Abstract
Environmental pollution related to anthropogenic pressures, and the associated repercussions on public health, represent a worldwide problem. Thus, the study of the effects that environmental contaminants can pose to natural ecosystems and human health is of vital importance. Laboratory model organisms such as Caenorhabditis elegans have played a significant role in clarifying multi-level effects of those agents. Although the evaluation of contaminants effects at the behavioral level of organisms is an emerging approach in ecotoxicology, studies assessing chemotaxis behavior in C. elegans within the ecotoxicological research context are still scarce. Chemotaxis studies in C. elegans have contributed to the understanding of both the neuronal mechanisms involved in the behavioral effects triggered by environmental cues, and the impact of contaminants on natural ecosystems. Its compact and well-characterized nervous system, as well as the availability of transgenic strains and molecular tools, allow a detailed examination of behavioral, molecular, and genetic chemosensation mechanisms. This overview provides a summary and general comparison of methods used to measure chemotaxis behavior in C. elegans, with the aim of helping researchers select the most suitable approach in their chemotaxis studies. We compare methods based on the type of chemical tested, advantages and drawbacks of the different approaches, and specific experimental goals. Lastly, we hope to encourage the evaluation of C. elegans’ chemotaxis behavior in ecotoxicology studies, as well as its potential integration in standardized protocols assessing environmental quality.
Keywords: chemotaxis, avoidance, attraction, ecotoxicology, Caenorhabditis elegans
1. INTRODUCTION
Environmental pollution caused by anthropogenic activities like metal-related industry and pesticide-dependent agriculture, as well as related public health issues, represent a major worldwide problem (e.g. Ali, Khan, & Ilahi, 2019; Vryzas et al., 2018). Therefore, it is critical to investigate the risks that environmental contaminants can pose to biota (e.g. Vryzas et al., 2018; van Gestel, 2012). Nematodes are one of the most abundant and ubiquitous animals on earth (Hagerbaumer et al., 2015), and the nematode Caenorhabditis elegans is an invaluable model in developmental biology and neurobiology research, and has also been gaining interest in ecotoxicology. Studies in C. elegans have contributed to clarify the toxicant effects of environmental contaminants at the behavioral, reproductive, physiological, morphological, biochemical, and genetic levels (e.g. Queirós et al., 2019).
Caenorhabditis elegans, as most multi-cellular organisms, uses its ability to perceive chemical signals (cues) (chemosensation) to interact with its environment, adjusting its behavior accordingly. Chemotaxis behavior has a key role in C. elegans survival, since it is used to forage for food, find mates, lay eggs, enter into the alternative dauer larva stage, and also to avoid pathogens and toxic substances (Prasad et al. 1999; Bargmann, 2006). Therefore, investigating potential effects of environmental contaminants on C. elegans’ chemotaxis behavior is highly relevant, given also the key environmental roles of nematodes in general (see below). In fact, efforts aimed at understanding the genetic, molecular, biochemical, neuronal, and behavioral mechanisms involved in the sensing, as well as in the reaction to each specific chemical cue, have provided useful information into C. elegans’ life-history strategies (e.g. Troemel et al., 1997; Kunitomo et al., 2013). These studies have additionally helped understand the mechanisms underlying related processes in other animals, including mammals (e.g. roles of specific molecules in common signal pathways; Prasad et al. 1999; Yuan et al. 2018). The utility of C. elegans in the investigation of genetic features and neuronal mechanisms involved in behavioral responses is indisputable (e.g. see Prasad et al. 1999). This is due to its compact and well-characterized nervous system, as well as the availability of transgenic strains and other molecular tools that have allow for the examination of these genetic and molecular mechanisms in detail (e.g. using transgenic strains lacking specific receptors or neurons to investigate its role in the triggered responses to each specific chemical cue; Bargmann, 2006).
Caenorhabditis elegans detects the presence of volatile and water-soluble chemicals through the action of a specific set of chemosensory neurons, with its chemosensory system being composed by amphid, phasmid, and inner labial neurons (Bargmann, 2006; Zou et al., 2017). The amphid neurons, which are eleven pairs in total, act primarily in detection. Each amphid neuron expresses a specific set of candidate receptor genes and detects a characteristic set of attractants, repellents, or pheromones. Different sensory neurons are required to detect volatile and water-soluble substances (Bargmann, 2006; Zhang et al., 2014). For instance, the ASE amphid neurons can sense salts and water-soluble attractants, while the AWA, AWB, and AWC amphid neurons can sense volatile substances, suggesting that C. elegans exhibits senses similar to taste and smell in mammals (Bargmann, 2006).
The work by Ward et al. (1973) pioneered chemotaxis assays with Caenorhabditis elegans. They observed that this nematode species was attracted to several substances from three different classes, specifically, cyclic nucleotides (e.g. cAMP and cGMP), anions (e.g. Cl− and Br−), and cations (e.g. Na+ and K+), as well as to conditions of high pH. They also observed that this behavioral response involved orientation and movement up gradients of the attractant, accumulation of the worms at the attractant area, and then, habituation. Thereafter, several studies have assessed chemotaxis in C. elegans in response to distinct bacterial food sources (e.g. Turner, Cox, Spellman, Stahl, & Bavari, 2020), as well as specific to water-soluble or volatile elements, such as volatile compounds released by bacteria, like isoamyl alcohol (e.g. Yoshida et al., 2012; Worthy et al., 2018) and benzaldehyde (e.g. Tsui & Kooy, 2008), but also other chemicals/molecules, like salts (Saeki et al., 2001; Tomioka et al., 2006), hormones (e.g. Luo, Xu, Tan, Zhang, & Ma, 2015), and metals (e.g. Sambongi & Nagae, 1999; Song et al., 2014; Wang et al., 2015; Wakabayashi et al., 2020). Other recent studies have also specifically assessed the effects of environmental contaminants of current concern on C. elegans’ chemotaxis behavior, like graphene oxide nanomaterials (Wang et al., 2019), nanopolystyrene nanoparticles (Qu et al., 2020), and pesticides (Hopewell et al., 2017; Sobkowiak et al., 2018).
The studies assessing the effects of both volatile and non-volatile chemicals on C. elegans’ chemotaxis behavior have contributed to: (1) generally characterize these chemicals as attractants or repellents to this nematode species (e.g. Bargmann et al., 1993); (2) understand specific neuronal mechanisms involved in observed behavioral responses (Prasad et al. 1999); (3) understand whether these substances may act as chemical cues in C. elegans natural environment (e.g. Worthy et al., 2018); and (4) clarify potential toxic effects of these chemicals on C. elegans’ chemosensation response (e.g. Hopewell et al., 2017). While the vast majority of studies assessing chemotaxis behavior in C. elegans have been performed in the neurobiological context, chemotaxis behavior seems to be still largely overlooked in the ecotoxicology context compared to other behavioral endpoints that have been studied in C. elegans, such as locomotor and feeding behavior (e.g. Leung et al., 2008; Queirós et al., 2019; Chen et al., 2019). Notably, avoidance behavior has been used as a reference sensitive endpoint to assess soil quality in other laboratory model species, like the earthworm Eisenia spp. and the springtail Folsomia candida (standardized guidelines based on this endpoint are available: ISO 17512–1, 2008 and ISO 17512–2, 2011, respectively), both regarding metals (e.g. Natal da Luz, 2004; Syed et al. 2017) and pesticides (e.g. García-Santos & Keller-Forrer, 2011; Santos et al., 2012) contamination. Thus, more widespread use of C. elegans’ chemotaxis behavior assays in ecotoxicological studies testing environmental pollutants of current concern (e.g. metals, nanomaterials and pesticides) may additionally provide relevant information about its environmental safety. For instance, such assays could help clarify the possible activity of these agents as chemical cues (i.e. by triggering a behavioral response) or even as interfering agents for other chemical cues (e.g. by provoking an alteration in sensorial perception to known chemical cues). Additionally, the use of C. elegans transgenic strains in these chemotaxis assays could further provide information about the mechanisms of neuronal action of these pollutants. This assessment is then relevant from both an environmental (i.e. safety to nematodes and potentially other animals in their natural environment) and human health (e.g. identification of potentially hazardous chemicals; identification of similar mechanisms of toxic action) point of view.
This overview provides a summary of studies describing different approaches to assess chemotaxis to volatile or water-soluble substances in C. elegans. A general discussion of their findings, a summary of the respective procedures, as well as their advantages and drawbacks are presented, with the aim of supporting researchers on the choice of the most suitable approach for their studies evaluating chemotaxis behavior in C. elegans. Specifically, approaches can vary with the characteristics of the chemical of interest and goals of each study. This work additionally intends to encourage a wider use of C. elegans chemotaxis assays to evaluate the effects of environmentally-relevant contaminants in ecotoxicological studies. With that in mind, comments on how the methods described could be employed in ecotoxicology contexts are made throughout the manuscript, as well as in a dedicated section.
2. CHEMOTAXIS ASSAYS: Overview
Chemotaxis assays with C. elegans are generally performed by placing a tester (attractant/repellent) at a point/area in one side of a Petri dish, a control on the opposite side, and the worms in a position equidistant to both the tester and control points/areas (see. Table 1 – “general scheme of the test plate”). The worms are then tracked and/or counted both at the tester and control areas at the end of a defined period of exposure. If worms are attracted, they move towards the area containing the tester, and if worms are repulsed, they move away from that area (i.e. more worms at the control side, than at the tester side; Cornelia I Bargmann, Hartwieg, & Horvitz, 1993; Dusenbery, 1983; Margie, Palmer, & Chin-sang, 2013). ‘Preference behavior’ between different chemicals can also be assessed in similar assays by placing two alternative attractants at the same time on the plate (i.e. instead of an attractant and the respective control), followed by the comparative assessment of the chemotaxis to each option. While ‘preference behavior’ assays are usually employed to assess food choice (dietary choice behavior; e.g. Shtonda & Avery, 2006), similar approaches have also been used to measure the effect of multiple chemicals on chemotactic behavior in C. elegans. For example, Matsuura et al. (2004; 2007) compared C. elegans preference between sodium acetate and diacetyl to investigate the neuronal mechanism and possible connections underlying behavioral selection.
Table 1 -.
Comparison of different methods that have been used to measure chemotaxis in Caenorhabditis elegans. The table includes the reference for the study, a general scheme of the test plate used in the assay, a brief overview of the procedure, the substances or compounds tested, the duration of the exposure, some advantages and drawbacks for each approach, and the respective chemotaxis index formula applied to classify the response as attraction or avoidance. The studies were grouped in the table according to the type of substance/compound tested, first listing those evaluating volatile toxicants, and then those studying water-soluble toxicants, including metal salts and NaCl. Figures are adapted from originals, with the exception of those shown for Sambongi et al. 1999 and Saeki et al. 2001, which were designed here for this article.
| Reference | General scheme of the test plate | Brief overview | Tested substances/compounds | Exposure duration | Advantages & Drawbacks | Chemotaxis index formula used |
|---|---|---|---|---|---|---|
| Volatile substances/compounds | ||||||
| Bargmann et al. 1993 |  |
Application of attractant and control plus NaN3 in opposite spots (A and C); Worms (100–200) placed at the plate’s center (Origin). Number of worms in A and C counted after 60 min. | Alcohols, ketones, esters, aldehydes, and aromatic compounds | 60 min |
Adv.
|
CI>0: worms attracted to the toxicant CI<0: worms repulsed by the toxicant CI=1.0: “perfect attraction” CI=−1.0: “perfect repulsion” |
| Troemel et al. 1997 |  |
Application of odorant and control plus NaN3 in two opposite sides of the plate; Worms placed at origin and counted in each sector after 60 min. | Diacetyl, 2-nonanone, pyrazine, 2,4,5-trimethylthiazole, isoamyl alcohol, benzaldehyde, 2,3-pentanedi- one, and 2-butanone | 60 min |
Adv.
|
CI>0: worms attracted to the odorant CI<0: worms repulsed by the odorant Lower the CI, higher the repulsion/avoidance |
| Matsuura et al. 2004, 2007 |  |
Application of attractant/repellent plus NaN3 differentially to test either attraction/ avoidance or preference. Attractant/repellent placed two times in A for a concentration gradient (15–18 hr and again 3 hr before the assay). Worms (15 in each spot) placed in C and D and counted every 10 min, during a 90-min interval. | Diacetyl and sodium acetate Note: An odorant (diacetyl) and a water-soluble substance (sodium acetate) have been jointly tested in this assay |
90 min |
Adv.
|
CI>0: worms attracted to the toxicant tested/ worms prefer toxicant tested in A CI<0: worms repulsed by the toxicant tested/ worms prefer toxicant tested in B |
| Yoshida et al. 2012 | 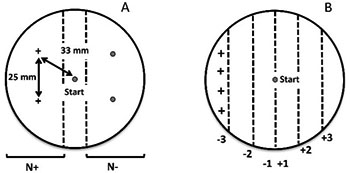 |
Application of odorant plus NaN3 or NaN3 only (control) in the plus and point marks, respectively; Worms placed at the plate’s center (“Start”) and counted in N+ and N- areas after 60 min. | Isoamylalcohol, benzaldehyde, pentanedione, trimetylthiazole, and diacetyl | 60 min |
Adv.
|
CI>0: worms attracted to the odorant CI<0: worms repulsed by the odorant |
| Margie et al. 2013 |  |
Application of attractant/repellent plus NaN3 at the test spots (T) and NaN3 only or mixed with the solvent used to dilute the attractant/repellent at the control spots (C). Worms (50–250) placed at the plate’s center and counted in each quadrant after 60 min. | Diacetyl | 60 min |
Adv.
|
CI>0: worms attracted to the substance CI<0: worms repulsed by the substance |
| Water-soluble substances/compounds | ||||||
| Metals | ||||||
| Sambongi et al. 1999 |  |
Application of medium containing the toxicant (pH = 7.2) in Test area and the uncontaminated medium (pH = 7.2) in Control and central areas. Worms (≈100) placed on the central area and counted in each area after 45 min. | Cd2+, Cu2+ and Ni2+ (acetate salts) | 45 min |
Adv.
|
CI>0: worms repulsed by the toxicant CI<0: worms attracted to the toxicant |
| Monteiro et al. 2014 |  |
Fill holes made with a sterile tube with 600 μL control K-medium (K), clean E. coli suspended in K-medium (EC), contaminated E. coli suspended in K-medium (M). Worms (30) placed at the plate’s center and counted in each spot after 1, 4, 6, and 24 hr. | Pb, Zn, and Ni (chloride salts) | 24 hr |
Adv.
|
_________________ |
| Chai et al. 2017 |  |
Application of attractant/repellent or the solvent control (1 μL drops) in Exp and Ctrl quadrants, respectively. Worms placed outside the treated circle; movement recorded inside a behavior chamber with infrared LED backlighting and a microscope camera. | CuCl2 | 2–3 min acclimation + 10 min video recording + 10 min video recording |
Adv.
|
*% worm pixels in CI>0: worms attracted to the toxicant tested CI<0: worms repulsed by the toxicant tested NOTE: The CI function counts the number of worm pixels in each ROI and calculates the percentage of worm pixels out of the total number of ROI pixels for each video frame. |
| Yuan et al. 2018 |  |
Attractant/repellent gradient is ensured by adding contaminated medium to one third of the plate (plate tilted), and then clean medium after solidification (plate flat). Worms (more than 150) placed at sector 3 and counted after 150 min. | CuCl2 | 150 min |
Adv.
|
CI>0: worms attracted to the substance CI<0: worms repulsed by the substance |
| NaCl | ||||||
| Saeki et al. 2001 |  |
Agar plug containing the attractant left overnight at B; NaN3 added to A and B, after plug removal. Worms (≈100) placed equidistantly from A and B and counted around 15 mm from the center of each spot. | NaCl and other water-soluble chemoattractants, such as ammonium chloride and sodium acetate | 30 min |
Adv.
|
CI>0: worms attracted to the substance tested CI<0: worms repulsed by the substance tested |
| Kunitomo et al. 2013 |  |
Two agar plugs with different concentrations of attractant added to create a gradient over an intermediate concentration-background. NaN3 added to plug areas. Worms (50–200) placed at Origin and counted within a 20 mm radius of the center of each area, after 45 min. Worms within a 10 mm radius of the Origin are not considered. | NaCl | 45 min |
Adv.
|
CI>0, worms attracted to the highest concentration of the substance CI<0: worms attracted to the lowest concentration of the substance |
A summary of the different steps typically involved in chemotaxis assays is presented in Table 2. Despite some variations, as detailed in the next section, all assays follow the same main steps, namely, plate preparation, assay initiation by placing the worms at specific spots on the plates, worms counting (and/or tracking) in each area at the end of each defined exposure period, and data analysis, which generally includes the calculation of chemotaxis indexes (CI).
Table 2 -.
General scheme followed in chemotaxis assays, highlighting some of the variations commonly found among methods, namely, type of plates used, method for the addition of the substances/compounds to the test plate, initial location of the worms, time of exposure, and procedure adopted for counting/analysis.
| Steps | Procedures | Some variations |
|---|---|---|
| 1. Plate Preparation | 1.1. Plates | Plates with different sizes, shapes, and sectors have been used (e.g. Table 1) |
| 1.2. Adding attractants/repellents plus the controls | Attractants/repellents have been added to the assay plates: | |
| ||
| 2. Exposure | 2.1. Placing the worms | Worms have been placed on:
|
| 2.2. Tracking and/or counting the worms at test and control areas | The time of exposure used has been study-specific (e.g. from 30 min to 24 hr; Table 1) | |
| Worms have been counted immediately at the defined timepoints, or later based on photos or videos recorded. | ||
| 3. Analysis | 3.1. Data analysis/ calculation of the chemotaxis index | Chemotaxis index formulas have been adapted for each specific plate design (e.g. Table 1) |
3. A GENERAL COMPARISON OF DIFFERENT APPROACHES TO ASSESS CHEMOTAXIS IN C. ELEGANS
In this section, we discuss studies describing different approaches to assess chemotaxis to different volatile and water-soluble chemicals in C. elegans. A summary of methods/approaches and respective variations that have been frequently used to measure chemotaxis in C. elegans is presented in Table 1. The methods will be discussed according to the type of substance/compound tested in the respective study/protocol: first, we will describe methods for assessing volatile toxicants, and then, those using water-soluble toxicants (metal salts and NaCl). The main advantages and drawbacks for each procedure are also discussed.
3.1. METHODS USED TO ASSESS CHEMOTAXIS TO VOLATILE SUBSTANCES/COMPOUNDS
The studies discussed in this section (Bargmann et al., 1993; Troemel et al., 1997, Matsuura et al., 2004; Matsuura et al., 2007 and Yoshida et al., 2012) have used chemotaxis assays in C. elegans to address the effects of volatile substances/compounds within the scope of neurobiology research, mostly evaluating attraction/avoidance behavioral responses and investigating the mechanisms involved in the response to specific chemical cues
3.1.1. Bargmann et al. (1993)
The method described in this study resulted from adaptations of previous methods, specifically those by Ward et al. (1973) and Bargmann et al. (1991). Bargmann et al. (1993) showed, for the first time, that C. elegans was attracted to numerous volatile organic molecules from different chemical groups such as alcohols, ketones, esters, aldehydes, and aromatic compounds (the attraction to water-soluble substances such as the cyclic nucleotide cAMP and NaCl had been shown previously; e.g. Ward et al. 1973; Bargmann & Horvitz, 1991). Interestingly, in some cases, this attraction was found to be concentration specific. For example, C. elegans was attracted to low concentrations of benzaldehyde but was repelled by high concentrations of this substance. C. elegans also exhibited an avoidance response to several tested substances, such as 1-octanol, 3-nonanone, ethyl heptanoate, butyl butyrate, and 2,4,5-trimethylthiazole. Additionally, some of the tested substances were neutral (e.g. thiazole and isobutyraldehyde), since they triggered neither an attraction nor an avoidance response in C. elegans.
The method used by Bargmann et al. (1993) is still currently applied, although mostly with some modifications (e.g. Luo et al. 2015; Nuttley et al. 2020). Briefly, it consists on the application of one attractant (or repellent) in one side of a round plate, and the respective control (e.g. the solvent used to dissolve the attractant/repellent) on the other side of the plate, followed by the addition of well-fed adult worms (adult worms with a fully developed nervous system) to the plate center in order to determine if the worms are attracted (or repelled) to the side containing the test substance/compound (Table 1). Sodium azide (NaN3) is added to both attractant and control spots to anesthetize worms within these areas - note that the rate at which animals accidentally wander into the anesthetic region is considered identical at the attractant and control. The anesthetic is used to minimize effects of adaptation, since C. elegans adapts to an attractant after prolonged exposure. A chemotaxis index (CI) is then determined by subtracting the number of worms in the attractant area by the number of worms in the control area, and dividing it by the total number of worms on the plate (see Table 1, Chemotaxis Index formula used). The CI index varies from 1.0 (“perfect attraction”) to −1.0 (“perfect repulsion”). This method is easy and fast (60 min) to perform, and requires the use of very low volumes of stock solution of the substance/compound tested for attraction (1 μL of attractant over the marked spot A, Table 1). Although this assay represents a very good option to run fast screenings of substances to assess attraction (or repulsion), it is not appropriate to study stimulus gradient navigation (i.e. movement decisions to drive directed navigation toward favorable conditions). Since C. elegans integrates environmental cues and uses behavioral components like turns, reversals, and curves to navigate along the stimulus gradients towards the most favorable condition (e.g. navigation towards a previously experienced concentration of a chemical, such as the salt concentration used in the culture growth medium; Kunitomo et al., 2013), studies focused on this response, including the neuronal circuits involved, are thus also important. The method used by Bargmann et al. (1993) was designed to assess attraction to volatile substances, which naturally diffuse quickly in the air, orienting the C. elegans movement to the attractant spot (the placement of the attractant on the lid of the petri plate instead of the agar surface triggered the accumulation of worms under the source of attractant, reinforcing that the detection occurs via air). This is distinct from the water-soluble substances that diffuse through the agar medium, and where a gradient might take hours to be established. Also, the proximity between the control and attractant/repellent in the round plate used may provoke a partial merging of the conditions, which can, for instance, create problems not observed when using a square plate, which allows for a more effective separation of the tested conditions and a more appropriate measurement of avoidance (method described below).
3.1.2. Troemel et al. (1997)
In this study, the authors used transgenic strains to investigate the involvement of specific C. elegans olfactory neurons and odorant receptors in the behavioral responses observed (e.g. attraction or avoidance) for different odorants (diacetyl, 2-nonanone, pyrazine, 2,4,5-trimethylthiazole, isoamyl alcohol, benzaldehyde, 2,3-pentanedione, and 2-butanone), most of them tested before by Bargmann et al. (1993). Troemel et al. (1997) specifically developed an assay to measure long-range avoidance of volatile repellents, since, as mentioned above, the one developed previously by Bargmann et al. (1993) is not suitable for this purpose, due to the proximity between the tested conditions. This assay is run on a square plate, divided into six equal sectors, with the repellent being placed along one edge (note the difference with the round plate used by Bargmann et al. 1993). Notably, in this method, the odorants and controls are placed not in one but two spots on the edge of each side of the plate, to allow its distribution over the entire sector’s length. As in Bargmann et al. (1993), NaN3 is added to all test and control spots, and the worms (population assay) are placed at the plate center. Only the four external sectors are considered for the calculation of the chemotaxis index (Table 1). The authors conducted this same approach in single-worm assays, by placing only one worm per plate (without adding NaN3), and tracking the worm’s movement in the different sectors. The response was classified as avoidance or attraction, by summing the scores of the sectors where the worm had travelled to, to calculate a final plate score (attributed nominal scores of 3, 2, 1, −1, −2 and −3 to sectors A–F, respectively; avoidance - negative final score, attraction - positive final score). The time of exposure in this adaptation is the same (60 min) as in Bargmann et al. (1993), and the required volume of the test substances is very similar (2 μL of attractant over the marked odorant spots, Table 1). This method can be a good option to specifically measure avoidance to volatile environmentally-relevant contaminants.
3.1.3. Matsuura et al. (2004, 2007)
Matsuura et al. (2004, 2007) were amongst the first to test C. elegans preference behavior when exposed to two chemical attractants (sodium acetate and diacetyl) simultaneously. They intended to assess the effect of multiple attractants on chemotaxis behavior, compared to single exposure, in order to contribute to the understanding of the overall mechanisms underlying the integration of multiple sensory stimuli in behavioral selection (Matsuura et al., 2004). In a subsequent study, they also investigated the effects of aging in the choice between these two attractants (Matsuura et al., 2007). Their results suggested the existence of excitatory and/or inhibitory connections in the neuronal circuit of C. elegans for attractant selection (Matsuura et al., 2004), and that this response varies with developmental stage (Matsuura et al., 2007). Specifically, the chemotactic responses to these attractants seemed to increase with development (higher chemotaxis indexes were obtained for young adult and 1-day adult worms) and, later, decline with increased age, possibly due to a decreased activity of spontaneous locomotion related to aging (Matsuura et al., 2007). When both attractants were presented, the chemotaxis response obtained for different development-stage worms was very different: the fraction of larval worms at the sodium acetate location was greater than that at the diacetyl location, the fraction of young adult worms was identical in both locations and, finally, the fraction of adults in the diacetyl location was higher than at the sodium acetate location (Matsuura et al., 2007). This reinforces the importance of studying choice responses (and integrated mechanisms) throughout different development stages, as well as possible interactions between the different variables (e.g. distinct bacterial food, or even uncontaminated vs contaminated food, or distinct attractants) and comparing that to single exposures, since in nature, the variables are not isolated as in the laboratory, and the mechanisms triggering specific choices are often unclear.
The approach applied to assess single attractant choice in the studies from Matsuura et al. (2004, 2007) is very similar to that used by Bargmann et al. (1993) (Table 1), except that the worms are originally placed not at the plate center, but at two distinct spots equidistant from the attractant and control spots, most likely to avoid an effect of population density. This represents an advantage of this method compared to that used by Bargmann et al., 1993, although it should be mentioned that the sample size was much smaller – only 30 worms, compared to 100–200 in Bargmann et al., 1993. The number of worms at the attractant and control spots (A and B, see general scheme of the test plate, Table 1) is determined every 10 min during a 90-min interval, which represents a longer exposure and more assessment timepoints than those in Bargmann et. al., 1993 (video-recording equipment may be useful to record the worms’ movement, and the counting can be made posteriorly). These parameters (i.e. exposure time and assessment timepoints), however, are easily adapted for any method depending on the study goals. Similar to Bargmann et. al., 1993, Matsuura et al. (2004, 2007) also used NaN3 in control and test spots to anesthetize worms at these areas and minimize effects of adaptation. Although the authors used this approach to test both a volatile substance (diacetyl) and a water-soluble substance (sodium acetate), it exhibits more similarities with existent methods testing volatile substances (e.g. Bargmann et al., 1993 and Matsuura et al. 2044, 2007), , hence its inclusion in this section. This method can be used, for example, to assess, in a more realistic scenario, if the existence of two different environmentally-relevant contaminants, present on the same plate, can alter the C. elegans behavioral response compared to independent and separate exposures.
3.1.4. Yoshida et al. (2012)
In 2012, Yoshida et al. reported the assessment of chemotaxis to the previously tested odorants isoamylalcohol, benzaldehyde, pentanedione, trimetylthiazole, and diacetyl in C. elegans, aiming to understand how olfactory preference in this model organism is shaped by odor concentration. The authors confirmed that C. elegans was attracted to all tested odorants at low concentrations, and repelled by all of them when tested at high concentrations. Moreover, they found that AWC neurons are involved in the response to the lowest concentrations, while the ASH neurons appear to mediate the response to the highest concentrations of the odorants. The chemotaxis assay used was based on a modified plate format assay following a previous protocol (Hirotsu et al., 2009). In this case, the odorants plus NaN3 and controls (NaN3 alone) are placed on opposite sides of a round plate, but at two spots on each side, separated by a distance of 25 mm (see general scheme of the test plate, Table 1). The worms are placed at the plate center, and their number in each side of the plate is assessed after 60 min to calculate the chemotaxis index (CI). The central part of the plate (width of 33 mm; general scheme of the test plate, Table 1) is not counted. A modified version of the assay plate based on the division of the plate into 6 equivalent parts/sectors (by drawing vertical lines to divide the plate into 2 equivalent parts first, and then, each part into 3 other equivalent parts) was also used in this study to perform single-animal chemotaxis assays. Note that the scoring scheme is similar to that used by Troemel et al. (1997) but adapted to a round plate. The track of each worm was manually recorded, and a chemotaxis index was calculated based on the sum of scores of the sectors in which the worm had travelled during 1 h. This results in nominal scores of −3, −2 and −1 for the sectors of the side containing the odor, and 1, 2 and 3 for the sectors at the other side; a negative final score means attraction and positive final score means avoidance (in this case, the odor was placed in four spots instead of two in the leftmost sector, with the score −3). This method exhibits some improvements when compared to that from Bargmann et al. (1993), since the odorants and controls are apparently better distributed on each side of the plate, and each corresponding area for the counting of the worms is better delimited. Particularly, the radial diffusion of the odorants and controls from the two spots on each side of the plate should allow good odorant distribution over each correspondent area (“N+” and “N-”; general scheme of the test plate, Table 1). The delimited central area, with potential merging of conditions, is not considered for the calculation of the CI.
3.1.5. Margie et al. (2013)
The authors designed a new chemotaxis assay by modifying the one developed by Bargmann et al. (1993), specifically, by dividing the Petri dish into four quadrants to optimize the worm starting location relative to both the control and test areas, and to minimize the interaction of worms with each other while ensuring a significant sample size (up to 250 worms can be used per plate; Table 1). First, the optimization of the worm starting location relative to both the control and test areas is achieved by the introduction of the four quadrants, creating an alternating pattern of test and control areas to prevent any bias that may occur from the initial placement of the worms (they are equidistant from testers and controls, independently from the side of the origin they begin to move). Second, less interaction between worms is important to circumvent the problem of the worms being forced to travel through clusters of other worms during the response to the odorant. As mentioned above, the problem of worms’ density and possible interaction in the response to the odorant had also been addressed before, by Matsuura et al. (2004), where the creation of two distinct spots for the initial placing of the worms was suggested, instead of duplicating the control and test spots. Specifically, in the method from Margie et al. (2013), the substances/compounds being tested are applied at two test and two control spots (T and C, respectively; see general scheme of the test plate, Table 1), and the number of worms, which are originally placed at the plate center (50–250 worms), is counted in each of the four quadrants after 60 min (as in Bargmann et al., 1993). NaN3 is added to both test and control spots. Margie et al. (2013) validated the method by testing the chemoattractant diacetyl and comparing the response of a wild-type strain vs. a transgenic strain lacking the receptor for diacetyl. The authors addressed the possibility of using a video camera attached to the microscope to record the worms’ movement towards one quadrant of the 5-cm assay plate (note that this recording procedure can be adapted for use in any of the other approaches described; the main constrain is the size of the plates used, because the field of view captured by the camera will correspond to smaller test areas for bigger plates). This is important, as this approach might provide information regarding the chemotaxis behavior of the organisms throughout the whole exposure period that could not be obtained by assessing at a definitive moment in time, such as a switch from attraction to avoidance. This protocol can be a good option to assess chemotaxis to environmentally-relevant volatile contaminants in C. elegans, as it was carefully designed and optimized to test volatile chemicals, allowing to classify them as attractants or repellents.
3.2. METHODS USED TO ASSESS CHEMOTAXIS TO WATER-SOLUBLE SUBSTANCES/COMPOUNDS IN C. ELEGANS
The studies discussed in this section (Sambongi et al., 1999; Monteiro et al., 2014; Yuan et al., 2018; Saeki et al., 2001 and Kunitomo et al., 2013; and the protocol from Chai et al., 2017; see Table 1) have used chemotaxis assays in C. elegans to address the effects of water-soluble substances/compounds (specifically metal salts and NaCl) on chemosensation. The methods/experimental designs described here can similarly be applied in ecotoxicological research, to assess, for instance, C. elegans chemotaxis to environmentally-relevant water-soluble contaminants. In fact, the study from Monteiro et al. (2014), discussed below, has been developed within this context.
3.2.1. METALS
3.2.1.1. Sambongi et al. (1999)
Sambongi et al. (1999) assessed avoidance behavior as a function of metal ion concentration (acetate salts of Cd2+, Cu2+ and Ni2+) in C. elegans. The authors found that C. elegans avoided toxic concentrations of Cd2+ and Cu2+, but not that of Ni2+. In this study, a special assay plate formed from a plastic tray (60 × 50 mm) divided into three sectors (two external of 25 mm and one central of 10 mm) was used in one of the assays performed to quantify avoidance behavior. Two thin plastic plates were used to divide the plastic tray into the three sectors, and the agar medium used (1.5% agarose solutions containing 10mM HEPES-NaOH, pH 7.2) was added to the external sectors. This resulted in the following sectors: control, without the metal (sector 1); and test, mixed with the metal (sector 3; general scheme of the test plate, Table 1). In addition, clean agar medium was added to a central area (sector 2; general scheme of the test plate, Table 1) after the medium in the wider sectors had dried, and the thin plates were then removed. Such preparation can be time-consuming, which could be considered a drawback of the approach. The worms (~100) were then placed at the center and counted in each sector after 45 min, including the central area. The number of worms in the central area is not considered for the calculation of the chemotaxis index (see chemotaxis index formula, Table 1), however, its assessment is important to ensure that most tested animals are motile/viable and able to respond to the chemical in order to validate the test. NaN3 was not used. This method is suitable to measure avoidance, providing an additional indication of stimulus gradient navigation for the specific case of water-soluble substances/compounds, such as it is the case of the metal salts. In this method, the tester is incorporated directly in the agar medium, during its preparation, and its diffusion will occur from sector 3 to the adjacent sectors (unidirectional diffusion - first to sector 2, and then to sector 1, to a lesser degree) in a “continuous horizontal way” (not in a radial way, as it occurs in applications of the testers in specific central points/areas on the agar medium surface), and this diffusion will start only after the addition of the agar medium to the central sector (sector 2). The CI is calculated similarly to the other assays described, but the formula used exhibits an inverted order of the variables in the numerator and denominator (chemotaxis index formula used, Table 1), which means that a CI higher or lower than 0 means repulsion and attraction, respectively. Larger quantities of test solutions are required in this experimental procedure compared to the previously mentioned methods assessing chemotaxis to volatile substances, since the toxicant is mixed with the agar during the medium preparation. Sambongi et al. (1999) assessed avoidance as described here with the final goal of studying the neuronal mechanisms involved in this behavioral response. The authors used cilia-defective mutants and laser microbeam surgery to kill specific sensory neurons, and compared the behavioral responses to those of wild-type C. elegans. Although this study was performed within a neurobiological context (authors found that the avoidance response to cadmium and copper was elicited by the amphid chemosensory neurons ADL, ASE, and ASH), the avoidance assay, as designed, can be used in ecotoxicological studies to address the chemotaxis response to metals or other water-soluble contaminants, and to look for avoidance behavior.
3.2.1.2. Monteiro et al. (2014)
Monteiro et al. studied the toxicant effects of metals (Pb, Zn, and Ni) in the reproduction, growth, and behavior of C. elegans. Here, those endpoints were combined to better characterize the potential biological effects of heavy metals in soil and aquatic environments. Behavioral assays were performed in agar plates (artificial substrate), assessing taxis to food in contaminated vs. uncontaminated food spots, to evaluate if worms exhibited any avoidance of metal-contaminated food. The authors compared the fraction of worms in metal-contaminated vs. uncontaminated food spots, and observed that taxis of C. elegans to food decreased when it was contaminated with Zn and Pb, even at the lowest tested concentrations (2 and 1 mg L−1, respectively). However, in the case of nickel, C. elegans only avoided food contaminated with very high concentrations of this metal (2916 mg L−1). In fact, the organism even preferred food contaminated with 1 mg L−1 nickel than uncontaminated food. In contrast to most of the studies assessing chemotaxis in C. elegans, this study falls within an environmental context. The method used, which is briefly summarized below, is slightly different from those previously described, but can be a good option to preliminarily studying metal contamination interference in taxis-to-food behavior in C. elegans. It allows a comparative analysis of different exposure conditions on the same plate, in holes containing clean or contaminated liquid medium. The holes are made in the agar medium with a sterile tube. The metals salts tested are dissolved in K-medium and mixed with the bacterial food suspension (Escherichia coli suspended in K-medium), and then added to the respective holes. In detail, the multiple-choice plate design of this assay comprises a total of 3 different options tested in duplicate [control K-medium (K), clean Escherichia coli suspended in K-medium (EC), and metal-contaminated Escherichia coli suspended in K-medium (M); Table 1, see general scheme of the test plate]. A total of 30 worms are placed at the plate center and counted in each spot after 1, 4, 6 and 24 hr. NaN3 is not used in this method, but the authors mentioned that when nematodes reached a food spot (contaminated or uncontaminated), they almost always stayed there. The total exposure time in this assay is long (24 hr) and has multiple assessment timepoints, which can be a drawback in comparison to other chemotaxis assays, since it is more demanding. Its main applicability is also different from the other approaches described, as it allows assessing the effects of contamination (metal-contamination, in this specific case) in the chemotaxis behavior to a known attractant to C. elegans – E. coli OP50 (its main laboratorial food source). This multiple-choice assay can be a good alternative to evaluate the effects of metals and other water-soluble contaminants on taxis-to-food behavior in C. elegans. However, it does not allow to classify the behavioral response as attraction or avoidance (a chemotaxis index cannot be calculated).
3.2.1.3. Chai et al. (2017)
Chai et al. (2017) identified the need for a robust and reproducible behavioral assay to decipher the neural circuits underlying behavior in C. elegans. They sought to combine the complementary strengths of three types of behavior assays: (i) drop test, consisting on placing a small drop of the test substance at the tail of a moving worm and once it reaches the anterior sensory apparatus, the response is scored as forward or backward movement; (ii) chemotaxis assay, consisting on adding an attractant/repellent on specific areas of the test plate and scoring the number of worms in these areas at the end of a defined exposure period (as performed in several studies described throughout in this article); and (iii) retention assay, which is dependent on the use of a video recording apparatus to record the worms’ behavior to minimize error during analysis and scoring. Briefly, Chai et al. (2017) developed a method that consists in placing a 35-mm assay plate into a specifically designed behavior chamber with infrared LED backlighting (which minimizes the effects of short-wavelength light on the worms’ behavior) for video recording of the worms’ movement and automated video analysis, allowing the immediate generation of a chemotaxis index (CI), designated as ‘preference index’. In this method, a small circle drawn in the middle of the assay plate is divided as in Margie et al. (2013), i.e. the controls (Ctrl) and experimental solutions (Exp) are alternately placed in 4 quadrants (Table 1), but the worms are placed outside the treated regions in two opposite locations (i.e. outside the drawn central circle, specifically between quadrants 1–2 and 3–4, or between quadrants 1–3 and 2–4 – see “extended lines”, general scheme of the test plate, Table 1), and the movement of the worms is recorded with a camera (10 min-videos). NaN3 is not used in this method. The CI is calculated based on the number of pixels in the regions of interest (Table 1, chemotaxis index formula used). This method uses smaller quantities of the experimental substances/compounds (2 μL per plate) than other methods that have been used to assess chemotaxis to water-soluble substances/compounds. Additionally, this method is less susceptible to errors related to manual counting of the worms. However, this automated method requires the construction of a behavior chamber and the use of video-recording equipment. Moreover, it is not very adequate to study stimulus gradient navigation due to the proximity between conditions (small test areas in the middle of small 35-mm plates). Authors showed the utility of the method for conducting behavioral/genetic screenings by testing a known repellent (copper ions) and comparing the response of C. elegans wild-type with that of two transgenic strains (osm-3 mutants with deficient sensory cilia; ocr-2 mutants with deficient ASH-mediated nociceptive response to copper). This method can also be used in ecotoxicological studies as an automatized alternative to screen avoidance to environmentally-relevant contaminants (both water-soluble and volatiles).
3.2.1.4. Yuan et al. (2018)
Yuan et al. investigated the role of the C. elegans Ctr1 homolog, CHCA-1, in avoidance behavior (along with other vital functions, namely copper uptake, and worms’ growth and development). Specifically, they studied whether altered copper homeostasis affects avoidance behavior. They concluded that animals lacking CHCA-1 exhibited significantly reduced avoidance behavior in response to toxic copper conditions compared with wild-type worms. In this study, the authors used a different technique to add the toxicant to the test assay plates (rectangular plates divided in 5 sectors, see Table 1). Specifically, the plates were tilted for adding contaminated agar medium to one end of the plate (approximately one third of the plate), and brought flat after solidification to add the remaining medium, but this time, clean agar medium. This ensured a gradient of the toxicant (CuCl2), i.e. from no copper supplementation on one end, to toxic copper levels on the other. NaN3 was not used in this method. The worms were placed on the central sector and counted in each one of the five areas after 150 min (worms from the central area of the plate - sector 3 - were not considered for the calculation of the chemotaxis index (CI); general scheme of the test plate, Table 1). This method exhibits some similarities with that used by Sambongi et al. (1999), however, this alternative form to add the toxicant to the plate should allow a more effective diffusion/distribution of the toxicant at the end of the plate where the contaminated medium is added, due to a bigger exchange surface. Additionally, the division of the plate into more sectors may be useful for observing, and possibly quantifying, different levels of avoidance (e.g. in the study from Yuan et al., 2018, the fraction of worms in sector 4 was higher than in sector 5, but lower than in all the other sectors). Still, the time of exposure is longer than that used in the assay performed by Sambongi et al. (1999). In general, it seems to be a suitable method to assess chemotaxis to water-soluble substances/compounds in C. elegans, providing additional information on stimulus gradient navigation, and can be used in ecotoxicological studies assessing chemotaxis to metals or other water-soluble contaminants (very suitable to assess/measure avoidance).
3.2.2. NaCl
3.2.2.1. Saeki et al. (2001)
Behavioral plasticity is the capacity of organisms to modify their behavior over time, by adapting to environmental conditions and learning to survive (e.g. Gannon et al., 1995). C. elegans exhibits different forms of behavioral plasticity, like taste and olfactory adaptation, as discussed, for instance, in Gannon et al., 1995. Saeki et al. developed a system to investigate C. elegans behavioral plasticity to NaCl, namely sensory adaptation by associative learning. In order to assess learning, worms were placed on plates for chemotaxis assessment after a conditioning procedure on: (1) NGM plates containing NaCl and bacteria; (2) NGM plates containing NaCl but without bacteria; (3) NGM plates without NaCl and without bacteria. A fourth treatment included the naive worms, which were directly placed on the chemotaxis-plates. It was observed that chemotaxis towards volatile chemoattractants (e.g. isoamylalcohol) did not significantly change after conditioning with NaCl, but chemotaxis towards some of the tested water-soluble attractants (e.g. ammonium chloride) decreased. In this study, an alternative technique was used to add NaCl and the other water-soluble compounds (e.g. ammonium chloride and sodium acetate) to the test plates. Extra plates with the intended concentrations of the test compounds were prepared, and plugs were cut from these plates and left overnight on the chemotaxis-plates at defined spots, to create salt gradients. Then, the plugs were removed just before the assay and NaN3 was added to both test and control spots. In this assay, worms were not originally placed at the plate center, but at a spot equidistant to the attractant and control areas (Table 1, general scheme of the test plate). Although the time of exposure of this method is very short (30 min), its implementation might require a slightly complex and prolonged experimental apparatus compared with other methods. Briefly, extra plates with the intended concentrations need to be prepared to cut plugs, and these plugs have to be left on the assay plates overnight. This assay preparation period is particularly long when compared with that of other methods used to assess chemotaxis towards NaCl, for example those consisting of (1) the addition of drops of concentrated salt solutions directly to specific spots on the top of the agar medium in the chemotaxis plate (e.g. Adachi et al., 2008); or (2) the addition of NaCl directly to the agar medium (e.g. Hukema et al., 2006; Watteyne et al., 2020). For instance, in this later case, purchased 4-compartments round dishes have been used to test buffered agar medium vs. buffered agar medium supplemented with NaCl in each combination of opposite quadrants (e.g. Wicks et al., 2000; Hukema et al., 2006; Hukema et al., 2008; Watteyne et al., 2020. Note, however, that this method is not suitable to assess worms’ navigation in concentration gradients). The number of worms in each quadrant is determined after the exposure period, and a chemotaxis index is calculated, similarly to the method described in Margie et al. (2013). The methodology used by Saeki et al. (1999) to assess behavioral plasticity to NaCl can be very useful in ecotoxicological studies, namely, for the assessment of C. elegans chemotaxis behavior and adaptation to increased environmental levels of NaCl (or other water-soluble contaminants). In fact, salinization of water and soil ecosystems is currently of high concern related to climate change (e.g. Su & Hock, 2016).
3.2.2.2. Kunitomo et al. (2013)
The authors identified a gap in knowledge regarding how sensory systems memorize the intensity of sensory stimulus, compare it with a newly sensed stimulus, and regulate its orientation behavior based on memory. In order to shed light on this matter, a modified assay was designed to explore how environmental NaCl concentration in the culture relates to the subsequent preference pattern towards NaCl levels. Overall, it was concluded that C. elegans ‘memorizes’ the environmental salt concentration in the culture and exhibits a strong behavioral preference for that concentration subsequently. The experimental design used to assess chemotaxis to NaCl after conditioning to the defined concentrations of NaCl is shortly summarized in Table 1. It has many similarities with that used by Saeki et al. (2001), except that in this case, two agar plugs containing 0 mM and 150 mM of NaCl are temporarily added to two opposite spots to create a gradient of attractant over a background concentration included in the agar medium (50 mM NaCl). By including a background concentration of NaCl, chemotaxis at a broader range of salt concentrations can be assessed when compared to the method from Saeki et al. (2001). This method allows creating a concentric salt gradient on the chemotaxis-plate, with the highest concentration at the center of the most concentrated plug, lowest concentration at the opposite plug, and a background concentration around the center of the plate. NaN3 is added to the plug areas, after their removal. The worms within a 20 mm radius from each spot (15 mm in Saeki et al. 2001) are counted after 45 min (30 min in Saeki et al. 2001). The number of animals within a 10 mm radius from the point in which worms are placed is not considered for the calculation of the chemotaxis index (Table 1). This method may also require a slightly complex and prolonged experimental apparatus preparation compared with the methods based on the addition of drops to specific spots in the agar medium or on the addition of NaCl directly to the agar medium (extra plates for plugs cutting, which are left 18 hr at the assay plates, before the start of the assay). Ecotoxicological studies addressing chemotaxis and adaptation to NaCl (or other water-soluble contaminants) in C. elegans, can make use of this methodology.
4. CHOOSING THE MOST APPROPRIATE APPROACH TO ASSESS CHEMOTAXIS IN C. ELEGANS
Caenorhabditis elegans has been used as a model organism for studying chemotaxis behavior in different research contexts, covering different fields such as neurophysiology, neurobiology, and toxicology, and has certainly contributed to clarify chemosensation mechanisms. Therefore, different assays have been applied in C. elegans and adapted over the years, evaluating chemotaxis to a multitude of agents, including volatile and water-soluble substances. As discussed in previous sections, most of these studies aimed to clarify neurobiology research questions. Despite this primary focus, many of these methods could also be applied in ecotoxicological studies (discussed in next section), namely, to assess chemotaxis behavior of C. elegans to environmentally-relevant contaminants, and behavioral ecotoxicology is gathering increased attention within environmental assessment frameworks, as it reflects relevant ecological responses of the biota to putative environmental stressors (Gerhardt, 2007). Some of the discussed approaches can be most adequate to test volatile contaminants (e.g. Bargmann et al. 1993 and Yoshida et al., 2012), while others are best suited to test water-soluble toxicants (e.g. Sambongi et al. 1999 and Yuan et al. 2018). C. elegans’ chemotaxis response has been shown to be substance-dependent and sometimes concentration-dependent (e.g. attracted to low concentrations of benzaldeyde and repelled by high concentrations of it; Bargmann et al. 1993; Yoshida et al. 2012). Thus, attention should be paid not only to the choice of method, but also to the concentrations to test. Besides considering the chemical properties of the test substance (e.g. volatile or water-soluble), the specific goals of each study should also be carefully analyzed, to choose the most appropriate approach to use, since, for instance, some methods can be more suitable to perform screenings to classify the tested substance as an attractant or repellent, and others, to specifically measure and study avoidance behavior. While the methodology used by Bargmann et al. 1993 is suitable to perform a screening of volatile substances/contaminants, allowing their classification as attractants or repellents, the methods used in Yoshida et al. (2012) and Margie et al. (2013) can be a better alternative by providing updated and more rigorous plate design versions. For instance, the plate design in Yoshida et al., 2012 comprises delimited areas for the application of the tester, control, and worms, and includes two points of application in each tester and control areas, which facilitates, respectively, the counting of the worms at the end of the exposure, and the distribution of the tester and control substances over the respective sectors. Additionally, the 4-quadrants design with alternate conditions in Margie et al., 2013 also includes a delimitation of the different areas, and minimizes bias in the worms’ movement through the optimization of the worms’ starting location relative to the tester and controls. Similarly, the automated method used by Chai et al. (2017) can also be applied for rapid screenings for both volatile and water-soluble contaminants, eliminating errors related to manual counting of worms. The method used by Troemel et al. (1997) can be applied for specifically measuring avoidance behavior of C. elegans to volatile substances/contaminants, while those reported in Sambongi et al. (1999) and Yuan et al. (2018) are adequate for testing this response to water-soluble substances. The approach followed by Matsuura et al. (2004, 2007) can be used as a basis for assessing chemotaxis behavior to simultaneous attractants (to assess preference or mixture interferences by comparison with independent exposures). Additionally, the methodology used by Monteiro et al. (2014) is appropriate for screening the interference effects of contaminants (e.g. metals) on the chemotaxis behavior to food in C. elegans. Finally, the methods reported in Saeki et al. (2001) and Kunitomo et al. (2013) are appropriate to test chemotaxis and adaptation to NaCl.
5. CHEMOTAXIS BEHAVIOR ASSAYS IN ECOTOXICOLOGY
The methods discussed throughout this article can be readily applied to assess chemotaxis to any type of volatile or water-soluble substance in C. elegans. Thus, these behavioral assays are a good complementary option to assess the toxicity of environmentally-relevant toxicants (e.g. metals, nanomaterials, and pesticides) in ecotoxicological studies (e.g. Hopewell et al., 2017; Sobkowiak et al., 2018; Wang et al., 2019; Qu et al. 2020). It is relevant to understand whether C. elegans is able to avoid toxicant environmental contaminants, or whether an alteration of its sensorial perception is triggered by the correspondent exposure. For instance, a few studies have described the ability of C. elegans to avoid certain metals, i.e.: media contaminated with toxic levels of cadmium (Sambongi & Nagae, 1999), copper (Sambongi & Nagae, 1999; Chai et al., 2017; Yuan et al., 2018), lead, or zinc (Monteiro et al. 2014; both singly tested). A more recent study, from Wakabayashi et al. (2020), showed that C. elegans also avoids rare elements, like yttrium and lanthanides. The effects of nanoparticles on C. elegans’ chemotaxis behavior has also been recently investigated, namely of graphene oxide (Wang et al., 2019; avoidance behavior observed after 90-min of exposure to concentrations equal or higher than 50 mg/L of graphene oxide) and nanopolystyrene (Qu et al., 2020; sensorial perception was altered). These findings reinforce the applicability of C. elegans’ chemotaxis assays in the assessment of chemosensation effects of emerging environmental contaminants. Additionally, to the best of our knowledge, only two studies assessing the effects of pesticides in C. elegans’ chemosensation are available in the literature (Hopewell et al., 2017; Sobkowiak et al., 2018). Specifically, Hopewell et al. (2017) assessed the effects of groundwater containning residual levels of the neonicotinoid thiacloprid (an insecticide) on the chemosensory ability of C. elegans, concluding that its ability to be attracted to the known chemoattractant NaCl was lost following exposure to environmentally relevant levels of this insecticide. Sobkowiak et al. (2018) tested commercial synthethic nematocides (oxamyl and fosthiazate) and natural nematocides consisting of plant secondary metabolites (trans-anethole, (E,E)-2,4-decadienal, (E)-2- decenal and 2-undecanone) in C. elegans and another nematode species (Meloidogyne incognita), with the goal of comparing the efficacy of these substances against nematodes by measuring their effects on the nematodes’ chemotaxis behavior. Altough some distinct responses were obtained for the two nematodes (i.e. the nematodes were not attracted/repulsed to exactly the same substances), this study shows an alternative application of these assays, namely in the investigation of environment-friendly nematocides.
Hazardous environmental contaminants can be identified by chemotaxis behavioral assays in C. elegans following two strategies (Figure 1), namely: (i) through avoidance records, i.e. the exposure to the contaminant(s) induces an avoidance response in this model organism and, consequently, avoidance behavior might be used as an indicator of a toxic condition (e.g. Chai et al. 2017; Wakabayashi et al., 2020); (ii) and/or through sensory perception alteration, i.e. pre-exposure to the tested contaminant(s) changes the chemotaxis behavior of C. elegans to known attractants/repellents (e.g. NaCl and diacetyl), and consequently, the sensory perception alteration might be used as an indicator of toxicity (e.g. Qu et al. 2020; Hopewell. et al. 2017). Both approaches can be widely explored in ecotoxicological studies to signalize potential hazardous environmental contaminants in C. elegans.
Figure 1 –

Diagram illustrating the described strategies to identify potential hazardous contaminants in chemotaxis assays using C. elegans as model study system.
6. CLOSING REMARKS
This overview article provides a general contextualization of methodology typically used to assess chemotaxis behavior in C. elegans, discussing some of their advantages and drawbacks, with the goal of assisting the reader in the selection of the most adequate method for their test setting, particularly for studies focusing on ecotoxicological assessment. We described assays suitable for testing different types of chemical substances and/or work hypothesis, and highlighted that the chemotaxis behavior of C. elegans could be a promising assessment tool in ecotoxicology. In fact, chemotaxis behavior is already extensively considered in environmental assessment fields with other model organisms for which standardized protocols are available, such as the earthworm Eisenia fetida (ISO 17512–1, 2008) and the springtail Folsomia candida (ISO 17512–2, 2011). However, the logistics involved in the assays with these model organisms are markedly more complex (e.g. larger infrastructure and human effort for organism’s maintenance and testing), and the counterpart assays are longer (typically 48 hr). Moreover, the readily available transgenic strains allowing detailed insights on the neuronal basis of behavioral patterns is a relevant advantage of C. elegans over these traditional models, which can help obtain a more comprehensive understanding of the mechanistic aspects underlying toxicant-driven behaviors.
To conclude, nematodes are ubiquitous in contaminated environments, where toxicants can act as chemical cues or interfere with their chemosensation response, ultimately affecting the survival of these ecologically important organisms. Nematodes occupy distinct positions in the food web, from bacterial feeders to predators (Yeates et al., 1993), and play important roles in the ecosystems, including in organic matter decomposition and nutrient regeneration (Schratzberger & Ingels, 2018; Ferris, 2010). Thus, alterations in nematodes’ chemotaxis response may compromise their survival, consequently disturbing trophic chains and ecosystems (Ferris et al., 2010), which can ultimately also affect human well-being (Haines-Young & Potschin, 2012). In this way, it is very relevant to study the effects of environmental contaminants in nematodes chemotaxis behavior, as it can provide an early warning system for public health intervention. The methodologies that have been largely applied in C. elegans under other research contexts can be very useful for such an endeavor. We hope this Overview will encourage a wider use of C. elegans chemotaxis assays to evaluate the effects of environmentally-relevant contaminants on chemotaxis behavior, and for the correspondent potential/sensitivity of this endpoint to be included in standardized guidelines to appraise environmental quality.
ACKNOWLEDGEMENTS
Thanks are due to FCT/MCTES for the financial support to (UIDP/50017/2020+UIDB/50017/2020), through national funds. Libânia Queirós was supported by individual research grant by FCT (SFRH/BD/129871/2017). Joana L. Pereira and Patrícia Pereira are funded by national funds (OE), through FCT - Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of the article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19, or under the Scientific Employment Stimulus - Individual Call - [CEECIND/01144/2017], respectively. Michael Aschner was supported in part by grants from the National Institute of Environmental Health Sciences (NIEHS), R01 ES07331 and R01 ES10563.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
LITERATURE CITED
- Adachi R, Osada H, & Shingai R (2008). Phase-dependent preference of thermosensation and chemosensation during simultaneous presentation assay in Caenorhabditis elegans. BMC Neuroscience, 9, 1–15. 10.1186/1471-2202-9-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H, Khan E, & Ilahi I (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 1–15. 10.1155/2019/6730305 [DOI] [Google Scholar]
- Bargmann CI (1998). Neurobiology of the Caenorhabditis elegans genome. Science, 282(5396), 2028–2033. 10.1126/science.282.5396.2028 [DOI] [PubMed] [Google Scholar]
- Bargmann CI (2006). Chemosensation in C. elegans. In WormBook: The Online Review of C. elegans Biology [Internet]. Pasadena (CA): WormBook; 2005–2018. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK19746/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, & Horvitz HR (1991). Chemosensory Neurons with Overlapping Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron, 7, 729–742. [DOI] [PubMed] [Google Scholar]
- Bargmann Cornelia I, Hartwieg E, & Horvitz HR (1993). Odorant-Selective Genes and Neurons Mediate Olfaction in C. elegans. Cell, 74, 515–527. [DOI] [PubMed] [Google Scholar]
- Bargmann Cornelia I, & Horvitz HR (1991). Chemosensory Neurons with Overlapping Direct Chemotaxis to Multiple Chemicals in C. elegans. Neuron, 7, 729–742. [DOI] [PubMed] [Google Scholar]
- Chai CM, Cronin CJ, & Sternberg PW (2017). Automated Analysis of a Nematode Population-based Chemosensory Preference Assay. Journal of Visualized Experiments, 125(e55963), 1–9. 10.3791/55963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wang C, Li H, Ma R, Yu Z, Li L, Xiang M, Chen X, Hua X, & Yu Y (2019). A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. Journal of Environmental Management, 237(September 2018), 519–525. [DOI] [PubMed] [Google Scholar]
- Dusenbery DB (1983). Chemotactic Behavior of Nematodes. Journal of Nematology, 15(2), 168–173. [PMC free article] [PubMed] [Google Scholar]
- Félix MA, & Braendle C (2010). The natural history of Caenorhabditis elegans. Current Biology, 20(22), R965–R969. 10.1016/j.cub.2010.09.050 [DOI] [PubMed] [Google Scholar]
- Ferris H (2010). Contribution of nematodes to the structure and function of the soil food web. Journal of Nematology, 42(1), 63–67. http://www.ncbi.nlm.nih.gov/pubmed/22736838%0A [PMC free article] [PubMed] [Google Scholar]
- Gannon TN, & Rankin CH (1995). Methods of Studying Behavioral Plasticity in Caenorhabditis elegans. Methods in Cell Biology, 48(C), 205–223. 10.1016/S0091-679X(08)61389-8 [DOI] [PubMed] [Google Scholar]
- García-Santos G, & Keller-Forrer K (2011). Avoidance behaviour of Eisenia fetida to carbofuran, chlorpyrifos, mancozeb and metamidophos in natural soils from the highlands of Colombia. Chemosphere, 84(5), 651–656. 10.1016/j.chemosphere.2011.03.036 [DOI] [PubMed] [Google Scholar]
- Gerhardt A (2007). Aquatic behavioral ecotoxicology - Prospects and limitations. Human and Ecological Risk Assessment, 13(3), 481–491. 10.1080/10807030701340839 [DOI] [Google Scholar]
- Hagerbaumer A, Sebastian H, Heininger P, & Traunspurger W (2015). Experimental Studies with Nematodes in Ecotoxicology: An Overview. Journal of Nematology, 47(1), 11–27. [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Bergamasco C, Arbucci S, Plasterk RHA, & Bazzicalupo P (2004). Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO Journal, 23(5), 1101–1111. 10.1038/sj.emboj.7600107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell H, Floyd KG, Burnell D, Hancock JT, Allainguillaume J, Ladomery MR, & Wilson ID (2017). Residual ground-water levels of the neonicotinoid thiacloprid perturb chemosensing of Caenorhabditis elegans. Ecotoxicology, 26(7), 981–990. 10.1007/s10646-017-1826-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukema RK, Rademakers S, Dekkers MPJ, Burghoorn J, & Jansen G (2006). Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO Journal, 25(2), 312–322. 10.1038/sj.emboj.7600940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukema RK, Rademakers S, & Jansen G (2008). Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learning and Memory, 15(11), 829–836. 10.1101/lm.994408 [DOI] [PubMed] [Google Scholar]
- ISO 17512–1. (2008). Soil quality - Avoidance test for determining the quality of soils and effects of chemicals on behaviour - Part 1: Test with earthworms (Eisenia fetida and Eisenia andrei).
- ISO 17512–2. (2011). Soil quality — Avoidance test for determining the quality of soils and effects of chemicals on behaviour — Part 2: Test with collembolans (Folsomia candida).
- Kunitomo H, Sato H, Iwata R, Satoh Y, Ohno H, Yamada K, & Iino Y (2013). Concentration memory-dependent synaptic plasticity of a taste circuit regulates salt concentration chemotaxis in Caenorhabditis elegans. Nature Communications, 4:2210, 1–11. 10.1038/ncomms3210 [DOI] [PubMed] [Google Scholar]
- Leung MCK, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, & Meyer JN (2008). Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicological Sciences, 106(1), 5–28. 10.1093/toxsci/kfn121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Xu Z, Tan Z, Zhang Z, & Ma L (2015). Neuropeptide Receptors NPR-1 and NPR-2 Regulate Caenorhabditis elegans Avoidance Response to the Plant Stress Hormone Methyl Salicylate. Genetics, 199(February), 523–531. 10.1534/genetics.114.172239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margie O, Palmer C, & Chin-sang I (2013). C. elegans Chemotaxis Assay. Journal of Visualized Experiments, 74(e50069), 1–6. 10.3791/50069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura T, Endo S, Iwamoto R, Takahashi H, & Ichinose M (2007). Developmental changes in chemotactic response and choice of two attractants, sodium acetate and diacetyl, in the nematode Caenorhabditis elegans. Comparative Biochemistry and Physiology, Part A, 147, 920–927. 10.1016/j.cbpa.2007.02.023 [DOI] [PubMed] [Google Scholar]
- Matsuura T, Oikawa T, Wakabayashi T, & Shingai R (2004). Effect of simultaneous presentation of multiple attractants on chemotactic response of the nematode Caenorhabditis elegans. Neuroscience, 48, 419–429. 10.1016/j.neures.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Monteiro L, Brinke M, Traunspurger W, & Moens T (2014). Effects of heavy metals on free-living nematodes: A multifaceted approach using growth, reproduction and behavioural assays. European Journal of Soil Biology, 62, 1–7. 10.1016/j.ejsobi.2014.02.005 [DOI] [Google Scholar]
- Natal da Luz T, Ribeiro R, & Sousa JP (2004). Avoidance tests with collembola and earthworms as early screening tools for site-specific assessment of polluted soils. Environmental Toxicology and Chemistry, 23(9), 2188–2193. [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Harbinder S, & Van Der Kooy D (2020). Regulation of Distinct Attractive and Aversive Mechanisms Mediating Benzaldehyde Chemotaxis in Caenorhabditis elegans. Learning & Memory, 8, 170–181. 10.1101/lm.36501.coupled [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, & Wang D (2020). Toxicity comparison between pristine and sulfonate modified nanopolystyrene particles in affecting locomotion behavior, sensory perception, and neuronal development in Caenorhabditis elegans. Science of the Total Environment, 703, 134817. 10.1016/j.scitotenv.2019.134817 [DOI] [PubMed] [Google Scholar]
- Queirós L, Pereira JL, Gonçalves FJM, Pacheco M, & Aschner M (2019). Critical Reviews in Toxicology Caenorhabditis elegans as a tool for environmental risk assessment: emerging and promising applications for a “ nobelized worm ” and promising applications for a “nobelized worm.” Critical Reviews in Toxicology, 0(0), 1–19. 10.1080/10408444.2019.1626801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, & Iino Y (2001). Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. The Journal of Experimental Biology 204, 204, 1757–1764. [DOI] [PubMed] [Google Scholar]
- Sambongi Y, & Nagae T (1999). Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Chemical Neuroscience, 10(4), 753–757. [DOI] [PubMed] [Google Scholar]
- Santos MJG, Ferreira MFL, Cachada A, Duarte AC, & Sousa JP (2012). Pesticide application to agricultural fields: Effects on the reproduction and avoidance behaviour of Folsomia candida and Eisenia andrei. Ecotoxicology, 21(8), 2113–2122. 10.1007/s10646-012-0963-7 [DOI] [PubMed] [Google Scholar]
- Schratzberger M, & Ingels J (2018). Meiofauna matters: The roles of meiofauna in benthic ecosystems. Journal of Experimental Marine Biology and Ecology, 502, 12–25. 10.1016/j.jembe.2017.01.007 [DOI] [Google Scholar]
- Shtonda BB, & Avery L (2006). Dietary choice behavior in Caenorhabditis elegans. Journal of Experimental Biology, 209(1), 89–102. 10.1242/jeb.01955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowiak R, Bojarska N, Krzyżaniak E, Wągiel K, & Ntalli N (2018). Chemoreception of botanical nematicides by Meloidogyne incognita and Caenorhabditis elegans. Journal of Environmental Science and Health - Part B Pesticides, Food Contaminants, and Agricultural Wastes, 53(8), 493–502. 10.1080/03601234.2018.1462936 [DOI] [PubMed] [Google Scholar]
- Song S, Guo Y, Zhang X, Zhang X, Zhang J, & Ma E (2014). Changes to cuticle surface ultrastructure and some biological functions in the nematode Caenorhabditis elegans exposed to excessive copper. Archives of Environmental Contamination and Toxicology, 66(3), 390–399. 10.1007/s00244-013-9991-4 [DOI] [PubMed] [Google Scholar]
- Su YT, & Hock LK (2016). Climate change and soil salinization: impact on agriculture, water and food security. International Journal of Agriculture, Forestry and Plantation, 2(February), 1–9. [Google Scholar]
- Syed Z, Alexander D, Ali J, Unrine J, & Shoults-Wilson WA (2017). Chemosensory cues alter earthworm (Eisenia fetida) avoidance of lead-contaminated soil. Environmental Toxicology and Chemistry, 36(4), 999–1004. 10.1002/.3619 [DOI] [PubMed] [Google Scholar]
- Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, & Iino Y (2006). The Insulin/PI 3-Kinase Pathway Regulates Salt Chemotaxis Learning in Caenorhabditis elegans. Neuron, 51, 613–625. 10.1016/j.neuron.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Troemel ER, Kimmel BE, & Bargmann CI (1997). Reprogramming Chemotaxis Responses: Sensory Neurons Define Olfactory Preferences in C. elegans. Cell, 91, 161–169. [DOI] [PubMed] [Google Scholar]
- Tsui D, & Van Der Kooy D.. (2008). Serotonin mediates a learned increase in attraction to high concentrations of benzaldehyde in aged C. elegans. Learning & Memory, 15, 844–855. 10.1101/lm.1188208.more [DOI] [PubMed] [Google Scholar]
- Turner MJ, Cox JK, Spellman AC, Stahl C, & Bavari S (2020). Avoidance behavior independent of innate-immune signaling seen in Caenorhabditis elegans challenged with Bacillus anthracis. Developmental and Comparative Immunology, 102(September 2018), 103453. 10.1016/j.dci.2019.103453 [DOI] [PubMed] [Google Scholar]
- van Gestel CAM (2012). Soil ecotoxicology: State of the art and future directions. ZooKeys, 176(SPECIAL ISSUE), 275–296. 10.3897/zookeys.176.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vryzas Z (2018). Pesticide fate in soil-sediment-water environment in relation to contamination preventing actions. Current Opinion in Environmental Science and Health, 4, 5–9. 10.1016/j.coesh.2018.03.001 [DOI] [Google Scholar]
- Wakabayashi T, Nojiri Y, & Takahashi-Watanabe M (2020). Multiple Chemosensory Neurons Mediate Avoidance Behavior to Rare Earth Ions in Caenorhabditis elegans. Biological Trace Element Research. 10.1007/s12011-020-02375-6 [DOI] [PubMed] [Google Scholar]
- Wang W, Xu Z, Wu Y, Qin L, Li Z, & Wu Z (2015). Off-response in ASH neurons evoked by CuSO4 requires the TRP channel OSM-9 in Caenorhabditis elegans. Biochemical and Biophysical Research Communications, 461, 463–468. 10.1038/srep29284 [DOI] [PubMed] [Google Scholar]
- Wang D, & Wang D (2019). Avoidance Behavior of Nematodes to Environmental Toxicants or Stresses. In Target Organ Toxicology in Caenorhabditis elegans. 10.1007/978-981-13-6010-7_2 [DOI] [Google Scholar]
- Ward S (1973). Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proceedings of the National Academy of Sciences of the United States of America, 70(3), 817–821. 10.1073/pnas.70.3.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watteyne J, Peymen K, Van Der Auwera P, Borghgraef C, Vandewyer E, Damme S Van, Rutten I, Lammertyn J, Jelier R, Schoofs L, & Beets I (2020). Neuromedin U signaling regulates retrieval of circuit. Nature Communications, 11(2076), 1–16. 10.1038/s41467-020-15964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, De Vries CJ, Van Luenen HGAM, & Plasterk RHA (2000). CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Developmental Biology, 221(2), 295–307. 10.1006/dbio.2000.9686 [DOI] [PubMed] [Google Scholar]
- Worthy SE, Haynes L, Chambers M, Bethune D, Kan E, Chung K, Ota R, Taylor CJ, & Glater EE (2018). Identification of attractive odorants released by preferred bacterial food found in the natural habitats of C. elegans. PLoS ONE, 13(7), 1–14. 10.1371/journal.pone.0201158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Hirotsu T, Tagawa T, Oda S, Wakabayashi T, Iino Y, & Ishihara T (2012). Odour concentration-dependent olfactory preference change in C. elegans. Nature Communications, 3, 711–739. 10.1038/ncomms1750 [DOI] [PubMed] [Google Scholar]
- Yeates GW, Bongers T, De Goede RGM, Freckman DW, & Georgieva SS (1993). Feeding Habits in Soil Nematode Families and Genera-An Outline for Soil Ecologists. Journal of Nematology, 25(3), 315–331. [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Sharma AK, Richart A, Lee J, & Kim B-E (2018). CHCA-1 is a copper-regulated Ctr1 homolog required for normal development, copper accumulation, and copper-sensing behavior in Caenorhabditis elegans. JBC Papers, 1–27. 10.1074/jbc.RA118.003503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yan J, Chen Y, Chen C, Zhang K, & Huang X (2014). The olfactory signal transduction for attractive odorants in Caenorhabditis elegans. Biotechnology Advances, 32(2), 290–295. 10.1016/j.biotechadv.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Zou W, Cheng H, Li S, Yue X, Xue Y, Chen S, & Kang L (2017). Polymodal responses in C. elegans phasmid neurons rely on multiple intracellular and intercellular signaling pathways. Scientific Reports, 7(February), 1–8. 10.1038/srep42295 [DOI] [PMC free article] [PubMed] [Google Scholar]


