Abstract
Purpose of review.
To discuss the association between the common dominantly inherited genetic trait hereditary alpha-tryptasemia (HαT) and Hymenoptera venom-induced anaphylaxis (HVA).
Recent findings.
Elevated BST has been correlated with more severe systemic anaphylaxis in humans in a number of settings – most notably in HVA. Clonal mast cell disease, in particular systemic mastocytosis, is frequently associated with elevated BST, and is a major risk factor for severe HVA. However, clonal mast cell diseases are believed to be rare, whereas HVA is relatively more common. HαT affects an estimated 3-5% of Western populations and is the common cause for elevated BST in these individuals. An association between HαT and severe HVA, as well as clonal mast cell disease has recently been demonstrated wherein this trait modifies reaction severity in venom allergic individuals. A mechanism underlying this association has been proposed through the identification of naturally occurring heterotetrameric tryptases and characterization of their unique physical attributes.
Summary.
Here we discuss the long-standing association between elevated BST and HVA severity, how HαT fits into this landscape, and review the clinical and mechanistic evidence that supports HαT as a modifier of HVA.
Keywords: HαT, mastocytosis, TPSAB1, insect sting, tetramer
Introduction
Elevated basal serum tryptase (BST) levels have been associated with increased severity of anaphylaxis in a number of settings (1, 2) (3) (4) (5) (6-8). This association has been shown to be preeminent among individuals with Hymenoptera venom-induced anaphylaxis (HVA) (9-14). Clonal mast cell diseases such as systemic mastocytosis (SM), have been demonstrated as causative of both BST elevation and HVA in many of these individuals – indeed this may be the presenting symptom of SM in otherwise healthy individuals (6). However, these disorders are believed to be too rare to account fully for the clinical association of elevated BST and severe HVA (15).
The recently described common cause for elevated BST, hereditary alpha-tryptasemia (HαT), has also been reported to impact HVA – imparting independent risk for severe HVA and accounting for elevated BST in the vast majority of individuals without clonal disease (16) (5). This review will examine the clinical data supporting an association between elevated BST and HVA severity, discuss HαT as a potential modifier of HVA and clonal mast cell disease, and describe a recently discovered mechanism that may partially explain these clinical associations.
Hereditary alpha-tryptasemia (HαT) is a common genetic trait
Mast cells are tissue-resident myeloid-lineage cells that produce a large number of mediators which contribute to allergic inflammatory responses, and are critical to the development of systemic immediate hypersensitivity reactions (17). Clinical symptoms manifest following mast cell activation and release of preformed mediators present in secretory granules through a process called degranulation. The most abundant secretory granule protein by mass is the serine proteinase tryptase (18). Due to this fact, tryptase has been used as a biomarker of mast cell activation (19) (20) (21) (22). However, under normal physiological conditions, pro-tryptases are constitutively secreted as monomers from mast cells into the blood stream. These are measured as total serum tryptase in clinical assays of healthy individuals. The median tryptase level in the serum of such individuals with wild-type tryptase genotypes is approximately 5ng/mL. Whereas tryptase protomers are enzymatically inactive, and currently have no known biological function, mature tetrameric tryptases result from step-wise proteolytic processing that involves removal of the pre- and pro-peptide fragments prior to their assembly into catalytically active, heparin-stabilized, molecules that are stored in mast cell secretory granules and only released upon mast cell activation. This occurs most commonly via cross-linking of specific IgE bound to the high affinity IgE receptor FcεRI by cognate antigen on the surface of tissue mast cells (23). Such a degranulation event leads to an acute rise in serum tryptase levels, and can be measured clinically to confirm the diagnosis of anaphylaxis; an increase of 20% + 2ng/mL over basal levels is currently considered diagnostic of mast cell activation (24).
Baseline serum tryptase (BST) levels have been examined and a level above the upper limit of the normal range, 11.4 ng/mL, is present in approximately 5–7% of the populations in which this has been studied (15) (25). Historically, the reason for this was not understood. However, hereditary alpha-tryptasemia (HαT) – a genetic trait caused by inherited structural genetic variation of the tryptase locus – has been demonstrated to account for the vast majority of individuals with BST > 8ng/mL, affecting an estimated 5.5% of these populations. The elevated BST levels observed in HαT are caused by increased germline copies of the gene TPSAB1 that encode for alpha-tryptase (16). Current reports demonstrate HαT to be transmitted with near complete penetrance based upon consistently elevated BST levels in carriers. Thus far these gene replications have only been reported for α-tryptase-encoding loci, with as many as four extra copies of TPSAB1 being present (26). A gene–dosage effect on BST levels in patient with HαT has been reported as well, with duplications yielding average BST levels of 15 +/− 5 ng/mL, triplications 24 +/− 6 ng/mL, and the identified quintuplication 37 +/− 14 ng/mL (27). However, expressivity of associated phenotypes is quite variable with some individuals reporting few if any overt symptoms (27). Of the phenotypes reported in the initial studies in which individuals were recruited for suspected HαT, some symptoms have been validated in at least one unselected cohort; these include irritable bowel syndrome-like symptoms, flushing/pruritus, retained primary dentition, and systemic venom reactions. Other symptoms that have been reported in association with HαT but that have not been validated in other cohorts include chronic gastroesophageal reflux, arthralgia, body pain and headaches, sleep disruption, systemic immediate hypersensitivity reaction, congenital skeletal abnormality, and joint hypermobility (4) (Table 1).
Table 1.
Clinical features reported in association with hereditary alpha-tryptasemia (HαT).
| Manifestation | Reported Prevalence* | Association Supported in an Unselected Cohort‡ |
|---|---|---|
| Basal serum tryptase >8ng/mL | 100% | Yes |
| Chronic gastroesophageal reflux symptoms | 56-77% | No |
| Arthralgia | 44-45% | No |
| Body pain/Headache | 33-47% | No |
| Flushing/Pruritus | 32-55% | Yes |
| Irritable bowel syndrome (Rome III) | 28-49% | Yes |
| Sleep disruption | 22-39% | No |
| Systemic immediate hypersensitivity reaction | 21-28% | No |
| Retained primary dentition | 20-33% | Yes |
| Systemic venom reaction | 14-22% | Yes |
| Congenital skeletal abnormality | 11-26% | No |
| Joint Hypermobility | 0-28% | No |
| Positive Tilt-table test | 0-11% | No |
Elevated basal serum tryptase (BST) and anaphylaxis severity
Whereas the association between HαT and elevated BST is now well established, there are currently very few other known causes for elevated BST. Among Mendelian disorders, GATA2 haploinsufficiency has been associated with elevated BST in some affected individuals. This rare disorder manifests with multiple protean manifestations such as lymphedema, bronchoalveolar proteinosis, and immunodeficiency (28). However, in these individuals, elevated BST may actually herald clonal myeloid disease due to the tendency of these patients to develop neoplasms of that lineage (29) (30, 31). A single case report also exists of a patient suffering from Gaucher’s disease, due to heterozygous loss-of-function in GBA encoding Glucocerebrosidase, with elevated BST (32). However, this may have been partially a result of type I hypersensitivity to ongoing enzymatic replacement. Finally, the acquired clonal disorder systemic mastocytosis – frequently resulting from the acquired gain-of-function missense variant p.D816V in KIT leading to clonal expansion of mast cell and increased reactivity – can be associated with elevated BST. Other clonal myeloid neoplasms have also been associated with elevated BST (33).
Despite the relatively few causes for elevated BST, there are a number of studies that have described a positive association between elevated BST of unknown cause(s) and both prevalence and severity of anaphylaxis(1, 2) (3-6) (7) (8). Clonal mast cell disorders account for some of this association as they are associated with both idiopathic and antigen-induced anaphylaxis. In adults with systemic mastocytosis, the lifetime prevalence of systemic anaphylaxis is estimated to approach 50% (34) (35), a prevalence much higher than the general population. However, several studies have demonstrated this association among individuals without clonal mast cell disease. In one cohort study of food allergic children a BST of 14.5 ng/mL was reported to have a 90% positive-predictive value for moderate-severe anaphylaxis . Two additional studies demonstrated that 12–17% of individuals presenting with idiopathic anaphylaxis had elevated BST in the absence of detectable clonal mast cell disease in bone marrow (36) (37).
Hymenoptera venom-induced anaphylaxis (HVA) severity positively correlates with BST levels
Type I IgE-mediated immediate hypersensitivity to Hymenoptera species – which include bees, yellow jackets, wasps, and hornets – is one of the most common causes of anaphylaxis in adults in the United States and is frequently associated with severe anaphylaxis resulting in hypotensive syncope (38) (34). HVA results in significant morbidity and impairment in quality of life for those affected, with individuals experiencing anxiety, engaging in avoidance behavior, and restricting outdoor physical activities (39) (40). The early and accurate diagnosis of venom allergy is vital as patients with venom allergy are candidates for venom immunotherapy, a treatment which can dramatically reduce the risk of recurrent severe reactions (41).
The grading scale for clinical anaphylaxis severity has evolved over time. The seminal grading system was developed by Ring and Messmer in 1977 (42) and modified by a Scandinavian guideline added a fifth severity category (43). A simpler alternative was developed in 2004 but was limited in the context of a perioperative setting as ‘severe’ did not distinguish between patients who have relatively mild or life-threatening reactions (44). In 2006, an attempt to standardize diagnostic criteria for anaphylaxis was published by The National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network (NIAID/FAAN). An international consensus was reached on definition and management of acute hypersensitivity (45) with the advantage of facilitating diagnosis of anaphylaxis (IgE or non-IgE mediated) at the time of reaction. However, there still remained disagreement as to what constitutes a grade 1 reaction. Currently, the majority of physicians use the Mueller grading system of anaphylaxis (grade 1: urticaria or erythema, itching, malaise or anxiety; grade 2: edema, tightness in the chest, wheezing, abdominal pain, nausea and vomiting, and dizziness; grade 3: dyspnea, dysarthria, hoarseness, weakness, confusion, and a feeling of impending doom; grade 4: loss of consciousness, incontinence of urine or feces, or cyanosis) (46).
Because HVA results from immediate hypersensitivity, with virtually all affected individuals demonstrating sensitization to Hymenoptera species via skin testing or serum venom-specific IgE (47) modifying diseases or traits were at least partially over-looked for many years. However, many studies have now shown a correlation between elevated BST and more severe – principally grade IV – HVA (13) (48) (2) (Table 2). Because of this correlation serum tryptase measurements have been proposed as a method for stratifying risk among Hymenoptera-allergic patients. Patients classified as low risk have tryptase values of <4 ng/mL (35.5% [n=50/141]), intermediate-risk ranging from ≥4 to 7.5 ng/mL (62.5% [n=107/172]), and high risk had tryptase values >7.5 ng/mL (88.5% [n=46/52]) (11). In these studies, it had been suggested that the patients with very high BST (>13.8 ng/ml), might be affected by a mast cell activation disorder.
Table 2.
Major studies examining the relationship between elevated BST and HVA.
| Study Population | Major Finding | Reference |
|---|---|---|
| 15, 298 atopic | Patients with elevated BST have a higher risk of severe anaphylaxis, with 24% reporting HVA | Fellinger et al, 2014 |
| 962 venom allergic | In patients with venom allergy, BST concentrations are associated with the risk for severe anaphylactic reactions | Rueff et al, 2009 |
| 548 venom allergic 82 SM 47 IA |
HαT accounts for >80% of individuals with elevated BST and severe HVA and IA who do not have a CMD, is significantly more prevalent in CMDs, and having concomitant HαT with a CMD is associated with greater anaphylaxis risk | Lyons et al, 2020 |
| 365 venom allergic 214 ACS |
Tryptase levels contribute to the severity of HVA and ACS, two completely different clinical conditions | Farioli et al, 2019 |
| 500 venom allergic | In addition to BST and age, risk factors for severe anaphylaxsis include an absence of urticaria or angioedema and a short sting to reaction interval | Chapsa et al, 2020 |
| 480 venom allergic | Indicators for severe systemic sting reactions include latency time, the absence of skin symptoms, age, and elevated BST | Fehr et al, 2019 |
| 379 venom allergic | Anaphylaxis with hypotension in HVA without urticaria/angioedema/typical skin lesions, regardless of BST level, may be a factor for identifying CMDs | Bonadonna et al, 2015 |
| 274 venom allergic | Elderly patients with increased serum tryptase levels are at increased risk for life-threatening anaphylactic reactions | Guenova et al, 2010 |
| 259 venom allergic | Increased BST is a risk factor for severe systemic allergic reactions to Hymenoptera stings | Haeberli et al, 2003 |
| 109 venom allergic | BST levels were elevated in 11% of venom allergic patients and correlated with sting reaction severity and age | Kucharewicz et al, 2007 |
| 10 venom allergic | HVA in patients with urticaria pigmentosa most often are IgE-mediated but can occur in a non-IgE manner | Fricker et al, 1997 |
ACS – acute coronary syndrome; CMDs – clonal mast cell disorders; SM – systemic mastocytosis; IA – idiopathic anaphylaxis.
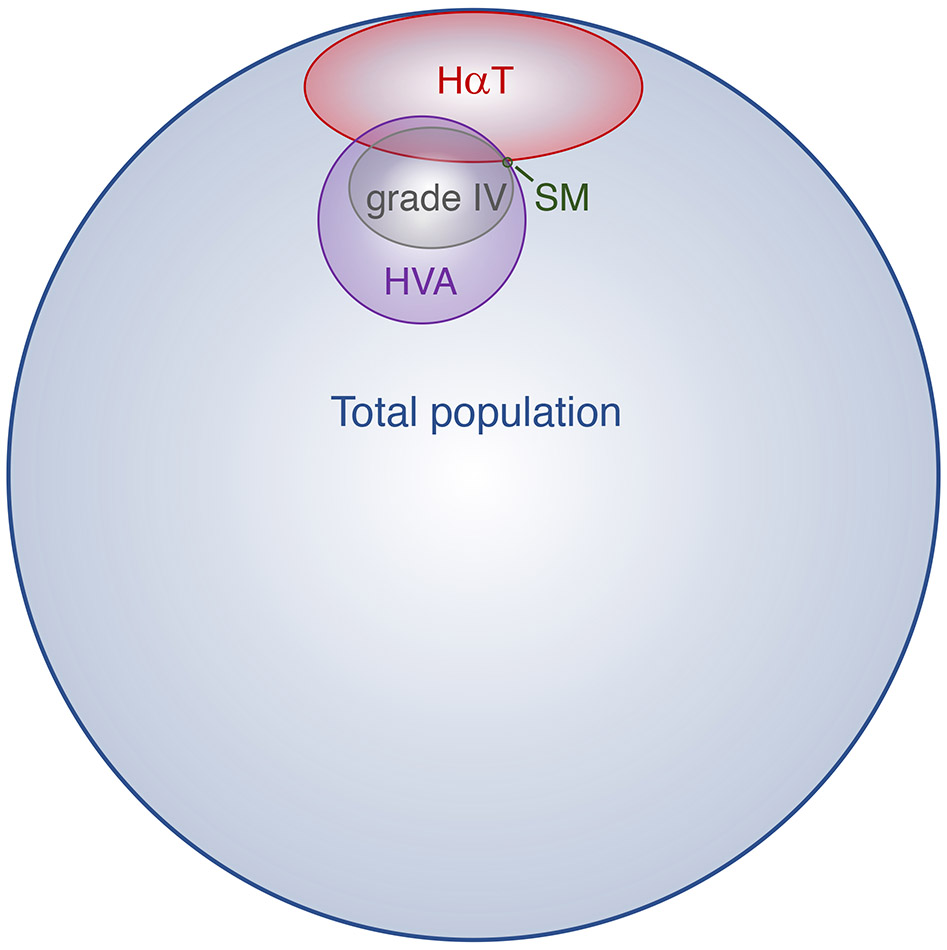
HVA among adults with cutaneous or systemic mastocytosis was initially described in case reports and small series (49) (50) (51) (52) (53) (54). Two larger studies subsequently demonstrated a higher incidence of HVA in adults with mastocytosis than in the general population, whereas in children with mastocytosis who frequently do not have systemic involvement, no such association was observed (55) (7). While this association in adults contributes to the risk for severe HVA among venom allergic individuals with elevated BST, it appears insufficient to account for it entirely. In one study of 15,298 individuals evaluated in a general allergy clinical in Austria, 900 (5.9%) presented with increased BST – 24% of those having elevated BST reporting HVA (15). Were clonal mast cell disease to fully account for this association, the prevalence would be ~1.4% in this cohort (N=216/15,298) – approximately 100 times more common than estimated in the general population (56) (Fig. 1).
Figure 1.
Scale Venn diagram depicting the measured prevalence of hereditary alpha tryptasemia (HαT), the estimated prevalence of systemic mastocytosis (SM), Hymenoptera venom-induced anaphylaxis (HVA), grade IV (Mueller scale) HVA reactions, and their respective overlap with one another.
HαT as a modifier of HVA
Although it has been well-established that elevated BST is associated with more severe HVA only recently was HαT identified as the common cause for elevated BST and a heritable modifier of anaphylaxis severity in humans. Moreover, there may be an additive or synergistic effect of HαT and clonal mast cell disease on anaphylaxis severity (5). In a small cohort analysis of ostensibly healthy volunteers enrolled in the ClinSeq® study, individuals that screened positive for HαT were estimated to be 11 times more likely (OR 11.4, range 1.4–94.0) to suffer from systemic venom reactions compared to individuals without HαT (16). Based on this preliminary study, the prevalence of HαT in two large cohorts of HVA patients from Italy and Slovenia has subsequently been examined (5). In the Italian cohort of individuals with grade IV HVA (N=282) all individuals had been extensively screened for clonal mast cell disease. While only a fraction (16.7%) of the entire cohort was available for genetic testing, the prevalence of HαT was found to be as least ≥8.5% in the entire cohort – accounting for 21/26 (81%) of those tested with elevated BST and no evidence of clonal disease. Moreover, 14.3% (N=3/21) of those screened with ISM and HVA were found to have concomitant HαT. In the Slovenian cohort which had not been universally screened for clonal mast cell disease, but included individuals with grades I-IV anaphylaxis, HαT was found to be significantly more prevalent in individuals with grade IV anaphylaxis compared to the other grades (21/229 vs. 12/278, P<0.05) (Fig. 1). Furthermore, all individuals identified with clonal mast cell disease and HαT (N=5) presented with grade IV HVA. Importantly, the prevalence among all individuals with HVA in the Slovenian cohort was not greater than expected for the general population (6.5%, N=33/507) indicating that HαT is unlikely to affect allergic sensitization to Hymenoptera species, but rather modify reactivity among allergic individuals. The authors also found that HαT was more prevalent in both severe idiopathic anaphylaxis (17%) and SM (12.2%), relative to controls. Furthermore, amongst patients with SM, concomitant HαT was associated with an increased risk for systemic anaphylaxis (P=0.007) including HVA, suggesting that HαT may potentially modify immediate hypersensitivity symptoms more generally (5).
Alpha-tryptase dependent mechanisms that may potentiate anaphylaxis severity
As previously mentioned, tryptase protomers are enzymatically inactive and undergo a step-wise proteolytic processing that results in heparin-stabilized mature tryptases that assemble into tetrameric complexes (23). Both α- and β-tryptases are expressed and secreted by human mast cells, but α-tryptase tetramers lack enzymatic activity, whereas β-tryptase tetramers have trypsin-like serine proteinase activity (57). Recently, the natural formation of α/β-tryptase heterotetramers has been discovered with tetramers existing in a 2:2 stoichiometry, exhibiting enhanced stability and expanded proteolytic activity (57). In contrast to homotetramers, α/β-tryptase heterotetramers have been shown as both uniquely able to contribute to vibration-induced mast cell degranulation via cleavage of the mechanosensing adhesion GPCR, Adhesion G protein-coupled receptor E2 (or EMR2 encoded for by ADGRE2). EMR2 is expressed by dendritic cells, monocytes and macrophages, and all types of granulocytes (58). A missense gain-of-function variant in ADGRE2 is associated with a very severe form of familial vibratory urticaria (59) and a common clinical finding of patients with HαT is cutaneous flushing, pruritus, and in some cases hives, with vibration often being reported as a trigger (16). It has also been shown that, when adhered to dermatan sulfate, cultured mast cells can be uniquely activated by a vibratory stimulus, when α/β-tryptase heterotetramers are present (57).
In contrast to β-tryptase homotetramers, and inactive α-tryptase tetramers, heterotetrameric α/β-tryptases have also been shown as uniquely able to cleave and activate protease activated receptor 2 (PAR2) in Jurkat cells (57). PAR2 is encoded by the F2RL1 gene and is involved in modulations of inflammatory responses, metabolism (60), obesity (61), and detects proteolytic enzymes generated during infection (62). It is a 7-transmembrane receptor, activated by trypsin, leading to proteolytic cleavage of its extracellular domain, resulting in a new amino terminus that functions as a tethered ligand and activates the receptor. PAR2 has been implicated in paracellular permeability in the gut (63), and it has recently been shown that α/β-tryptase heterotetramers selectively promote human endothelial cell permeability in a PAR2-dependent manner (5). Together, these findings of the unique activities of heterotetrameric tryptases, and their ability to augment mast cell activation and target cell response to mediators respectively, provide a potential mechanism whereby α-tryptase over-expression as observed in HαT may modify HVA. Moreover, in a humanized mouse model of systemic IgE-mediated anaphylaxis, inhibition of tryptase enzymatic activity was shown to reduce anaphylaxis severity (64). Thus, tryptase inhibition may prove to be a useful therapeutic strategy to limit anaphylaxis severity in selected individuals in the future.
Conclusions
HαT is an autosomal dominant genetic trait that is the common cause for elevated BST in Western populations. While a major factor contributing to the established association between elevated BST and severe HVA is clonal mast cell disease, HαT appears to also contribute to this clinical finding. Furthermore, when both HαT and clonal mast cell disease are present, the risk for severe HVA and anaphylaxis appears to be even greater. While mechanistic studies are ongoing to understand these clinical observations, the recent identification of α/β-tryptase heterotetramers and their unique in vitro properties provides potential insight into how α-tryptase over-expression might modify mast cell reactivity.
Key Points.
Elevated BST is most commonly caused by HαT in Western populations.
HαT is the first identified common heritable genetic modifier of HVA in humans.
Having concomitant clonal mast cell disease and HαT is associated with greater likelihood of severe HVA.
α/β-Tryptase heterotetramers have unique functional activities that may potentiate immediate hypersensitivity reactions.
Acknowledgments
Financial support and sponsorship
This research was supported by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Conflicts of interest
The authors have no potential conflicts of interest to disclose.
References
- 1.Guenova E, Volz T, Eichner M, Hoetzenecker W, Caroli U, Griesinger G, et al. Basal serum tryptase as risk assessment for severe Hymenoptera sting reactions in elderly. Allergy. 2010;65(7):919–23. [DOI] [PubMed] [Google Scholar]
- 2.Rueff F, Przybilla B, Bilo MB, Muller U, Scheipl F, Aberer W, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol. 2009;124(5):1047–54. [DOI] [PubMed] [Google Scholar]
- 3.Castells MC, Hornick JL, Akin C. Anaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both? J Allergy Clin Immunol Pract. 2015;3(3):350–5. [DOI] [PubMed] [Google Scholar]
- 4.Khoury P, Lyons JJ. Mast cell activation in the context of elevated basal serum tryptase: genetics and presentations. Curr Allergy Asthma Rep. 2019;19(12):55. [DOI] [PubMed] [Google Scholar]
- **5.Lyons JJ, Chovanec J, O'Connell MP, Liu Y, Selb J, Zanotti R, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;In press.Demonstrated for the first time HαT is associated with increased risk of severe HVA and clonal and non-clonal mast cell disorders, potentially resulting from unique activities of α/β-tryptase heterotetramers.
- 6.Bonadonna P, Zanotti R, Muller U. Mastocytosis and insect venom allergy. Curr Opin Allergy Clin Immunol. 2010;10(4):347–53. [DOI] [PubMed] [Google Scholar]
- 7.Brockow K, Jofer C, Behrendt H, Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63(2):226–32. [DOI] [PubMed] [Google Scholar]
- 8.Gulen T, Hagglund H, Dahlen B, Nilsson G. High prevalence of anaphylaxis in patients with systemic mastocytosis - a single-centre experience. Clin Exp Allergy. 2014;44(1):121–9. [DOI] [PubMed] [Google Scholar]
- 9.Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S, Dal Fior D, et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J Allergy Clin Immunol. 2009;123(3):680–6. [DOI] [PubMed] [Google Scholar]
- 10.Chapsa M, Roensch H, Langner M, Beissert S, Bauer A. Predictors of severe anaphylaxis in Hymenoptera venom allergy: The importance of absence of urticaria and angioedema. Ann Allergy Asthma Immunol. 2020;125(1):72–7. [DOI] [PubMed] [Google Scholar]
- 11.Farioli L, Losappio LM, Schroeder JW, Preziosi D, Scibilia J, Caron L, et al. Basal Tryptase Levels Can Predict Clinical Severity in Hymenoptera Venom Anaphylaxis and Ischemic Cardiovascular Disorders. J Investig Allergol Clin Immunol. 2019;29(2):162–4. [DOI] [PubMed] [Google Scholar]
- 12.Fehr D, Micaletto S, Moehr T, Schmid-Grendelmeier P. Risk factors for severe systemic sting reactions in wasp (Vespula spp.) and honeybee (Apis mellifera) venom allergic patients. Clin Transl Allergy. 2019;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeberli G, Bronnimann M, Hunziker T, Muller U. Elevated basal serum tryptase and hymenoptera venom allergy: relation to severity of sting reactions and to safety and efficacy of venom immunotherapy. Clin Exp Allergy. 2003;33(9):1216–20. [DOI] [PubMed] [Google Scholar]
- 14.Fricker M, Helbling A, Schwartz L, Muller U. Hymenoptera sting anaphylaxis and urticaria pigmentosa: clinical findings and results of venom immunotherapy in ten patients. J Allergy Clin Immunol. 1997;100(1):11–5. [DOI] [PubMed] [Google Scholar]
- 15.Fellinger C, Hemmer W, Wohrl S, Sesztak-Greinecker G, Jarisch R, Wantke F. Clinical characteristics and risk profile of patients with elevated baseline serum tryptase. Allergol Immunopathol (Madr). 2014;42(6):544–52. [DOI] [PubMed] [Google Scholar]
- 16.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48(12):1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman P, Garvey LH. Mast Cells and Anaphylaxis. Curr Allergy Asthma Rep. 2016;16(3):20. [DOI] [PubMed] [Google Scholar]
- 18.Robyn J, Metcalfe DD. Systemic mastocytosis. Adv Immunol. 2006;89:169–243. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, McRae BJ, Cho K, Cook R, Fraki JE, Johnson DA, et al. Mammalian tissue trypsin-like enzymes. Comparative reactivities of human skin tryptase, human lung tryptase, and bovine trypsin with peptide 4-nitroanilide and thioester substrates. J Biol Chem. 1983;258(22):13552–7. [PubMed] [Google Scholar]
- 20.Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci U S A. 1990;87(10):3811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kido H, Fukusen N, Katunuma N. Chymotrypsin- and trypsin-type serine proteases in rat mast cells: properties and functions. Arch Biochem Biophys. 1985;239(2):436–43. [DOI] [PubMed] [Google Scholar]
- 22.Harvima IT, Schechter NM, Harvima RJ, Fraki JE. Human skin tryptase: purification, partial characterization and comparison with human lung tryptase. Biochim Biophys Acta. 1988;957(1):71–80. [DOI] [PubMed] [Google Scholar]
- 23.Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol. 2006;117(6):1411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valent P, Bonadonna P, Hartmann K, Broesby-Olsen S, Brockow K, Butterfield JH, et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int Arch Allergy Immunol. 2019;180(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Quintela A, Vizcaino L, Gude F, Rey J, Meijide L, Fernandez-Merino C, et al. Factors influencing serum total tryptase concentrations in a general adult population. Clin Chem Lab Med. 2010;48(5):701–6. [DOI] [PubMed] [Google Scholar]
- 26.Sabato V, Chovanec J, Faber M, Milner JD, Ebo D, Lyons JJ. First Identification of an Inherited TPSAB1 Quintuplication in a Patient with Clonal Mast Cell Disease. J Clin Immunol. 2018;38(4):457–9. [DOI] [PubMed] [Google Scholar]
- 27.Lyons JJ. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical Features. Immunol Allergy Clin North Am. 2018;38(3):483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A, Sowerwine K, Liu Y, Lawrence MG, Chovanec J, Hsu AP, et al. GATA-2-deficient mast cells limit IgE-mediated immediate hypersensitivity reactions in human subjects. J Allergy Clin Immunol. 2019;144(2):613–7 e14. [DOI] [PubMed] [Google Scholar]
- 29.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118(10):2656–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schussler E, Yang A, Lyons JJ, Milner JD, Wang J. Persistent tryptase elevation in a patient with Gaucher disease. J Allergy Clin Immunol Pract. 2018;6(2):697–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperr WR, El-Samahi A, Kundi M, Girschikofsky M, Winkler S, Lutz D, et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: a novel diagnostic approach and screen marker in clinical haematology. Eur J Clin Invest. 2009;39(10):914–23. [DOI] [PubMed] [Google Scholar]
- 34.Wood RA, Camargo CA Jr., Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–7. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008;101(3):139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahiner UM, Yavuz ST, Buyuktiryaki B, Cavkaytar O, Yilmaz EA, Tuncer A, et al. Serum basal tryptase may be a good marker for predicting the risk of anaphylaxis in children with food allergy. Allergy. 2014;69(2):265–8. [DOI] [PubMed] [Google Scholar]
- 37.Akin C, Scott LM, Kocabas CN, Kushnir-Sukhov N, Brittain E, Noel P, et al. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with "idiopathic" anaphylaxis. Blood. 2007;110(7):2331–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carter MC, Desai A, Komarow HD, Bai Y, Clayton ST, Clark AS, et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J Allergy Clin Immunol. 2018;141(1):180–8 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäfer T Epidemiology of insect venom allergy. Allergo. 2016;18:353–8(2009). [Google Scholar]
- 40.Oude Elberink JN, De Monchy JG, Van Der Heide S, Guyatt GH, Dubois AE. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol. 2002;110(1):174–82. [DOI] [PubMed] [Google Scholar]
- 41.Koschel D Impaired quality of life in patients with insect venom allergy. Allergo Journal International 2017;26:88–92. [Google Scholar]
- 42.Franken HH, Dubois AE, Minkema HJ, van der Heide S, de Monchy JG. Lack of reproducibility of a single negative sting challenge response in the assessment of anaphylactic risk in patients with suspected yellow jacket hypersensitivity. J Allergy Clin Immunol. 1994;93(2):431–6. [DOI] [PubMed] [Google Scholar]
- 43.Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1(8009):466–9. [DOI] [PubMed] [Google Scholar]
- 44.Kroigaard M, Garvey LH, Gillberg L, Johansson SG, Mosbech H, Florvaag E, et al. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51(6):655–70. [DOI] [PubMed] [Google Scholar]
- 45.Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–6. [DOI] [PubMed] [Google Scholar]
- 46.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr., Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–80. [DOI] [PubMed] [Google Scholar]
- 47.UR M Clinical presentation and pathogenesis. In: Mueller UR, editor. Insect sting allergy. . Stuttgart: Gustav Fischer; 1990.
- 48.Golden DBK, Carter MC. Insect Sting Anaphylaxis-Or Mastocytosis-Or Something Else? J Allergy Clin Immunol Pract. 2019;7(4):1117–23. [DOI] [PubMed] [Google Scholar]
- 49.Ludolph-Hauser D, Rueff F, Fries C, Schopf P, Przybilla B. Constitutively raised serum concentrations of mast-cell tryptase and severe anaphylactic reactions to Hymenoptera stings. Lancet. 2001;357(9253):361–2. [DOI] [PubMed] [Google Scholar]
- 50.Oude Elberink JN, de Monchy JG, Kors JW, van Doormaal JJ, Dubois AE. Fatal anaphylaxis after a yellow jacket sting, despite venom immunotherapy, in two patients with mastocytosis. J Allergy Clin Immunol. 1997;99(1 Pt 1):153–4. [DOI] [PubMed] [Google Scholar]
- 51.Florian S, Krauth MT, Simonitsch-Klupp I, Sperr WR, Fritsche-Polanz R, Sonneck K, et al. Indolent systemic mastocytosis with elevated serum tryptase, absence of skin lesions, and recurrent severe anaphylactoid episodes. Int Arch Allergy Immunol. 2005;136(3):273–80. [DOI] [PubMed] [Google Scholar]
- 52.Muller UR, Horat W, Wuthrich B, Conroy M, Reisman RE. Anaphylaxis after Hymenoptera stings in three patients with urticaria pigmentosa. J Allergy Clin Immunol. 1983;72(6):685–9. [DOI] [PubMed] [Google Scholar]
- 53.Kors JW, van Doormaal JJ, de Monchy JG. Anaphylactoid shock following Hymenoptera sting as a presenting symptom of systemic mastocytosis. J Intern Med. 1993;233(3):255–8. [DOI] [PubMed] [Google Scholar]
- 54.Biedermann T, Rueff F, Sander CA, Przybilla B. Mastocytosis associated with severe wasp sting anaphylaxis detected by elevated serum mast cell tryptase levels. Br J Dermatol. 1999;141(6):1110–2. [DOI] [PubMed] [Google Scholar]
- 55.Pumphrey RS. Fatal anaphylaxis in the UK, 1992-2001. Novartis Found Symp. 2004;257:116–28; discussion 28-32, 57-60, 276-85. [PubMed] [Google Scholar]
- 56.Gonzalez de Olano D, de la Hoz Caballer B, Nunez Lopez R, Sanchez Munoz L, Cuevas Agustin M, Dieguez MC, et al. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA). Clin Exp Allergy. 2007;37(10):1547–55. [DOI] [PubMed] [Google Scholar]
- 57.Brockow K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol Allergy Clin North Am. 2014;34(2):283–95. [DOI] [PubMed] [Google Scholar]
- *58.Le QT, Lyons JJ, Naranjo AN, Olivera A, Lazarus RA, Metcalfe DD, et al. Impact of naturally forming human alpha/beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J Exp Med. 2019;216(10):2348–61.Identify and characterize for the first time naturally forming α/β-tryptase heterotetramers and implicate clinical their relavence in cutaneous mast cell symptoms.
- 59.Lin HH, Stacey M, Hamann J, Gordon S, McKnight AJ. Human EMR2, a novel EGF-TM7 molecule on chromosome 19p13.1, is closely related to CD97. Genomics. 2000;67(2):188–200. [DOI] [PubMed] [Google Scholar]
- 60.Boyden SE, Desai A, Cruse G, Young ML, Bolan HC, Scott LM, et al. Vibratory Urticaria Associated with a Missense Variant in ADGRE2. N Engl J Med. 2016;374(7):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Badeanlou L, Furlan-Freguia C, Yang G, Ruf W, Samad F. Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat Med. 2011;17(11):1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim J, Iyer A, Liu L, Suen JY, Lohman RJ, Seow V, et al. Diet-induced obesity, adipose inflammation, and metabolic dysfunction correlating with PAR2 expression are attenuated by PAR2 antagonism. FASEB J. 2013;27(12):4757–67. [DOI] [PubMed] [Google Scholar]
- 63.Lee SE, Jeong SK, Lee SH. Protease and protease-activated receptor-2 signaling in the pathogenesis of atopic dermatitis. Yonsei Med J. 2010;51(6):808–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacob C, Yang PC, Darmoul D, Amadesi S, Saito T, Cottrell GS, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280(36):31936–48. [DOI] [PubMed] [Google Scholar]
- **65.Maun HR, Jackman JK, Choy DF, Loyet KM, Staton TL, Jia G, et al. An Allosteric Anti-tryptase Antibody for the Treatment of Mast Cell-Mediated Severe Asthma. Cell. 2019;179(2):417–31 e19.Identifies mature tryptase as a critical mediator of immediate hypersensitivity and successfully target it to reduce anaphylaxis severity in a humanized mouse model.
- 66.Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O'Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133(5):1471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]