Abstract
Background
Cognitive impairment in chronic kidney disease (CKD) is common and underrecognized [1, 2]. Determining risk factors for cognitive impairment and whether speed of CKD progression is an important consideration may help identify cognitive impairment by nephrologists. Vascular disease is thought to underpin cognitive impairment in CKD and by segregating CKD patients with proven vascular disease, we may also be able to discover other important associations with cognitive impairment in CKD patients.
Method
A total of 250 patients in a UK prospective cohort of CKD patients underwent two cognitive assessments: Montreal Cognitive Assessment test and Trail Making Test. Cognitive impairment was defined using validated population cut-offs (cognitive impairment) and relative cognitive impairment. Relative cognitive impairment was defined by <1 standard deviation below the mean Z-score on any completed test. Two multivariable logistical regression models identified variables associated with cognitive impairment and realtive cognitive impairment.
Results
About 44 and 24.8% of patients suffered cognitive impairment and relative cognitive impairment, respectively. Depression, previous stroke and older age were significantly associated with cognitive impairment. Older age was significantly associated with relative cognitive impairment (P ≤ 0.05) and higher proteinuria and the use of psychodynamic medications were also significantly associated with relative cognitive impairment (P = 0.05). Delta estimated glomerular filtration rate (eGFR) in patients with cognitive impairment and relative cognitive impairment compared with those having normal cognition was similar (−0.77 versus −1.35 mL/min/1.73 m2/year, P = 0.34 for cognitive impairment and −1.12 versus −1.02 mL/min/1.73 m2/year, P = 0.89 for relative cognitive impairment).
Conclusion
Risk factors for cognitive impairment in CKD include previous stroke, depression or anxiety, higher proteinuria and prescription of psychodynamic medications. Patients with a faster eGFR decline do not represent a group of patients at increased risk of cognitive impairment.
Keywords: age, CKD, depression, elderly, GFR, proteinuria, quality of life
INTRODUCTION
Cognitive impairment is common and often unrecognized [1, 2]. Chronic kidney disease (CKD) confers an increased risk of cognitive impairment [3–6]. Nephrologists are poor at recognizing patients with cognitive impairment [7]. The speed of CKD progression has not previously been investigated as a risk factor for cognitive impairment in patients with moderate non-dialysis CKD (ND-CKD). The primary objective of this study was to determine if the speed of estimated glomerular filtration rate (eGFR) decline is a significant risk factor for cognitive impairment after accounting for other clinically important variables. If faster declining renal function is associated with cognitive impairment, then this could help identify patients with cognitive impairment and those who require additional support with decision-making or medication adherence [8]. There may be an association with increased burden of cardiovascular disease, cognitive impairment and rapidly declining eGFR [9]. Alternatively, the duration of CKD, associated oxidative stress, endothelial dysfunction and inflammation may be a more pertinent consideration underpinning cognitive disease in CKD.
The diagnosis of cognitive impairment is typically made using absolute validated numerical cut-off scores in brief cognitive screening assessments [10–13]. The diagnosis can also be made using a relative score that is significantly lower than the rest of the studied population [5, 14–16]. A second element of our study aimed to determine a difference in cardiovascular and psychosocial risk factor profiles according to the method of diagnosis used. Furthermore, we wanted to better understand the overlap between our primary cognitive impairment measure, the Montreal Cognitive Assessment (MoCA) test and the Trail Making Test (TMT). The TMT is quicker and easier to administer and examines executive function, which is proposed as the most common cognitive deficit in CKD [17].
Finally, we postulated that traditional cardiovascular risk factors for cognitive disease might underpin any model of cognitive impairment in CKD. We therefore investigated the risk factor profiles for cognitive impairment in patients with and without established vascular disease.
MATERIALS AND METHODS
Salford Kidney Study
Cognitive and quality of life assessments were undertaken in consecutive patients as part of the Salford Kidney Study (SKS) annual review between December 2016 and August 2018. The SKS is a prospectively collected longitudinal epidemiological cohort study of >3000 patients with all-cause ND-CKD recruited since 2002. Ethical approval was granted by the regional ethics committee (REC15/NW/0818). The SKS was previously known as the Chronic Renal Insufficiency Standards Implementation Study, details of which have been published previously [18, 19]. The SKS recruit patients >18 years old referred to a tertiary renal centre (catchment population 1.55 million) with an eGFR <60 mL/min/1.73 m2 and not requiring immediate renal replacement therapy. Patients with known dementia, suspected dementia or severe psychological illness were not included in this study. Demographic, comorbidity and laboratory data were recorded at baseline and annually. All previous cerebrovascular and cardiovascular events were validated following a review of clinical notes, radiology reports, general practice records and clinical coding. Laboratory parameters were included from the same study visit as the cognitive assessment or within 7 days if collected in the outpatient setting. Psychodynamic medications were recorded and included opiate and neuropathic analgesics, anticholinergics, benzodiazepines and antihistamines. The change in eGFR was determined using linear regression of eGFR measures for patients who had more than two outpatient eGFR results >2 years prior to cognitive assessments. Fast progression of CKD was defined as an eGFR decline >3 mL/min/1.73 m2/year [9]. Alternative methods of rapid decline in renal function were also used and included >20% decline in eGFR within the 12 months antecedent to the cognitive assessment and >20 and >50% decline in eGFR from baseline during the study.
Cognitive assessments
The MoCA [10] and the TMT A and B [12] were performed on each patient by a trained research nurse. The MoCA is a brief cognitive impairment screening tool that covers the cognitive domains of episodic memory, executive function, language, attention, visuospatial ability and orientation. It takes <15 min to administer, with a maximum score of 30. A score <26 after adjustment for education defined cognitive impairment. Recent data have suggested a score <19 as the optimal cut-off to categorize possible dementia as opposed to cognitive impairment, so these patients were highlighted to their treating clinician with advice for further referral [20].
The TMT is a short test made up of two parts [12, 21]. Part A tests visual awareness and motor speed. Part B tests executive function due to its requirement for mental flexibility, although visual scanning and psychomotor speed are also assessed [22, 23]. The TMT was also categorized as a binary variable whereby cognitive impairment is defined as a Trail-A score >75 s or a Trail-B score >180 s [13].
The cognitive outcome measures used in this study were as follows: cognitive impairment (binary and continuous output from MoCA); cognitive impairment (binary and continuous output from TMT A or TMT B); relativd cognitive impairment continuous, which is the sum of the Z-score of completed cognitive tests divided by the number of cognitive tests fully completed (used in correlation analysis only); and relative cognitive impairment binary, which is any Z-score on either the MoCA or TMT >1 standard deviation (SD) below the mean (Z-score >−1).
Quality of life assessments
Health-related quality of life measurements were assessed using the EuroQol 5-dimension 5-level (EQ-5D-5L) questionnaire [24]. The EQ-5D-5L questionnaire uses an ordinal scale of 1–5 for most domains of quality of life. Depression was categorized if patients self-reported depression or anxiety was moderate, severe or extreme (3–5 on the scale).
Statistical analysis
Data distribution analysis was performed using the Shapiro–Wilk test. All continuous variables were non-parametric and test results are presented as median and interquartile range (IQR). The MoCA and TMT scores were standardized to produce three cognitive Z-scores such that a higher Z-score describes better cognitive performance. Spearman rank correlations were used to analyse correlations between TMT A and B times. Relative cognitive impairment was operationally defined as a cognitive score for any of the cognitive measures falling >1 SD below mean standardized test scores. This criterion for relatively poor performance has been used in previous studies [5, 25], including publication of population norms [26–28].
Two multivariable models were created for both cognitive impairment and relative cognitive impairment outcome variables, one examining psychosocial factors and one examining physical comorbidity variables. This was an exploratory analysis of factors associated with cognitive impairment and relative cognitive impairment. The two models avoided overfitting the regression models, which may mask genuine relationships between variables. Age and eGFR were included in both model types due to their significant correlations with cognitive impairment. We analysed psychosocial covariates with relative cognitive impairment and cognitive impairment in (i) an unadjusted model, (ii) an age-adjusted model, (iii) a parsimonious model including age and eGFR and (iv) a fully adjusted psychosocial model. In the comorbidity model, we tested the association of fast eGFR decline with cognitive impairment and relative cognitive impairment in the same models (i–iii), (iv) the fully adjusted comorbidity model and (v) the same model including fast eGFR progression (eGFR at the time of cognitive assessment was not included in this final model due to collinearity with fast eGFR progression).
All variables were then analysed unadjusted and with adjustment for eGFR and age in two subgroups:,patients with known vascular disease [previous coronary bypass grafting, angioplasty, myocardial infarction (MI), stroke, transient ischaemic attack and peripheral vascular disease] and patients without known vascular disease. A P-value <0.05 was considered statistically significant for all analyses. There is scant evidence investigating comorbid, demographic and psychosocial associations of cognitive impairment and relative cognitive impairment in moderate to severe CKD, so multiplicity adjustments were not performed, as these may have resulted in a type 2 error [29]. Analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA) licensed to the University of Manchester.
RESULTS
All 251 participants attending an SKS annual review visit were consecutively approached and agreed to a cognitive assessment. A total of 250 participants completed the MoCA [median age 55 years (IQR 53–74)], of which 239 participants also undertook both parts of the TMT. One patient was excluded due to missing demographic data; 217 patients also completed the quality of life assessments. The MoCA found 111 (44.4%) patients were classified as cognitively impaired and 62 (24.8%) as suffering relative cognitive impairment (of whom 79.0% had been classified as cognitive impairment using the MoCA). Thirteen patients were classified as having relative cognitive impairment but not cognitive impairment as defined using MoCA. In these 13 patients, cognitive deficiencies were driven by poor performance on TMT A and/or B. Eighteen (7.2%) patients were classified as cognitively impaired using the binary cut-off values of the TMT A and/or B, 12 of whom were already identified as having cognitive impairment using the MoCA. Six patients were categorized as having relative cognitive impairment but did not score <26 on the MoCA, >75 s on TMT A or >180 s on TMT B (Figure 1).
FIGURE 1:
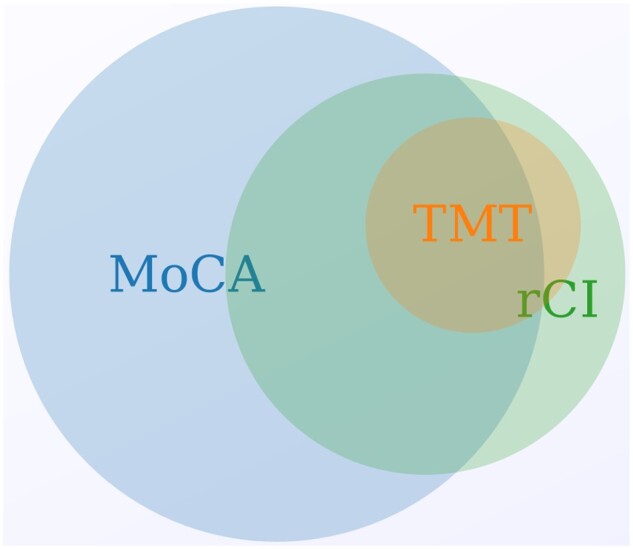
A proportionate Venn diagram demonstrating the overlapping definitions of cognitive impairment according to the different cognitive tests and definitions used. Created using meta-chart.com.
Participant characteristics split by cognitive impairment definition are presented in Table 1. Patients who did not have cognitive impairment on any test performed (n = 127) had significantly better renal function [median eGFR 39 mL/min/1.73 m2 (IQR 23–50)] and significantly lower proteinuria [28 mg/mmol (IQR 7–100)]. Patients identified with cognitive impairment using any method of definition were significantly older, more likely to be retired or widowed and suffer/had suffered from atrial fibrillation, peripheral vascular disease, MI, stroke or coronary heart disease than patients with normal cognitive performance. Significantly fewer patients whose primary renal disease was glomerulonephritis demonstrated cognitive impairment (P = 0.031).
Table 1.
Demographics, comorbidity and laboratory data of participants on the day of study
| Variable | Total (N = 250) | Normal cognitive performance ( n = 127) | Cognitive impairment (MoCA < 26/30) ( n = 111) | Cognitive impairment (TMT A or B) (n = 18) | Relative cognitive impairment (>1 SD below the mean Z-score on any test) (n = 62) | Significance (normal versus cognitive impairment using any measure) |
|---|---|---|---|---|---|---|
| Age (years) | 66 (53–74) | 59 (49–69) | 70 (58–77) | 77 (75–84) | 76 (67–81) | 0.000 |
| Sex, n (%) | ||||||
| Male | 164 | 81 (63.8) | 74 (66.7) | 11 (61.1) | 39 (62.9) | 0.538 |
| Ethnic group, n (%) | ||||||
| Caucasian | 241 (96.4) | 124 (97.6) | 105 (94.6) | 18 (100) | 61 (98.4) | 0.204 |
| Non-Caucasian | 9 (3.6) | 3 (2.4) | 6 (5.4) | 1 (1.6) | ||
| Employment status, n (%) | ||||||
| Full time | 82 (32.8) | 57 (44.9) | 23 (20.7) | 1 (5.6) | 5 (8.1) | |
| Part time | 20 (8.0) | 7 (5.5) | 12 (10.8) | 2 (11.1) | 6 (9.7) | |
| Homemaker | 2 (0.8) | 1 (0.8) | 1 (0.9) | 0 | 1 (1.6) | 0.006 |
| Retired | 124 (49.6) | 51 (40.2) | 65 (58.6) | 15 (83.3) | 46 (74.2) | |
| Never employed | 0 (0) | 0 (0) | 0 (0) | 0 | 0 (0) | |
| Unemployed | 6 (2.4) | 3 (2.4) | 3 (2.7) | 0 | 1 (1.6) | |
| Unable to work due to health | 12 (4.8) | 6 (4.7) | 5 (4.5) | 0 | 3 (4.8) | |
| Other | 2 (0.84) | 2 (1.6) | 2 (1.8) | 0 | 0 (0) | |
| Living alone, n (%) | ||||||
| Yes | 46 (18.4) | 20 (15.7) | 24 (21.6) | 6 (33.3) | 13 (21.0) | 0.272 |
| Marital status, n (%) | ||||||
| Married | 175 (70) | 92 (72.4) | 74 (66.7) | 12 (66.7) | 41 (66.1) | |
| Widowed | 14 (5.6) | 3 (2.4) | 10 (9) | 2 (11.1) | 6 (9.7) | |
| Separated | 4 (1.6) | 3 (2.4) | 1 (0.9) | 0 | 1 (1.6) | 0.008 |
| Single | 35 (14.0) | 19 (15.0) | 14 (12.6) | 3 (16.7) | 8 (12.9) | |
| Divorced | 10 (4) | 1 (0.8) | 9 (8.1) | 1 (5.6) | 5 (8.1) | |
| Cohabitation | 12 (4.8) | 9 (7.1) | 3 (2.7) | 0 | 1 (1.6) | |
| Education, n (%) | ||||||
| ≤12 years | 132 (52.8) | 64 (50.4) | 60 (54.1) | 12 (66.7) | 38 (61.3) | 0.439 |
| Current or previous smoker | ||||||
| Yes, n (%) | 128 (51.2) | 62 (48.8) | 60 (54.1) | 13 (72.2) | 33 (53.2) | 0.444 |
| BMI | 27.8 (24.8–31.9) | 27.4 (24.8–32.0) | 27.8 (24.1–31.7) | 25.1 (25.5–28.4) | 28.2 (25.8–31.8) | 0.897 |
| Units of alcohol/week | 0 (0–8) | 1 (0–7) | 0 (0–10) | 5 (0–2) | 0 (0–2) | 0.682 |
| Primary renal disease, n (%) | ||||||
| Diabetic nephropathy | 33 (13.2) | 15 (11.8) | 15 (13.5) | 3 (16.7) | 10 (23.3) | 0.510 |
| Renovascular | 43 (17.2) | 18 (14.2) | 22 (19.8) | 5 (27.8) | 9 (20.9) | 0.198 |
| Glomerulonephritis | 55 (22.0) | 35 (27.6) | 18 (16.2) | 3 (16.6) | 7 (16.3) | 0.031 |
| ADPKD | 32 (12.8) | 17 (13.4) | 15 (13.5) | 1 (5.6) | 3 (7.0) | 0.778 |
| Obstructive | 9 (3.6) | 4 (3.1) | 4 (3.6) | 0 | 0 (0) | 0.698 |
| Vasculitis | 10 (4.0) | 8 (6.3) | 2 (1.8) | 0 | 1 (2.3) | 0.059 |
| Pyelonephritis | 12 (4.8) | 5 (3.9) | 7 (6.3) | 1 (5.6) | 1 (2.3) | 0.517 |
| Other | 37 (14.8) | 18 (14.2) | 16 (14.4) | 3 (16.7) | 8 (18.6) | 0.777 |
| Unknown | 19 (7.6) | 7 (5.5) | 12 (10.8) | 2 (11.1) | 4 (9.3) | 0.205 |
| Psychodynamic mediations, n (%) | ||||||
| Yes | 29 (11.6) | 11 (8.7) | 17 (15.3) | 4 (22.2) | 11 (17.7) | 0.140 |
| Hypertension | 225 (90.4) | 111 (88.1) | 103 (92.8) | 15 (83.3) | 57 (91.9) | 0.220 |
| Diabetes | 60 (24) | 26 (20.5) | 31 (27.9) | 5 (27.8) | 23 (37.1) | 0.184 |
| Previous MI | 32 (12.8) | 8 (6.3) | 23 (20.7) | 4 (22.2) | 12 (19.4) | 0.002 |
| Coronary heart disease (including MI) | 57 (22.8) | 20 (15.7) | 34 (30.6) | 10 (55.6) | 22 (35.5) | 0.007 |
| Heart failure | 37 (14.9) | 10 (7.9) | 24 (21.6) | 7 (38.9) | 16 (26.2) | 0.002 |
| Atrial fibrillation | 28 (11.2) | 6 (4.7) | 18 (16.2) | 5 (27.8) | 15 (24.2) | 0.001 |
| Previous stroke | 17 (6.8) | 2 (1.6) | 14 (12.6) | 3 (16.7) | 9 (14.5) | 0.001 |
| Previous TIA | 7 (2.8) | 3 (2.4) | 3 (2.7) | 1 (5.6) | 2 (3.2) | 0.670 |
| Cerebrovascular disease (previous stroke or TIA) | 23 (9.2) | 5 (3.9) | 16 (14.4) | 3 (16.7) | 6 (14.0) | 0.003 |
| Peripheral vascular disease | 22 (8.8) | 6 (4.7) | 15 (13.5) | 2 (11.1) | 9 (14.5) | 0.013 |
| COPD | 28 (11.2) | 14 (11.0) | 13 1(1.7) | 2 (11.1) | 6 (9.7) | 0.928 |
| Depression or anxietya | 28 (12.9) | 8 (7.0) | 18 (19.1) | 3 (20) | 4 (7.4) | 0.006 |
| eGFR (mL/min/1.73 m2) | 33 (21–46) | 39 (23–50) | 29 (19–42) | 27 (18–37) | 26 (18–37) | 0.003 |
| Haemoglobin (g/L)b | 127 (114–140) | 129 (114–140) | 126 (113–137) | 114 (106–117) | 119 (111–1213) | 0.211 |
| Corrected calcium ( mmol/L)c | 2.38 (2.31–2.45) | 2.38 (2.31–2.45) | 2.39 (2.30–2.46) | 2.38 (2.30–2.43) | 2.38 (2.32–2.48) | 0.895 |
| Phosphate (mmol/L)c | 1.09 (0.95–1.27) | 1.06 (0.89–1.27) | 1.13 (0.97–1.27) | 1.21 (1.07–1.21) | 1.13 (0.98–1.23) | 0.056 |
| Parathyroid hormone ( ng/L)d | 10.4 (5.9–18.0) | 9.2 (6.0–17.2) | 11 (5.7–18.8) | 23.6 (9.4–24.5) | 12.1 (8.4–21.3) | 0.271 |
| Albumin (g/L)e | 43 (40–44) | 43 (41–45) | 43 (40–44) | 39 (38–42) | 42 (39–44) | 0.196 |
| Urine PCR (g/mol)f | 35 (10–107) | 28 (7–100) | 43 (11–100) | 150 (17–224) | 69 (17–157) | 0.052 |
| Delta eGFR (mL/min/ 1.73 m2/year)g | −1.032 (−2.844 to −0.408) | −1.23 (−3.22–0.59) | −0.77 (−2.28–0.32) | −1.10 (−1.64–0.34) | −1.02 (−2.06–0.55) | 0.574 |
| >20% drop in eGFR in 12 months prior to cognitive assessment | 26 (18.4) | 24 (23.1) | 20 (21.5) | 5 (29.4) | 11 (22.0) | 0.824 |
| >20% decline in eGFR during studyg | 109 (45.5) | 55 (47.8) | 48 (47.1) | 6 (35.3) | 27 (49.1) | 0.953 |
| >50% decline in eGFR during studyg | 34 (14.9) | 15 (14.7) | 2 (11.8) | 6 (16.2) | 6 (10.9) | 0.649 |
| Months in study | 46 (12–72) | 40 (11–68) | 49 (18–85) | 50 (18–61) | 46 (14–74) | 0.177 |
Values are presented as median (IQR) unless stated otherwise.
Psychodynamic medications included opiates, anticholinergics, benzodiazepines, antihistamines and neuropathic analgesic medications.
ADPKD, autosomal dominant polycystic kidney disease; CPOD, chronic obstructive pulmonary disease; TIA, transient ischaemic attack; PCR, protein creatinine ratio. Results based on
217 results,
249 results,
244 results,
192 results,
246 results,
247 results and
227 patients with >2 years of blood tests prior to cognitive test and >2 blood tests, median number of antecedent months covered by calculation was 58 months. This was based on a median of 19 (IQR 11–31) outpatient blood tests prior to the cognitive questionnaire.
Spearman rank correlations (Supplementary data, Table S1) demonstrated a strong inverse correlation between age and a combined relative cognitive impairment Z-score (R = −609, P = 0.000), age and TMT A Z-score (−0.528, P = 0.000), age and TMT B Z-score (R = −0.504, P = 0.000) and a weakly inverse significant correlation with age and MoCA Z-score (−0.316, P = 0.000). There were also significant correlations between cognitive scores, haemoglobin and albumin. Patients global health rating on the EQ-5D-5L weakly correlated with the MoCA Z-score (R = 0.147, P = 0.030) but did not correlate with any other cognitive variable. Univariate associations between comorbidities, lifestyle factors, demographic factors, primary renal disease and cognitive impairment defined by the MoCA and TMT and relative cognitive impairment are displayed in Supplementary data, Table S2. The same variables, after adjustment for eGFR and age, are displayed in Supplementary data, Table S3. In the multivariable logistic regression, psychosocial model depression and older age were significantly associated with cognitive impairment (defined by the MoCA) after adjustment for other psychosocial variables. Older age was associated with relative cognitive impairment (Table 2).
Table 2.
Psychosocial model
| Cognitive impairment (using MoCA) |
Realtive cognitive impairment |
|||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
OR (95% CI) |
|||||||
| Variable | Unadjusted | Model 1 | Model 2 | Model 3 | Unadjusted | Model 1 | Model 2 | Model 3 |
| eGFR (per mL/min /1.73 m2 increase) | 0.984 (0.970–0.999)* | 0.988 (0.973–1.003) | 0.988 (0973–1.003) | 0.991 (0.973–1.008) | 0.969 (0.950-0.988)* | 0.976 (0.956–0.997)* | 0.976 (0.956–0.997)* | 0.982 (0.959–1.006) |
| Age (per year) | 1.037 (1.017–1.057)* | X | 1.035 (1.015–1.055)* | 1.045 (1.018–1.072)* | 1.095 (1.062–1.130)* | 1.088 (1.055–1.122)* | 1.097 (1.055–1.140)* | |
| Male sex | 1.089 (0.643–1.843) | 1.060 (0.616–1.824) | 1.098 (0.635–1.901) | 1.100 (0.571–2.119) | 0.855 (0.470–1.554) | 0.785 (0.404–1.527) | 0.875 (0.444–1.725) | 0.687 (0.313–1.509) |
| Living alone | 1.467 (0.772–2.787) | 0.798 (0.412–1.546) | 1.232 (0.634–2.394) | 1.497 (0.696–3.222) | 1.246 (0.608–2.554) | 0.908 (0.412–1.998) | 0.878 (0396–1.947) | 1.118 (0.456–2.745) |
| Raised BMI | 0.643 (0.341–1.210) | 0.546 (0.281–1.063) | 0.559 (0.287–1.090) | 0.486 (0.226–1.046) | 1.717 (0.778–3.791) | 1.600 (0.670–3.823) | 1.654 (0.688–3.980) | 1.980 (0.704–5.573) |
| Smoking history | 1.228 (0.745–2.025) | 1.127 (0.672–1.889) | 1.083 (0.643–1.825) | 0.856 (0.461–1.588) | 1.114 (0.627–1.980) | 0.857 (0.451–1.629) | 0.835 (0.435–1.600) | 0.833 (0.395–1.757) |
| Alcohol excess | 0.954 (0.460–1.977) | 0.959 (0.445–2.022) | 0.945 (0.446–2.004) | 0.993 (0.406–2.431) | 1.51 (0.621–3.701) | 1.163 (0.411–3.294) | 1.470 (0.593–3.647) | 1.978 (0.694–5.635) |
| ≤12-year education | 1.095 (0.664–1.805) | 0.804 (0.469–1.379) | 0.770 (0.446–1.329) | 0.742 (0.386–1.429) | 1.583 (0.882–2.844) | 0.933 (0.483–1.803) | 0.874 (0.447–1.710) | 0.772 (0.351–1.696) |
| Anxiety or depression | 2.676 (1.172–6.112)* | 3.536 (1.463–8.548)* | 3.425 (1.408–8.332)* | 4.325 (1.694–11.044)* | 3.180 (0.767–13.181) | 6.801 (1.248–37.068)* | 5.049 (0.918–27.784) | 4.921 (0.827–29.266) |
P ≤ 0.05. Model 1 is adjusted for age, Model 2 is adjusted for age and eGFR and Model 3 is adjusted for all variables listed.
In the physical comorbidity model after full adjustment, older age and previous stroke were significantly associated with cognitive impairment (Table 3). In the model using relative cognitive impairment as the dependent variable after adjustment for comorbidities, older age was associated with relative cognitive impairment (P < 0.05), high proteinuria and the use of psychodynamic medications (P = 0.05; Table 3).
Table 3.
Physical comorbidity model
| Cognitive impairment (using MoCA) |
Relative cognitive impairment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
OR (95% CI) |
|||||||||
| Variable | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 |
| eGFR (per mL /min/1.73 m2 increase) | 0.984 (0.970–0.999)* | 0.988 (0.973–1.003) | 0.988 (0.973–1.003) | 0.989 (0.972–1.006) | 0.969 (0.950–0.988)* | 0.976 (0.956–0.997) | 0.976 (0.956–0.997) | 0.992 (0.969–1.015) | ||
| Age (per year) | 1.037 (1.017–1.057)* | 1.035 (1.015–1.055)* | 1.027 (1.003–1.050)* | 1.035 (1.009–1.062)* | 1.095 (1.062–1.130)* | 1.088 (1.055–1.122)* | 1.088 (1.050–1.128) | 1.114 (1.064–1.165) | ||
| Stroke | 6.543 (1.830–23.389)* | 5.461 (1.491–20.001)* | 5.351 (1.452–19.716)* | 4.946 (1.286–19.025)* | 6.430 (1.257–32.888)* | 3.821 (1.405–10.390) | 2.640 (0.861–8.098)* | 2.536 (0.810–7.936) | 2.203 (0.623–7.788) | 1.496 (0.343–6.531) |
| MI | 3.775 (1.668–8.544)* | 2.896 (1.251–6.704)* | 2.806 (1.209–6.515)* | 1.933 (0.764–4.886) | 1.547 (0.582–4.108) | 2.016 (0.922–4.408) | 1.051 (0.443–2.492) | 1.006 (0.419–2.413) | 1.055 (0.390–2.850) | 0.882 (0.299–2.608) |
| Heart failure | 2.653 (1.280–5.495)* | 1.857 (0.866–3.983) | 1.738 (0.804–3.754) | 1.516 (0.655–3.511) | 1.280 (0.532–3.079) | 2.828 (1.364–5.861) | 1.397 (0.627–3.110) | 1.264 (0.560–2.850) | 1.079 (0.428–2.721) | 1.179 (0.436–3.186) |
| Atrial fibrillation | 2.497 (1.102–5.656)* | 1.532 (0.642–3.660) | 1.447 (0.602–3.480) | 1.284 (0.493–3.345) | 1.176 (0.422–3.278) | 4.296 (1.912–9.653) | 1.647 (0.680–3.987) | 1.526 (0.619–3.760) | 1.453 (0.536–3.942) | 1.135 (0.385–3.348) |
| Peripheral vascular disease | 2.559 (1.043–6.278)* | 2.045 (0.814–5.135) | 2.151 (0.836–5.534) | 1.732 (0.627–4.788) | 1.430 (0.462–4.427) | 2.111 (0.865–5.150) | 1.336 (0.501–3.561) | 1.271 (0.458–3.528) | 0.863 (0.282–2.640) | 0.681 (0.175–2.648) |
| Diabetes | 1.47 (0.821–2.632) | 1.049 (0.584–1.953) | 0.956 (0.506–1.805) | 0.780 (0.393–1.548) | 0.764 (0.366–1.594) | 2.407 (1.284–4.511)* | 1.307 (0.650–2.627) | 1.121 (0.545–2.305) | 0.873 (0.402–1.896) | 1.109 (0.481–2.561) |
| uPCR >70 mg/mmol | 1.254 (0.743–2.116) | 1.385 (0.804–2.386) | 1.208 (0.672–2.169) | 1.287 (0.686–2.415) | 1.649 (0.877–3.102) | 2.246 (1.248–4.041)* | 3.076 (1.563–6.054)* | 2.599 (1.253–5.391)* | 2.450 (1.128–5.323) | 2.244 (0.992–5.074) |
| Anaemia | 1.315 (0.791–2.184) | 1.078 (0.633–1.835) | 0.921 (0.520–1.625) | 0.836 (0.459–1.524) | 0.869 (0.471–1.601) | 2.713 (1.505–4.892)* | 1.931 (1.011–3.686) | 1.594 (0.806–3.155) | 1.651 (0.810–3.366) | 1.404 (0.655–3.008) |
| Psychodynamic medication | 1.914 (0.872–4.199) | 2.006 (0.889–4.527) | 1.972 (0.869–4.476) | 1.473 (0.602–3.605) | 1.391 (0.55–3.487) | 2.037 (0.904–4.592) | 2.443 (0.965–6.184) | 2.332 (0.905–6.008) | 2.572 (0.915–7.224) | 3.044 (0.990–9.362) |
| Fast progression (more than −3 mL/min/ year) | 0.589 (0.313–1.109) | 0.820 (0.418–1.608) | 0.730 (0.350–1.522) | 0.552 (0.250–1.218) | 1.406 (0.550–3.595) | 1.247 (0.481–3.234) | 0.954 (0.333–2.730) | |||
P ≤ 0.05. Psychodynamic medications included opiates, anticholinergics, benzodiazepines, antihistamines and neuropathic analgesic medications. Model 1 is adjusted for age, Model 2 is adjusted for age and eGFR and Model 3 is adjusted for all variables listed except fast progression. Model 4 is adjusted for all variables listed except eGFR. uPCR, urinary protein:creatinine ratio.
The prevalence of cognitive impairment and relative cognitive impairment was higher in patients with vascular disease than in patients without (58.5% versus 35.9% for cognitive impairment and 35.1% versus 18.6% for relative cognitive impairment, respectively). Unadjusted odds ratios (ORs) for all variables are presented in Supplementary data, Table S4 and ORs adjusted for age and eGFR are presented in Table 4. In patients without known vascular disease, the significant variables associated with cognitive impairment after adjustments were the prescription of psychodynamic medications and depression or anxiety. The use of psychodynamic medications was also significantly associated with relative cognitive impairment. In patients with known vascular disease, prior stroke was a significant comorbidity variable associated with cognitive impairment. A body mass index (BMI) >25 was associated with a reduced OR for cognitive impairment. Greater proteinuria (>70 g/mol) was significantly associated with relative cognitive impairment but not cognitive impairment.
Table 4.
Logistic regression adjusted for age and eGFR split by known vascular disease
| Cognitive impairment [n = 56/156 (35.9%)] |
Relative cognitive impairment [n = 29/156 (18.6%)] |
Cognitive impairment [n = 55/94 (58.5%)] |
Relative cognitive impairment [n = 33/94 (35.1%)] |
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| No vascular disease, n = 156 | Vascular disease, n = 94 | |||||||
| Male | 1.591 (0.758–3.341) | 0.220 | 0.942 (0.66–2.425) | 0.901 | 0.675 (0.278–1.639) | 0.385 | 0.759 (0.282–2.045) | 0.759 |
| BMI >25 | 0.780 (0.343–1.776) | 0.555 | 1.467 (0.453–4.754) | 0.523 | 0.175 (0.040–0.773) | 0.021 | 1.804 (0.477–6.825) | 0.385 |
| Living alone | 1.541 (0.622–3.821) | 0.350 | 0.963 (0.295–3.139) | 0.963 | 0.962 (0.354–2.616) | 0.940 | 0.857 (0.286–2.568) | 0.783 |
| >14 units of alcohol per week | 1.323 (0.512–3.416) | 0.563 | 1.333 (0.371–4.790) | 0.660 | 0.523 (0.148–1.851) | 0.315 | 1.500 (0.395–5.687) | 0.551 |
| Smoking history | 0.943 (0.478–1.861) | 0.866 | 0.630 (0.252–1.575) | 0.324 | 1.089 (0.456–2.602) | 0.848 | 1.086 (0.407–2.893) | 0.869 |
| Psychodynamic medications | 5.139 (1.318–20.038) | 0.018 | 7.994 (1.573–40.634) | 0.012 | 0.904 (0.313–2.620) | 0.853 | 1.405 (0.426–4.631) | 0.577 |
| Depression | 4.117 (1.088–15.581) | 0.037 | 5.122 (0.586–44.71) | 0.140 | 2.304 (0.692–7.671) | 0.174 | 5.510 (0.380–79.787) | 0.211 |
| Education ≤12 years | 0.344–1.437 | 0.344 | 1.311 (0.511–3.365) | 0.574 | 0.880 (0.365–2.121) | 0.776 | 0.589 (0.221–1.569) | 0.290 |
| Comorbidities | ||||||||
| Diabetes | 1.122 (0.458–2.753) | 0.801 | 1.044 (0.356–3.062) | 0.938 | 0.754 (0.276–1.919( | 0.553 | 1.237 (0.457–3.352) | 0.676 |
| Hypertension | 1.049 (0.388–2.838) | 0.925 | 0.664 (0.177–2.491) | 0.543 | 1.844 (0.106–32.123) | 0.675 | 0.578 (0.022–14.935) | 0.741 |
| Stroke | 4.364 (1.12–17.002) | 0.034 | 2.763 (0.818–9.327) | 0.102 | ||||
| TIA | 0.514 (0.106–2.498) | 0.409 | 0.787 (0.126–4.933) | 0.798 | ||||
| Atrial fibrillation | 0.920 (0.196–4.328) | 0.916 | 1.071 (0.195–5.877) | 0.937 | 1.453 (0.476–4.434) | 0.512 | 1.656 (0.559–4.901) | 0.363 |
| MI | 2.294 (0.901–5.839 | 0.082 | 0.949 (0.357–2.523) | 0.916 | ||||
| Cardiovascular disease | 0.970 (0.404–2.328) | 0.946 | 0.951 (0.353–2.566) | 0.921 | ||||
| Heart failure | 1.370 (0.564–3.329) | 0.488 | 1.311 (0.506–3.396) | 0.577 | ||||
| Peripheral vascular disease | 1.441 (0.528–3.935) | 0.476 | 1.287 (0.437–3.788) | 0.647 | ||||
| Chronic obstructive pulmonary disease | 1.199 (0.343–4.187) | 0.776 | 1.060 (0.213–5.263) | 0.943 | 0.678 (0.225–2.038) | 0.488 | 0.341 (0.081–1.435) | 0.142 |
| Anaemic | 0.613 (0.282–1.330) | 0.215 | 1.340 (0.514–3.491) | 0.550 | 1.549 (0.623–3.855) | 0.347 | 1.826 (0.682–4.889) | 0.231 |
| uPCR ≥70 g/mol | 1.122 (0.509–2.470) | 0.766 | 1.994 (0.721–5.516) | 0.184 | 1.161 (0.470–2.865) | 0.746 | 3.400 (1.165–9.920) | 0.025 |
| Fast progression (> −3 mL/min/ 1.73 m2/year) | 0.641 (0.282–1.461) | 0.290 | 1.190 (0.382–3.705) | 0.764 | 1.260 (0.306–5.185) | 0.749 | 1.328 (0.243–7.264) | 0.744 |
Psychodynamic medications included opiates, anticholinergics, benzodiazepines, antihistamines and neuropathic analgesic medications. uPCR, urinary protein:creatinine ratio; TIA, transient ischaemic attack. Fast progression was not adjusted for eGFR due to collinearity.
A total of 227 patients had at least two blood tests with >2 years of SKS follow-up prior to cognitive testing. Fast decline in renal function was not associated with the presence of cognitive impairment or relative cognitive impairment in unadjusted or fully adjusted models. There was no significant difference between the eGFR slope in patients with cognitive impairment and relative cognitive impairment compared with those having normal cognition (−0.77 versus −1.35 mL/min/1.73 m2/year, P = 0.344 for cognitive impairment and −1.12 versus −1.02 mL/min/1.73 m2/year, P = 0.887 for relative cognitive impairment). Patients with known vascular disease had a similar rate of eGFR decline as those without known vascular disease (−0.91 versus −1.15 mL/min/1.73 m2/year, P = 0.077). Rapidly declining eGFR classified by any of the alternative definitions of 20% decline in eGFR in the antecedent 12 months or by 20 or 50% declines in eGFR during the entire SKS study follow-up was not associated with cognitive impairment (defined by the MoCA, TMT or relative cognitive impairment).
DISCUSSION
Our prospective study in ND-CKD patients is the first to find a faster eGFR decline was not associated with the presence of cognitive impairment measured by three independent cognitive assessments in moderate to severe CKD. Such a finding persisted even in patients with a higher vascular comorbid burden. The timing of the eGFR decline does not appear to affect the likelihood of discovering cognitive impairment either. Patients with a rapid decrease in eGFR (>20% decline) in the 12 months prior to cognitive assessments were not more likely to demonstrate cognitive impairment.
A Taiwanese community population study performed in an elderly cohort suggested eGFR decline (defined as 20% annual reduction in eGFR) was independently associated with cognitive decline. In this study, the majority (93%) did not suffer from CKD (eGFR >60 mL/min); only 0.74% of the study population had an eGFR <30 mL/min/1.73 m2 [30]. The accuracy of GFR estimates by serum creatinine estimating equations is known to be reduced at higher eGFRs, especially in the elderly, hence the significance of a 20% decline in eGFR in elderly patients whose eGFR is >60 mL/min/1.73 m2 is questionable [31].
In another study, 259 of 2383 French patients >65 years old who had a rapid decline in eGFR (>4 mL/min/1.73 m2) had an increased relative risk of cognitive decline and dementia with a vascular component. However, the mean eGFR at baseline in that study was 76.7 mL/min/1.73 m2 [32]. In our study, 43.6% of patients had an eGFR <30 mL/min/1.73 m2. Etgen et al. [33] demonstrated that ‘newly acquired’ CKD (creatinine clearance rate <60 mL/min/1.73 m2) was independently associated with newly acquired cognitive impairment when compared with participants with normal or near-normal renal function during a 2-year follow-up [OR 1.73 (95% CI 1.26–2.43), P < 0.001]. These three studies are at odds with the outcome of our study, potentially due to differences in CKD severity between the study populations. Our study cohort had significant CKD, which is more representative of patients cared for in secondary care and relevant to practising nephrologists [34]. Organ performance in the kidney and brain may not decline in parallel. Current published literature suggests eGFR decline may predict future cognitive decline. Once eGFR has already declined, other pathological drivers of cognitive impairment may predominate and further eGFR decline may not be an important risk factor.
Our study shows the significant impact of previous stroke on cognitive impairment in an ND-CKD population, which is consistent with other CKD studies [35], and in the general population [36, 37]. Our study supports the notion that patients with CKD and previous stroke should undergo cognitive assessment as part of standard CKD care. In the psychosocial model, depression or anxiety was significantly associated with cognitive impairment. Depression is a frequently detected (and often overlooked) psychiatric comorbidity in patients with renal disease [38, 39]. Our results support screening for depression at the time of cognitive assessment to establish the presence or absence of ‘pseudo cognitive impairment’. If depression is discovered, there may be potential therapeutic strategies to improve cognitive performance [40].
We investigated important factors associated with cognitive impairment in ND-CKD in patients without known vascular comorbidity. In our model adjusted for age and eGFR, the prescription of psychodynamic medications and depression or anxiety were independent risk factors for cognitive impairment. However, the direction of causality is difficult to adjudicate for the relationship with medications, which were prescribed to ameliorate sleep disturbance, pain and restless legs. Psychodynamic medications should be used cautiously in CKD patients. If cognitive impairment is caused by psychodynamic medications, then this relationship should be reversible when the medication is removed. Cognitive assessments before and after commencement of psychodynamic medications may be of use. Determination of which psychodynamic medications have the most significant impact on cognitive impairment in CKD is an area for future study. Due to small numbers, this aspect could not be investigated in this study.
Patients with BMI >25 and with a known vascular comorbidity were less likely to demonstrate cognitive impairment. Sarcopenia is independently associated with cognitive impairment [41] and may reflect the absence of sarcopenia rather than a higher BMI providing a protective mechanism against cognitive impairment.
Cognitive impairment was defined using different methods. Figure 1 demonstrates a significant overlap between the two definitions. While age was strongly associated with both cognitive impairment and relative cognitive impairment, stroke and depression were not associated with relative cognitive impairment despite being acknowledged as important within the general population. This study might therefore suggest that cognitive disease in ND-CKD should be measured and defined using methods applicable to the general population, otherwise important associations and confounders may be overlooked. Alternatively, if 44% of the CKD population suffer from cognitive impairment, all of these patients may require additional resources and support in relation to decision-making, medication compliance and consent for procedures. Adopting a relative cognitive impairment definition may help to highlight patients who might benefit the most from additional support. The MoCA alone may overestimate clinically significant cognitive impairment in the CKD population. Using a lower cut-off for cognitive impairment, e.g. <24/30, as has been suggested in the haemodialysis population, may be more appropriate [42].
The strengths of this study include detailed characterization of the study population with thorough comorbidity assessment, multiple measures of cognitive functioning covering most domains of cognition, minimal missing data and a long antecedent follow-up period before cognitive assessment for accurate calculation of eGFR slopes.
One potential limitation is the majority of patients in this study were invited to participate at an SKS follow-up visit. The competing risk of death or dialysis commencement and cognitive impairment means that some rapidly progressive CKD patients in the SKS cohort may have died or commenced dialysis, potentially biasing our cognitive impairment study cohort to patients with more stable disease. However, the median eGFR decline in this subgroup was −1.032 mL/min/1.73 m2/year, whereas the median eGFR decline in the whole of the SKS (>3000 patients) is not markedly different (−1.37 mL/min/1.73 m2/year). Second, cognitive assessments were not performed prior to study enrolment, although patients with known dementia are not recruited to the SKS, as most are unable to provide informed consent. eGFR decline was assessed using linear regression, but not all patients with CKD decline in a linear fashion. eGFR decline was therefore analysed using a binary 20% decrease in eGFR in the antecedent 12 months prior to cognitive assessment [30] and by a 20 and 50% decrease in eGFR during the study (Supplementary data, Tables S3 and S4). Cognitive impairment (or relative cognitive impairment) was not significantly associated with any of these eGFR decline variables. The sample size of this study is comparable with many other studies investigating CKD and cognitive impairment. However, due to this sample size and slow eGFR decline, this study lacks statistical power to definitely confirm that fast eGFR decline is not associated with cognitive impairment. Finally, this study was performed in a largely Caucasian UK CKD cohort. Patients from Southeast Asia are at higher risk of CKD progression and reaching end-stage renal disease [43]. It may not be appropriate to translate the findings of this study into non-Caucasian patients with CKD.
In conclusion, we found that faster eGFR decline is not associated with the presence of cognitive impairment in patients with moderate to severe ND-CKD. Older patients with a previous stroke, depression or anxiety, higher proteinuria and patients prescribed psychodynamic medications are at higher risk of cognitive impairment in ND-CKD.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the renal patients enrolled in the SKS who contributed to this study. The authors would also like to acknowledge the work of the renal research nurses who have compiled the database and Emma Flanagan for compiling electronic patient record data.
FUNDING
There is no funding to declare.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Murray AM, Tupper DE, Knopman DS. et al. Cognitive impairment in hemodialysis patients is common. Neurology 2006; 67: 216–223 [DOI] [PubMed] [Google Scholar]
- 2. Iyasere O, Okai D, Brown E.. Cognitive function and advanced kidney disease: longitudinal trends and impact on decision-making. Clin Kidney J 2017; 10: 89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Etgen T, Chonchol M, Förstl H. et al. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012; 35: 474–482 [DOI] [PubMed] [Google Scholar]
- 4. Kurella-Tamura M, Wadley V. et al. Kidney function and cognitive impairment in US adults: the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis 2008; 52: 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elias MF, Elias PK, Seliger SL. et al. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 2009; 24: 2446–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seliger SL, Siscovick DS, Stehman BC. et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004; 15: 1904–1911 [DOI] [PubMed] [Google Scholar]
- 7. Tyrrell J, Paturel L, Cadec B. et al. Older patients undergoing dialysis treatment: cognitive functioning, depressive mood and health-related quality of life. Aging Ment Health 2005; 9: 374–379 [DOI] [PubMed] [Google Scholar]
- 8. Tollitt J, Odudu A, Montaldi D. et al. Cognitive impairment in chronic kidney disease and dialysis. J Kidney Care 2018; 3: 23–29 [Google Scholar]
- 9. Rifkin DE, Shlipak MG, Katz R. et al. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008; 168: 2212–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasreddine ZS, Phillips NA, Bédirian V. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699 [DOI] [PubMed] [Google Scholar]
- 11. Kurella M, Chertow GM, Fried LF. et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005. ; 16: 2127–2133 [DOI] [PubMed] [Google Scholar]
- 12. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–214 [DOI] [PubMed] [Google Scholar]
- 13. Yeudall LT, Reddon JR, Gill DM. et al. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol 1987; 43: 346. [DOI] [PubMed] [Google Scholar]
- 14. Kurella Tamura M, Tam K, Vittinghoff E. et al. Inflammatory markers and risk for cognitive decline in chronic kidney disease: the CRIC study. Kidney Int Reports 2017; 2: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaffe K, Kurella-Tamura M, Ackerson L. et al. Higher levels of cystatin C are associated with worse cognitive function in older adults with chronic kidney disease: the Chronic Renal Insufficiency Cohort Cognitive Study. J Am Geriatr Soc 2014; 62: 1623–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAdams-DeMarco MA, Tan J, Salter ML. et al. Frailty and cognitive function in incident hemodialysis patients. Clin J Am Soc Nephrol 2015. ; 10: 2181–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannan M, Steffen A, Quinn L. et al. The assessment of cognitive function in older adult patients with chronic kidney disease: an integrative review. J Nephrol 2018; 32: 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoefield RA, Kalra PA, Lane B. et al. Associations of baseline characteristics with evolution of eGFR in a referred chronic kidney disease cohort. QJM 2013; 106: 915–924 [DOI] [PubMed] [Google Scholar]
- 19. Ritchie J, Rainone F, Green D. et al. Extreme elevations in blood pressure and all-cause mortality in a referred CKD population: results from the CRISIS study. Int J Hypertens 2013; 2013:597906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milani SA, Marsiske M, Cottler LB. et al. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimers Dement (Amst) 2018; 10: 773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bowie CR, Harvey PD.. Administration and interpretation of the Trail Making Test. Nat Protoc 2006; 1: 2277–2281 [DOI] [PubMed] [Google Scholar]
- 22. Clark HB, Boyd SB, Macrae JW.. A classroom program teaching disadvantaged youths to write biographic information. J Appl Behav Anal 1975; 8: 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arbuthnott K, Frank J.. Trail Making Test, Part B as a measure of executive control: Validation using a set-switching paradigm. J Clin Exp Neuropsychol 2000; 22: 518–528 [DOI] [PubMed] [Google Scholar]
- 24. Herdman M, Gudex C, Lloyd A. et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao Y, Zhang Y, Yang Z. et al. Sleep disorders and cognitive impairment in peritoneal dialysis: a multicenter prospective cohort study. Kidney Blood Press Res 2019; 44: 1115–1127 [DOI] [PubMed] [Google Scholar]
- 26. Dore GA, Elias MF, Robbins MA. et al. Cognitive performance and age: norms from the Maine–Syracuse study. Exp Aging Res 2007; 33: 205–271 [DOI] [PubMed] [Google Scholar]
- 27. Au R, Seshadri S, Wolf PA. et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res 2004; 30: 333–358 [DOI] [PubMed] [Google Scholar]
- 28. Elias PK, Elias MF, D'Agostino RB. et al. NIDDM and blood pressure as risk factors for poor cognitive performance: the Framingham study. Diabetes Care 1997; 20: 1388–1395 [DOI] [PubMed] [Google Scholar]
- 29. Streiner DL, Norman GR.. Correction for multiple testing: is there a resolution? Chest 2011; 140: 16–18 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y-C, Weng S-C, Liu J-S. et al. Severe decline of estimated glomerular filtration rate associates with progressive cognitive deterioration in the elderly: a community-based cohort study. Sci Rep 2017; 7: 42690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellam T, Twohig H, Khwaja A.. Chronic kidney disease in elderly people: disease or disease label? BMJ 2016. ; 352: h6559. [DOI] [PubMed] [Google Scholar]
- 32. Helmer C, Stengel B, Metzger M. et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011; 77: 2043–2051 [DOI] [PubMed] [Google Scholar]
- 33. Etgen T, Sander D, Chonchol M. et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant 2009; 24: 3144–3150 [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. Chronic Kidney Disease in Adults: Assessment and Management. London: National Institute for Health and Care Excellence, 2014. https://www.nice.org.uk/guidance/cg182 [PubMed] [Google Scholar]
- 35. Foster R, Walker S, Brar R. et al. Cognitive impairment in advanced chronic kidney disease: the canadian frailty observation and interventions trial. Am J Nephrol 2016; 44: 473–480 [DOI] [PubMed] [Google Scholar]
- 36. Godefroy O, Bogousslavsky J.. The behavioral and cognitive neurology of stroke. Godefroy O, Bogousslavsky J (eds). The Behavioral and Cognitive Neurology of Stroke. Cambridge: Cambridge University Press, 2007. [Google Scholar]
- 37.Leys D, Pasquier F. Poststroke dementia. In: Caplan L, Van Gijn J (eds.). Stroke Syndromes, 3 edn. Cambridge: Cambridge University Press, 2012: 245–254. [Google Scholar]
- 38. Marazziti D, Consoli G, Picchetti M. et al. Cognitive impairment in major depression. Eur J Pharmacol 2010; 626: 83–86 [DOI] [PubMed] [Google Scholar]
- 39. Chilcot J, Wellsted D, Farrington K.. Depression in end-stage renal disease: current advances and research. Semin Dial 2010; 23: 74–82 [DOI] [PubMed] [Google Scholar]
- 40. Tamura MK, Yaffe K.. Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int 2011; 79: 14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chang K-V, Hsu T-H, Wu W-T. et al. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc 2016; 17: 1164.e7–1164.e15 [DOI] [PubMed] [Google Scholar]
- 42. Tiffin-Richards FE, Costa AS, Holschbach B. et al. The Montreal Cognitive Assessment (MoCA) – a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One 2014; 9: e106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barbour SJ, Er L, Djurdjev O. et al. Differences in progression of CKD and mortality amongst Caucasian, Oriental Asian and South Asian CKD patients. Nephrol Dial Transplant 2010; 25: 3663–3672 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


