Abstract
Background
The Coronavirus disease 2019 (COVID-19) pandemic has led to a dramatic crisis in health care systems worldwide. These may have significant implications for the management of cardiometabolic diseases. We conducted a systematic review of published evidence to assess the indirect impact of the COVID-19 pandemic on hospitalisations for cardiovascular diseases and their management.
Methods
Studies that evaluated volume of hospitalisations for cardiometabolic conditions and their management with comparisons between the COVID-19 and pre-COVID periods were identified from MEDLINE, Embase and the reference list of relevant studies from January 2020 to 25 February 2021.
Results
We identified 103 observational studies, with most studies assessing hospitalisations for acute cardiovascular conditions such as acute coronary syndrome, ischemic strokes and heart failure. About 89% of studies reported a decline in hospitalisations during the pandemic compared to pre-pandemic times, with reductions ranging from 20.2 to 73%. Severe presentation, less utilization of cardiovascular procedures, and longer patient- and healthcare-related delays were common during the pandemic. Most studies reported shorter length of hospital stay during the pandemic than before the pandemic (1–8 vs 2–12 days) or no difference in length of stay. Most studies reported no change in in-hospital mortality among hospitalised patients.
Conclusion
Clinical care of patients for acute cardiovascular conditions, their management and outcomes have been adversely impacted by the COVID-19 pandemic. Patients should be educated via population-wide approaches on the need for timely medical contact and health systems should put strategies in place to provide timely care to patients at high risk.
Systematic review registration
PROSPERO 2021: CRD42021236102
Keywords: COVID-19, Impact, Hospitalisation, Diabetes, Acute coronary syndrome, Stroke cardiovascular disease, Systematic review
1. Introduction
Coronavirus disease 2019 (COVID-19), a respiratory infectious disease caused severe acute respiratory syndrome coronavirus 2 (SARS CoV-2), was declared a global public health emergency on 30 January 2020 and it has since caused so much morbidity and mortality [1]. The COVID-19 virus spreads primarily through droplets generated when an infected person coughs or sneezes, or through droplets of saliva or discharge from the nose. The majority of patients with COVID-19 are asymptomatic or exhibit mild symptoms and never require hospitalisation [2], with a few progressing to severe illness with extrapulmonary manifestations, leading to multiorgan failure and death [[3], [4], [5], [6]]. Accumulating evidence suggests that older and obese patients, males, Black, Asian and Minority Ethnic groups and those with pre-existing comorbidities such as cardiovascular disease (CVD), hypertension, chronic kidney and liver diseases and diabetes are more likely to be infected with SARS CoV-2 and are at highest risk for severe illness or death from COVID-19 [[7], [8], [9]].
Several public health response strategies have been introduced to prevent or slow down the transmission of COVID-19 and these include social distancing, quarantine, use of personal protective equipment, and personal hygiene. Since the World Health Organization (WHO) declared COVID-19 a pandemic on 12 March 2020, other strategies introduced to mitigate the spread of the virus have included shutting down entire cities or communities (“lockdowns”) and banning international or domestic travel. During the period of March 2020, most countries all over the world announced nationwide lockdowns, which severely restricted movement among citizens, though people were still allowed to leave their homes for essential reasons, including seeking medical care.
The COVID-19 pandemic has led to a dramatic crisis of health care systems worldwide directly or indirectly. In pre-pandemic times, large proportions of health service budgets of countries were spent treating chronic health conditions such as CVD, diabetes and their complications. With the pandemic, large proportions of health budgets have been earmarked for COVID-19 management. Healthcare systems have also reorganised their management pathways for acute cardiovascular conditions such as stroke and acute coronary syndromes (ACSs). In addition, the social isolation measures put in place have led to losses in employment and income [9], with changes in human behaviour, including being house-bound and physically inactive because of fear of contracting the virus. All these factors are likely to increase the incidence of cardiometabolic conditions, cause delays in seeking medical care and also adversely affect in-hospital management of these patients. However, a comprehensive synthesis of the evidence of these likely trends is non-existent.
In this context, using a systematic review of all available published observational evidence, our primary aim was to assess the indirect impact of COVID-19 on hospitalisations (including emergency room attendance) and management for cardiovascular conditions. Our specific objectives were to (i) assess the different cardiometabolic conditions that have been impacted by the COVID-19 pandemic; (ii) assess prevalence and trends in hospitalisations for these cardiovascular conditions as a result of the COVID-19 pandemic; (iii) assess COVID-19 related reasons provided by patients for delaying medical contact; and (iv) assess if in-hospital management of these patients has been affected. We also sought to explore if there are gaps in the existing evidence.
2. Methods
2.1. Eligibility criteria
The review was conducted based on a predefined protocol and in accordance with PRISMA and MOOSE guidelines [10,11] (Supplementary Appendix 1–2). The protocol has been registered in the PROSPERO prospective register of systematic reviews (CRD42021236102). We searched for clinical observational studies (prospective cohort, retrospective cohort, case-cohort, nested case-control, case-control and cross-sectional) that have evaluated the indirect impact of COVID-19 on hospitalisations (including emergency room attendances) for cardiovascular diseases and management of these conditions. We included studies that reported comparisons between the COVID-19 pandemic period vs. pre-pandemic and/or historical periods (or other comparisons such as lockdown vs pre-lockdown and/or pre-COVID-19 periods; post-pandemic declaration vs pre-pandemic declaration; or in temporal relation to COVID-19 related restrictions). Henceforth, this is referred to as pandemic vs. pre-pandemic period. Cardiovascular conditions included myocardial infarction (MI), ACS (ST-elevation MI (STEMI), non-ST segment elevation MI (NSTEMI) or angina), stroke and acute heart failure (HF); hypertension; venous thromboembolism (VTE); diabetes and related complications; and mortality. The following exclusions were applied: (i) randomised control trials (RCTs); (ii) studies in which patients were selected and matched between the pandemic and pre-pandemic periods; (iii) studies that had evaluated the direct effects of COVID-19 on cardiovascular conditions and their related complications; and (iv) studies with no pre-pandemic controls for comparison or did not evaluate outcomes in temporal relation to COVID-19 related restrictions.
2.2. Data sources and search strategy
We searched MEDLINE and Embase from January 2020 to 25 February 2021. The computer-based searches combined free and MeSH search terms related to impact (e.g., “impact”, “effect”, “delay”, “reduction”), COVID-19 (e.g., “COVID-19”, “SARS-CoV-2”) and cardiometabolic condition (e.g., “diabetes”, “myocardial infarction”, “acute coronary syndrome”, “cardiac arrest”, “stroke”, “heart failure”). There were no restrictions on language. Titles and abstracts of retrieved citations were initially screened by one author (SKK) to assess their suitability for potential inclusion, followed by the acquisition of full texts for detailed evaluation. Full-text evaluation was independently conducted by two authors (SKK and SS). Reference lists of retrieved articles were manually scanned for all relevant additional studies and review articles missed by the original search. Citing references were also checked in Web of Science. Full details on the search strategy are presented in Supplementary Appendix 3.
2.3. Data extraction and risk of bias assessment
One author (S.K.K.) independently extracted data and performed risk of bias assessments using a standardized predesigned data collection form. A second reviewer (S.S.) checked extracted data with that in the original articles. Data were extracted on publication year, study design, geographical location, pandemic and pre-pandemic periods evaluated, baseline age, proportion of males, cardiometabolic condition and/or related complication, number of hospitalisations for outcome during pandemic and pre-pandemic periods or similar frame, trends in the number of hospitalisations, severity of presentation on hospitalisation, in-hospital management, patient- and system-related delays, COVID-19 related reasons provided for delaying medical contact, length of hospital stay, and outcomes related to in-hospital management. Methodological quality of observational cohort studies was assessed using the nine-star Newcastle–Ottawa Scale (NOS) [12], which uses pre-defined criteria namely: selection (population representativeness), comparability (adjustment for confounders), and ascertainment of outcome. Nine points on the NOS reflects the highest study quality. For cross-sectional studies, methodological quality was evaluated using the NOS modified for cross-sectional studies [13] (Supplementary Appendix 4). It uses three pre-defined domains namely: selection of participants (population representativeness), comparability (adjustment for confounders), and ascertainment of outcomes of interest. A maximum score of 8 reflected the highest study quality.
2.4. Data synthesis
Where possible and appropriate, percentages were calculated [(n/N)*100], where n denotes number of a particular condition and N refers to the total number of conditions. Pooled analysis could not be conducted for any of the outcomes because of the heterogeneous nature of the data. All studies reported trends in hospitalisations and provided data on outcomes, so these were summarised in tables and narrative synthesis was performed.
3. Results
3.1. Study identification and selection
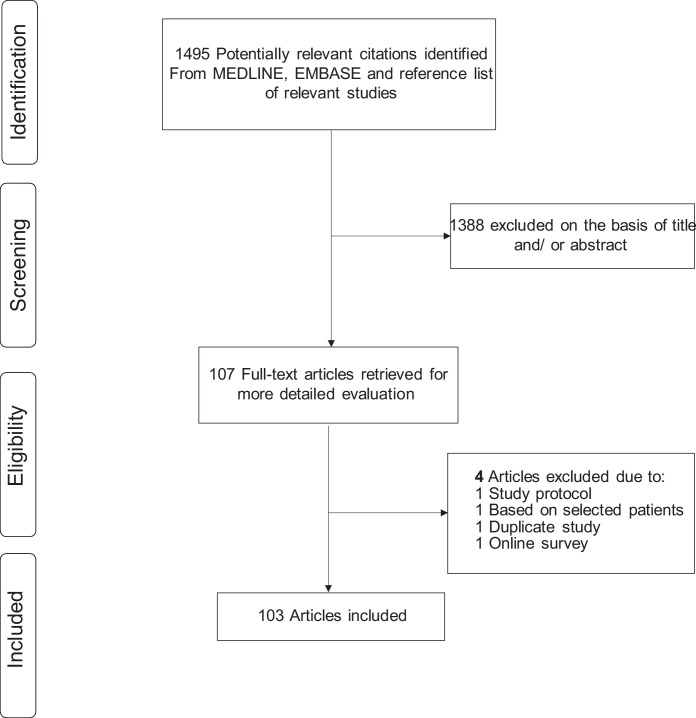
Fig. 1 shows the flow of studies through the review. The literature search identified 1495 potentially relevant citations. After the initial screen based on titles and abstracts, 107 articles were selected for full text evaluation. Following detailed assessment of the full articles, 4 were excluded because (i) was based on a study protocol (n = 1); (ii) based on selected patients (n = 1); (iii) was a duplicate study (n = 1); and (iv) was based on an online survey (n = 1). The remaining 103 articles met the inclusion criteria and were included in the review (Supplementary Appendix 5).
Fig. 1.
Selection of studies included in the review.
3.2. Study characteristics and study quality
Table 1 summarises the key baseline characteristics of the included studies. Most studies were published in 2020, with only 8.7% in 2021. Studies were conducted across 6 continents: 56 in Europe; 25 in North America; 13 in Asia; 3 in South America and 2 each in North America and Oceania. One study was conducted in 17 countries across 4 continents and one in 4 countries across 3 continents. Most studies were retrospective cohort designs (n = 78), followed by cross-sectional designs (n = 15), prospective cohort designs (n = 9) and one ecological retrospective study. Sources of data were healthcare institutions/centres, registries and established databases. Except for one, all studies reported data for a period during the pandemic (ranging from January to October 2020) and compared this to data in the pre-pandemic period (ranging from October 2019 to March 2020) and/or a historical period (similar periods ranging from 2014 to 2019) or compared lockdown with pre-lockdown data based on date of lockdown in the particular country of the study. In the study by Metzler and colleagues [14], data was collected from 2 to 29 March 2020 and comparisons were made between the first and last week of the period surveyed. For studies that provided data on age, mean age of patients ranged from 49 to 78 years and the mean ages were similar comparing patients between the pandemic and pre-pandemic periods. The percentage of males also appeared to be similar across both periods. Among the observational cohort studies, quality score using NOS ranged from 5 to 8 and that for the cross-sectional studies ranged from 4 to 6 (Table 1).
Table 1.
Baseline characteristics of included studies.
| Author, year of publication | Period of data collection | Pandemic period/lockdown | Pre-pandemic period/pre-lockdown | Historical control period/pre-COVID-19 | Study design | Country | Location | Source of data | Male % | Average age | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Araiza-Garaygordobil, 2021 | 12 Mar to 15 Apr, 2020 | 1 Dec 2019 to 11 Mar 2020 | Dec 2018 to April 2019 | Ecological retrospective study | 17 countries | Intercontinental | NA | ||||
| Mafham, 2020 | 1 Jan 2019 to 24 May, 2020 | March to May 2020 | Jan 2019 to Feb 2020 | Retrospective cohort | England | Europe | Acute NHS hospital trusts | 6 | |||
| Perrin, 2020 | 13 Mar to 30 Apr 2020 | 13 Mar to 30 Apr 2019 and pre-pandemic period (7 Jan to 24 Feb 2020) | Retrospective cohort | Switzerland | Europe | Geneva University Hospitals | Pandemic (80); Control (75) | Pandemic (63.8); Control (68.0) | 6 | ||
| Tam, 2020 | 1 Nov 2019 to 31 Mar 2020 | After 25 Jan 2020 | Before 25 Jan 2020 | Cross-sectional study | Hong Kong | Asia | Accident and Emergency Department | Pandemic (70.3); Pre-pandemic (65.9) | Pandemic (69.4); Pre-pandemic (69.0) | 6 | |
| Toniolo, 2020 | Mar-20 | Mar-19 | Retrospective cohort | Italy | Europe | Cardiology Division of Udine University Hospital | 6 | ||||
| Huet, 2020 | 17−22 Mar 2020 | 2−6 Mar 2020 | Cross-sectional study | France | Europe | Intensive cardiac units of 9 cardiology centres | 5 | ||||
| Kwok, 2020b | Jan 2017 to April 2020 | After 23 March 2020 | Before 23 March 2020 | Retrospective cohort | UK | Europe | British Cardiovascular Intervention Society-National Institute of Cardiovascular Outcomes Research database | 7 | |||
| Boukhris, 2020 | 1 Jan to 30 April 2020 | 1 Jan to 30 April 2019 | Cross-sectional study | Russia, Brazil, Saudi Arabia, Tunisia | Intercontinental | 5 | |||||
| Braiteh, 2020 | March to April 2020 | March to April 2019 | Retrospective cohort | USA | North America | Upstate New York Hospitals | Pandemic (70.1); Control (61.9) | Pandemic (65.1); Control (72.3) | 6 | ||
| Butt, 2020 | January and March 2020 | Mar-19 | Retrospective cohort | Qatar | Asia | Hamad Medical Corporation | 6 | ||||
| De Filippo, 2020 | 20 Feb to 31 March 2020 | 1 Jan to 19 Feb 2020 | 20 Feb to 31 March 2019 | Retrospective cohort | Italy | Europe | 15 Hospitals | Pandemic (76.8) | Pandemic (68) | 6 | |
| De Rosa, 2020 | 12−19 March 2020 | 12−19 March 2019 | Cross-sectional study | Italy | Europe | Cardiac care units | Pandemic (68); Control (67.1) | 6 | |||
| Fileti, 2020 | 10 March to 10 April 2020 | 10 March to 10 April 2019 | Retrospective cohort | Italy | Europe | 2 Hospitals with cardiac catheterization facilities | Pandemic (65.3); Control (64.9) | Pandemic (69.7); Control (70.4) | 6 | ||
| Folino, 2020 | 2020 | 2019 | Cross-sectional study | Italy | Europe | 10 Cardiological centres in Northern Italy | 6 | ||||
| Haddad, 2020 | mid-March to mid-May 2020 | Jan to mid-March 2020 | mid-March to mid-May 2019 | Retrospective cohort | Canada | North America | Hospitals in the Greater Montreal area | Pandemic (44); Pre-pandemic (42); Control (42) | Pandemic (60.6); Pre-pandemic (61.1); Control (69.5) | 6 | |
| Hauguel-Moreau, 2020 | Weeks 8−7, 2018−2020 | 17 Feb to 26 April 2020 | 17 Feb to 26 April 2018; 17 Feb to 26 April 2019 | Retrospective cohort | France | Europe | High volume PCI coronary unit | 6 | |||
| Holy, 2020 | Before and after 16 March 2020 (lockdown) | Before and after 16 March 2019 | Cross-sectional study | Switzerland | Europe | University Heart Center Zurich | 5 | ||||
| Metzler, 2020 | 2−29 March 2020 | Cross-sectional study | Austria | Europe | PCI centres | 4 | |||||
| Montagnon, 2021 | 23 March to 5 April 2020 | 23 March to 5 April 2019 | Cross-sectional study | France | Europe | Hospital Sainte Anne | ACS - Pandemic (71.4); Control (75)/Stroke plus TIA - Pandemic (70); Control (55.6) | ACS - Pandemic (67); Control (72)/Stroke plus TIA - Pandemic (73); Control (76) | 6 | ||
| Showkathali, 2020 | 25 March to 31 May 2020 | 25 March to 31 May 2018; 25 March to 31 May 2019 | Retrospective cohort | India | Asia | Tertiary referral hospital | Pandemic (59); Control (72.3) | 6 | |||
| Sokolski, 2020 | 1 March to 30 April 2020 | 1 March to 30 April 2019 | Retrospective cohort | 12 countries | Europe | 15 centres in Europe | 7 | ||||
| Solomon, 2020 | Before and after 4 March 2020 | Before and after 4 March 2019 | Retrospective cohort | USA | North America | Kaiser Permanente Northern California | 6 | ||||
| Vacanti, 2020 | 1 Jan to 30 June 2020 | 2 Jan to 30 June 2018; 1 Jan to 30 June 2019 | Retrospective cohort | Germany | Europe | Cardiology Dept | 6 | ||||
| Yalamanchi, 2020 | 22 March to 1 August 2020 | 22 March to 1 August 2018; 22 March to 1 August 2019 | Retrospective cohort | India | Asia | Cardiac intensive care unit | 2020 (59); 2019 (63); 2018 (62) | 6 | |||
| Tsioufis, 2020 | 1 Jan to 30 April 2020 | 1 Jan to 30 April 2018; 1 Jan to 30 April 2019 | Retrospective cohort | Greece | Europe | Cardiology Dept of a Tertiary General Hospital | 6 | ||||
| Gasior, 2020 | 9 March to 16 April 2020 | 9 March to 16 April 2019 | Retrospective cohort | Poland | Europe | Polish National Health Fund | 6 | ||||
| Dreger, 2020 | Weeks 2−21 2020 | Weeks 2−21 2017 to 2019 | Retrospective cohort | Germany | Europe | Hospitals in Berlin | 6 | ||||
| Anderson, 2020 | 11 March to 28 April 2020 | 11 March to 28 April 2019 | Retrospective cohort | USA | North America | Hospital in Boston | 6 | ||||
| Gluckman, 2020 | 30 Dec 2018 to 16 May 2020 | 29 March 2020 to 16 May 2020 | 23 Feb 2020 to 28 March 2020 | 30 Dec 2018 to 22 Feb 2020 | Cross-sectional study | USA | North America | 49 hospitals in the Providence St Joseph Health system | Later COVID-19 period (66); Early COVID-19 period (68); Historical control (66) | Later COVID-19 period (67); Early COVID-19 period (67); Historical control (68) | 6 |
| Mohammad, 2020 | 1 March to 7 May 2020 | 1 March to 7 May 2015−2019 | Retrospective cohort | Sweden | Europe | Swedish Coronary Angiography and Angioplasty Registry | Pandemic (67.4); Control (67.4) | Pandemic (70); Control (70) | 6 | ||
| Piccolo, 2020 | 30 Jan to 26 March 2020 | 4 weeks after 27 Feb 2020 | 4 weeks before 27 Feb 2020 | Retrospective cohort | Italy | Europe | PCI centres | Pandemic (75%); Pre-pandemic (72%) | Pandemic (65.6); Pre-pandemic (65.8) | 6 | |
| Secco, 2020 | Mar-20 | Mar-19 | Retrospective cohort | Italy | Europe | 3 High volume hospitals | Pandemic (67.4); Control (67.4) | Pandemic (67.4); Control (67.4) | 6 | ||
| Ayad, 2021 | 1 Feb to October 2020 | 1 Feb to October 2019 | Retrospective cohort | Egypt | Africa | International Cardiac Center hospital | Pandemic (81.5); Control (85.7) | Pandemic (57.1); Control (58.9) | 6 | ||
| Bhatt, 2020 | 1 Jan 2019 to 31 March 2020 | Mar-20 | Mar-19 | Retrospective cohort | USA | North America | Mass General Brigham health system | Pandemic (55.4); Control (57.8) | Pandemic (70.3); Control (71.1) | 6 | |
| Daoulah, 2021 | 1 January to 30 April 2020 | 1 January to 30 April 2018−2019 | Retrospective cohort | Saudi Arabia | Asia | 16 centres | 2018 (89.8); 2019 (84.9); 2020 (90.8) | 2018 (56.7); 2019 (56.5); 2020 (55.4) | 6 | ||
| Desai, 2020 | Mar-20 | March 2017−2019 | Retrospective cohort | USA | North America | Stroke center | 6 | ||||
| Diegoli, 2020 | After 17 March 2020 | 2019 | Retrospective cohort | Brazil | South America | Joinville Stroke Registry | 6 | ||||
| Gitt, 2020 | 1 March to 21 April 2020 | 1 March to 21 April 2017−2019 | Retrospective cohort | Germany | Europe | Heart Center Ludwigshafen | 6 | ||||
| Hammad, 2020 | 1 Jan to 15 April 2020 | After 23 March 2020 | Before 23 March 2020 | Retrospective cohort | USA | North America | Integrated 18-hospital system | Pandemic (49); Pre-pandemic (67) | Pandemic (66); Pre-pandemic (61.8) | 6 | |
| Kerleroux, 2020 | 15 Feb to 30 March 2020 | 15 Feb to 30 March 2019 | Prospective cohort | France | Europe | 32 stroke centres | Pandemic (51.2); Control (58.4) | Pandemic (70.6); Control (71.8) | 7 | ||
| Montaner, 2020 | Before and after 31 March 2020 | Before and after 31 March 2019 | Prospective cohort | Spain | Europe | Stroke units | 7 | ||||
| Neves Briard, 2020 | 30 March to 31 May 2020 | 30 March to 31 May 2019 | Prospective cohort | Canada | North America | Stroke center | Pandemic (48); Control (51) | Pandemic (69.4); Control (72.1) | 8 | ||
| Pop, 2020 | 1−31 March 2020 | 1−31 March 2019 | Retrospective cohort | France | Europe | 3 Stroke units | Pandemic (65.5); Control (64) | 6 | |||
| Popovic, 2020 | 26 Feb to 10 May 2020 | 2008−2017 | Prospective cohort | France | Europe | University Hospital of Nancy | Pandemic (56.3); Control (76.1) | Pandemic (62.6); Control (59.6) | 7 | ||
| Range, 2020 | 15 Jan 2019 to 14 April 2020 | 15 March to 14 April 2020 | Before 15 March 2020 | Prospective cohort | France | Europe | France PCI registry | Pandemic (70.5); Control (76.1) | Pandemic (62.9); Control (63.6) | 7 | |
| Reinstadler, 2020 | 24 Feb (week 9) to 5 April 2020 (week 14) | Retrospective cohort | Austria | Europe | 7 tertiary care hospitals | 73 | 61 | 5 | |||
| Sarfo, 2020 | January to June 2020 | January to June 2019 | Retrospective cohort | Ghana | Africa | Komfo Anokye Hospital | Pandemic (57.1); Control (53.4) | Pandemic (60.6); Control (59.7) | 7 | ||
| Teo, 2020 | 23 Jan to 24 March 2020 | 23 Jan to 24 March 2019 | Retrospective cohort | Hong Kong | Asia | Queen Mary Hospital | Pandemic (43.8); Control (50.6) | Pandemic (70.1); Control (73.6) | 6 | ||
| Toner, 2020 | 16 March to 15 April 2020 | 16 March to 15 April 2014−2019 | Prospective cohort | Australia | Oceania | Tertiary hospital | Pandemic (65.0); Control (71.6) | Pandemic (68.1); Control (65.0) | 7 | ||
| Abdelaziz, 2020 | 1−31 March 2020 | 1−31 March 2019 | Retrospective cohort | UK | Europe | Tertiary cardiac center | Pandemic (69.6); Control (76.8) | Pandemic (63.2); Control (66.6) | 6 | ||
| Agarwal, 2020 | 01 June 2019 to 15 May 2020 | 1 March 2020 to 15 May 2020 | 1 June 2019 to 29 Feb 2020 | Retrospective cohort | USA | North America | Langone Health Stroke Center | Pandemic (49.2); Pre-pandemic (54.7) | Pandemic (68); Pre-pandemic (72) | 6 | |
| Burgos, 2020 | Feb to March 2020 | Feb to March 2019 | Retrospective cohort | Argentina | South America | Cardiology center | 6 | ||||
| Aldujeli, 2020 | 11 March to 20 April 2020 | 11 March to 20 April 2019 | Retrospective cohort | Lithuania | Europe | Lithuanian University of Health Sciences Kaunas Clinics | NSTEMI: Pandemic (73); Control (60)/STEMI: Pandemic (72); Control (65) | NSTEMI: Pandemic (70); Control (69.5)/STEMI: Pandemic (67); Control (68.5) | 6 | ||
| Andersson, 2020 | 1 Jan to 11 March 2020/12–31 Mar 2020 | 1 Jan to 11 March 2019/12–31 Mar 2019 | Retrospective cohort | Denmark | Europe | Danish Nationwide Patient Registry | Pandemic: New-onset HF (61−62); Worsening HF (69−72)/Control: New-onset HF (62); Worsening HF (66−67) | Pandemic: New-onset HF (73.3−74.8); Worsening HF (74.0−75.2)/Control: New-onset HF (73.4−74.3); Worsening HF (75.2−75.3) | 7 | ||
| Ball, 2020 | 28 Oct 2019 to 10 May 2020 | 28 Oct 2019 to 10 May 2018−2019 | Cross-sectional study | UK | Europe | 9 Hospitals | 6 | ||||
| Boeddinghaus, 2020 | March to April 2020 | Jan to Feb 2020 | March to April 2019 | Retrospective cohort | Switzerland | Europe | Tertiary University Hospital | Pandemic (77.5); Pre-pandemic (76.8) | Pandemic (66); Pre-pandemic (68) | 6 | |
| Bromage, 2020 | 2 March to 19 April 2020 | 2 March to 19 April 2017−2019 | Retrospective cohort | UK | Europe | King’s College Hospital | Pandemic (54); Control (58) | Pandemic (73); Control (71) | 6 | ||
| Bryndza, 2021 | March to April 2020 | March to April 2020 | Retrospective cohort | Poland | Europe | Cardiology center | 6 | ||||
| Cammalleri, 2020 | 1−31 March 2020 | Mar-19 | Retrospective cohort | Italy | Europe | Cardiology Department | Pandemic (85); Control (87) | Pandemic (65); Control (62) | 6 | ||
| Candelaresi, 2021 | 9 March to 12 April 2020 | 2 Feb to 8 March 2020 | Same period in 2019 | Retrospective cohort | Italy | Europe | 5 Campania stroke hubs | 6 | |||
| Chew, 2021 | 7 Feb to 31 March 2020 | 1 October 2019 to 6 Feb 2020 | Retrospective cohort | Singapore | Asia | National University Hospital Singapore | Pandemic (56.8); Pre-pandemic (64.4) | Pandemic (59); Pre-pandemic (57) | 7 | ||
| Choudhary, 2020 | 25 March to 24 April 2020 | 25 Feb to 24 March 2020 | 25 Jan to 24 Feb 2020 | Retrospective cohort | India | Asia | 4 Tertiary regional Eds | 6 | |||
| Çinier, 2020 | 5 March to 6 April 2020 | 5 March to 6 April 2020 | Retrospective cohort | Turkey | Europe | Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital | Pandemic (81.1); Control (85.6) | Pandemic (59.3); Control (63.7) | 7 | ||
| Colivicchi, 2020 | 20 Feb to 20 April 2020 | 20 Feb to 20 April 2019 | Retrospective cohort | Italy | Europe | San Filippo Neri Hospital | Pandemic (79); Control (57) | Pandemic (78); Control (73) | 7 | ||
| Cummings, 2020 | March to April 2020 | March 2019 to Feb 2020 | Retrospective cohort | USA | North America | Telestroke registry at Medical University of South Carolina | Pandemic (44); Control (46.9) | Pandemic (69); Control (67) | 6 | ||
| Del Pinto, 2020 | 1 January to 31 March 2020 | 1 January to 31 March 2019 | Retrospective cohort | Italy | Europe | 5 Hospitals in L'Aquila | 6 | ||||
| Enache, 2020 | First 3 months of 2020 | First 3 months of 2019 | Retrospective cohort | Monaco | Europe | Cardiology Departments | 6 | ||||
| Erol, 2020 | 17 April to 2 May 2020 | 1−15 November 2018 | Prospective cohort | Turkey | Europe | Registries | Pandemic (76.2); Control (73.7) | Pandemic (60); Control (62) | 8 | ||
| Frisullo, 2020 | 11 March to 11 April 2020 | 11 March to 11 April 2019 | Retrospective cohort | Italy | Europe | ED of Policlinico A. Gemelli Hospital | Pandemic (59.6); Control (46.3) | Pandemic (71.6); Control (73.7) | 6 | ||
| Giannouchos, 2021 | 1 Jan to 31 Aug 2020 | 1 Jan to 31 Aug 2019 | Cross-sectional study | USA | North America | University of Utah Healthcare Systems | 6 | ||||
| Hoyer, 2020 | Weeks 1−15 2020 | Weeks 1−15 2019 | Retrospective cohort | Germany | Europe | 4 stroke centers | 6 | ||||
| Hsiao, 2020 | Weeks 11−15 2020 | Weeks 1−10 2020 | mid-March to mid-April 2019 | Retrospective cohort | USA | North America | 30 Healthcare facilities | 6 | |||
| JF Huang, 2020 | 11 March to 9 April 2020 | 10 Feb to 10 March 2020 | Cross-sectional study | USA | North America | Mayo Clinic telestroke network | 6 | ||||
| Ikenberg, 2020 | 1 Jan to 19 April 2020 | 21 March to 19 April 2020 | 1 Jan to 20 March 2020 | Retrospective cohort | Germany | Europe | Bavarian Comprehensive Stroke Center | 6 | |||
| Jasne, 2020 | 1 Jan to 28 April 2020 | 1 Jan to 28 April 2019 | Cross-sectional study | USA | North America | 3 Connecticut hospitals | 49.7 | 70 | 6 | ||
| John, 2020 | 1 March to 10 May 2020 | 1 March to 10 May 2019 | Retrospective cohort | UAE | Asia | Cleveland Clinic Abu Dhabi | Pandemic - Ischemic stroke (72.3); hemorrhagic stroke (66.7); Control - Ischemic stroke (67.9); hemorrhagic stroke (80.8) | Pandemic - Ischemic stroke (57.5); hemorrhagic stroke (48.9); Control - Ischemic stroke (58.4); hemorrhagic stroke (49.3) | 6 | ||
| Kobo, 2020 | 20 March to 30 April 2020 | 20 March to 30 April 2019 | Prospective cohort | Israel | Asia | 4 Cardiac centers | Pandemic (84.1); Control (81.5) | Pandemic (63); Control (61) | 8 | ||
| Kuitunen, 2020 | 1 February to 30 April 2020 | 6 weeks after 16 March 2020 | 6 weeks before 16 March 2020 | Corresponding period in 2019 | Retrospective cohort | Finland | Europe | 3 Emergency Departments | 6 | ||
| Lauridsen, 2020 | 12 March to 13 May 2020 | 12 March to 13 May 2015−2019 | Retrospective cohort | Denmark | Europe | Danish Civil Population Registry | Pandemic (71); Control (75) | Pandemic (69); Control (70) | 7 | ||
| Little, 2020 | 1 March to 30 April 2020 | 1 March to 30 April 2019 | Retrospective cohort | UK | Europe | 7 Heart Attack Centres | Pandemic (80); Control (78) | Pandemic (63); Control (63) | 6 | ||
| Nagamine, 2020 | March to April 2020 | March to April 2019 | Retrospective cohort | USA | North America | Stroke center | Pandemic (75); Control (61) | Pandemic (65.3); Control (69) | 6 | ||
| Mitra, 2020 | 26 March to 23 April 2020 | 26 March to 23 April 2019 | Cross-sectional study | Australia | Oceania | Tertiary cardiology and neurosciences centre | Pandemic (67.3); Control (84.2) | Pandemic (71.1); Control (74.7) | 6 | ||
| Nguyen-Huynh, 2020 | 15 March to 9 May 2020 | 1 Jan 2019 to 14 March 2020 | Retrospective cohort | USA | North America | Kaiser Permanente Northern California | Pandemic (47.9); Pre-pandemic (47.1) | Pandemic (69); Pre-pandemic (68.8) | 7 | ||
| Oseran, 2020 | 1 March to 30 April 2020 | 1 Jan to 29 Feb 2020 | 1 March to 30 April 2019/1 Jan to 28 Feb 2019 | Cross-sectional study | USA | North America | 8 Acute care hospitals | 6 | |||
| Paliwal, 2020 | November 2019 to April 2020 | 7 Feb to 30 April 2020 | 1 Nov 2019 to 7 Feb 2020 | Retrospective cohort | Singapore | Asia | Stroke center | Pandemic (57); Pre-pandemic (20) | Pandemic (64.6); Pre-pandemic (65.6) | 6 | |
| Papafaklis, 2020 | 2 March to 12 April 2020 | 2 March to 12 April 2019 | Retrospective cohort | Greece | Europe | Public hospitals | Pandemic (79.1); Control (76.2) | Pandemic (64.3); Control (64) | 6 | ||
| Piuhola, 2020 | Mar-20 | Jan to Feb 2020 | January to Feb 2017−2019 | Retrospective cohort | Finland | Europe | 5 Tertiary centers | 6 | |||
| Rashid Hons, 2020 | 1 Feb to 14 May 2020 | 1 Feb to 14 May 2019 | Retrospective cohort | UK | Europe | Myocardial Ischaemia National Audit Project/British Cardiovascular Intervention Society | Pandemic (71.2); Control (79.5) | Pandemic (67.1); Control (63.1) | 7 | ||
| Richter, 2021 | 16 March to 15 May 2020 | 16 Jan to 15 March 2020 | 16 March to 15 May 2019 | Retrospective cohort | Germany | Europe | 1463 Hospitals | 6 | |||
| Rodríguez-Leor, 2020 | 16 March to 14 April 2020 | 1−30 April 2019 | Retrospective cohort | Spain | Europe | 75 STEMI care centers | Pandemic (78.4); Control (78.4) | Pandemic (63.1); Control (63.7) | 7 | ||
| Ruparelia, 2020 | After 20 March 2020 | Jan to Feb 2020 | Jan to March 2019 | Retrospective cohort | UK | Europe | 2 Large Hospitals | Pandemic (65.3); Control (68.9) | Pandemic (75.4); Control (73.2) | 6 | |
| Schirmer, 2020 | Feb to March 2020 | Feb to March 2019 | Retrospective cohort | USA | North America | 12 Stroke centers | Pandemic (67); Control (70) | 6 | |||
| Seiffert, 2020 | 1 Jan 2019 to 31 May 2020 | Jan to May 2020 | Jan to May 2019 | Retrospective cohort | Germany | Europe | Insurance Claims Data | 6 | |||
| Sharma, 2020 | 30 Dec 2019 to 19 April 2020 | 31 Dec 2018 to 21 April 2019 | Retrospective cohort | USA | North America | 5 Tertiary stroke centers | 6 | ||||
| Siegler, 2020 | 1 March 2020 to 15 April 2020 | 1 October 2019 to 29 Feb 2020 | Retrospective cohort | USA | North America | Stroke center | Pandemic (57); Control (59) | Pandemic (68); Control (68) | 7 | ||
| Tejada Meza, 2020 | 30 Dec 2019 to 3 May 2020 | After 14 March 2020 | Before 14 March 2020 | Retrospective cohort | Spain | Europe | Tertiary hospitals of the NORDICTUS network | 6 | |||
| Uchino, 2020 | 9 March to 2 April 2020 | 1 Jan to 8 March 2020 | Retrospective cohort | USA | North America | 19 Emergency departments | 6 | ||||
| Vensentini, 2020 | March to April 2020 | March to April 2010−2019 | Prospective cohort | Argentina | South America | 6 Cardiovascular Intensive Care Units | 7 | ||||
| Wadhera, 2021 | 18 March to June 2020 | 1 Jan to 17 March 2020 | Jan to June 2019 | Retrospective cohort | USA | North America | National Center for Health Statistics | 6 | |||
| J Wang, 2020 | 12 March to 30 June 2020 | 1 Dec 2019 to 11 Mar 2020 | Retrospective cohort | USA | North America | Inova Fairfax Medical Campus | Pandemic (53.3); Pre-pandemic (51.9) | Pandemic (73); Pre-pandemic (70) | 6 | ||
| Yang, 2020 | 23 Jan to 7 March 2020 | 1 December 2019 to 14 Jan 2020 | Retrospective cohort | China | Asia | Stroke center | Pandemic (71.4); Pre-pandemic (64.7) | Pandemic (62.3); Pre-pandemic (65.2) | 6 | ||
| Zhao, 2020 | Feb-20 | Feb-19 | Retrospective cohort | China | Asia | Big Data Observatory Platform for Stroke of China & 280 stroke centers | 6 | ||||
| Cox, 2020 | 22 March to 20 April 2020 | 22 March to 20 April 2019 | Retrospective cohort | USA | North America | Vanderbilt University Medical Center | 6 |
3.3. Cardiovascular diseases
Table 2 provides details of various cardiovascular diseases that were assessed by eligible studies and specific outcomes reported. The majority of studies (n = 65) assessed hospitalisations for ACS (STEMI, NSTEMI or both) only, ACS patients undergoing percutaneous coronary intervention (PCI) or a combination of ACS and other cardiovascular conditions such as HF, strokes. Other outcomes assessed were stroke hospitalisations (n = 30); acute HF (n = 5); cardiometabolic conditions such as hypertension, diabetes, VTE and arrhythmias (n = 2) and CVD deaths (n = 1).
Table 2.
Trends in hospitalisations of cardiometabolic conditions and severity at presentation.
| Author, year of publication | Cardiometabolic condition | Historical control/pre-COVID-19 hospitalisations | Pre-pandemic/pre-lockdown hospitalisations | Pandemic/lockdown hospitalisations | Trends in hospitalisations for cardiometabolic outcomes | Severity of presentation |
|---|---|---|---|---|---|---|
| Araiza-Garaygordobil, 2021 | ACS admissions | 8750 | 5923 | 1444 | Compared to the pre-pandemic period, a significant overall trend for reduction of 20.2% in the weekly number of ACS hospitalizations was observed during the pandemic period. There were also reductions when compared to the historical period. | |
| Mafham, 2020 | ACS admissions | Monthly average for 2019 (13,075); Jan 2020 (13,645); Feb 2020 (12,443) | Mar 2020 (10,118); Apr 2020 (8739); May 2020 (9756) | From mid-February 2020, hospital admissions fell from a 2019 baseline rate of 3017 admissions per week to 1813 per week by the end of March, 2020 (reduction of 40%). This decline was partly reversed during April and May, 2020, such that by the last week of May, 2020, there were 2522 admissions, representing a 16% reduction from baseline. | ||
| Perrin, 2020 | Undergoing PCI for ACS | 140 | 45 | The incidence rate of ACS was lower during the COVID-19 period than the control period (0.7 vs 1.1 per 1000 person-years, p < 0.01). There were significantly more patients presenting with out-of-hospital cardiac arrest during the COVID-19 period compared with the control period (22.2% vs 7.1%, p < 0.01). | ACS patients presented higher cardiac enzymes during the COVID-19 period compared with the control period. | |
| Tam, 2020 | MI admissions | 85 | 64 | There was a reduction in daily emergency room attendance since January 25, 2020 (231 per day compared to 327 per day before the pandemic) | ||
| Toniolo, 2020 | Severe emergent CVD admissions | 71 | 34 | A decrease was observed in all SECDs hospital admissions comparing the pandemic to the control period: 27 versus 19 for STE-ACS (−30%); 44 versus 15 for non-STE-ACS (−66%) and 46 versus 23 for atrioventricular-block/acute sinus node dysfunction (−50%). | ||
| Huet, 2020 | Acute myocardial infarction or acute heart failure admissions | Before containment, the nine participating intensive cardiac care units admitted 4.8 ± 1.6 patients per day, versus 2.6 ± 1.5 after containment. | ||||
| Kwok, 2020b | PCI procedures for STEMI | 33,255 | 683 | A 43% decline in monthly average procedures was recorded between 2017 and 2019 (865) to 497 in April 2020 | ||
| Boukhris, 2020 | Volume of ACS and ischemic strokes | ACS (2398); ischemic stroke (4027) | ACS (2215); ischemic stroke (3905) | ACS volume tended to increase in January and February 2020 in comparison to the same period in 2019. In March and April 2020, STEMI and NSTEMI decreased in comparison with March and April 2019. There was a gradual decrease in stroke cases from January to March 2020 compared to 2019, followed by an increase in April 2020. | ||
| Braiteh, 2020 | ACS admissions | 113 | 67 | Drop by 40.7% in total ACS cases during the pandemic in comparison to 2019 | ||
| Butt, 2020 | ACS, other CVDs, stroke | ACS (171); other CVDs (116); stroke (109) | March 2020 - ACS (114); other CVDs (64); stroke (83) | Compared to March 2019, there was a decrease in ACS, other CVDs, and stroke by 50%, 81.3% and 31.3% respectively, in March 2020 | ||
| De Filippo, 2020 | ACS admissions | ACS (756) | ACS (899) | ACS (547) | 13.3 ACS admissions/day during the pandemic period compared to 18.0 for the pre-pandemic period and 18.9 for the historical period. The corresponding values for STEMI were 6.1, 7.8 and 8.0. That for NSTEMI were 4.2, 7.1 and 7.5. Unstable angina was 3.1, 3.1 and 3.4. | |
| De Rosa, 2020 | AMI admissions | AMI (618); STEMI (268); NSTEMI (350) | AMI (319); STEMI (197); NSTEMI (122) | 48.4% reduction in AMI admissions during the pandemic period compared to 2019. Reductions were significant for both STEMI and NSTEMI. The reductions for STEMIs were higher for women compared to men. Reductions in admissions for HF, AF and PE during the pandemic compared to 2019. | ||
| Fileti, 2020 | ACS admissions | ACS (94); STEMI (36); NSTEMI (58) | ACS (72); STEMI (34); NSTEMI (38) | 23.4% reduction in ACS admissions during 2020 compared to 2019, with a decrease for both STEMI and NSTEMI | ||
| Folino, 2020 | Access to coronary care unit for ACS | NSTEMI in first 8 weeks of 2019 (260) and 221 in the following 5 weeks. Corresponding values for STEMI were 22 and 21 | After the eighth week of 2020, there was a significant reduction in access to CCU for NSTEMI compared to the same period of the previous year, but not for STEMI. | |||
| Haddad, 2020 | STEMI admissions | 60 | 54 | 53 | Number of STEMI admissions were unaffected during the pandemic period | |
| Hauguel-Moreau, 2020 | ACS admissions | In 2020, there were two distinct phases in ACS admissions - a first significant fall, with a relative reduction of 73%, from the week of lockdown (week 12) to 3 weeks later followed by an increase | ||||
| Holy, 2020 | ACS and OHCA referrals | Four weeks after March 16th 2020 ACS referrals decreased by 42% (NSTEMI: –49%, STEMI: –56%, unstable angina: +37%) while OHCA referrals declined by 57% | ||||
| Metzler, 2020 | ACS admissions | Comparing the first and last calendar week of the period surveyed, there was a relative reduction of 39.4% in admissions for ACS. STEMI admissions reduced from 94 to 70 and NSTEMI from 132 to 67 | ||||
| Montagnon, 2021 | Admissions for ACS and strokes | ACS (12); Stroke or TIA (27) | ACS (7); Stroke or TIA (30) | There were five fewer cases of ACS in 2020, a reduction of 41.7% compared with 2019. In 2020, an increase was observed in the number of strokes and TIAs, with 27 cases in 2019 as opposed to 30 in 2020. | ||
| Showkathali, 2020 | ACS admissions | 183 | 104 | During the study period in 2020, 104 patients were admitted with ACS, which is a 43% decline in admissions compared to the same time period in the previous 2 years (183). The decline in STEMI, NSTEMI and unstable angina admissions were 47%, 33%, and 54% respectively | ||
| Sokolski, 2020 | Cardiovascular admissions | 4452 | 3007 | In 2020, there were fewer admissions for ACS, acute HF, arrhythmia, and others. There was a relatively higher percentage of pulmonary embolism admissions in 2020 | ||
| Solomon, 2020 | AMI admissions | The weekly rates of hospitalization for AMI decreased by up to 48% during the Covid-19 period | ||||
| Vacanti, 2020 | ACS admissions | 2018 (326); 2019 (353) | 270 | Decline in ACS admissions in 2020 compared to 2019 and 2018, representing a decline of 24% and 19%, respectively. | ||
| Yalamanchi, 2020 | ACS admissions | 2018 (307); 2019 (322) | 216 | Decline in ACS admissions in 2020 compared to 2019 and 2018, representing a decline of 33% and 30%, respectively.There was a decline in admissions for acute decompensated heart failure, arrhythmia, and other diagnoses in 2020, which were 38%, 62%, and 59%, respectively; while there was a 50% increase in acute pulmonary embolism admission compared to the mean admission in 2018 and 2019 | ||
| Tsioufis, 2020 | AMI admissions | The number of AMI cases in March 2020 was the lowest compared to the entire three year period. Similar significant findings were observed for STEMI and NSTEMI. Cases of HF and CAD were also lower in 2020 compared to the preceeding periods | ||||
| Gasior, 2020 | AMI admissions | The number of admissions for AMI dropped on average by 43.6% | ||||
| Dreger, 2020 | AMI admissions | 2017 (255); 2018 (250); 2019 (257) | 207 | There was a reduction in AMI admissions in 2020 compared with the same time period in the three previous years. | ||
| Anderson, 2020 | Admissions for cardiometabolic conditions | Overall decrease in cardiometabolic conditions (CVD, strokes, VTE, HF and diabetes) | ||||
| Gluckman, 2020 | AMI admissions | 13,329 | Early COVID-19 period (860) | Later COVID-19 period (1055) | Beginning February 23, 2020, AMI-associated hospitalizations decreased for 5 weeks (early COVID-19 period). Thereafter, AMI-associated hospitalizations increased during the later COVID-19 period | |
| Mohammad, 2020 | MI cases referred for coronary angiography | 15,213 | 2443 | The incidence of MI referred for invasive treatment was reduced during the COVID-19 pandemic | ||
| Piccolo, 2020 | PCI procedures for ACS | 178 cases/100,000 residents per year | 120/100,000 residents per year | During the 8 week period, there was a decline by 32% in the number of PCIs for ACS. In the last 2 weeks of the observational period, PCIs for ACS were reduced by 50%. The reduuction was similar for STEMI and NSTEMI | ||
| Secco, 2020 | ACS admissions | 162 | 84 | Hospitalization for ACS decreased from 162 patients in 2019 to 84 patients in 2020 | ||
| Ayad, 2021 | STEMI patients requiring PCI | 364 | 270 | During the COVID-19 period, the number of PCI procedures was reduced by 25.7% compared with previous year | ||
| Bhatt, 2020 | Acute cardiovascular conditions | 404 | 231 | There were 43.4% fewer estimated daily hospitalizations in March 2020 compared with March 2019 | ||
| Daoulah, 2021 | STEMI admissions | 2018 (650); 2019 (635) | 500 | STEMI volumes were reduced by 28% during the pandemic period | ||
| Desai, 2020 | Stroke and TIA admissions | Strokes - 2017 (163); 2018 (161); 2019 (159)/TIA – 2017 (11); 2018 (18); 2019 (16) | Stroke (96); TIA (6) | Number of acute ischemic strokes and TIAs decreased by 40% and 60% respectively, from March 2017−2019 to March 2020 | ||
| Diegoli, 2020 | Stroke admissions | 12.9 cases/100,000 | 8.3 cases/100,000 | When compared with the same period in 2019, there was a 36.4% reduction in stroke admissions in 2020 | No differences in admissions for stroke severity | |
| Gitt, 2020 | ACS admissions | STEMI (49); NSTEMI (95); UA (94) | STEMI (46); NSTEMI (50); UA (48) | During the pandemic, there was a 50% reduction in both unstable angina and NSTEMI | ||
| Hammad, 2020 | STEMI admissions | 108 | 35 | Lower STEMI admissions during the pandemic period. | Post-COVID-19 presentation was severe compared to pre-COVID-19 | |
| Kerleroux, 2020 | Stroke patients receiving mechanical thrombectomy | 844 | 668 | There was a 21% significant decrease in MT case volumes during the pandemic period | ||
| Montaner, 2020 | Stroke admissions | 25% reduction in stroke admissions during the pandemic period. 40% reductions in TIAs attending the emergency department | ||||
| Neves Briard, 2020 | Stroke admissions | 138 | 156 | The first two months of the COVID-19 pandemic were not associated with a decrease in acute stroke evaluations | ||
| Pop, 2020 | Stroke admissions | 167 | 122 | Compared to the same period in 2019, there were 39.6% fewer stroke alerts in 2020 | ||
| Popovic, 2020 | STEMI patients undergoing PCI | 1552 | 83 | |||
| Range, 2020 | STEMI patients undergoing PCI | 1942 | 122 | There was a significant drop (12%) in mean number of STEMI/month in the lockdown group compared with prelockdown (139 vs 122, p < 0.04). | ||
| Reinstadler, 2020 | STEMI patients referred for PCI | 163 | Rates of STEMI admissions decreased (calendar week 9/10 (n = 69, 42%); calendar week 11/12 (n = 51, 31%); calendar week 13/14 (n = 43, 26%) | |||
| Sarfo, 2020 | Stroke admissions | 401 | 431 | Stroke admissions were higher during the pandemic period (increase of 7.5%). Recurrent stroke admissions were also higher during the pandemic period | ||
| Teo, 2020 | Stroke admissions | 89 | 73 | Fewer stroke admissions in pandemic period | No differences in stroke severity | |
| Toner, 2020 | ACS undergoing PCI | 102 | 20 | The case volume for the number of ACS patients undergoing PCI was not significantly different in the COVID and non-COVID eras | ||
| Abdelaziz, 2020 | STEMI patients undergoing PCI | 69 | 46 | Fewer STEMI admissions in the pandemic period | Higher cardiac troponin-I levels on admission in STEMI patients during pandemic than pre-COVID era | |
| Agarwal, 2020 | Acute ischemic stroke care | 634 | 120 | Fewer admissions during pandemic compared to pre-pandemic | Pandemic patients presented with a higher median admission NIHSS scores. | |
| Burgos, 2020 | HF admissions | 49 | 36 | 26.5% decrease in number of HF admissions during the pandemic period | ||
| Aldujeli, 2020 | AMI admissions | NSTEMI (62); STEMI (60) | NSTEMI (30); STEMI (47) | Fewer admissions during pandemic compared to pre-pandemic | ||
| Andersson, 2020 | New-onset and worsening HF admissions | New-onset HF (2819); Worsening HF (1419) | New-onset HF (2595); Worsening HF (1364) | In the lockdown period, rates of new-onset HF diagnoses and of hospitalizations for worsening HF were significantly lower in 2020 versus 2019 | ||
| Ball, 2020 | CVDs admissions | Activity for cardiac, cerebrovascular and other vascular conditions started to decline 1–2 weeks before lockdown and fell by 31%–88% after lockdown, with the greatest reductions observed for coronary artery bypass grafts, carotid endarterectomy, aortic aneurysm repair and peripheral arterial disease procedures, compared with the previous year. | ||||
| Boeddinghaus, 2020 | ACS admissions | 220 | 178 | Compared to January/February 2020, there was a dramatic reduction of ED presentations after the COVID-19 outbreak on March 1st (31% relative reduction). Comparing March/April 2020 to that of 2019, there was a 38.7% reduction in ED presentations | ||
| Bromage, 2020 | HF admissions | 78 | 26 | Significantly lower admission rate for HF was observed during the study covid-19 pandemic compared to all other included time periods. | Patients admitted during the COVID-19 pandemic had higher rates of NYHA III or IV symptoms and severe peripheral oedema | |
| Bryndza, 2021 | AMI admissions | 1055 | 827 | In comparison to the control period, there was a 21.6% decrease in the total number of AMI cases (a 18.6% decrease in the number of patients with STEMI and a 23.9% decrease in the number of patients with NSTEMI). | ||
| Cammalleri, 2020 | STEMI patients undergoing PCI | 35 | 13 | During March 2020, there was a 63% reduction of patients with STEMI admitted for PCI, when compared with the same period of 2019 | Patients in 2020 had higher levels of cardiac biomarkers and a worse left ventricular ejection fraction at baseline | |
| Candelaresi, 2021 | Stroke admissions | The global number of patients presenting with acute stroke did not significantly differ between the periods | Baseline NIHSS score was significantly more severe during the lockdown compared to the same period of 2019 and tended to be more severe compared to the immediate prelockdown phase | |||
| Chew, 2021 | STEMI patients undergoing PCI | 208 | 95 | Fewer admissions during pandemic compared to pre-pandemic | ||
| Choudhary, 2020 | Cardiovascular emergencies (ACS, acute decompensated HF and high degree AV block) | 1488 | 830 | 289 | Fewer emergency cardiovascular admissions during the lockdown period than the pre-lockdown and pre-COVID periods. | Risk factors associated with poorer prognosis in ACS were higher in patients during the lockdown and pre-lockdown period compared to pre-COVID period. |
| Çinier, 2020 | STEMI patients undergoing PCI | 174 | 90 | Significant reduction in STEMI cases during COVID-19 pandemic compared to previous year | ||
| Colivicchi, 2020 | Acute HF admissions | 6060 | 2711 | The number of patients with acute HF decreased by 49% during the pandemic period | Risk factors associated with poorer prognosis were higher in patients during the pandemic compared to the pre-COVID period | |
| Cummings, 2020 | Tele stroke consulations | 5239 | 613 | Fewer stroke patients were seen during the pandemic. The median number of weekly consults dropped from 112 to 77 during the pandemic. Black patients were less likely to present with strokes during the pandemic | ||
| Del Pinto, 2020 | CVDs admissions | Less cardiovascular hospitalizations occurred in 2020 than in 2019 | ||||
| Enache, 2020 | CVDs admissions | 419 | 346 | Compared to March 2019, the total cardiovascular admissions were lower by 17%. Similarly, compared to March 2019, cardiovascular emergency admissions were down by 21%. | ||
| Erol, 2020 | AMI admissions | 1872 | 991 | There was a 47.1% decrease in acute MI admissions during the pandemic. This reduction in admission was more prominent in patients with NSTEMI compared with STEMI | ||
| Frisullo, 2020 | Ischemic stroke admissions | 41 | 52 | No significant difference observed between 2019 and 2020 in number of admissions | ||
| Giannouchos, 2021 | ED visits for medical conditions (included hypertension and diabetes) | Hypertension (461); diabetes (300) | Hypertension (251); diabetes (209) | Decrease in ED visits for both hypertension and diabetes | ||
| Hoyer, 2020 | Ischemic stroke admissions | A significant decrease in the number of admissions for transient ischemic attack was observed in 3 of 4 centers during the pandemic | ||||
| Hsiao, 2020 | Stroke consultations | Compared with the 10 weeks prior, stroke consultations declined by 39% in the 5 weeks after announcement of COVID-19 mitigation measures. Results compared with the prior year and time trend analyses were consistent | ||||
| JF Huang, 2020 | Tele stroke activations | 142 | 71 | There was a 50% reduction in stroke volume activations during the post-pandemic declaration period | ||
| Ikenberg, 2020 | Stroke referrals | 171 | 70 | The absolute daily number of Code Stroke referrals and the portion of patients with stroke mimics remained stable. The portion of female stroke patients decreased (55% to 33%; p = 0.03) during the lockdown. | Stroke severity as measured by the NIHSS increased during the lockdown. | |
| Jasne, 2020 | Stroke code activations | 786 | 756 | There was a significant decline in weekly stroke code volumes at the 3 hospitals from January to April, 2020. 30% decrease in total stroke codes during the pandemic weeks in 2020 versus 2019 | There was no difference in stroke severity | |
| John, 2020 | Stroke admissions | 148 | 210 | There was a 41.9% increase in stroke admissions in 2020. The difference in 2020 was driven by significant increases in ischemic stroke, intracerebral hemorrhage and stroke mimics. | Ischemic stroke: Severity of stroke presentation was higher in 2020 as recorded by the NIHSS. | |
| Kobo, 2020 | STEMI patients undergoing PCI | 136 | 107 | There was a 22% decrease in STEMI admissions | Patients admitted in 2020 had higher admission and peak troponin levels | |
| Kuitunen, 2020 | ED visits and inpatient admissions for medical conditions (included AMI, strokes and other heart disease) | Stroke (553); AMI (650); other heart disease (1837) | Stroke (558); AMI (645); other heart disease (1513) | The visit rate and inpatient admissions due to AMI and strokes remained stable throughout the study period. | ||
| Lauridsen, 2020 | AMI admissions | AMI (11,769); AMI-CS (342) | AMI (2132); AMI-CS (60) | The total number of MI patients decreased by 15% during lockdown comparing the average number of MI admissions in 2015–2019 with the number of MIs in 2020 | The incidence proportions of AMI-related cardiogenic shock were similar during lockdown comparing 2015–2019 and 2020 | |
| Little, 2020 | STEMI admissions | 440 | 348 | There was a 21% reduction in STEMI admissions in 2020 vs in 2019 | ||
| Nagamine, 2020 | Ischemic stroke admissions | 68 | 48 | There was reduction in stroke admissions in 2020 compared to 2019 | ||
| Mitra, 2020 | Acute stroke and AMI admissions | 57 | 52 | There was a 9.6% reduction in stroke and AMI admissions in 2020 compared to 2020 | ||
| Nguyen-Huynh, 2020 | Acute stroke presentations | 8337 | 783 | Stroke volumes decreased significantly post lockdown compared with pre-lockdown | Post-lockdown patients had higher NIHSS scores, lower comorbidity score, and arrived more often by ambulance. Post-lockdown patients also had large vessel occlusions. | |
| Oseran, 2020 | Cardiovascular admissions | During the pandemic period, there was a decrease in admission rates for all conditions including cardiovascular conditions | ||||
| Paliwal, 2020 | Stroke admissions | 206 | 144 | Decline in stroke activations | In terms of stroke severity, the median NIHSS on arrival was similar | |
| Papafaklis, 2020 | ACS admissions | 1077 | 771 | ACS admissions in the COVID-19 period were reduced by 28.4% compared to 2019 | During the COVID-19 period, patients admitted with ACS presented more frequently with left ventricular systolic impairment | |
| Piuhola, 2020 | STEMI admissions | During 2017–2019, there were no marked differences in STEMI incidence between January, February and March. During 2020, there was an average drop of 32% in STEMI incidence in March. | ||||
| Rashid Hons, 2020 | AMI admissions with OHCA | 731 | 524 | AMI hospitalizations during COVID-19 period were reduced by >50% | ||
| Richter, 2021 | Stroke admissions | Decline in hospitalizations during the pandemic compared to the pre-pandemic period | ||||
| Rodríguez-Leor, 2020 | STEMI admissions | 1305 | 1009 | Suspected STEMI patients treated in STEMI networks decreased by 27.6% with a reduction in confirmed STEMI cases by 22.7% during the pandemic | ||
| Ruparelia, 2020 | ACS admissions | 376 | 280 | There was a significant reduction in the entire spectrum of ACSs following the beginning of the COVID-19 pandemic | ||
| Schirmer, 2020 | Stroke admissions | 320 | 163 | In the COVID period in 2020, there was a drop in the absolute number of cases per calendar week | There was no difference in the severity of the presentation between groups | |
| Seiffert, 2020 | Admissions for AMI, acute limb ischemia, aortic rupture, stroke or TIA | 78.6/100,000 | 70.6/100,000 | Monthly admission rates declined from pre-COVID to COVID periods. The lowest admission rate was observed in April 2020 | ||
| Sharma, 2020 | Stroke and TIA admissions | There was a decline in stroke/TIA admissions and ED stroke alerts during 30 December 2019 to 19 April 2020. The greatest decline in hospital admissions was observed between 23 March and 19 April 2020 | Baseline NIHSS score was higher in the pandemic period | |||
| Siegler, 2020 | Stroke admissions | 275 | 53 | There was a mean fall of 38% in new stroke diagnoses | No difference with respect to severity of stroke | |
| Tejada Meza, 2020 | Stroke admissions | 173/week | 124/week | There was a decrease in the weekly mean admitted patients during the pandemic | ||
| Uchino, 2020 | Stroke presentations | 10 alerts/day | 8 alerts/day | There was a significant decrease in acute stroke presentations by 30% across emergency departments during the COVID-19 period | Stroke severity measured by NIHSS was unchanged. | |
| Vensentini, 2020 | CVDs admissions | Average (595) | Average (348) | The average number of CVD admissions decreased by 46.8% during the COVID-19 period. Reductions in cardiovascular surgery 72.3%, electrophysiological interventions 67.8%, NSTEMI 52.6%, angioplasties 47.6%, arrhythmias 48.7%, heart failure 46%, atrial fibrillation 35.7%, STEMI 34.7%, non cardiac chest pain 31.8% and others 51.6% during the COVID-19 period. Hypertensive crisis increased by 89% | ||
| Wadhera, 2021 | CVD deaths | 199,311 | 197,731 | Deaths caused by ischemic heart disease and hypertensive disease increased nationally after the onset of the pandemic in 2020, compared with changes over the same period in 2019, but not for heart failure, cerebrovascular disease, or other diseases of the circulatory system. | ||
| J Wang, 2020 | Acute ischemic stroke admissions | 320 | 255 | There was a 22.1% and 39.5% decline in admission for acute ischemic stroke in April and May 2020, respectively. | Stroke severity at presentation measured by NIHSS was unchanged. | |
| Yang, 2020 | Acute stroke patients undergoing endovascular thrombectomy | 34 | 21 | Decline in acute stroke patients undergoing endovascular thrombectomy during the pandemic era | Stroke severity at presentation measured by NIHSS was unchanged. | |
| Zhao, 2020 | Stroke care | Hospital admissions related to stroke dropped by 40% | ||||
| Cox, 2020 | Acute HF admissions | 62% decrease in HF admissions during the pandemic period relative to last year | Severity at admission was unchanged |
3.4. Trends in hospitalisations for cardiovascular diseases
Trends in hospitalisations for cardiovascular diseases are reported in Table 2. Except for 12 studies, all others reported a decline in the outcomes assessed during the pandemic period compared to the pre-pandemic period. The declines ranged from 20.2 to 73%. Eleven of the 12 studies reported mostly an increase in stroke hospitalisations during the pandemic period compared to the pre-pandemic period or observed no changes. In the single study by Wadhera et al. which evaluated CVD deaths in the USA [15], deaths caused by ischemic heart disease and hypertensive disease increased nationally after the onset of the pandemic in 2020, compared with changes over the same period in 2019, but not for HF, cerebrovascular disease, or other diseases of the circulatory system.
3.5. Severity of presentation
The severity of presentation on hospitalisation was reported by 26 studies (Table 2). Most studies (n = 15) reported that presentation was severe during the pandemic period compared to the pre-pandemic period. A variety of presentations were reported which included higher cardiac enzymes and worse left ventricular ejection fraction for ACS patients, higher admission National Institutes of Health Stroke Scale (NIHSS) scores for stroke patients, higher rates of New York Heart Association (NYHA) III or IV symptoms and severe peripheral oedema for HF patients and higher prevalence of risk factors associated with poorer prognosis in stroke or HF patients. Eleven studies reported no differences in severity of presentation comparing the two periods.
3.6. Management on hospitalisation
Thirty-six studies reported data on procedures performed during hospitalisation (Table 3 ). The majority of studies (n = 20) reported that less cardiovascular procedures were performed during the pandemic compared to the pre-pandemic periods. Procedures reported included coronary angiographies, PCI and thrombolysis for ACS patients and magnetic resonance imaging, acute revascularization treatments, thrombolysis and thrombectomies for stroke. Fifteen studies reported no differences in these procedures between the periods compared. Only one study reported that stroke patients admitted during the pandemic period were more likely to undergo intravenous thrombolysis and mechanical thrombectomy.
Table 3.
Management, patient- and system-related delays and outcomes.
| Author, year of publication | Management | Patient- and system-related delays | Reasons for delays in seeking medical care | Length of stay | Outcomes related to management |
|---|---|---|---|---|---|
| Araiza-Garaygordobil, 2021 | Significant reduction of patients undergoing pPCI was observed (81.8% pre-pandemic vs. 76.2% pandemic, difference: −5.6%, p = 0.041). | The proportion of patients who developed any mechanical complication during the pandemic period was higher when compared with the pre-pandemic period (1.98% [23/1161] vs. 0.98% [41/4143], p = 0.006) and compared to the historical control (1.98% [23/1161] vs. 1.17% [30/2547], p = 0.057) | |||
| Mafham, 2020 | There were reductions in the number of PCI procedures for patients with both STEMI (438 PCI procedures per week in 2019 vs 346 by the end of March, 2020; percent reduction 21%) and NSTEMI (383 PCI procedures per week in 2019 vs 240 by the end of March, 2020; percent reduction 37%). | The median length of stay among patients with ACS fell from 4 days (IQR 2–9) in 2019 to 3 days (1–5) by the end of March, 2020. | No apparent change in in-hospital mortality among patients admitted with ACS in the period | ||
| Perrin, 2020 | Delay from symptom onset to first medical contact was longer among patients suffering from STEMI in the COVID-19 period compared with the control period (112 min vs 60 min, p = 0.049). Delayed presentations were reported in 18.2% and 9% of patients in the COVID-19 and control periods, respectively (p = 0.3) | ACS patients delayed their call to the emergency services mainly because of fear of contracting or spreading COVID-19 following hospital admission, as well as of adding burden to the healthcare system | Hospital length of stay was significantly shorter for the COVID-19 period as compared to the control period (6 vs 7 days, p = 0.03). | ||
| Tam, 2020 | Delay from symptom onset to first medical contact was longer among patients suffering from STEMI in the COVID-19 period compared with the pre-pandemic period. The proportion of patients who presented out of the revasculrization window during the pandemic period was higher when compared with the pre-pandemic period (33% vs 27.8%) | The primary composite outcome of in-hospital death, cardiogenic shock, sustained ventricular tachycardia or fibrillation and use of mechanical circulatory support was significantly higher during the pandemic period compared to pre-pandemic period (29.7% vs 14.1%, p = 0.02) | |||
| Toniolo, 2020 | |||||
| Huet, 2020 | |||||
| Kwok, 2020b | Compared with 2017–2019, patients admitted with primary PCI for STEMI in the month of April 2020 were more likely to have longer time from symptom-to-hospital (median 135 min vs 153 min, p = 0.004) and they also had a longer door-to-balloon time (48 (21–112) vs 37 (16–94) min, p < 0.001). | There was a shorter median length of stay postlockdown compared to prelockdown: 2 (1–3) days vs 3 (2−4) days, p < 0.001). | No significant differences in in-hospital death and MACE were observed overall | ||
| Boukhris, 2020 | There was increase in patients with >2 h delays in the setting of STEMI in the pandemic period compared to the same period in 2019. Delays in ischemic strokes were similar between the two periods | ||||
| Braiteh, 2020 | In NSTEMI patients, 36.4% presented late (>24 h of symptoms) during the COVID-19 pandemic in comparison with 2019 (27.1%, p = .033). | ||||
| Butt, 2020 | Overall length of stay was shorter during the pandemic period compared to March 2019. | Deaths - Compared to March 2019 (179), there was 19% increase in in-hospital deaths in March 2020 (221) (p = 0.05) | |||
| De Filippo, 2020 | |||||
| De Rosa, 2020 | Both patient- and system-related declared delays were substantially increased during the COVID-19 outbreak. Time from symptom onset to coronary angiography was increased by 39.2% in 2020 compared with the equivalent week in 2019, while the time from first medical contact to coronary revascularization was increased by 31.5%. | Case fatality rate during the pandemic was increased compared with 2019. | |||
| Fileti, 2020 | Among those admitted for ACS, 57 (79.1%) were treated with PCI in 2020, and 67 (71.2%) in 2019, with an overall 14.9% reduction. | Among STEMI patients, the rate of those with a time delay presentation from symptoms onset longer than 180 min was significantly higher during the pandemic period compared to 2019 | PCI procedural success and in-hospital mortality were not significantly different between the two periods | ||
| Folino, 2020 | |||||
| Haddad, 2020 | Longer delays between symptom onset and first medical contact were noted during the pandemic compared to pre-pandemic and control period | There were worse in-hospital outcomes (MACE, mechanical complications, death, other cardiac complications) during the pandemic compared to pre-pandemic and control period | |||
| Hauguel-Moreau, 2020 | Median symptom-onset-to-first medical contact time was significantly higher in 2020 than in the two previous years (600 min [298–632] versus 121 min [55–291], p < 0.001). There was also a delay in STEMI management (3-fold increase in ischemic time) | ||||
| Holy, 2020 | |||||
| Metzler, 2020 | |||||
| Montagnon, 2021 | For patients with ACS, the average time interval between the first symptoms and the consultation was shorter in 2020. However, the average time lapse between the consultation and subsequent cerebral imaging increased in 2020 compared with 2019. | ||||
| Showkathali, 2020 | The symptom to door time was prolonged in 2020 compared to 2019 | The duration of hospital stay was longer in 2020 compared to previous years | There was no difference in in-hospital mortality between the two study periods of 2020 and 2019 respectively | ||
| Sokolski, 2020 | The mean length of stay was significantly shorter in 2020 (4.9 days) in comparison to 2019 (5.9 days) | There was no statistically significant difference in death rates between studied periods: 107 (3.6%) in 2020 versus 175 (3.9%) deaths in 2019 | |||
| Solomon, 2020 | |||||
| Vacanti, 2020 | The total number of coronary angiographies and PCIs were lower in 2020 compared to 2019 and 2019 | ||||
| Yalamanchi, 2020 | The in-hospital mortality of patients was also similar in all 3 years | ||||
| Tsioufis, 2020 | |||||
| Gasior, 2020 | |||||
| Dreger, 2020 | Number of PCI in AMI patients also fell. | ||||
| Anderson, 2020 | |||||
| Gluckman, 2020 | Median length of stay for patients with AMI was shorter in the early COVID-19 period by 7 h and in the later COVID-19 period by 6 h compared with the before period. Similar trends were observed for STEMI and NSTEMI | Patients with STEMI had a statistically greater risk of mortality during the later COVID-19 period | |||
| Mohammad, 2020 | PCI was equally performed during the two periods | Time from symptom onset to PCI was shorter during the pandemic compared to the control period | No differences in all-cause mortality rates between the two periods | ||
| Piccolo, 2020 | |||||
| Secco, 2020 | Longer door-to-balloon and symptoms to PCI times in 2020 compared to 2019 | No difference in in-hospital mortality between the two periods. However, in 2020, patients had a lower discharged residual left ventricular function and an increased predicted late cardiovascular mortality | |||
| Ayad, 2021 | Time from first medical contact to needle was longer during the pandemic period. | Hospital length of stay was longer during the pandemic | In-hospital mortality, incidence of re-infarction and need for revascularization were higher during the pandemic period. Incidence of HF, stroke and bleeding was not different between the periods | ||
| Bhatt, 2020 | Hospital length of stay was shorter in March 2020: 4.8 (2.4−8.3) days compared with March 2019: 6.0 (3.1−9.6) days | In-hospital mortality was not significantly different between the two periods | |||
| Daoulah, 2021 | Timing from the onset of symptoms to the balloon of more than 12 h was higher during 2020 comparing to pre-COVID 19 | No differences in length of hospital stay | There were no differences with respect to in-hospital events (mortality, thrombosis, bleeding etc) | ||
| Desai, 2020 | Number of patients undergoing endovascular thrombectomy remained constant | ||||
| Diegoli, 2020 | No differences in number of patients provided with reperfusion therapies | No differences in time from onset to admission. | |||
| Gitt, 2020 | |||||
| Hammad, 2020 | Door-to-balloon time were not significantly different | (i) Fear of contracting COVID-19 (27%); (ii) Symptoms were COVID-19 related (18%); (iii) Did not want to burden the emergency dept (9%) | Shorter ICU duration and length of stay during the pandemic period: 2.3 vs 3.6 days | ||
| Kerleroux, 2020 | There was a significant increase in delays between imaging and groin puncture during the pandemic period | No difference in outcomes (successful reperfusion and in-hospital mortality) | |||
| Montaner, 2020 | Time from symptoms onset to arrival at hospital was delayed during the pandemic period. Door-to-needle time was delayed during the pandemic. However, mean times of arrival to thrombectomy reference center from symptoms onset improved during the pandemic | ||||
| Neves Briard, 2020 | Time from symptom onset to hospital presentation was longer during the pandemic period. Door-to-needle and door-to-recanalization metrics were also longer during the pandemic. A significantly smaller proportion of ischemic stroke patients was treated with thrombolysis or thrombectomy during the pandemic | ||||
| Pop, 2020 | There were 33.3% fewer acute revascularization treatments, 40.9% less intravenous thrombolysis and 27.6% less mechanical thrombectomy in 2020 | No significant differences in patient- and system-related delays. | |||
| Popovic, 2020 | Delayed hospital presentation in the pandemic period compared to control period | Higher in-hospital mortality in the pandemic period | |||
| Range, 2020 | Time from symptom onset to first medical contact was longer for lockdown group | Length of hospital stay was similar in both periods | There were higher rates of in-hospital MACE and mortality in the lockdown group but the differences were not significant. | ||
| Reinstadler, 2020 | Door-to-balloon times were constant during the period. Total ischemic times increased from 164 min (calendar week 9/10) to 237 min (calendar week 11/12) and to 275 min (calendar week 13/14) (p = 0.006). | Rates of in-hospital death and re-infarction were similar between groups | |||
| Sarfo, 2020 | Case fatality rate during the pandemic was increased compared with 2019. | ||||
| Teo, 2020 | Stroke onset-to-door arrival time was longer during the pandemic. There were no significant differences in the ambulance scene arrival to hospital arrival time, proportion of patients receiving reperfusion therapy, door-to-needle time, and mechanical thrombectomy procedural times during the 2 periods | ||||
| Toner, 2020 | Symptom-to-door time was longer during the COVID-19 period (4-fold increase). Proportion of patients presenting late was also higher during the pandemic period. | ||||
| Abdelaziz, 2020 | Delay in symptom-to-first medical contact during pandemic vs pre-COVID era. The door-to-balloon time was similar between both groups. | ||||
| Agarwal, 2020 | The time from symptom onset to presentation was not significantly different the two groups. There were longer median door to head CT and door to groin puncture times during the pandemic compared to pre-pandemic times. Time to alteplase administration, door to reperfusion times and defect-free care were similar in the pandemic and pre-pandemic groups | There was no difference in the length of hospital stay between the pandemic and pre-pandemic cohorts | Successful recanalization rates were similar between the two groups. Pandemic patients had increased discharge mortality in multivariable analysis compared to pre-pandemic patients | ||
| Burgos, 2020 | |||||
| Aldujeli, 2020 | NSTEMI: The median pain-to-door time was longer during the pandemic compared to pre-pandemic era. There was a significant delay in door-to-reperfusion time during the pandemic. There were 24 (80%) and 25 (42%) patients who presented after 12 h of pain onset in pandemic and pre-pandemic eras, respectively (p = 0.0006). STEMI: The median pain-to-door time during the pandemic was longer than that of the pre-pandemic. There were 22 (47%) and 14 (24%) patients who presented after 12 h of pain onset in the pandemic and prepandemic eras, respectively (p = 0.0127). There was no difference in delay in door-to-reperfusion time. | There were no differences in length of hospitalization between pandemic and pre-pandemic eras. | There were no differences in in-hospital death, or stroke between pandemic and pre-pandemic eras. | ||
| Andersson, 2020 | Mortality was similar before and after the national lockdown for the population with HF | ||||
| Ball, 2020 | |||||
| Boeddinghaus, 2020 | 220/398 PCIs (55.3%) PCIs were performed before versus 178/398 PCIs (44.7%) after the outbreak. | Time from chest pain onset to ED presentation, postinfarction LVEF, and median door-to-balloon time remained unchanged. | |||
| Bromage, 2020 | There were no differences in inpatient management, including place of care and pharmacological management of heart failure with reduced ejection fraction | In-hospital mortality rates were low in both periods | |||
| Bryndza, 2021 | There was a significant increase (90.7%) in the number of patients who experienced pain longer than 12 h prior to presentation to the hospital | There was a 100% increase in mechanical complications during pandemic period compared to control period | |||
| Cammalleri, 2020 | In March 2020, there was longer median time in symptom-to-first medical contact, spoke-to-hub, and the cumulative symptom-to-wire delay compared to March 2019 | Length of hospitalization was longer in 2020 | Procedural data and in-hospital outcomes were similar between the 2 groups. Patients in 2020 had a worse left ventricular ejection fraction at discharge. | ||
| Candelaresi, 2021 | Compared to the pre-lockdown, there was a significant reduction in the number of acute reperfusion treatments for stroke. | The time to reach medical attention was significantly longer in the lockdown phase. For patients who underwent acute reperfusion treatment, there was a significantly longer time-to-imaging and a trend to longer time-to-needle (75 versus 90 min P 0.23), but not time-to-groin. | Discharge neurological status was not significantly different between the periods | ||
| Chew, 2021 | Fewer patients in the pandemic group achieved door-to-baloon time <90 min compared with the pre-pandemic group. | There was no difference in hospital admission duration between groups. | In-hospital mortality was similar between groups. The 30-day readmission rate was lower in the pandemic group compared with the pre-pandemic group. The rates of sepsis and acute mitral regurgitation were higher in the pandemic group compared with the pre-pandemic group. | ||
| Choudhary, 2020 | Percentage of STEMI patients undergoing emergent catheterisation was lower in the lockdown and pre-lockdown period compared to pre-COVID period. Percentage of STEMI patients having thrombolysis was higher in the lockdown and pre-lockdown period compared to pre-COVID period. | The percentage of STEMI patients who presented outside the window period (presentation after 12 h of symptom onset) was 6.1% in the pre-COVID period, 17.4% during the pre-lockdown period and 25.0% during the lockdown period. | In-hospital mortality was 7.3%, 3.5% and 2.7%, in the lockdown, pre-lockdown, and pre-COVID periods, respectively. | ||
| Çinier, 2020 | Prolonged ischemic time, longer pain-to-balloon and door-to-balloon time during the pandemic. | ||||
| Colivicchi, 2020 | In-hospital all-cause mortality was 17.2% in 2020 and 6.3% in 2019 | ||||
| Cummings, 2020 | There was a higher percentage of patients receiving intravenous tPA during the pandemic, and the number of thrombectomies per week was lower during the pandemic | No differences in door-to-needle and door-in-door-out times | No differences in in-hospital mortality | ||
| Del Pinto, 2020 | Less daily cardiovascular procedures performed in 2020 than in 2019 | More in-hospital cardiovascular deaths occurred in 2020 compared with 2019. Less in-hospital all-cause mortality occurred in 2020 than 2019 | |||
| Enache, 2020 | |||||
| Erol, 2020 | There was a significant reduction in the overall frequency of coronary angiography during the pandemic period compared to the pre-pandemic period. Frequency of PCI decreased during the pandemic. | EMS transport significantly increased during the pandemic period. Median time from symptom-onset to hospital-arrival was increased during the pandemic. The total ischemic time for patients with STEMI who were treated with PCI was significantly longer during the pandemic period compared with the pre-pandemic period. Door-to-balloon time was similar in the two periods. | In-hospital major adverse cardiac events were significantly increased during the pandemic period | ||
| Frisullo, 2020 | Significant reduction of the total number of thrombolysis and a non-significant increase of thrombectomy during the pandemic | Significant increase in onset-to-door time and door-to-groin time during pandemic | Significant reduction in length of hospitalization during pandemic | ||
| Giannouchos, 2021 | |||||
| Hoyer, 2020 | There was a significant drop in the thrombolysis rate by 60% and in the thrombectomy rate by 61% during the pandemic in one of the centers | ||||
| Hsiao, 2020 | Reperfusion treatments also appeared to decline by 31% and specifically thrombolysis by 33% | ||||
| JF Huang, 2020 | Recommendation for acute stroke intervention (IV-tPA and/or thrombectomy) occurred at a lower rate for our postepandemic declaration group | The last known normal/symptom onset time to telestroke activation in the ED was significantly shorter for the postepandemic declaration group | |||
| Ikenberg, 2020 | There was no difference of daily numbers of patients receiving thrombolysis and thrombectomy | ||||
| Jasne, 2020 | No difference in rate of Thrombolysis in Cerebral Infarction 2b or greater revascularization | There was no difference in time to presentation, door-to-needle and door-to-reperfusion times | No difference in median length of stay | There was no difference in discharge modified Rankin Scale. | |
| John, 2020 | Ischemic stroke: The rate of treatment with intravenous thrombolysis was similar in both years; Haemorrhagic stroke: Surgical treatment including placement of an external ventricular drain, endovascular embolization and microsurgical clipping/resection or hematoma evacuation occurred at similar rates. | Ischemic stroke: Presentation to the hospital from last known well time and door-to-needle times for intravenous thrombolysis was similar. However, door-to-groin puncture times for endovascular thrombectomy was significantly longer in 2020. | There was no difference in in-hospital mortality, discharge disposition or discharge/30-day modified Rankin Score | ||
| Kobo, 2020 | Patients admitted in 2020 had greater likelihood of door-to-balloon times> 90 min and greater likelihood of pain-to-balloon times> 12 h | Patients admitted in 2020 had longer hospital stay | Patients admitted in 2020 experienced higher rates hemodynamic instability and fewer early (<72 h) discharge | ||
| Kuitunen, 2020 | |||||
| Lauridsen, 2020 | The proportion of patients who underwent CAG, PCI, CABG, and extra corporeal membrane oxygenation (ECMO) were similar between 2015 and 2019 and 2020 during lockdown | No difference in 7-day mortality was observed between study periods. | |||
| Little, 2020 | Aspiration thrombectomy and rates of cases completed with TIMI flow less than 3 were similar between both groups | There was no significant difference in pain to first call for help or door-to-balloon. There was longer ambulance response times in 2020 than in 2019. | Length of stay was similar | There was no significant difference in ICU admission or in-hospital all-cause mortality | |
| Nagamine, 2020 | The average last known well to arrival time for stroke codes was longer in 2020 than in 2019. Mean time from patient arrival to administration of tPA (door-to-needle) was similar for both periods. Mean time from patient arrival to vessel puncture for endovascular therapy (door to puncture) was shorter in 2020 compared to 2019. | In-hospital mortality was higher in 2020 than in 2019 | |||
| Mitra, 2020 | Median time from symptom onset to presentation was shorter in 2020 compared to 2019. Proportion of patients who presented >12 h after onset of symptoms were similar in both periods. Median time to primary reperfusion intervention was longer in 2020 compared to 2019. | No differences in hospital length of stay | There were no differences in mortality at hospital discharge | ||
| Nguyen-Huynh, 2020 | The percentage of patients receiving alteplase was not significantly different. | Median door-to-needle time among noncanceled stroke alerts was unchanged. The median times from LTKW-to-needle time or alteplase treatment time were not significantly different. | Length of stay was similar | Stroke discharges decreased significantly post lockdown compared with pre-lockdown. No difference in in-hospital mortality | |
| Oseran, 2020 | |||||
| Paliwal, 2020 | Proportion of activations receiving acute recanalization therapy remained stable. | In patients undergoing acute intervention, door-to-activation and door-to-neurologist review time were longer during the lockdown compared to pre-lockdown. Symptom-to-door-time was similar | For patients that received acute recanalization therapy, early neurological outcomes in terms of change in median NIHSS at 24 h were largely similar | ||
| Papafaklis, 2020 | There was no difference in the rate of cardiac deaths between the two periods, while the rates of in-hospital repeat MI and stent thrombosis were numerically higher during the COVID-19 | ||||
| Piuhola, 2020 | |||||
| Rashid Hons, 2020 | The overall rates of invasive coronary angiography were significantly lower during COVID-19 period. The use of PCI was also lower across COVID-19 months in 2020 compared with pre–COVID-19 months in 2019. Patients admitted during the COVID-19 period were slightly less likely to be seen by a cardiologist | Increased time to reperfusion in STEMI patients during the COVID-19 period | Increased in-hospital mortality during the COVID-19 period | ||
| Richter, 2021 | IVT rate in patients with stroke was comparable, whereas mechanical thrombectomy rate was significantly higher during the pandemic | In-hospital mortality was significantly increased in patients with stroke during the pandemic period | |||
| Rodríguez-Leor, 2020 | There were no differences in reperfusion strategy (> 94% treated with primary PCI in both groups) | Patients treated with primary PCI during the COVID-19 outbreak had a longer ischemic time but showed no differences in the time from first medical contact to reperfusion. | In-hospital mortality was higher during the COVID-19 period | ||
| Ruparelia, 2020 | |||||
| Schirmer, 2020 | The mean interval from last-known-well to the presentation was significantly longer in the COVID period | ||||
| Seiffert, 2020 | The percentage of patients treated with interventional or open-surgical procedures remained similar over time | In-hospital mortality in hospitalizations for stroke increased from pre-COVID to COVID | |||
| Sharma, 2020 | There was no difference in time from symptom onset to hospital arrival. | ||||
| Siegler, 2020 | Fewer brain MRIs were performed during the COVID-19 period when compared to pre-COVID-19 | There was no significant delay from the time patients were last known well to ED arrival, arrival to computed tomography scan or to thrombolysis. | Patients treated during COVID-19 had a shorter hospital length of stay when compared to patients admitted during the pre-COVID-19 period: 2.5 (2–7) vs 4 (2−8) | No difference in in-hospital mortality | |
| Tejada Meza, 2020 | There were no differences in the proportion of patients undergoing intravenous and endovascular treatments procedures | In-hospital mortality of stroke patients increased significantly | |||
| Uchino, 2020 | Thrombolysis decreased during the COVID-19 period but thrombectomy remained unchanged | Time to presentation and time to treatment were unchanged. Door-to-needle, CT completion and puncture times were unchanged. | |||
| Vensentini, 2020 | |||||
| Wadhera, 2021 | |||||
| J Wang, 2020 | Patients admitted during the COVID-19 pandemic were more likely to undergo intravenous thrombolysis and mechanical thrombectomy | There were no differences in patients’ disposition including home, short-term, and longterm facility | |||
| Yang, 2020 | Onset to hospital arrival was not different. The time from hospital arrival to puncture and time from hospital arrival to reperfusion were significantly prolonged in the pandemic group compared with the pre-pandemic group. | The rate of successful reperfusion was not significantly different between the two groups | |||
| Zhao, 2020 | The total number of thrombolysis and thrombectomy cases dropped by 26.7% and 25.3%, respectively, in 2020 | Patients’ and their families’ fear of contracting virus in hospital (87.2%); Insufficient transportation resources (43.2%); Lack of first aid knowledge (42.3%); Lack of family support (31.7%); Insufficient ambulance resources (15.4%) | |||
| Cox, 2020 | No difference in length of stay | In-hospital mortality rate was not different between the two periods |
3.7. Patient- and system-related delays
Fifty-five studies reported on patient- and health system-related delays during presentation and management (Table 3). The majority of studies (n = 37) reported more patient- and health system-related delays during the pandemic compared to pre-pandemic times. The most common patient-delay was a longer delay between symptom onset and first medical contact. The longest delay was a median of 10 h for symptom onset to first medical contact for an acute coronary syndrome during the pandemic compared to a median of about 2 h pre-pandemic (600 min [298–632] versus 121 min [55–291], p < 0.001) [16].
Other measures of delay that were reported included: delays in emergency transport, longer time from symptom onset to consultation and intervention (coronary angiography, PCI, reperfusion or thrombectomy), larger proportion of patients presenting outside of the revascularization window, increase in ischemic time, longer symptom-to-door time, longer medical contact to needle, longer door-to-balloon time, and longer door-to-needle time. Fifteen studies reported no significant differences in patient- and system-related delays. Only three studies reported shorter times between symptom onset and consultation or PCI procedure during the pandemic compared to the pre-pandemic periods.
3.8. Reasons for delays in seeking medical care
Only three studies were identified to have assessed the reasons patients provided for their delay in seeking medical care (Table 3). The main reasons provided was because of the fear of contracting COVID-19 in hospital as well as adding extra burden to the healthcare system.
3.9. Length of stay
Twenty-four studies reported data on length of hospital stay (Table 3). Of this number, 10 studies reported a shorter length of stay during the pandemic period (1–8 days) compared with the pre-pandemic period (2–12 days); 10 reported no difference in length of stay between the two periods; and 4 reported a longer length of stay during the pandemic period.
3.10. Outcomes related to management
Fifty-four studies reported outcomes related to management (Table 3). The majority of studies (n = 31) reported no change in in-hospital mortality or discharge disposition among hospitalised patients comparing the pandemic period with pre-pandemic times. Twenty-three studies reported an increase in worse in-hospital outcomes (major adverse cardiac events (MACE), stroke, cardiac and mechanical complications and the need for revascularisation) and in-hospital death during the pandemic compared to the pre-pandemic period.
4. Discussion
4.1. Key findings
Using systematic review methodology, we have assessed the indirect impact of COVID-19 on hospitalisations for cardiometabolic conditions and their management. Based on 103 studies conducted in more than 34 countries across six continents, our results demonstrated the following: (i) the majority of studies had evaluated trends in hospitalisations for ACS followed by acute strokes and HF, with only two studies on hypertension and diabetes; (ii) majority of studies reported declines in hospitalisations during the pandemic compared to pre-pandemic times, with the reductions ranging from 20.2 to 73% and the results were not suggestive of age-specific and gender-related declines; (iii) severe presentation, less utilization of cardiovascular procedures such as coronary angiographies and PCI, and longer patient- and healthcare-related delays were common during the pandemic; (iv) the fear of contracting COVID-19 was the main reason patients provided for the delay in seeking medical care; (v) most studies reported shorter length of hospital stay during the pandemic or no difference in length of stay; and (vi) finally, the majority of studies reported no change in in-hospital mortality among hospitalised patients comparing the pandemic period with pre-pandemic times.
We identified only one relevant systematic review which assessed the extent to which health services related to the care and management of acute cardiovascular events had been impacted during the COVID-19 pandemic. Kiss and colleagues in a review of 27 studies reported a decrease in stroke and ACS admissions (declines ranging from 12 to 50%) during the COVID-19 period compared to non-COVID-19 periods, a decrease in number of reperfusion procedures, a shortening in the lengths of hospital stay, and longer symptom-to-door times [17]. Whiles the authors only evaluated acute cardiovascular conditions, we adopted a broader approach and focused on all cardiometabolic conditions and evaluated other outcome measures such as severity of presentation, utilization of cardiovascular procedures, both patient and system-related delays, reasons provided by patients for delays in seeking care, and outcomes related to management such as complications, in-hospital mortality and discharge disposition. We have also identified some gaps in the evidence. None of the studies provided specific guidance on how the adverse impact of the pandemic on patients’ health-seeking behaviour and response of health systems could be mitigated.
As expected, infectious disease outbreaks have the potential to cause volume changes in emergency department attendances and hospitalisations. These changes have been witnessed in previous epidemics and pandemics such as the 2003 severe acute respiratory syndrome (SARS) outbreak [18], the 2009 novel influenza A (H1N1) pandemic [19,20] and the 2015 Middle East respiratory syndrome (MERS) outbreak [21]. The unprecedented surge in COVID-19 infection rates and associated substantial morbidity and mortality rates across the globe led policy makers to institute several containment measures ranging from social distancing to lockdowns, to mitigate the disease. Obviously, these measures came at a cost as they impacted on patients’ health-seeking behaviors’ and the delivery of health services. Several reasons could explain the decline in hospitalisations for cardiometabolic conditions, especially the acute ones. The major reason being the reluctance of patients and their families to initiate medical contact to avoid exposure to COVID-19, as evaluated and reported by three of the included studies in the present review. Other potential reasons include patients not wanting to burden the health system; lack of family support; insufficient ambulance support due to the pressure on the health system and lack of adequate personnel; and restructuring of the healthcare system in response to the pandemic which involved increased hospital capacity for patients infected with COVID-19 and deferral or cancellation of nonessential procedures, routine patient visits and diagnostic evaluations. Despite these reasons, we found that among hospitalised patients, comparing the pandemic period with pre-pandemic times no change in in-hospital mortality was observed. This may suggests that once in the hospital, the care patients received for cardio-metabolic conditions was not compromised. Patients suffering acute cardio-metabolic conditions are not therefore to be encouraged to seek medical help.
It is possible the stay-at-home campaigns by governments and the constant information provided by the media were alarming enough for patients to delay seeking medical care [22,23]. Even though out-of-hospital mortality is out of the scope of this review, it is not inconceivable that this could have been adversely affected as a result of the stay-home-messaging. Three studies conducted in France, United Arab Emirates (UAE) and Ghana observed an increase in stroke hospitalisations during the pandemic, which could be attributed to an increasing burden of stroke and the fact that the COVID-19 pandemic was not severe especially in Ghana and the UAE. Increased severity at presentation during the pandemic period compared to the pre-pandemic period is likely related to the delays in seeking medical care, effects of the social restrictions such as loneliness, stress, depression, mental health issues and physical inactivity [[24], [25], [26]]. Less utilization of cardiovascular procedures, healthcare system delays for procedures and shorter lengths of hospital stay during the pandemic period are likely due to the pressure on healthcare systems, shortage of health personnel because of sickness due to COVID-19 and early discharges to avoid too much exposure in the hospital environment.
These findings have several implications for both patient care and healthcare systems all over the world, given that cardiovascular conditions account for substantial morbidity and over 17 million deaths each year, being the leading cause of mortality in the world [27]. As we come out of the pandemic, preparations for delivering services for cardiovascular diseases needs to take these findings into consideration. The findings should also inform preparations for future pandemics. The declines in hospitalisations for acute cardiovascular conditions are irrespective of age and sex and largely due to fear of contracting COVID-19. This has also been fueled by media and anecdotal reports about patients contracting COVID-19 in the hospital. While there is some truth to this, patients need to be educated on the need to seek medical help promptly and adopt healthy lifestyles in the areas of nutrition and physical activity. Furthermore, given that the decrease in hospitalisations and delays in management could also be due to the adaptations of healthcare systems in response to the pandemic, further restructuring needs to be done to maintain high standards of care and also prepare for future pandemics of this nature.
4.2. Strengths and limitations
There are several strengths and limitations of this study that deserve consideration. Compared to the only relevant previous review on the topic [16], our review was more comprehensive and focussed on cardiometabolic conditions and a wide range of measures related to the management of patients. Our literature search was detailed and spanned multiple databases, yielding over 100 articles conducted across 6 continents. The findings may be generalisable globally given that the findings were based on data across 6 out of 7 continents. Though there were a number of limitations, these were all inherent to the included studies and not the actual review. The outcome measures were heterogenous and not consistently reported, hence we were unable to conduct any meta-analysis as originally planned in our published protocol (CRD42021236102). However, we were able to summarise the evidence according to identified consistent themes using narrative synthesis and tables. The findings reported by included studies were based on a diversity of observational study designs which were generally not of high methodological quality. The pandemic and pre-pandemic periods utilised were not consistent across studies, hence it is challenging to make any head-to-head comparisons. Only three out of the 103 studies formally assessed the reasons patients provided for their delay in seeking medical care. Given these limitations, the findings should be interpreted with caution.
5. Conclusion
Data based on available real-world evidence clearly indicates that health-seeking behaviour of patients for cardiometabolic conditions (particularly the acute ones), their management and outcomes have been adversely impacted by the COVID-19 pandemic. Though the pandemic seemed to have adversely impacted on the management of patients, in-hospital mortality rates were not significantly affected as reported by the majority of studies. None of the studies provided specific guidance on how the adverse impact of the pandemic on patients’ health-seeking behaviour and response of health systems could be mitigated. Patients should be educated via population-wide approaches on the need for timely medical contact and health systems should put adequate response strategies in place to provide timely care to patients at high risk and also manage future outbreaks of infectious disease.
Data sharing
The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study is based in data from published articles.
Authors contribution
Samuel Seidu: Literature search, data collection, study design, data interpretation, writing.
Setor K. Kunutsor: Literature search, data collection, analysis, data interpretation, writing.
Xavier Cos: Data interpretation, writing, editing.
Kamlesh Khunti: Study design, literature search, data interpretation, writing.
Funding
This study was supported with a research grant from Primary Care Diabetes Europe (PCDE). PCDE as a society, has received sponsorship from Novo Nordisk, Eli Lilly and Roche Diagnostics, but the companies had no input in the study.
Declarations of interest
None.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.pcd.2021.05.011.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J. Characteristics of and important lessons from the 367 coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 368 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;369 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Kunutsor S.K., Laukkanen J.A. Renal complications in COVID-19: a systematic review and meta-analysis. Ann. Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunutsor S.K., Laukkanen J.A. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81(Aug (2)):e139–41. doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunutsor S.K., Laukkanen J.A. Hepatic manifestations and complications of COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81(Sep (3)):e72–4. doi: 10.1016/j.jinf.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunutsor S.K., Laukkanen J.A. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J. Infect. 2021;82(Jan (1)):159–198. doi: 10.1016/j.jinf.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidu S., Gillies C., Zaccardi F., Kunutsor S.K., Hartmann‐Boyce J., Yates T. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: a systematic review and meta-analysis. Endocrinol. Diabetes Metab. 2021;4(1) doi: 10.1002/edm2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(Mar (10229)):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M. 2000. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [Google Scholar]
- 13.Kunutsor S.K., Apekey T.A., Laukkanen J.A. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: systematic review and meta-analysis of observational evidence. Eur. J. Epidemiol. 2015;30(8):599–614. doi: 10.1007/s10654-015-0058-x. [DOI] [PubMed] [Google Scholar]
- 14.Metzler B., Siostrzonek P., Binder R.K., Bauer A., Reinstadler S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur. Heart J. 2020;41(19):1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadhera R.K., Shen C., Gondi S., Chen S., Kazi D.S., Yeh R.W. Cardiovascular deaths during the COVID-19 pandemic in the United States. J. Am. Coll. Cardiol. 2021;77(2):159–169. doi: 10.1016/j.jacc.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauguel-Moreau M., Pillière R., Prati G., Beaune S., Loeb T., Lannou S. Impact of Coronavirus Disease 2019 outbreak on acute coronary syndrome admissions: four weeks to reverse the trend. J. Thromb. Thrombolysis. 2021;51(1):31–32. doi: 10.1007/s11239-020-02201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss P., Carcel C., Hockham C., Peters S.A. The impact of the COVID-19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur. Heart J. Qual. Care Clin. Outcomes. 2021;7(1):18–27. doi: 10.1093/ehjqcco/qcaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Yen D.H., Huang H., Kao W., Wang L., Huang C. Impact of severe acute respiratory syndrome (SARS) outbreaks on the use of emergency department medical resources. J. Chinese Med. Assoc. 2005;68(6):254–259. doi: 10.1016/S1726-4901(09)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fagbuyi D.B., Brown K.M., Mathison D.J., Kingsnorth J., Morrison S., Saidinejad M. A rapid medical screening process improves emergency department patient flow during surge associated with novel H1N1 influenza virus. Ann. Emerg. Med. 2011;57(1):52–59. doi: 10.1016/j.annemergmed.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Miroballi Y., Baird J.S., Zackai S., Cannon J., Messina M., Ravindranath T. Novel influenza A (H1N1) in a pediatric health care facility in New York City during the first wave of the 2009 pandemic. Arch. Pediatr. Adolesc. Med. 2010;164(1):24–30. doi: 10.1001/archpediatrics.2009.259. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.Y., Khang Y.H., Lim H.K. Impact of the 2015 Middle East Respiratory syndrome outbreak on emergency care utilization and mortality in South Korea. Yonsei Med. J. 2019;60(Aug (8)):796–803. doi: 10.3349/ymj.2019.60.8.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moroni F., Gramegna M., Ajello S., Beneduce A., Baldetti L., Vilca L.M. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. Case Rep. 2020;2(10):1620–1624. doi: 10.1016/j.jaccas.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessler B.S., Kent D.M., Konstam M.A. Fear of coronavirus disease 2019—an emerging cardiac risk. JAMA Cardiol. 2020;5(9):981–982. doi: 10.1001/jamacardio.2020.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holt-Lunstad J., Smith T.B., Baker M., Harris T., Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect. Psychol. Sci. 2015;10(2):227–237. doi: 10.1177/1745691614568352. [DOI] [PubMed] [Google Scholar]
- 25.Lima C.K.T., de Medeiros Carvalho P.M., de Araújo Araruna Silva Lima I., de Oliveira Nunes J.V.A., Saraiva J.S., de Souza R.I. The emotional impact of Coronavirus 2019-nCoV (new Coronavirus disease) Psychiatry Res. 2020;287 doi: 10.1016/j.psychres.2020.112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redfors P., Isaksén D., Lappas G., Blomstrand C., Rosengren A., Jood K. Living alone predicts mortality in patients with ischemic stroke before 70 years of age: a long-term prospective follow-up study. BMC Neurol. 2016;16(1):1–8. doi: 10.1186/s12883-016-0599-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann D.L., Zipes D., Libby P., Bonow R. 9th ed. 2012. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.