Abstract
The first total synthesis of the resveratrol tetramers vitisin A and vitisin D is reported. Electrochemical generation and selective dimerization of persistent radicals is followed by thermal isomerization of the symmetric C8b–C8c dimer to the C3c–C8b isomer, providing rapid entry into the vitisin core. Computational results suggest that this synthetic approach mimics Nature’s strategy for constructing these complex molecules. Sequential acid-mediated rearrangements consistent with the proposed biogenesis of these compounds afford vitisin A and vitisin D. The rapid synthesis of these complex molecules will allow for further study of their pharmacological potential.
Graphical Abstract
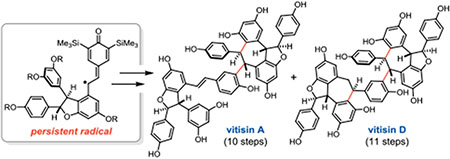
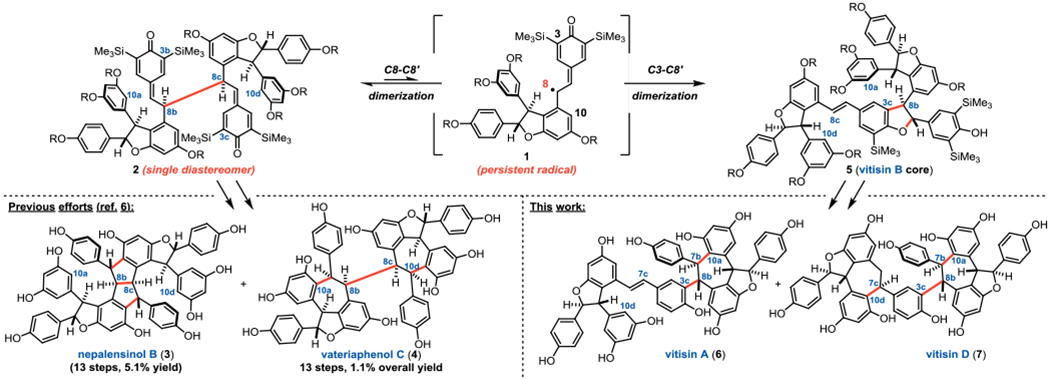
Resveratrol and its oligomers comprise a natural product class of hundreds of structurally distinct compounds; however, challenges associated with isolating individual molecules in adequate supply and purity have precluded rigorous evaluation of their pharmacological potential.1 Numerous research groups have devised innovative synthetic approaches to access these complex molecules, including cationic cyclizations,2 transition metal catalysis,3 and reagent-controlled bromination.4 It has been proposed that in nature, oligomerization proceeds via phenoxyl radical intermediates.1 Indeed, this biosynthetic hypothesis has inspired our own efforts in this area. We recently reported the use of persistent phenoxyl radicals (e.g. 1) for the synthesis of C8–C8′ resveratrol dimers pallidol and quadrangularin A,5 and tetramers nepalensinol B (3) and vateriaphenol C (4, Figure 1).6,7 It has further been proposed that interconversion of the C8–C8′ and C3–C8′ constitutional isomers is the key step in the divergent biogenesis of related natural products possessing C3–C8′ connectivity.1 Herein, we leverage the facile equilibrium between persistent phenoxyl radicals and their corresponding quinone methide dimers (i.e. 1 and 2, respectively, Figure 1) to achieve the first total synthesis of vitisin A and vitisin D (6 and 7, Figure 1).
Figure 1.
Divergent reactivity of persistent radicals in the synthesis of resveratrol tetramers.
A new opportunity for C3–C8′ resveratrol oligomer synthesis was discovered while investigating the 8A–9A equilibrium (Figure 2A). Discontinuity in the Van’t Hoff analysis above 50 °C suggested an alternative and irreversible reaction path was accessible to 9A (see Figure S3 of the Supporting Information).6 Importantly, no such discontinuity was observed in corresponding experiments with dimers featuring tert-butyl groups (8B/9B) in lieu of the TMS groups in 8A/9A. Subsequent NMR analysis revealed that upon heating the C8–C8′ dimer 8A had rearranged to the δ-viniferin core 11. We speculated that this rearrangement proceeded via the intermediacy of the C3–C8′ dimer 10A, which was assumed to be less energetically favorable than the C8–C8′ dimer 8A but primed to lose the trimethylsilyl group and aromatize the phenolic moiety. Indeed, computations suggest that the C3–C8′ bond in 10A is 7.4 kcal/mol weaker than the C8–C8′ bond in 8A.8 However, the simple fact that 9A is in equilibrium with 8A (for which the C8–C8′ bond dissociation enthalpy was previously determined to be 16.4 kcal/mol6), implies that it is also in equilbrium with the C3–C8′ dimer 10A. Presumably, 10A is not observed due to its rapid decomposition to the δ-viniferin core (11). Computations predict that unimolecular expulsion of the TMS cation proceeds with a significant barrier,9 suggesting that adventitious water in the acetone promotes desilylation and concomitant aromatization. Remarkably, the C8–C8′ to C3–C8′ isomerization and subsequent cyclization to 11 occurs in nearly quantitative yield (Figure 2A).
Figure 2.
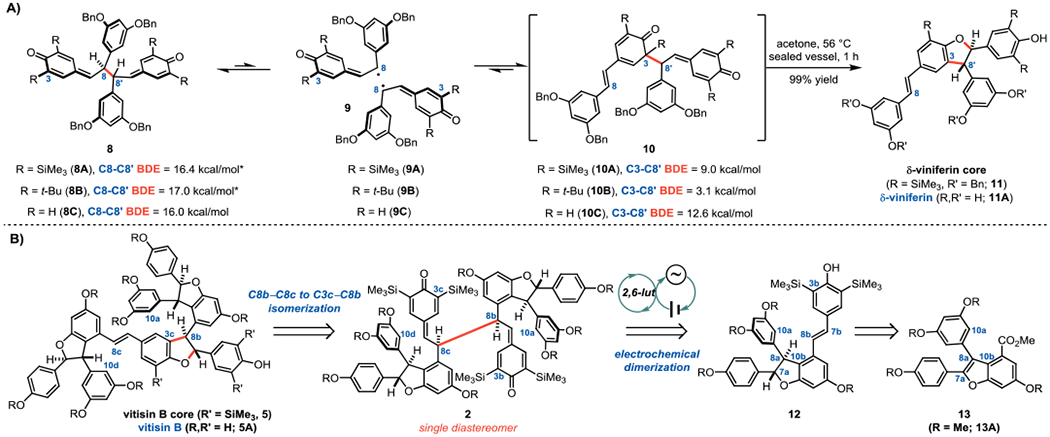
A) Discovery of C8b–C8c to C3c–C8b isomerization from thermodynamic study of persistent radical equilibria. B) Retrosynthetic analysis for the synthesis of vitisin tetramers based on this late stage homolytic isomerization. *C8-C8′ BDEs determined experimentally;6 BDEs for the corresponding C3-C8′ dimers are given relative to these experimental values.8
The difference in the C-C BDEs of the corresponding tert-butylated dimers (8B/10B) is nearly twice as large (13.9 kcal/mol), reflecting the greater steric repulsion in the C3–C8′ dimer resulting from the shorter C–C bonds relative to C–Si bonds. Indeed, the C3–C8′ and C8–C8′ BDEs in the dimers which lack any ortho substitution (8C/10C) are computed to be only 3.6 kcal/mol apart, funneling both of these intermediates toward the δ-viniferin core. These computations are consistent with the hypothesis that resveratrol oligomerization relies upon equilibration of the C8–C8′ and C3–C8′ constitutional isomers.1 In fact, numerous research groups have realized the direct conversion of resveratrol (S1) to δ-viniferin (11A, Figure 2A) through single electron oxidation strategies (see Table S1 of the Supporting Information for a summary of these efforts).10 While excellent yields for conversion to 11A have been realized, extension of this strategy to higher-order oligomers has not achieved the same degree of success. The most noteworthy example was reported by Sako and co-workers in their semi-synthesis of vitisin B (5A).10g By treating isolated (+)-ε-viniferin (S4) with silver acetate in methanol at elevated temperatures (50 °C), they observed conversion to vitisin B (5A) in 40% yield on 20 milligram scale (see Figure S2 of the Supporting Information). The C3c–C8b-fused resveratrol tetramers exhibit some of the most compelling biological activities among members of the resveratrol oligomer class reported to date; therefore, an approach to their synthesis reliant on readily available materials is desirable. For example, vitisin B (5A) was found by Lee and co-workers to be a potent inhibitor of the NS3 helicase of hepatitis C (IC50 = 3 nM), while vitisin A (6) was also active against the same target (IC50 = 35 nM).11 To the best of our knowledge, similarly rigorous biological analysis of vitisin D (7) has not yet been reported.
Quinone methide dimer 2 serves as the linchpin for the proposed isomerization approach to the C3c–C8b resveratrol tetramers (Figure 2B). Importantly, this scaffold forms exclusively as a single diastereomer upon oxidation of racemic starting material 12, meaning dimerization occurs selectively between the same enantiomeric precursors in the same fashion required to access vitisin B (5A). Preservation of this stereochemical integrity during C8b–C8c to C3c–C8b isomerization would directly convert 2 to the core of vitisin B (5). As 5A is proposed to be the biogenic precursor to both 6 and 7,12 this novel formal [1,5]-shift could in principle provide access to multiple biologically active members of the C3c–C8b tetramers. Hypothesizing that the late-stage C8b–C8c to C3c–C8b isomerization would be preceded by our recently reported method for the electrochemical dimerization of phenylpropenoid scaffolds,13 attention turned to the synthesis of the dimerization starting material – protected ε-viniferin analog 12. This scaffold was previously prepared using an approach developed by the Snyder group in one of their seminal contributions to this field;4c however, it was envisioned that a benzofuran precursor (i.e. 13) might offer a more direct route to 9. Kim and Choi recently reported an approach to 13A to access permethylated resveratrol dimers,3b providing an excellent starting point for our synthesis.
Execution of this plan commenced with the preparation of the requisite benzofuran precursor while adopting a new protecting group strategy, and gratifyingly, cyclodehydration of 14A afforded benzofuran 15A in 85% yield (Figure 3). Unfortunately, C–H arylation of 13A did not occur – instead these efforts were plagued by silyl deprotection. Benzyl ethers could be replaced for silyl ethers at this stage to allow for elaboration to 16 (see Figure S5 of the Supporting Information for details). Alternatively, isopropyl ethers have been demonstrated to be similarly robust to methyl, yet more readily cleaved, so this protection strategy was employed to access 16 in a shorter sequence with fewer protecting group manipulations (Figure 3). After C–H arylation,14 13B was converted to 16 by Lewis acid-mediated deprotection,15 Kishi reduction,16 and silyl protection in a three-step sequence that did not require intermediate purification. Reduction of the C8b-ester and a Parikh-Doering oxidation17 delivered aldehyde 17, and a Wittig olefination18 smoothly afforded the silyl protected ε-viniferin analog 12A. At this point, our dimerization method utilizing anodic oxidation was employed to access 2A.13 Deviation from the published conditions was required to ensure full solubility, and, after adding dichloromethane as a co-solvent and decreasing the electrolyte concentration by half, 12A was converted to the desired quinone methide tetramer 2A in 63% yield (Figure 3).
Figure 3.
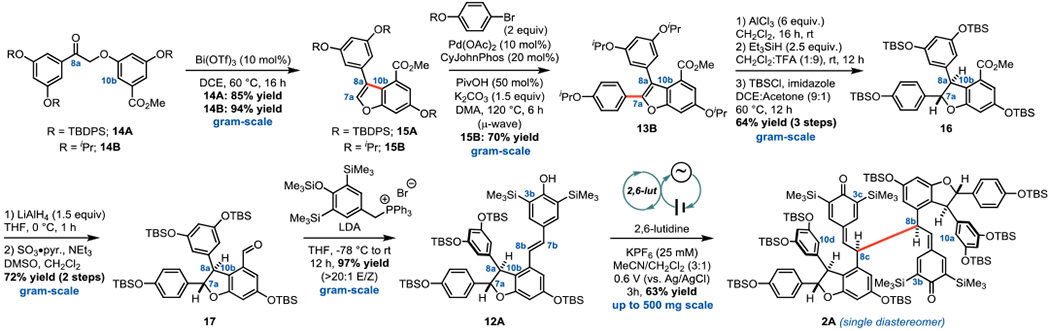
Synthesis of silyl-protected bis-quinone methide 2A from the electrochemical dimerization of 12A.
With the silyl-protected tetrameric material in hand, the key C8b–C8c to C3c–C8b isomerization step was next investigated. In order to determine if the persistent radicals would escape the solvent cage prior to the formal [1,5]-shift, possibly leading to mismatched C3c–C8b oligomers, a crossover experiment was performed with 8A and a differentially protected analog (see Figure S4 of Suppporting Information). Indeed, the crossover products were observed, suggesting C–C fragmentation and diffusion is competitive with isomerization. However, the dimer model system was not sufficient to determine if the stereochemical integrity of 2A would be completely eroded. Subjecting 2A to the thermal isomerization conditions readily accessed the vitisin B core (5B), but as a mixture of four C3c–C8b dihydrobenzofuran (DHB) isomers (Sequence 1, Figure 4). Increasing the temperature improved the trans/cis ratio of the DHB rings, presumably due to thermal epimerization; however, the facial selectivity of C3c–C8b recombination remained unchanged. Gratifyingly, the TBS ethers were readily cleaved with HF-triethylamine to afford two compounds – 19 and 20. These trans-DHB isomers arise from each possible facial addition of C8b to C3c during the formation of 18 (Sequence 1, Figure 4) suggesting that the stereochemical integrity is preserved during the thermal isomerization. The O-silyl deprotection conditions also resulted in epimerization of cis-DHB isomers to the corresponding trans-DHBs, which is well precedented in the literature,3f,g thereby delivering only the two observed products. To support the hypothesis that the formal [1,5]-shift only occurs through the relative configuration depicted by 2A, a second crossover experiment between 2A and the corresponding TIPS-protected analog 2B was performed (Figure 4). After thermal isomerization, the crossover product was observed, further supporting that C–C fragmentation and diffusion is competitive with in-cage recombination to yield the isomer. However, upon TBS deprotection with HF-triethylamine, 19 and 20 were the only observed products, suggesting that thermal isomerization proceeds without loss of stereochemical integrity afforded by C8b–C8c dimerization.
Figure 4.
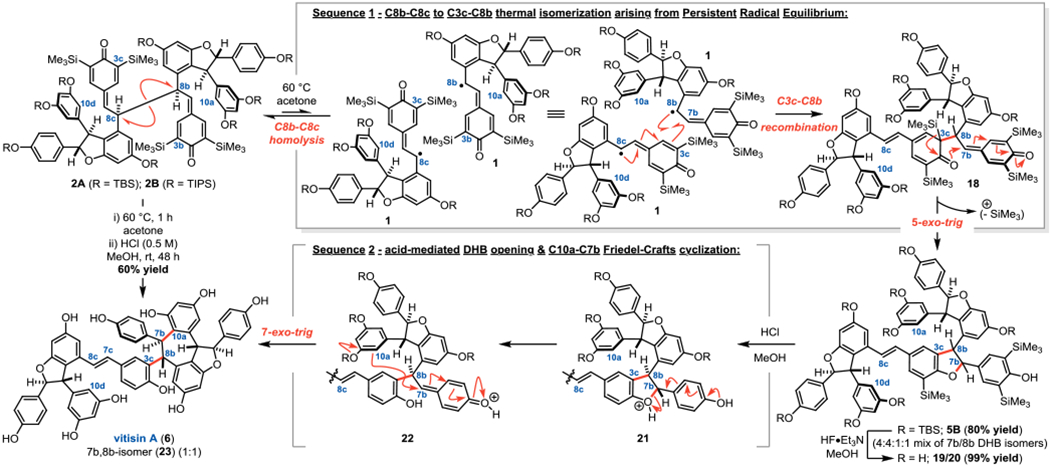
C8b–C8c to C3c–C8b isomerization, Friedel-Crafts cyclization, and deprotections deliver vitisin A (3).
Inspired by these results, we set out to develop conditions for O-silyl deprotection and protodesilylation to complete the synthesis. Global desilylation was achieved by addition of a methanolic solution of hydrochloric acid upon completion of the isomerization of 2A yielding vitisin A (6) and its C7b, C8b-isomer (23). Vitisin A (6) is proposed to arise from vitisin B (5A) in the biosynthesis of these compounds.12 Acid-mediated cleavage of the C7b–O bond upon protonation of the DHB (21) affords a quinone methide (22) to which C10a of the adjacent resorcinol ring adds in a 7-exo-trig cyclization (Sequence 2, Figure 4). Of note, the C10a–C7b bond formation exclusively delivers the relative configuration depicted in Figure 4, with the C8b configuration dictating the facial selectivity of the cyclization.
In an attempt to prevent Friedel-Crafts rearrangement and directly reveal vitisin B (5A), intermediates 19/20 were exposed to milder protodesilylation conditions.19 Remarkably, vitisin D (7) and the isomer 26 were isolated after treating 19/20 with SiMe3Cl, KI, and H2O in MeCN for one hour (Figure 5A). These compounds are the result of two acid-promoted cyclizations; after cyclization to vitisin A (6) in situ (Sequence 2, Figure 4), protonation of the stilbene at C8c results in formation of the quinone methide tautomer 24/25, which is trapped by 7-exo-trig cyclization by C10d of the adjacent resorcinol ring (Sequence 3, Figure 5A). Acid-mediated conversion of vitisin A (6) to vitisin D (7) has been proposed in the biosynthesis of these compounds,12 thus it is perhaps unsurprising that the in-situ generation of hydroiodic acid would give rise to 7 and 26.
Figure 5.
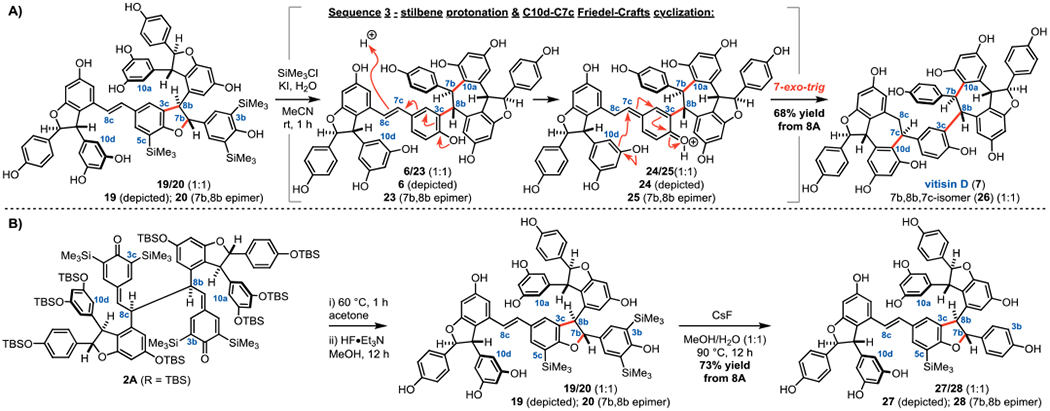
A) C8b–C8c to C3c–C8b isomerization, Friedel-Crafts cyclizations, and deprotections deliver vitisin D (4). B) C5c-TMS group survives fluoride-mediated desilylation attempts.
Alkaline conditions were next attempted in an effort to target vitisin B (5A).20 It was quickly determined that the C3/5b aryl silyl groups are cleaved via fluoride-mediated-desilylation; however, the C5c-TMS group remains intact (27/28, Figure 5B). Our investigations have demonstrated that the C3c–C8b DHB is quite labile, and in fact, conversion to vitisin A (6) occurs under a range of conditions (see Table S6 of the Supporting Information). Despite an extensive evaluation of desilylation conditions, access to vitisin B (5A) from this approach remains an outstanding challenge, the solution to which will be reported in due course.
Herein we have reported the first total synthesis of the resveratrol tetramers vitisin A (3, 3.3% overall yield) and vitisin D (4, 3.7% overall yield) in 10 and 11 steps from 14A, respectively. Our approach utilizes persistent radicals to enable a unique, late-stage, formal [1,5]-shift that is consistent with the biosynthetic hypothesis of these complex molecules. The persistent radicals arise from mild anodic oxidative conditions, and the subsequent biomimetic transformations rapidly convert dimeric intermediates to tetrameric structures, which will enable the investigation of the pharmacological potential of these complex molecules.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the financial support for this research from the NIH NIGMS (R01-GM121656), the Camille Dreyfus Teacher-Scholar Award Program, and the University of Michigan. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1256260 (for K. J. R.). This work was also supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Canada Foundation for Innovation. The authors thank Dr. Daryl Staveness, Dr. Rory McAtee, and Mr. Matthew Galliher for helpful suggestions in the preparation of this manuscript.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and characterization data for all new compounds (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).Keylor MH; Matsuura BS; Stephenson CRJ Chemistry and Biology of Resveratrol-Derived Natural Products. Chem. Rev 2015, 115 (17), 8976–9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Snyder SA; Zografos AL; Lin Y Total Synthesis of Resveratrol-Based Natural Products: A Chemoselective Solution. Angew. Chem. Int. Ed 2007, 46 (43), 8186–8191. [DOI] [PubMed] [Google Scholar]; (b) Snyder SA; Breazzano SP; Ross AG; Lin Y; Zografos AL Total Synthesis of Diverse Carbogenic Complexity within the Resveratrol Class from a Common Building Block. J. Am. Chem. Soc 2009, 131 (5), 1753–1765. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC; Wu TR; Kang Q; Chen DY-K Total Synthesis of Hopeahainol A and Hopeanol. Angew. Chem. Int. Ed 2009, 48 (19), 3440–3443. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC; Kang Q; Wu TR; Lim CS; Chen DY-K Total Synthesis and Biological Evaluation of the Resveratrol-Derived Polyphenol Natural Products Hopeanol and Hopeahainol A. J. Am. Chem. Soc 2010, 132 (21), 7540–7548. [DOI] [PubMed] [Google Scholar]; (e) Chen DY-K; Kang Q; Wu TR Modular Synthesis of Polyphenolic Benzofurans, and Application in the Total Synthesis of Malibatol A and Shoreaphenol. Molecules 2010, 15 (9), 5909–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Snyder SA; Brill ZG Structural Revision and Total Synthesis of Caraphenol B and C. Org. Lett 2011, 13 (20), 5524–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Snyder SA; Wright NE; Pflueger JJ; Breazzano SP Total Syntheses of Heimiol A, Hopeahainol D, and Constrained Analogues. Angew. Chem. Int. Ed 2011, 50 (37), 8629–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Snyder SA; Thomas SB; Mayer AC; Breazzano SP Total Syntheses of Hopeanol and Hopeahainol A Empowered by a Chiral Brønsted Acid Induced Pinacol Rearrangement. Angew. Chem. Int. Ed 2012, 51 (17), 4080–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Choi YL; Kim BT; Heo J-N Total Synthesis of Laetevirenol A. J. Org. Chem 2012, 77 (19), 8762–8767. [DOI] [PubMed] [Google Scholar]
- (3).(a) Jeffrey JL; Sarpong R Concise Synthesis of Pauciflorol F Using a Larock Annulation. Org. Lett 2009, 11 (23), 5450–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kim I; Choi J A Versatile Approach to Oligostilbenoid Natural Products – Synthesis of Permethylated Analogues of Viniferifuran, Malibatol A, and Shoreaphenol. Org. Biomol. Chem 2009, 7 (13), 2788–2795. [DOI] [PubMed] [Google Scholar]; (c) Yang Y; Philips D; Pan S A Concise Synthesis of Paucifloral F and Related Indanone Analogues via Palladium-Catalyzed α-Arylation. J. Org. Chem 2011, 76 (6), 1902–1905. [DOI] [PubMed] [Google Scholar]; (d) Lee BH; Choi YL; Shin S; Heo J-N Stereoselective Palladium-Catalyzed α-Arylation of 3-Aryl-1-Indanones: An Asymmetric Synthesis of (+)-Pauciflorol F. J. Org. Chem 2011, 76 (16), 6611–6618. [DOI] [PubMed] [Google Scholar]; (e) Klotter F; Studer A Total Synthesis of Resveratrol-Based Natural Products Using a Palladium-Catalyzed Decarboxylative Arylation and an Oxidative Heck Reaction. Angew. Chem. Int. Ed 2014, 53 (9), 2473–2476. [DOI] [PubMed] [Google Scholar]; (f) Soldi C; Lamb KN; Squitieri RA; González-López M; Di Maso MJ; Shaw JT Enantioselective Intramolecular C–H Insertion Reactions of Donor–Donor Metal Carbenoids. J. Am. Chem. Soc 2014, 136 (43), 15142–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lindgren AEG; Öberg CT; Hillgren JM; Elofsson M Total Synthesis of the Resveratrol Oligomers (±)-Ampelopsin B and (±)-ϵ-Viniferin. Eur. J. Org. Chem 2016, 2016 (3), 426–429. [Google Scholar]
- (4).(a) Snyder SA; Gollner A; Chiriac MI Regioselective Reactions for Programmable Resveratrol Oligomer Synthesis. Nature 2011, 474 (7352), 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jepsen TH; Thomas SB; Lin Y; Stathakis CI; de Miguel I; Snyder SA Harnessing Quinone Methides: Total Synthesis of (±)-Vaticanol A. Angew. Chem. Int. Ed 2014, 53 (26), 6747–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wright NE; Snyder SA 9-Membered Carbocycle Formation: Development of Distinct Friedel–Crafts Cyclizations and Application to a Scalable Total Synthesis of (±)-Caraphenol A. Angew. Chem. Int. Ed 2014, 53 (13), 3409–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Matsuura BS; Keylor MH; Li B; Lin Y; Allison S; Pratt DA; Stephenson CRJ A Scalable Biomimetic Synthesis of Resveratrol Dimers and Systematic Evaluation of Their Antioxidant Activities. Angew. Chem. Int. Ed 2015, 54 (12), 3754–3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Keylor MH; Matsuura BS; Griesser M; Chauvin J-PR; Harding RA; Kirillova MS; Zhu X; Fischer OJ; Pratt DA; Stephenson CRJ Synthesis of Resveratrol Tetramers via a Stereoconvergent Radical Equilibrium. Science 2016, 354 (6317), 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Romero KJ; Galliher MS; Pratt DA; Stephenson CRJ Radicals in Natural Product Synthesis. Chem. Soc. Rev 2018, 47 (21), 7851–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Computations were carried out with B3LYP-D3/6-311G(d,p). This approach significantly overestimates the C-C BDEs for which comparison to experiment is possible (8A and 8B) due to an overestimation of the dispersion interactions between the trimethylsilyl and t-butyl groups with neighboring aryl rings. Omission of these substituents brings the C8-C8’ BDE into good agreement with experiment. Omission of dispersion corrections to any of these calculations leads to massive underestimations in the C-C BDEs (see Supporting Information for additional information). These observations will be expanded upon in a future report.
- (9).Incorporation of a SMD solvent model parameterized for acetone sug-gests a barrier of 21.7 kcal/mol, implying that this reaction cannot compete with C3–C8′ bond homolysis and return to the C8–C8′ dimer.
- (10).(a) Fan G-J; Liu X-D; Qian Y-P; Shang Y-J; Li X-Z; Dai F; Fang J-G; Jin X-L; Zhou B 4,4′-Dihydroxy-Trans-Stilbene, a Resveratrol Analogue, Exhibited Enhanced Antioxidant Activity and Cytotoxicity. Bioorg. Med. Chem 2009, 17 (6), 2360–2365. [DOI] [PubMed] [Google Scholar]; (b) Wang M; Jin Y; Ho C-T Evaluation of Resveratrol Derivatives as Potential Antioxidants and Identification of a Reaction Product of Resveratrol and 2,2-Diphenyl-1-Picryhydrazyl Radical. J. Agric. Food Chem 1999, 47 (10), 3974–3977. [DOI] [PubMed] [Google Scholar]; (c) Takaya Y; Terashima K; Ito J; He Y-H; Tateoka M; Yamaguchi N; Niwa M Biomimic Transformation of Resveratrol. Tetrahedron 2005, 61 (43), 10285–10290. [Google Scholar]; (d) Nicotra S; Cramarossa MR; Mucci A; Pagnoni UM; Riva S; Forti L Biotransformation of Resveratrol: Synthesis of Trans-Dehydrodimers Catalyzed by Laccases from Myceliophtora Thermophyla and from Trametes Pubescens. Tetrahedron 2004, 60 (3), 595–600. [Google Scholar]; (e) Shang Y-J; Qian Y-P; Liu X-D; Dai F; Shang X-L; Jia W-Q; Liu Q; Fang J-G; Zhou B Radical-Scavenging Activity and Mechanism of Resveratrol-Oriented Analogues: Influence of the Solvent, Radical, and Substitution. J. Org. Chem 2009, 74 (14), 5025–5031. [DOI] [PubMed] [Google Scholar]; (f) Song T; Zhou B; Peng G-W; Zhang Q-B; Wu L-Z; Liu Q; Wang Y Aerobic Oxidative Coupling of Resveratrol and Its Analogues by Visible Light Using Mesoporous Graphitic Carbon Nitride (Mpg-C3N4) as a Bioinspired Catalyst. Chem. – Eur. J 2014, 20 (3), 678–682. [DOI] [PubMed] [Google Scholar]; (g) Sako M; Hosokawa H; Ito T; Iinuma M Regioselective Oxidative Coupling of 4-Hydroxystilbenes: Synthesis of Resveratrol and ε-Viniferin (E)-Dehydrodimers. J. Org. Chem 2004, 69 (7), 2598–2600. [DOI] [PubMed] [Google Scholar]; (h) Li C; Lu J; Xu X; Hu R; Pan Y pH-Switched HRP-Catalyzed Dimerization of Resveratrol: A Selective Biomimetic Synthesis. Green Chem. 2012, 14 (12), 3281–3284. [Google Scholar]
- (11).Lee S; Yoon KD; Lee M; Cho Y; Choi G; Jang H; Kim B; Jung D-H; Oh J-G; Kim G-W; Oh J-W; Jeong Y-J; Kwon HJ; Bae SK; Min D-H; Windisch MP; Heo T-H; Lee C Identification of a Resveratrol Tetramer as a Potent Inhibitor of Hepatitis C Virus Helicase. Br. J. Pharmacol 2016, 173 (1), 191–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Takaya Y; Yan K-X; Terashima K; He Y-H; Niwa M Biogenetic Reactions on Stilbenetetramers from Vitaceaeous Plants. Tetrahedron 2002, 58 (45), 9265–9271. [Google Scholar]
- (13).Romero KJ; Galliher MS; Raycroft MAR; Chauvin J-PR; Bosque I; Pratt DA; Stephenson CRJ Electrochemical Dimerization of Phenylpropenoids and the Surprising Antioxidant Activity of the Resultant Quinone Methide Dimers. Angew. Chem. Int. Ed 2018, 57 (52), 17125–17129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Liégault B; Lapointe D; Caron L; Vlassova A; Fagnou K Establishment of Broadly Applicable Reaction Conditions for the Palladium-Catalyzed Direct Arylation of Heteroatom-Containing Aromatic Compounds. J. Org. Chem 2009, 74 (5), 1826–1834. [DOI] [PubMed] [Google Scholar]
- (15).Banwell MG; Flynn BL; Stewart SG Selective Cleavage of Isopropyl Aryl Ethers by Aluminum Trichloride†. J. Org. Chem 1998, 63 (24), 9139–9144. [Google Scholar]
- (16).Zhang J; Zhang J; Kang Y; Shi J; Yao C A Facile and Practical Total Synthetic Route for Ampelopsin F and Permethylated ε-Viniferin. Synlett 2016, 27 (10), 1587–1591. [Google Scholar]
- (17).Parikh JR; Doering W. v. E. Sulfur Trioxide in the Oxidation of Alcohols by Dimethyl Sulfoxide. J. Am. Chem. Soc 1967, 89 (21), 5505–5507. [Google Scholar]
- (18).Wittig G; Schöllkopf U Über Triphenyl-phosphin-methylene als olefinbildende Reagenzien (I. Mitteil. Chem. Ber 1954, 87 (9), 1318–1330. [Google Scholar]
- (19).Radner F; Wistrand L-G A Convenient Method for the Protodesilylation of Aryltrimethylsilanes. Tetrahedron Lett. 1995, 36 (28), 5093–5094. [Google Scholar]
- (20).(a) Corey EJ; Fleet GWJ; Kato M A Biogenetic Approach to the Synthesis of a Prostanoid Precursor. Tetrahedron Lett. 1973, 14 (40), 3963–3966. [Google Scholar]; (b) Clive DLJ; Tao Y; Bo Y; Hu Y-Z; Selvakumar N; Sun S; Daigneault S; Wu Y-J Synthetic Studies on calicheamicinγ1I—synthesis of (−)-Calicheamicinone and Models Representing the Four Sugars And the Aromatic System. Chem. Commun 2000, No. 15, 1341–1350. [Google Scholar]; (c) Kraus GA; Bae J Synthesis of N-(2-Methylpropyl)-2E-Undecene-8,10-Diynamide, a Novel Constituent of Echinacea Angustifolia. Tetrahedron Lett. 2003, 44 (29), 5505–5506. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


