Abstract
Background
Clinical trials of the BNT162b2 vaccine, revealed efficacy and safety. We report six cases of myocarditis, which occurred shortly after BNT162b2 vaccination.
Methods
Patients were identified upon presentation to the emergency department with symptoms of chest pain/discomfort. In all study patients, we excluded past and current COVID-19. Routine clinical and laboratory investigations for common etiologies of myocarditis were performed. Laboratory tests also included troponin and C-reactive protein levels. The diagnosis of myocarditis was established after cardiac MRI.
Findings
Five patients presented after the second and one after the first dose of the vaccine. All patients were males with a median age of 23 years. Myocarditis was diagnosed in all patients, there was no evidence of COVID-19 infection. Laboratory assays excluded concomitant infection; autoimmune disorder was considered unlikely. All patients responded to the BNT162b2 vaccine. The clinical course was mild in all six patients.
Interpretation
Our report of myocarditis after BNT162b2 vaccination may be possibly considered as an adverse reaction following immunization. We believe our information should be interpreted with caution and further surveillance is warranted.
Keywords: Covid-19, Vaccine, BNT162b2, Myocarditis, Adverse reaction
Abbreviations: BNT162b2, Pfizer-BioNTech mRNA Vaccine; CMR, Cardiac Magnetic Resonance Imaging; Covid-19, SARS-COV-2; CT, Computerized Tomography; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; RT-PCR, Reverse Transcription-Polymerase Chain Reaction
1. Introduction
1.1. Background
On December 2020 the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the Pfizer-BioNTech mRNA vaccine (BNT162b2) for prevention of COVID-19 disease. The vaccine’s EUA relied on data which were obtained from several clinical trials [1], [2]. The results of these trials revealed that the vaccine’s efficacy is 95% and its safety profile is good and similar to that of other vaccines [1], [2], [3]. Systemic reactions to the vaccine, which were usually mild and transient, were reported more commonly among the younger population and more often after the second dose [1], [2].
In Israel, the nationwide rollout of the 2-dose BNT162b2 vaccination program started in December 2020. More than 4 million people have received two doses of the vaccine, by the time of writing. These include persons 16 years old and older. In this report, we inform on the unforeseen occurrence of myocarditis in five male persons shortly after they received two doses of the BNT162b2 vaccine and in one male person 16 days after he received the first dose of the BNT162b2 vaccine. We suspect that these adverse events were related to the vaccine.
1.2. Patients and Clinical, laboratory and imaging Assessments
In a three-week interval (January 30th through February 20th 2021, six men were hospitalized with suspected myocarditis, all shortly after the vaccination. The COVID-19 status of the six patients was assessed by reverse transcription-polymerase chain reaction (RT-PCR) of nasopharyngeal swabs (TaqPath™ COVID-19 Combo Kit, Thermo Fisher Scientific) and serological determination of the antibody levels against viral nucleocapsid (anti N Ab) (Anti-Nucleocapsid antibodies- Elecsys® N Anti-SARS-CoV-2, Cobas®, Roche Diagnostics) and spike proteins (anti S Ab) (Anti-Spike antibodies, LIAISON® SARS-CoV-2 S1/S2 IgG DiaSorin).
The laboratory tests also included a routine complete blood count, complete metabolic panel, troponin, C-reactive protein, PCR testing and serological determination of antibodies against common infectious pathogens related to myocarditis (for details see supplementary).
1.3. Cardiac imaging
When a coronary event was suspected, a computerized tomography (CT) coronary angiogram or a diagnostic coronary angiogram was done.
transthoracic echocardiography and cardiac magnetic resonance imaging (CMR)) were done in all patients.
Routine ECG-gated CMR was performed on a 1.5 Tesla magnet (Philips Ingenia 1.5 T MRI system) equipped with phased array body coils for signal reception using commercially available cardiac software (for CMR protocol see supplementary).
CMR analyses were performed offline using Philips Intellispace Portal by an experienced radiologist.
The CMR diagnosis was made in accordance with prior published recommendations in the presence of typical non-ischemic myocardial injury [4].
2. Results
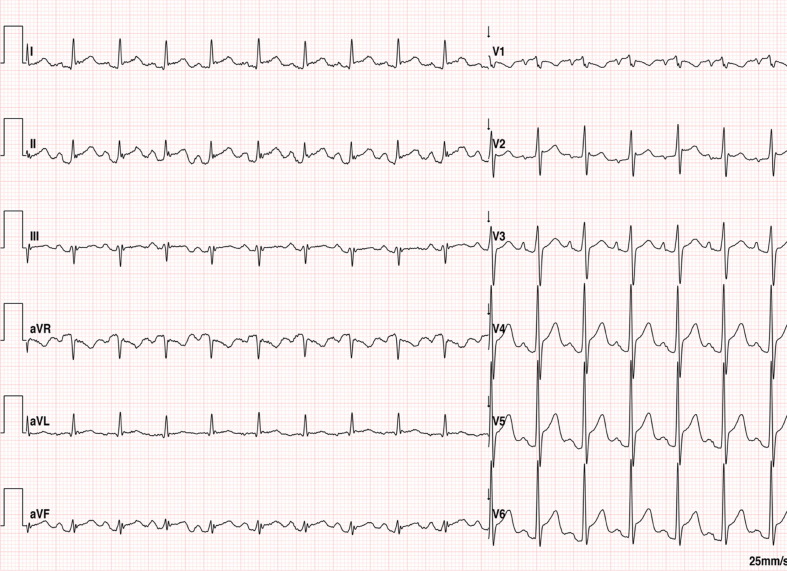
Six patients (16–45 years; median 22 years) were evaluated because of chest pain/discomfort (Table 1 ). Five patients presented 24–72 h after receiving the second dose of the vaccine and one patient presented 16 days after receiving the first dose of the vaccine. All patients had an abnormal electrocardiogram (Fig. 1 ) and elevated serum C-reactive protein levels (56–347 mg/L: normal level < 5.0 mg/L). Five patients had elevated serum troponin T levels (peak levels 392–1062 ng/L; normal level < 13 ng/L) and one patient had an elevated high-sensitivity cardiac troponin-I level (14350 ng/L; normal level < 34 ng/L). The supplementary material details the laboratory results of each patient.
Table 1.
Clinical and laboratory summary of the study population.
| No. | Age/sex/ Time of presentation | ECG | Peak CRP* Peak Troponin-T*Serology**RT-PCR *** | COVID-19 status1. RT-PCR2. nucleocapsid antibodies3.Spike protein antibodies | TTE | Coronary imaging | Cardiac MRI |
|---|---|---|---|---|---|---|---|
| Case 1 | 24 y, male,72 h after receiving the2nd dose of the vaccine | Diffuse ST elevation Inverted T lead III | CRP - 58.1 mg/L;Troponin T - 589 ng/L;Serology – negativeRT-PCR - negative | 1.negative2.negative3.positive | Normal study | NA | T2 sequence showed mild myocardial edema of the basal septum and inferolateral wall.Subepicardial and mid myocardial LGE of the same affected segments |
| Case 2 | 20 y, male,24 h after 2nd vaccine | Sinus tachycardiaST elevation V2-6 | 100.0 mg/lTroponin T - 1062 ng/lSerology – negativeRT-PCR- negative | 1.negative2.negative3.positive | LVEF-50-55%Apical hypokinesis | CT Angiography: NCA | T2 sequence showed mild myocardial edema with LGE in the subepicardial region of the basal and middle anterolateral and inferolateral walls |
| Case 3 | 29 y, male,48 h after receiving the 2nd dose of the vaccine | Diffuse PR depressionDiffuse ST elevation | CRP - 86.0 mg/LTroponin T - 876 ng/LSerology – negativeRT-PCR- negative | 1.negative2.negative3.positive | Normal study | NA | T2 sequences showed mild diffuse myocardial edemaand LGE of the basal, inferolateral, anterolateral and anteroseptal walls |
| Case 4 | 45 y, male,16 days after receiving the1st dose of the vaccine | ST elevation: I, aVL, V3-5Inverted T, ST depression:III, aVF | CRP - 56.2 mg/LTroponin T - 392 ng/LSerology – negativeRT-PCR- negative | 1.negative2.negative3.positive | LVEF-50-55% | Coronary angiogram:NCA | LVEF 50-55%T2 sequence showed subepicardial edema of the middle anterolateral, inferolateral and of the apical anterior walls with LGE of the affected walls |
| Case 5 | 16 y, male24 h after receiving the2nd dose of the vaccine | ST elevation V2-4 | CRP -1.6 mg/L Hs troponin-I 14350 ng/LSerology – negativeRT-PCR- negative | 1.negative2. nonreactive3. positive | Normal study | NA | Normal LV size LVEF 59%T2 sequence show midmyocardial and subepicardial edema of the basal inferolateral and middle anterolateral segments. LGE present in the same segments |
| Case 6 | 17 y, male72 h after receiving the 2nd dose of the vaccine | ST elevation I II aVL, V2-6SI QIII TIII | CRP - 54.7 mg/LTroponin T 1130 ng/LSerology - negativeRT-PCR - negative | 1.Negative2.Nonreactive3.positive | Normal study | NA | T2 sequence showed subepicardial edema of the basal inferolateral, middle inferolateral and infero-septal and apical lateral, anterior and inferior walls. LGE present in the same segments and mid-myocardial enhancement of the middle inferolateral and anterolateral and apical anterior and lateral walls. Findings consistent with myo-pericarditis. |
ECG - electrocardiography; EF - Ejection Fraction; TTE - transthoracic echocardiography; NA - not assessed; NCA - normal coronary arteries; LV - Left Ventricle; LGE - late gadolinium enhancement.
* Serum C-reactive protein (CRP) level (normal range (0.0–5.0 mg/L); serum troponin T level (normal range 0.0–13.0 ng/L; Above 50 – positive).
** Serology: Epstein-Barr virus (EBV), cytomegalovirus (CMV), hepatitis B virus (HBV), Coxsackie viruses, parvovirus B19, Coxiella burnetii, Mycoplasma pneumoniae.
*** RT-PCR: adenovirus, parainfluenza, respiratory syncytial virus (RSV), influenza A virus, influenza B virus, enterovirus, human metapneumovirus, Bordetella pertussis, Bordetella parapertussis, Chlamydia pneumoniae, Mycoplasma pneumoniae.
Fig. 1.
Abnormal ECG recording, showing diffuse PR depression and diffuse ST segment elevation.
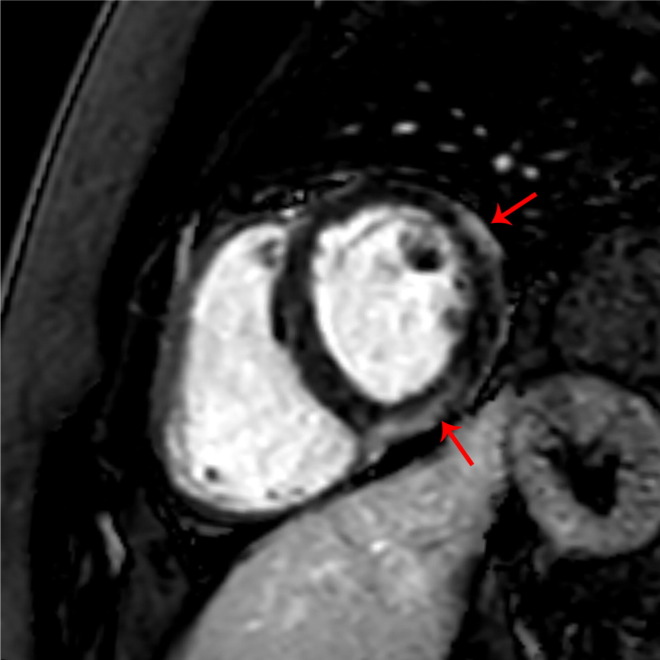
The echocardiographic examination was normal in four patients, and revealed a borderline left ventricular ejection fraction in two patients. The CMR revealed findings which were compatible with myocarditis (myocardial edema and late gadolinium enhancement) in all patients (Fig. 2 , and supplementary material).
Fig. 2.
Short axis late gadolinium enhancement image. Mid-myocardial enhancement of the middle inferolateral and anterolateral wall consistent with myo-pericarditis. (Red arrows point to LGE). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
No significant narrowing of the coronary arteries was noted in one patient who had a cardiac CT and another patient who had coronary angiography.
All six patients presented in less than a month period. We investigated the background rate of myocarditis during winter months (December through March) in our hospital in the past 5 years, and found a mean of 1.17 cases of myocarditis per month (range 0–3 cases per month).
The disease course was mild in all patients. They were treated with a nonsteroidal anti-inflammatory drug and colchicine and were discharged 4–8 days after admission for outpatient follow-up (see supplementary material for detailed case report of each patient).
3. Discussion
We identified six cases of clinically suspected myocarditis in otherwise healthy male individuals shortly after they received the BNT162b2 mRNA COVID-19 vaccine. Five patients presented shortly after the second vaccine dose and one patient presented 16 days after receiving his first vaccine dose. We included this patient because of identical clinical investigation results and clinical course. We considered and assessed a wide range of differential diagnoses for these cases.
3.1. COVID-19 associated myocarditis
Myocarditis is reported to be one of the manifestations of COVID-19 infection. The proposed pathophysiology involves direct damage by (a) invasion of the virus into cardiomyocytes, (b) the host immune response of cytotoxic T lymphocytes, and (c) the presence of over-inflammation and the cytokine storm [4], [5].
Although protection from COVID-19 infection takes a few days to develop after receiving the second dose of the vaccine, COVID-19 infection can still occur in this early period. We ruled out COVID-19 infection in our patients. All patients were COVID-19 RT-PCR negative and nonreactive for nucleocapsid protein (anti N Ab Negative). All patients responded to the vaccine, as evidenced for being found positive for antibodies against the spike protein (anti S Ab positive).
3.2. Infectious autoimmune and toxic/drug exposure
We performed antibody and PCR testing for common pathogens associated with myocarditis and pericarditis and found no indication of any concomitant infection. None of our patients had any clinical sign or laboratory finding compatible with autoimmune disease. None of the patients had a history of an exposure to new drugs or toxins prior to onset of their symptoms.
3.3. Myocarditis as an adverse reaction to the COVID-19 vaccine
In general, myocarditis, is considered an uncommon adverse event following immunization [6], [7], [8]. An exception to this generalization is myocarditis following smallpox vaccination in adults with a reported incidence of 1:10,000 [7], [9], [10], [11], [12].in a prospective safety surveillance study of perimyocarditis following smallpox vaccine, the authors report that most of the identified cases of perimyocarditis were subclinical and were identified through routine pre and post vaccination troponin level measurments [13]. This finding raises concern that we identified only the “tip of the icberg” i.e. symptomatic cases and other asymptomatic cases may had not been detected.
An important consideration is a comparison of number of cases of suspected myocarditis post vaccination to the background rate of myocarditis. Our hospital serves a population of approximately 500,000 people. The mean number of myocarditis cases in winter months (December through March) for the past 5 years was 1.17 cases per month. Our six patients presented in less than a month period, indicating a higher rate.
The development and approval via Emergency Use Authorization of vaccines for the prevention of infection with COVID-19 is a huge step forward to control the disease and decrease morbidity and mortality.
It is possible that uncommon adverse reactions to the COVID-19 vaccine may have not been captured during the clinical trials due to low rate of myocarditis and the relatively limited size of the population that received the vaccine before its authorization. In Israel, nationwide vaccination rollout started at the end of December 2020. Near 4 million people had already received two doses of the BNT162b2 vaccine at the time of writing. The vaccination of a large population in a short period may allow detection of less common vaccination-related events.
Immunization against COVID-19 and other viral pathogens using mRNA-based vaccines is a new and promising technology [13], [14]. In the clinical trials, systemic adverse reactions after the second dose were reported more frequently mainly in the younger population with a median onset time of 1–2 days [1], [2]. This time pattern fits five of our six cases. Also, the relatively young age profile for the systemic drug reactions described for the vaccine fits all our patients.
The course of the disease was mild in our patients. However, myocarditis may have a more severe clinical presentation, may impose limitations on physical activity and may require long-term medical treatment, and follow-up [15], [16].
4. Conclusion
Our report on the occurrence of myocarditis after BNT162b2 mRNA COVID-19 vaccination in six male patients may be possibly considered to be an adverse reaction following immunization. Accordingly, it is important to collect more data on this entity as the vaccine becomes more widely distributed, and more data on the vaccine’s efficacy and safety is obtained. It is important to interpret our data with caution because public acceptance of COVID-19 vaccines is much needed. The individual and public benefit from COVID-19 vaccination outweighs these rare findings described herein.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.05.087.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The Advisory Committee on Immunization Practices' Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 5.Siripanthong B., Nazarian S., Muser D., Deo R., Santangeli P., Khanji M.Y., et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbasi J. Researchers Investigate What COVID-19 Does to the Heart. JAMA. 2021;325:808–811. doi: 10.1001/jama.2021.0107. [DOI] [PubMed] [Google Scholar]
- 7.Cassimatis D.C., Atwood J.E., Engler R.M., Linz P.E., Grabenstein J.D., Vernalis M.N. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43:1503–1510. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Eckart R.E., Love S.S., Atwood J.E., Arness M.K., Cassimatis D.C., Campbell C.L., et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–205. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Mei R., Raschi E., Forcesi E., Diemberger I., De Ponti F., Poluzzi E. Myocarditis and pericarditis after immunization: Gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183–186. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 11.Engler R.J., Nelson M.R., Collins L.C., Jr., Spooner C., Hemann B.A., Gibbs B.T., et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuntz J., Crane B., Weinmann S., Naleway A.L. Vaccine Safety Datalink Investigator T. Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine. 2018;36:1524–1527. doi: 10.1016/j.vaccine.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faix D.J., Gordon D.M., Perry L.N., Raymond-Loher I., Tati N., Lin G., et al. Prospective safety surveillance study of ACAM2000 smallpox vaccine in deploying military personnel. Vaccine. 2020;38:7323–7330. doi: 10.1016/j.vaccine.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Chilamakuri R., Agarwal S. COVID-19: Characteristics and Therapeutics. Cells. 2021;10 doi: 10.3390/cells10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caforio A.L.P., Adler Y., Agostini C., Allanore Y., Anastasakis A., Arad M., et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur Heart J. 2017;38:2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.