Abstract
Background:
Granulomas caused by infectious lung diseases can present as indeterminate pulmonary nodules (IPN). This study aims to validate an enzyme immunoassay (EIA) for histoplasma immunoglobulins G and M (IgG, IgM) for diagnosing benign IPN in areas with endemic histoplasmosis.
Methods:
Prospectively collected serum samples from patients at Vanderbilt University Medical Center (VUMC, n=204), University of Pittsburgh Medical Center (UPMC, n=71), and University of Cincinnati (UC, n=51) with IPN measuring 6–30mm were analyzed for histoplasma IgG and IgM with EIA. Diagnostic test characteristics were compared to results from VUMC pilot cohort (n=127). A multivariable logistic regression model was developed to predict granuloma in IPN.
Results:
Cancer prevalence varied by cohort: VUMC pilot 60%, VUMC validation 65%, UPMC 35%, UC 75%. Across all cohorts, 19% of patients had positive IgG titers, 5% positive IgM, and 3% both positive IgG and IgM. Of patients with benign disease, 33% were positive for at least one antibody. All patients positive for both IgG and IgM antibodies at acute infection levels had benign disease (n=13), with a positive predictive value of 100%. The prediction model for granuloma in IPN demonstrated an area under receiver operating curve 0.84 and Brier score of 0.10.
Conclusions:
This study confirmed that histoplasma EIA testing can be useful for diagnosing benign IPN in areas with endemic histoplasmosis in a population at high risk for lung cancer. Integrating histoplasma EIA testing into the current diagnostic algorithm where histoplasmosis is endemic could improve management of IPN and potentially decrease unnecessary invasive biopsies.
Introduction
The United States is currently facing an epidemic of indeterminate pulmonay nodules (IPN) largely due to the increased use of high resolution chest imaging.1 IPN may be found incidentally on imaging or on computed tomography (CT) scans done to screen for lung cancer. Once an IPN is discovered, clinicians must determine the likelihood that the nodule is malignant and whether further diagnostic evaluation is warranted.2
Determining whether an IPN is benign or malignant is more difficult in regions with endemic fungal disease. Fungal diseases such as histoplasmosis, blastomycosis, and coccidiomycosis cause granulomas and can masquerade as early stage lung cancer on imaging studies, leading to unnecessary invasive biopsies in patients with benign disease (see Supplemental Figure 1). For example, an analysis of CT scans performed in the Ohio and Mississippi River Valleys (areas of endemic histoplasmosis with 60–90% of residents exposed3) found three times as many false positives when trying to diagnose lung cancer when compared to patients from non-endemic regions.4 Additionally, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), which is the guideline recommended test for evaluation of intermediate risk IPN, has elevated false positive rates in areas of endemic fungal disease.5 As imaging can produce indeterminate results, invasive biopsies are often required to ultimately diagnose the IPN as malignant. When patients in endemic fungal regions proceed to invasive biopsy for IPN, granulomatous disease (consistent with prior fungal infection) is the most common benign etiology found.5–7
Relying on imaging alone to discriminate fungal disease from lung cancer in areas of endemic fungal disease is not adequate. Additional non-invasive tools are needed to assist clinicians, such as blood-based fungal biomarkers, to avoid possible harms arising from invasive tests. Our group previously evaluated a newly available serum enzyme immunoassay (EIA) test for histoplasma immunoglobulins G and M (IgG, IgM) for the diagnosis of benign IPN in areas with endemic histoplasmosis.8 In this pilot study, Histoplasma antibodies were found in 12% of patients with benign disease. Histoplasma EIA was more sensitive than older serologic tests (immunodiffusion) and more specific than imaging (FDG-PET) for benign disease in this population. All patients with both positive IgG and IgM antibodies (n=8) had benign disease (positive predictive value 100%) consistent with granuloma.8
Our previously published pilot study demonstrated that integrating a novel fungal biomarker into the evaluation of IPN for lung cancer could be useful in areas of endemic fungal disease. The aims of the study reported here were to validate the use of histoplasma IgG and IgM as measured by EIA in the diagnosis of benign IPN across populations with different local histoplasmosis exposure levels and different prevalences of lung cancer. Additionally, this study explored whether histoplasma biomarkers can be useful in a prediction model for granuloma diagnosis in IPN.
Methods
Study population
Clinical information and serum was prospectively collected from patients with IPN measuring 6–30mm in diameter. These patients were followed for the ultimate diagnosis of the nodule which was either diaganosed via pathologic tissue specimen (biopsy obtained from imaging- or bronchoscopic-guided fine needle aspiration or from surgical resection) or via radiologic follow up for 2 years. Nodules stable over 2 years were considered benign. Blood samples were drawn within 120 days of the CT scan done prior to tissue acquisition or study enrollment. Exclusion criteria included a diagnosis of lung cancer within five years before enrollment, concern for metastatic disease at the time of presentation, or a calcified nodule or lymph nodes consistent with benign disease.
The patient serum samples used in this study came from four different cohorts. Patients in Cohort 1 (n=127) presented to Vanderbilt University Medical Center (VUMC) for evaluation of their IPN, and were included in the previously published analysis of the histoplasmosis EIA serologic test (hereafter referred to as VUMC Pilot). Patients in Cohort 2 (n=204) also presented to VUMC for the evaluation of IPN but were not included in the original pilot analysis. Patients in Cohort 3 (n=71) presented to the University of Pittsburgh Medical Center (UPMC) for evaluation of IPN; specific pathologic diagnosis other than cancer or benign disease was not available for this cohort. Patients in Cohort 4 (n=51) presented to the University of Cincinnati (UC) thoracic surgical service for evaluation of IPN. Patients in cohorts 1, 2, and 4 were considered to be from highly endemic regions for histoplasmosis infections while patients in Cohort 3 were considered to be from an area of low histoplasmosis prevalence, as observed in Edwards et. al 1969.9 This study was approved by each site’s Institutional Review Board and conducted in accordance with the U.S. Common Rule.
Serologic testing
Frozen serum was shipped to MiraVista Diagnostics which performed all serologic tests and was blinded to all clinical data. Each participant was tested for histoplasmosis by MiraVista’s proprietary EIA for IgG and IgM antibodies, the methodology of which has been reported elsewhere8,10. EIA results were standardized to enzyme immunoassay antibody units (EU), with values ≥10 EU indicating a positive test consistent with active disease. These cutoffs were determined based on the manufacturer’s recommendation. For analysis using continuous values, 0 EU was used for an undetectable result and 80 EU was used for any values ≥80 EU.
The cost for both serologic tests is approximately 100 U.S. dollars. Once MiraVista Diagnostic’s central laboratory receives the samples, results are typically available in 3–5 days.
Statistical analysis
All analyses were performed in R v3.6.0. Despcriptive statistics were performed for each cohort. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive values were estimated for each histoplasma immunoglobin in each cohort and in combination using the above cutoff values to determine positive or negative results.
Prediction model
A multivariable logistic regression model was developed11,12 to predict the presence of granuloma in patients with IPN. Patients were excluded from model development if no detailed pathologic diagnosis was available to define those with or without granulomatous disease, which excluded 51 patients from cohorts 1 and 2 and all patients from Cohort 3. These patients were excluded as the purpose of the model was to predict the present of granuloma rather than all-cause benign disease. Predictor variables were pre-specified from clincal expertise and available information and included age, smoking history, nodule size, upper lobe location, histoplasma IgG, and histoplasma IgM EUs. Continuous values were used for age, nodule size, IgG, and IgM. Predictor variables were assessed for nonlinearity and interactions association with the outcome. The binary outcome was granuloma or other disease (which included both cancer and benign diseases) based off of pathologic results; the etiology of granuloma was not commented on in the majority of pathology results hence the decision was made to predict any granuloma rather than granuloma caused by histoplasmosis specifically. The model was internally validated using bootstrap with replacement (n=500) and corrected for optimism.11 The discrimination and calibration of the model was estimated using the area under the receiver operating curve (AUC) and Brier score and compared to a simplified prediction model for granuloma that did not include histoplasma antibody results.
Results
The charactersitics of patients included in each cohort are presented in Table 1. The patients were predominantly older smokers, with cancer prevalence ranging from 35–75% across the four cohorts. Among the entire study population, patients with benign disease were younger (64 vs. 67 years old, p<0.001), less likely to be current or former smokers (83% vs. 94%, p>0.001), and had smaller pulmonary nodules (14mm vs. 18mm, p<0.001).
Table 1.
Patient characteristics
| Cohort 1: VUMC Pilot n=127 | Cohort 2: VUMC 2 n=204 | Cohort 3: UPMC n=71 | Cohort 4: UC n=51 | |
|---|---|---|---|---|
| Age median (IQR) | 65 (59, 71) | 66 (59, 72) | 68 (64, 86) | 64 (60, 70) |
| Gender male | 76 (60%) | 105 (51%) | 46 (63%) | 21 (41%) |
| Ever smoker | 104 (82%) | 184 (90%) | 71 (100%) | 50 (98%) |
| Nodule size (mm) median (IQR) | 16 (11.5, 21.5) | 17 (12, 22) | 13 (10, 20) | 16 (13,19) |
| Upper lobe location | 66 (52%) | 114 (56%) | 33 (46%) | 31 (61%) |
| Spiculated edge | 37 (29%) | 68 (33%) | 5 (7%) | 25 (49%) |
| Final diagnosis cancer | 76 (60%) | 134 (66%) | 25 (35%) | 38 (75%) |
VUMC, Vanderbilt University Medical Center
UPMC, University of Pittsburgh Medical Center
UC, University of Cincinnati
IQR, Interquartile Range
Of patients determined to have benign IPN (Table 2), 46% were found to have a granuloma on pathology. Of those diagnosed with a granuloma, 32% were found to have histoplasmosis on pathologic specimen, while in almost 60% a causative etiology or organism was not identified. Notably, 36% of patients with benign disease had non-diagnostic biopsy results showing normal tissue or non-specific changes.
Table 2.
Pathologic diagnoses
| Cohort 1: VUMC 1 n=127 | Cohort 2: VUMC 2 n=204 | Cohort 3: UPMC* n=71 | Cohort 4: UC n=51 | |
|---|---|---|---|---|
| Cancer | 76 (60%) | 134 (66%) | 25 (35%) | 38 (75%) |
| Benign pathology | 51 (40%) | 65 (33%) | 46 (65%) | 13 (25%) |
| Granuloma | 29 (56%) | 22 (34%) | - | 8 (62%) |
| Histoplasmosis | 10 (34%) | 2 (9%) | - | 7 (88%) |
| Other infectious | 3 (10%) | 2 (9%) | - | - |
| Non-specific | 16 (55%) | 18 (82%) | - | 1 (13%) |
| Active infection | 3 (6%) | 8 (12%) | - | 2 (4%) |
| Fibrosis | 2 (4%) | 1 (1.5%) | - | 0 |
| Non-diagnostic | 15 (27%) | 31 (48%) | - | 0 |
| Other | 2 (6%) | 3 (5%) | - | 3 (6%) |
| Benign by imaging | 0 | 5 (2%) | - | 0 |
pathology information unavailable for UPMC cohort
VUMC, Vanderbilt University Medical Center
UPMC, University of Pittsburgh Medical Center
UC, University of Cincinnati
Histoplasma serologic results
Across all cohorts, 19% of patients had positive IgG titers, 5% had positive IgM titers, and 3% had both positive IgG and IgM titers. Cohort 4, from Cincinnati, had the highest rate of positive serology and Cohort 3, from Pittsburgh, had the lowest rate of positive serology. Overall, 33% of patients with benign disease were positive for at least one antibody, and 14% of patients with cancer were positive for at least one antibody. The test characteristics of VUMC Cohort 2 and Cincinnati, both from highly endemic regions, were most similar to Cohort 1 used in the pilot study (Table 3). All patients with both histoplasma IgG and IgM positive titers (n=13) had benign disease, with a PPVof 100%. The clinical, radiographic, and pathologic characteristics of the thirteen patients positive for both histoplasma IgG and IgM can be found in Supplemental Table 1.
Table 3:
Test characteristics of histoplasma EIA
| IgG+ | IgM+ | IgG+ & IgM+ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VUMC Pilot | VUMC 2 | UPMC | UC | VUMC Pilot | VUMC 2 | UPMC | UC | VUMC Pilot | VUMC 2 | UPMC | UC | |
| Positive test | 28 | 25 | 6 | 16 | 9 | 8 | 4 | 3 | 6 | 6 | 0 | 1 |
| Sens | 39% | 32% | 9% | 54% | 13% | 11% | 4% | 8% | 12% | 8% | - | 8% |
| Spec | 89% | 91% | 92% | 76% | 97% | 100% | 92% | 95% | 100% | 100% | - | 100% |
| PPV | 71% | 66% | 67% | 44% | 77% | 100% | 50% | 33% | 100% | 100% | - | 100% |
| NPV | 69% | 72% | 35% | 83% | 63% | 68% | 34% | 75% | 63% | 67% | - | 76% |
VUMC, Vanderbilt University Medical Center
UPMC, University of Pittsburgh Medical Center
UC, University of Cincinnati
Sens, sensitivity
Spec, specificity
PPV, positive predictive value
NPV, negative predictive value
Granuloma prediction model
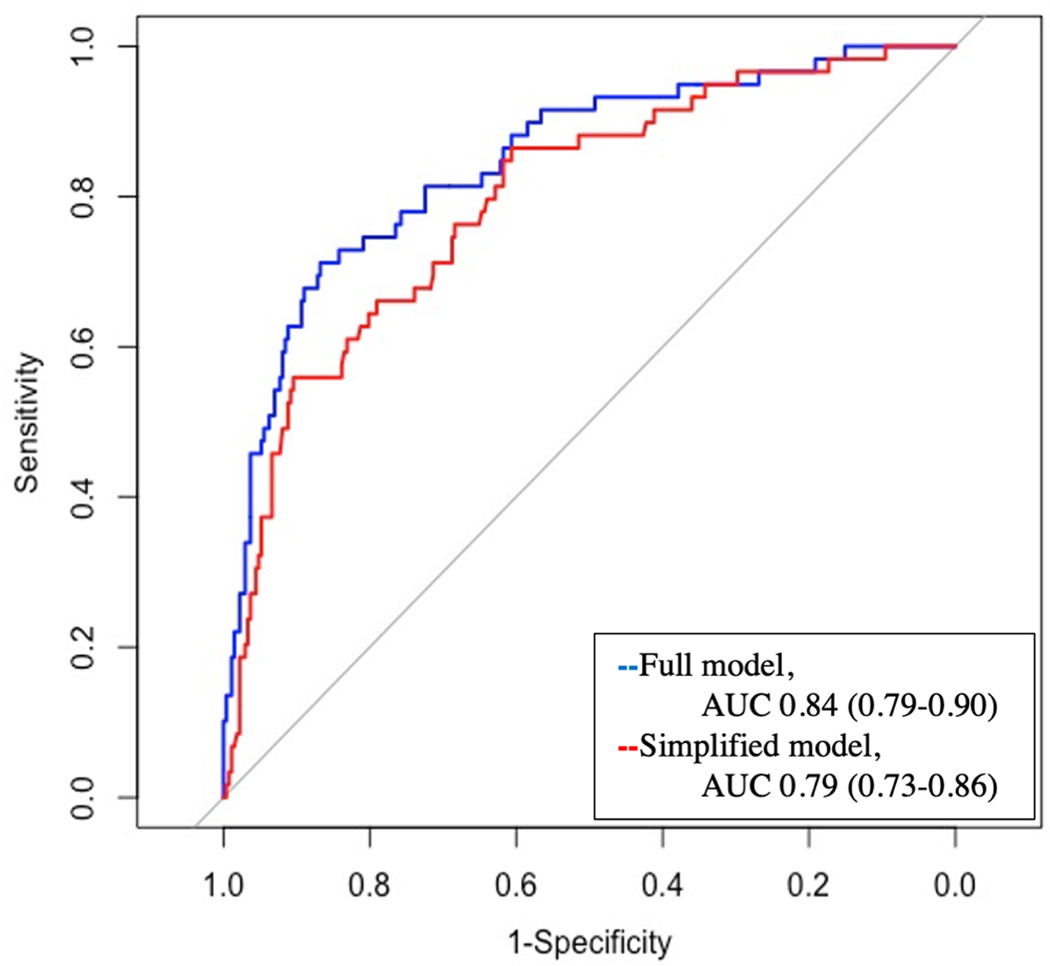
The relative effect of the variables included in the prediction model for granuloma are presented in Table 4; age, smoking, IgG, and IgM were significant predictors of a granuloma diagnosis. The AUC for the prediction model was 0.85 (95% Confidence Interval 0.79–0.90), which was corrected for optimism to 0.84. The model was well calibrated with the Brier score of 0.10. This full model was compared to a simplified version of the model containing only clinical and radiographic factors (age, smoking history, nodule size, and upper lobe location). The simplified model had an AUC of 0.80 (95% Confidence Interval 0.73–0.86), which was corrected for optimism to 0.79, and a Brier score of 0.12. When the full model was compared to the simplified model, the discrimination was significantly improved (p<0.01).
Table 4:
Results of granuloma prediction model
| Variable | Odds Ratio |
|---|---|
| Age (years) | 0.50 (0.3–0.7)* |
| Ever smoker | 0.08 (0.03–0.2)* |
| Nodule size (mm) | 1.2 (0.7–2.2) |
| Upper lobe | 1.4 (0.7–2.9) |
| IgG (EU) | 1.3 (1.1–1.5)* |
| IgM (EU) | 1.4 (1.0–1.7)* |
p<0.05
Comment
The increasing number of patients presenting with IPN requires improved non-invasive diagnostic techniques to avoid unnecessary squaele of diagnostic operations while not delaying diagnosis and treatment. This study validated the use of a blood-based fungal biomarker, histoplasma IgG and IgM antibodies measured using EIA, in the diagnosis of benign IPN. While this test was a commercially available, CLIA certified test indicated to help diagnose acute or diseminated histoplasmosis, we observed positive antibodies in over 30% of patients with benign IPN who live in endemic regions suggesting a broader use of the test could be clinically useful.
In agreement with the findings of our pilot study, having positive results for both IgG and IgM antibodies at acute infection levels only occurred in patients with benign disease across all cohorts (PPV 100% for benign disease). While this study did not investigate the clinical utility of integrating these results into a diagnostic algorithm for IPN, one could argue that patients with dually positive serologic tests could be followed with close observation and interval radiographic imaging. For example, while we do not have detailed information on the diagnostic evaluations for each of the thirteen patients, positive serologic results could have potentially prevented FDG-PET scans in the nine patients or invasive biopsies in twelve patients from the validation group at our institution. Implementing serologic testing in endemic areas while avoiding expensive tests and biopsies could improve time to diagnosis and save resources for patients who need them. Further research is needed in this area.
However, based on the results of this study the use of either IgG or IgM results alone should not be used clinically without other clinical or radiographic information to support the diagnosis of benign disease. A number of patients with cancer tested positive for IgG or IgM, indicating recent or historic exposure to histoplasmin, which led to lower positive predictive values when these tests were used in isolation. As stated in our earlier work and shown again here, a negative EIA test does not rule out the presence of benign disease and would ideally be coupled with an accurate cancer biomarker test.
The results of Histoplasma IgG and IgM EIA tests were incorporated into a prediction model for granuloma using levels of histoplasma antibodies in a population of IPN with pathology specimens. This model was well calibrated and discriminatory for the presence of granuloma. Some limitations include the small population size used to develop the model (59 granulomas among 331 patients) and by the lack of external validation of the model, as all patients were used for model development. Patients without pathology results were excluded from model development which could have impacted the results of the model. Additionally, many benign pathologic results in this study were non-diagnostic, and a proportion of these patients likely had unidentified granulomas that may not be caused by Histoplasma capsulatum and could change risk estimates along with model performance.
In conclusion, histoplasma IgG and IgM antibodies as measured by EIA can be a useful addition to the diagnostic algorithm for IPN in areas with endemic histoplasmosis. Further research, including prospective clinical trials, is needed to determine how fungal biomarkers should be tested along with cancer biomarkers and advanced imaging techniques for the diagnosis of IPN.
Supplementary Material
Figure 1:
Area under the receiver operating curve (AUC) for fungal granuloma prediction model which included IgG and IgM compared to the simplified model. The simplified model contained the clinical variables only.
Acknowledgments
Funding: Dr. Shipe is supported by the Agency for Healthcare Research (AHRQ) under Award Number T32 HS026122. Drs. Massion, Grogan and Deppen are funded by the NIH grant EDRN U01 CA152662. The authors declare that they have no conflicts of interest.
Abbreviations
- AUC
Area under the receiver operating curve
- CLIA
Clinical laboratory improvement amendments
- CT
Computed tomography
- EIA
Enzyme immunoassay
- EU
Enzyme immunoassay antibody units
- FDG-PET
18F-fluorodeoxyglucose positron emission tomography
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IPN
Indeterminate pulmonary nodules
- NPV
Negative predictive value
- PPV
Positive predictive value
- UC
University of Cincinnati
- UPMC
University of Pittsburgh Medical Center
- VUMC
Vanderbilt University Medical Center
Footnotes
Disclosures
None of the authors had any conflicts of interest. Dr. Shipe is supported by the Agency for Healthcare Research (AHRQ) under Award Number T32 HS026122. Drs. Massion, Grogan and Deppen are supported by NIH grant EDRN U01 CA152662. Dr. Grogan is a former recipient of the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service Career Development Award (10–024). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
References
- 1.Gould MK, Tang T, Lee J, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192(10):1208–1214. doi: 10.1164/rccm.201505-0990oc [DOI] [PubMed] [Google Scholar]
- 2.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: When is it lung cancer? Diagnosis and management of lung cancer? Chest. 2013;143(5 SUPPL):93–120. doi: 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MANOS NE,FEREBEE SH, KERSCHBAUM WF Geographic variation in the prevalence of histoplasmin sensitivity. Dis Chest. 1956;29(6):649–668. doi: 10.1378/chest.29.6.649 [DOI] [PubMed] [Google Scholar]
- 4.Starnes SL, Reed MF, Meyer CA, et al. Can lung cancer screening by computed tomography be effective in areas with endemic histoplasmosis? J Thorac Cardiovasc Surg. 2011;141(3):688–693. doi: 10.1016/j.jtcvs.2010.08.045 [DOI] [PubMed] [Google Scholar]
- 5.Grogan EL, Deppen SA, Ballman KV., et al. Accuracy of fluorodeoxyglucose-positron emission tomography within the clinical practice of the American College of Surgeons Oncology Group Z4031 trial to diagnose clinical stage i non-small cell lung cancer. Ann Thorac Surg. 2014;97(4):1142–1148. doi: 10.1016/j.athoracsur.2013.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maiga AW, Deppen SA, Mercaldo SF, et al. Assessment of fluorodeoxyglucose F18–Labeled positron emission tomography for diagnosis of high-Risk lung nodules. JAMA Surg. 2018;153(4):329–334. doi: 10.1001/jamasurg.2017.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deppen SA, Blume JD, Kensinger CD, et al. Accuracy of FDG-PET to diagnose lung cancer in areas with infectious lung disease: A meta-analysis. JAMA - J Am Med Assoc. 2014;312(12):1227–1236. doi: 10.1001/jama.2014.11488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deppen SA, Massion PP, Blume J, et al. Accuracy of a novel histoplasmosis enzyme immunoassay to evaluate suspicious lung nodules. Cancer Epidemiol Biomarkers Prev. 2019;28(2):321–326. doi: 10.1158/1055-9965.EPI-18-0169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards L, Acquaviva F, Livesay V, Cross F, Palmer C. An atlas of sensitivity to tuberculin, PPD-B, and histoplasmin in the United States. Am Rev Respir Dis. 1969;99(Suppl):1–132. [PubMed] [Google Scholar]
- 10.Richer S, Smedema M, Durkin M, Herman K, Hage C, Fuller D. mproved diagnosis of acute pulmonary histoplasmosis by combin- ing antigen and antibody detection. Clin Infect Dis. 2016;62:896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrell FE. Regression Modeling Strategies. 2nd ed. New York City, NY: Springer Science+Business Media; 2015. doi: 10.1007/978-3-319-19425-7 [DOI] [Google Scholar]
- 12.Steyerberg EW. Clinical Prediction Models. Springer Science+Business Media; 2009. doi: 10.1007/978-0-387-77244-8 [DOI] [Google Scholar]
- 13.Swensen S, Silverstein M, Ilstrup D, Schlek C, Edell E. The Probability of Malignancy in Solitary Pulmonary Nodules. Arch Intern Med. 1997;157(8):849–855. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.