Abstract
Background & Aims:
Obesity in adulthood has been associated with increased risk of liver-related mortality. Whether higher levels of physical activity counteract the excess risk conferred by obesity remains unknown. We simultaneously evaluated the long-term impact of physical activity and adiposity on liver-related mortality, within two nationwide populations.
Methods:
We conducted a prospective cohort study of 77,238 women and 48,026 men, with detailed, validated assessments of weekly physical activity (metabolic equivalent task [MET]-hours]), adiposity (body mass index [BMI], waist circumference), and diet, alcohol use and clinical comorbidities, biennially from 1986 through 2012. Using Cox proportional hazards regression models, we calculated multivariable adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for liver-related mortality, including death from hepatocellular carcinoma (HCC) and other cirrhosis complications.
Results:
Over 1,856,226 person-years, we recorded 295 liver-related deaths (108 HCC; 187 cirrhosis). Risk of liver-related mortality increased monotonically with higher BMI during adulthood (Ptrend<0.0001) and with weight gain during early-adulthood (Ptrend<0.0001). The risk of liver-related mortality also declined progressively, with increasing physical activity (Ptrend=0.0003); the aHRs across increasing physical activity quintiles were: 1.0, 0.70 (95%CI=0.51-0.96), 0.59 (95%CI=0.42-0.84), 0.52 (95%CI=0.36-0.74) and 0.46 (95%CI=0.31-0.66). Compared to lean-active adults (BMI<25; ≥18 MET-hours/week), the aHRs for obese-active, lean-sedentary, and obese-sedentary adults were: 1.04 (95%CI=0.73-1.37), 2.08 (95%CI=1.21-3.33) and 3.40 (95%CI=2.06-5.56), respectively. Findings were similar for HCC-specific and cirrhosis-specific mortality. Overall, engaging in average-pace walking for >3 hours/week could have prevented 25% of liver-related deaths (95%CI=0.12-0.38).
Conclusions:
In two prospective, nationwide cohorts, both excess adiposity and reduced PA were significant predictors of liver-related mortality. Achieving higher PA levels counteracted the excess liver-related risks associated with obesity.
Keywords: cirrhosis, lifestyle, modifiable risk factor, prevention
Lay Summary:
This is the first large, prospective cohort study to simultaneously evaluate the impact of obesity and physical activity on the long-term risk of liver-related mortality, in two nationwide populations of U.S. men and women. The study demonstrated that obesity predicted significantly increased risk of liver-related mortality, while physical activity predicted significantly lower risk of liver-related mortality. Importantly, the excess risk of liver-related mortality observed with obesity was no longer statistically significant among adults who engaged in the equivalent of average-pace walking for 3 hours or more, per week.
Introduction
In 2016, more than 34,000 Americans died from chronic liver disease, which includes death from hepatocellular carcinoma (HCC) and from other complications of cirrhosis1, 2. Over the past 20 years, the prevalence of cirrhosis in the U.S. has doubled, and rates of liver-related mortality have increased by more than 65%2, due in part to the growing epidemics of obesity and non-alcoholic fatty liver disease (NAFLD). Despite this, knowledge of modifiable lifestyle factors remains incomplete, and effective strategies to prevent liver-related mortality are lacking.
Obesity and physical inactivity represent major public health problems for patients with chronic liver disease. Approximately 80% of Americans with NAFLD are overweight3, and most do not engage in physical activity4, even when their liver disease is mild5, 6. Obesity is a risk factor for cirrhosis7, 8, HCC9-12 and liver-related death7, 13. Furthermore, physical activity is inversely associated with HCC incidence9, and in short-term clinical studies, physical activity interventions improve liver fat, inflammation and fibrosis, even in the absence of weight loss14-17. However, whether this translates to long-term reductions in liver-related mortality is unknown. While it has been suggested that achieving higher levels of fitness might eliminate the adverse effects of obesity on long-term hepatic outcomes18, 19, evidence to support this hypothesis is lacking. To date, no prospective epidemiologic study has simultaneously evaluated the long-term influence of physical activity and adiposity on liver-related mortality.
Numerous national subspecialty societies provide specific physical activity recommendations for the prevention of cancer20, heart disease21, and other chronic conditions22, 23. In contrast, no recommendations currently exist for the prevention of major adverse hepatic events, including death from cirrhosis and from HCC. Given the growing burden of chronic liver disease, understanding the optimal level of activity to prevent liver-related mortality remains an important unmet need.
Thus, we prospectively evaluated the relationships between physical activity, adiposity and liver-related mortality, within two nationwide populations of U.S. men and women. Specifically, we assessed whether achieving higher levels of activity might counteract the association between excess body weight and liver-related mortality.
Methods
Participants
The Nurses’ Health Study (NHS) prospectively enrolled 121,700 female registered nurses, aged 30-55 years, in 1976, and the Health Professionals Follow-up Study (HPFS) prospectively enrolled 51,529 male health professionals, aged 40-75 years, in 198624, 25. Since enrollment, participants have returned detailed biennial questionnaires, providing prospectively-updated data on lifestyle, medical history, physical activity and disease outcomes, with follow-up that consistently exceeds 90%26. We included all individuals from both cohorts who completed the first comprehensive physical activity assessment, in 198627, 28. We excluded anyone with missing information on baseline physical activity or body mass index (BMI), self-reported cirrhosis (n=775), viral hepatitis (n=305), or prior cancer, except nonmelanoma skin cancer (n=8,366), consistent with prior work29. This left 125,264 participants (77,238 women, 48,026 men), eligible for analysis.
The NHS and HPFS cohorts were approved by the Institutional Review Boards of the Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health (1999-P-011114), and those of participating registries as required. Return of questionnaires was considered as informed consent.
Assessments of Adiposity
BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2) in 1986, and biennially thereafter. The validity and reliability of these measurements in these cohorts have previously been demonstrated (r=0.97 for men and women)30. The baseline questionnaire queried early adulthood weight (i.e. 21 years [men] and 18 years [women]), which has been validated (r=0.87)31. We constructed three BMI exposures: cumulative average updated BMI, calculated from all questionnaires up to the start of each 2-year interval, early adulthood BMI, and mid-life BMI (in 1986).
Abdominal adiposity was defined using validated waist circumference (WC) and waist-to-hip ratio (WHR) measurements, reported in HPFS in 1987 and 1996, and in NHS in 1986, 1996 and 200030. The correlation coefficients between self-reported and technician-measured WC and hip circumferences were 0.95 and 0.88, respectively, among men, and 0.89 and 0.84, respectively, among women30.
Physical Activity and Covariate Assessment
Detailed physical activity assessments were first obtained in 1986 and updated biennially thereafter32, 33. Prior studies have demonstrated the validity and reproducibility of physical activity data in these cohorts25, 34. With each questionnaire, participants report average weekly time engaged in leisure activities, including walking, jogging, running, bicycling, swimming, calisthenics, rowing, squash, racquet ball and tennis, heavy outdoor work (since 1988), and weight training (since 1990). This permits calculation of weekly energy expenditure in metabolic equivalent task–hours (MET-hours), using validated approaches (eMethods)35.
Cumulative average updated activity was defined as average weekly time spent engaged in moderate or vigorous physical activity (i.e. requiring ≥3 MET-hours) from all questionnaires up to each 2-year interval, consistent with prior studies32, 33. We also calculated baseline (1986) physical activity. Physical activity was not assessed in the 1990 NHS questionnaire, therefore we carried these data forward by 1 interval (i.e. we applied 1988 data to the 1990-92 interval). Otherwise, missing data were not carried forward. As in prior work29, covariates were selected a priori (eMethods). Incident cirrhosis or viral hepatitis diagnosed during the prior 2-year interval were ascertained from each questionnaire.36
Outcomes
Deaths were reported by next-of-kin, the postal authorities or the National Death Index, with >98% follow-up for both cohorts37, 38. For all deaths, we sought death certificates and, when appropriate, requested permission to review medical records, from which the cause of death was confirmed and classified according to the International Classification of Diseases, 8th Revision. Major surgeries were ascertained and updated with each biennial questionnaire. The primary endpoint was liver-related mortality, defined as death from HCC or from a non-HCC complication of cirrhosis, consistent with recommendations39 (eMethods). As previously described, all HCC cases were confirmed through blinded review of medical records29.
Statistical Analysis
To best represent long-term patterns and minimize within-person variation, our primary analysis used cumulative average updated exposures32, 33. To account for potential changes in physical activity after a diagnosis of cirrhosis, we suspended updating of exposures after incident cirrhosis was reported in follow-up. For the primary analysis, we applied an 8-year latency period, with follow-up beginning in 1994; this permits the assessment of updated exposures, while minimizing potential reverse causation40. The 8-year period was selected a priori, given the prolonged time that may elapse between the development of cirrhosis and decompensation or death41. In additional analyses, we used baseline and extended latency exposures (with a lag of 12 years). BMI was modeled in World Health Organization categories (<19, 19-<25, 25-<30, 30-<35 and ≥35kg/m2). Physical activity was categorized in quintiles based upon its distribution, and further in clinically-informative categories (e.g. <3, 3-<9, 9-<18, 18 to <27 and ≥27 MET-hours/week), corresponding to the equivalent of average-pace walking for <1, 1 to <3, 3 to <6, 6 to <9 and ≥9 hours/week. These categories were selected because walking is readily adoptable and was the most commonly-reported form of exercise, as it is in the broader U.S. population42. Early adulthood weight change was calculated as the difference between weight in mid-life (1986) and early adulthood weight, and grouped into 5 categories33. WC and WHR were modeled continuously and in quartiles. For all models, linear trend was assessed using continuous variables.
Person-time of follow-up accrued from return of the baseline questionnaire to the date of death or end of follow-up (January 31, 2012 [HPFS]; June 1, 2012 [NHS]), whichever came first. We used Cox proportional hazards regression conditioned on age (years), questionnaire cycle and sex to calculate age- and multivariable-adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for liver-related mortality, accounting for a priori, time-varying covariates (see eMethods).
In stratified models, we assessed the influence of BMI or physical activity according to pre-specified sub-groups, and we tested the significance of interactions using the log likelihood ratio test. We also compared HCC-specific and cirrhosis/decompensation-specific mortality. To understand whether physical activity might counteract the excess risk conferred by adiposity, we jointly evaluated physical activity and BMI, as well as physical activity and WC. Finally, we calculated the population attributable risk conferred by low (<9 MET-hours/week) and very low (<3 MET-hours/week) levels of activity, to estimate the percentage of liver-related deaths that might have been prevented if all participants had engaged in regular physical activity, assuming a causal relationship between factors43.
Sensitivity Analyses
We tested the robustness of our results in numerous sensitivity analyses. First, we extended the latency period to 12 years (from 8 years). Second, because severe underlying disease or obesity may limit exercise, we excluded anyone reporting no baseline physical activity. Third, because incident viral hepatitis or cirrhosis may represent intermediates that impact both adiposity and exercise4, 19, we constructed separate models further accounting for those factors. Fourth, we continued updating exposures after the date of diagnosis of cirrhosis. Fifth, to more completely address the influence of alcohol use on liver-related mortality, we repeated the analysis after excluding anyone with significant alcohol consumption (i.e. ≥1 drink per day among women, or ≥2 drinks/day among men). In exploratory analyses, we compared resistance training and aerobic activity, and we repeated our primary analysis with liver transplantation included as a competing event. Finally, in an exploratory analysis, we compared the relative influence of obesity and physical activity on liver-related mortality, among adults with known cirrhosis in 1986 (n=775), who previously were excluded from the analysis.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with a 2-sided significance P-value<0.05.
Results:
Overall, we recorded 383 liver-related deaths among 77,238 women and 48,026 men. Table 1 shows the age-standardized characteristics according to BMI category, in 1986. Table S1 outlines the corresponding characteristics by physical activity quintile. After applying the 8-year latency, there were 105,894 subjects (70,810 women; 35,084 men), and 295 liver-related deaths (187 cirrhosis; 108 HCC) over 1,856,226 person-years of follow-up.
Table 1:
Age-standardized Characteristics of NHS (n=77,238) and HPFS (n=48,026) participants according to BMI Category, in 1986
| BMI Category, kg/m2 | |||||
|---|---|---|---|---|---|
| Cohort | <19 | 19 to <25 | 25 to <30 | 30 to <35 | ≥35 |
| Women (NHS) | 3,533 | 40,275 | 21,976 | 6,897 | 4,557 |
| median BMI, kg/m2 [IQR] | 18.3 [17.5, 18.9] | 23.4 [22.2, 24.1] | 26.6 [25.8, 27.9] | 31.2 [30.5, 32.3] | 36.0 [35.1, 38.5] |
| Age, years, SD | 52.8 (7.3) | 52.1 (7.1) | 53.3 (7.0) | 53.2 (6.9) | 52.1 (6.9) |
| White race, % | 96.2 | 96.3 | 95.8 | 95.8 | 95.2 |
| Waist-to-hip ratio, SD | 0.78 (0.09) | 0.80 (0.14) | 0.84 (0.11) | 0.86 (0.11) | 0.86 (0.11) |
| Physical Activity, MET-hrs/wk [IQR] | 7.9 [2.7, 20.5] | 8.6 [3.1, 20.4] | 7.1 [2.5, 16.8] | 5.2 [2.1, 13.7] | 3.9 [1.5, 10.9] |
| Hypertension, % | 15.2 | 25.1 | 37.9 | 47.1 | 51.8 |
| Dyslipidemia, % | 17.1 | 30.3 | 38.3 | 39.8 | 36.0 |
| Type 2 diabetes, % | 2.8 | 3.6 | 9.7 | 18.1 | 26.1 |
| Smoking status, % | |||||
| •Current | 23.3 | 13.4 | 9.7 | 8.2 | 6.4 |
| •Former | 36.3 | 42.4 | 44.9 | 45.3 | 44.7 |
| •Never | 40.4 | 44.2 | 45.4 | 46.5 | 48.9 |
| Alcohol intake, g/day [IQR] | 1.8 [0, 7.3] | 1.8 [0, 8.5] | 1.1 [0, 4.7] | 0 [0, 2.5] | 0 [0, 1.8] |
| Regular aspirin use1, % | 34.0 | 39.7 | 43.2 | 43.8 | 43.6 |
| Adherence to a healthy diet2, % | 22.8 | 26.6 | 24.6 | 21.7 | 18.2 |
| Men (HPFS) | 1,744 | 13,550 | 20,166 | 8,794 | 3,772 |
| median BMI, kg/m2 [IQR] | 17.9 [16.9, 18.5] | 22.3 [21.1, 23.8] | 26.9 [25.8, 28.2] | 31.5 [30.7, 32.5] | 36.7 [35.2, 39.8] |
| Age, years, SD | 54.0 (9.9) | 52.5 (9.6) | 53.4 (9.3) | 52.9 (8.6) | 52.1 (8.9) |
| White race, % | 94.9 | 95.1 | 95.9 | 96.2 | 96.6 |
| Waist-to-hip ratio, SD | 0.91 (0.06) | 0.93 (0.06) | 0.96 (0.06) | 0.98 (0.07) | 1.00 (0.08) |
| Physical Activity, MET-hrs/wk [IQR] | 10.9 [3.1, 25.9] | 15.1 [5.0, 32.3] | 10.8 [3.6, 25.9] | 7.6 [2.3, 19.4] | 5.0 [1.6-15.4] |
| Hypertension, % | 25.9 | 30.6 | 42.6 | 57.1 | 65.0 |
| Dyslipidemia, % | 28.9 | 40.2 | 46.2 | 49.4 | 48.0 |
| Type 2 diabetes, % | 5.3 | 4.8 | 8.2 | 17.2 | 25.5 |
| Smoking status, % | |||||
| •Current | 11.9 | 6.5 | 6.4 | 6.1 | 6.1 |
| •Former | 37.5 | 39.5 | 45.3 | 47.9 | 46.5 |
| •Never | 50.6 | 54.0 | 48.3 | 46.0 | 47.4 |
| Alcohol intake, g/day [IQR] | 1.8 [0, 8.3] | 2.6 [1.8, 13.1] | 2.0 [1.8, 12.7] | 1.8 [1.3, 9.6] | 1.8 [0.9, 5.1] |
| Regular aspirin use1, % | 34.3 | 43.9 | 47.4 | 49.0 | 49.0 |
| Adherence to a healthy diet2, % | 32.3 | 29.5 | 22.2 | 18.6 | 17.1 |
Abbreviations: No., number; BMI, body mass index; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; MET-hrs/wk, metabolic equivalent task hours per week; g/day, grams per day; IQR, interquartile range; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drug
All data reported as percentage (%) or mean±standard deviation (SD), unless noted otherwise. Except for mean of age, all data were age-standardized to the age distribution of participants. Physical activity was defined according to expended MET-hours/week (see Methods).
Regular use of aspirin was defined as consumption of at least 2 or more standard-dose (325-mg) tablets per week (vs. non-regular or non-use).
A healthy dietary pattern was defined using the Alternative Healthy Eating Index 2010 (AHEI-2010), modeled in quartiles. Adherence was defined as the highest quartile of the continuous AHEI score
Total and Central Adiposity
After multivariable adjustment, each 1-unit increase in cumulative average updated BMI predicted a 5.1% higher risk of liver-related mortality (95% CI 1.03-1.07; Ptrend<0.0001). Compared to normal BMI (19-<25), the aHRs for BMI 25-<30, 30-<35 and ≥35kg/m2 were 1.40 (95% CI 1.06-1.84), 2.22 (95% CI 1.56-3.16) and 2.97 (95% CI 1.84-4.79), respectively (Table 2). These findings were similar in men and women (Table S2), and they were consistent across all strata defined by pre-specified risk factors, including age, race/ethnicity, diabetes, alcohol use, smoking and diet (all Pinteractions>0.05; Tables S3). Moreover, this strong, positive gradient of risk was similar for HCC-specific and cirrhosis-specific mortality (Pheterogeneity=0.34; Table S4).
Table 2.
Liver-Related Mortality according to Body-Mass Index and Physical Activity, in the Pooled NHS and HPFS Cohorts
| BMI or PA Category | ||||||
|---|---|---|---|---|---|---|
| Lowest | Second | Third | Fourth | Highest | P for Trend | |
| Updated 8-Year Latency1 | ||||||
| Body Mass Index (kg/m2) | BMI <19 | BMI 19 to <25 | BMI 25 to <30 | BMI 30 to <35 | BMI ≥35 | -- |
| Liver-Related Deaths / Person-Years | 23 / 222,779 | 50 / 543,500 | 92 / 566,877 | 58 / 305,736 | 72 / 217,334 | -- |
| Crude Incidence / 100,000 Person-Years | 10 | 9 | 16 | 19 | 33 | -- |
| Age-adjusted HR (95%CI) | 1.24 (0.572.67) | 1 (ref.) | 1.55 (1.18-2.03) | 2.92 (1.08-4.10) | 4.37 (2.77-6.91) | <0.0001 |
| Multivariable adjusted HR‡; (95%CI) | 1.19 (0.55-2.58) | 1 (ref.) | 1.40 (1.06-1.84) | 2.22 (1.56-3.16) | 2.97 (1.84-4.79) | <0.0001 |
| Physical Activity (MET-hour / week) | Lowest Quintile | Second | Third | Fourth | Highest Quintile | -- |
| Median [IQR] | 1.2 [0.4 to 2.3] | 5.1 [3.6 to 7.7] | 11.0 [8.6 to 15.6] | 21.3 [17.2 to 28.3] | 46.9 [34.6 to 65.5] | -- |
| Liver-Related Deaths / Person-Years | 93 / 341,257 | 66 / 370,359 | 55 / 388,307 | 44 / 384,463 | 37 / 371,840 | -- |
| Crude Incidence / 100,000 Person-Years | 27 | 18 | 14 | 11 | 10 | -- |
| Age-adjusted HR (95%CI) | 1 (ref.) | 0.70 (0.51-0.96) | 0.59 (0.42-0.84) | 0.52 (0.36-0.74) | 0.46 (0.31-0.66) | <0.0001 |
| Multivariable adjusted HR‡; (95%CI) | ||||||
| •Without BMI | 1 (ref.) | 0.73 (0.53-1.01) | 0.64 (0.45-0.90) | 0.58 (0.40-0.84) | 0.50 (0.34-0.73) | <0.0001 |
| •With BMI | 1 (ref.) | 0.75 (0.54-1.03) | 0.67 (0.47-0.95) | 0.63 (0.44-0.91) | 0.54 (0.37-0.78) | 0.0003 |
| Baseline1 | ||||||
| Body Mass Index (kg/m2) | <19 | 19 to <25 | 25 to <30 | 30 to <35 | ≥35 | -- |
| Liver-Related Deaths / Person-Years | 10 / 64,737 | 131 / 1,489,374 | 148 / 1,032,255 | 64 / 257,102 | 30 / 95,528 | -- |
| Crude Incidence / 100,000 Person-Years | 15 | 9 | 14 | 25 | 31 | |
| Age-adjusted HR (95%CI) | 1.93 (1.01-3.69) | 1 (ref.) | 1.56 (1.23-1.98) | 2.89 (2.15-3.90) | 3.83 (2.57-5.71) | <0.0001 |
| Multivariable adjusted HR1; (95%CI) | 1.66 (0.87-3.17) | 1 (ref.) | 1.35 (1.06-1.72) | 1.90 (1.38-2.60) | 2.04 (1.34-3.11) | <0.0001 |
| Physical Activity (MET-hour / week) | Lowest Quintile | Second | Third | Fourth | Highest Quintile | -- |
| Median [IQR] | 0.9 [0.4 to 1.5] | 3.6 [2.9 to 4.4] | 8.4 [6.7 to 10.2] | 17.9 [14.3 to 21.1] | 39.4 [31.5 to 55.4] | -- |
| Liver-Related Deaths / Person-Years | 108 / 560,489 | 81 / 579,411 | 65 / 592,292 | 62 / 604,128 | 67 / 602,677 | -- |
| Crude Incidence / 100,000 Person-Years | 19 | 14 | 11 | 10 | 11 | -- |
| Age-adjusted HR (95%CI) | 1 (ref.) | 0.73 (0.54-0.97) | 0.58 (0.42-0.78) | 0.57 (0.42-0.77) | 0.53 (0.39-0.73) | <0.0001 |
| Multivariable adjusted HR1; (95%CI) | ||||||
| •Without BMI | 1 (ref.) | 0.79 (0.59-1.05) | 0.72 (0.53-0.98) | 0.65 (0.48-0.89) | 0.64 (0.47-0.87) | 0.009 |
| •With BMI | 1 (ref.) | 0.80 (0.60-1.07) | 0.77 (0.56-1.05) | 0.68 (0.50-0.92) | 0.68 (0.49-0.93) | 0.009 |
Abbreviations: BMI, body mass index; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; CI, confidence interval; MET-hour, metabolic equivalent task hour; PA, physical activity.
For the baseline analyses, BMI and PA were ascertained in 1986, with follow-up from 1986-2012 (n=125,264). For the latency analyses, an 8-year period was used, with a follow-up beginning in 1994 (n=105,894). For details see Methods.
Multivariable Cox proportional hazards regression model conditioned on age (years), sex and year of questionnaire return, with further adjustment for race (white, black, hispanic, asian or other), alcohol intake (0 – 4.9 g/day, 5-14.9 g/day, ≥15 g/day), smoking status (current vs. prior vs. never), type 2 diabetes (yes vs. no), hypertension (yes vs. no), dyslipidemia (yes vs. no), statin medication use (yes vs. no), regular aspirin use (≥2 tablets per week vs. no), coffee consumption (continuous), adherence to a healthy diet, defined by the continuous alternative healthy eating index (AHEI).
To minimize potential reverse causation, we conducted additional analyses using BMI exposures from early adulthood (i.e. age 18 years [women], or 21 years [men]) and from mid-life (in 1986). Consistent with the primary analysis, higher mid-life BMI predicted significantly increased risk of liver-related mortality in later life (Ptrend <0.0001); specifically, compared to mid-life BMI 19-<25, the highest risk was observed with mid-life BMI ≥35kg/m2 (aHR 2.04, 95% CI 1.34-3.11; Table 2). These findings remained similar after further accounting for early adulthood BMI (Ptrend <0.0001). Additionally, we observed that a higher early-adulthood BMI also was independently associated with increased risk of liver-related mortality (Ptrend=0.0002), even after further adjusting for mid-life BMI (Ptrend=0.0003).
In analyses focused on central adiposity, each 1cm increase in WC predicted a significant, 5.6% higher risk of liver-related mortality (95% CI 1.02-1.09; Ptrend =0.003). Compared to the lowest WC quartile, the aHR in the highest quartile was 2.09 (95% CI 1.16-3.77). These patterns of association were similar when WHR was used in place of WC (Ptrend<0.0001).
Physical Activity
Overall, every 3 MET-hours/week of exercise – the equivalent of walking at an average pace for 1 hour/week – conferred a 3.5% reduction in risk of liver-related mortality (95% CI 0.95-0.98). The multivariable aHRs across increasing physical activity quintiles were: 1.0, 0.70 (95% CI 0.51-0.96), 0.59 (95% CI 0.42-0.84), 0.52 (95% CI 0.36-0.74) and 0.46 (95% CI 0.31-0.66), respectively (Ptrend<0.0001; Table 2). This inverse gradient was not materially altered after further accounting for BMI (Ptrend=0.0003), and it was similar when we considered only baseline exposures (Ptrend=0.009). We observed consistent, inverse associations among both women and men (Table S2), and in analyses comparing HCC-specific and cirrhosis-specific mortality (Pheterogeneity=0.42; Table S4). Our findings also remained similar when physical activity was modeled in clinically relevant categories (i.e. <3, 3-<9, 9-<18, 18-<27 and ≥27 MET-hours/week), corresponding to walking at an average pace for <1, 1 to <3, 3 to <6, 6 to <9 and ≥9 hours per week (Ptrend<0.0001; Table S5).
At baseline, 32% of participants had low physical activity (<9 MET-hours/week), and 11% had very low physical activity (<3 MET-hours/week). Accordingly, we estimated that exercising for at least 3 MET-hours/week or more could have prevented 16% of liver-related deaths (95% CI 0.07-0.26), and exercising for >9 MET-hours/week could have prevented 25% of liver-related deaths (95% CI 0.12-0.38).
Joint Associations
At all levels of BMI, increasing level of physical activity predicted significantly reduced risk of liver-related mortality (all Ptrend<0.0001; Figure 1). When examined jointly, the risk was lowest among lean-active adults (BMI<25, PA≥18 MET-hours/week). Compared to this lean-active group, the highest risk was found among obese-sedentary adults (BMI≥30; <3 MET-hours/week), who had aHR, 3.40 (95% CI 2.06-5.56). Importantly, physical activity appeared to attenuate the excess risk associated with obesity: specifically, the aHRs were 1.04 (95% CI 0.73-1.37) for obese-active adults (BMI≥30; ≥18 MET-hours/week), and 1.13 (95% CI 0.83-1.52) for obese-moderately active adults (BMI≥30; 9-<18 MET-hours/week). In contrast, being lean did not fully attenuate the excess risk associated with sedentary behavior: compared to lean-active adults, the aHR among lean-sedentary adults was 2.08 (95% CI 1.21-3.33). These findings were similar when we used WC in place of BMI (Figure S1).
Figure 1. Multivariate ‡ Risk of Liver-Related Mortality according to Body Mass Index and Physical Activity Level.
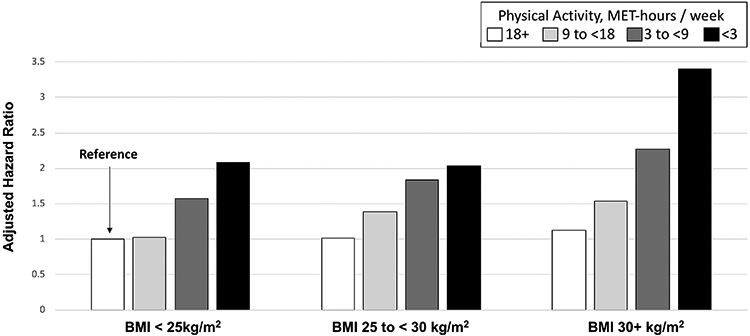
Each column shows the multivariable-adjusted hazard ratio for the joint association of body mass index (BMI) and physical activity on the risk of liver-related mortality.
Abbreviations: BMI, body mass index (kg/m2); MET-hours/ week, metabolic equivalent task hours per week; HR, hazard ratio.
‡ Multivariable Cox proportional hazards regression model conditioned on age (years), sex and year of questionnaire return, with adjustment for time-updated covariates (see footnotes to Table 2).
Exposure variables included cumulative updated BMI and physical activity, with an 8-year latency period, as outlined in the Methods
Early-Adulthood Weight Gain
Weight gain between early adulthood and mid-life contributed to significantly higher risk of liver-related mortality in later life (Ptrend<0.0001), even after accounting for early-adulthood weight (Ptrend<0.0001) (Table 3). Compared to participants with stable weight (+/− 5kg), the highest risk was observed with weight gain ≥15kg (aHR 1.82, 95% CI 1.32-2.51). This association was significantly modified by physical activity level: compared to persons with stable weight between early adulthood and mid-life, the aHRs for weight gain ≥15kg was 1.41 (95% CI 0.79-2.51) for active adults, and 2.19 (95% CI 1.30-3.69) for sedentary adults (Pinteraction=0.014). These associations were similar for both HCC- and cirrhosis-specific mortality (Table S6).
Table 3.
Weight Change in Early Adulthood, Physical Activity and Risk of Liver-Related Mortality in Later Life1
| Weight Loss |
Weight Gain |
|||||
|---|---|---|---|---|---|---|
| Variable | ≥ −5kg | -5 to < 5kg | 5 to <10kg | 10 to <15kg | ≥15 kg | P for Trend |
| No. of liver-related deaths | 116 | 71 | 42 | 69 | 83 | -- |
| Person-years | 605,371 | 726,687 | 510,191 | 629,496 | 464,630 | -- |
| Multivariable adjusted HR1; (95%CI) | 1.87 (1.38-2.52) | 1 (ref.) | 0.98 (0.74-1.21) | 1.26 (0.83-1.91) | 1.82 (1.32-2.51) | <0.0001 |
| Multivariable-adjusted HR by PA strata (95%CI) | ||||||
| •Sedentary: <3 MET-hours / week | 2.16 (1.31-3.58) | 1 (ref.) | 1.12 (0.66-1.89) | 1.28 (0.63-2.56) | 2.19 (1.30-3.69) | 0.002 |
| •Moderate: 3 to <9 MET-hours / week | 1.91 (1.12-3.27) | 1 (ref.) | 1.06 (0.48-2.31) | 1.08 (0.62-1.88) | 1.91 (1.09-3.36) | 0.02 |
| •Active: ≥9 MET-hours / week | 1.43 (0.89-2.31) | 1 (ref.) | 0.76 (0.46-1.27) | 1.01 (0.49-1.87) | 1.41 (0.79-2.41) | 0.15 |
Abbreviations: No., number; kg, kilogram; HR, hazard ratio; CI, confidence interval; MET, metabolic equivalent task
This analysis included the baseline study population (n=125,264) and excluded any participant with missing data regarding weight in early adulthood (defined as age 18 years [women] or 21 years [men]). Weight change was the difference in weight in kilograms between early adulthood and study baseline (in 1986). Physical activity (MET-hours/week) was defined using baseline data from 1986.
Multivariable Cox proportional hazards regression model conditioned on age (years), sex and year of questionnaire return, with further adjustment for weight in early adulthood (continuous kilograms), race (white, black, hispanic, asian or other), alcohol intake (0 – 4.9 g/day, 5-14.9 g/day, ≥15 g/day), smoking status (current vs. prior vs. never), type 2 diabetes (yes vs. no), hypertension (yes vs. no), dyslipidemia (yes vs. no), statin medication use (yes vs. no), regular aspirin use (≥2 tablets per week vs. no), and adherence to a healthy diet, defined by the alternative healthy eating index 2010 (AHEI), with all relevant covariates ascertained in 1986.
Sensitivity Analyses:
Our findings were robust across all sensitivity analyses, including: (1) after applying a 12-year latency period (Table S7); (2) after excluding any participant who reported no exercise (n=14,364 excluded; Table S8); (3) after further adjusting for incident viral hepatitis or cirrhosis (Table S9); (4) after continuing to update exposures, after a diagnosis of incident cirrhosis or viral hepatitis (Table S10); and (5) after excluding any participant with significant alcohol consumption (i.e. ≥1 drink per day among women, or ≥2 drinks/day among men) (Table S11). In an exploratory analysis comparing resistance training and aerobic activity, the observed magnitude of benefit appeared similar (Table S12). Additionally, including liver transplantation as a competing event did not materially impact our results (not shown). Finally, in an exploratory analysis of adults with underlying cirrhosis in 1986 (n=775), we observed a similar, significant, inverse association between increasing physical activity level and reduced liver-related mortality risk (Ptrend =0.002).
Discussion
In two prospective cohorts of U.S. men and women, low physical activity levels and elevated BMI were each independently associated with increased risk of liver-related mortality, including death from HCC and cirrhosis. Risk of liver-related mortality was consistently and significantly elevated with obesity in early adulthood, in mid-life, and even with modest weight gain during early adulthood. However, increased physical activity during adulthood predicted significantly lower risk of liver-related mortality, regardless of BMI. Importantly, we found that the excess risk conferred by obesity was substantially attenuated among adults who engaged in >9 MET-hours per week of physical activity, the equivalent of walking at an average pace for 3 hours per week or more.
Short-term clinical studies have demonstrated that physical activity is associated with reductions in liver fat, inflammation and insulin resistance14, 15, 19, 36, 44; further, among patients with HCC, therapeutic exercise may increase muscle mass45, while exercise capacity may predict post-hepatectomy survival46. However, to date, no large-scale, prospective study has evaluated whether this might translate to improved long-term hepatic outcomes19. Published epidemiological evidence regarding physical activity and long-term hepatic events derive from just three prior studies, that linked higher physical activity levels to reduced incidence of hepatobiliary cancer9-11. However, those prior studies did not assess liver-related mortality, nor did they include prospectively updated exposures, or account for key clinical and lifestyle factors, including underlying cirrhosis, alcohol use, diet and smoking, which are essential to accurately estimate the long-term effects of physical activity and adiposity on hepatic outcomes. Specifically, failing to address underlying cirrhosis can lead to reverse causation, because cirrhosis contributes to weight loss and to increased mortality. In contrast, by excluding individuals with cirrhosis at baseline, and including detailed and updated clinical and lifestyle data over 26 years of follow-up, the current study is able to more precisely characterize the relationships between physical activity, adiposity and liver-related mortality.
We observed strong, inverse associations between increasing physical activity and reduced risk of liver-related mortality, that were consistent regardless of BMI or WC. Importantly, the excess risk associated with obesity was substantially attenuated in persons who exercised for at least 9 MET-hours/week, the equivalent of average-pace walking for just 3 hours/week. Given the lack of published data or clinical guidelines regarding the optimal weight or physical activity level to improve major hepatic outcomes, these findings are both timely and important. Our data demonstrate for the first time that exercise is an independent, modifiable determinant of long-term hepatic outcomes. As such, our findings support additional research to identify the optimal type, intensity and duration of physical activity, for incorporation into prevention guidelines.
Several mechanisms might explain the shared effects of exercise and adiposity on liver disease progression and mortality. Obesity is marked by disproportionately increased visceral adipose tissue (VAT) volume, which promotes fibrosis progression47, 48, while exercise preferentially reduces VAT, even when weight loss is not achieved36, 49, 50. Further, exercise interventions can attenuate liver fat and inflammation14, 15, 19, 36, 44, normalize pro-inflammatory biomarkers50, 51 and modulate bile acids52, which may impact the natural history of liver disease. Finally, because physical activity and obesity may operate on similar inflammatory and carcinogenic pathways, it is possible that a key benefit of physical activity is the minimization of long-term weight gain, over the lifespan53. Accordingly, it has been recommended that studies examining adiposity and physical activity leverage prospectively-updated longitudinal data, to best elucidate these relationships53.
This study is strengthened by the prospective design, large sample size, and by the inclusion of two independent cohorts with high rates of follow-up and updated information regarding numerous risk factors for liver-related mortality. The prospective design minimizes potential recall bias, and any errors in recall would most likely have attenuated rather than exaggerated a true association. The use of repeated assessments reduces measurement error, while simultaneously addressing real-life changes in adiposity and physical activity patterns over time. Further, accounting for a range of times between exposure assessment and death minimizes the possibility of reverse causation.
We acknowledge several limitations. First, we cannot exclude the possibility of residual confounding, and we lacked detailed data regarding histological fibrosis stage, non-invasive estimates of fibrosis or adherence to HCC screening guidelines. However, the observed benefits of physical activity were robust across all sensitivity analyses, after careful accounting for viral hepatitis and alcohol use, and they were similar in persons with and without underlying cirrhosis. Second, although reverse causation is possible, we conducted baseline and extended latency analyses in which exposures were assessed many years prior to study outcomes, rendering it highly unlikely that symptoms of undiagnosed liver disease could have biased our results. Third, we acknowledge that our participants are predominantly white, highlighting the need for future research in more diverse, prospective populations; however, our age- and sex-specific rates of HCC and cirrhosis-specific mortality39, and our well-validated covariate data24, 25, closely approximate other epidemiological cohorts, supporting the generalizability of our findings. Fourth, specific types of physical activity may be more beneficial than others, underscoring the need for future studies with detailed, prospectively updated physical activity data and long-term outcomes, to define the optimal type and intensity of physical activity to maximize risk reduction. Finally, active participants may be more likely to adhere to other healthy behaviors; while our results were similar after carefully accounting for health-promoting factors like diet, alcohol and smoking, future studies are needed that focus on lifestyle overall.
In conclusion, within two prospective cohorts of U.S. adults without established liver disease at enrollment, both low physical activity and obesity were independent predictors of liver-related mortality, including death from cirrhosis and HCC. Significantly elevated risk was apparent with obesity in early adulthood, in mid-life, and with weight gain during early adulthood. However, the excess risk conferred by obesity was markedly attenuated by physical activity, and it was no longer statistically significant among adults who engaged in >9 MET-hours per week of physical activity. These findings demonstrate that physical activity is a major modifiable determinant of long-term hepatic outcomes. They also support further research to characterize the optimal type and intensity of activity for the prevention of liver-related mortality.
Supplementary Material
Acknowledgements:
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Role of the Funding Source:
No sponsor had a role in the study design, data collection, analysis or interpretation of the data, or in the writing of the manuscript or the decision to submit the paper for publication. All authors had full access to the study data and had final responsibility for the decision to submit for publication.
Grant Support:
UM1 CA186107 (Nurses’ Health Study infrastructure grant)
P01 CA87969 (Nurses’ Health Study program grant for cancer research)
UM1 CA167552 (Health Professionals Follow-up Study infrastructure grant)
NIH K24 DK078772 (RTC)
NIH K24 DK098311 (ATC)
NIH K07 CA188126 (XZ)
NIH K23 DK122104 (TGS)
Dr. Simon is supported by the American Association of the Study of Liver Diseases (AASLD) Clinical and Translational Research Award (CTORA), and by the Boston Nutrition Obesity Research Council (BNORC; NIH P30DK046200-26).
Dr. Chan is a Stuart and Suzanne Steele MGH Research Scholar
Footnotes
Disclosures and conflicts of interest:
Dr. Chan has previously served as a consultant for Bayer Pharma AG for work unrelated to this manuscript.
Dr. Meyerhardt has received institutional research funding from Boston Biomedical; JAM has served as an advisor/consultant to Ignyta, Array Pharmaceutical and Cota.
The remaining authors have no disclosures and no conflicts of interest to disclose.
REFERENCES
- 1.Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology 2015;149:1471–1482 e5; quiz e17-8. [DOI] [PubMed] [Google Scholar]
- 2.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999-2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 4.Dunn MA, Josbeno DA, Schmotzer AR, et al. The gap between clinically assessed physical performance and objective physical activity in liver transplant candidates. Liver Transpl 2016;22:1324–32. [DOI] [PubMed] [Google Scholar]
- 5.Long MT, Pedley A, Massaro JM, et al. Hepatic steatosis is associated with lower levels of physical activity measured via accelerometry. Obesity (Silver Spring) 2015;23:1259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber L, Otgonsuren M, Mishra A, et al. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther 2012;36:772–81. [DOI] [PubMed] [Google Scholar]
- 7.Hagstrom H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut 2018;67:1536–1542. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Balkwill A, Reeves G, et al. Body mass index and risk of liver cirrhosis in middle aged UK women: prospective study. BMJ 2010;340:c912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumeister SE, Schlesinger S, Aleksandrova K, et al. Association of Physical Activity and Risk of Hepatobiliary Cancers: A Multinational Cohort Study. J Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 10.Behrens G, Matthews CE, Moore SC, et al. The association between frequency of vigorous physical activity and hepatobiliary cancers in the NIH-AARP Diet and Health Study. Eur J Epidemiol 2013;28:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore SC, Lee IM, Weiderpass E, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016;176:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell PT, Newton CC, Freedman ND, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res 2016;76:6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ioannou GN, Weiss NS, Kowdley KV, et al. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology 2003;125:1053–9. [DOI] [PubMed] [Google Scholar]
- 14.Kantartzis K, Thamer C, Peter A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009;58:1281–8. [DOI] [PubMed] [Google Scholar]
- 15.Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157–66. [DOI] [PubMed] [Google Scholar]
- 16.Wong VW, Chan RS, Wong GL, et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2013;59:536–42. [DOI] [PubMed] [Google Scholar]
- 17.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Windt DJ, Sud V, Zhang H, et al. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr 2018;18:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol 2018;69:1164–1177. [DOI] [PubMed] [Google Scholar]
- 20.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2012;62:30–67. [DOI] [PubMed] [Google Scholar]
- 21.Varghese T, Schultz WM, McCue AA, et al. Physical activity in the prevention of coronary heart disease: implications for the clinician. Heart 2016;102:904–9. [DOI] [PubMed] [Google Scholar]
- 22.Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13–64. [DOI] [PubMed] [Google Scholar]
- 24.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology 1996;7:81–6. [DOI] [PubMed] [Google Scholar]
- 26.Rimm EB, Stampfer MJ, Colditz GA, et al. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am J Epidemiol 1990;131:1068–71. [DOI] [PubMed] [Google Scholar]
- 27.Chan AT, Giovannucci EL, Meyerhardt JA, et al. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 2008;134:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan AT, Manson JE, Feskanich D, et al. Long-term aspirin use and mortality in women. Arch Intern Med 2007;167:562–72. [DOI] [PubMed] [Google Scholar]
- 29.Simon TG, Ma Y, Ludvigsson JF, et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol 2018;4:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 31.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–2. [PubMed] [Google Scholar]
- 32.Hu FB, Leitzmann MF, Stampfer MJ, et al. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8. [DOI] [PubMed] [Google Scholar]
- 33.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694–703. [DOI] [PubMed] [Google Scholar]
- 34.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 35.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25:71–80. [DOI] [PubMed] [Google Scholar]
- 36.Johnson NA, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- 37.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140:1016–9. [DOI] [PubMed] [Google Scholar]
- 38.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol 1984;119:837–9. [DOI] [PubMed] [Google Scholar]
- 39.Asrani SK, Larson JJ, Yawn B, et al. Underestimation of liver-related mortality in the United States. Gastroenterology 2013;145:375–82 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keum N, Bao Y, Smith-Warner SA, et al. Association of Physical Activity by Type and Intensity With Digestive System Cancer Risk. JAMA Oncol 2016;2:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Tsao G, Friedman S, Iredale J, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010;51:1445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai S, Carroll DD, Watson KB, et al. Participation in Types of Physical Activities Among US Adults--National Health and Nutrition Examination Survey 1999-2006. J Phys Act Health 2015;12 Suppl 1:S128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothman KJ, Greenland S Modern epidemiology. 2nd ed. Philadelphia: Lippincott-Raven, 1998.: [Google Scholar]
- 44.Lee S, Deldin AR, White D, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. Am J Physiol Endocrinol Metab 2013;305:E1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koya S, Kawaguchi T, Hashida R, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol 2019;34:580–588. [DOI] [PubMed] [Google Scholar]
- 46.Kaibori M, Matsui K, Yoshii K, et al. Perioperative exercise capacity in chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. PLoS One 2019;14:e0221079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petta S, Amato MC, Di Marco V, et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012;35:238–47. [DOI] [PubMed] [Google Scholar]
- 48.Kim Y, Chang Y, Cho YK, et al. Obesity and Weight Gain Are Associated With Progression of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2019;17:543–550 e2. [DOI] [PubMed] [Google Scholar]
- 49.O'Leary VB, Marchetti CM, Krishnan RK, et al. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol (1985) 2006;100:1584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol (1985) 2005;99:1220–5. [DOI] [PubMed] [Google Scholar]
- 51.Malaguarnera R, Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front Endocrinol (Lausanne) 2014;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morville T, Sahl RE, Trammell SA, et al. Divergent effects of resistance and endurance exercise on plasma bile acids, FGF19, and FGF21 in humans. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovannucci E An Integrative Approach for Deciphering the Causal Associations of Physical Activity and Cancer Risk: The Role of Adiposity. J Natl Cancer Inst 2018;110:935–941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


