Abstract
In this study, we compared the incidence of pneumomediastinum in coronavirus disease (COVID-19) patients during the ascending phases of the 1st and 2nd epidemic waves. Crude incidence was higher during the 2nd wave at a quasi-significant level (0.68/1000 vs. 2.05/1000 patient-days, p = 0.05). When restricting the analysis to patients who developed pneumomediastinum during noninvasive ventilation, the difference became clearly significant (0.17/1000 vs 1.36/1000 patient-days, p = 0.039). At logistic regression, predisposing factors (p = 0.031), and COVID-19 radiological severity (p = 0.019) were independently associated with pneumomediastinum. Mortality in patients with pneumomediastinum was 87.5%. However, pneumomediastinum seemed to be related to a generally worse disease presentation in hospitalized patients during the 2nd wave, rather than to a separate pattern of disease.
Keywords: COVID-19, Pneumomediastinum, Barotrauma, Pneumothorax
1. Introduction
Pneumomediastinum and pneumothorax have been consistently reported in patients with COVID-19 [1,2]. Barotrauma due to mechanical or noninvasive ventilation is one of the main causative mechanisms, even though it can also occur in patients without any breathing support. Furthermore, COVID-19 itself can cause emphysema-like changes [[3], [4], [5]]. Such changes seem to be disease-specific and may explain the higher incidence of pneumomediastinum and pneumothorax in COVID-19 compared to other pneumonias at equal severity [3,4].
In March 2020, our hospital become a reference COVID-19 center for a catchment area of 1.2 million inhabitants. During the early recrudescence of the COVID-19 epidemic in Italy (October to November 2020), we audited a higher number of pneumomediastinum episodes compared to that of the 1st wave. We therefore analyzed whether this observation reflected a random drift or a statistically significant change, mirroring a possible pathomorphosis of the disease.
2. Patients and methods
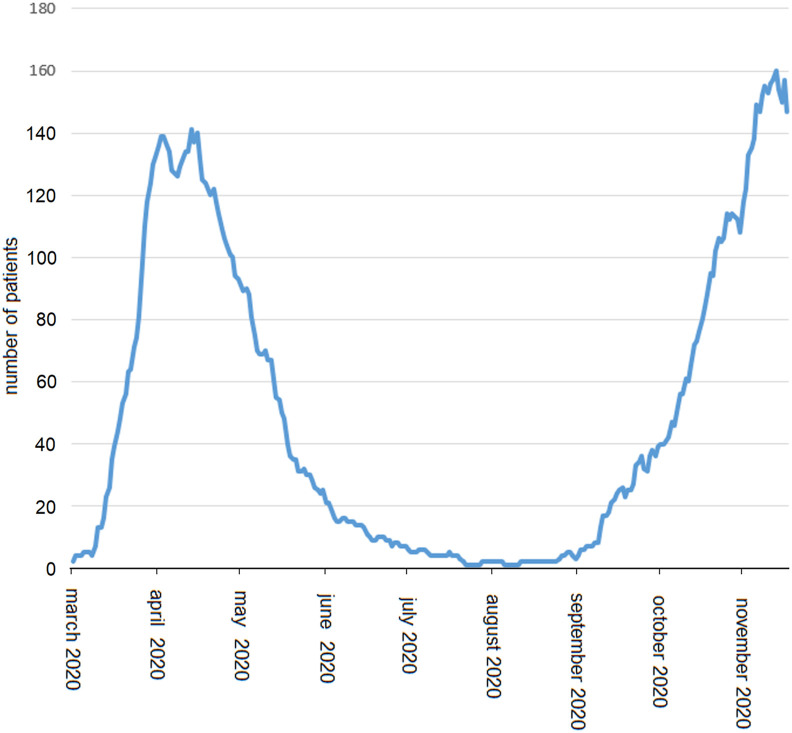
We restricted the analysis to the ascending phases of the 1st and 2nd epidemic waves in order to compare time periods with a similar incremental trend in daily hospitalizations (Fig. 1 ) and a similar workload to health-care personnel. Indeed, the latter factor has been shown to affect pneumomediastinum frequency [6], thus possibly acting as a confounder. Crude incidence rates were reported as events/1000 patient-days, and the mid-p adjusted exact test was applied for comparison. We also analyzed predictors of pneumomediastinum, as well as independent prognosticators of in-hospital mortality. Variables of interest included age, sex, mechanical ventilation, Charlson Comorbidity Index, and a simplified five-point radiological severity score derived from a semi-quantitative method [7]. Furthermore, predisposing factors to pneumomediastinum were considered, including emphysema, blebs/bullae, interstitial lung disease, bronchiectasis, and history of asthma. Logistic regressions were conducted on parsimonious models based on Akaike's Information Criterion scores. Finally, Kaplan-Meier analysis was applied to analyzed survival differences between waves. The SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and the OpenEpi app (www.openepi.com) were used for statistics. A p-value < 0.05 was considered significant. Odds ratios (ORs) and confidence intervals (CIs) were reported where appropriate. The internal review board waived the need for a formal approval process to analyze and report these data, given their non-experimental nature and possible relevance in a public health emergency of international concern.
Fig. 1.
Cumulative daily hospitalizations during 1st and 2nd COVID-19 epidemic waves at the Tor Vergata Polyclinic of Rome, Italy.
3. Results
The trimmed dataset included 687 patients. No differences between the 1st and 2nd epidemic waves were found in terms of mean ages (66.1 ± 16.9 vs. 65.8 ± 15.3 years, respectively; p = 0.821), comorbidity indices (3.4 ± 2.3 vs. 3.7 ± 2.2, respectively; p = 0.130), and the need for mechanical ventilation (35/252 vs. 54/346, respectively; p = 0.615). The male-to-female ratio (172/115 vs. 284/116, respectively; p = 0.002) and mean severity score (3.0 ± 1.4 vs, 3.4 ± 1.3, respectively; p = 0.001) were higher during the 2nd wave. Pneumomediastinum was reported in 16 (2.3%) patients. Clinical details are reported in Table 1 . In 7 patients, pneumomediastinum manifested during invasive ventilation, whereas in the remaining patients, it occurred under noninvasive ventilation (n = 7) or unassisted breathing (n = 2). In-hospital mortality in the subgroup of pneumomediastinum patients was 14/16 (87.5%), a value consistent with previous reports [1,4]. All deaths occurred because of rapid progression toward severe acute respiratory distress syndrome and multiorgan failure. Overall pneumomediastinum incidence was 1.37/1000 patient-days. The incidence was higher during the 2nd wave at a quasi-significant level (p = 0.05): 0.68/1000 (95% CI: 0.18–1.75) vs. 2.05/1000 patient-days (95% CI: 1.06–3.58). When restricting the analysis to patients without invasive ventilation, the difference became significant (p = 0.039): 0.17/1000 (95% CI: 0–0.95) vs. 1.36/1000 patient-days (95% CI: 0.47–2.46). Logistic regression revealed that predisposing factors (OR: 3.94, 95% CI: 1.13–13.6, p = 0.031) and radiological severity (OR: 6.63, 95% CI: 1.36–32.3, p = 0.019) were independently associated with the risk of pneumomediastinum. Unexpectedly, a higher comorbidity index was related to a reduced pneumomediastinum risk (OR: 0.28, 95% CI: 0.092–0.86, p = 0.027). When restricting the analysis to patients who developed pneumomediastinum off-ventilator, the presence of predisposing factors became the unique independent predictor (OR: 8.74, 95% CI: 2.15–35.44, p = 0.002).
Table 1.
Clinical details of patients with COVID-19 pneumomediastinum, divided by epidemic wave.
| Pt No. | Age, Sex | COVID-19 severitya | PM Extentb | LDH (U/L) | Ferritin (ng/mL) | D-dimer (ng/mL) | Breathings support | RRc | PEEP (CmH2O) | P/F Ratio (mm/Hg) | Vt (mL) | FiO2 | Predisposing factor | Predisposing factor location | Medications | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wave 1 | ||||||||||||||||
| 1 | 66, M | 5 | S | 603 | 1289 | 58000 | MV | NA | 10 | 220 | NA | 0.8 | Blebs/bullae | RUL, LUL | None | |
| 2 | 58, M | 5 | S,P | 283 | 969 | 1297 | MV | 22 | 12 | 99 | 550 | 1.0 | Diffuse emphysema | All lobes | Dex | |
| 3 | 56, M | 5 | S,P | 462 | 2423 | 745 | MV | 28 | 8 | 133 | 600 | 0.5 | None | – | None | |
| 4 | 79, M | 4 | M | 452 | 340 | 983 | HFNC | 21 | NA | 120 | NA | N | Bullous emphysema | RUL (5 cm) | Dex | |
| Wave 2 | ||||||||||||||||
| 5 | 19, F | 3 | S | 226 | NA | 129 | Unassisted | 19 | 260 | 280 | NA | 0.4 | Asthma | – | None | |
| 6 | 60, M | 5 | L | 723 | NA | 3492 | CPAP | 18 | 6 | 113 | NA | 1.0 | Blebs/bullae | RUL, LUL | Rem, Dex | |
| 7 | 79, M | 5 | S | 377 | 3992 | 5002 | MV | NA | 12 | 78 | NA | 0.9 | None | – | Dex | |
| 8 | 68, M | 4 | L | 405 | 666 | 1597 | CPAP | 20 | 8 | 111 | NA | 1.0 | Traction bronchiectasis | ML,RLL, LLL | Rem, Dex | |
| 9 | 38, M | 5 | S | 615 | NA | 2518 | CPAP | NA | 12.5 | 153 | NA | 0.6 | None | – | NA | |
| 10 | 54, M | 5 | S | 358 | 1250 | 1000 | CPAP | 24 | 10 | 132 | NA | 1.0 | None | – | NA | |
| 11 | 78, M | 5 | S | 335 | 1976 | 5740 | CPAP | 18 | 8 | 114 | 560 | 1.0 | Paraseptal emphysema | RUL,LUL, RLL | Dex | |
| 12 | 65, M | 5 | S,P | 416 | 1289 | 997 | MV | NA | 8 | 110 | 570 | 1.0 | None | – | NA | |
| 13 | 59, M | 4 | S | 413 | 3027 | 1373 | Unassisted | 22 | 8 | 160 | NA | 0.5 | None | – | None | |
| 14 | 90, M | 5 | S | 376 | 840 | 1156 | CPAP | NA | 7.5 | 148 | NA | 0.1 | Bullae/blebs | RUL, LUL, LLL, RLL | Dex | |
| 15 | 30, F | 5 | S,P | 356 | 103 | 13000 | CPAPd | 20 | 8 | 225 | 400 | 0.6 | None | – | Rem | |
| 16 | 70, M | 5 | S | 380 | 698 | 502 | MV | NA | 10 | 96 | 550 | 1.0 | ILD, traction bronchiectasis | RLL | Dex | |
Legend: CPAP: continuous positive airway pressure (helmet); HFNC: high-flow nasal cannulae; LDH: lactate dehydrogenase; LUL: left upper lobe; LLL: left lower lobe; ML: middle lobe; MV: mechanical ventilation; NA: not available: NTL: neutrophil-to-lymphocyte ratio; PEEP: positive end-expiratory pressure; PM: pneumomediastinum; RLL: right lower lobe; RR: respiratory rate.
All the other reported variables refer to the time when pneumomediastinum occurred. Medications legend: Dex: dexamethasone; Rem: remdesivir (other supportive medications with less specific relation to COVID-19 evolution and pneumomediastinum are not reported).
based on computed tomography scan [7], expressed in quintiles.
legend for extent of pneumomediastinum: L: light (detected on chest X-ray or at chest palpation), S: severe (obvious swelling extended to chest and neck); M: massive (extending to face and eyelid; required subcutaneous drainage); P: associated pneumothorax.
In some patient, respiratory rate refers to the last available record under spontaneous breathing, before orotracheal intubation and mechanical ventilation.
in this patient, intubation and mechanical ventilation was initiated very soon after occurrence of pneumomediastinum.
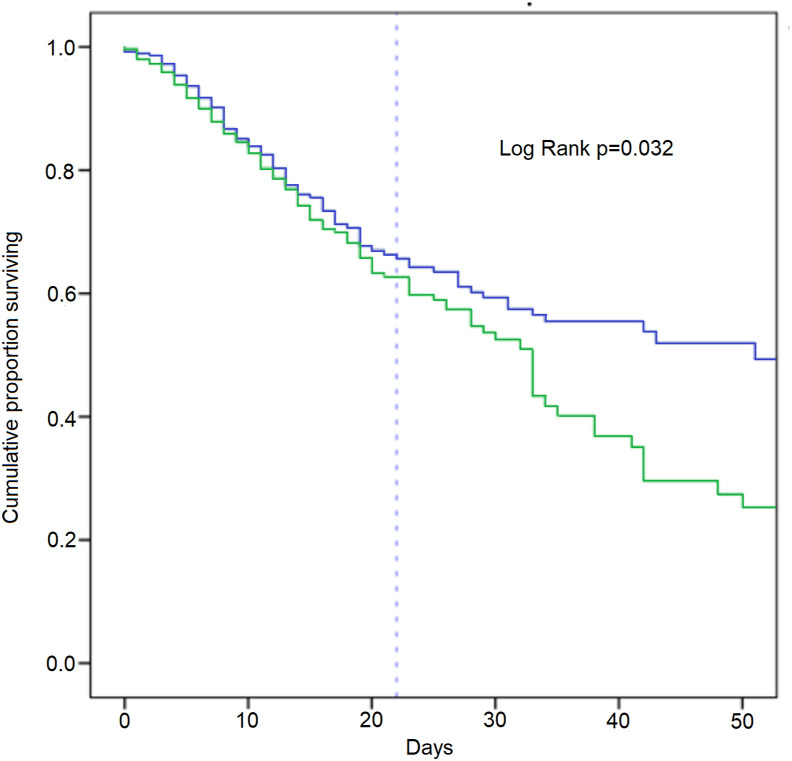
Pneumomediastinum was independently associated with a higher risk of in-hospital mortality (OR: 11.70, 95% CI: 2.74–50.1, p = 0.001). Other negative predictors were advanced age (p = 0.001), a higher COVID-19 radiological severity score, and a higher comorbidity index (both p < 0.001). Although the epidemic wave was not independently associated with in-hospital mortality, time-event analysis showed a globally worse cumulative survival rate in patients with a longer hospital stay (Fig. 2 ).
Fig. 2.
Kaplan-Meier analysis of cumulative survival proportion during 1st (blue line) and 2nd epidemic wave (green line). The dashed vertical line indicates the mean hospital stay (22 days). Day intervals: 1–10, 11–20, 21–30, 31–40. Number of patients entering each interval (1st wave): 286, 204, 106, 65; number of patients entering each interval (2nd wave): 400, 230, 106, 47; number censored per interval (1st wave): 42, 63, 30, 56); number censored per interval (2nd wave): 115, 83, 43, 27; deaths per interval (1st wave): 40, 35, 11, 1; deaths per interval (2nd wave): 55, 41, 16, 11. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this study, we found a trend toward a higher incidence of pneumomediastinum among hospitalized COVID-19 patients during the 2nd epidemic wave. The nature of this observation is, however, difficult to interpret. Higher pneumomediastinum incidence might just reflect a generally worse presentation of the disease during the 2nd wave rather than a separate pattern of disease. Furthermore, admission bias might have had a role in the trend. Indeed, it is possible that, during 2nd wave, an increasing proportion of patients with less severe clinical presentations were diverted toward non-specialized hospitals, as a result of local policies aimed at optimizing COVID-19 patients flows. Interestingly, however, during the 2nd wave, we received two requests for online consultation from other zonal hospitals owing to pneumomediastinum, compared to none in the 1st wave. These cases were excluded from the present analysis.
During the 2nd wave, a larger proportion of patients developed pneumomediastinum while on noninvasive ventilation or even unassisted breathing. This observation suggests that the culprit lesion of COVID-19 pneumomediastinum is more often located at the distal airways, although post-intubation tracheal injury can occasionally occur in a considerable proportion of patients [8]. Therefore, we have now adopted a watchful waiting strategy rather than offering upfront investigations, such as bronchoscopy, unless obviously indicated. Indeed, the latter may be superfluous in many instances, while increasing the risk of hospital personnel's exposure.
The analysis of data also allowed some speculation on mechanisms of pneumomediastinum formation in COVID-19. The disease itself it is known to cause cystic lung changes [3]. Given the relevant contribution of the microthrombotic component to the development of COVID-19 [8], it is possible that such changes occur as a consequence of focal ischemia and necrosis. A similar mechanism has been also called into question to explain some cases of pneumomediastinum related to dermatomyositis [9]. Application of positive pressure in these patients may then lead to alveolar disruption and air escape. However, other complex pathophysiological events may come into play. We hypothesize that labored breathing resulted in a heterogeneous alveolar strain, possibly contributing to rupture, in patients who were on noninvasive or unassisted ventilation. This mechanism has been described in both animal and human studies [10], and might be particularly relevant in patients with an uneven distribution of lung elastance due to preexisting emphysematous and/or fibrotic changes. Transition from low-to high-elastance due to COVID-19 itself may result in further heterogeneity of alveolar stress [8], thus acting as a triggering factor for the development of pneumomediastinum. This might explain why, in our experience, the onset of pneumomediastinum often anticipated the need of mechanical ventilation. Unfortunately, we were not able to investigate whether severe acute respiratory syndrome coronavirus 2 variants might be implicated in either mechanism.
Contrary to expectations, we observed that patients with a higher comorbidity profile were less prone to develop pneumomediastinum. We hypothesize that this finding might reflect a competing risk paradigm. Indeed, in the high comorbidity population, there was a significant number of patients with extremely deteriorated conditions due to advanced cancer and/or end-stage organ failure. It is possible that, in these patients, fatalities occurred independently of the extent of lung injury and before escalation to mechanical ventilation.
5. Conclusions
To conclude, we reported a trend of a higher pneumomediastinum rate during the 2nd COVID-19 epidemic wave. This finding seemed associated with a globally worsening disease presentation in hospitalized patients; however, data is still insufficient to support the hypothesis of an emerging pattern of disease.
Funding
This study has no funding support.
Conflict of Interest
None of the author has any relevant conflict of interest of any nature to disclose. Prof. Paola Rogliani has received research grants from a few pharmaceutical companies, outside of the present study.
Acknowledgments
The authors wish to thank Dr. Mauro De Chicchis, from the Department of Human Resources and Clinical Governance, for his precious technological and logistic support.
References
- 1.Mart M.F., Norfolk S.G., Flemmons L.N., Stokes J.W., Bacchetta M.D., Trindade A.J. Pneumomediastinum in acute respiratory distress syndrome from COVID-19. Am J Respir Crit Care Med. 2021;15:237–238. doi: 10.1164/rccm.202008-3376IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinelli A.W., Ingle R., Newman J., Nadeem I., Jackson K., Lane N.D. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J. 2020 Nov;56 doi: 10.1183/13993003.02697-2020. https://erj.ersjournals.com/content/early/2020/09/03/13993003.02697-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K., Zeng Y., Xie P., Ye X., Xu G., Liu J. Covid-19 with cystic features on computed tomography. A case report. Medicine. 2020 May;99 doi: 10.1097/MD.0000000000020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miro O., LLorens P., Jimenez S., Pinera P., Burillo-Putze G., Martin A. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159:1241–1255. doi: 10.1016/j.chest.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemmers D., Hilal M.A., Bnà C., Prezioso C., Cavallo E., Nencini N. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? Eur Resp J Open Res. 2020 Nov;6 doi: 10.1183/23120541.00385-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kangas-Dick A., Gazivoda V., Ibrahim M., Sun A., Shaw J.P., Brichov I. Clinical characteristics and outcome of pneumomediastinum in patients with COVID-19 pneumonia. J Laparoendosc Adv Surg Tech. 2020 Sept doi: 10.1089/lap.2020.0692. https://www.liebertpub.com/doi/pdfplus/10.1089/lap.2020.0692 0. [DOI] [PubMed] [Google Scholar]
- 7.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA Insights. 2020;323:2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 9.Kono H., Inokuma S., Nakayama H., Suzuki M. Pneumomediastinum in dermatomyositis: association with cutaneous vasculopathy. Ann Rheum Dis. 2000;59:372–376. doi: 10.1136/ard.59.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruces P., Retamal J., Hurtado D., Erranz B., Iturrieta P., Gonzales C. A physiological approach to understand the role of respiratory effort in the progression of lung inury in SARS-CoV-2 infection. Crit Care. 2020 Aug doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]