Abstract
The growth factor progranulin plays a critical role in bladder cancer by modulating tumor cell motility and invasion. Progranulin regulates remodeling of the actin cytoskeleton by interacting with drebrin, an actin binding protein that regulates tumor growth. We previously discovered that progranulin depletion inhibits epithelial-to-mesenchymal transition and markedly reduces in vivo tumor growth. Moreover, progranulin depletion sensitizes urothelial cancer cells to cisplatin treatment, further substantiating a pro-survival function of progranulin. Until recently, the progranulin signaling receptor remained unidentified, precluding a full understanding of progranulin action in tumor cell biology. We recently identified EphA2, a member of a large family of receptor tyrosine-kinases, as the functional receptor for progranulin. However, it is not established whether EphA2 plays an oncogenic role in bladder cancer. Here we demonstrate that progranulin, and not ephrin-A1, the canonical ligand for EphA2, is the predominant EphA2 ligand in bladder cancer. Progranulin evoked Akt- and Erk1/2-mediated EphA2 phosphorylation at Ser897, which could drive bladder tumorigenesis. We discovered that EphA2 depletion severely blunted progranulin-dependent motility and anchorage-independent growth, and sensitized bladder cancer cells to cisplatin treatment. We further defined the mechanisms of progranulin/EphA2-dependent motility by identifying liprin-α1 as a novel progranulin-dependent EphA2 interacting protein and establishing its critical role in cell motility. The discovery of EphA2 as the functional signaling receptor for progranulin and the identification of novel downstream effectors offer a new avenue for understanding the underlying mechanism of progranulin action and may constitute novel clinical and therapeutic targets in bladder cancer.
Keywords: Bladder cancer, Progranulin, EphA2, Motility, Liprinα−1
Introduction
Bladder cancer is a major health issue with >80,000 new cases in the USA and nearly 18,000 estimated deaths in 2019 [1]. Comparatively, bladder cancer has the highest cost/patient ratio due to disease prevalence and long-term monitoring [2]. Surprisingly, bladder cancer is significantly understudied and underfunded in respect to the mortality it causes [3,4]. The prognosis for early stage bladder tumors is generally good; however, patients often progress to invasive disease resulting in a much less favorable outcome. Radical cystectomy with cisplatin-based chemotherapy is the clinical benchmark for treating muscle-invasive bladder cancers. A glaring hole in the field is that no substantial advances in targeted therapy have been made since the introduction of cisplatin-based regimens [5]. Therefore, there is a substantial and unmet medical need to identify novel therapeutic targets and prognosticators of disease progression that will significantly improve the treatment of bladder cancer patients.
Progranulin is a pluripotent growth factor containing 7 and a half highly-conserved granulins [6] that is sensitive to elastase and MMPs to produce granulin peptides A-G and paragranulin (p) [7,8]. Progranulin is implicated in several human diseases including cancer, neurodegenerative diseases, and rheumatoid arthritis [9,10]. Importantly, progranulin promotes in vivo tumorigenesis, and its elevated expression in various solid tumors correlates with an invasive phenotype and poor survival [9, 11–16]. Moreover, progranulin is a key adipokine that regulates insulin-resistance in adipose tissue [17].
Our laboratories have established that progranulin plays a critical role in bladder cancer by promoting tumor cell motility and invasion [18–20]. Progranulin regulates actin remodeling by interacting with the F-actin binding protein drebrin, which modulates in vivo tumor growth [21], and its expression correlates with bladder cancer progression [21]. We also discovered that progranulin depletion markedly reduces in vivo tumor growth of both orthotopic and subcutaneous bladder cancer xenografts, and sensitizes urothelial cancer cells to cisplatin [22]. Thus, our findings suggest that the progranulin pathway represents a novel therapeutic axis in bladder cancer.
A major drawback of the research on progranulin was the lack of the identification of a functional signaling receptor. Although previous studies have shown progranulin-binding proteins at the cell surface [23,24], these putative receptors were not further investigated. Sortilin, a single-pass Type I transmembrane protein of the Vps10 family, was identified as a novel progranulin-interacting protein [25]. However, sortilin lacks kinase activity and acts as a negative regulator of progranulin by targeting progranulin for internalization and subsequent lysosomal degradation [25]. We further demonstrated that sortilin-mediated progranulin downregulation inhibits tumorigenesis [26, 27]. TNF receptors 1 and 2 (TNFR1/2) have also been proposed as progranulin receptors [28]. However, progranulin/TNFR1/2 interaction blocks TNFα-evoked activation of MAPK [19,28], in sharp contrast with the established progranulin role in MAPK activation [18,29], thereby not supporting TNFRs as functional progranulin signaling receptors.
We recently provided a crucial conceptual advance in the field by identifying a functional receptor for progranulin, EphA2 [30]. Using an unbiased antibody array for differential RTK Tyr-phosphorylation, we demonstrated that progranulin promoted rapid and robust EphA2 Tyr-phosphorylation in urothelial carcinoma cells. By using microscale thermophoresis, a technique that measures binding affinities in solution, we found a high-affinity (~1.2 nM) interaction between progranulin–and the ectodomain of EphA2. These findings are important as correlate with the established biological capability of progranulin when assayed as a growth factor for cancer cells [31].
The Eph receptors constitute the largest family of receptor tyrosine-kinase (RTK) and together with their plasma membrane-bound ephrin ligands are important regulators of development and disease [32]. EphA2 activation by its natural ligand ephrin-A1 (canonical signaling) regulates the physiology of cellular repulsion and adhesion, but the role of EphA2 in cancer is more complex with data suggesting either pro- or anti-oncogenic functions [33–45]. For example, in the presence of ephrin-A1, EphA2 is dephosphorylated at Ser897 leading to inhibition of cancer cell motility and invasion. Conversely, ephrin-A1-independent Akt or RSK activation (non-canonical signaling), evokes EphA2 phosphorylation at Ser897 enhancing EphA2 oncogenic activity [46–48].
There are only a few reports suggesting a potential role of EphA2 and ephrin-A1 in bladder cancer showing opposite effects of the ligand [49,50]. In one case, cell growth inhibitory action of ephrin-A1 was reported [49], whereas pro-angiokine activity for ephrin-A1 was shown [50]. However, the molecular mechanisms of EphA2 activation and action in bladder cancer remain elusive.
Our results demonstrate a mechanistic model whereby in bladder cancer progranulin induces non-canonical EphA2-driven oncogenesis governed by receptor Ser-phosphorylation triggered by a progranulin-evoked Akt/Erk1/2 positive feedback loop. Our results provide a deeper understanding of progranulin /EphA2 biological signaling axis in bladder cancer and could identify new and clinically relevant targets for therapy.
Results
Progranulin and Ephrin-A1 expression in bladder cancer
We recently demonstrated that progranulin is a high-affinity ligand for EphA2 [30]. However, it is not established whether progranulin is the predominant ligand for EphA2 in bladder cancer, and we do not know the role of the progranulin/EphA2 axis in this cancer.
As an initial approach, we compared public available mRNA levels of progranulin (GRN) and ephrin-A1 (EFNA1) in bladder cancer by interrogating the Oncomine database [51,52]. GRN mRNA levels were dramatically upregulated in urothelial tumors compared to those of EFNA1, which are not statistically different from normal controls (Fig. 1A). Steady state GRN levels were significantly higher than EFNA1 in T24 and UMUC-3 bladder cancer cells (p<0.001, Fig. 1B) indicating the preferential expression of progranulin over ephrin-A1 in bladder cancer.
Fig. 1.
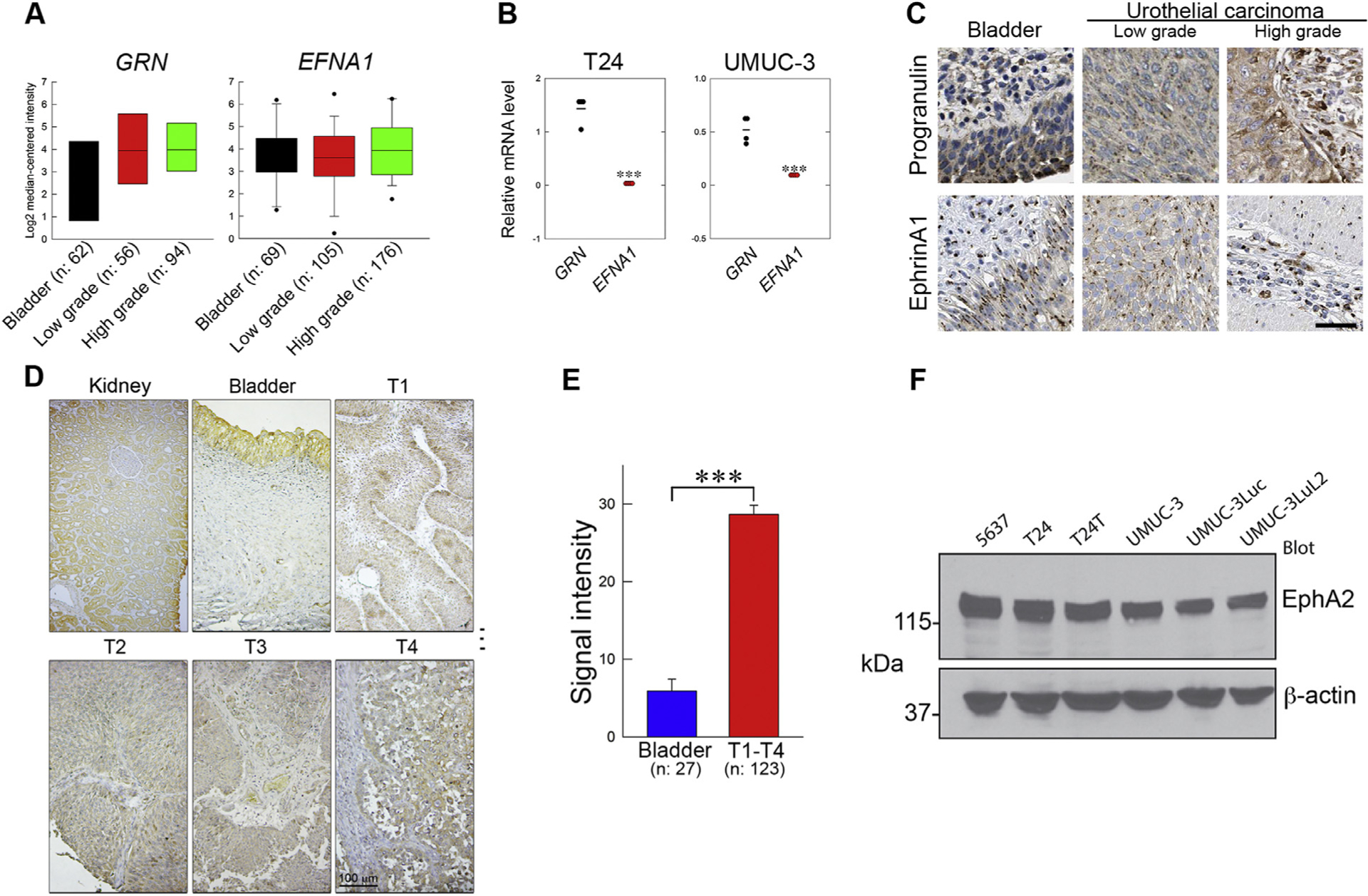
Progranulin, Ephrin-A1, and EphA2 expression in bladder cancer. (A) mRNA levels of GRN and EFNA1 in normal or urothelial carcinomas of low and high grade (Oncomine). (B) Steady state levels of progranulin (GRN) and ephrin-A1 (EFNA1) in T24 and UMUC-3 cells; mean ±SEM, n = 3. (C) Micrographs from the human protein Atlas depicting representative images of normal bladder and low or high-grade urothelial carcinomas. (D) EphA2 expression levels in bladder cancer tissues using IHC on a bladder cancer TMA. (E) Quantification of EphA2 expression in tissues using ImageJ; bladder, n = 27; T1-T4 urothelial carcinomas, n = 123; ***p<0.001. (F) EphA2 expression in various urothelial cancer cells by immunoblot using anti-EphA2 and anti-β-actin antibodies.
We than queried the Human Protein Atlas (https://www.proteinatlas.org/) and discovered that progranulin was upregulated in bladder cancer vis-a-vis ephrin-A1, which expression levels did not diverge from those of healthy bladder tissue as demonstrated by immunohistochemistry (Fig. 1C) in agreement with previous work [22]. We found that EphA2 expression was highly enriched in tissue microarrays (Biomax BL208) representing 69 cases of urothelial carcinomas at various clinical stages and normal bladder embedded in 208 cores (Fig. 1D, E). Moreover, we found that EphA2 was highly expressed in all tested urothelial carcinoma cell lines derived from either male (5637, UMUC-3 and derivatives) or female (T24 and T24T) patients [53] (Fig. 1F). Thus, we propose that in bladder cancer progranulin is the prevalent ligand for EphA2 and the progranulin/EphA2 axis may drive bladder cancer progression and could be a clinically relevant target for therapy.
Progranulin evokes EphA2 phosphorylation
To further dissect the mechanism of progranulin-evoked activation of EphA2, we performed a detailed phospho-proteomic mass-spec analysis of the EphA2 intracellular domains [54, 55] in T24 cells, the bladder cancer cells where this interaction was originally discovered [30]. We identified multiple EphA2 residues (Tyr588, Ser897 and Ser901) significantly phosphorylated upon progranulin stimulation (Fig. 2A, B). In contrast, constitutively phosphorylated Ser899 and Ser570 were de-phosphorylated upon progranulin stimulation (Fig. 2A). Notably, phospho-Tyr594 and Thr647 were unchanged (Fig. 2A). This differential phosphorylation indicates a degree of specificity for soluble progranulin and EphA2 interactions.
Fig. 2.
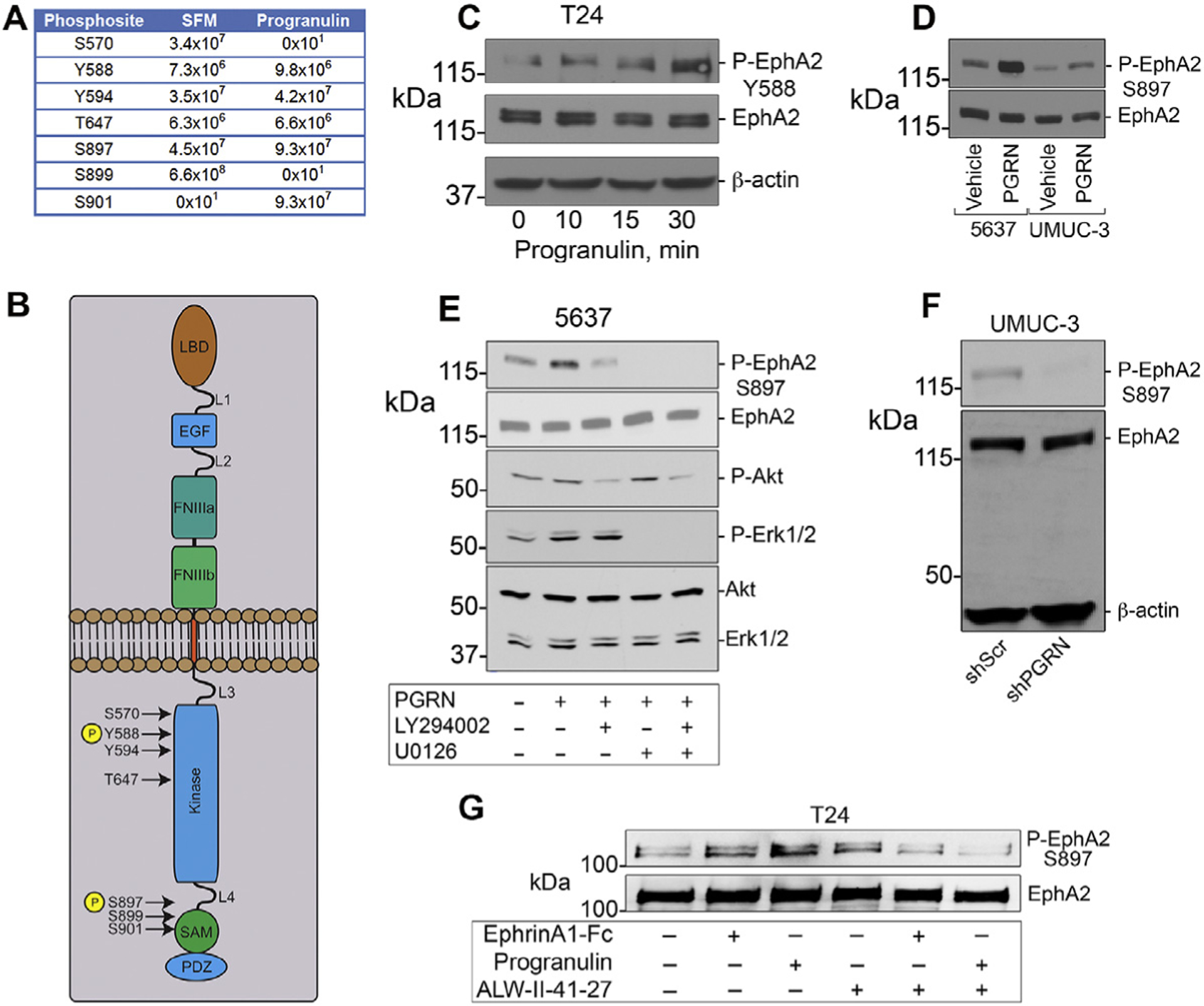
Progranulin evokes EphA2 phosphorylation. Serum-starved T24 cells were exposed to progranulin (150 nM, 15 min) and immunoprecipitated with anti-EphA2 antibodies. Coomassie-stained bands of interest were trypsin-digested and analyzed by LC-MS/MS on a Q Exactive™ Plus mass spec. The mass spec data were probed against the UniProt human database for STY phosphorylation. False discovery rate for peptides/site identifications was set at 1%. (A) Modifications of various residues shown as the sum of MS peptides intensities. (B) EphA2 schematic showing the location of phosphorylated residues. (C-D) Western immunoblots of serum-starved T24 cells exposed to 150 nM progranulin using several Phospho-specific and total antibodies. (E) Western immunoblots of serum-starved 5637 cells after progranulin stimulation ±specific inhibitors for PI3K pathway (LY294002, 20 μM) or Erk1/2 (U0126, 10 μM). (F) Phospho-EphA2 (Ser897) assessed by immunoblot in UMUC-3/shScr (control) and UMUC-3/shPGRN cells. (G) Inhibition of EphA2 kinase activity blocks progranulin-induced phosphorylation. Western immunoblots of serum-starved T24 cells exposed to Ephrin-A1-Fc (2.1 nM) or progranulin (150 nM) for 15 min ±ALW-II-41–27 (1 μM), and probed with Phospho-EphA2 (Ser897) and EphA2 antibodies.
Next, we validated some of these phosphosites using phopho-specific antibodies. We found that progranulin evoked rapid and dynamic phosphorylation at Tyr588 (Fig. 2C). We then confirmed that progranulin stimulated EphA2 phosphorylation at Ser897 in both 5637 and UMUC-3 bladder cancer cells (Fig. 2D). This effect was strongly dependent on progranulin-mediated activation of Erk1/2 and, to a lesser extent, Akt (Fig. 2E) as demonstrated by using specific inhibitors of both pathways. To prove specificity, we utilized UMUC-3 cells stably depleted of endogenous progranulin, previously generated in our laboratory [22], and found that lack of progranulin in this cells is sufficient to prevent EphA2 phosphorylation at Ser897 (Fig. 2F).
Inhibition of the EphA2 kinase abrogates progranulin-induced phosphorylation
To validate the specificity of progranulin-induced EphA2 phosphorylation we used ALW-II-41–27, a potent small- molecule multi-kinase inhibitor that inhibits EphA2 tyrosine-kinase and that was shown to hinder the growth of EphA2-overexpressing breast cancer xenografts in vivo [33]. We found that both Ephrin-A1-Fc and progranulin stimulated EphA2 phosphorylation at Ser897 within 15 min, but this was completely abolished by co-incubating the two ligands with ALW-II-41–27 (Fig. 2G). Collectively, these novel findings corroborate our hypothesis that endogenous and exogenous progranulin affects EphA2 activation and phosphorylation in an autocrine and paracrine fashion, thereby leading to enhanced cancer growth.
EphA2 is required for progranulin-evoked biological activity and cisplatin sensitivity
Next, we needed to provide a proof-of-principle that urothelial carcinoma cells utilize EphA2 as the functional progranulin receptor. To this end, we used shRNA to stably deplete endogenous EphA2 in invasive UMUC-3 cells (Fig. 3A) and observed a dramatic inhibition of progranulin-dependent motility (Fig. 3B) and anchorage-independent growth (Fig. 3 C). These findings validate EphA2 as the required receptor for progranulin-evoked cell motility. Further, we recently demonstrated that stable progranulin depletion sensitized urothelial cancer cells to cisplatin treatment [22]. To expand these findings, we performed additional experiments and discovered that loss of EphA2 significantly enhanced cisplatin-dependent cell death as compared to control cells with an IC50 of 2.8 μM and ~14.4 μM, respectively (Fig. 3D clone B5). These results strongly suggest that targeting the progranulin/EphA2 axis could have clinical relevance by decreasing chemo-resistance in invasive bladder cancer.
Fig. 3.
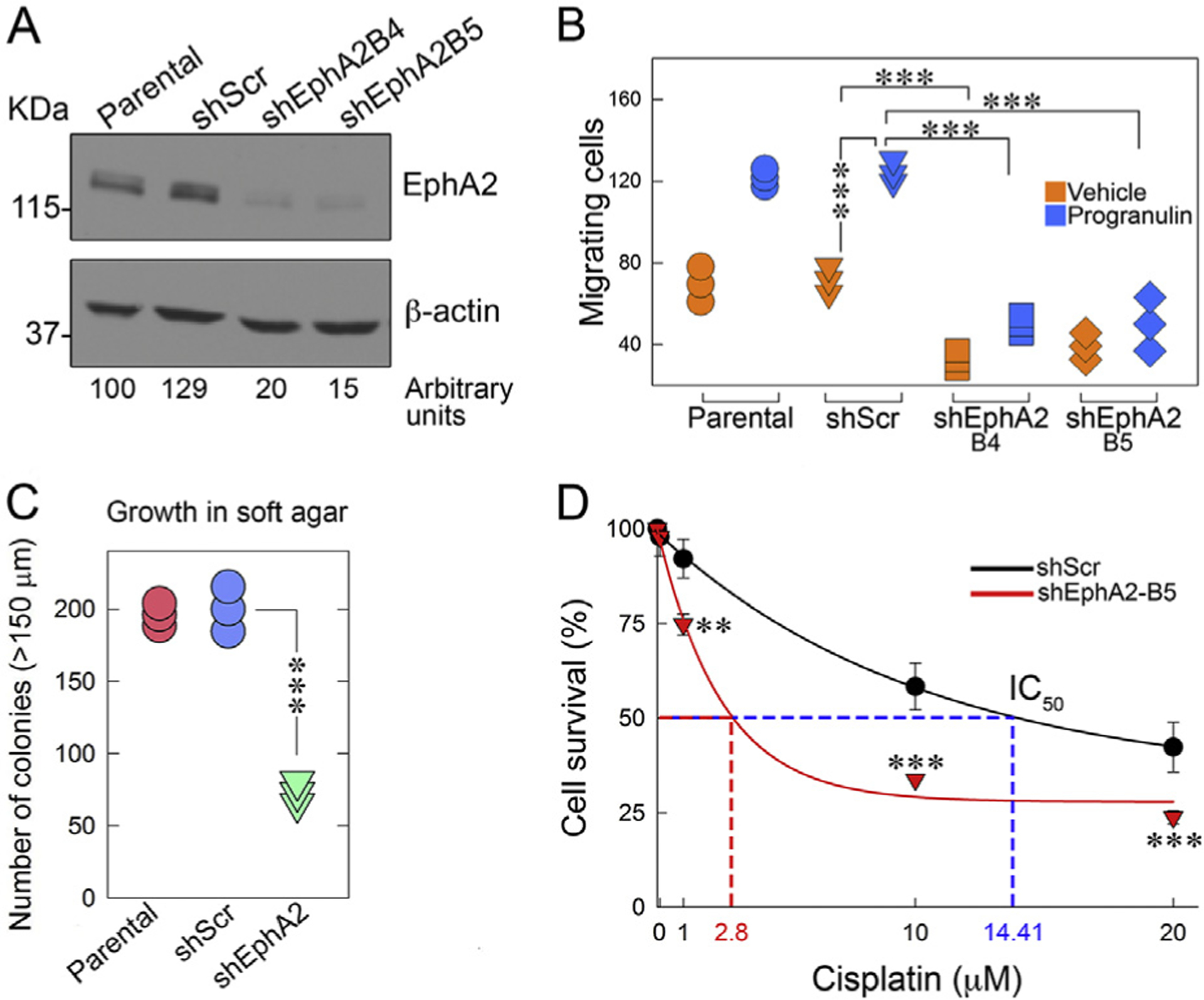
EphA2 is required for progranulin-evoked activity and cisplatin sensitivity. (A) EphA2 was depleted in UMUC-3 cells by siRNA approaches and EphA2 expression levels were assessed by immunoblot with anti-EphA2 polyclonal antibodies and normalized over β-actin content. Densitometric analysis was performed using ImageJ (National Institutes of Health) and expressed as arbitrary units. (B) Motility assays in Boyden chambers ±progranulin, ***p<0.001. (C) Anchorage-independent growth in soft-agar was performed as described in Materials and Methods ***p<0.001. (D) Cell survival as assessed by a Colorimetric Cell Cytotoxicity Assay Kit at various cisplatin concentrations. Mean ±SD; n = 3 biological replicates run in duplicates. **p<0.005; ***p<0.001.
EphA2 interacts with liprinα–1, a critical factor for progranulin-evoked cell motility
While the role of progranulin in motility and invasion has been clearly established [18–22], the molecular mechanism regulating progranulin/EphA2-evoked cell motility is not fully defined. Thus, we investigated the EphA2 interactome in order to identify novel EphA2 binding proteins that might have roles in regulating cell motility via a proteomics approach. We used pull-down experiments with an anti-EphA2 specific antibody in T24 cells and identified novel progranulin-dependent EphA2-binding partners. We discovered several proteins involved in cell motility including liprinα–1, liprinβ–1, vinculin and ERC1 [56–60] (Fig. 4A). Significantly, this protein complex includes paxillin [58], which we previously discovered as activated by progranulin and subsequently translocated to focal adhesions [18]. We focused first on liprinα–1, encoded by the PPF1A1 gene, and vinculin since their corresponding peptide fragments were the most highly enriched. Moreover, liprinα–1 mRNA is significantly upregulated in bladder cancer [61, 62] and liprinα–1 protein is highly expressed in urothelial cancer according to the Human Protein Atlas (not shown). Using CoIP, we confirmed that both proteins complexed with EphA2 upon progranulin exposure (Fig. 4B). Already evident at baseline, the vinculin/EphA2 interaction was enhanced after 5 min of progranulin stimulation. In contrast, EphA2/liprinα–1 complexes displayed slower kinetics and formed after 15 min (Fig. 4B), suggesting that vinculin may bind first and then recruit liprinα–1 to EphA2. Moreover, both liprinα–1 and vinculin were abundantly expressed in multiple bladder cancer cell lines (Fig. 4C) and EphA2 and liprinα–1 strongly co-localized at the plasma membrane upon progranulin stimulation (Fig. 4D).
Fig. 4.
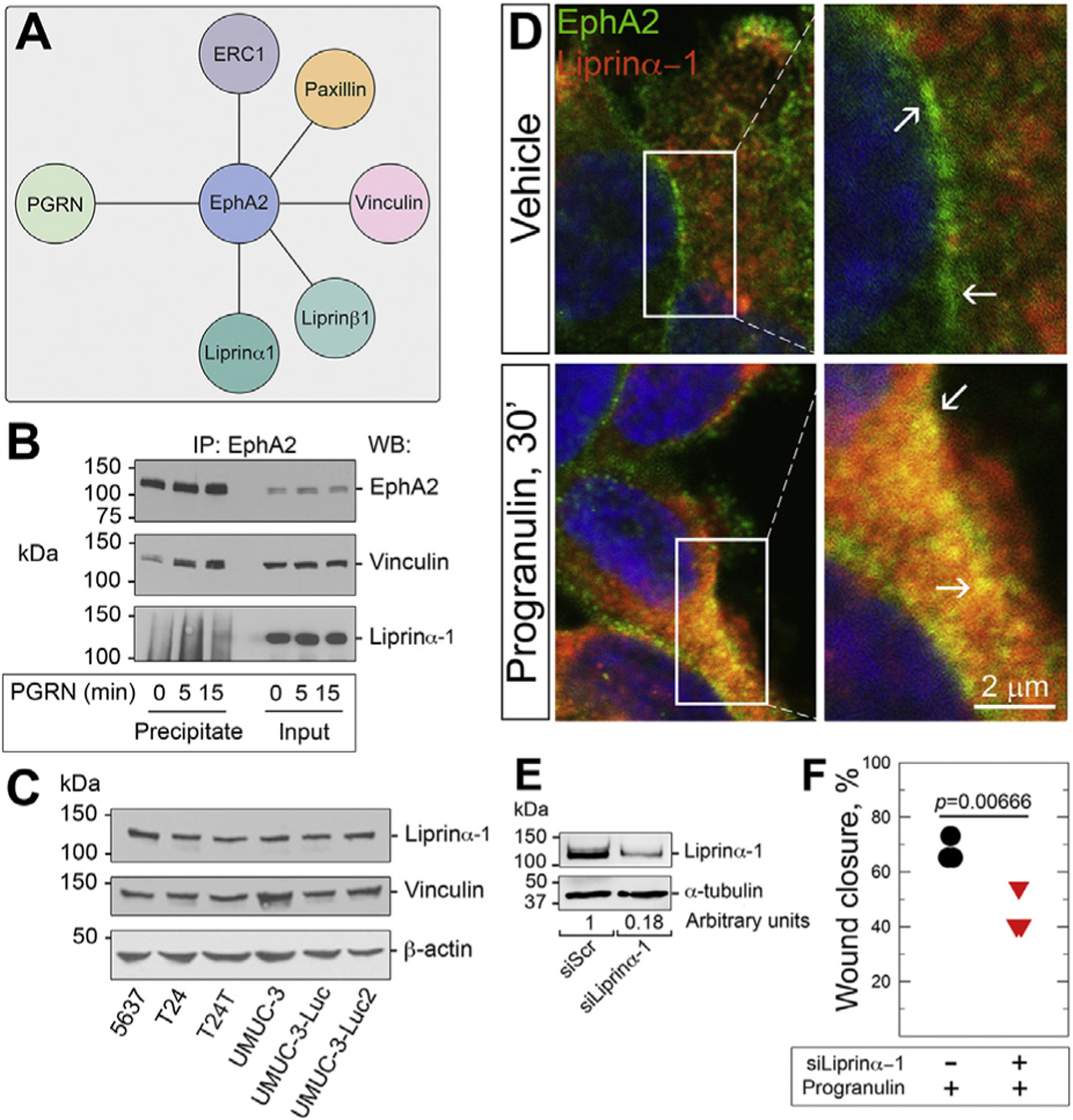
EphA2 interacts with liprinα–1 and vinculin. (A) EphA2 interactome involved in cell motility as identified by proteomics. (B) Co-immunoprecipitation and western immunoblot of serum-starved T24 cells ±progranulin (150 nM) with the indicated antibodies. (C) Immunoblot of Liprinα–1 and vinculin in various urothelial carcinoma cells. (D) Co-localization of EphA2/liprinα–1 assessed by confocal microscopy. Insets = higher magnification of the relative areas within the white boxes. Bar = 10 μm. (E) Western immunoblot of liprinα–1- depleted T24 cells. (F) Quantification of T24 cell lateral motility determined by wound healing assays as previously described [18,19,26]. p = 0.000666.
To further define the functional relevance of the EphA2/liprinα–1 interaction, we transiently depleted liprinα–1 by siRNA and tested cell motility. Liprinα–1 depletion (Fig. 4E) significantly inhibited progranulin-dependent lateral motility of T24 cells (Fig. 4F), supporting the hypothesis that liprinα–1 is critical for progranulin-dependent urothelial cancer cell motility. Thus, our findings suggest that upon progranulin stimulation, EphA2 mediates motility and invasion through the assembly and disassembly of a pro-migratory plasma membrane-associated protein network.
Discussion
Bladder cancer is among the most common solid tumors in USA with an alarming 80,470 new cases and a predicted 17,670 deaths in 2019 [1]; however, very little is known about the molecular mechanisms that govern and drive tumor formation and progression.
Progranulin is a secreted, pleiotropic growth factor that plays a key role in cell proliferation, angiogenesis, wound healing, and transformation [9,12,13]. It is secreted by both stromal and cancer cells, and thus contributes to the development of a tumor stroma [63] supportive of tumorigenesis. We recently discovered EphA2, a member of the largest known family of receptor tyrosine-kinases (RTK) [33–35,39,64,65], as the functional signaling receptor for progranulin [30]. Progranulin bound to the EphA2 ectodomain with a KD ~1.2 nM, >60 times higher than the natural ligand Ephrin-A1 [30], and co-localized with EphA2 in both tumor and primary endothelial cells [30]. Soluble EphA2 ectodomain competed with endogenous EphA2 for progranulin binding, an interaction lost by EphA2 silencing [30]. Notably, EphA2 is highly expressed in bladder cancer mechanistically linking this receptor to our discovery that progranulin promotes cell motility and invasion of urothelial cancer cells by activating Akt/MAPK pathways [18,19,29], similar to downstream EphA2 signaling. We further discovered that progranulin modulates F-actin remodeling by interacting with drebrin [21,66,67], a protein that stabilizes actin filaments through effects on interstrand and intrastrand contacts [68] critical for progranulin-dependent motility and in vivo tumor growth [21]. Finally, we found that progranulin expression is upregulated in invasive bladder cancer and is essential for in vivo tumorigenesis and cisplatin resistance [22]. These data together with the fact that progranulin is detected in the urine of normal and bladder cancer patients [19, 69], provide a robust hypothesis for investigating the progranulin/EphA2 axis in bladder cancer.
In the present study, we demonstrate that progranulin is the predominant ligand for EphA2, which is essential to promote bladder cancer cell motility, anchorage-independent growth and resistance to cisplatin. Our results can be summarized as follows: A) Progranulin (GRN) mRNA levels are dramatically upregulated in urothelial tumors relative to ephrin A1 (EFNA1), which are statistically not different from normal controls, as per Oncomine [51,52] B) Steady-state GRN levels are significantly higher than EFNA1 in T24 and UMUC-3 cells. C) Progranulin is upregulated in bladder cancer vis-a-vis Ephrin-A1, which was not significantly different from normal tissue. D) EphA2 expression is highly enhanced in bladder tissue microarrays compared to normal bladder controls. Moreover, EphA2 is highly expressed in all tested urothelial carcinoma cell lines. E) Using phospho-proteomics, we identified multiple EphA2 residues specifically phosphorylated by progranulin. F) EphA2 is required for progranulin-evoked biological activity and cisplatin sensitivity. G) Upon progranulin stimulation, EphA2 interacts with liprinα–1, which is critical for progranulin-evoked cell motility.
Our unbiased proteomic approach has identified several EphA2 residues specifically phosphorylated by progranulin (cfr. Fig. 2A, B), indicating that these residues may be critical for progranulin/EphA2-mediated signaling and cell motility. Of particular significance is the identification of Ser897 and Ser901 as residues phosphorylated upon progranulin stimulation. Significantly, Ser897 phosphorylation is severely compromised in bladder cancer cells with progranulin loss indicating that this residue is specifically phosphorylated in an autocrine manner by progranulin. Since ephrin-A1-independent Ser897 phosphorylation drives EphA2 oncogenic action [46–48] and given that progranulin is overexpressed in bladder cancer, it is reasonable to envision a mechanism through which progranulin might activate an oncogenic signaling pathway by binding ephrin-free EphA2 and thereby counteract the tumor suppressive function of ephrin-EphA2 coupling [49,70]. Thus, our results support a mechanistic model whereby progranulin induces non-canonical EphA2-driven oncogenesis governed by receptor Ser-phosphorylation and triggered by progranulin-evoked Akt/Erk1/2 positive feedback loop (Fig. 5). Experiments are currently under way to determine whether progranulin-mediated Ser897 phosphorylation is necessary and sufficient to drive EphA2 action in bladder cancer.
Fig. 5.
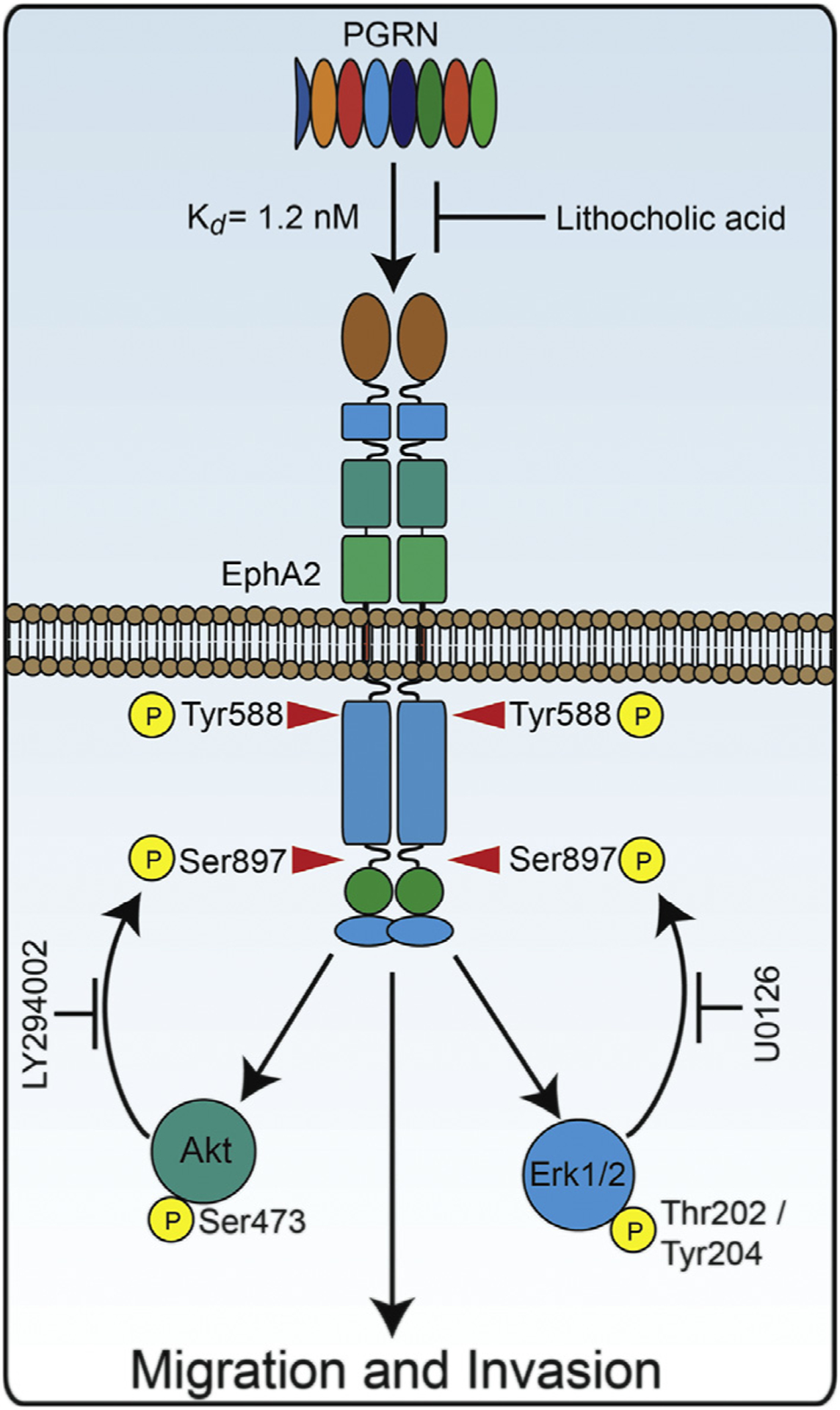
Mechanism of progranulin-dependent EphA2 activation. Scheme depicting progranulin-induced EphA2 activation at Tyr588 and resultant Akt/MAPK feedback loop to phosphorylate Ser897.
One possible caveat is that the engagement of EphA2 by progranulin may trigger cross talk among other receptor tyrosine kinases, including other Eph receptors and EGFR, which are weakly phosphorylated upon progranulin [30,70]. This possible cross talk may contribute to enhance MAPK and Akt activation thereby possibly supporting progranulin-evoked EphA2 Ser897 phosphorylation and biological responses.
Progranulin action in promoting bladder cancer cell motility and invasion is quite complex and may work at multiple levels. Progranulin regulates actin remodeling by interacting with the F-actin binding protein drebrin, a crucial factor for progranulin-evoked signaling and biological responses [21]. Progranulin is also critical for focal adhesion dynamics by recruiting paxillin at focal adhesions at the leading edge of migrating bladder cancer cells [18]. The results of our proteomic approach further demonstrates that upon progranulin stimulation, EphA2 mediates motility and invasion through the assembly and disassembly of a pro-migratory plasma membrane-associated protein complex, which in addition to paxillin includes liprinα–1 and/or liprinβ–1, vinculin and ERC1/ELKs. Notably, these four proteins have never been linked to EphA2, and act to promote the turnover of adhesions/invadosomes and stimulate protrusion of migrating cells [56–60,71,72]. Liprinα–1, LL5, and ERC1 constitute a novel dynamic membrane-less compartment that regulates matrix degradation by affecting invadosome motility [73]. In addition, liprinα–1 and ERC1 are part of cytoplasmic condensates with a behavior that is consistent with liquid phases [71]. These condensates specifically host partners of a network relevant to cell motility [71]. Phase separation at specific sites of the cell periphery may constitute a novel mechanism to regulate the assembly and turnover of dynamic scaffolds needed for the spatial localization and processing of molecules critical for various biological functions, including cell motility. Whether progranulin-activated EphA2 resides with liprinα–1 in these liquid-phase condensates remains to be experimentally determined.
Our results demonstrated that liprinα–1 depletion severely inhibits progranulin-dependent migration of bladder cancer cells. Moreover, liprinα–1 mRNA is significantly upregulated in bladder cancer as reported in the Oncomine analysis tool [61,62] and liprinα–1 is highly expressed in urothelial cancer as per the Human Protein Atlas (not shown). Collectively, these data suggest that liprinα–1 may be critical for motility of bladder cancer cells and could represent a novel biomarker for bladder tumor progression.
There is also robust evidence that liprin-α1 modulates breast cancer cell signaling by regulating the transmembrane protein CD82 in adhesive membrane domains linked to the cytoskeleton [60]. These results suggest an alternate mechanisms of liprinα–1 action at the plasma membrane, whereby progranulin-mediated liprinα–1 recruitment to EphA2 might regulate receptor membrane availability or trafficking to a particular cellular compartment, where progranulin-activated EphA2 may drive signaling leading to enhanced motility. Our confocal analysis indicates progranulin-dependent membrane colocalization between EphA2 and liprinα–1, thereby supporting this hypothesis.
Recent data have shown that liprinα–1 interacts with leukocyte common antigen-related receptor protein tyrosine phosphatases (LAR-RPTPs), cell adhesion molecules involved in neuronal development, and enhances its clustering at the plasma membrane [74]. Structural analysis revealed homophilic interactions of LAR and liprinα–1 via the catalytic D1 domain of LAR, and disruption of D1/D1 attenuated the liprina-promoted LAR clustering and increased Tyr-dephosphorylation [74] Thus, it is plausible that liprinα–1 could affect the tyrosine kinase activity of EphA2 in response to progranulin, although this is only speculative at the moment.
Of more general interest is the recent discovery that liprinα–1 controls the delivery of fibronectin to the basolateral face of endothelial cells and the recycling of endcytosed active α5β1 integrin to the cell surface [75]. Notably, silencing of liprinα–1 has no effects on the apical secretion of fibronectin but significantly inhibits fibronectin delivery to the basolateral region of polarized endothelial cells monolayers [76]. Indeed, loss of liprinα–1 causes a significant disruption of the normal polarity of secretion for a subset of proteins including dystroglycan, lysyl oxidase, multimerin-2, phospholipase transfer protein and fibronectin [76]. Thus, our discovery of a physical interaction between EphA2 and liprinα–1 offers novel avenues of research with implications in secretory sorting and the polarization of endothelial and perhaps epithelial cells.
In terms of clinical relevance of this work, targeting progranulin [22] or EphA2 (cfr. Fig. 3D) inhibits tumorigenesis and sensitized bladder cancer cells to cisplatin treatment thereby suggesting that the short-term outcome of this research will be the identification of novel targets and additional treatment options for bladder cancer therapy. EphA2 inhibitors are already in the clinic for treatment of other solid cancer types [77–79] (ClinicalTrials.gov). Thus, the long-term outcomes of this study will be to establish treatment regimens that block progranulin/EphA2-signaling alone or in combination with cisplatin as an effective clinical approach for bladder cancer therapy.
The complexity of progranulin regulatory mechanisms has been additionally highlighted by previous work from our laboratories, which demonstrated extracellular matrix proteins as additional modulators of progranulin tumorigenic action. Endorepellin, the C-terminal domain of the heparin sulfate proteoglycan perlecan, binds progranulin and inhibits progranulin activity in tumor angiogenesis [80]. Progranulin and perlecan are both highly expressed in many types of cancer where they co-localize within the stroma and perivascular compartments [80], strongly supporting the hypothesis that progranulin could interact in vivo with perlecan within the provisional matrix of newly formed vasculature. This interaction might constitute a regulatory mechanism of fine-tuning each other’s biological activity, thereby regulating tumor angiogenesis and continued tumor growth [81, 82], further underscoring the role of ECM-driven phenotypic heterogeneity [83]. Interestingly, endorepellin functions via dual receptor mechanisms through the binding to VEGFR2 and α2β1. Similarly, EphA2, the newly discover binding partner of progranulin, in the invasive protrusions of glioblastoma, was found to be co-localized with another integrin, α3β1, suggesting a cross-talk between EphA2 and α3β1 that could enhance adhesion-dependent signal cascades and therefore, contribute to the invasive phenotype [84]. Moreover, in the damaged adult brain, progranulin aided by perlecan may help overcoming axonal dystrophy, and orchestrate regenerative processes in synergy with the proteoglycan NG2/CSPG4 [85].
Moreover, considering the identification of liprinα–1 in coordinating basolateral polarization to ensure correct cargo composition and secretion, the progranulin/EphA2 axis may influence the deposition of important matrix factors to sustain angiogenesis and tumorigenesis. Indeed, dystroglycan, for example, is a perlecan interacting protein [86] has been implicated in angiogenesis [87].
The oncosuppressive small leucine rich proteoglycan decorin [88] regulates key downstream signaling processes indirectly by sequestering growth factors or directly antagonizing receptor tyrosine- kinases [88–93]. We have previously shown that decorin expression inversely correlated with IGF-IR in low-and high-grade bladder cancers. Decorin bound with high affinity to IGF-IR and IGF-I at distinct sites and negatively regulated IGF-IR activity in urothelial cancer cells [89]. The involvement of decorin in cancer has been shown in decorin null mice [94–96] and in double knockouts of decorin and p53 [97,98]. Systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer [99]. Because progranulin plays an important role in regulating cancer cell motility of castration-resistant prostate cancer cells [26,27], we can reasonably speculate that the anti-oncogenic function of decorin in prostate cancer might relate, in part, to an inhibitory action on the progranulin/EphA2 tumorigenic axis. Collectively, these results suggest that decorin loss may contribute to increased RTKs activity in the progression of several solid tumors, including prostate and bladder cancer. However, more studies need to be done to establish whether decorin may regulate the progranulin/EphA2 axis by interfering with either the ligand or the receptor.
In conclusion, the characterization of EphA2 as the functional progranulin receptor in bladder cancer will not only provide important information to further define the mechanisms regulating tumor formation but also yield valuable insight for translational research. Furthermore, the characterization of the progranulin/EphA2 signaling axis may contribute to the identification of novel and clinically relevant targets for therapeutic intervention in bladder neoplasia along with more accurate diagnostic and prognostic markers for patients afflicted with this devastating disease.
Materials and methods
Cell lines
Urothelial carcinoma-derived human 5637, T24 and UMUC-3 cells were obtained by ATCC. T24T, UMUC-3Luc and UMUC-3LuL2 cells (a kind gift from Dr. Dan Theodorescu, University of Colorado Cancer Center) are more aggressive isogenic derivatives of T24 and UMUC-3 cells respectively [100–102]. 5637,T24 and T24T cells were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS). UMUC-3 cells and derivatives were maintained in MEM with EARL medium with 10% fetal bovine serum (FBS). Serum-free medium (SFM) is DMEM supplemented with 0.1% bovine serum albumin and 50 μg/ml of transferrin (Sigma-Aldrich).
Progranulin and ephrin-A1 microarray and mRNA expression analysis
The Oncomine database and gene microarray analysis tool, a repository for published cDNA microarray data (http://www.oncomine.org) [51,52], was explored (September 2018) for mRNA levels of GRN and EFNA1 in normal or urothelial carcinomas of low and high grade. Statistical analysis of the differences in proepithelin expression between the aforementioned tissues was accomplished through use of ONCOMINE algorithms, which allow for multiple comparisons among different studies [51,52,103]. Only studies with +analysis results with p<0.05 were considered. For quantitative real-time PCR analysis, T24 and UMUC-3 cells were lysed in either 1 ml of TRIzol reagent (Invitrogen) to extract total RNA. Next, ~1 μg of total RNA was annealed with oligo(dT18–20) primers, and cDNA was synthesized with SuperScript Reverse Transcriptase II (SSRT II, Invitrogen). The primers used to determine the relative levels of GRN and EFNA1 were validated for amplification efficiency and found they were suitable for comparison. PCR amplicons representing target genes and the endogenous house-keeping gene, ACTB, were amplified in quadruplicate, independent reactions with the Brilliant SYBR Green Master Mix II reagent (Agilent Technologies, Cedar Creek, TX). All samples were run on the Roche LightCycler 480-II Real Time PCR platform (Roche Applied Sciences), and cycle number (Ct) was recorded for each independent reaction. Data represent three independent trials run in quadruplicate for both GRN and EFNA1 genes.
Immunohistochemical detection of EphA2 expression in bladder cancer tissues
Human Protein Atlas (https://www.proteinatlas.org) was interrogated for progranulin and Ephrin-A1 expression in bladder and urothelial carcinoma tissues in September 2018, and representetive images were acquired and compared to highlight different expression levels among the analyzed tissues. EphA2 expression was analyzed by immunohistochemistry as described [21,22,104] on a Biomax human bladder cancer tissue microarray (BL208) at the Translational Core Facility of the Sidney Kimmel Cancer Center. The antibody used was an anti-EphA2 rabbit polyclonal antibody (Santa Cruz Biotechnology (C-20): sc-924) at a dilution of 1:100. Detailed specifications of the bladder tissue array can be found at: http://www.biomax.us/tissue-arrays/Bladder/BL208. The array contains 43 cases of urothelial carcinoma, 7 adenocarcinoma, 6 carcinoma in situ, 4 metastatic carcinoma and 9 bladder tissue, triplicate cores per case. Quantification of EphA2 staining in the microarray was done utilizing ImageJ. The threshold of staining of each image acquired was adjusted in order to show only the specific staining. The representative areas of staining were then quantified and a representative plot was created in SigmaPlot.
EphA2 pull-down assays and proteomic analysis
T24 cells were serum-starved for 24 h and then stimulated for 15 min with 150 nM human recombinant progranulin [80]. Cells were lysed and collected in NP-40 lysis buffer containing Protease and Phosphatase Inhibitor Cocktail (Thermo Scientifics, Waltham, MA USA). After preclearing, 20 mg of lysates were IP O.N. with anti-EphA2 antibodies. NP-40 lysis buffer containing Protease and Phosphatase Inhibitor Cocktail (Thermo Scientifics, Waltham, MA USA). Beads were washed with lysis buffer and suspended in Laemmli buffer. Samples were separated by SDS-PAGE and Coomassie-stained bands of interest were trypsin-digested and analyzed for mass spectrometry at the Proteomic Core Facility of Wistar by LC-MS/MS on a Q Exactive™ Plus mass spectrometry. The mass spec data were probed against the UniProt human database for STY phosphorylation. All peptides were identified with a confidence of at least 95%. False discovery rate for peptides/site identifications was set at 1%.
Total and Phospho-EphA2 antibodies used in western immunoblots were from Cell Signaling Technology. Anti-MEK (U0126, 10 μm) and anti-PI3K (LY294002, 20 μM) inhibitors were from Calbiochem. The specific EphA2 inhibitor ALW-II-41–27 (1 μM) was from Cayman Chemical and was resuspended and utilized according to the manufacturer’s instructions.
Generation of EphA2-depleted bladder cancer cells
Cell lines (UMUC-3) stably depleted of endogenous EphA2 were generated by transfecting the pRS-shScr (scrambled shRNAs) and pRS/shEphA2 plasmids expressing shRNAs against human EphA2 (OriGene Technologies, Inc.) using the TransIT®-Prostate Transfection Kit (Mirus). Cells were selected in medium supplemented with 2 μg/ml of Puromycin as described [21,22]. After selection, pools of EphA2-depleted UMUC-3 cells were tested for EphA2 expression levels by immunoblot using specific polyclonal antibodies as previously described [90, 105, 106].
Migration assays and colony formation in soft agar
Cell motility was assessed in Boyden chambers as described [19,21,22]. Briefly, HTS FluoroBloks™ inserts (Becton Dickinson) were saturated with PBS-1% bovine serum albumin for 2 h at room temperature. Serum-starved cells were labeled with DiI (Molecular Probes) for 20 min at 37 °C and then seeded in the HTS FluoroBloks™ upper chamber in either SFM or SFM supplemented with progranulin (150 nM) and incubated at 37 °C for 18 h. After fixing in 4% paraformaldehyde, membranes were mounted on a slide, and migrated cells were counted and photographed with a Zeiss Axiovert 200 M cell live microscope at the Kimmel Cancer Center Confocal Microscopy Core Facility. Anchorage-independent grow was measured by colony formation in soft-agar as described in detail in previous work from our laboratories [21,26,107]. Cells were seeded in soft-agar at a density of 5 × 103 cells/35 mm plate and counted after three weeks in culture. Colonies > 150 μm were scored as positive.
Cell cytotoxicity assay
UMUC-3/shScr and UMUC-3/shEphA2 were plated onto six-well plate at a density of 3.5 × 105 cells/well in media supplemented with 10% FBS. After 24 h, cells were washed twice with PBS 1X and transfer to 5% FBS-supplemented media with or without cisplatin (Sigma-Aldrich, St Louis, MO, USA) at the concentrations of 1, 10 and 20 μM. Cells were then counted after 24 h using the Cell Cytotoxicity Assay Kit – Colorimetric (ab112118) from Abcam following the manufacturer’s instruction.
Coimmunoprecipitations and western immunoblots
T24 cells were serum-starved over-night and then stimulated ±progranulin for the indicated time points (150 nM). After lysis, 2 mg of proteins were immunoprecipitated over-night with anti EphA2 antibodies. Liprinα–1 and vinculin in various urothelial carcinoma cells were detected by western immunoblot with specific antibodies. β-actin was used as loading control.
Confocal microscopy
Colocalization between EphA2 and liprinα–1 was assessed by confocal microscopy analysis performed as described [30]. Serum-starved T24 cells plated on four-well chamber slides (Nunc, Thermo Fisher Scientific) were stimulated with progranulin (150 nm for 30 min). After treatment, cells were rinsed twice with DPBS (Thermo Fisher Scientific) and fixed in 4% (wt/vol) paraformaldehyde for 30 min. After washing, EphA2 and liprinα–1 were detected using specific polyclonal antibodies from Cell Signaling Technology followed by immunoreaction with Alexa-Fluor secondary antibodies (Thermo Fisher). Confocal analysis was performed using a 63 ×, 1.3 oil-immersion objective of an LSM-780 confocal laser-scanning microscope (ZEISS) with filters set at 450/594 nm for dual-channel imaging. All images were analyzed using ImageJ (National Institute of Health) and Adobe Photoshop CS6 (Adobe Systems). Images are representative of 10 independent fields from three independent experiments.
Transient liprinα–1 depletion and wound healing assay
Transient liprinα–1 depletion in T24 cells was achieved by siRNA strategies. Liprinα–1-specific siRNA were SMART pool oligos designed by Dharmacon. Cells were transfected using the TransIT®-Prostate Transfection Kit (Mirus) and targeting and scrambled oligos (80 and 20 pM, respectively) were used. Lateral motility was assessed by a wound healing assay 72 h post-transfection. Briefly, T24 cells transfected onto 35 mm plates were scratched with a thin disposable tip to generate a wound (500 μM) in the cells monolayer [18,19,26]. Cells were analyzed and photographed after 24 h with a Leica inverted microscope. Wound closure was quantified utilizing ImageJ. Data are representative of three independent experiments.
Statistical analysis
Results of multiple experiments are expressed as mean ± SD. All statistical analyses were carried out with SigmaStat for Windows version 3.10 (Systat Software, Inc., Port Richmond, CA). Significance of the differences was evaluated by Student’s t-test with significance at p < 0.05. All data presented herein were collected from a minimum of three independent experiments. Where more than two experimental conditions were compared one-way ANOVA was performed.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 CA164462 (A.Morr., R. V.I.), RO1 CA39481 (R.V.I.), RO1 CA47282 (R.V.I.) and grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) (grant n. AIRC IG 19242) (A.B.). The Bioimaging Core Facility of the Sidney Kimmel Cancer Center is supported by NIH/NCI (P30CA056036).
Abbreviations:
- EphA2
erythropoietin-producing human hepatocellular receptor type-A2
- RTK
receptor tyrosine-kinase
- MAPK
mitogen-activated protein kinase
- EGFR
epidermal growth factor receptor
- SFM
serum-free medium
- Liprinα1
protein tyrosine phosphatase receptor type F interacting protein α1
- LAR-RPTP
leukocyte common antigen-related receptor protein tyrosine phosphatase
Footnotes
Disclosure
The authors declare no competing financial interests.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, 69(1) (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R, The health economics of bladder cancer: a comprehensive review of the published literature, Pharmacoeconomics 21 (18) (2003) 1315–1330. [DOI] [PubMed] [Google Scholar]
- [3].Boormans JL, Zwarthoff EC, Limited funds for bladder cancer research and what can we do about it, Bladder Cancer 2 (1) (2016) 49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB, Bergren SK, Pietzak EJ, Anderson CB, Benson MC, Coleman JA, Taylor BS, Abate-Shen C, McKiernan JM, Al-Ahmadie H, Solit DB, Shen MM, Tumor evolution and drug response in patient-derived organoid models of bladder cancer, Cell 173 (2) (2018) 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bladder cancer: diagnosis and management of bladder cancer: (c) NICE (2015) Bladder cancer: diagnosis and management of bladder cancer, BJU Int. 120 (6) (2017) 755–765. [DOI] [PubMed] [Google Scholar]
- [6].Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G, Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration, J. Biol. Chem 287 (39) (2012) 32298–32306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rollinson S, Young K, Bennion-Callister J, Pickering-Brown SM, Identification of biological pathways regulated by PGRN and GRN peptide treatments using transcriptome analysis, Eur. J. Neurosci 44 (5) (2016) 2214–2225. [DOI] [PubMed] [Google Scholar]
- [8].Kumar-Singh S, Progranulin and TDP-43: mechanistic links and future directions, J. Mol. Neurosci. MN 45 (3) (2011) 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].He Z, Bateman A, Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis, J. Mol. Med 81 (10) (2003) 600–612. [DOI] [PubMed] [Google Scholar]
- [10].Bateman A, Bennett HP, Granulins: the structure and function of an emerging family of growth factors, J. Endocrinol 158 (2) (1998) 145–151. [DOI] [PubMed] [Google Scholar]
- [11].He Z, Bateman A, Progranulin gene expression regulates epithelial cell growth and promotes tumor growth in vivo, Cancer Res. 59 (13) (1999) 3222–3229. [PubMed] [Google Scholar]
- [12].He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A, Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival, Cancer Res. 62 (19) (2002) 5590–5596. [PubMed] [Google Scholar]
- [13].He Z, Ong CH, Halper J, Bateman A, Progranulin is a mediator of the wound response, Nat. Med 9 (2) (2003) 225–229. [DOI] [PubMed] [Google Scholar]
- [14].Tkaczuk KHR, Hawkins D, Yue B, Hicks D, Tait N, Serrero G, Association of serum progranulin levels with disease progression, therapy response and survival in patients with metastatic breast cancer, Clin. Breast Cancer (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tkaczuk KR, Yue B, Zhan M, Tait N, Yarlagadda L, Dai H, Serrero G, Increased circulating level of the survival factor GP88 (progranulin) in the serum of breast cancer patients when compared to healthy subjects, Breast Cancer Basic Clin. Res 5 (2011) 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abdulrahman A, Eckstein M, Jung R, Guzman J, Weigelt K, Serrero G, Yue B, Geppert C, Stohr R, Hartmann A, Wullich B, Wach S, Taubert H, Lieb V, Expression of GP88 (Progranulin) protein is an independent prognostic factor in prostate cancer patients, Cancers (Basel) 11 (12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, Tamori Y, Yokoi N, Watanabe M, Matsuo E, Nishimura O, Seino S, PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue, Cell Metab. 15 (1) (2012) 38–50. [DOI] [PubMed] [Google Scholar]
- [18].Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A, Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex, Cancer Res. 66 (14) (2006) 7103–7110. [DOI] [PubMed] [Google Scholar]
- [19].Lovat F, Bitto A, Xu SQ, Fassan M, Goldoni S, Metalli D, Wubah V, McCue P, Serrero G, Gomella LG, Baffa R, Iozzo RV, Morrione A, Proepithelin is an autocrine growth factor for bladder cancer, Carcinogenesis 30 (5) (2009) 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanimoto R, Lu KG, Xu SQ, Buraschi S, Belfiore A, Iozzo RV, Morrione A, Mechanisms of progranulin action and regulation in genitourinary cancers, Front. Endocrinol. (Lausanne) 7 (2016) 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu SQ, Buraschi S, Morcavallo A, Genua M, Shirao T, Peiper SC, Gomella LG, Birbe R, Belfiore A, Iozzo RV, Morrione A, A novel role for drebrin in regulating progranulin bioactivity in bladder cancer, Oncotarget 6 (13) (2015) 10825–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buraschi S, Xu SQ, Stefanello M, Moskalev I, Morcavallo A, Genua M, Tanimoto R, Birbe R, Peiper SC, Gomella LG, Belfiore A, Black PC, Iozzo RV, Morrione A, Suppression of progranulin expression inhibits bladder cancer growth and sensitizes cancer cells to cisplatin, Oncotarget 7 (26) (2016) 39980–39995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xia X, Serrero G, Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts, Biochem. Biophys. Res. Co 245 (2) (1998) 539–543. [DOI] [PubMed] [Google Scholar]
- [24].Culouscou JM, Carlton GW, Shoyab M, Biochemical analysis of the epithelin receptor, J. Biol. Chem 268 (14) (1993) 10458–10462. [PubMed] [Google Scholar]
- [25].Hu F, Padukkavidana T, Vaegter CB, Brady OA, Zheng Y, Mackenzie IR, Feldman HH, Nykjaer A, Strittmatter SM, Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin, Neuron 68 (4) (2010) 654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tanimoto R, Morcavallo A, Terracciano M, Xu SQ, Stefanello M, Buraschi S, Lu KG, Bagley DH, Gomella LG, Scotlandi K, Belfiore A, Iozzo RV, Morrione A, Sortilin regulates progranulin action in castration-resistant prostate cancer cells, Endocrinology 156 (1) (2015) 58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tanimoto R, Palladino C, Xu SQ, Buraschi S, Neill T, Gomella LG, Peiper SC, Belfiore A, Iozzo RV, Morrione A, The perlecan-interacting growth factor progranulin regulates ubiquitination, sorting, and lysosomal degradation of sortilin, Matrix Biol. 64 (2017) 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, Su J, Yin F, Ding AH, Zanin-Zhorov A, Dustin ML, Tao J, Craft J, Yin Z, Feng JQ, Abramson SB, Yu XP, Liu CJ, The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice, Science 332 (6028) (2011) 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu S, Buraschi S, Tanimoto R, Stefanello M, Belfiore A, Iozzo RV, Morrione A, Analysis of Progranulin-Mediated Akt and MAPK Activation, Methods Mol. Biol 1806 (2018) 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Neill T, Buraschi S, Goyal A, Sharpe C, Natkanski E, Schaefer L, Morrione A, Iozzo RV, EphA2 is a functional receptor for the growth factor progranulin, J. Cell Biol 215 (5) (2016) 687–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lisabeth EM, Falivelli G, Pasquale EB, Eph receptor signaling and ephrins, Cold Spring Harb. Perspect. Biol 5 (9) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kullander K, Klein R, Mechanisms and functions of Eph and ephrin signaling, Nat. Rev. Mol. Cell Biol 3 (2002) 475–486. [DOI] [PubMed] [Google Scholar]
- [33].Amato KR, Wang S, Hastings AK, Youngblood VM, Santapuram PR, Chen H, Cates JM, Colvin DC, Ye F, Brantley-Sieders DM, Cook RS, Tan L, Gray NS, Chen J, Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC, J. Clin. Invest 124 (5) (2014) 2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Amato KR, Wang S, Tan L, Hastings AK, Song W, Lovly CM, Meador CB, Ye F, Lu P, Balko JM, Colvin DC, Cates JM, Pao W, Gray NS, Chen J, EPHA2 blockade overcomes acquired resistance to EGFR kinase inhibitors in lung cancer, Cancer Res. 76 (2) (2016) 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pasquale EB, Eph receptors and ephrins in cancer: bidirectional signalling and beyond, Nat. Rev. Cancer 10 (3) (2010) 165–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Annamalai B, Liu X, Gopal U, Isaacs JS, Hsp90 is an essential regulator of EphA2 receptor stability and signaling: implications for cancer cell migration and metastasis, Mol. Cancer Res 7 (7) (2009) 1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, Lin C, Chen J, Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo, Oncogene 21 (46) (2002) 7011–7026. [DOI] [PubMed] [Google Scholar]
- [38].Brantley-Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J, EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3-kinase-mediated Rac1 GTPase activation, J. Cell Sci 117 (Pt 10) (2004) 2037–2049. [DOI] [PubMed] [Google Scholar]
- [39].Brantley-Sieders DM, Chen J, Eph receptor tyrosine kinases in angiogenesis: from development to disease, Angiogenesis 7 (1) (2004) 17–28. [DOI] [PubMed] [Google Scholar]
- [40].Brantley-Sieders DM, Fang WB, Hicks DJ, Zhuang G, Shyr Y, Chen J, Impaired tumor microenvironment in EphA2-deficient mice inhibits tumor angiogenesis and metastatic progression, FASEB J 19 (13) (2005) 1884–1886. [DOI] [PubMed] [Google Scholar]
- [41].Brantley-Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J, Ephrin-A1 facilitates mammary tumor metastasis through an angiogenesis-dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice, Cancer Res. 66 (21) (2006) 10315–10324. [DOI] [PubMed] [Google Scholar]
- [42].Brantley-Sieders DM, Jiang A, Sarma K, Badu-Nkansah A, Walter DL, Shyr Y, Chen J, Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome, PLoS One 6 (9) (2011) e24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, Chen J, The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling, J. Clin. Invest 118 (1) (2008) 64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Song W, Hwang Y, Youngblood VM, Cook RS, Balko JM, Chen J, Brantley-Sieders DM, Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers, Oncogene 36 (40) (2017) 5620–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dunaway CM, Hwang Y, Lindsley CW, Cook RS, Wu JY, Boothby M, Chen J, Brantley-Sieders DM, Cooperative signaling between Slit2 and Ephrin-A1 regulates a balance between angiogenesis and angiostasis, Mol. Cell. Biol 31 (3) (2011) 404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B, EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt, Cancer Cell 16 (1) (2009) 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Paraiso KH, Das Thakur M, Fang B, Koomen JM, Fedorenko IV, John JK, Tsao H, Flaherty KT, Sondak VK, Messina JL, Pasquale EB, Villagra A, Rao UN, Kirkwood JM, Meier F, Sloot S, Gibney GT, Stuart D, Tawbi H, Smalley KS, Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype, Cancer Discov. 5 (3) (2015) 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhou Y, Yamada N, Tanaka T, Hori T, Yokoyama S, Hayakawa Y, Yano S, Fukuoka J, Koizumi K, Saiki I, Sakurai H, Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2, Nat. Commun 6 (2015) 7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed SI, Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder, Clin. Cancer Res 12 (2) (2006) 353–360. [DOI] [PubMed] [Google Scholar]
- [50].Chu M, Zhang C, Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer, Sci. Rep 8 (1) (2018) 1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM, ONCOMINE: a cancer microarray database and integrated data-mining platform, Neoplasia 6 (1) (2004) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM, Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression, Proc. Natl. Acad. Sci. USA 101 (25) (2004) 9309–9314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nickerson ML, Witte N, Im KM, Turan S, Owens C, Misner K, Tsang SX, Cai Z, Wu S, Dean M, Costello JC, Theodorescu D, Molecular analysis of urothelial cancer cell lines for modeling tumor biology and drug response, Oncogene 36 (1) (2016) 35–46, doi: 10.1038/onc.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Old WM, Shabb JB, Houel S, Wang H, Couts KL, Yen CY, Litman ES, Croy CH, Meyer-Arendt K, Miranda JG, Brown RA, Witze ES, Schweppe RE, Resing KA, Ahn NG, Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma, Mol. Cell 34 (1) (2009) 115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Witze ES, Old WM, Resing KA, Ahn NG, Mapping protein post-translational modifications with mass spectrometry, Nat. Methods 4 (10) (2007) 798–806. [DOI] [PubMed] [Google Scholar]
- [56].Astro V, Asperti C, Cangi MG, Doglioni C, de Curtis I, Liprin-alpha1 regulates breast cancer cell invasion by affecting cell motility, invadopodia and extracellular matrix degradation, Oncogene 30 (15) (2011) 1841–1849. [DOI] [PubMed] [Google Scholar]
- [57].Astro V, Tonoli D, Chiaretti S, Badanai S, Sala K, Zerial M, de Curtis I, Liprin-alpha1 and ERC1 control cell edge dynamics by promoting focal adhesion turnover, Sci. Rep 633653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Astro V, Tonoli D, Chiaretti S, Badanai S, Sala K, Zerial M, de Curtis I, Liprin-alpha1 and ERC1 control cell edge dynamics by promoting focal adhesion turnover, Sci. Rep 6 (2016) 33653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chiaretti S, Astro V, Chiricozzi E, de Curtis I, Effects of the scaffold proteins liprin-alpha1, beta1 and beta2 on invasion by breast cancer cells, Biol. Cell 108 (3) (2016) 65–75. [DOI] [PubMed] [Google Scholar]
- [60].Pehkonen H, Lento M, von Nandelstadh P, Filippou A, Grenman R, Lehti K, Monni O, Liprin-alpha1 modulates cancer cell signaling by transmembrane protein CD82 in adhesive membrane domains linked to cytoskeleton, Cell Commun. Signal 16 (1) (2018) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C, Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligo-nucleotide microarrays, J. Clin. Oncol 24 (5) (2006) 778–789. [DOI] [PubMed] [Google Scholar]
- [62].Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, Devries S, Koppie T, Pejavar S, Carroll P, Waldman FM, Bladder cancer outcome and subtype classification by gene expression, Clin. Cancer Res 11 (11) (2005) 4044–4055. [DOI] [PubMed] [Google Scholar]
- [63].Alexander J, Cukierman E, Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions, Curr. Opin. Cell Biol 42 (2016) 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pasquale EB, Eph receptor signalling casts a wide net on cell behaviour, Nat. Rev. Mol. Cell Biol 6 (6) (2005) 462–475. [DOI] [PubMed] [Google Scholar]
- [65].Pasquale EB, Eph-ephrin bidirectional signaling in physiology and disease, Cell 133 (1) (2008) 38–52. [DOI] [PubMed] [Google Scholar]
- [66].Shirao T, Inoue HK, Kano Y, Obata K, Localization of a developmentally regulated neuron-specific protein S54 in dendrites as revealed by immunoelectron microscopy, Brain Res. 413 (2) (1987) 374–378. [DOI] [PubMed] [Google Scholar]
- [67].Shirao T, Kojima N, Kato Y, Obata K, Molecular cloning of a cDNA for the developmentally regulated brain protein, drebrin, Brain Res. 464 (1) (1988) 71–74. [DOI] [PubMed] [Google Scholar]
- [68].Mikati MA, Grintsevich EE, Reisler E, Drebrin-induced stabilization of actin filaments, J. Biol. Chem 288 (27) (2013) 19926–19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Selmy MA, Ibrahim GH, El Serafi TI, Ghobeish AA, Evaluation of urinary proepithelin as a potential biomarker for bladder cancer detection and prognosis in Egyptian patients, Cancer Biomark 7 (3) (2010) 163–170. [DOI] [PubMed] [Google Scholar]
- [70].Chitramuthu B, Bateman A, Progranulin and the receptor tyrosine kinase EphA2, partners in crime? J. Cell Biol 215 (5) (2016) 603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sala K, Corbetta A, Minici C, Tonoli D, Murray DH, Cammarota E, Ribolla L, Ramella M, Fesce R, Mazza D, Degano M, de Curtis I, The ERC1 scaffold protein implicated in cell motility drives the assembly of a liquid phase, Sci. Rep 9 (1) (2019) 13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chiaretti S, de Curtis I, Role of liprins in the regulation of tumor cell motility and invasion, Curr. Cancer Drug Targets 16 (3) (2016) 238–248. [DOI] [PubMed] [Google Scholar]
- [73].Sala K, Raimondi A, Tonoli D, Tacchetti C, de Curtis I, Identification of a membrane-less compartment regulating invadosome function and motility, Sci. Rep 8 (1) (2018) 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Xie X, Luo L, Liang M, Zhang W, Zhang T, Yu C, Wei Z, Structural basis of liprin-alpha-promoted LAR-RPTP clustering for modulation of phosphatase activity, Nat. Commun 11 (1) (2020) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mana G, Clapero F, Panieri E, Panero V, Bottcher RT, Tseng HY, Saltarin F, Astanina E, Wolanska KI, Morgan MR, Humphries MJ, Santoro MM, Serini G, Valdembri D, PPFIA1 drives active alpha5beta1 integrin recycling and controls fibronectin fibrillogenesis and vascular morphogenesis, Nat. Commun 7 (2016) 13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wei H, Sundararaman A, Dickson E, Rennie-Campbell L, Cross E, Heesom KJ, Mellor H, Characterization of the polarized endothelial secretome, FASEB J. 33 (11) (2019) 12277–12287. [DOI] [PubMed] [Google Scholar]
- [77].Wykosky J, Debinski W, The EphA2 receptor and ephrin-A1 ligand in solid tumors: function and therapeutic targeting, Mol. Cancer Res 6 (12) (2008) 1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Tandon M, Vemula SV, Mittal SK, Emerging strategies for EphA2 receptor targeting for cancer therapeutics, Expert Opin. Ther. Targets 15 (1) (2011) 31–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hasegawa J, Sue M, Yamato M, Ichikawa J, Ishida S, Shibutani T, Kitamura M, Wada T, Agatsuma T, Novel anti-EPHA2 antibody, DS-8895a for cancer treatment, Cancer Biol. Ther 17 (11) (2016) 1158–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV, A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth, J. Biol. Chem 278 (40) (2003) 38113–38116. [DOI] [PubMed] [Google Scholar]
- [81].Wight TN, A role for proteoglycans in vascular disease, Matrix Biol. 71-72 (2018) 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Theocharis AD, Skandalis SS, Tzanakakis GN, Karamanos NK, Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting, FEBS J 277 (19) (2010) 3904–3923. [DOI] [PubMed] [Google Scholar]
- [83].Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Pure E, Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts, Matrix Biol. 67 (2018) 90–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ramovs V, Te Molder L, Sonnenberg A, The opposing roles of laminin-binding integrins in cancer, Matrix Biol 57–58 (2017) 213–243. [DOI] [PubMed] [Google Scholar]
- [85].Schafer MKE, Tegeder I, NG2/CSPG4 and progranulin in the posttraumatic glial scar, Matrix Biol. 68–69 (2018) 571–588. [DOI] [PubMed] [Google Scholar]
- [86].Baiocchi M, Di Rico C, Di Pietro R, Di Baldassarre A, Migliaccio AR, 5-azacytidine reactivates the erythroid differentiation potential of the myeloid-restricted murine cell line 32D Ro, Exp. Cell Res 285 (2) (2003) 258–267. [DOI] [PubMed] [Google Scholar]
- [87].Korenaga T, Fu X, Xing Y, Matsusita T, Kuramoto K, Syumiya S, Hasegawa K, Naiki H, Ueno M, Ishihara T, Hosokawa M, Mori M, Higuchi K, Tissue distribution, biochemical properties, and transmission of mouse type A AApoAII amyloid fibrils, Am. J. Pathol 164 (5) (2004) 1597–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Neill T, Schaefer L, Iozzo RV, Decorin: a guardian from the matrix, Am. J. Pathol 181 (2) (2012) 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Iozzo RV, Buraschi S, Genua M, Xu SQ, Solomides CC, Peiper SC, Gomella LG, Owens RC, Morrione A, Decorin antagonizes IGF-IR function by interfering with IGF-IR activity and attenuating downstream signaling, J. Biol. Chem 286 (40) (2011) 34712–34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morcavallo A, Buraschi S, Xu SQ, Belfiore A, Schaefer L, Iozzo RV, Morrione A, Decorin differentially modulates the activity of insulin receptor isoform A ligands, Matrix Biol. 35 (2014) 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Morrione A, Neill T, Iozzo RV, Dichotomy of decorin activity on the insulin-like growth factor-I system, FEBS J 280 (10) (2013) 2138–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Buraschi S, Neill T, Iozzo RV, Decorin is a devouring proteoglycan: remodeling of intracellular catabolism via autophagy and mitophagy, Matrix Biol. 75–76 (2019) 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Iozzo RV, Gubbiotti MA, Extracellular matrix: the driving force of mammalian diseases, Matrix Biol. 71–72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV, Yang W, Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice, Carcinogenesis 33 (2) (2011) 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bi X, Tong C, Dockendorff A, Bancroft L, Gallagher L, Guzman G, Iozzo RV, Augenlicht LH, Yang W, Genetic deficiency of decorin causes intestinal tumor formation through disruption of intestinal cell maturation, Carcinogenesis 29 (7) (2008) 1435–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bi X, Xia X, Fan D, Mu T, Zhang Q, Iozzo RV, Yang W, Oncogenic activin C interacts with decorin in colorectal cancer in vivo and in vitro, Mol. Carcinog 55 (11) (2016) 1786–1795. [DOI] [PubMed] [Google Scholar]
- [97].Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I, Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis, Proc. Natl. Acad. Sci. USA 96 (6) (1999) 3092–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Neill T, Schaefer L, Iozzo RV, Decorin as a multivalent therapeutic agent against cancer, Adv. Drug. Deliv. Rev 97 (2016) 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Xu W, Neill T, Yang Y, Hu Z, Cleveland E, Wu Y, Hutten R, Xiao X, Stock SR, Shevrin D, Kaul K, Brendler C, Iozzo RV, Seth P, The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer, Gene Ther. 22 (3) (2015) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gildea JJ, Golden WL, Harding MA, Theodorescu D, Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer, Genes Chromosom. Cancer 27 (3) (2000) 252–263. [DOI] [PubMed] [Google Scholar]
- [101].Gildea JJ, Harding MA, Seraj MJ, Gulding KM, Theodorescu D, The role of Ral A in epidermal growth factor receptor-regulated cell motility, Cancer Res. 62 (4) (2002) 982–985. [PubMed] [Google Scholar]
- [102].Gildea JJ, Seraj MJ, Oxford G, Harding MA, Hampton GM, Moskaluk CA, Frierson HF, Conaway MR, Theodorescu D, RhoGDI2 is an invasion and metastasis suppressor gene in human cancer, Cancer Res. 62 (22) (2002) 6418–6423. [PubMed] [Google Scholar]
- [103].Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA, Dysregulation of the annexin family protein family is associated with prostate cancer progression, Am. J. Pathol 162 (1) (2003) 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Vecchione A, Fassan M, Anesti V, Morrione A, Goldoni S, Baldassarre G, Byrne D, D’Arca D, Palazzo JP, Lloyd J, Scorrano L, Gomella LG, Iozzo RV, Baffa R, MITOSTATIN, a putative tumor suppressor on chromosome 12q24.1, is downregulated in human bladder and breast cancer, Oncogene 28 (2) (2009) 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Morcavallo A, Genua M, Palummo A, Kletvikova E, Jiracek J, Brzozowski AM, Iozzo RV, Belfiore A, Morrione A, Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A, J. Biol. Chem 287 (14) (2012) 11422–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, Spatuzza M, Xu SQ, Iozzo RV, Vigneri R, Morrione A, Belfiore A, Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses, Oncotarget 6 (18) (2015) 16084–16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Monami G, Emiliozzi V, Bitto A, Lovat F, Xu SQ, Goldoni S, Fassan M, Serrero G, Gomella LG, Baffa R, Iozzo RV, Morrione A, Proepithelin regulates prostate cancer cell biology by promoting cell growth, migration, and anchorage-independent growth, Am. J. Pathol 174 (3) (2009) 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]


