Abstract
Mathematical models describing SARS-CoV-2 dynamics and the corresponding immune responses in patients with COVID-19 can be critical to evaluate possible clinical outcomes of antiviral treatments. In this work, based on the concept of virus spreadability in the host, antiviral effectiveness thresholds are determined to establish whether or not a treatment will be able to clear the infection. In addition, the virus dynamic in the host – including the time-to-peak and the final monotonically decreasing behavior – is characterized as a function of the time to treatment initiation. Simulation results, based on nine patient data, show the potential clinical benefits of a treatment classification according to patient critical parameters. This study is aimed at paving the way for the different antivirals being developed to tackle SARS-CoV-2.
Keywords: SARS-CoV-2, In-host model, Dynamic characterization, Antiviral effectiveness
1. Introduction
With more than 140 million cases confirmed so far (April 2021) in 220 countries (WHO, 2020a, WHO, 2020b), coronavirus disease COVID-19, caused by SARS-CoV-2 virus, continues spreading around the globe without neither effective treatment available to date. In fact, the estimated worldwide case-fatality rate (CFR) for COVID-19 is about 2%–3%, which is almost 15 times higher than the CFR of seasonal influenza (0.0962%) (Cao et al., 2020, Khan et al., 2020).
Currently, several clinical studies about the potentiality of repurposed antiviral agents (i.e., Remdesivir, Favipiravir, Lopinavir/Ritonavir, Ribavirin, etc.) to ameliorate the viral spreading in the host are underway (Mitjà & Clotet, 2020). The clinical observations suggest that prophylaxis with approved doses could prevent SARS-CoV-2 infection and reduce viral shedding (Gao et al., 2020, Yao et al., 2020), but these reports suffer from a number of limitations. For instance, there is no certainty that the undefined benefits are not outweighed by the acute toxicity of the specific antiviral agents. To overcome this impasse, randomized clinical trials with adequate potency should be performed (Kalil, 2020). On the other hand, despite some reported dosing recommendation to treat COVID-19 (Colson et al., 2020, Commission et al., 2020), the efficacy of these proposed therapies cannot be guaranteed due to a paucity of data regarding the optimal dose of the antivirals (Sanders, Monogue, Jodlowski, & Cutrell, 2020). In a race to find medical therapies to improve outcomes in patients with COVID-19 further studies are needed to elucidate the benefits of adapting antiviral agents.
An important strategy to help to find the optimal dose of drugs is the pharmacological modeling based on in-vitro drug testing. This approach could suggest whenever the prophylaxis with an appropriate doses of antiviral agents could prevent SARS-CoV-2 infection or control the replication cycle of the virus (Tett, Cutler, Day, & Brown, 1989). In this regard, recent studies have concentrated on the potential of a quantitative comprehension of COVID-19 dynamics (Giordano et al., 2020, Gonçalves et al., 2020, Hernandez-Vargas and Velasco-Hernandez, 2020, Kim et al., 2020). Within-host mathematical models demonstrated useful insights about SARS-CoV-2 infection dynamics and its interactions with the immune system. More important, such results invoke the potential utility of assessing targets for drug development.
The target cell-limited model, widely used to represent several diseases such as Influenza (Baccam et al., 2006, Hernandez-Vargas et al., 2014), Ebola (Nguyen, Binder, Boianelli, Meyer-Hermann, & Hernandez-Vargas, 2015), HIV (Perelson and Ribeiro, 2013, Rong and Perelson, 2009), Hepatitis viruses (Graw & Perelson, 2016), among others, has been linked to adjust the viral kinetics in infected patients with COVID-19 reported by Wölfel et al. (2020). While a complete analysis of the main dynamic characteristic for the target cell model was developed to evaluate SARS-CoV-2 infection (Abuin, Anderson, Ferramosca, Hernandez-Vargas, & Gonzalez, 2020), there is no extensive analysis of the effects of the existent pharmacological therapy for this model, with some remarkable exceptions (Gonçalves et al., 2020, Ke et al., 2020, Kim et al., 2020, Wang and Lu et al., 2020).
The main contribution of this work – that can be seen as an extension of Abuin et al. (2020) – is to provide a formal mathematical analysis of the SARS-CoV-2 dynamics under the effect of antiviral treatments, which may help to understand how to schedule the different therapies in function of the host parameters. A quantitative classification stating whether or not an antiviral will be effective – in terms of its capability to died out the virus in a reasonable period of time – is made, showing that their effectiveness could vary significantly between subjects.
After the introduction given in Section 1 the article is organized as follows. Section 2 proposes a general three-states “in-host” model to represent COVID-19 infection dynamics under the effect of antivirals drugs and formalizes the concepts of virus spreadability and critical values for the uninfected cells. Then, in Section 3, the antiviral effectiveness is analyzed, as the ability to avoid the virus to spread in the host. Both, the inhibition effect on the replication rate of the virus and the inhibition effect on the infection rate of susceptible healthy cells are studied. In Section 5, the results of Section 3 are extensively demonstrated by simulating different treatments scenarios for nine patients from the literature. Finally, Section 6 gives the discussion of the work, while several mathematical formalism – necessary to support the main results of article – are given in Appendix.
2. Within-host COVID-19 infection model under antiviral effect
Mathematical models of within-host virus dynamic helps to improve the understanding of the interactions that govern infections and permits the human intervention to moderate their effects (Hernandez-Vargas, 2019). Basic models usually include the cells that the pathogen infects, the pathogen particles, and their life cycle (Ciupe & Heffernan, 2017) and, opposite to one can expect, they vary little in its structure from one infectious disease to another.
The goal of this section is to formally consider the effect of antivirals into an acute infection model (Abuin et al., 2020, Baccam et al., 2006, Hernandez-Vargas and Velasco-Hernandez, 2020) to obtain a controlled system, i.e. a system with certain control actions – given by the administered antiviral drugs – that allows one to (even partially) modified the whole system dynamic according to some control objectives. Antivirals have the potential to inhibit the virus replication, reducing the advance of the infection over the target cells population of the infected host (i.e.: epithelial cells in the respiratory tract for H1N1 (Baccam et al., 2006), cells with ACE2-TMPRSS2 complex for SARS-CoV-2, mainly nasal and bronchial epithelial cells due to their high expression of ACE2 (Gupta et al., 2020)). Several antivirals are being tested for COVID-19 treatment, with different results concerning both, their inhibition effect on the virus replication and their toxicity. Based on their potential therapeutic targets over the SARS-CoV-2 lifecycle, they can be classified in those which prevent virus entry into the host cell (i.e.: potential antiviral drugs – Umifenovir, Camostat/Nafamostat-) and those which inhibit virus replication into the infected cells, mainly blocking viral protease and/or viral RNA replicase (i.e.: potential antiviral drugs – Lopinavir, Remdesivir, Favipiravir, Penciclovir) (Dobrovolny, 2020, Jomah et al., 2020, Liu et al., 2020, Sanders et al., 2020, Wang and Cao et al., 2020). Consequently, the antiviral effect can be modeled as a reduction of the virus infectivity in the presence of inhibitors (by reducing the infection rate) and/or as a reduction in the replication of infectious virions (by reducing the virus replication rate) (Kim et al., 2020, Mitjà and Clotet, 2020). In any case, the effectiveness of a treatment is limited and depends on the patient parameters (which in turn depends on his/her clinical state). Next, the following closed-loop mathematical model – based on the open-loop ones presented in Abuin et al., 2020, Hernandez-Vargas and Velasco-Hernandez, 2020 – is considered:
where (cell), (cell) and (copies/mL) represent the uninfected cells, the infected cells, and the virus concentration; parameter ((copies/mL)−1 day−1) is the infection rate; (day−1) is the death rates of , ((copies/mL) day−1 cells−1) is the viral replication rate, and (day−1) is the viral clearance rate. The effects of immune responses are not explicitly described in this model, but they are implicitly included in the death rate of infected cells () and the clearance rate of virus () (Baccam et al., 2006). The antiviral inhibition effects and - one affecting the infection rate and the other affecting the replication rate - are assumed to jump from to the values and – representing the full inhibition treatment effects – at the treatment time (i.e., the pharmacokinetic of the antivirals is assumed to have time constants significantly smaller than the ones of the open-loop system, and, so, it is neglected):
| (2.2) |
Remark 1
The assumption that the antiviral treatment is maintained indefinitely for relies on the further assumption that once the virus load is under a given low threshold value (for instance ) it cannot grow up again, independently of value of . However, it is worth to remark that according to the stability analysis made in Abuin et al. (2020), if the antiviral treatment is interrupted before reaches the threshold value (defined next, in Definition 3), a virus rebound may occur. To model better viral eradication, it can be either assumed that the amount of virus is zero after certain threshold, or to change the ODE model for the stochastic version which can abstract viral eradication.
System (2.1) is positive, which means that , and , for all . We denote the state vector, and
| (2.3) |
the state constraints set.
The initial conditions of (2.1), which represent a healthy steady state before the infection, are assumed to be , , and , for . Then, at time , a small quantity of virions enters the host body and, so, a discontinuity occurs in . Indeed, jumps from to a small positive value at (formally, has a discontinuity of the first kind at , i.e., while ). This way, for the time after the infection, the virus may spread or being cleared depending on its infection effectiveness. To properly determine what such a spread means, the following (mathematical) definition is given (Abuin et al., 2020).
Definition 1 Spreadability of the Virus in the Host —
Consider system (2.1), constrained by the positive set , with , , and , for . Consider also that at time , jumps from to a small positive value . Then, it is said that the virus spreads (in some degree) in the host for if for some . If the virus does not spread in the host, it is said that it is cleared for .
Definition 1 states that the virus spreads in the host, for , if increases at some time , and so, given that (as it is shown in Abuin et al. (2020)), it reaches at least one local maximum at some positive time. On the other hand, the virus is cleared for if is strictly decreasing for all , which means that has neither local minima nor local maxima at any (as stated in (Abuin et al., 2020), has at most one minimum and one maximum, provided and for all ). An infectious disease can be related to the virus peak and/or the period of permanence of the virus in the host (Nguyen et al., 2015), and both effects are related to an increase of the virus load at some time after the infection. This is the reason why the spreadability is defined based on the virus positive derivative. A second reason supporting Definition 1 is its use in the determination of antiviral effectiveness (in Section 3): antivirals producing values of and able to clear the virus can be considered effective.
2.1. Reproduction number and critical value for the uninfected cells
To formally establish conditions under which the virus does or does not spread for , some basic concepts need to be defined. Let consider, at this stage, that and for all (i.e., untreated case).
Definition 2
The within-host basic reproduction number is defined as the number of infected cells (or virus particles) that are produced by one infected cell (or virus particle), over a course of its life-span. Its mathematical expression is given by . Particularly, for , this number describes the number of virus particles produced by a virus particle when a small amount of virus, , is introduced into a healthy stationary population of uninfected target cells, , .
A second number, which is closely related to the first one, is the critical value for the uninfected/susceptible cells such that the virus starts to decrease.
Definition 3
The critical number of susceptible cells , , is defined as , which for fixed system parameters , , and , is a constant.
Note that if and only if , for every . The basic reproduction number and the critical number completely describe dynamic (2.1). In Abuin et al. (2020), a full characterization of the equilibrium sets, their stability and the full behavior of each variable are made. There, it is stated that the (trivial and asymptotically stable) equilibrium set can be divided into two sets, determined by , being the first the smallest asymptotically stable equilibrium set and, the second, unstable (Theorem 3.3, in Abuin et al. (2020)). Furthermore, provided that virus spread for , it is also stated (Theorem 4.1) that there exist positive times , , and , such that , where and are the times at which reaches a local minimum and a local maximum, respectively, is the time at which reaches a local maximum, and is the time at which reaches . In addition, for all , which completely characterizes the system behavior.
3. Antiviral treatment effectiveness
The time to initiate treatment is assumed to be between the minimum and maximum time of , i.e., (although some simulations will be performed for , for the sake of completeness). The full antiviral effect and are limited by the inhibitory potential of the drug (expressed in terms of EC50, or drug concentration for inhibiting 50% of antigen particles) and its cytotoxic effect (expressed in terms of IC50, or drug concentration which causes death to 50% of susceptible cells) (Vergnaud and Rosca, 2005, Wang and Cao et al., 2020).
As the antiviral treatment reduces the system parameter in some amount, it will quantitatively modify the virus behavior. Particularly, the virus peak time will be modified from (untreated patient case) to (treated patient case). However, given that the treatment is initiated when the virus is increasing (i.e., between and ), then the new peak will occur at the same time or after the treatment time, i.e., . This way, even when the virus peak will always be smaller with a treatment (smaller peaks are obtained for smaller values of and ), the time at which this peak takes place can be smaller or greater than the one without any treatment. This effect, usually disregarded in many studies concerning the effectiveness of antivirals, could be critical to define whether or not a given antiviral is able to prevent a severe disease. Indeed, in some cases, antivirals significantly delay the virus peaks, largely increasing the time of permanence of the virus in the host. In order to qualitatively assess antiviral effectiveness according to the time of the virus peak, the following classification is made:
Definition 4 Antiviral Treatment Effectiveness —
Consider system (2.1), constrained by the positive set , such that the virus spreads in the host from time , which implies that: , and . Consider also that, at time , with , an antiviral treatment is initiated such that and/or jump from to and/or , respectively (as stated in (2.2)). Then, the treatment is said to be effective if the virus peaks at a time , being the latter the virus peak time for the untreated viral dynamics (i.e., when ). Otherwise, if , it is said that the treatment is ineffective.
Definition 4 is closely related to the capacity of the antiviral drug to clear the infection (or, the same, cutting off its spread) in such a way that it could: (a) decline the viral grow at the treatment time, and, so, the virus clearance starts when the therapy is initiated, or (b) hasten the virus peak, and, so, even though the virus clearance is not started at treatment time, it begins prior to the untreated case. Note also that Definition 4 accounts for three typical antiviral effect metrics: the area under the virus curve (AUC) and the duration of infection (DI) (Hadjichrysanthou, Cauët, Lawrence, Vegvari, De Wolf, & Anderson, 2016), in a direct way, and the difference of viral loads at the time-to-peak (Gonçalves et al., 2020), in an indirect way.
3.1. Antiviral effectiveness characterization
In this subsection it is shown that the effectiveness of antivirals depends on weather and/or are greater or smaller than a specified threshold, which is a function of the parameters and the time of the treatment initiation. In order to characterize such thresholds, the within-host basic reproduction number at treatment time is computed as follows:
| (3.1) |
where . The critical values of and are the ones that make , i.e.:
| (3.2) |
| (3.3) |
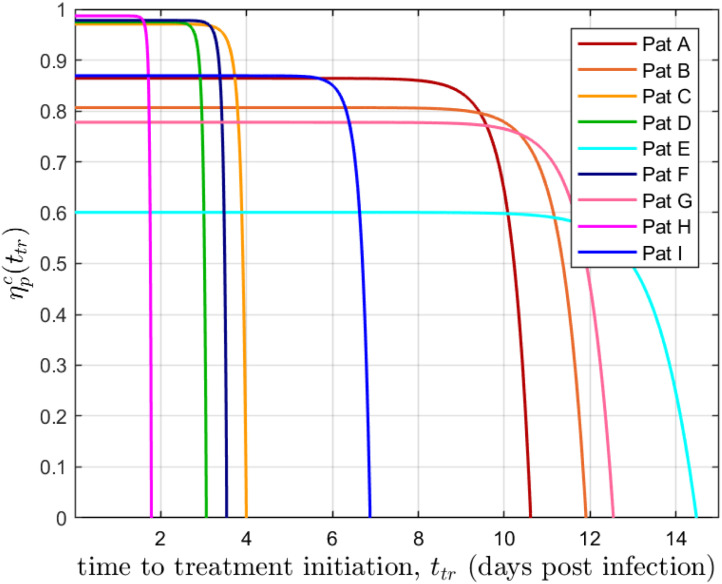
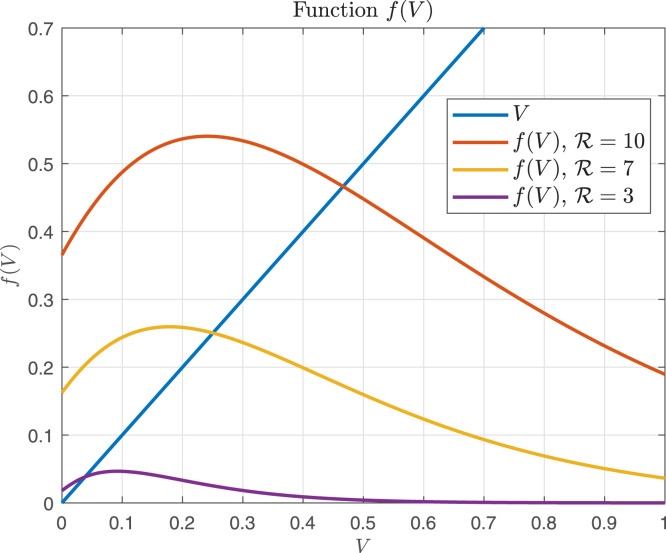
From Eqs. (3.2), (3.3) it can be inferred that and are increasing functions of . Fig. 1 shows the time behavior of for the nine COVID-19 patients identified in Section 4. Note that for and for (). Similar results concerning the critical drug efficacy with respect to the availability of target cells at the treatment time were reached in Dobrovolny, Gieschke, Davies, Jumbe, and Beauchemin (2011), for acute models, although the authors focused the analysis on treatment starting at the beginning of the infection.
Fig. 1.
vs corresponding to the nine patients simulated in Section 4. . The nine COVID-19 patients (A, B, C, D, E, F, G, H and I) identified from data sets in (Wölfel et al. (2020)) are represented here as ”pat”.
The following theorem determines the effectiveness of antivirals in terms of and , by considering them separately. In the next subsection, the combined effect will be studied.
Theorem 3.1
Consider system (2.1) , constrained by the positive set . Consider that the virus spreads in the host for , which implies that: , and . Consider also that, at time , with (when the virus is increasing), an antiviral treatment is initiated such that or jumps from to or , respectively (as stated in (2.2) ). Then,
- i.
if the inhibition effect is such that (or ) then the maximum of occurs at , which is smaller than by hypothesis. In this case the antiviral treatment is effective.
- ii.
for two antiviral treatment, 1 and 2, with inhibition effects such that (or two inhibition effects such that ), there exist a time (large enough) such that , for all , being and the virus corresponding to treatments and , respectively. In this case both treatments are effective, and treatment 2 is more efficient than treatment 1.
- iii.
if the inhibition effect is such that (or ), then the virus reaches a maximum at a time . Furthermore, there exists a time – denoted as early treatment time – such that for . In this case the antiviral treatment is ineffective.
- iv.
for two antiviral treatment, 1 and 2, with inhibition effects such that with (or for two inhibition effects such that ), it is , being and the virus maximum time corresponding to treatments and , respectively. In this case both treatments are ineffective, but treatment 1 is more efficient than treatment 2, which is a rather counter-intuitive fact.
Sketch of Proof
For the sake of simplicity and clarity the proof is based on the approximation of system (2.1) described in Appendix A.2. In such a case, system (2.1) is approximated by , and . Only antiviral treatments affecting parameter are tackled , since the results follows from conditions on and parameters and affect in the same way.
- i.
Since (by hypothesis) and , then by Theorem 4.1 in Abuin et al. (2020), for . Given that , then by (3.1) jumps to a value smaller than one at , i.e., and (where ). Given that is strictly decreasing, for . From equation , it follows that for all . So, given that is assumed that is increasing when the treatment is initiated, its maximum occurs at .
- ii.
Let us consider that for all , and are the virus and the susceptible cells for treatment , i.e., the treatment under the inhibition effect , and with and . By hypothesis , then and for all and . Since is a continuous function for , there is a positive time such that for all . From equation it follows that for all (note that is not defined on ). As it is shown in Section 5.1, for real data patients, is large enough such that .
- iii.
Since (by hypothesis) and , then by Theorem 4.1 in Abuin et al. (2020), for . Given that , then by (3.1) jumps, at , from to the smaller value , which is still greater than one; i.e., . From the fact that , it follows that , and given that is decreasing for , there exists a time such that , in which case, it is
(3.4) This means that reaches a maximum at time . Now, we need to prove that there exists a time such that for it is . According to Lemma 1 in Appendix A.1, there exists a time , smaller than , such that for a treatment time , is a decreasing function of , for . This implies that smaller values of (greater values of ) correspond to larger times at which , , reaches , i.e., smaller values of , correspond to larger values of . So, from the fact that (being the value of at if no treatment is applied), it follows that , for . As it is shown in Sections 5.1, 5.2 , for real data patients, (the maximal) is close to .
(3.5) - iv.
Since , by hypothesis, then , being and . Therefore, by following the same steps of the previous item, it follows that , being and the maximum times of the virus corresponding to and , respectively. □
Remark 2
Item (iii) of Theorem 3.1 establishes just the existence of such that for treatments starting at , the new virus peak time is larger than the one corresponding to the untreated case, i.e., . However, it should be noted that for parameters coming from real patient data, (the maximal) is indeed close to . This means that the time period where treatments can be ineffective is in most of the cases similar to , i.e., comparable with the time period where the virus is growing. Figures in Section 5 confirm this fact.
A main consequence of item (iii) of Theorem 3.1 is that early treatments, if no effective, could be more detrimental than late ones. Another critical point to be remarked is that when an early treatment is not strong enough to avoid the virus spreadability right after , the highest the antiviral effectiveness (or ) is, the longer time the virus remains in the host, since the maximum time is delayed, as established in item (iv). As a result, antivirals could be detrimental as treated patients would need to be isolated for larger periods of time than untreated ones.
Note that even when virus peak time can be delayed for some treatments, the virus peak will be always smaller than the one without any treatment. Furthermore, the fraction of dead cells at the end of the infection () will be always greater if no antiviral is administrated. Indeed, and, according to Abuin et al. (2020), , with , and being the Lambert function. Therefore:
| (3.6) |
Consequently, if treatment is started early, such that , then the fraction of dead cells can be approximated as , which is equal to 0 if . Note that, by definition, for , so . On the other hand, if then is monotonically increasing with (see Figure 1, in Abuin et al. (2020)). Hence, the fraction of dead cells at the end of infection is a monotonically increasing function of , for . Moreover, as the treatment time is delayed, grows, being a monotonically increasing function of , even for .
3.2. Antiviral effectiveness considering the combined effect on and
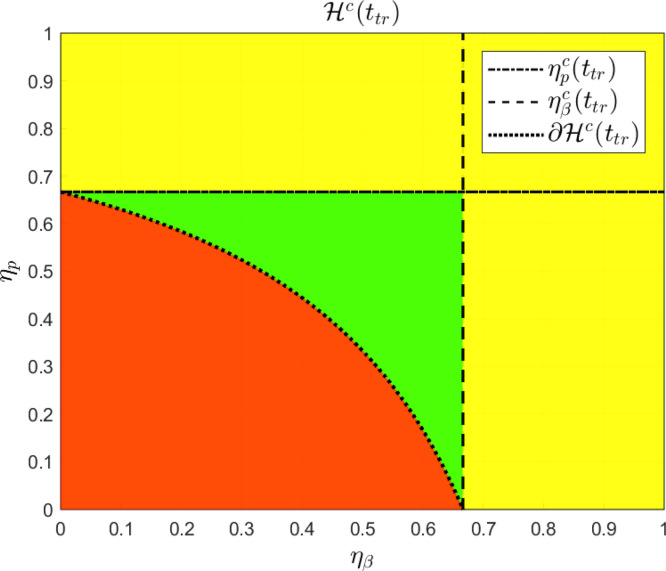
Theorem 3.1 describes the behavior of the virus under the effect of an inhibitor reducing the infection rate or the replication rate . If both effects ( and ) are simultaneously included in the model, it can be computed a region – in the space – for which condition is fulfilled. This way, instead of independent critical values and corresponding to each parameter, there is an entire set of critical combinations that makes a treatment effective. This set depends on treatment time and is placed on the boundary of the effective set
| (3.7) |
Clearly, every pair fulfills condition and, reciprocally, every pair fulfills condition . Fig. 2 shows a plot of set in the plane , corresponding to parameters , , and , with .
Fig. 2.
is given by the yellow and the green regions, considering the following system parameters: , , and , with . The critical boundary of , denoted by , represents the critical pairs of and such that . (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
As it can be seen, for every inhibition effect pair (i.e. a point inside the red region in Fig. 2) the antiviral treatment is ineffective. On the other hand, as it is shown in Theorem 3.1, for every pair such that or (the yellow region in Fig. 2) the antiviral treatment is effective. Finally, for pairs in the region inside with and (which does not fit conditions in Theorem 3.1 and it is represented by the green region in Fig. 2) the antiviral treatment is still effective.
4. Within-host modeling of COVID-19
In this section, the parameters of model (2.1) are estimated using viral load data of nine patients, labeled as A, B, C, D, E, F, G, H and I, reported by Wölfel et al. (2020). We follow a similar procedure as in Hernandez-Vargas and Velasco-Hernandez (2020). Since the viral load is measured in Log10 scales, the model fitting was fulfilled by minimizing the root mean squared of logarithmic error (RMSLE), denoted as:
| (4.1) |
where is the number of measurements, the model predictive output, and the experimental measurement. Since the minimization of (4.1) implies a nonlinear optimization problem, with highly dependence on initial conditions, the Differential Evolution (DE) algorithm (Hernandez-Vargas, 2019, Storn and Price, 1995) was employed as a global optimization algorithm, which has shown to be robust to initial guesses of parameters (Torres-Cerna, Alanis, Poblete-Castro, Bermejo-Jambrina, & Hernandez-Vargas, 2016).
Even though it is still debatable which compartments SARS-CoV-2 can infect, there is a common agreement that the viral shedding take places mainly in the respiratory epithelial cells (due to the high expression of ACE2) with direct viral toxicity of the infected cells (Gupta et al., 2020). Therefore, following previous works of mathematical modeling for influenza infection in humans, the value of was taken as about (cells) for all patients (Baccam et al., 2006). Furthermore, was assumed to be (cells) and was estimated (using a regression model, since the viral at the day of infection was not provided) (Hernandez-Vargas & Velasco-Hernandez, 2020) to be about 0.31 (copies/mL). Moreover, in order to avoid identifiability problems related with the fact that only viral titters are employed to model fitting, the viral clearance parameter (c) was set in 2.4 (day−1), which is in accordance with previous estimates for influenza and HIV (Baccam et al., 2006, Hernandez-Vargas and Middleton, 2013). The parameters and initial conditions at the time of infection, days post infection (dpi), of each patient are collected in Table 1, Table 2. Since the viral load was measured after the onset of the symptoms, an incubation period of 7 days was assumed from the time of infection, according to Hernandez-Vargas and Velasco-Hernandez (2020).
Table 1.
Target cell-limited model parameter values for COVID-19 patients (Hernandez-Vargas & Velasco-Hernandez, 2020).
| Patient | ||||
|---|---|---|---|---|
| A | 0.61 | 0.2 | 2.4 | |
| B | 0.81 | 0.2 | 2.4 | |
| C | 0.51 | 0.2 | 2.4 | |
| D | 1.21 | 361.6 | 2.4 | |
| E | 2.01 | 0.2 | 2.4 | |
| F | 0.81 | 382 | 2.4 | |
| G | 0.91 | 0.2 | 2.4 | |
| H | 1.61 | 278.2 | 2.4 | |
| I | 2.01 | 299 | 2.4 |
Table 2.
Parameter Characterization of COVID-19 patients. The times are given in days post infection (dpi).
| Patient | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | 0.54 | 0.26 | 7.35 | 0.22 | 10.53 | 10.61 | 10.95 | 1.50 |
| B | 0.77 | 0.23 | 5.17 | 0.24 | 11.81 | 11.90 | 12.20 | 1.18 |
| C | 0.12 | 0.00 | 34.22 | 0.06 | 3.94 | 3.99 | 4.42 | 2.10 |
| D | 0.10 | 0.00 | 39.47 | 0.02 | 3.00 | 3.05 | 3.39 | 2.90 |
| E | 1.60 | 4.27 | 2.50 | 0.23 | 14.31 | 14.47 | 14.69 | 0.42 |
| F | 0.09 | 0.00 | 45.12 | 0.03 | 3.48 | 3.53 | 3.93 | 3.55 |
| G | 0.90 | 0.47 | 4.50 | 0.25 | 12.44 | 12.53 | 12.83 | 1.04 |
| H | 0.05 | 0.00 | 75.57 | 0.00 | 1.73 | 1.77 | 2.11 | 2.02 |
| I | 0.52 | 0.19 | 7.64 | 0.07 | 6.77 | 6.86 | 7.09 | 1.50 |
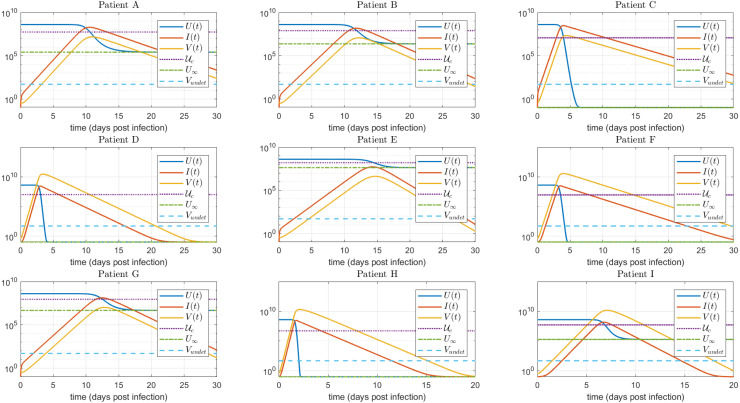
The time evolution of , and is shown in Fig. 3, for each patient. As it can be seen, the plot confirms the results given in Theorem 4.1 (see Abuin et al., 2020) concerning the minimum and maximum times of , the maximum time of and the time when reaches (i.e., when reaches ). Fig. 3 also supports the approximation made in Appendix A.2, by showing that the peaks of virus concentration and infected cells occur approximately at the same time at which reaches . All these temporal metrics , together with the values of (the critical target cell value), (the final value of at the end of infection), and (virus concentration at time-to-peak) are shown in Table 2, for the nine simulated patients.
Fig. 3.
, , time evolution for the untreated case. As stated in Theorem 4.1, in Abuin et al. (2020), , where and are the times at which reaches a local minimum and a local maximum, respectively, is the time at which reaches a local maximum, and is the time at which reaches . Furthermore, as stated in Appendix A.2, and . (copies/mL) stands for the undetectable level of the virus load.
Remark 3
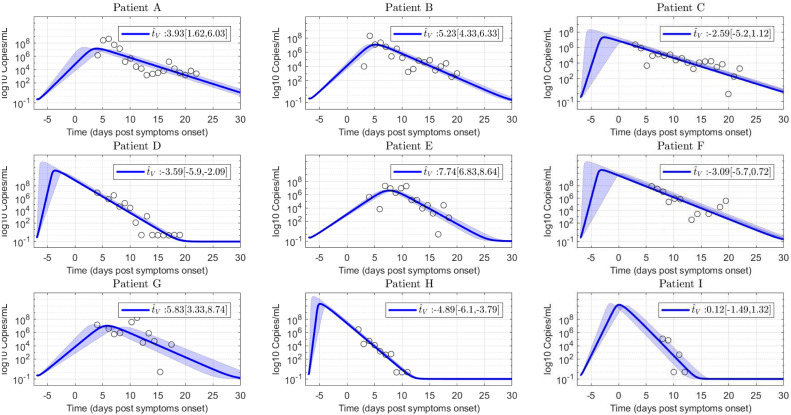
In comparison with other coronavirus diseases, such as MERS and SARS, where the virus load peak takes place after the onset of symptoms ( days post infection) (He et al., 2020), for SARS-CoV-2 infection it is not clear the temporal interval where the viral load reaches a peak. A recent study linking epidemiological and viral load data, suggests that the viral load peak occurs during the day of symptom onset (He et al., 2020). However, observation of viral load in infected macaques (Munster et al., 2020, Williamson et al., 2020) denotes that the viral peak from nose and throat swabs happens during the 1–3 days post infection. Therefore, since the target cell model fitting was conducted using SARS-CoV-2 viral load measured after the onset of the symptoms (Wölfel et al., 2020), the estimated time-to-peak () in Table 2 is subject to practical identifiability problems, which as was indicated in Theorem 3.1 and will be shown in Section 5, is a main parameter to evaluate antiviral effectiveness. Due to this reason, an uncertainty analysis computing the likelihood-based confidence intervals (Raue et al., 2009) for each parameter was done. In Fig. 4 such analysis is shown for the 95% confidence interval of parameter p, where it can be seen the high degree of uncertainty in the estimated for patients A, C, F and G (approximately days).
Fig. 4.
Uncertainty analysis on viral load evolution for -parameter 95% confidence interval. Note the uncertainty in viral load peak. Empty dots are data of COVID-19 patients.
5. Simulation results
To evaluate the results concerning the antiviral treatment effectiveness, several simulations involving the nine patients introduced in Section 4 were performed. First, the virus spreading interval – the time between the estimated day of infection ( days post symptoms onset (dpo)) and the time-to-peak of viral load () – are considered in Section 5.1, to assess the results of Theorem 3.1. Due to the reported variability on the estimated time-to-peak (Fig. 4), we decided to initialize the treatments taking into account the relative time with respect to the estimated , instead of using a fixed time for all the population. Furthermore, the (maximal) early treatment time () of each patient was computed numerically, being of the order of .
Then, in Section 5.2, the case when the antiviral therapy is started before and after the untreated time-to-peak is simulated, in order to analyze the effectiveness of a subpotent/potent antiviral drug as treatment time is delayed. Finally, in Section 5.3 the synergistic effects of antiviral therapies blocking the viral replication and the host cell infection are studied, taking into account the combined drug effect analysis presented in Section 3.2.
To numerically assess the viral kinetics evolution, the following infected-related metrics are employed: (i) the difference of viral loads with and without treatment at time-to-peak, , which is a measure of the viral reduction at time-to-peak respect to the untreated case (Gonçalves et al., 2020); (ii) the duration of infection, , defined as the time spent by the viral titer curve over a detection limit (i.e.: 100 copies/ml), which is a measure of the viral shedding interval and can also be used, as an indicator of the time a patient is infectious, requiring isolation or quarantine to prevent onward infection (Dobrovolny, Reddy, Kamal, Rayner, & Beauchemin, 2013); and (iii) the time-to-peak , which is an indicator of the viral replication rate and can be employed, as an estimation of the time window available for effective treatment (Dobrovolny, Baron, Gieschke, Davies, Jumbe, & Beauchemin, 2010). For the sake of simplicity, unless otherwise stated, the approximation presented in Appendix A.2, in the Appendix, is used for simulation analysis.
5.1. Treatment initiated at different times, during viral spreading interval ()
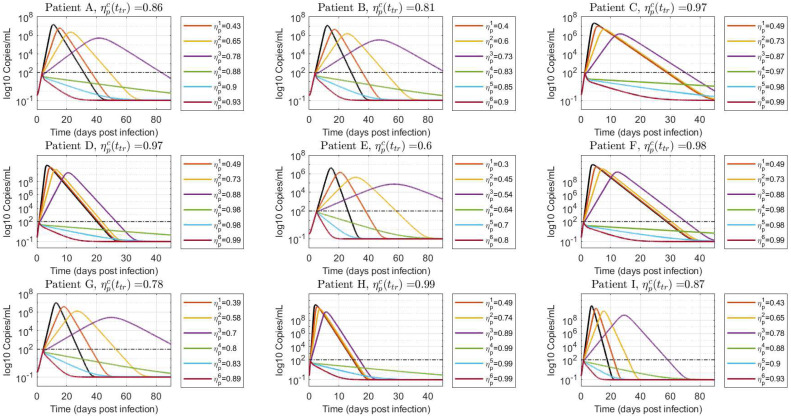
Scenario 1: treatment is initiated at viral load detection level ()
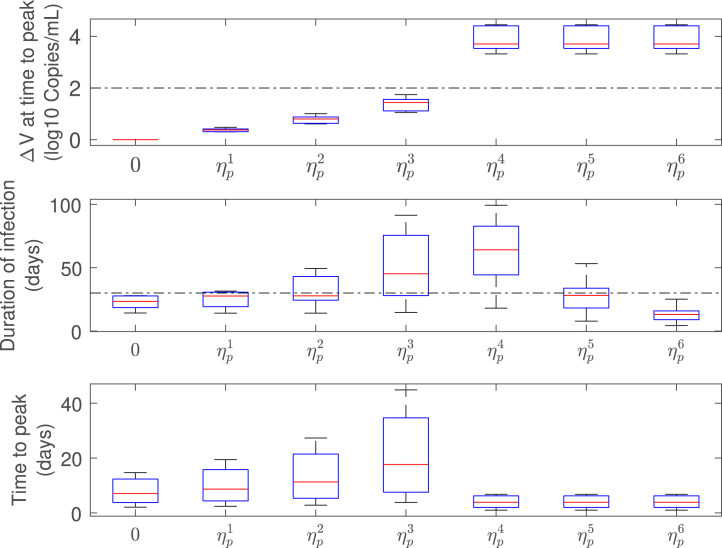
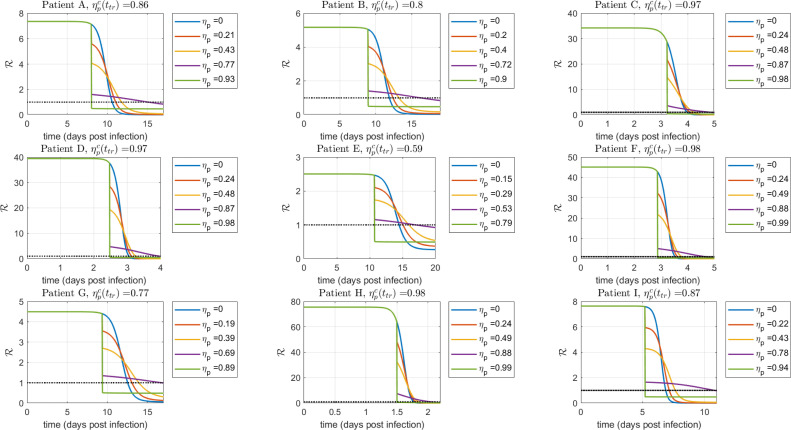
Fig. 5 shows the simulated virus load evolution corresponding to each of the nine patients, when the antiviral therapy is started at the first positive PCR test time (, being the time at which the virus reaches the value copies/mL), which is about . Consequently, all the patients fall in the case which means that we are under the hypothesis of Theorem 3.1 and, furthermore, the treatment time belongs to the early treatment time interval. To properly assess the antiviral replication inhibition effect, increasing values of were used. First we started with small values of , and , fulfilling the condition (item iii in Theorem 3.1, ineffective treatment). Then was set to values approximately equal to , and , to reinforce the counter-intuitive fact that larger inhibition effects fulfilling produce larger virus peak times (item iv in Theorem 3.1). Finally, was increased to two values larger than , and , (items i and ii in Theorem 3.1), to show that higher inhibition effects produce faster eradication of the virus . For the simulations, efficacies equally spaced from were used, such that: , , , , , and , for all patients.
Fig. 5.
Free virus behavior when treatment is started at viral load detection level (). Values of smaller (), approximately equal () and greater () than are simulated to demonstrate the results in Theorem 3.1. The black line denotes the untreated case ().
As expected, effective treatments produce an instantaneous decline of the viral load, with a monotonically decreasing viral shedding interval as the antiviral efficacy is incremented. On the other hand, ineffective treatments cause a delay in time to peak, significantly increasing the duration of viral shedding as is augmented from to . Even when the viral load peak is a monotonically decreasing function of (by following similar steps than the ones in Lemma 1 it can be shown that , for , with ), the patient will be PCR-positive for longer periods of time. This means that isolation and precautions measures with treated patients should be carefully considered, depending on the antiviral effectiveness.
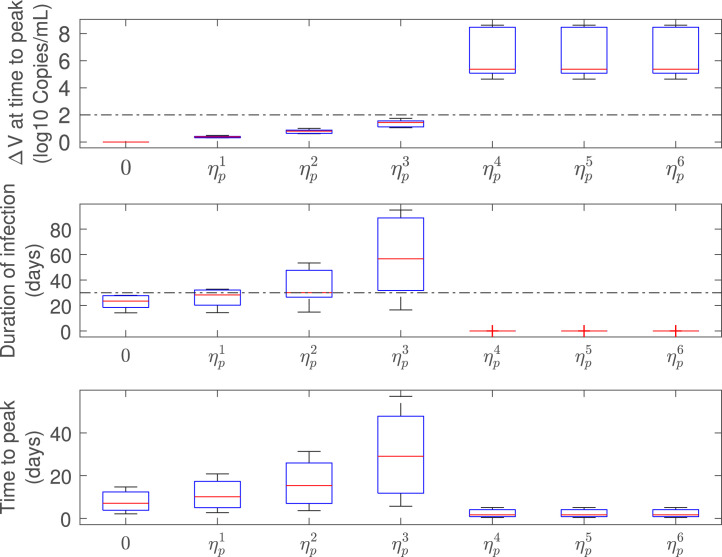
Fig. 6 shows a box-plot of the infected-related metrics for the antiviral effectiveness assessment. For an effective antiviral therapy (), the difference of viral loads at time-to-peak () is above the 2 logs threshold (Gonçalves et al., 2020) and the duration of infection () is below the 30 days limit (according Zhou, She, Wang, and Ma (2020), the viral shedding interval for untreated COVID-19 patients is in order of 30 days) which is in conformity with an effective viral clearance strategy. On the other hand, for an ineffective antiviral therapy (), even tough is monotonically increasing with , the duration of infection is increased as a subpotent drug efficacy is employed. This means that the delay of the virus peak associated to ineffective treatments is significant in terms of the infected-related metrics.
Fig. 6.
Infection-related metrics as function of (), for antiviral effectiveness assessment (all patients). Note that, , , , , , and , being the critical drug efficacy of each patient at time to treatment initiation, .
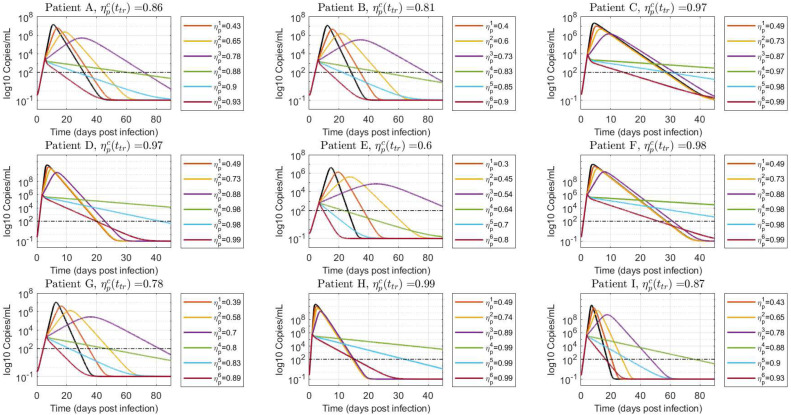
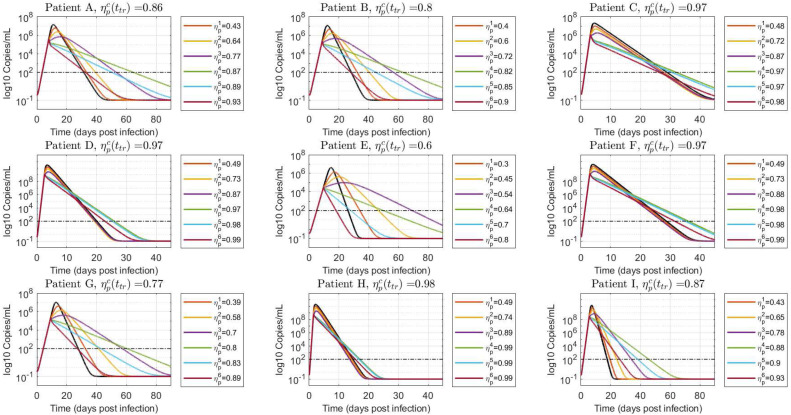
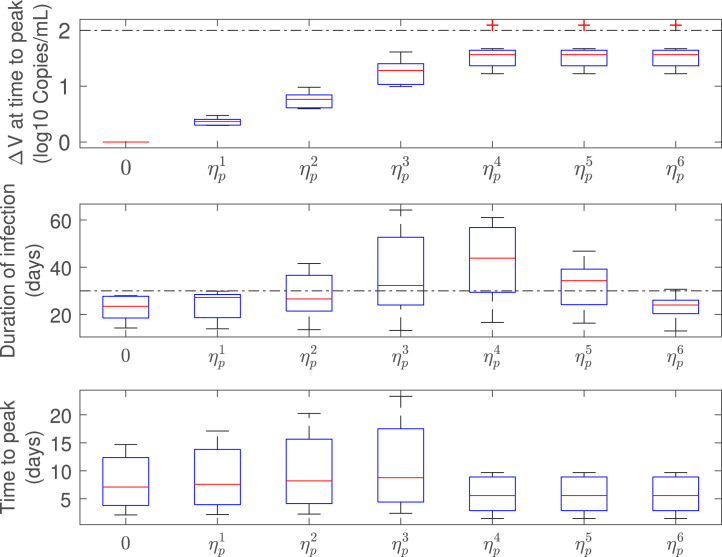
Scenario 2: treatment initiated in the course of viral spreading
Fig. 7, Fig. 8 show the virus load evolution when the antiviral therapy is started in the course of viral spreading, at and , respectively. The viral load over the time has the same qualitative behavior than in the previous case. However, the duration of the infection is longer as the inhibition effect approaches from below and from above (i.e.: and cases, violet and green lines in Fig. 7, Fig. 8). Indeed, for values of drug effectiveness in the vicinity of , the viral duration interval is augmented – even for effective therapies – since has already reached a relatively high value at the treatment time. This behavior is confirmed in box-plots Fig. 9, Fig. 10, where a sudden increase of the duration of infection happens when the antiviral efficacy is near the critical value. However, as the treatment time is delayed this behavior is mitigated (see box-plot 10) due to the natural increment of (recall that , for , so larger values of produce larger values of ). It is important to remark that, even when an increased duration of the viral shedding is reported for the effective treatment case in the boundary of critical drug effectiveness (), the viral load peak occurs previous to , in accordance with items i and ii of Theorem 3.1.
Fig. 7.
Viral load time evolution with treatment initial time given by . Values of smaller, approximately equal and greater than are simulated to demonstrate the results in Theorem 3.1. The black line denotes the untreated case ().
Fig. 8.
Viral load time evolution with treatment initial time given by . Values of smaller, approximately equal and greater than are simulated to demonstrate the results in Theorem 3.1. The black line denotes the untreated case ().
Fig. 9.
Infection-related metrics as function of (), for antiviral effectiveness assessment (all patients).
Fig. 10.
Infection-related metrics as function of (), for antiviral effectiveness assessment (all patients).
5.2. Treatment initiated at different times, with the same effectiveness
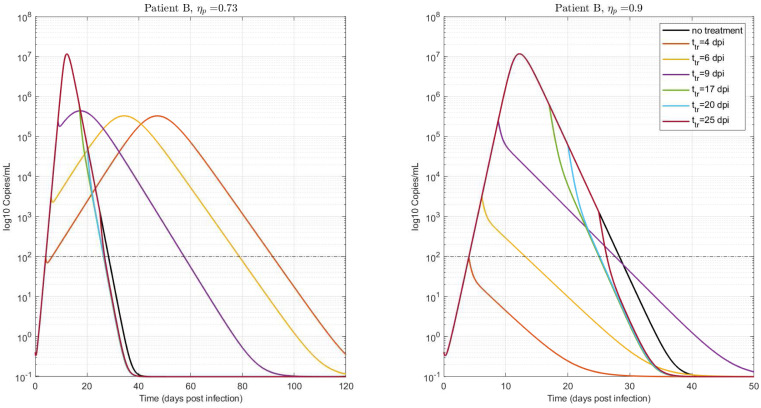
In order to analyze the viral kinetics when the antiviral therapy is initiated at different times over the infection period (i.e., before and after ), we studied the temporal dependence of infection-related metrics corresponding to fixed subpotent/potent drug effectiveness. Based on the uncertainty analysis presented in Section 4, patient B was selected as a representative patient, whose critical drug efficacy for (see Fig. 1, being dpi). The treatment initial times were: , , , , and dpi. Fig. 11 (left) shows that if the treatment is initiated with an ineffective therapy (), the time-to-peak () and the duration of infection () decrease as the treatment time is delayed. This can be explained by the fact that, for the same effectiveness, is reduced as is augmented. Eqs. (A.4), (A.5) in Lemma 1 show that for a fixed , and are increasing functions of (i.e.: and for while and for , being ). Therefore, since (according to Eq. (A.3)), it can be inferred that is monotonically decreasing with . Moreover, comparing the viral load at the time-to-peak, it can be deduced that for an ineffective therapy started at two treatment times during the beginning of the infection (assuming that ).
Fig. 11.
Virus time evolution for different treatment times, (dpi). Two fixed values of were used, smaller and bigger than : (left) and (right), respectively. for . Patient B. .
By following similar steps as in Lemma 1, the viral load at the time-to-peak can be written as: and, therefore, . However, from Fig. 11 (left) it can be seen that is of the order of (copies/ml), independently of the treatment initiation time. This can be explained by the fact that the difference of viral load at , for therapies started at different times, depends mainly on the deviation between the viral loads at treatment times, which normally are several order of magnitude below . For example, , and copies/ml while , and copies/ml. Consequently, the increment on the viral peak is not significant as the treatment is delayed, although the duration of infection is considerably reduced (i.e.: from to days). Note that the target cell-model assumes that the viral clearance in the convalescent phase is proportional to the virus load concentration at time-to-peak, which is in the same order of magnitude for the three cases. As a result, and taking into account that the viral peak is reached early as the treatment is delayed, a quickly viral deletion is observed for the postponed case. As a consequence, if a fixed subpotent drug is employed, delaying the treatment initiation time could reduce viral shedding interval, without significantly increasing the viral load at time-to-peak. It is important to remark that if is reduced even more (), the viral at time-to-peak follows the same behavior, although, the viral shedding interval is not-considerably decreased, since as (). Hence, although a non-significant improvement on the duration of infection is achieved if the treatment is delayed, the viral outcomes continues to be acceptable.
On the other hand, if the ineffective therapy is initiated after the viral load peak, the duration of infection is slightly reduced with respect to the previous case, since at this time and, therefore, is a decreasing function of . In contrast to this, Fig. 11 (right) shows that if an effective antiviral therapy is applied () the duration of the infection is still decreased, since , being the within-host basic reproduction number under a treatment with inhibition effect (note that, and for patient B).
5.3. Treatment with antiviral inhibiting both viral infection () and viral replication rate ()
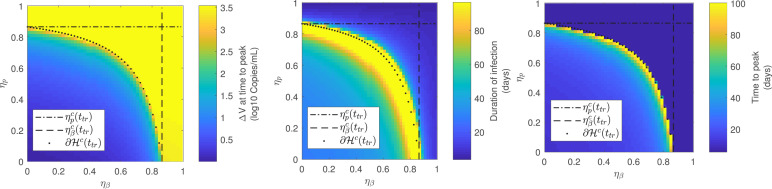
Finally, the virus behavior was considered when a combined antiviral therapy inhibiting both, the viral infection rate () and the viral replication rate (), was applied. Since the critical drug efficacy for acute models is defined during the viral spreading interval (Section 3.2), the treatment was initiated at , which is a value fulfilling .
For the sake of clarity, only Patient A was considered and the 3 previous infection-related metrics were assessed as functions of the antiviral inhibition effects and . Fig. 12 shows that the antiviral is effective for every inhibition effect pair fulfilling (i.e.: yellow region of ), in accordance with the results in Section 3.2. Moreover, as for the single inhibition cases, the duration of infection and the time-to-peak increase as the combined drug efficacy pair tends to the boundary of ( in Fig. 12). The difference of viral load at time-to-peak remains constant for every since for effective treatments. In conclusion, the synergistic effect of drug effectiveness in combined therapies produces a reduction on the critical effectiveness with respect to single-therapies cases, with the same dynamical behavior over the boundary of .
Fig. 12.
Infection-related metrics for antiviral effectiveness assessment as function of and (). Patient A.. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
6. Discussion
While several vaccines have been developed to prevent COVID-19 disease, it is imperative to evaluate potential therapies against SARS-CoV-2 infection. Among them, antiviral treatments are promissory strategies to increment the viral clearance “within-host”, decreasing disease severity. In this sense, the drug effectiveness concept is crucial to evaluate the drug effect threshold above which the viral load starts declining (Callaway & Perelson, 2002). Although this critical value has been described for chronic infections (e.g. HIV (Callaway and Perelson, 2002, Huang et al., 2003), HCV (Dahari, Lo, Ribeiro, & Perelson, 2007)) it has not been studied yet for COVID-19. The critical drug efficacy can be understood in terms of the antiviral potency to decline viral spreading, in spite of guiding the system to an uninfected equilibrium, as it was introduced in Definition 4, Section 3.2. In addition, the critical inhibition effect of an antiviral depends on the treatment initiation time, and it has shown to be a monotonically decreasing function during the viral spreading interval (roughly speaking, during the time interval before the untreated virus peak).
Regarding the virus behavior for effective therapies (), the viral load at time-to-peak is practically equal to (i.e., a treatment is considered effective if the virus starts to decrease at the very moment the treatment is initiated, by modifying either , , or both), so is larger for earlier treatments, being greater than the 2 log threshold for effective antiviral therapies started at (). Nevertheless, for approaching (from above), the viral shedding interval is enlarged as the treatment is initiated during the infection growth. Therefore, if the therapeutic objective is to reduce both, the viral shedding interval and the viral peak, the efficacy level would need to overpass the critical value in a given quantity. From Fig. 1 it can be seen that for most of patients.
Simulations results suggest that for ineffective therapies, the virus would take longer to be cleared – rather than a monotonic decline, as in the previous case – reaching a peak and finally decreasing to zero. In spite of the fact that the viral load at time-to-peak decreases monotonically with ( increases as the inhibition effect jumps from to ), the time-to-peak increases as approaches (from below), which results in a longer duration of infection, potentially requiring additional isolation measures for the treated patient.
Although an effective reduction of the viral load peak and the duration of infection could be achieved with early treatments (treatments started before the untreated peak), minor effects are attained with late ones. Particularly, Fig. 11 corroborates a slight reduction on the viral shedding interval if a subpotent/potent drug therapy is employed after the time-to-peak. Consequently, taking into account that the viral load in COVID-19 patients presumably reaches the peak prior to the symptom onset, no further clinical improvements may be obtained if the therapy is started in this symptomatic phase (i.e.: notice that SARS-CoV-2 pathophysiology is characterized by a direct cytotoxic effect, endothelial cell damage, dysregulation of immune response, among others (Gupta et al., 2020)).
The effectiveness of combined treatments affecting both, and , was also studied and an interdependent critical drug efficacy level was computed. Mathematically, the critical combination of values of and is placed on the boundary of the effective set , which is a set in the space. In comparison with single treatment cases (represented by the horizontal and vertical dash–dot lines in Fig. 12), a reduced critical antiviral efficacy was reported for the combined case, denoting a reduction on the necessary drug effectiveness to reduce viral spreading. Moreover, the viral characteristic behavior in the vicinity of the critical drug efficacy (i.e.: increase of time-to-peak and duration of infection) was preserved for the combined case, as the pair belongs to the critical boundary of , which implies that the antiviral effectiveness characterization made in Theorem 3.1 for single treatments could be extended to combined therapies.
In sum, this work formalizes the existence of a critical drug efficacy for acute infection models, which could have implications in the extended viral shedding observed “in-silico” by Goyal, Cardozo-Ojeda, and Schiffer (2020) (for SARS-CoV-2 infections) and by Cao and McCaw (2017) (for influenza) when the antiviral therapy is initiated early but with a subpotent drug efficacy. Moreover, it was shown the importance of initialization the antiviral therapy early (before viral load peak) in order to achieve a significant reduction of and (Gonçalves et al., 2020, Wu et al., 2020). Although a time dependence was noticed for the critical drug efficacy (), since it is a decreasing function of (Fig. 1), its behavior does not compromise the antiviral success if an effective therapy is started later.
The main clinical implications for acute infections, and, particularly, for the SARS-CoV-2 infection are: (a) importance of viral load monitoring on probably infected COVID-19 patients (prophylactic use of antiviral therapy, previous to onset of symptoms, although adverse effects have been reported for potential antiviral drugs (Jomah et al., 2020), which could limited their prophylactic usage in risk patients), (b) isolation of treated patient, (c) possible explanation of the increase of duration of infection showed in immune compromised COVID-19 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix.
A.1. Virus peak time behavior under ineffective treatments
The next Lemma establishes conditions on the virus peak time as a function of .
Lemma 1
Consider (2.1) , constrained by the positive set , at the beginning of the infection , with , and . Consider that at some time , jumps from to , being the critical value defined in (3.2) . Then, there exists such that if , the virus peak time (considering the treatment effect) is a decreasing function of .
Proof
Consider the approximation (A.10), in Appendix A.2. Since jumps from to , at , then jumps from to , with and . Then it is possible to approximate the explicit solutions for and (by approximating the function in the time interval before the virus peak by , being and arbitrary constants, fulfilling (Kaushal et al., 2020)). So, , for , can be written as
(A.1) where
A reasonable approximation, however, can be obtained by selecting and (also and gives a good result, but clearly the former selection significantly simplifies the expressions). In such a case, , , , and read:
being . This way, can be simplified as
(A.2) The time at which reaches - i.e., the time at which reaches and reaches its peak, denoted as (with ) - can be explicitly computed as
(A.3) where
being the critical value for corresponding to the treatment parameter . Now, recalling that , and denoting , and as , and , respectively, for the sake of simplicity, , can be rewritten as:
(A.4)
(A.5) This way, represents the time of the virus peak in terms of . Note that is defined only for ; indeed, since , while for .
The idea now is to consider the derivative of with respect to , to show that it is negative for treatment times small enough. This derivative reads:
where
(A.6)
(A.7) Since for , condition can be written as
(A.8) Furthermore, given that is an increasing function on , it follows that
(A.9) Then, by replacing (A.4), (A.5), (A.6), (A.7) in inequality (A.9), we have:
Now, for the exponent is negative, so function
starts at the positive value , for , then increases to a maximum and finally decreases asymptotically to zero, for . So, there exists some interval of , maybe small, such that . Fig. 13 shows a plot of for different values of .
Finally, since we are considering the treatment time to belong to the increasing period of (i.e., , with , then small values of correspond to small values of . So, a time interval exists such that is a decreasing function of , and the proof is complete. Fig. 14 shows the time evolution of when the antiviral treatment is initiated at and different antiviral inhibition effects are considered, for the real data patients simulated in Sections 4, 5. As it can be seen, larger values of (or smaller values of ) corresponds always with larger values of . □
Fig. 13.
Qualitative plot of function (arbitrary parameters) for different values of . As it can be seen – independently of the parameter values – if it there exists an interval of values of , and a corresponding period of time such that .
Fig. 14.
Evolution of when an antiviral treatment is initiated at a time of approximately , and different antiviral inhibition effects are considered, smaller and greater than the critical value . The black dashed line represents . As it can be seen, for values of , crosses at larger times for larger values of as it is stated in Lemma 1. This implies that if , larger values of delays the virus peak time, as it is stated in Theorem 3.1.iii. Furthermore, the figure confirms that, for real patient date, is close to .
A.2. System approximation
A characteristic of system (2.1) – that shows to be useful in the development of the main results of the work – is that for the untreated case ( and for all ), it can be simplified. Indeed, if we assume that virus is in quasi-steady state during viral spreading interval, which is a good assumption as long as (see (Kim et al., 2020), Equation (4), (Dahari et al., 2007, Ikeda et al., 2016, Nowak and May, 2000)), the three-states system (2.1) can be approximated by the following two-state equations:
where the infected cells state are given by .
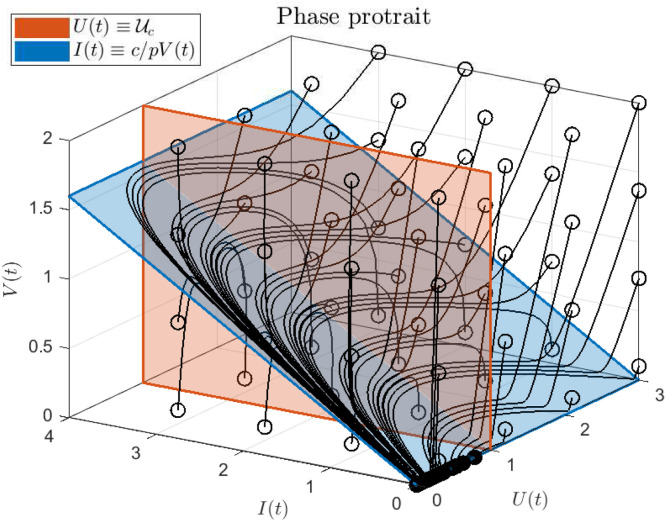
Note that Eq. (A.10b) can be written as . Then, according to Theorem 4.1 in Abuin et al. (2020), it is easy to see that from the right and from the left when , i.e., the peaks of and tend to occur simultaneously, at time . Fig. 15 shows a phase portrait of system (2.1), without antiviral treatment, with a rather unrealistic parameter values (, , and ) but useful to exemplify how, after a relatively short time (and provided that ), the system reaches the manifold defined by the condition .
Fig. 15.
Phase portrait of system (2.1) with parameters , , and , for different initial conditions not necessarily representing realistic cases. Empty circles represent the initial state, while solid circles represent final states. The red hyperplane corresponds to (i.e., the critical value of , when ) while the blue hyperplane corresponds to the fast manifold in which and are proportional (.i.e, ). Note that only the initial states with corresponds to scenarios with .
References
- Abuin P., Anderson A., Ferramosca A., Hernandez-Vargas E.A., Gonzalez A.H. Characterization of SARS-CoV-2 dynamics in the host. Annual Reviews in Control. 2020;50:457–468. doi: 10.1016/j.arcontrol.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccam P., Beauchemin C., Macken C.A., Hayden F.G., Perelson A.S. Kinetics of influenza a virus infection in humans. Journal of Virology. 2006;80(15):7590–7599. doi: 10.1128/JVI.01623-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway D.S., Perelson A.S. HIV-1 infection and low steady state viral loads. Bulletin of Mathematical Biology. 2002;64(1):29–64. doi: 10.1006/bulm.2001.0266. [DOI] [PubMed] [Google Scholar]
- Cao Y., Hiyoshi A., Montgomery S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country-level data. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-043560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P., McCaw J.M. The mechanisms for within-host influenza virus control affect model-based assessment and prediction of antiviral treatment. Viruses. 2017;9(8):197. doi: 10.3390/v9080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciupe S.M., Heffernan J.M. In-host modeling. Infectious Disease Modelling. 2017;2(2):188–202. doi: 10.1016/j.idm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. International Journal of Antimicrobial Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission N.H., et al. Translation: Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Infectious Microbes & Diseases. 2020:48–54. [Google Scholar]
- Dahari H., Lo A., Ribeiro R.M., Perelson A.S. Modeling hepatitis c virus dynamics: liver regeneration and critical drug efficacy. Journal of Theoretical Biology. 2007;247(2):371–381. doi: 10.1016/j.jtbi.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolny H.M. Quantifying the effect of remdesivir in rhesus macaques infected with SARS-CoV-2. Virology. 2020 doi: 10.1016/j.virol.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolny H.M., Baron M.J., Gieschke R., Davies B.E., Jumbe N.L., Beauchemin C.A. Exploring cell tropism as a possible contributor to influenza infection severity. Plos One. 2010;5(11) doi: 10.1371/journal.pone.0013811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrovolny H.M., Gieschke R., Davies B.E., Jumbe N.L., Beauchemin C.A. Neuraminidase inhibitors for treatment of human and avian strain influenza: A comparative modeling study. Journal of Theoretical Biology. 2011;269(1):234–244. doi: 10.1016/j.jtbi.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Dobrovolny H.M., Reddy M.B., Kamal M.A., Rayner C.R., Beauchemin C.A. Assessing mathematical models of influenza infections using features of the immune response. Plos One. 2013;8(2) doi: 10.1371/journal.pone.0057088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends. 2020;14 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- Giordano G., Blanchini F., Bruno R., Colaneri P., Di Filippo A., Di Matteo A., et al. 2020. A SIDARTHE model of COVID-19 epidemic in Italy. ArXiv Preprint arXiv:2003.09861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A., Bertrand J., Ke R., Comets E., De Lamballerie X., Malvy D., et al. Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load. CPT: Pharmacometrics & Systems Pharmacology. 2020;9(9):509–514. doi: 10.1002/psp4.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A., Cardozo-Ojeda E.F., Schiffer J.T. Potency and timing of antiviral therapy as determinants of duration of SARS-CoV-2 shedding and intensity of inflammatory response. MedRxiv. 2020 doi: 10.1126/sciadv.abc7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw F., Perelson A. Modeling viral spread. Annual Review of Virology. 2016;3 doi: 10.1146/annurev-virology-110615-042249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nature Medicine. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjichrysanthou C., Cauët E., Lawrence E., Vegvari C., De Wolf F., Anderson R.M. Understanding the within-host dynamics of influenza a virus: from theory to clinical implications. Journal of the Royal Society Interface. 2016;13(119) doi: 10.1098/rsif.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas E.A. Academic Press; 2019. Modeling and control of infectious diseases in the host: with MATLAB and R. [Google Scholar]
- Hernandez-Vargas E.A., Middleton R.H. Modeling the three stages in HIV infection. Journal of Theoretical Biology. 2013;320:33–40. doi: 10.1016/j.jtbi.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vargas E.A., Velasco-Hernandez J.X. In-host modelling of COVID-19 kinetics in humans. Annual Reviews in Control. 2020;50:448–456. doi: 10.1016/j.arcontrol.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Vargas E.A., Wilk E., Canini L., Toapanta F., Binder S., Uvarovskii A., et al. Effects of aging on influenza virus infection dynamics. Journal of Virology. 2014;88 doi: 10.1128/JVI.03644-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Rosenkranz S.L., Wu H. Modeling HIV dynamics and antiviral response with consideration of time-varying drug exposures, adherence and phenotypic sensitivity. Mathematical Biosciences. 2003;184(2):165–186. doi: 10.1016/s0025-5564(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Nakaoka S., de Boer R.J., Morita S., Misawa N., Koyanagi Y., et al. Quantifying the effect of vpu on the promotion of HIV-1 replication in the humanized mouse model. Retrovirology. 2016;13(1):23. doi: 10.1186/s12977-016-0252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomah S., Asdaq S.M.B., Al-Yamani M.J. Clinical efficacy of antivirals against novel coronavirus (COVID-19): A review. Journal of Infection and Public Health. 2020 doi: 10.1016/j.jiph.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. 2020;323 doi: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- Kaushal S., Rajput A.S., Bhattacharya S., Vidyasagar M., Kumar A., Prakash M.K., et al. 2020. Estimating hidden asymptomatics, herd immunity threshold and lockdown effects using a COVID-19 specific model. ArXiv Preprint arXiv:2006.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke R., Zitzmann C., Ribeiro R.M., Perelson A.S. Kinetics of SARS-CoV-2 infection in the human upper and lower respiratory tracts and their relationship with infectiousness. MedRxiv. 2020 [Google Scholar]
- Khan M.S., Shahid I., Anker S.D., Solomon S.D., Vardeny O., Michos E.D., et al. Cardiovascular implications of COVID-19 versus influenza infection: a review. BMC Medicine. 2020;18(1):1–13. doi: 10.1186/s12916-020-01816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.S., Ejima K., Ito Y., Iwanami S., Ohashi H., Koizumi Y., et al. Modelling SARS-CoV-2 dynamics: Implications for therapy. MedRxiv. 2020 [Google Scholar]
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., et al. ACS Publications; 2020. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. The Lancet Global Health. 2020 doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster V.J., Feldmann F., Williamson B.N., van Doremalen N., Pérez-Pérez L., Schulz J., et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020:1–5. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V., Binder S., Boianelli A., Meyer-Hermann M., Hernandez-Vargas E.A. Ebola virus infection modelling and identifiability problems. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M., May R.M. Oxford University Press, UK; 2000. Virus dynamics: mathematical principles of immunology and virology. [Google Scholar]
- Perelson A.S., Ribeiro R.M. Modeling the within-host dynamics of HIV infection. BMC biology. 2013;11(1):96. doi: 10.1186/1741-7007-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue A., Kreutz C., Maiwald T., Bachmann J., Schilling M., Klingmüller U., et al. Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics. 2009;25(15):1923–1929. doi: 10.1093/bioinformatics/btp358. [DOI] [PubMed] [Google Scholar]
- Rong L., Perelson A. Modeling HIV persistence, the latent reservoir, and viral blips. Journal of Theoretical Biology. 2009;260:308–331. doi: 10.1016/j.jtbi.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Storn R., Price K. Tech. Rep. TR-95-012, International Computer Science Institute, Berkeley …; 1995. Differential evolution-a simple efficient adaptive scheme for global optimization. [Google Scholar]
- Tett S., Cutler D., Day R., Brown K. Bioavailability of hydroxychloroquine tablets in healthy volunteers. British Journal of Clinical Pharmacology. 1989;27:771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cerna C.E., Alanis A.Y., Poblete-Castro I., Bermejo-Jambrina M., Hernandez-Vargas E.A. 2016 IEEE congress on evolutionary computation. IEEE; 2016. A comparative study of differential evolution algorithms for parameter fitting procedures; pp. 4662–4666. [Google Scholar]
- Vergnaud J.-M., Rosca I.-D. CRC press; 2005. Assessing bioavailablility of drug delivery systems: mathematical modeling. [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Lu Z., Wang X., Li H., Li H., Lin D., et al. Current trends and future prediction of novel coronavirus disease (COVID-19) epidemic in China: a dynamical modeling analysis. Mathematical Bioscience and Engineering. 2020;17(4):3052–3061. doi: 10.3934/mbe.2020173. [DOI] [PubMed] [Google Scholar]
- WHO K. 2020. Coronavirus disease 2019 (COVID-19) situation reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed 28 December 2020) [Google Scholar]
- WHO K. 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, https://coronavirus.jhu.edu/map.html (Accessed 28 April 2020) [Google Scholar]
- Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. BioRxiv. 2020 doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) Journal of Internal Medicine. 2020 doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B., She J., Wang Y., Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa451. [DOI] [PMC free article] [PubMed] [Google Scholar]