Abstract
Background
While there are many cross-sectional studies of glomerulonephritis (GN) incidence, changes in incidence over time, particularly in the 21st century have received less attention. Similarly, little is known about temporal changes in GN prognosis. The presence in Denmark of comprehensive registries for renal biopsy results, end-stage renal disease (ESRD), comorbidity and mortality permit these questions to be addressed.
Methods
Data for all renal biopsies in Denmark between 1985 and 2014 were extracted from the Danish Renal Biopsy Registry and Patobank registries. The date of first dialysis or transplantation was extracted from the Danish Nephrology Registry for those patients developing ESRD. Dates of death and presence of chronic comorbid conditions at date of biopsy were extracted from the National Patient Registry. The incidence of GN, adjusted to the 2013 European standard population, was calculated. ESRD incidence and mortality were calculated, both in absolute terms and after correction for age, comorbidity and presence of renal tubulointerstitial fibrosis.
Results
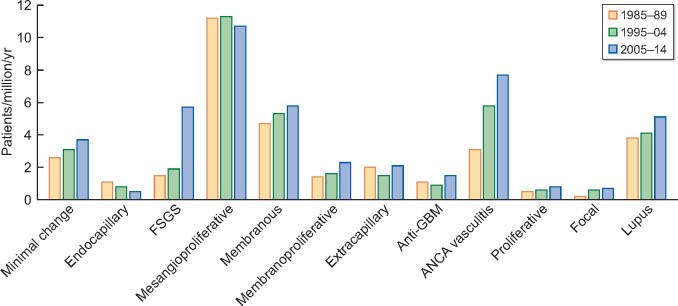
The incidence rose from 33.3 patients per million (ppm)/year in 1985–94 to 46.5 ppm in 2005–14. The increase could in part be related to changes in renal biopsy policy. Large increases in Anti-neutropil cytoplasmic antibody (ANCA) vasculitis (ANCAV) (3.1–7.7 ppm/year) and focal segmental glomerulosclerosis (FSGS) (1.5–5.7 ppm/year) incidence were noted. The biopsy-proven prevalence of GN in 2014 was 748 ppm of which 155 ppm were being treated with dialysis or transplantation. Adjusted ESRD incidence fell by 25% during the study period, mortality by 62% and combined ESRD/mortality by 46%. The fall in ESRD incidence was limited to minimal change GN, FSGS, membranous GN and lupus nephritis, while reductions in mortality, and the combination of ESRD and/or death, were seen for nearly all GN diagnoses.
Conclusions
This study suggests that the incidence of GN has generally increased between 1985 and 2014, but some of the increase may be related to changes in renal biopsy policy. Major increases in FSGS and ANCAV incidence have occurred. The prognosis of GN, both as regards ESRD and mortality, has improved.
Keywords: ANCA, epidemiology, FSGS, glomerulonephritis, IgA nephropathy, lupus nephritis, membranous nephropathy, membranoproliferative glomerulonephritis, minimal change disease, prognosis
INTRODUCTION
While there are many studies concerning the incidence and prevalence of glomerulonephritis (GN), fewer studies have been published concerning changes in incidence over time [1–15]. Most of these studies only cover the period before 2000 [1–4, 6, 9, 10, 13, 14], while others only have a short period of observation [5, 8, 11]. Many of the studies do not have a defined background population, and thus express relative frequencies rather than absolute incidence. Two large reviews of the studies have been published [12, 16]. The studies are characterized by considerable regional heterogeneity, but in general document a falling frequency of mesangioproliferative GN (MesPGN) and membranous GN (MGN), and an increase in focal segmental glomerulosclerosis (FSGS). The frequency of IgA nephropathy seems to have fallen in recent years.
Only three studies have presented long-term studies with recent data [12, 15, 17]. The existence of a national, comprehensive renal biopsy registry in Denmark since 1985 permits an evaluation of changes in absolute GN incidence over a long period of time. Furthermore, by linking these data with national registries of end-stage renal disease (ESRD), comorbidity and death, estimates of GN prognosis can be performed.
MATERIALS AND METHODS
All patients with GN, as confirmed by renal biopsy, and residing in Denmark between the years 1985 and 2014 inclusive, were included. The study was an observational study in epidemiology and followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for reporting observational studies [18].
Renal biopsies
The renal biopsy information was derived from two registries:
Danish Renal Biopsy Registry (DANYRBI). This registry recorded all biopsies performed in Denmark between 1985 and 1999 [19]. The reproducibility of the glomerular diagnosis has been investigated and found acceptable with a kappa value of 0.61 [20].
Since 2000 renal biopsy results have been registered by the National Pathology Data Bank (Patobank).
The following Systematized Nomenclature of Medicine (SNOMED) diagnoses were included (SNOMED codes in parentheses): minimal changes disease (MCGN), (M00100, combined with proteinuria or nephrosis: S65080, S67020, or S67550), endocapillary GN (EndGN, M46870), FSGS (M53341), MesPGN (M46811, M46862), MGN (M68130), membranoproliferative GN (MPGN) (M46842), proliferative GN (ProlGN, M46810), Focal GN (M46861), extracapillary (crescentic) GN (M46880), anti-glomerular basement GN (AntiGBM) (S67400), ANCA vasculitis (ANCAV) (S76950), lupus nephritis (LN) (S38720). Due to inaccurate diagnosis of IgA nephropathy in early years, this diagnosis was combined with MesPGN, and classified as MesPGN. For most of these biopsies, the correct diagnosis will probably have been IgA GN and not primarily MesPGN. Most cases of EndGN will have been infection-related GN. For each patient, only one biopsy was included, being the first biopsy with a GN diagnosis. Patients <15 years old were excluded. For biopsies with multiple GN diagnoses, the first mentioned diagnosis was chosen, with the following exceptions: AntiGBM, ANCAV and LN were given first priority; MCGN was ignored in the presence of a more specific diagnosis. Microscopic diagnoses were supplemented with the following clinical diagnoses [International Classification of Diseases (ICD)] (vide infra) if these were compatible with microscopy: lupus (ICD-8 734.19, ICD-10 32.1–32.9), ANCAV (ICD-8 446.29, ICD-10 31.3), antiGBM (ICD-8 446.19, ICD-10 M31.0). The presence in the biopsy of tubulointerstitial fibrosis (M49000-M49005) was noted, but the registries do not contain information concerning degree of fibrosis.
The abbreviations used in this article are shown in Table 1.
Table 1.
Abbreviations
| ANCA | Antineutrophil cytoplasmic antibody |
| ANCAV | ANCA vasculitis |
| AntiGBM | Anti-glomerular basement membrane glomerulonephritis |
| CCI | Charlson comorbidity index |
| DANYRBI | Danish Renal Biopsy Registry |
| DM | Diabetes mellitus |
| EndGN | Endocapillary glomerulonephritis |
| ESRD | End-stage renal disease |
| FSGS | Focal segmental glomerulosclerosis |
| GN | Glomerulonephritis |
| ICD | International Classification of Diseases |
| LN | Lupus nephritis |
| LPR | National Patient Registry |
| LTF | Lost to follow-up |
| MCGN | Minimal change glomerulonephritis |
| MGN | Membranous glomerulonephritis |
| MesPGN | Mesangioproliferative glomerulonephritis |
| MemPGN | Membranoproliferative glomerulonephritis |
| ProlGN | Proliferative glomerulonephritis |
| SNOMED | Systematized Nomenclature of Medicine |
| STROBE | STrengthening the Reporting of OBservational studies in Epidemiology |
Patient data
Patient sex and birthday were calculated from the national identity number. Dates of emigration and death were extracted from the National Patient Registry (LPR). The presence of the following clinical comorbidities at biopsy was also extracted: diabetes mellitus (DM), chronic heart disease, previous myocardial infarction, heart failure, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, chronic hepatic disease, cancer (excluding basocellular). Chronically reduced renal function was registered (SNOMED S65050, S65150, S65110, ICD-8 582.09, ICD-10 N03.x, N18.0, N18.3-18.9). ESRD was defined as requirement for maintenance dialysis or renal transplantation. Date of first dialysis or transplantation for these patients was extracted from the Danish Nephrology Registry. National population statistics by age were extracted from Statistics Denmark.
Statistics
Charlson comorbidity index (CCI) [21] was calculated from the registered comorbidity. Incidence rates were calculated for the whole population and by 10-year age group. Temporal changes were assessed using three cohorts: 1986–94, 1995–2004 and 2005–14. Age-standardized incidence rates were calculated from the European Standard population 2013 published by Eurostat, the Statistical Office of the European Union [22].
Normally distributed variables were compared using Student’s t-test. Categorical and non-parametric variables were compared using Chi-square and Mann–Whitney.
Patients were followed until death, emigration or 1 January 2015. Patient survival [censored for lost to follow-up (LTF)], renal survival (time to ESRD, censored for patient death or LTF) and combined survival (time to death or ESRD, censored for LTF) were calculated using Kaplan–Meier analysis and Cox proportional hazards iterative regression analysis. Relative risks for the cohorts 1995–2004 and 2005–14, compared with 1985–94 as referent, were calculated after adjusting for patient age, sex, comorbidities and tubulointerstitial fibrosis. It was assumed that tubulointerstitial fibrosis was a marker of changes in biopsy indications, in that increased biopsy incidence of uraemic patients with reduced kidney size would increase the incidence of tubulointerstitial fibrosis. The Statistica (Tulsa, USA) program was used for the statistical analysis.
RESULTS
Patient details are shown in Table 2 and Figure 1. The mean age at biopsy rose from 46.3 ± 18 to 50.6 ± 18 years and CCI from 1.5 to 2.3 during the period of observation. The incidence of GN diagnoses is shown in Table 3. For most diagnoses, the absolute number and population-adjusted incidence of GN increased. However, the incidence of MesPGN, extracapillary GN and AntiGBM was unchanged, and EndGN incidence fell. Large increases in ANCAV [3.1–7.7 patients/million/year (ppm/year)] and FSGS (1.5–5.7 ppm/year) incidence were noted. The incidence of GN overall rose from 33.3 to 46.5 ppm/year. The biopsy-proven prevalence of GN in 2014 was 748 ppm of which 155 ppm were being treated with dialysis or transplantation. The relative frequency of the diagnoses for the period 2005–14 is compared with other published series in Table 4. Comparison between the studies is difficult due to differences in histological classification, non-inclusion of secondary GN and subdivision of the results into cohorts and age groups. The published figures have therefore been adjusted to facilitate comparison.
Table 2.
Distribution of patient number, relative per cent, age, sex and Charlson comorbidity index classified by renal diagnosis and cohort
| Number |
Relative per cent |
Age (years) |
CCI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 |
| Minimal change | 113 | 135 | 166 | 8.1 | 8.5 | 8.0 | 43.0 ±19 | 43.2 ±19 | 43.7 ±18 | 0.8 | 1.1a | 1.5c |
| Endocapillary | 49 | 31 | 24 | 3.5 | 2.0 | 1.2 | 42.6 ±1 | 52.2 ±20a | 43.9 ±18 | 1.0 | 1.7a | 2.0b |
| FSGS | 62 | 83 | 255 | 4.5 | 5.3 | 12.3 | 45.3 ±16 | 46.7 ±16 | 50.7 ±17a | 1.7 | 1.9 | 2.4b |
| Mesangioproliferative | 480 | 490 | 487 | 34.6 | 31.0 | 23.5 | 43.5 ±17 | 45.8 ±18a | 44.6 ±19 | 1.4 | 1.8c | 2.2c |
| Membranous | 189 | 216 | 257 | 13.6 | 13.7 | 12.4 | 50.0 ±17 | 54.0 ±16a | 56.0 ±16c | 1.3 | 1.7b | 2.3c |
| Membranoproliferative | 59 | 70 | 103 | 4.3 | 4.4 | 5.0 | 45.3 ±19 | 48.9 ±16 | 56.4 ±15c | 1.5 | 2.1b | 2.9c |
| Extracapillary | 76 | 59 | 92 | 5.5 | 3.7 | 4.4 | 59.3 ±15 | 56.4 ±20 | 53.9 ±19a | 2.1 | 2.1 | 2.5 |
| AntiGBM | 46 | 35 | 63 | 3.3 | 2.2 | 3.0 | 48.5 ±21 | 53.8 ±23 | 58.2 ±20b | 2.1 | 2.8 | 2.9b |
| ANCAV | 116 | 222 | 332 | 8.4 | 14.1 | 16.0 | 57.9 ±13 | 61.3 ±14 | 61.7 ±15a | 1.8 | 2.5c | 2.6c |
| Proliferative | 19 | 23 | 35 | 1.4 | 1.5 | 1.7 | 55.8 ±18 | 46.9 ±21 | 44.1 ±19a | 2.2 | 2.4 | 2.1 |
| Focal | 7 | 24 | 29 | 0.5 | 1.5 | 1.4 | 43.2 ±21 | 51.3 ±18 | 54.2 ±16 | 2.0 | 2.0 | 2.9 |
| Lupus | 172 | 191 | 233 | 12.4 | 12.1 | 11.2 | 38.5 ±17 | 37.0 ±15 | 41.1 ±16 | 1.5 | 1.8 | 1.9b |
| Total | 1388 | 1579 | 2076 | 100 | 100 | 100 | 46.3 ±18 | 48.8 ±18c | 50.6 ±18c | 1.5 | 1.9c | 2.3c |
P < 0.05.
P < 0.01.
P < 0.001 (versus 1985–94).
CCI shown as mean values, but analysed by Mann–Whitney.
FIGURE 1:
Adjusted incidence of renal diagnoses by cohort.
Table 3.
Relative frequency of diagnoses for the period 2005–14, compared with selected publications
| Author | Present studya | Chiu et al. [23] | Polito et al. [24] | Hanko et al. [10] | Hou et al. [11] | Jegatheesan et al. [8] | Rychlik et al. [25] | Schena et al. [26] | Woo et al. [12] | Xu et al. [17] | Rivera et al. [27] | Wang et al. [15] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Land | Denmark | Taiwan | Brazil | UK | China | Australia | Czechoslovakia | Italy | Singapore | China | Spain | China |
| Publication date | 2020 | 2018 | 2010 | 2009 | 2018 | 2016 | 2004 | 1997 | 2019 | 2016 | 2002 | 2019 |
| Minimal change | 8 | 19 | 12 | 11 | 12 | 5 | 10 | 6 | 20 | 5 | 12 | 12 |
| Endocapillary | 1 | 2 | 7 | 1 | 3 | 1 | 3 | 1 | ||||
| FSGS | 12 | 22 | 19 | 4 | 8 | 19 | 9 | 10 | 25 | 16 | 5 | |
| Mesangioproliferative/IgAb | 24 | 29 | 19 | 43 | 51 | 30 | 31 | 40 | 31 | 56 | 20 | 39 |
| Membranous | 12 | 21 | 16 | 15 | 12 | 8 | 17 | 15 | 12 | 16 | 13 | |
| Membranoproliferative | 5 | 3 | 3 | 10 | 1 | 3 | 4 | 5 | 2 | 9 | ||
| Extracapillary | 4 | 5 | 12 | 3 | 4 | 1 | ||||||
| AntiGBM | 3 | 1 | 0.3 | |||||||||
| ANCAV | 16 | 2 | 5 | 5 | 11 | |||||||
| Proliferative | 2 | 4 | ||||||||||
| Focal | 1 | 9 | ||||||||||
| Lupus | 11 | 16 | 11 | 13 | 8 | 9 | 7 | 14 |
2005–14.
Including Henoch–Schönlein.
Published figures adjusted for comparison. Figures in per cent.
Table 4.
Absolute and adjusted incidence of GN classified by renal diagnosis and cohort
| Incidence (ppm) |
Adjusted incidence (ppm#) |
|||||
|---|---|---|---|---|---|---|
| Cohort | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 |
| Minimal change | 2.7 | 3.1 | 3.7b | 2.6 | 3.1 | 3.7 |
| Endocapillary | 1.2 | 0.7a | 0.5b | 1.1 | 0.8 | 0.5b |
| FSGS | 1.5 | 1.9 | 5.7c | 1.5 | 1.9 | 5.7c |
| Mesangioproliferative | 11.3 | 11.3 | 10.8 | 11.2 | 11.3 | 10.7 |
| Membranous | 4.4 | 5.0 | 5.7a | 4.7 | 5.3 | 5.8a |
| Membranoproliferative | 1.4 | 1.6 | 2.3b | 1.4 | 1.6 | 2.3b |
| Extracapillary | 1.8 | 1.4 | 2.0 | 2.0 | 1.5 | 2.1 |
| AntiGBM | 1.1 | 0.8 | 1.4 | 1.1 | 0.9 | 1.5 |
| ANCAV | 2.7 | 5.1c | 7.4c | 3.1 | 5.8c | 7.7c |
| Proliferative | 0.4 | 0.5 | 0.8a | 0.5 | 0.6 | 0.8a |
| Focal | 0.2 | 0.6b | 0.6b | 0.2 | 0.6b | 0.7c |
| Lupus | 4.1 | 4.4 | 5.2b | 3.8 | 4.1 | 5.1b |
| Total | 32.7 | 36.3 | 46.2 | 33.3 | 37.3 | 46.5 |
P < 0.05.
P < 0.01.
P < 0.001 (versus 1985–94).
ppm: patients per million inhabitants/year. Adjusted for European standard population 2013.
The relationship of incidence to patient age is shown in Table 5 and Figure 2. The increase in incidence was particularly marked in age groups >50 years, but was also present in younger age groups.
Table 5.
Absolute incidence of GN classified by patient age and cohort
| Absolute incidence (ppm) |
|||
|---|---|---|---|
| Cohort |
|||
| Age (years) | 85-94 | 95-04 | 05-14 |
| 15–19 | 26.1 | 35.0 | 36.3a |
| 20–29 | 30.9 | 31.4 | 37.4 |
| 30–39 | 29.0 | 27.3 | 39.1c |
| 40–49 | 28.4 | 28.2 | 39.6c |
| 50–59 | 42.0 | 45.1 | 46.9 |
| 60–69 | 48.7 | 55.9 | 66.0c |
| 70–79 | 37.5 | 51.4b | 70.0c |
| >79 | 7.0 | 17.4b | 30.4c |
P < 0.05.
P < 0.01.
P < 0.001 (versus 1985–94).
FIGURE 2:
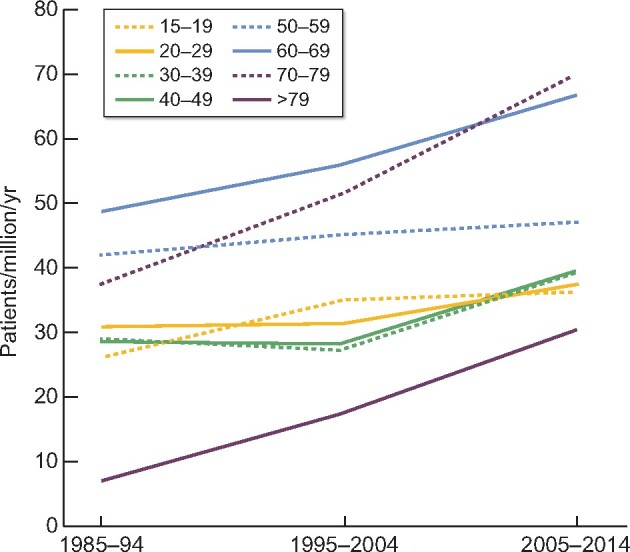
Absolute incidence of glomerulonephritis by age.
In total, 692 (13.7%) biopsies contained tubulointerstitial fibrosis. This was evenly divided between diagnoses with some outliers: MCGN 16%, EndGN 5%, FSGS 28%. The proportion of biopsies with tubulointerstitial fibrosis rose during the period of observation (1985–94 5%; 1995–2004 14%; 2005–14 20%). The prevalence of chronically reduced renal function at biopsy increased (1985–94 47%; 1995–2004 57%; 2005–14 65%). The standardized incidence rate for biopsies without tubulointerstitial fibrosis also increased (1985–94 31.9 ppm/year; 1995–2004 32.0; 2005–14 37.3). This increase was common for most diagnoses, except for MesPGN, where incidence fell from 10.7 ppm/year to 8.0.
The changes in 1-, 5- and 10-year absolute incidence of ESRD, death and ESRD/death combined are shown in Table 6. An unadjusted bivariate Kaplan–Meier analysis, including the two cohorts 1995–2004 and 2005–14 was performed. With one (stated) exception, the significance values shown refer to the overall significance of the analyses. The number of patients with EndGN, ProlGN and Focal GN was too small for statistical analysis. The incidence of ESRD showed a heterogeneous pattern. For some diagnoses incidence rose in the period 1995–2004, and then fell. Only MCGN, ANCAV and LN showed a consistent fall in ESRD incidence. There was no overall change in absolute ESRD incidence.
Table 6.
The 1-, 5- and 10-year incidence of end-stage renal disease (ESRD), death and the combination, classified by renal diagnosis and cohort
| 1 år |
5 år |
10 år |
Significance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ESRD | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 | 85-94 | 95-04 | 05-14 | |
| Minimal change | 0 | 2 | 0 | 3 | 2 | 2 | 12 | 3 | 0 | P = 0.05 |
| Endocapillary | 0 | 16 | 18 | 2 | 16 | 18 | 2 | 16 | 18 | |
| FSGS | 5 | 7 | 2 | 24 | 21 | 16 | 40 | 38 | 33 | |
| Mesangioproliferative | 6 | 8 | 8 | 18 | 17 | 20 | 26 | 26 | 34 | |
| Membranous | 2 | 6 | 2 | 10 | 16 | 3 | 19 | 21 | 19 | P < 0.01 |
| Membranoproliferative | 16 | 14 | 18 | 34 | 22 | 26 | 50 | 42 | 42 | |
| Extracapillary | 28 | 43 | 20 | 44 | 55 | 37 | 48 | 57 | 45 | |
| AntiGBM | 43 | 69 | 51 | 63 | 69 | 61 | 72 | 73 | 64 | |
| ANCAV | 12 | 20 | 7 | 18 | 30 | 18 | 38 | 37 | 24 | P < 0.01 |
| Proliferative | 22 | 27 | 15 | 29 | 43 | 19 | 43 | 54 | 46 | |
| Focal | 33 | 13 | 22 | 50 | 18 | 22 | 50 | 21 | 22 | |
| Lupus | 12 | 18 | 13 | 14 | 12 | 6 | 20 | 18 | 9 | P < 0.05* |
| Total | 7 | 12 | 9 | 18 | 20 | 16 | 27 | 27 | 25 | P < 0.05 |
| Death | ||||||||||
| Minimal change | 6 | 4 | 1 | 15 | 8 | 3 | 23 | 14 | 15 | P < 0.05 |
| Endocapillary | 28 | 36 | 8 | 21 | 22 | 8 | 12 | 10 | 8 | |
| FSGS | 7 | 0 | 4 | 18 | 10 | 14 | 34 | 26 | 25 | |
| Mesangioproliferative | 7 | 6 | 4 | 18 | 16 | 10 | 28 | 28 | 23 | |
| Membranous | 6 | 12 | 5 | 18 | 24 | 12 | 29 | 33 | 29 | P < 0.05 |
| Membranoproliferative | 10 | 7 | 12 | 23 | 18 | 26 | 35 | 32 | 35 | |
| Extracapillary | 42 | 27 | 9 | 63 | 39 | 20 | 75 | 54 | 20 | P < 0.001 |
| AntiGBM | 35 | 18 | 14 | 48 | 35 | 33 | 54 | 47 | 46 | |
| ANCAV | 20 | 18 | 11 | 41 | 34 | 23 | 57 | 47 | 43 | P < 0.01 |
| Proliferative | 21 | 13 | 6 | 37 | 22 | 14 | 52 | 39 | 14 | |
| Focal | 14 | 4 | 8 | 57 | 8 | 20 | 57 | 42 | 48 | |
| Lupus | 7 | 3 | 4 | 20 | 13 | 11 | 27 | 19 | 12 | P < 0.05 |
| Total | 11 | 9 | 6 | 24 | 20 | 14 | 34 | 30 | 25 | P < 0.001 |
| Combined | ||||||||||
| Minimal change | 6 | 6 | 1 | 17 | 9 | 5 | 38 | 14 | 16 | P < 0.01 |
| Endocapillary | 12 | 26 | 20 | 21 | 36 | 26 | 28 | 45 | 26 | |
| FSGS | 10 | 8 | 6 | 37 | 30 | 24 | 57 | 51 | 41 | |
| Mesangioproliferative | 12 | 12 | 11 | 30 | 26 | 26 | 42 | 40 | 44 | |
| Membranous | 8 | 15 | 6 | 26 | 32 | 13 | 39 | 42 | 35 | P < 0.001 |
| Membranoproliferative | 24 | 19 | 28 | 47 | 36 | 44 | 67 | 59 | 61 | |
| Extracapillary | 61 | 56 | 37 | 79 | 69 | 48 | 86 | 73 | 52 | P < 0.01 |
| AntiGBM | 63 | 76 | 61 | 81 | 80 | 71 | 85 | 82 | 74 | |
| ANCAV | 26 | 32 | 20 | 49 | 46 | 34 | 67 | 58 | 49 | P < 0.01 |
| Proliferative | 37 | 35 | 21 | 47 | 52 | 29 | 63 | 65 | 52 | |
| Focal | 42 | 17 | 25 | 71 | 25 | 39 | 71 | 49 | 50 | |
| Lupus | 10 | 10 | 6 | 30 | 20 | 16 | 40 | 31 | 20 | P < 0.01 |
| Total | 17 | 18 | 14 | 36 | 32 | 26 | 48 | 44 | 40 | P < 0.001 |
1995–2004 versus 2005–14 only.
Figures in per cent.
For all diagnoses except MGN, mortality fell, and this was significant for MCGN, extracapillary GN, ANCAV and LN. MGN showed a heterogeneous pattern, with an initial rise followed by a significant fall. The overall 5-year mortality fell from 24% to 14%. Except for MesPGN, falls in combined ESRD and death were also seen and were significant for MCGN, extracapillary GN, ANCAV and LN. The combined 5-year ESRD/mortality fell from 36% to 26%.
Multivariate Cox proportional hazards analysis including age, sex and comorbidity was performed to investigate whether changes were independent of changes in patient age and comorbidity. The results are shown in Table 7. The number of patients with EndGN,AntiGBM, ProlGN and Focal GN was generally too small for statistical analysis. The adjusted ESRD incidence fell by 25% during the period. This reduction was considerable for the period 2005–14, where incidence fell 20% compared with 1995–2004. The fall was particularly notable for MCGN, FSGS, MGN and LN, but was only significant for the latter two diagnoses. No change in MesPGN ESRD incidence was seen. For nearly all diagnoses, significant falls in adjusted mortality were noted. The mortality fell by 38% in 1995–2004 compared with 1985–94, and by a further 39% after 2004. The total mortality fell by 62%. No fall in ProlGN mortality was seen, and the reductions for EndGN, MemPGN and Focal GN were insignificant. The incidence of ESRD or death in combination also fell by 46%: 24% in the first period of observation, and a further 29% in the second. The reduction was common for most major diagnoses. No reduction in the combination incidence was seen for EndGN, MemPGN, AntiGBM, ProlGN or Focal GN.
Table 7.
Risk of ESRD, death and the combination, relative to the cohort 1985–94
| ESRD |
Death |
Combination |
||||
|---|---|---|---|---|---|---|
| 95-04 | 05-14 | 95-04 | 05-14 | 95-04 | 05-14 | |
| Minimal change | 0.44 (0.17–1.13) | 0.21 (0.04–1.02) | 0.53 (0.30–0.93)a | 0.29 (0.13–0.66)b | 0.54 (0.32–0.90)a | 0.31 (0.15–0.63)b |
| Endocapillary | 2.52 (0.51–12.5) | 2.56 (0.42–15.6) | 0.51 (0.17–1.50) | 0.37 (0.08–1.78) | 1.15 (0.47–2.82) | 0.99 (0.34–2.90) |
| FSGS | 0.87 (0.51–1.49) | 0.59 (0.34–1.01)p=0.055 | 0.49 (0.28–0.87)a | 0.35 (0.19–0.65)c | 0.75 (0.48–1.15) | 0.48 (0.31–0.74)b |
| Mesangioproliferative | 0.83 (0.65–1.06) | 1.00 (0.74–1.34) | 0.69 (0.55–0.86)b | 0.44 (0.31–0.61)c | 0.77 (0.64–0.92)b | 0.76 (0.60–0.97)a |
| Membranous | 1.01 (0.64–1.62) | 0.32 (0.15–0.68)b | 0.79 (0.58–1.08) | 0.31 (0.19–0.49)c | 0.88 (0.66–1.17) | 0.32 (0.21–0.48)c |
| Membranoproliferative | 0.73 (0.43–1.22) | 0.76 (0.43–1.35) | 0.75 (0.43–1.31) | 0.56 (0.30–1.05) | 0.83 (0.55–1.27) | 0.78 (0.49–1.23) |
| Extracapillary | 1.20 (0.68–2.12) | 0.58 (0.31–1.08) | 0.52 (0.34–0.81)b | 0.20 (0.10–0.39)c | 0.79 (0.53–1.19) | 0.36 (0.22–0.59)c |
| AntiGBM | 1.64 (0.89–3.05) | 1.39 (0.79–2.44) | 0.33 (0.17–0.66)b | 0.26 (0.13–0.51)c | 1.09 (0.64–1.87) | 0.86 (0.53–1.39) |
| ANCAV | 1.11 (0.73–1.71) | 0.69 (0.43–1.09) | 0.57 (0.42–0.76)c | 0.37 (0.26–0.51)c | 0.72 (0.55–0.95)a | 0.45 (0.33–0.60)c |
| Proliferative | 1.57 (0.52–4.80) | 1.01 (0.28–3.68) | 0.42 (0.14–1.31) | 0.98 (0.17–5.75) | 1.21 (0.52–2.85) | 0.87 (0.32–2.38) |
| Focal | 1.65 (0.18–14.9) | 1.06 (0.10–11.4) | 0.20 (0.04–0.93)a | 0.38 (0.07–2.18) | 0.74 (0.18–2.95) | 0.67 (0.15–3.06) |
| Lupus | 1.04 (0.65–1.65) | 0.50 (0.26–0.96)a | 0.59 (0.40–0.88)b | 0.36 (0.21–0.61)c | 0.76 (0.55–1.05) | 0.43 (0.28–0.65)c |
| Total | 0.94 (0.82–1.08) | 0.75 (0.63–0.88)c | 0.62 (0.55–0.69)c | 0.38 (0.33–0.69)c | 0.76 (0.69–0.85)c | 0.54 (0.48–0.61)c |
P < 0.05.
P < 0.01.
P < 0.001.
Adjusted for age, sex and comorbidity.
DISCUSSION
Cross-sectional results from the DANYRBI have previously been published [28]. Detailed reviews of previously published incidence studies are available [12, 16]. Comparison between the studies is difficult due to differences in histological classification, non-inclusion of secondary GN, and subdivision of the results into cohorts and/or age groups. A comparison of our latest results with selected publications is shown in Table 3. The published figures have been adjusted to facilitate comparison. The distribution in the present study is generally not atypical. IgA nephropathy is the most common diagnosis. MCGN, FSGS, MGN and LN are common diagnoses. EndGN, MPGN and crescentic GN are rare, while AntiGBM GN is excessively rare. The main difference is the high rate of ANCAV in recent years compared with most other countries (vide infra).
The primary aim of this study was to assess changes in the incidence and prognosis over time. This study has several advantages. The possibility of linking renal biopsy diagnoses to patient comorbidity, consequent death and/or ESRD, and general population statistics is unique. All registries involved are comprehensive. However, there are considerable methodological problems associated with assessing changes over so long a period.
The incidence of GN generally increased during the study period, except for EndGN, which fell. However, it would appear that the indications for renal biopsy were also increased. The average age of patients increased, and the increase in incidence was particularly marked among elderly patients. This is not the whole explanation, in that the incidence also increased among younger age groups (Table 5, Figure 2). Another possibility is that the indication for biopsy was increased to include patients with some degree of reduced renal size. Assuming that shrunken kidneys will have an increased degree of tubulointerstitial fibrosis, and vice versa, this will result in an increased incidence of tubulointerstitial fibrosis in the biopsies, which is what we found. However, the standardized incidence of fibrosis-free GN also increased, so this is not the entire explanation.
These considerations are not relevant for FSGS and ANCAV, where the increases were dramatic. The rising incidence of FSGS is well described in the literature [1–3, 5, 6], with some exceptions [8, 11, 15], and is often ascribed to increases in FSGS due to environmental and lifestyle changes, in particularly the general increase in obesity [4, 5]. The increasing ANCAV incidence has also been previously described [29–31]. The cause is unknown. The increase in MCGN has previously been described [3, 15, 17], although one study has shown a stable incidence [5]. Similarly, a rising MGN has generally been seen after 2000 [11, 12, 15, 17]. We were unable to confirm previous studies, which have observed a falling incidence of MPGN [9, 13, 14, 17] and LN [11, 17]. Studies of IgA nephropathy incidence are heterogeneous, some finding an increase over time [5, 6, 10, 17], others an unchanged or falling incidence [9, 12, 15]. We found an unchanged incidence; interpretational difficulties are discussed below.
The overall absolute incidence of ESRD did not change during the study period. The incidence of ESRD generally rose from 1985–94 to 1995–2004, and then fell. The indication for dialysis and/or transplantation therapy has been widened to include elderly patients and those with Type 2 DM. Thus, the national incidence of ESRD rose from 62 to 139 ppm/year between 1990 and 2000 [32] after which it stabilized. This may explain the observed pattern. After adjusting incidence for age and comorbidity, the incidence of ESRD fell by 25%. This fall was significant for MGN, and LN, and borderline significant for MCGN and FSGS.
No change in ESRD incidence was seen for ProlGN, Focal GN, EndGN, AntiGBM and MesPGN. While the first four diagnoses were too rare to permit reliable statistical analysis, the observation is probably true for MesPGN. The estimates of incidence and prognosis of MesPGN are problematic. No reliable estimates of the proportion of IgA nephritis in this study could be made. Younger patients with monosymptomatic haematuria and a normal renal function will rarely be biopsied, since the presumptive diagnosis is IgA nephropathy, and treatment non-specific. Renal biopsy is reserved for patients with progressive renal failure or a high degree of proteinuria. This probably explains the poor prognosis of MesPGN in this study compared with other series [33, 34]. The incidence of non-fibrotic MesPGN fell from 10.7 to 8.0 ppm/year; the findings are not incompatible with a falling MesPGN incidence, as found by Woo et al. in a meta-analysis [12].
A reduction in the incidence of ESRD in recent years has been observed in Denmark for a number of renal diagnoses other than GN [35], and also a number of other countries [36]. This can possibly be ascribed to an increase in the use of antihypertensive therapy, especially renin–angiotensin system (RAS) blockers. These therapies will also have been used extensively for GN. Many forms of GN can now be treated with immunosuppressive therapy. However, effective immunosuppressive treatments for LN, MCGN, ANCAV, AntiGBM and MGN were already available at the start of the study period [37–44]. No data are available concerning possible increases in the use of immunosuppressive therapy in Denmark.
A general reduction in mortality and in combined mortality or ESRD was seen, both in absolute terms and after adjusting for age and comorbidity. The increased dialysis/transplantation indication could explain the reduced mortality, but not the combination. Any treatment that reduces uraemia progression (vide supra) can also be expected to reduce both ESRD incidence and mortality. It is also possible that this improvement is independent of renal disease, and just an expression of the increased longevity of the general population. Cardiovascular morbidity and mortality in the general population have fallen considerably, secondary to smoking cessation, antihypertensive therapy, RAS blockade, statin treatment, and improved therapies for acute coronary injury and heart failure. This may be particularly relevant for renal patients, who have a massively increased risk of cardiovascular disease [45].
Several criticisms of this study can be made. As previously mentioned, changes in biopsy indication and indications for dialysis therapy imply that the reality of observed changes over time can be questioned. Due to previous registration problems, the incidence of IgA nephropathy could not be included. The limited indications for renal biopsy in patients with MesPGN suggest that the results of this group will be unreliable. Data after 2014 were unavailable.
With these caveats, this study suggests that the incidence of GN has generally increased between 1985 and 2014, but some of the increase may be related to changes in renal biopsy policy. Major increases in FSGS and ANCAV have occurred. The prognosis of GN, both as regards ESRD and mortality, has improved despite an increased burden of comorbidity.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Braden GL, Mulhern JG, O'Shea MH. et al. Changing incidence of glomerular diseases in adults. Am J Kidney Dis 2000; 35: 878–883 [DOI] [PubMed] [Google Scholar]
- 2. Choi IJ, Jeong HJ, Han DS. et al. An analysis of 4,514 cases of renal biopsy in Korea. Yonsei Med J 2001; 42: 247–254 [DOI] [PubMed] [Google Scholar]
- 3. Narasimhan B, Chacko B, John GT. et al. Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J Nephrol 2006; 19: 205–210 [PubMed] [Google Scholar]
- 4. Dragovic D, Rosenstock JL, Wahl SJ. et al. Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol 2005; 63: 1–7 [DOI] [PubMed] [Google Scholar]
- 5. Sim JJ, Batech M, Hever A. et al. Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000-2011 among a racially and ethnically diverse US population. Am J Kidney Dis 2016; 68: 533–544 [DOI] [PubMed] [Google Scholar]
- 6. Swaminathan S, Leung N, Lager DJ. et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol 2006; 1: 483–487 [DOI] [PubMed] [Google Scholar]
- 7. Zhou FD, Zhao MH, Zou WZ. et al. The changing spectrum of primary glomerular diseases within 15 years: a survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 2008; 24: 870–876 [DOI] [PubMed] [Google Scholar]
- 8. Jegatheesan D, Nath K, Reyaldeen R. et al. Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrology (Carlton) 2016; 21: 28–34 [DOI] [PubMed] [Google Scholar]
- 9. Simon P, Ramee MP, Boulahrouz R. et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int 2004; 66: 905–908 [DOI] [PubMed] [Google Scholar]
- 10. Hanko JB, Mullan RN, O’Rourke DM. et al. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant 2009; 24: 3050–3054 [DOI] [PubMed] [Google Scholar]
- 11. Hou JH, Zhu HX, Zhou ML. et al. Changes in the spectrum of kidney diseases: an analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis 2018; 4: 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo KT, Chan CM, Lim C. et al. A global evolutionary trend of the frequency of primary glomerulonephritis over the past four decades. Kidney Dis 2019; 5: 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honma M, Toyoda M, Umezono T. et al. An investigation of 2,093 renal biopsies performed at Tokai University Hospital between 1976 and 2000. Clin Nephrol 2008; 69: 18–23 [DOI] [PubMed] [Google Scholar]
- 14. Stratta P, Segoloni GP, Canavese C. et al. Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis 1996; 27: 631–639 [DOI] [PubMed] [Google Scholar]
- 15. Wang P, Tang L, Yao J. et al. The spectrum of biopsy-proven secondary glomerular diseases: a cross-sectional study in China. Clin Nephrol 2017; 88: 270–276 [DOI] [PubMed] [Google Scholar]
- 16. McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011; 26: 414–430 [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Ning Y, Shang W. et al. Analysis of 4931 renal biopsy data in central China from 1994 to 2014. Ren Fail 2016; 38: 1021–1030 [DOI] [PubMed] [Google Scholar]
- 18. von EE, Altman DG, Egger M. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457 [DOI] [PubMed] [Google Scholar]
- 19. Heaf J. The Danish Renal Biopsy Register. Kidney Int 2004; 66: 895–897 [DOI] [PubMed] [Google Scholar]
- 20. Marcussen N, Olsen S, Larsen S. et al. Reproducibility of the WHO classification of glomerulonephritis. Clin Nephrol 1995; 44: 220–224 [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL. et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 22.Revision of the European Standard Population. Report of Eurostat's task force 2013. 2012; 25: 30. https://ec.europa.eu/eurostat [Google Scholar]
- 23. Chiu HF, Chen HC, Lu KC. et al. , Taiwan Society of Nephrology. Distribution of glomerular diseases in Taiwan: preliminary report of National Renal Biopsy Registry-publication on behalf of Taiwan Society of Nephrology. BMC Nephrol 2018; 19: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polito MG, de Moura LA, Kirsztajn GM.. An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant 2010; 25: 490–496 [DOI] [PubMed] [Google Scholar]
- 25. Rychlik I, Jancova E, Tesar V. et al. on behalf of the Czech Registry of Renal Biopsies. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol Dial Transplant 2004; 19: 3040–3049. [DOI] [PubMed] [Google Scholar]
- 26. Schena FP. Survey of the Italian Registry of Renal Biopsies. Frequency of the renal diseases for 7 consecutive years. The Italian Group of Renal Immunopathology. Nephrol Dial Transplant 1997; 12: 418–426 [DOI] [PubMed] [Google Scholar]
- 27. Rivera F, Lopez-Gomez JM, Perez-Garcia R.. Frequency of renal pathology in Spain 1994-1999. Nephrol Dial Transplant 2002; 17: 1594–1602 [DOI] [PubMed] [Google Scholar]
- 28. Heaf J, Lokkegaard H, Larsen S.. The epidemiology and prognosis of glomerulonephritis in Denmark 1985-1997. Nephrol Dial Transplant 1999; 14: 1889–1897 [DOI] [PubMed] [Google Scholar]
- 29. Knight A, Ekbom A, Brandt L. et al. Increasing incidence of Wegener's granulomatosis in Sweden, 1975-2001. J Rheumatol 2006; 33: 2060–2063 [PubMed] [Google Scholar]
- 30. Koldingsnes W, Nossent H.. Epidemiology of Wegener's granulomatosis in northern Norway. Arthritis Rheum 2000; 43: 2481–2487 [DOI] [PubMed] [Google Scholar]
- 31. Nilsen AT, Karlsen C, Bakland G. et al. Increasing incidence and prevalence of ANCA-associated vasculitis in Northern Norway. Rheumatology (Oxford) 2019; doi: 10.1093/rheumatology/kez597 [DOI] [PubMed] [Google Scholar]
- 32.Danish Nephrology Registry Annual Report 2011. www nephrology dk2012; 30 (16 August 2020, date last accessed)
- 33. Barbour SJ, Espino-Hernandez G, Reich HN. et al. The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 34. Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 35. Heaf JG, Wehberg S.. Reduced incidence of end stage renal disease among the elderly in Denmark: an observational study. BMC Nephrol 2012; 13: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heaf J. Current trends in European renal epidemiology. Clin Kidney J 2017; 10: 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heaf JG, Jorgensen F, Nielsen LP.. Treatment and prognosis of extracapillary glomerulonephritis. Nephron 1983; 35: 217–224 [DOI] [PubMed] [Google Scholar]
- 38. Ponticelli C, Zucchelli P, Imbasciati E. et al. Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 1984; 310: 946–950 [DOI] [PubMed] [Google Scholar]
- 39. Black DA, Rose G, Brewer DB.. Controlled trial of prednisone in adult patients with the nephrotic syndrome. Br Med J 1970; 3: 421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coggins CH. Adult minimal change nephropathy: experience of the collaborative study of glomerular disease. Trans Am Clin Climatol Assoc 1986; 97: 18–26 [PMC free article] [PubMed] [Google Scholar]
- 41. Austin HA III, Klippel JH, Balow JE. et al. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 1986; 314: 614–619. [DOI] [PubMed] [Google Scholar]
- 42. Johnson JP, Moore J Jr, Austin HA III. et al. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985; 64: 219–227 [DOI] [PubMed] [Google Scholar]
- 43. Glockner WM, Sieberth HG, Wichmann HE. et al. Plasma exchange and immunosuppression in rapidly progressive glomerulonephritis: a controlled, multi-center study. Clin Nephrol 1988; 29: 1–8 [PubMed] [Google Scholar]
- 44. Levy JB, Turner AN, Rees AJ. et al. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression. Ann Intern Med 2001; 134: 1033–1042 [DOI] [PubMed] [Google Scholar]
- 45. Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32: S112–S119 [DOI] [PubMed] [Google Scholar]