Abstract
Background
Coexistence of fibrillary glomerulonephritis (FGN) and immunoglobulin A (IgA) nephropathy (IgAN) in the same kidney biopsy (FGN–IgAN) is rare, and the clinicopathologic characteristics and outcome of this dual glomerulopathy are unknown.
Methods
In this study, 20 patients with FGN–IgAN were studied and their characteristics were compared with 40 FGN and 40 IgAN control patients.
Results
Concurrent IgAN was present in 1.8% of 847 consecutive FGN cases and was the second most common concurrent glomerulopathy after diabetic nephropathy. FGN–IgAN patients were overwhelmingly White (94%) and contrary to FGN patients were predominantly (60%) males. Compared with IgAN patients, FGN–IgAN patients were older, had higher proteinuria, a higher incidence of renal insufficiency, and a lower incidence of microhematuria and gross hematuria at diagnosis. Six (30%) patients had malignancy, autoimmune disease or hepatitis C infection, but none had a secondary cause of IgAN or clinical features of Henoch–Schonlein purpura. Histologically, all cases exhibited smudgy glomerular staining for immunoglobulin G and DnaJ homolog subfamily B member 9 (DNAJB9) with corresponding fibrillary deposits and granular mesangial staining for IgA with corresponding mesangial granular electron-dense deposits. On follow-up (median 27 months), 10 of 18 (56%) FGN–IgAN patients progressed to end-stage kidney disease (ESKD), including 5 who subsequently died. Serum creatinine at diagnosis was a poor predictor of renal survival. The proportion of patients reaching ESKD or died was higher in FGN–IgAN than in IgAN. The median Kaplan–Meier ESKD-free survival time was 44 months for FGN–IgAN, which was shorter than IgAN (unable to compute, P = 0.013) and FGN (107 months, P = 0.048).
Conclusions
FGN–IgAN is very rare, with clinical presentation and demographics closer to FGN than IgAN. Prognosis is guarded with a median renal survival of 3.6 years. The diagnosis of this dual glomerulopathy requires careful evaluation of immunofluorescence findings, and electron microscopy or DNAJB9 immunohistochemistry.
Keywords: DNAJB9, electron microscopy, fibrillary deposits, fibrillary glomerulonephritis, glomerulonephritis, IgA nephropathy
INTRODUCTION
Fibrillary glomerulonephritis (FGN) is a rare primary glomerular disease historically defined by glomerular deposition of Congo red negative, randomly oriented straight fibrils that lack a hollow center and stain with immunoglobulin G (IgG) by immunofluorescence (IF) [1–3]. The recent identification of DnaJ homolog subfamily B member 9 (DNAJB9) as a highly sensitive and specific marker for FGN has revolutionized the diagnosis of this disease [4–6] and now allows for distinction from amyloidosis (including the distinction between congophilic FGN and amyloidosis) [7, 8], immunotactoid glomerulopathy [4, 8] and other glomerulopathies characterized by organized deposits [4]. Most reported patients with FGN are White [2, 3, 8, 9] and the disease is diagnosed more frequently in North America (0.6–1.4% of native kidney biopsies) [1, 3, 9–11] than in other continents (0.03–0.4%) [11–13], possibly due to routine performance of electron microscopy (EM) on native kidney biopsies in North America and/or due to genetic or environmental factors [14]. FGN mainly affects middle-aged patients, with a reported average age at diagnosis ranging from 49 to 61 years [8, 9], with approximately a 2:1 female-to-male predominance [8, 9, 11]. It was initially considered to be an idiopathic disease but subsequent studies highlighted association in some cases with autoimmune disease, solid malignancy or hepatitis C [2, 15, 16]. Most patients present with hematuria, proteinuria and renal insufficiency. Prognosis is poor, with about half of patients progressing to end-stage kidney disease (ESKD) within 2–4 years from diagnosis [2, 8]. There is currently no effective therapy, aside from kidney transplantation.
Immunoglobulin A (IgA) nephropathy (IgAN) is the most common primary glomerulonephritis (GN) worldwide, with the highest frequency in East Asians and Whites. It accounts for 40% of glomerular disease diagnoses in Asia, 22% in Europe and 12% in North America [11]. IgAN is defined immunohistologically by IgA-dominant or -codominant glomerular deposits [17]. The IgA staining should be ≥1+ in intensity [18]. The deposits in IgAN are primarily localized to the mesangium and appear granular (i.e. without substructure) on EM. The light microscopy (LM) findings are variable and range from no or minimal mesangial alterations to mesangial proliferative GN to crescentic GN. The Oxford Classification of IgAN was introduced in 2009 and updated in 2017 to standardize the grading of LM features of IgAN, and is currently widely used [18, 19]. Contrary to FGN, there is an approximately a 2:1 male-to-female predominance in North America [11]. While patients with IgAN can present at any age, there is a peak incidence in the second and third decades of life. The clinical presentation is variable and ranges from recurrent gross hematuria, to microscopic hematuria and mild proteinuria, to, rarely, rapidly progressive GN [20]. Prognosis is variable but better than that for FGN; ∼10–20% of patients progress to ESKD within 10 years.
The occurrence of FGN and IgAN in the same patient (FGN–IgAN) is quite rare. Concomitant IgAN was present in 2 of 84 (2.4%) FGN patients included in a single-center series [4] but not in any of 266 FGN patients included in a multi-institutional cohort reported by Andeen et al. [8]. Thus, the incidence, characteristics and prognosis of this dual glomerulopathy are unknown. Herein, we report the first clinicopathologic series on FGN–IgAN, with the aim of defining its clinical characteristics, pathologic features and outcome, and comparing these characteristics with FGN and with IgAN.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the Renal Biopsy Laboratory archives at the Mayo Clinic, Rochester, from January 2007 through March 2020 and identified 15 patients with a native kidney biopsy diagnosis of FGN–IgAN. Five additional patients with available clinical and follow-up data were identified from the pathology archives of Arkana Laboratories during the same time period and were also included in this study. FGN was defined by glomerular deposition of randomly oriented straight fibrils that lack a hollow center and exhibit smudgy staining for IgG by IF and DNAJB9 positivity by immunohistochemistry. IgAN was defined by granular mesangial IgA staining on IF (≥1+ in intensity on a scale of 0–3+) with corresponding mesangial electron deposits that exhibit granular texture (i.e. without substructure) on EM. Two patients included in this series were previously reported [4].
To determine the incidence of concurrent IgAN and other glomerulopathies in biopsies of FGN, we reviewed the pathology reports of all 847 consecutive cases of FGN accessioned at the Mayo Clinic Renal Biopsy Laboratory and Arkana Laboratories between 2015 and 2019.
Kidney biopsy evaluation
All renal biopsies were processed according to standard techniques for LM, IF and EM. For LM, all cases were stained with hematoxylin and eosin, periodic acid–Schiff, Masson’s trichrome and Jones methenamine silver. For DNAJB9 immunohistochemistry, slides were stained with an anti-DNAJB9 rabbit polyclonal antibody (catalog#PA5-59621, 1/200 titer, Thermo Fisher Scientific, Waltham, MA, USA) on a Ventana BenchMark XT system using Ventana OptiView Universal DAB Detection and OptiView Amplification kits. Standard IF on frozen tissue was performed on 3–4 µm cryostat sections using polyclonal fluorescein isothiocyanate (FITC)-conjugated antibodies to IgG, IgM, IgA, C3, C1q, kappa, lambda, fibrinogen and albumin (Dako Corporation, Carpinteria, CA, USA, for the Mayo cases; Kent Laboratories, Bellingham, WA, USA, for the Arkana cases). IF staining for IgG subclass was performed on 3–4 µm cryostat sections using monoclonal FITC-conjugated antibodies to IgG1, IgG2, IgG3 and IgG4 (Sigma-Aldrich Corp., St Louis, MO, USA).
Clinical data
Demographics, renal characteristics, laboratory findings, treatment and follow-up were obtained from patients’ medical records. Quantification of proteinuria was performed by 24-h collection or by spot urine protein-to-creatinine ratio when 24-h urine collection was not performed. The following clinical definitions were used: nephrotic-range proteinuria (NRP): ≥3.0 g/day; hypoalbuminemia: serum albumin <3.5 g/dL; renal insufficiency: serum creatinine >1.2 mg/dL; and nephrotic syndrome: NRP with hypoalbuminemia and peripheral edema. Tubular atrophy and interstitial fibrosis were graded based on an estimate of the percentage of renal cortex affected and recorded as: 0 (none), 1–25% (mild), 26–50% (moderate) or >50% (severe). The following outcome definitions were used: (i) remission: reduction in proteinuria by at least 50% with stable renal function (no more than 20% increase in serum creatinine); (ii) persistent renal dysfunction (PRD): failure to meet criteria for remission but not reaching ESKD; and (iii) ESKD: requiring renal replacement therapy. Institutional review board approval was obtained for this study, and it was conducted in accordance with the Declaration of Helsinki.
Control groups
We retrospectively reviewed the Renal Biopsy Laboratory archives at the Mayo Clinic, Rochester, during the same study period and randomly selected 40 Mayo Clinic patients with FGN and 40 Mayo Clinic patients with IgAN in whom complete clinical and follow-up data were available as two control groups at a ratio of 2:1.
Statistical analyses
Data were analyzed using SPSS Statistics, version 17.0 (Chicago, IL, USA) and StatXact/LogXact, version 11 (Cytel Software Corp., Cambridge, MA, USA). The one-sample Kolmogorv–Smirnov test was used to determine the normality of distribution. Continuous variables are reported as mean ± standard deviation or, for non-normally distributed variables, as median (25–75% interquartile range). Analysis was performed using nonparametric exact statistical methods using the Mann–Whitney–Wilcoxon test, the Kruskal–Wallis test, the Jonckheere–Terpstra test and the Fisher–Freeman–Halton exact test, as appropriate for variable type. Survival analysis for progression to ESKD was performed by using the Kaplan–Meier method for categorical variables and by Cox regression for continuous variables. Multivariate survival analyses were performed using the Cox proportional hazards model (Cox regression). Statistical significance was assumed at P < 0.05.
RESULTS
Clinical features
The demographics, renal characteristics and outcome for each of the 20 patients with FGN–IgAN are listed in Table 1, and their pathologic characteristics are listed in Table 2. Table 3 compares the pertinent clinicopathologic features and outcome of patients with FGN–IgAN with those of FGN and those of IgAN. The FGN–IgAN cohort was overwhelmingly White (94%), with male predominance (60%). The male predominance was in contrast to FGN patients, in whom 70% were females (P = 0.049), but not different from IgAN patients [68% males, P =not significant (NS)]. The mean age at diagnostic biopsy for FGN–IgAN was 57 years (range 35–78). Seven (35%) were elderly (⩾64 years of age). FGN–IgAN patients were older than IgAN patients (P = 0.03). Associated conditions included malignancy in 15% (non-Hodgkin’s lymphoma in one, prostate cancer in one and throat cancer in one), autoimmune disease in 10% (lupus in one and sarcoidosis in one) and hepatitis C infection (HCV) in one (5%). Coexistent conditions included hypertension in 65%, diabetes mellitus in 25% and hypothyroidism in 10%.
Table 1.
Demographics, renal characteristics and outcome of 20 patients with FGN–IgAN
| Case number | Age (years) | Gender | Race | S. Cr. (mg/dL) | Proteinuria (g/day) | S. albumin | Edema | Full nephrotic syndrome | Hematuria | Concurrent conditions | F/U duration in months | Treatment | Outcome | Last F/U S. Cr. | Last F/U proteinuria (g/day) | Time to ESKD in months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | W | 4.9 | 5.1 | 4.5 | No | No | Yes | None | 14 | Steroids, rituximab | ESKD | ESKD | ESKD | 3 |
| 2 | 64 | M | W | 0.9 | 1.7 | 3.6 | No | No | Yes | HTN, prostate cancer | 95 | None | ESKD | ESKD | ESKD | 52 |
| 3 | 54 | M | W | 1.3 | 4.1 | 4 | Yes | No | No | HTN | 123 | Steroids | ESKD/ death | ESKD | ESKD | 65 |
| 4 | 35 | M | W | 0.9 | 2.3 | 3.6 | No | No | Yes | None | 20 | Cyclophosphamide, rituximab | remission | 1 | 0.285 | |
| 5 | 57 | M | W | 4 | 7.7 | 3.4 | No | No | Yes | HTN, DM | 21 | None | ESKD/ death | ESKD | ESKD | 8 |
| 6 | 60 | F | W | 3.6 | 1 | 3.7 | No | No | Yes | HTN, non-Hodgkin lymphoma | 44 | Steroids | ESKD/ death | ESKD | ESKD | 44 |
| 7 | 78 | F | W | 2 | 3.9 | 4.2 | No | No | Yes | HTN | 13 | ARB (losartan) | Remission | 1.5 | 0.039 | |
| 8 | 53 | M | W | 2.1 | 4.1 | NA | NA | NA | yes | none | 133 | Steroids | ESKD/ death | ESKD | ESKD | 131 |
| 9 | 46 | F | W | 0.7 | 0.86 | 4.5 | Yes | No | Yes | None | 14 | ACE I (lisinopril), fish oil | Remission | 0.7 | 0.27 | |
| 10 | 64 | M | W | 1.7 | 7.3 | 3 | Yes | Yes | Yes | HTN, DM, ischemic cardiomyopathy | 25 | MMF | ESKD | ESKD | ESKD | 22 |
| 11 | 53 | M | W | 1.3 | 2.4 | 4.2 | Yes | No | Yes | Fatty liver, obesity | 5 | ACE I (lisinopril), ARB (losartan) | Remission | 1.3 | 0.8 | |
| 12 | 51 | M | H | 1 | 3.1 | 4.1 | No | No | No | HTN, lupus | 35 | None | PRD | 1.4 | 0.983 | |
| 13 | 58 | M | W | 2.4 | 14 | 3 | Yes | Yes | Yes | HTN, DM | 38 | CyBorD | ESKD/death | ESKD | ESKD | 23 |
| 14 | 49 | M | W | 2.1 | 0.75 | 3.2 | No | No | Yes | HCV, IV drug use | 28 | Zepatier (elbasvir and garzoprevir) for HCV | PRD | 4 | 13.6 | |
| 15 | 69 | F | W | 2.6 | 6.7 | 2 | No | No | Yes | HTN | 29 | ARB (losartan) | ESKD | ESKD | ESKD | 7 |
| 16 | 74 | F | NA | 1.7 | 5.6 | NA | No | No | Yes | HTN, nonischemic cardiomyopathy | NA | |||||
| 17 | 41 | F | W | 1.5 | 3.8 | NA | Yes | NA | No | HTN, sarcoidosis, hypothyroidism | 15 | ARB (temisartan) | PRD | 1.6 | 2.8 | |
| 18 | 73 | F | W | NA | 9 | Low | Yes | Yes | No | HTN, DM | 10 | Steroids, MMF, rituximab | NA | 1.3 | NA | |
| 19 | 43 | M | W | 3.5 | 3.8 | NA | NA | NA | NA | HTN, DM, throat cancer | 84 | NA | ESKD | ESKD | ESKD | 12 |
| 20 | 45 | F | NA | 0.6 | 1.6 | NA | No | No | Yes | Hypothyroidism | NA |
ACE I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CyBorD, cyclophosphamide+bortezomib+dexamethasone; DM, diabetes; F, female; F/U, follow-up; H, Hispanic; HTN, hypertension; IV, intravenous; M, male; NA, not available; S. albumin, serum albumin; S. Cr., serum creatinine; W, White.
Table 2.
Pathologic findings by LM, IF and EM in patients with FGN–IgAN
| Case number | LM | Pattern of injury | Number of glomeruli in LM | % GGS | % crescents | TA/IF | IFa | IgG | IgA | IgM | C1q | C3 | κ | λ | EM | Mes. fibrillary deposits | GBM fibrillary deposits | Mes. granular electron- dense deposits | GCW granular electron- dense deposits | Mean fibril thickness in nm | FPE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Crescentic GN | 33 | 61 | 24 | Mild | 3+ | 2+ | 0.5+ | 0 | 2+ | 0 | 2+ | Yes | Yes (G) | Yes | Yes | 15 | G | |||
| 2 | MesGN | 24 | 0 | 0 | Mild | 1+ | 1.5+ | 1.5+ | 0 | 1+ | 0.5+ | 1.5+ | Yes | Yes (S) | Yes | No | 17 | S | |||
| 3 | MesGN | 20 | 30 | 10 | Mild | 0.5+ | 1+ | 0 | 0 | 2+ | 1+ | 2+ | Yes | Yes (S) | Yes | No | 19 | G | |||
| 4 | MesGN | 19 | 37 | 0 | Mild | 2+ | 2+ | 1+ | 0.5+ | 2+ | 1+ | 2+ | Yes | Yes (G) | Yes | No | 17 | S | |||
| 5 | MesGN + nodular DGS | 44 | 66 | 0 | Marked | 1+ | 1.5+ | 0 | 1+ | 1.5+ | 1+ | 1+ | Yes | Yes (S) | Yes | No | 15 | G | |||
| 6 | MesGN | 11 | 18 | 9 | Mild | 1+ | 1+ | 0 | 0 | 1+ | 1+ | 1+ | Yes | Yes (S) | Yes | No | S | ||||
| 7 | MesGN | 14 | 43 | 0 | Mild | 1+ | 1+ | 0.5+ | 0 | 1.5+ | 1.5+ | 1+ | Yes | Yes (G) | Yes | No | 12 | S | |||
| 8 | MesGN | 5 | 60 | 0 | Moderate | 3+ | 3+ | 0.5+ | 0 | 3+ | 0.5+ | 2+ | Yes | Yes (S) | Yes | No | 18 | S | |||
| 9 | MesGN | 36 | 6 | 0 | None | 2.5+ | 1.5+ | 0.5+ | 0.5+ | 2.5+ | 1.5+ | 1+ | Yes | Yes (G) | Yes | No | 13 | S | |||
| 10 | MesGN + DGS | 3 | 67 | 0 | Moderate | 1+ | 1+ | 0 | 0 | 2+ | 1+ | 1+ | Yes | Yes (G) | Yes | No | 21 | G | |||
| 11 | MesGN | 7 | 14 | 0 | Mild | 2+ | 2+ | 2+ | 0.5+ | 2+ | 1.5+ | 2+ | Yes | Yes (S) | Yes | No | 14 | S | |||
| 12 | MesGN | 13 | 62 | 0 | Mild | 1.5+ | 2+ | 1.5+ | 1+ | 2+ | 1+ | 2+ | Yes | Yes (S) | Yes | No | 13 | S | |||
| 13 | MesGN | 16 | 50 | 0 | Marked | 2+ | 1+ | 0 | 0.5+ | 2+ | 1+ | 0.5+ | Yes | Yes (G) | Yes | Yes | 15 | G | |||
| 14 | MesGN | 27 | 15 | 4 | Mild | 1+ | 2.5+ | 0.5+ | 0 | 2.5+ | 0.5+ | 1+ | Yes | Yes (S) | Yes | No | 16 | S | |||
| 15 | MesGN | 50 | 80 | 0 | Marked | 2.5+ | 1.5+ | 1.5+ | 1+ | 3+ | 1+ | 1.5+ | Yes | Yes (G) | Yes | Yes | NM | G | |||
| 16 | MesGN | 10 | 90 | 0 | Moderate | 2+ | 3+ | 0 | 0 | 2+ | 2.5+ | 3+ | Yes | Yes (S) | Yes | No | 17 | G | |||
| 17 | EPGN | 15 | 33 | 0 | Moderate | 3+ | 2+ | 0.5+ | 0.5+ | 1.5+ | 2+ | 2+ | Yes | Yes (S) | Yes | No | 17 | G | |||
| 18 | MesGN | 23 | 8 | 0 | Moderate | 3+ | 3+ | 0 | 0 | 0 | 3+ | 3+ | Yes | Yes (S) | Yes | No | 19 | S | |||
| 19 | MesGN | 7 | 43 | 0 | Mild | 3+ | 2+ | 0 | 0 | 2+ | 2+ | 2+ | Yes | Yes (S) | Yes | No | 20 | S | |||
| 20 | MesGN | 18 | 0 | 0 | None | 3+ | 3+ | 1+ | 1+ | 3+ | 3+ | 3+ | Yes | Yes (S) | Yes | Yes | 17 | S |
DGS, diabetic glomerulosclerosis; EPGN, endocapillary proliferative glomerulonephritis; FPE, degree of podocyte foot process effacement; G, global; Mes., mesangial; NM, not measured; S, segmental.
IF intensities scale: 0–3+.
Table 3.
Comparison of clinical and pathologic characteristics among FGN–IgAN, FGN and IgAN
| Characteristics | FGN–IgAN | FGN | IgAN | P (FGN–IgAN vs FGN vs IgAN) | P (FGN–IgAN vs FGN | P (FGN–IgAN vs IgAN) |
|---|---|---|---|---|---|---|
| Number of patients | 20 | 40 | 40 | |||
| Clinical characteristics at diagnosis | ||||||
| Age in years | 56.7 ± 11.8 | 55.7 ±− 11.5 | 46.1 ±18.8 | 0.019 | NS | 0.033 |
| Gender (M:F) | 12:8 | 12:28 | 27:13 | 0.003 | 0.049 | NS |
| White race | 17/18 | 38/40 | 35/40 | NS | NS | NS |
| Proteinuria (g/day) | 3.85 (1.85, 6.42) | 3.00 (1.65, 7.25) | 1.55 (0.57, 3.08) | <0.001 | NS | 0.001 |
| Serum albumin (g/dL) | 3.64 ± 0.69 | 3.33 ± 0.72 | 3.83 ± 0.53 | 0.012 | NS | NS |
| Edema | 7/18 | 19/40 | 8/40 | 0.023 | NS | NS |
| Nephrotic syndrome | 3/17 | 10/40 | 1/40 | 0.008 | NS | NS |
| S. Cr. (mg/dL) | 1.70 (1.00, 2.60) | 1.40 (0.83, 1.80) | 1.65 (1.20, 2.18) | NS | NS | NS |
| Renal insufficiency | 14/19 | 22/40 | 27/40 | 0.013 | 0.052 | 0.005 |
| Microhematuria | 15/19 | 30/39 | 39/40 | 0.011 | NS | 0.033 |
| Gross hematuria | 0/16 | 2/40 | 12/40 | 0.001 | NS | 0.012 |
| Hypertension | 13/20 | 22/40 | 23/40 | NS | NS | NS |
| Hypocomplementemia | 0/16 | 1/31 | 2/35 | NS | NS | NS |
| Concurrent conditions | ||||||
| Diabetes | 5/20 | 6/40 | 6/40 | NS | NS | NS |
| Autoimmune disease | 2/20 | 9/40 | 8/40 | NS | NS | NS |
| HCV | 1/20 | 4/40 | 0/40 | NS | NS | NS |
| Malignancy | 3/20 | 8/40 | 2/40 | NS | NS | NS |
| Monoclonal gammopathy | 0/15 | 1/40 | 5/40 | NS | NS | NS |
| Pathologic characteristics | ||||||
| Pattern of glomerular injury on LM | NS | NS | NS | |||
| MesGN | 18 | 32 | 24 | 0.023 | NS | 0.019 |
| EPGN | 1 | 1 | 12 | 0.001 | NS | 0.043 |
| Crescentic GN | 1 | 3 | 1 | NS | NS | NS |
| MPGN | 0 | 3 | 1 | NS | NS | NS |
| Unremarkable | 0 | 1 | 2 | NS | NS | NS |
| % GGS on LM | 39.2 ± 27.4 | 23.9 ± 17.8 | 21.7 ± 17.9 | 0.036 | 0.037 | 0.018 |
| Presence of cellular or fibrocellular crescents | 4/20 | 6/40 | 12/40 | NS | NS | NS |
| Tubular atrophy and interstitial fibrosis (none, mild, moderate and marked) | 2:10:5:3 | 1: 27: 9: 3 | 6: 26: 7: 1 | NS | NS | NS |
| Arteriosclerosis (none, mild, moderate and marked) | 6: 6: 6: 1 | 1: 27: 9: 3 | 13: 14: 9: 3 | NS | NS | NS |
| IgG staining intensitya | 2.0 (1.0–3.0) | 3.0 (2.5–3.0) | 0.0 (0.0–0.0) | <0.001 | 0.001 | <0.001 |
| IgA staining intensitya | 2.0 (1.1–2.4) | 0.0 (0.0–0.4 ) | 2.5 (2.0–3.0) | <0.001 | <0.001 | 0.022 |
| IgM staining intensitya | 0.5 (0.0–1.0) | 0.0 (0.0–0.5) | 0.5 (0.1–1.0) | 0.047 | NS | NS |
| C3 staining intensitya | 2.0 (1.5–2.4) | 2.0 (1.6–3.0) | 2.0 (1.0–3.0) | NS | NS | NS |
| C1q staining intensitya | 0.0 (0.0–0.5) | 0.0 (0.0–0.5) | 0.0 (0.0–0.0) | 0.003 | NS | 0.001 |
| Kappa staining intensitya | 1.0 (1.0–1.9) | 2.0 (1.5–3.0) | 1.0 (1.0–2.0) | 0.004 | 0.007 | NS |
| Lambda staining intensitya | 2.0 (1.0–2.0) | 2.0 (1.0–2.5) | 2.0 (1.5–3.0) | NS | NS | NS |
| Follow-up | ||||||
| Duration of follow-up in months | 27 (14–54) | 28.5 (19–46) | 27 (15–36) | NS | NS | NS |
| IS therapy (steroids with/ without other IS agents) | 8/18 | 22/40 | 13/40 | NS | NS | NS |
| ESKD | 10/18 | 11/40 | 4/40 | 0.001 | NS | <0.001 |
| Death | 5/18 | 3/40 | 1/40 | 0.02 | NS | 0.009 |
EPGN, endocapillary proliferative glomerulonephritis; F, female; M, male; MPGN, membranoproliferative glomerulonephritis; NS, not significant. Values are presented as numbers, mean ± standard deviation for continuous variables and median (25–75% interquartile range) for non-normally distributed continuous variables.
IF staining intensity on a scale of 0–3+.
Most patients with FGN–IgAN presented with proteinuria, microhematuria and renal insufficiency. Thus, at the time of diagnostic biopsy, all patients had proteinuria (median 24-h urine protein 3.9 g), which was higher than in patients with IgAN (P = 0.001) but not patients with FGN (P = NS). Whereas proteinuria was within the nephrotic range in 65%, only 18% had full nephrotic syndrome. Microhematuria was documented in 79% of patients. No patient presented with recurrent gross hematuria, contrary to IgAN patients (30%, P = 0.012). Renal insufficiency at diagnosis was present in 74%; the mean serum creatinine was 1.9 mg/dL (range 0.6–4.9).
Serum complement C3 and C4 were normal in all 16 patients tested. Testing for hepatitis B surface antigen was done in 17 patients and was negative in all, while hepatitis C antibody was positive in only 1 of the 17 patients tested. ANA was positive in 3 of 14 (21%) patients tested. antineutrophil cytoplasmic antibody was negative in all 12 patients tested including one with crescentic GN on biopsy. Serum protein electrophoresis with immunofixation, performed in 18 patients, and urine protein electrophoresis with immunofixation, performed in 11, were negative for monoclonal protein.
Pathologic findings
Light microscopy
Sampling for LM included a mean of 20 glomeruli, a mean of 39% of which were globally sclerotic. The percentage of global glomerulosclerosis (GGS) was higher than in FGN (P = 0.037) and IgAN (P = 0.018). The most common pattern of glomerular injury was by far mesangial proliferative/sclerosing glomerulonephritis (MesGN), observed in 18 (90%) cases, with variable degrees of mesangial hypercellularity, sclerosis and expansion by immune deposits (Figure 1A andTable 2). One case (5%, Case #17 in Table 2) showed focal endocapillary proliferative GN. This pattern was less common than in patients with IgAN (P = 0.043). The remaining case (5%, Case #1 in Table 2) showed crescentic and necrotizing GN with crescents involving 24% of glomeruli (62% of nonsclerotic glomeruli) and fibrinoid necrosis involving 14% of nonsclerotic glomeruli. Two cases with MesGN pattern showed concurrent nodular (one case) or diffuse (one case) diabetic glomerulosclerosis. In addition to the one case of crescentic GN, three cases showed focal crescents involving 10, 9 and 4% of glomeruli. The degree of tubular atrophy and interstitial fibrosis (TA/IF) ranged from absent in 2 cases to mild in 10, to moderate in 5 and to marked in 3. Arteries were sampled in 19 cases. The degree of arteriosclerosis ranged from none in six cases to mild in six, to moderate in six and to marked in one. Glomeruli showed strong smudgy staining for DNAJB9 in all 20 cases (Figure 1B). Congo red stain was negative in all 15 cases tested.
FIGURE 1:
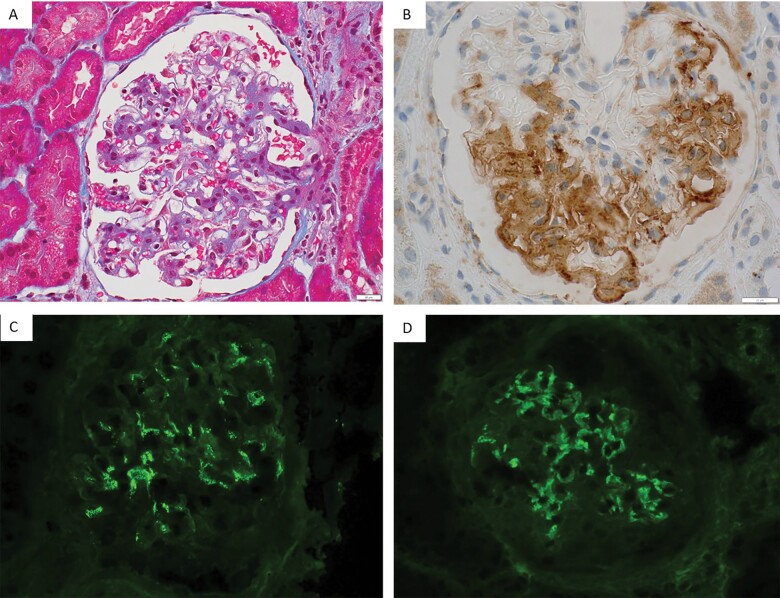
Pathologic findings on LM and IF (A) A representative glomerulus from Case #3 showing mild global mesangial hypercellularity and matrix expansion (trichrome stain, ×400). (B) A representative glomerulus from Case #2 showing smudgy mesangial and GBM positivity for DNAJB9 immunohistochemical stain (×600). (C) A representative glomerulus from Case #6 showing granular global mesangial staining for IgA (×400). (D) A representative glomerulus from the same case (#6) exhibiting smudgy mesangial and GBM staining for IgG (×400).
Immunofluorescence
On standard IF on frozen tissue, all cases showed granular mesangial staining for IgA with a median intensity of 2+ (on a scale of 0–3+; Figure 1C) and smudgy mesangial and glomerular capillary wall (GCW) staining for IgG with a median intensity of 2+ (Figure 1D and Tables 2 and 3). The intensity of IgG staining was lower in FGN–IgAN than in FGN (P = 0.001) and expectedly higher than in IgAN (P < 0.001), and the intensity of IgA staining was lower in FGN–IgAN than in IgAN (P = 0.022) and expectedly higher than in FGN (P < 0.001; Table 3). Glomerular deposition of C3 was detected in 95% of cases (median intensity 2+), IgM in 60% (median intensity 0.5+) and C1q in 45% (median intensity 0). All cases, except one, showed staining for both lambda light chain (median intensity 2+) and kappa light chain (median intensity 1+). The one exception (Case #1) stained 2+ for lambda with negative staining for kappa; however, by IF on paraffin tissue after pronase digestion, it stained for both lambda (3+) and kappa (1+), and this patient had negative serum immunofixation and normal serum-free light-chain ratio. Focal extraglomerular staining for IgG was present in 15% of cases (involving tubular basement membranes in two patients and arterioles in one). IF staining for IgG subtypes, performed on 11 cases, showed heavy chain isotype restriction (i.e. staining for a single IgG subclass) in six (55%, IgG4 in three and IgG1 in three) and staining for more than one subclass in the remaining five cases (IgG4 dominant in two, IgG1 dominant in two and IgG1 and IgG4 codominance in one).
Electron microscopy
All cases exhibited randomly oriented straight fibrils (mean thickness 16 nm, range 12–21 nm) permeating the mesangial matrix and the glomerular basement membranes (GBM), typical of FGN (Figure 2A and B; Table 2). The GBM fibrillar deposits were segmental (i.e. involving <50% of GCW) in 13 cases and global in the remaining 7 cases. In addition to the fibrillar deposits, all cases exhibited mesangial granular electron-dense deposits (i.e. without substructure). In contrast to the mesangial fibrillary deposits that intermingled with the mesangial matrix, the mesangial granular electron-dense deposits in most cases formed discrete deposits with a tendency to localize beneath the GBM reflection over the mesangium (so referred to as ‘paramesangial deposits’; Figure 2C and D), as seen in the majority of IgAN cases. Rare, small granular GCW deposits (intramembranous or subendothelial) were seen in four (20%) cases.
FIGURE 2:
EM findings in Case #11 (A) Fibrillary deposits permeating the GBM (×12 000). (B) On high magnification, infiltration of the GBM by randomly oriented straight fibrils (mean thickness 14 nm and range 11–18 nm) is evident (×30 000). (C) In the same biopsy, there were also discrete electron-dense deposits (arrows) localized beneath the GBM reflection over the mesangium (‘paramesangial deposits’; ×4000). (D) A higher magnification of the deposits depicted in (C) showing granular texture (i.e. without substructure; ×30 000).
Treatment and outcome
Follow-up was available on 18 patients with FGN–IgAN. The median duration of follow-up was 27 months (range 5–133 months). On follow-up, 10 (56%) progressed to ESKD (Table 1). Three of these patients did not receive immunosuppression therapy, three were treated with steroids only, one received steroids and rituximab, one received mycophenolate mofetil (MMF) only and one was treated with seven cycles of cyclophosphamide + bortezomib + dexamethasone. The latter patient initially partially responded to this chemotherapeutic regimen with reduction of proteinuria from 14 to 6 g/day and reduction of serum creatinine from 2.4 to 1.4 mg/dL, but subsequently advanced to ESKD. Data on treatment were not available on the remaining patient. Five patients with ESKD died, one of acute coronary artery disease, one of sepsis in the setting of Clostridium difficile colitis, one of pneumonia, one of metastatic lung cancer and one of undetermined cause. One of the patients with ESKD (Case #19) underwent a living-donor kidney transplant and had biopsy-proven recurrence of IgAN (without FGN) in the renal allograft. Seven-year postimplantation, his proteinuria was 7.5 g/day and serum creatinine was 1.0 mg/dL.
Of the remaining eight (44%) patients who did not progress to ESKD, three had PRD [none of whom received immunosuppressive (IS) agents], four had remission (three of whom did not receive IS agents and one received cyclophosphamide and rituximab) and one had an elevated serum creatinine at last follow-up but the degree of proteinuria was not available (who was treated with steroids, MMF and rituximab; Table 1).
Using Cox regression, the only predictor of reaching ESKD by univariate analysis in patients with FGN–IgAN was a higher serum creatinine at biopsy (P = 0.008). Using the Cox proportional hazards model, the only independent predictor of the rate of progression to ESKD by multivariate analysis was a higher serum creatinine at biopsy (P = 0.039; hazard ratio = 4.445; 95% confidence interval 1.077–18.349). Proteinuria at biopsy, serum albumin and degree of GGS, TA/IF and arteriosclerosis did not predict renal survival.
The duration of follow-up and proportion of patients who received IS agents (steroids and/or other IS agents) were not different among the FGN–IgAN, FGN and IgAN groups (Table 3). The proportion of patients reaching ESKD was higher in FGN–IgAN than in IgAN (56% versus 10%, P < 0.001) and tended to be higher than in FGN (28%), but this did not reach statistical significance. Likewise, the death rate in FGN–IgAN was higher than in IgAN (28% versus 3%, P = 0.009) and tended to be higher than in FGN (8%), but this did not reach statistical significance. The median Kaplan–Meier ESRD-free survival time was 44 months for FGN–IgAN, which was shorter than IgAN (unable to compute, P = 0.013) and FGN (107 months, P = 0.048; Figure 3).
FIGURE 3:
Kaplan–Meier ESKD-free survival curve for FGN–IgAN, FGN and IgAN.
Repeat native biopsies
Four patients had prior and/or follow-up native kidney biopsies. Patients #4 and #13 had kidney biopsies 5 and 3 years prior to the diagnostic biopsies that showed FGN without IgAN indicating that FGN preceded IgAN in these two patients. Patient #13 also had a third biopsy one and a half years following the diagnostic biopsy which revealed diffuse sclerosing GN with both fibrillary and granular deposits by EM but no glomeruli were sampled for IF.
Patient #8 had a repeat biopsy 11 years following the diagnostic biopsy that showed active IgAN (two of six sampled glomeruli with cellular crescents on LM, bright glomerular staining for IgA on IF and abundant granular deposits on EM) with burnt-out FGN (only rare remnant fibrillary deposits on EM and trace staining for IgG by IF).
Patient #14 had a repeat biopsy 2 years after the diagnostic biopsy that showed diffuse sclerosing GN with evidence of persistent FGN and IgAN on IF (with both smudgy staining for IgG and granular staining for IgA) and EM (with both fibrillary and granular deposits).
DISCUSSION
In our experience, the overall incidence of a concurrent glomerulopathy in FGN is 17.4% (147/847; Table 4). Concurrent IgAN appears to be very rare, present in only 1.8% of FGN cases, but it is the second most common concurrent glomerulopathy after diabetic nephropathy (Table 4) which is not surprising considering the higher frequency of IgAN than other primary glomerular diseases. This study describes the clinical features, pathologic characteristics and outcome of patients with FGN concurrent with IgAN. In contrast to FGN, which is more common in females [9, 11], FGN–IgAN was more common in males, similar to IgAN in North America [11]. Mean age at diagnosis was 57 years, which was comparable to FGN patients and higher than IgAN patients. FGN–IgAN patients had a higher degree of proteinuria, higher incidence of renal insufficiency and lower incidence of microhematuria at diagnosis than IgAN patients. Notably, no FGN–IgAN patient presented with recurrent macrohematuria accompanying upper respiratory tract infect ‘synpharyngitic hematuria’, which is a common presentation in IgAN in North America [21] and which was observed in 30% in our IgAN control group. Thus, FGN–IgAN has similar renal presentation and demographics (aside from male predominance) to FGN. Overall, 30% (6/20) of patients had underlying HCV, autoimmune disease or malignancy, conditions known to be present in some patients with FGN [2, 15, 16], and thus exclusion of such conditions in patients with FGN–IgAN is important. Noticeably, no patient had liver cirrhosis, celiac disease, inflammatory bowel disease, HIV infection or systemic features of Henoch–Schonlein purpura, favoring that IgAN in these patients is mostly idiopathic and renal limited.
Table 4.
Concurrent glomerulopathies in 847 consecutive cases of FGN accessioned between 2015 and 2019 at the Mayo Clinic Renal Biopsy Laboratory and Arkana Laboratories
| Number of FGN cases | Number of cases with concurrent glomerulopathy (%) | Type of concurrent lesion (%) |
|---|---|---|
| 847 | 147 (17.4) | |
|
Diabetic glomerulosclerosis = 107 (12.6%) IgA nephropathy = 15 (1.8%) ANCA-associated crescentic GN = 5 (0.6%) Thrombotic microangiopathy = 5 (0.6%) Membranous nephropathy = 4 (0.5%) Smoking and HTN-associated mesangial sclerosing glomerulopathy = 3 (0.4%) Collapsing glomerulopathy = 2 (0.2%) Thin basement membrane disease = 2 (0.2%) Anti-GBM nephritis = 1 (0.1%) Bacterial infection-associated GN = 1 (0.1%) Lupus nephritis class IV = 1 (0.1%) Minimal change disease = 1 (0.1%) |
The prognosis of FGN–IgAN seems to be guarded, as over a half of patients progressed to ESKD within a median follow-up of 27 months. The proportion of FGN–IgAN patients reaching ESKD was higher than in IgAN patients, and the ESKD-free survival was shorter in FGN–IgAN than in IgAN and FGN; thus, the accurate diagnosis of this dual glomerulopathy is clinically relevant.
Of note, a repeat native biopsy in one patient showed active IgAN with burnt-out FGN and an allograft biopsy in another patient revealed IgAN without FGN; thus, repeat biopsies might be needed to determine the evolution of this lesion in patients who are clinically suspected to have a disease relapse.
Due to the limited sample size, we cannot draw conclusions about the appropriate therapy for FGN–IgAN. We note, however, that three of the four patients who had disease remission were not treated with IS agents, suggesting that conservative therapy alone may be appropriate for mild cases. Rituximab, which may preserve kidney function in some patients with FGN [8, 22], was given (together with other IS agents) to three patients, one of whom had remission, one had persistently elevated serum creatinine and one progressed to ESKD. Larger studies with longer follow-up are needed to determine the optimal therapy.
The diagnosis of FGN–IgAN requires careful evaluation of IF findings, which will demonstrate dual pattern of glomerular positivity: smudgy mesangial and GCW staining for IgG and granular mesangial staining for IgA. The diagnosis is confirmed by the ultrastructural findings of both randomly oriented fibrils involving the mesangium and GBM and granular electron-dense deposits that tend to localize in the paramesangial areas. This dual glomerulopathy is likely underdiagnosed as EM is not often available in renal pathology laboratories in developing countries and is not routinely performed in the evaluation of native renal biopsies in the developed countries, including some countries in Europe [23]. Thus, 70% (14/20) of our FGN–IgAN had IgG staining intensity weaker (six cases) or equal to (eight cases) IgA staining intensity, and these cases could be misdiagnosed as IgAN alone if careful evaluation of the pattern of IF staining at high magnification and EM were not performed. The availability of DNAJB9 immunohistochemical stain, a highly sensitive and specific marker for FGN [4–6], now allows for easier diagnosis of FGN. Of note, there have been two reported cases of IgA-dominant FGN [24, 25], which are different than our cases in that they did not exhibit IgG staining on IF or granular electron-dense deposits on EM. Thus, these two cases do not represent FGN–IgAN but rather exceptional cases of an IgA variant of FGN.
Given the different morphology on IF and EM and pathophysiology of these two forms of GN, we favor that the occurrence of FGN and IgAN in the same biopsy likely represents a chance occurrence of two unrelated conditions. Furthermore, all 20 cases were positive for DNAJB9 and, when tested, exhibited IgG4 and/or IgG1 restriction or dominance (as seen in FGN alone), arguing against a different pathogenesis for FGN in these cases. Since IgA deposits without clinical evidence of kidney disease are present in 5–16% of healthy individuals [26, 27], it is tempting to speculate that FGN–IgAN probably represents FGN superimposed on preexisting mild IgAN. However, in two of our FGN–IgAN patients prior biopsies showed FGN without IgAN, indicating that in at least some patients, the development of FGN precedes that of IgAN.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no relevant financial interests. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Fogo A, Qureshi N, Horn RG.. Morphologic and clinical features of fibrillary glomerulonephritis versus immunotactoid glomerulopathy. Am J Kidney Dis 1993; 22: 367–377 [DOI] [PubMed] [Google Scholar]
- 2. Nasr SH, Valeri AM, Cornell LD. et al. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol 2011; 6: 775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenstock JL, Markowitz GS, Valeri AM. et al. Fibrillary and immunotactoid glomerulonephritis: distinct entities with different clinical and pathologic features. Kidney Int 2003; 63: 1450–1461 [DOI] [PubMed] [Google Scholar]
- 4. Nasr SH, Vrana JA, Dasari S. et al. DNAJB9 is a specific immunohistochemical marker for fibrillary glomerulonephritis. Kidney Int Rep 2018; 3: 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dasari S, Alexander MP, Vrana JA. et al. DnaJ heat shock protein family B member 9 is a novel biomarker for fibrillary GN. J Am Soc Nephrol 2018; 29: 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andeen NK, Yang HY, Dai DF. et al. DnaJ homolog subfamily B member 9 is a putative autoantigen in fibrillary GN. J Am Soc Nephrol 2018; 29: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander MP, Dasari S, Vrana JA. et al. Congophilic fibrillary glomerulonephritis: a case series. Am J Kidney Dis 2018; 72: 325–336 [DOI] [PubMed] [Google Scholar]
- 8. Andeen NK, Troxell ML, Riazy M. et al. Fibrillary glomerulonephritis: clinicopathologic features and atypical cases from a multi-institutional cohort. Clin J Am Soc Nephrol 2019; 14: 1741–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nasr SH, Fogo AB.. New developments in the diagnosis of fibrillary glomerulonephritis. Kidney Int 2019; 96: 581–592 [DOI] [PubMed] [Google Scholar]
- 10. O’Shaughnessy MM, Hogan SL, Poulton CJ. et al. Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol 2017; 12: 614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Shaughnessy MM, Hogan SL, Thompson BD. et al. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant 2018; 33: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalbermatter SA, Marone C, Casartelli D. et al. Outcome of fibrillary glomerulonephritis. Swiss Med Wkly 2012; 142: w13578. [DOI] [PubMed] [Google Scholar]
- 13. Li LS, Liu ZH.. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int 2004; 66: 920–923 [DOI] [PubMed] [Google Scholar]
- 14. Bijol V, Farag YMK, Harris DCH. et al. Renal pathology practice globally: identifying needs and meeting the challenge. Kidney Int 2019; 96: 258–261 [DOI] [PubMed] [Google Scholar]
- 15. Payan Schober F, Jobson MA, Poulton CJ. et al. Clinical features and outcomes of a racially diverse population with fibrillary glomerulonephritis. Am J Nephrol 2017; 45: 248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Javaugue V, Karras A, Glowacki F. et al. Long-term kidney disease outcomes in fibrillary glomerulonephritis: a case series of 27 patients. Am J Kidney Dis 2013; 62: 679–690 [DOI] [PubMed] [Google Scholar]
- 17. Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol 2014; 10: 445–454 [DOI] [PubMed] [Google Scholar]
- 18. Cattran DC, Coppo R, Cook HT. et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 19. Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 20. Wyatt RJ, Julian BA.. IgA nephropathy. N Engl J Med 2013; 368: 2402–2414 [DOI] [PubMed] [Google Scholar]
- 21. Schena FP. A retrospective analysis of the natural history of primary IgA nephropathy worldwide. Am J Med 1990; 89: 209–215 [DOI] [PubMed] [Google Scholar]
- 22. Hogan J, Restivo M, Canetta PA. et al. Rituximab treatment for fibrillary glomerulonephritis. Nephrol Dial Transplant 2014; 29: 1925–1931 [DOI] [PubMed] [Google Scholar]
- 23. Pullman JM, Ferrario F, Nast CC.. Actual practices in nephropathology: a survey and comparison with best practices. Adv Anat Pathol 2007; 14: 132–140 [DOI] [PubMed] [Google Scholar]
- 24. Calls Ginesta J, Torras A, Ricart MJ. et al. Fibrillary glomerulonephritis and pulmonary hemorrhage in a patient with renal transplantation. Clin Nephrol 1995; 43: 180–183 [PubMed] [Google Scholar]
- 25. Nebuloni M, Genderini A, Tosoni A. et al. Fibrillary glomerulonephritis with prevalent IgA deposition associated with undifferentiated connective tissue disease: A case report. NDT Plus 2010; 3: 57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waldherr R, Rambausek M, Duncker WD. et al. Frequency of mesangial IgA deposits in a non-selected autopsy series. Nephrol Dial Transplant 1989; 4: 943–946 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki K, Honda K, Tanabe K. et al. Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 2003; 63: 2286–2294 [DOI] [PubMed] [Google Scholar]