Abstract
Objective:
In tissue engineering, biomaterials create a 3D scaffold for cell-to-cell adhesion, proliferation and tissue formation. Because of their similarity to extracellular matrix and architectural adaptability, nanofibers are of particular interest in tissue engineering. Electrospinning is a well-documented technique for nanofiber production for tissue engineering scaffolds. Here we present literature on the applications of electrospinning in the field of otolaryngology.
Review Methods:
A PubMed database search was performed to isolate articles published about applications of electrospun nanofibers for tissue engineering in otolaryngology. Study design, size, material tested, site of application within the head and neck, and outcomes were obtained for each study.
Results:
Almost all data on electrospinning in otolaryngology was published in the last 6 years (84%), highlighting its novelty. A total of 25 pre-clinical studies were identified: 9 in vitro studies, 5 in vivo animal studies, and 11 combination studies. Sites of application included: tracheal reconstruction (n = 16), tympanic membrane repair (n = 3), cranial nerve regeneration (n = 3), mastoid osteogenesis (n = 1) and ear/nose chondrogenesis (n = 2).
Implications for Practice:
Tissue engineering is a burgeoning field, with recent innovative applications in the field of otolaryngology. Electrospun nanofibers specifically have relevant applications in the field of otolaryngology, due in part to their similarity to native extracellular matrix, with emerging areas of interest being tympanic membrane repair, cranial nerve regeneration and tracheal reconstruction.
Keywords: electrospinning, otolaryngology, tissue engineering, nanofibers, extracellular matrix, head and neck
Introduction
In tissue engineering, biomaterials are used to create synthetic 3-dimensional scaffolds for cell-to-cell adhesion, proliferation and tissue formation. Because of their similarity to extracellular matrix (ECM) and architectural adaptability, nanofibers are of particular interest in tissue engineering.1 Electrospinning has recently emerged as a well-documented technique for nanofiber production for tissue engineered scaffolds.1 It is a highly versatile and robust technique in which fibers can be fabricated with diameters spanning from several nanometers to tens of micrometers. Electrospun fibers can act a functional environment for cells during tissue regeneration.2
The process of electrospinning for nanofiber production is relatively new, introduced in the 1990s.3 Since then, electrospun materials have shown promise in a range of tissue engineering applications.3 Electrospinning is particularly appealing due to its relatively low production costs and simple setup, in addition to the large amount of customizability.2 The ability to manipulate various process parameters, including polymer choice, concentration of polymer, flow rate, needle tip distance and temperature of the solution, can lead to subtle alterations in nanofiber characteristics.3 This allows precise control over scaffold characteristics, such as pore size, fiber diameter, fiber alignment, as well as surface chemistry.4
Tissue engineering is particularly germane to the field of otolaryngology, given the paucity of existing tissue analogues for structures within the head and neck. Due to their architectural adaptability and customizability, electrospun nanofiber scaffolds may be uniquely applied to tissue engineering applications within otolaryngology. Maintaining integral structure and function within the head and neck is imperative; thus the comparability of electrospun nanofibers to the native extracellular matrix is ideal for the creation of tissue engineered scaffolds that promote growth and regeneration of tissues within the head and neck (Figure 1).
Figure 1.
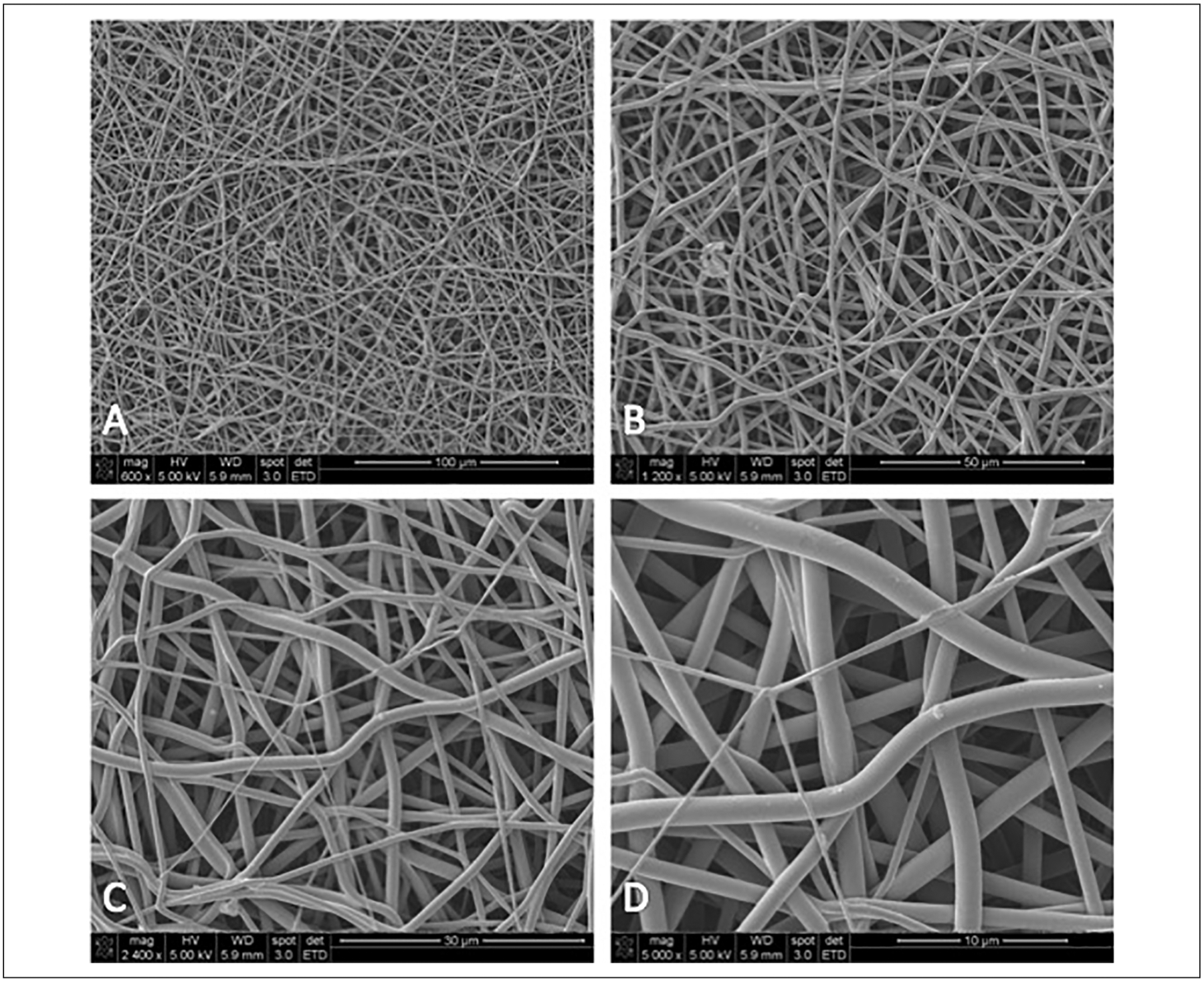
Scanning electron microscope images showing the similarity of electrospun nanofibers to native extracellular matrix. (A) 600× magnification, (B) 1200× magnification, (C) 2400× magnification, (D) 5000× magnification.
Here, we present a synopsis of the published literature regarding applications of tissue engineered electrospun nanofiber scaffolds for tissue replacement and regeneration within the head and neck. To date, a review of the applications of electrospinning for tissue engineering applications within the head and neck has yet to exist. Our ultimate goal is to elucidate nanofiber characteristics and polymer compositions that are most ideal for various applications within otolaryngology.
Review Methods
A comprehensive search of the United States National Library of Medicine (PubMed) and Scopus databases was performed to identify all publications about electrospinning with applications in otolaryngology. Study design, study size, polymer choice, presence of seeded cells or growth factors, site of application within the head and neck and outcomes were recorded for each study.
Search terms “electrospinning,” “nanofibers,” “otolaryngology,” “head and neck and “tissue engineering” were used to isolate pertinent studies. All studies were reviewed and narrowed based on the following inclusion criteria: (1) in vitro studies, in vivo studies or a combination of the 2 testing electrospun nanofibers scaffolds for applications within the head and neck. Exclusion criteria included: (1) studies about electrospinning applications outside the head and neck, (2) studies about nanofibers created from other tissue engineering techniques besides of electrospinning and (3) studies about electrospinning applications other than tissue engineering (ie, drug delivery).
Given the lack of statistically robust studies, we did not factor in meaningful statistical analysis into our inclusion criteria. We included studies assessing the addition of seeded cells or growth factors to the tissue engineered scaffold. We isolated a total of 25 studies about applications of electrospinning for tissue engineering within the head and neck.
Results
A total of 25 studies were identified, with almost all data on electrospinning applications in otolaryngology being published in the last 6 years (84%), highlighting the novelty of this data. All studies were pre-clinical: 9 in vitro studies, 5 in vivo animal studies, and 11 combination studies. Approximately 80% (20 of 25) of studies tested electrospun nanofiber scaffolds with the addition of seeded cells or growth factors. Anatomic sites of application are represented in Figure 2. Table 1 contains a brief synopsis of each study, broken down by anatomical site.
Figure 2.
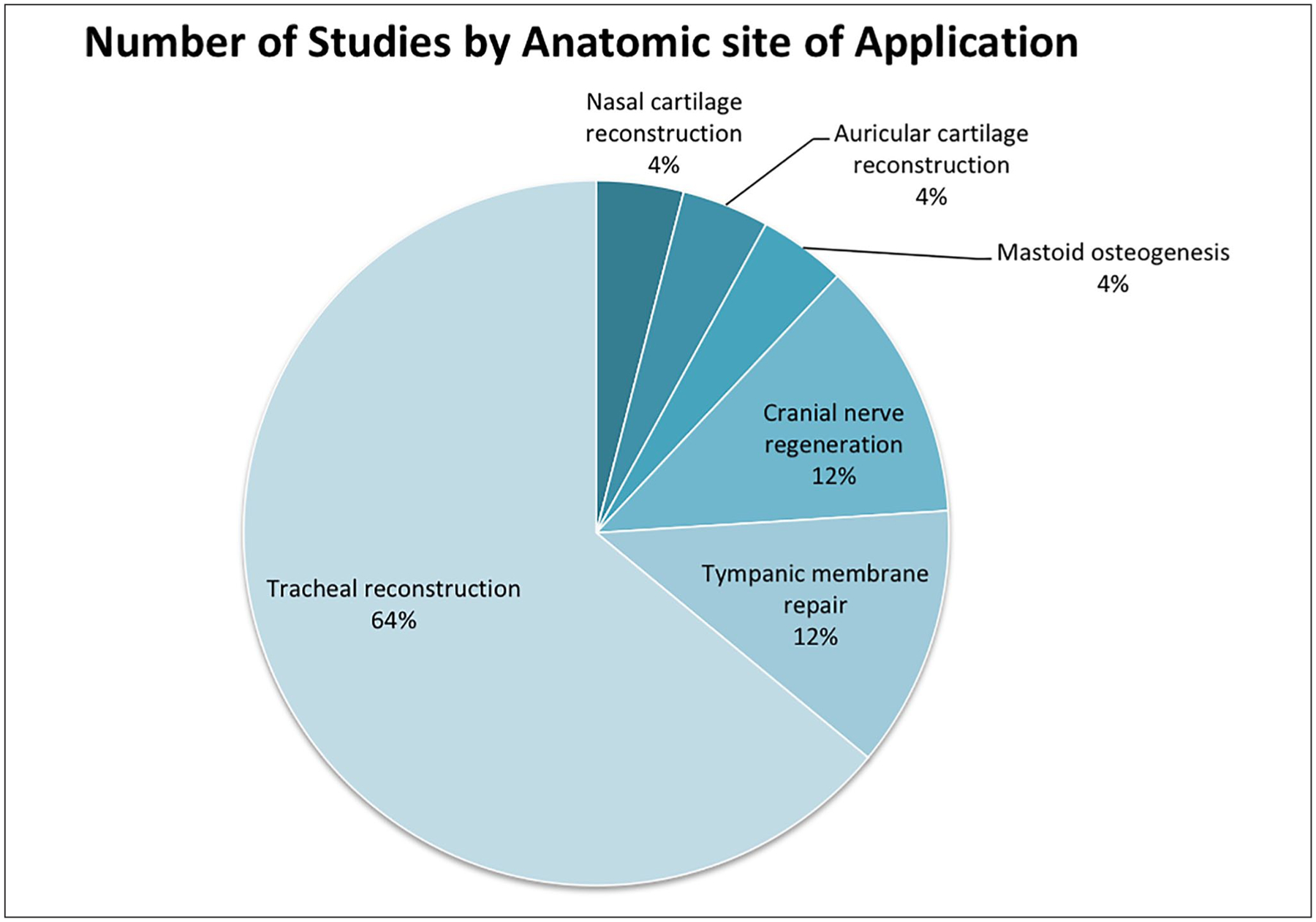
Proportion of studies by anatomic site of application.
Table 1.
Summary Table Detailing Each Study, by Anatomic Site of Application.
| Study Design | Seeded Cells/Growth Factors | |||||
|---|---|---|---|---|---|---|
| Study (Year) | Size (n) | In vivo | In vitro | Nanofiber Material | Results | |
| Tracheal Reconstruction | ||||||
| Dharmadhikari et al5 | 50 | Yes, mice | Yes | PET:PU 20:80 percent weight | BM-MNCs |
|
| Wu et al6 | 55 | Yes, rabbits | Yes | PGS/PCL with pedicled fascia | N/A |
|
| Pepper et al7 | 8 | Yes, sheep | No | PET:PU 20:80 percent weight | BM-MNCs |
|
| Romanova et al8 | N/A | No | Yes | Chitosan-gelatin-PLLA | Airway epithelial cells, tracheal/dermal fibroblasts, MSCs |
|
| Wiet et al9 | 4 | Yes, mice | No | PET:PU 20:80 percent weight | BM-MNCs |
|
| Dharmadhikari et al10 | 25 | Yes, mice | Yes | PET:PU and resorbable PLCLPGA | N/A |
|
| Best et al11 | N/A | No | Yes | PET and PU in ratios of either 8:2 or 2:8 percent weight | BM-MNCs |
|
| Townsend et al12 | 5 | Yes, sheep | No | PCL nanofibers layered with PCL C-rings | N/A |
|
| Pepper et al13 | 7 | Yes, sheep | No | PET:PU 20:80 percent weight | BM-MNCs |
|
| Wu et al14 | 27 | Yes, rats | Yes | PLCL/collagen | Rat tracheal epithelial cells and chondrocytes |
|
| Ghorbani et al15 | 6 | Yes, rabbits | Yes | PCL, collagen coated PCL, PCL blended with collagen | Chondrocytes, adipose-derived MSCs |
|
| Clark et al16 | 5 | Yes, sheep | Yes | PET:PU 70:30percent weight | BM-MNCs |
|
| Mahoney et al17 | N/A | No | Yes | PCL/chitosan | Porcine TBE cells |
|
| Bridge et al4 | N/A | No | Yes | PET (8%, 30% and 10%) | Epithelial, fibroblast and smooth muscle cells |
|
| Jang et al1 | 14 | Yes, guinea pigs | Yes | PCL/collagen | hUCS |
|
| Hinderer et al18 | N/A | No | Yes | PCL/gelatin/dec-orin | hPAECs |
|
| Tympanic Membrane Repair | ||||||
| Seonwoo et al19 | 108 | Yes, rats | Yes | 8% PCL | EGF |
|
| Li et al20 | N/A | No | Yes | Gelatin/genipin | Skin fibroblasts, hUVEC |
|
| Mota et al21 | N/A | No | Yes | PLGA and PEOT/PBT | Human MSCs |
|
| Cranial nerve regeneration | ||||||
| Hackelberg et al22 | 2 | Yes, guinea pigs | Yes | 4:1 blend of PLLA and PCL | NPCs |
|
| Jang et al23 | 16 | Yes, rats | Yes | PCL/collagen | hUCS |
|
| Hu et al24 | 30 | Yes, rats | No | Silk fibroin | N/A |
|
| Osteogenesis | ||||||
| Jang et al25 | 40 | Yes, guinea pigs | Yes | PCL/β-TCP/collagen versus PCL/β-TCP | N/A |
|
| Chondrogenesis | ||||||
| Dahl et al26 | N/A | No | Yes | PLGA | hUCMSCs |
|
| San Marina et al27 | N/A | No | Yes | PLCL, PDO, PHBV-PCL, PH BY, PS | AD-MSC |
|
Abbreviations: AD-MSC, Adipose-derived mesenchymal stem cells; BM-MNCs, Bone marrow-derived mononuclear cells; EGF, epidermal growth factor; hPAECs, human primary airway epithelial cells; hUCS, Human umbilical cord serum; hUVEC, Human umbilical vein endothelial cells; MSC, Mesenchymal stem cells; NPC, Neural precursor cells; PBT, Poly(butylene terephthalate); PCL, Polycaprolactone; PDO, Polydioxanone; PET, Polyethylene terephthalate; PEOT, Poly(ethylene oxide terephthalate); PGA, Polyglycolic acid; PGS, poly glycerol sebacate; PHBV, Poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PLCL, Poly(L-lactide-co-caprolactone); PLGA, Poly(lactic-co-glycolic) acid; PLLA, poly(L-lactic) acid; PS, Polystyrene; PU, Polyurethane; TBE, tracheobronchial epithelial; β-TCP, Beta tri-calcium phosphate.
Tracheal reconstruction
Sixteen studies were isolated pertaining to the application of electrospun nanofibers as tissue engineered tracheal grafts (TETG) for reconstruction of long segment tracheal defects.1,4–18 Although the specifics of scaffold design and fabrication vary for each study, Clarke and colleagues describe a representative process: First a custom mandrel was created from stainless steel with dimensions matching those obtained from native juvenile sheep tracheas.16 Once the desired polymer blend was selected, this solution was electrospun using a custom designed apparatus comprised of 20 gauge blunt tip needles and a high voltage direct current (DC) power supply (Figure 3). The scaffold was modeled using a stainless steel template.16 A medical grade polycarbonate sheet was cut to create C-rings, which were manually embedded into the graft during the electrospinning process.16 Grafts were then washed in deionized water and sterilized.16
Figure 3.
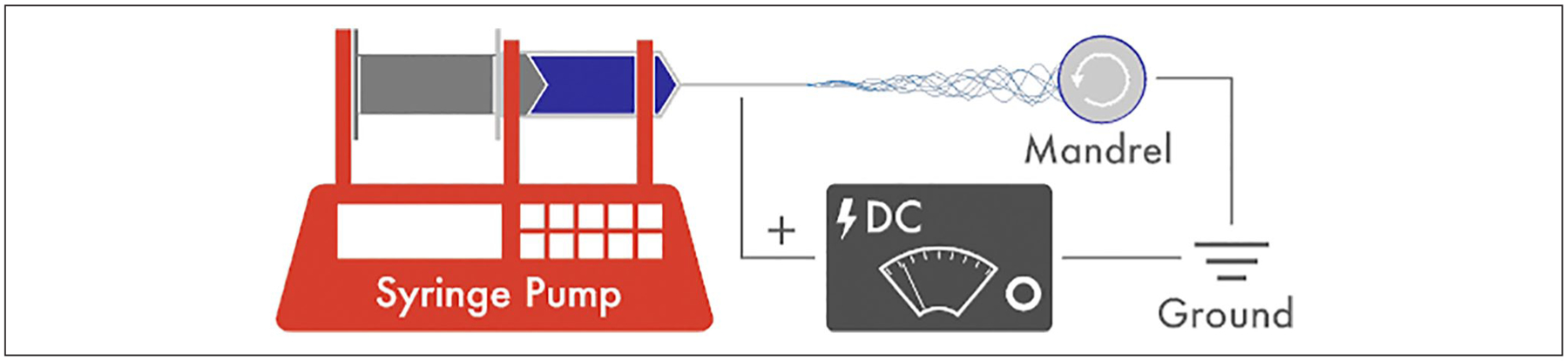
Depiction of the electrospinning process for nanofiber creation. The principle components are an injection pump with a syringe, which is attached to a high voltage power supply. An electric field is created between the tip of the needle and the grounded collector plate or mandrel, which is positioned a fixed distance away from the syringe tip. Once the polymer solution is injected, nanofibers are created as the solution passes through the electric field.
The combination of polymers polyethylene terephthalate (PET) and polyurethane (PU) in various ratios has been explored for use in TETGs.5,7,9–11,13,16 Clark and colleagues tested 70:30 PET:PU grafts seeded with bone marrow-derived mononuclear cells in an ovine model.16 Three centimeter TETGs were surgically implanted into the mid-cervical trachea of juvenile sheep.16 Bronchoscopy was used to evaluate TETG healing at 7 and 14 days after implantation, and at the study endpoint sheep were autop-sied and TETGs explanted.16 No cases of acute TETG failure were noted, and graft stenosis was noted in 2/2 unseeded scaffolds and 1/3 seeded scaffolds.16 Best and colleagues produced electrospun nanofiber scaffolds constructed of PET:PU in mixtures of 20:80 percent by weight and found that these TETGs closely approximate the compliance of native ovine trachea.11 Additionally, grafts comprised of this polymer ratio had superior cell seeding capacity compared to ratios of 80:20 PET:PU.11 Implantation of these scaffolds revealed graft stenosis within several weeks that ultimately required graft stenting.13 Despite significant improvement of respiratory symptoms and overall survival with endoscopic intervention and graft stenting, PET:PU TETGs demonstrated chronic inflammation, foreign body reaction, and poor epithelialization.7 Subsequent studies in a mouse model of tracheal replacement using a representative scaffold has demonstrated that PET:PU is capable of supporting a functional epithelium; however, graft epithelialization occurs in the presence of chronic inflammation.5
Both poly(L-lactic) acid (PLLA) and poly(L-lactide-co-caprolactone) (PLCL) have been preliminarily explored in tracheal reconstruction; in a rat model, pre-vascularized PLCL/collagen grafts demonstrated reduced immunogenicity in addition to tracheal tissue regeneration.8,14 Electrospun polycaprolactone (PCL) scaffolds have also been explored in TETGs.6,12,15,17,18 Townsend et al studied TETGs derived from PCL in an ovine model and showed that these grafts were suturable, airtight and durable.12 However, an overgrowth of fibrous tissue around the site of reconstruction was noted.12 Further, these grafts showed poor tissue integration, likely contributing to the rate of fibrous tissue overgrowth and stenosis.12
In fact, the most frequently reported issues with TETGs in the literature are stenosis and delayed epithelialization.5,7,10–12 Some evidence indicates TETGs seeded with bone marrow-derived mononuclear cells exhibit delayed stenosis and increased epithelial migration.7 Seok Jang et al studied a PCL and collagen nanofiber TETG seeded with human umbilical cord serum and found that it promoted the growth of cartilage and epithelial cells in guinea pigs without a simultaneous increase in inflammation.1 TETGs that are supraphysiologic in compression tests compared to native in vivo models may confer higher rates of graft stenosis; non-resorbable grafts may also contribute to more frequent stenosis (Table 1).10,11
Tympanic membrane repair
We isolated 3 studies pertaining to the application of electrospun nanofibers for tympanic membrane (TM) repair.19–21 A representative model of scaffold production described by Li and colleagues is as follows: The desired polymer solution was loaded into a plastic syringe with copper needle tip, which was connected to a high voltage DC generator (Figure 3).20 An aluminum foil collector plate was placed 10 cm from the tip of the needle.20 Once the web of fibers was created, it was vacuum dried, and a 5 mM glutaraldehyde solution was used to crosslink the fibers, maintaining morphology and preventing dissolution.20
Seonwoo and colleagues created an electrospun 8% PCL nanofibrous scaffold containing epidermal growth factor (EGF) and applied it in a rat model of chronic TM perforation.19 Perforation closure rate was observed weekly for 8 weeks in each of the study groups: random fibers with EGF, radially aligned fibers with EGF and a control group. Radially aligned nanofibers combined with EGF led to the quickest regeneration rates, with TM thickness approximating that of normal TMs.19 Two in vitro studies were also identified, showing promise for gelatin/genipin nanofibrous scaffolds and poly(lactic-co-glycolic) acid and poly(ethylene oxide terephthalate)/poly(butylene terephthalate) nanofibrous scaffolds.20,21 Both of these grafts were seeded with stem cells and showed appropriate mechanical characteristics for TM repair (Table 1).20,21
Cranial Nerve Regeneration
Three studies were isolated assessing the application of electrospun nanofibers for cranial nerve regeneration. Previous studies have shown efficacy of electrospun nanofiber scaffolds for peripheral nerve regeneration.28 Here we identified two studies assessing facial nerve regeneration and 1 study assessing auditory nerve regeneration. Each of the 3 studies had an in vivo component, although the 2 facial nerve studies had more robust sample sizes (n = 16 and n = 30) compared to the auditory nerve study with a sample size of 2.22–24
Hackleberg et al used a PCL-based scaffold comprised of a blend of 4:1 PCL:PLLA (poly (L-acetic) acid) to assess auditory nerve recovery in a sample size of 2 guinea pigs.22 Nanofibers were collected on a rotating disc placed 30 cm away from the tip of the needle, allowing the creation of a hollow PCL tube.22 Both with the addition of neural precursor cells and without, auditory nerves showed partial recovery.22 Hu and colleagues assessed silk fibroin scaffolds, which showed no sign of inflammatory response in the host Sprague Dawley rats with facial nerve regeneration activity comparable to nerve autografts.24 Jang and colleagues assessed various nanofibrous tube-shaped scaffolds and found that those created from the combination of PCL/collagen and human umbilical cord serum most successfully promoted facial nerve regeneration in rats (Table 1).23
Osteogenesis and Chondrogenesis
Applications of electrospinning for osteogenesis and chrondrogenesis have been explored in depth outside the head and neck. Germane to the field of otolaryngology, we identified one study assessing the application of electrospun nanofiber scaffolds for mastoid osteogenesis, and two studies pertaining to nasal and auricular chondrogenesis, respectively.25–27 Jang and colleagues studied a PCL/collagen and beta tri-calcium phosphate (β-TCP) electrospun nanofiber scaffold for mastoid bone regeneration in an in vivo guinea pig model.25 The scaffolds in which type 1 collagen was embedded within layered PCL/β-TCP struts displayed broader cell attachment sites and increased osteogenesis compared to scaffolds without collagen.25 Dahl and colleagues found that human umbilical cord mesenchymal stem cells (hUMSCs) grown on PLGA scaffolds had higher rates of auricular cartilage expression in an in vitro model.26 San Marina and colleagues constructed electrospun scaffolds of 5 different polymer blends, with preliminary findings suggesting polydioxanone (PDO) and poly-3-hydroxybutyrate-co-3-hydroxyvalerate (PHBV), with and without the addition of PCL, showed the greatest amount of matrix deposition when a nasal chondrogenic supplement was applied in an in vitro model (Table 1).27
Implications for Practice
Tissue engineering is a burgeoning field, with recent innovative applications in the field of otolaryngology. Particularly in the past 6 years, electrospinning has been explored as a means of producing nanofiber scaffolds for tissue regeneration within the head and neck. Electrospinning is a highly adaptable method of producing 3-dimensional fibrous scaffolds that can be tailored to have structural similarity to organ or tissue specific extracellular matrixes.4
Electrospun nanofibers are ideal for replicating the basement membrane of structures.1–3 The large specific surface area of electrospun nanofiber scaffolds can also be used to accommodate bioactive molecules, helping to further promote cellular responses.1 In fact, the majority of studies within the scope of this review assessed a uniquely constructed electrospun nanofiber scaffold with the addition of bioactive molecules for tissue regeneration (80%).
Electrospinning is relatively inexpensive, and the basic set up is straightforward compared to other methods of nanofiber and biomaterial manufacturing.3 The primary components include a high voltage power supply, a pump with a syringe attached and a grounded collector plate (Figure 3).1,3 The syringe is loaded with a natural or synthetic polymer solution.3 When voltage is applied at the needle tip, a droplet of solution will then form at the tip of the spinneret, forming a Taylor cone. As the solution passes through the electric field and makes its way to the collector plate, volatile components evaporate quickly. The end result is a scaffold of nonwoven fiber mesh, the geometry of which dictated by the collector and electrospinning setup.1,2 Fiber morphology depends on intrinsic properties of the solution, processing conditions and environmental conditions.2
A distinct advantage of the electrospinning process is the ability to manipulate process parameters.3 Based on these subtle variations, scaffold properties can be customized, making them particularly suited to promote tissue regeneration in challenging anatomical regions within the head and neck. Recent investigation has focused on extending the capabilities of electrospinning to produce scaffolds with more complex geometries and fiber morphologies.1–3 For example, the diameter of the fibers produced is primarily determined by the flow rate (Q), while the strength of the electric field is determined by voltage (V) and distance (D) between the spinneret and collector and is integral in tuning the morphology of the end product.1,2 Polymer concentration can be adjusted to modulate the viscosity of the solution, with increased viscosity resulting in larger fiber diameters and decreased bead formation along the fiber.2
Electrospun nanofiber scaffolds have relevant applications in the field of otolaryngology, due in part to their similarity to native ECM. Notable areas of application include TM repair, tracheal reconstruction and cranial nerve regeneration. The 25 studies presented in this review were all pre-clinical in nature, the majority of which were published within the last 6 years, highlighting the novelty of this data.
Long segment tracheal defects can result from con genital defects, trauma, infection or malignancy.11,16 Once >50% of the tracheal length is compromised due to diseased tissue or absence, it can no longer be reconstructed surgically.11,16 Accordingly, tissue engineered tracheal grafts (TETG) have been explored as an option for reconstruction.11,16 The first implantation of a tissue-engineered trachea was in 2008, with the appeal being that they are readily available, customizable and spare donor site morbidity.12 An ideal tracheal scaffold for the management of long segment tracheal defects is biocompatible, with low complication risk and comprised of a material that seamlessly integrates with the host.10 To date, PET:PU and PCL scaffolds have been the most rigorously studied for regeneration of tracheal defects, though complications of stenosis and delayed epithelialization have posed a challenge to broader application of such scaffolds.5,7,11–13,16 In cases of tracheal graft stenosis in scaffolds comprised of PET:PU nanofibers, Pepper and colleagues identified that tracheal stents were superior to dilation for the maintenance of graft patency, with 100% of dilations and 29% of stent placements requiring urgent follow up bronchoscopy.12
Chronic TM perforations can lead to recurrent otitis media, development of cholesteatoma and conductive hearing loss.19 Conventional treatment is tympanoplasty or myringoplasty; however, the requirement for general anesthesia and specialized surgeons make this a costly and sometimes unattainable option.19 More recent research has explored the role of biomaterials in the creation of tissue engineered scaffolds for TM repair in chronic perforations. To date, Seonwoo and colleagues have published the only combined in vitro and in vivo study assessing electrospun nanofiber scaffolds for TM repair, showing promise for radially aligned electrospun PCL nanofiber scaffolds containing EGF in effective repair of chronic TM perforations.19
Three studies assessed the role of electrospun nanofiber scaffolds in cranial nerve regeneration, where they are used to mimic the architecture of an acellular nerve graft and enhance neurite outgrowth.28 Success with this technique in peripheral nerve regeneration suggests it may be applied to cranial nerve regeneration.28 Grafts comprised of silk fibroin and PLLA (poly (L-acetic) acid) combined with PCL showed no overt signs of an inflammatory response in the host.22,24 PLLA has been explored in primary motor and sensory neurons.28,29 PCL is suggested to be an ideal scaffold given its biocompatibility and slower rate of degradation.23 PCL/collagen nanofibers have been shown to be efficacious in bone regeneration, as well.23,25
Electrospun nanofiber scaffolds show promise for the field of otolaryngology, particularly given the lack of existing analogues for tissue replacement. Its relatively simple and adaptable set up allows this technique to be utilized for a wide variety of applications. Further, numerous studies have utilized materials that are already FDA-approved for biomedical applications, such as polycaprolactone and EGF.19 Nevertheless, data regarding this new technology has several shortcomings, primarily due to the novelty of this technique. First, while existing research shows promise for feasibility of tissue regeneration, long-term data about the reproducibility of such grafts as well as the durability of the regenerated tissue is lacking. Regarding tracheal reconstruction, electrospun nanofiber grafts displayed excess fibrous and inflammatory tissue overgrowth, impacting the longevity of such scaffolds. Despite the ability of electrospinning to create ECM-like architecture, scaffolds may require additional modifications to fully recapitulate the biophysical and biochemical cues needed to represent a true analogue for biologic grafts. Further research assessing specific polymer and fiber coating combinations, as well as the ideal porosity of tracheal grafts, is necessary. Many of these studies are preliminary in nature and, accordingly, have relatively small sample sizes. Larger studies, with both in vitro and in vivo components, are necessary to further understand the physiologic reactions that take place once these grafts are introduced.
Biomaterials and tissue engineering research is a burgeoning field. Electrospinning is a relatively straightforward, well-described process by which fibers of nano- and micrometer size are constructed and can be used in the creation of scaffolds for tissue engineering. The customizability of electrospinning process parameters allows subtle alterations to both the mechanical and bioactive properties of scaffolds constructed.2 Electrospun nanofiber scaffolds are particularly suited for the unique anatomical and functional challenges within the head and neck, owing primarily to their architectural adaptability and customizability. Further preclinical studies of electrospun nanofiber scaffolds for tissue regeneration within the head and neck are warranted. In particular, in vivo models with a focus on methods of reducing host inflammatory response are crucial before these promising substrates can be tested in humans.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
This manuscript was presented as an oral presentation at the AAO-HNSF meeting on September 17, 2019 in New Orleans, LA, USA.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AH: No conflict of interest. No financial disclosures.
SS: No conflict of interest. No financial disclosures.
PM: No conflict of interest. No financial disclosures.
JJ: Affiliated with Nanofiber Solutions, Inc.
TC: No conflict of interest. No financial disclosures.
References
- 1.Jang YS, Jang CH, Cho YB, Kim M, Kim GH. Tracheal regeneration using polycaprolactone/collagen-nanofiber coated with umbilical cord serum after partial resection. Int J Pediatr Otorhinolaryngol. 2014;78(12):2237–2243. [DOI] [PubMed] [Google Scholar]
- 2.Kishan A, Cosgriff-Hernandez E. Recent advancements in electrospinning design for tissue engineering applications: a review. J Biomed Mater Res. 2017;105:2892–2905. [DOI] [PubMed] [Google Scholar]
- 3.Weng L, Xie J. Smart electrospun nanofibers for controlled drug release: recent advances and new perspectives. Curr Pharm Des. 2015;21:1944–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridge J, Aylott J, Brightling C, et al. Adapting the electrospinning process to provide three unique environments for a tri-layered in vitro model of the airway wall. J Vis Exp. 2015; 101:e52986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dharmadhikari S, Liu L, Shontz K, et al. Deconstructing tissue engineered trachea: assessing the role of synthetic scaffolds, segmental replacement and cell seeding on graft performance. Acta Biomater. 2020;102:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu W, Jia S, Chen W, Liu X, Zhang S. Fast degrading elastomer stented fascia remodels into tough and vascularized construct for tracheal regeneration. Mater Sci Eng C. 2019; 101:1–14. [DOI] [PubMed] [Google Scholar]
- 7.Pepper V, Best CA, Buckley K, et al. Factors influencing poor outcomes in synthetic tissue-engineered tracheal replacement. Otolaryngol Head Neck Surg. 2019;161:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romanova O, Tenchurin T, Demina T, et al. Non-woven bilayered biodegradable chitosan-gelatin-polylactide scaffold for bioengineering of tracheal epithelium. Cell Prolif. 2019; 52:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiet M, Dharmadhikarl S, White A, et al. Seeding and implantation of a biosynthetic tissue-engineered graft in mouse model. J Vis Exp. 2019;146:e59173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dharmadhikarl S, Best C, King N, et al. Mouse model of tracheal replacement with electrospun nanofiber scaffolds. Ann Otol Rhinol Laryngol. 2019;128:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Best CA, Pepper VK, Ohst D, et al. Designing a tissue engineered tracheal scaffold for pre-clinical evaluation. Int J Pediatr Otorhinolaryngol. 2018;104:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend J, Ott L, Salash J, et al. Reinforced electrospun polycaprolactone nanofibers for tracheal repair in an in vivo ovine model. TERMIS. 2018;24:1301–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepper V, Onwuka E, Best C, et al. Endoscopic management of tissue-engineered tracheal graft stenosis in an ovine model. Laryngoscope. 2017;127:2219–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu T, Zheng H, Chen J, et al. Application of a bilayer tubular scaffold based on electrospun poly(L-lactide-co-caprolactone)/collagen fibers and yarns for tracheal tissue engineering. J Mater Chem B. 2017;5:139–150. [DOI] [PubMed] [Google Scholar]
- 15.Ghorbani F, Moradi L, Shadmehr MB, Bonakdar S, Droodinia A, Safshekan F. In-vivo characterization of a 3D hybrid scaffold based on PCL/decellularized aorta for tracheal tissue engineering. Mater Sci Eng C. 2017;81:74–83. [DOI] [PubMed] [Google Scholar]
- 16.Clark E, Best C, Onwuka E, et al. Effects of cell seeding on neotissue formation in a tissue engineered trachea. J Pediatr. 2016;51:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahoney C, Conklin D, Waterman J, Sankar J, Bhattarai N. Electrospun nanofibers of poly(ε-caprolactone)/depolymerized chitosan for respiratory tissue engineering applications. J Biomater Sci Polym Ed. 2016;27:611–625. [DOI] [PubMed] [Google Scholar]
- 18.Hinderer S, Schesny M, Bayrak A, et al. Engineering of fibrillar decorin matrices for a tissue-engineered trachea. Biomaterials. 2012;33:5259–5266. [DOI] [PubMed] [Google Scholar]
- 19.Seonwoo H, Shin B, Kyoung-Je J, et al. Epidermal growth factor-releasing radially aligned electrospun nanofibrous patches for the regeneration of chronic tympanic membrane perforations. Adv Healthc Mater. 2018;8(2):e1801160. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zhang W, Huang M, et al. Preparation of gelatin/genipin nanofibrous membrane for tympanic membrane repair. J Biomater Sci Polym Ed. 2018;29:2154–2167. [DOI] [PubMed] [Google Scholar]
- 21.Mota C, Danti S, D’Alessandro D, et al. Multiscale fabrication of biomimetic scaffolds for tympanic membrane tissue engineering. Biofabrication. 2015;7:02005. [DOI] [PubMed] [Google Scholar]
- 22.Hackelberg S, Tuck S, He L, et al. Nanofibrous scaffolds for the guidance of stem cell-derived neurons for auditory nerve regeneration. PLoS One. 2017;12:e0180427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang C, Lee H, Kim M, Kim G. Effect of polycaprolactone/collagen/hUCS microfiber nerve conduit on facial nerve regeneration. Int J Biol Macromol. 2016;93:1575–1582. [DOI] [PubMed] [Google Scholar]
- 24.Hu A, Zuo B, Zhang F, Zhang H, Lan Q. Evaluation of electrospun silk fibroin-based transplants used for facial nerve repair. Otol Neurotol. 2013;34:311–318. [DOI] [PubMed] [Google Scholar]
- 25.Jang C, Cho Y, Yeo M, Kim G. Mastoid obliteration using 3-dimensional composite scaffolds consisting of polycaprolactone/β-tricalcium phosphate/collagen nanofibers: an in vitro and in vivo study. Macromol Biosci. 2013;13:660–668. [DOI] [PubMed] [Google Scholar]
- 26.Dahl J, Caballero M, Pappa A, Madan G, Shockley W, van Aalst J. Analysis of human auricular cartilage to guide tissue-engineered nanofiber-based chondrogenesis: implication for microtia reconstruction. Otolaryngol Head Neck Surg. 2011;145:915–923. [DOI] [PubMed] [Google Scholar]
- 27.San Marina S, Sharma A, Voss S, Janus J, Hamilton G. Assessment of scaffolding properties for chondrogenic differentiation of adipose-derived mesenchymal stem cells in nasal reconstruction. JAMA Facial Plast Surg. 2017;19:108–114. [DOI] [PubMed] [Google Scholar]
- 28.Frost H, Andersson T, Johansson S, et al. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci Rep. 2018;8:16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corey J, Gertz C, Wang B, et al. The design of electrospun PLLA nanofiber scaffolds compatible with serum-free growth of primary motor and sensory neurons. Acta Biomater. 2008;4:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]


