matias-Garcia PR, Wilson R, Mussack V, Reischl E, Waldenberger M, Gieger C, et al. (2020) Impact of long-term storage and freeze-thawing on eight circulating microRNAs in plasma samples. PloS one 15:e0227648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. (2005) Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum 52:3045–53. [DOI] [PubMed] [Google Scholar]
- 9.Crowson CS, Gabriel SE, Semb AG, van Riel P, Karpouzas G, Dessein PH, et al. (2017) Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology (Oxford) 56:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ormseth MJ, Solus JF, Sheng Q, Ye F, Wu Q, Guo Y, et al. (2020) Development and Validation of a MicroRNA Panel to Differentiate Between Patients with Rheumatoid Arthritis or Systemic Lupus Erythematosus and Controls. J Rheumatol 47:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata K, Yoshitomi H, Tanida S, Ishikawa M, Nishitani K, Ito H, et al. (2010) Plasma and synovial fluid microRNAs as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 12:R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condorelli G, Latronico MV, Cavarretta E. (2014) microRNAs in cardiovascular diseases: current knowledge and the road ahead. J Am Coll Cardiol 63:2177–87. [DOI] [PubMed] [Google Scholar]
- 13.Barwari T, Joshi A, Mayr M. (2016) MicroRNAs in Cardiovascular Disease. J Am Coll Cardiol 68:2577–84. [DOI] [PubMed] [Google Scholar]
- 14.Bonneau E, Neveu B, Kostantin E, Tsongalis GJ, De Guire V. (2019) How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC 30:114–27. [PMC free article] [PubMed] [Google Scholar]
- 15.Ormseth MJ, Solus JF, Vickers KC, Oeser AM, Raggi P, Stein CM. (2015) Utility of Select Plasma MicroRNA for Disease and Cardiovascular Risk Assessment in Patients with Rheumatoid Arthritis. J Rheumatol 42:1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–24. [DOI] [PubMed] [Google Scholar]
- 17.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38:44–8. [DOI] [PubMed] [Google Scholar]
- 18.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, Shintani A, Pincus T, Stein CM. (2009) Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum 60:1906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, Pincus T, Avalos I, Stein CM. (2005) Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum 52:3045–53. [DOI] [PubMed] [Google Scholar]
- 20.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–32. [DOI] [PubMed] [Google Scholar]
- 21.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63(25 Pt B):2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen RM, Zhao S, Ramirez Solano MA, Zhu W, Michell DL, Wang Y, et al. (2018) Bioinformatic analysis of endogenous and exogenous small RNAs on lipoproteins. J Extracell Vesicles 7:1506198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ormseth MJ, Solus JF, Sheng Q, Ye F, Song H, Wu Q, et al. (2020) The Endogenous Plasma Small RNAome of Rheumatoid Arthritis. ACR Open Rheumatology 2:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R Sr., O’Donnell CJ. (2008) C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes 1:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, et al. (2005) Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 112:572–7. [DOI] [PubMed] [Google Scholar]
- 26.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. (2018) Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 72:434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soifer HS, Rossi JJ, Saetrom P. (2005) MicroRNAs in disease and potential therapeutic applications. Mol Ther 15:2070–9. [DOI] [PubMed] [Google Scholar]
- 28.Gangwar RS, Rajagopalan S, Natarajan R, Deiuliis JA. (2018) Noncoding RNAs in Cardiovascular Disease: Pathological Relevance and Emerging Role as Biomarkers and Therapeutics. Am J Hypertens 31:150–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. (2008) MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105:1516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, et al. (2014) MicroRNA-126–5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med 20:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boon RA, Dimmeler S. (2014) MicroRNA-126 in atherosclerosis. Arterioscler Thromb Vasc Biol 34:e15–6. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Pedrera C, Barbarroja N, Patiño-Trives AM, Luque-Tévar M, Torres-Granados C, Aguirre-Zamorano MA, Collantes-Estevez E, Pérez-Sánchez C. (2020) Role of microRNAs in the Development of Cardiovascular Disease in Systemic Autoimmune Disorders. Int J Mol Sci 21:2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balderman JA, Lee HY, Mahoney CE, Handy DE, White K, Annis S, et al. (2012) Bone morphogenetic protein-2 decreases microRNA-30b and microRNA-30c to promote vascular smooth muscle cell calcification. J Am Heart Assoc 1:e003905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bidzhekov K, Gan L, Denecke B, Rostalsky A, Hristov M, Koeppel TA, et al. (2012) microRNA expression signatures and parallels between monocyte subsets and atherosclerotic plaque in humans. Thromb Haemost 107:619–25. [DOI] [PubMed] [Google Scholar]
- 35.Kim BG, Kang S, Han HH, Lee JH, Kim JE, Lee SH, et al. (2016) Transcriptome-wide analysis of compression-induced microRNA expression alteration in breast cancer for mining therapeutic targets. Oncotarget 7:27468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heo KS, Fujiwara K, Abe J. (2014) Shear stress and atherosclerosis. Mol Cells 37:435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinney EF, Lee JC, Jayne DR, Lyons PA, Smith KG. (2015) T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523:612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Huang GQ, Ke ZP. (2019) Silence of long intergenic noncoding RNA HOTAIR ameliorates oxidative stress and inflammation response in ox-LDL-treated human macrophages by upregulating miR-330–5p. J Cell Physiol 234:5134–42. [DOI] [PubMed] [Google Scholar]
- 39.Cai Y, Yang Y, Chen X, Wu G, Zhang X, Liu Y, et al. (2016) Circulating ‘lncRNA OTTHUMT00000387022’ from monocytes as a novel biomarker for coronary artery disease. Cardiovasc Res 112:714–24. [DOI] [PubMed] [Google Scholar]
- 40.Avazpour N, Hajjari M, Yazdankhah S, Sahni A, Foroughmand AM. (2018) Circulating HOTAIR RNA Is Potentially Up-regulated in Coronary Artery Disease. Genomics Inform 16:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Onat UI, Yildirim AD, Tufanli O, Cimen I, Kocaturk B, Veli Z, et al. (2019) Intercepting the Lipid-Induced Integrated Stress Response Reduces Atherosclerosis. J Am Coll Cardiol 73:1149–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wek RC, Jiang HY, Anthony TG. (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34(Pt 1):7–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


