Abstract
Coronavirus disease 2019 (COVID-19) has caused significant devastation globally. Despite the development of several vaccines, with uncertainty around global uptake and vaccine efficacy, the need for effective therapeutic agents remains. Increased levels of cytokines including tumour necrosis factor are significant in the pathogenesis of COVID-19 and associated with poor outcomes including ventilator requirement and mortality. Repurposing tumour necrosis factor blocker therapy used in conditions such as rheumatoid arthritis and inflammatory bowel disease seems promising, with early feasibility data showing a reduction in circulation of pro-inflammatory cytokines and encouraging the evaluation of such interventions in preventing disease progression and clinical deterioration in patients with COVID-19. Here, we examine the biological activities of tumour necrosis factor inhibitors indicative of their potential in COVID-19 and briefly outline the randomised control trials assessing their benefit-risk profile in COVID-19 therapy.
Keywords: COVID-19, Tumour necrosis factor (TNF), Cytokines, International standards, Clinical trials, Therapy, SARS-COV-2
Graphical Abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) is defined as a clinical syndrome caused by infection by an RNA virus, a beta-coronavirus, named Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2). The virus was originally identified in Wuhan, China and has since spread across the world, with the COVID-19 pandemic being officially accepted by the World Health Organisation on March 11, 2020. This disease results in a clinical picture of fever, dry cough, shortness of breath, myalgia and fatigue and radiological signs of pneumonitis which can progress to acute respiratory distress syndrome (ARDS). SARS-CoV-2 binds to angiotensin converting enzyme-2 (ACE-2) receptors to enter host cells, and then replicates within the host cells to form new virions. The host cell then disintegrates due to the cytopathic effect of the virus and the virus spreads to other cells. Virus antigens activate both the innate and adaptive immune systems resulting in production of vast amounts of pro-inflammatory chemokines and cytokines [1]. In some patients, hyperactivation of the inflammatory cascade results in a ‘cytokine storm’ and excessive thrombosis which can lead to multi-organ failure and ultimately death [2], [3].
One of the key cytokines identified as being fundamental in the cytokine storm is Tumour Necrosis Factor (or sometimes referred to as Tumour Necrosis Factor-alpha, TNF-α). Produced mainly by monocytes and macrophages but also by B-cells, T-cells and fibroblasts, TNF interacts through two receptors namely, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2), to initiate signal transduction pathways leading to numerous cellular processes including cellular differentiation, proliferation, migration, cell death and cell survival. In response to TNF, vascular endothelial cells undergo several changes to increase leukocyte adhesion, transendothelial migration, vascular permeability and encourage thrombosis [4]. Excessive production of TNF along with other pro-inflammatory cytokines such as Interleukin-1 (IL-1), Interleukin-6 (IL-6) contributes to the pathological processes associated with rheumatoid arthritis [5], inflammatory bowel disease [6], psoriasis [7] and sepsis. Since the approval of infliximab [8], the first monoclonal antibody against TNF for rheumatoid arthritis (RA), TNF inhibitors including adalimumab, certolizumab, golimumab and the fusion protein etanercept are increasingly utilised as therapy for rheumatoid arthritis (RA), inflammatory bowel disease (IBD) and other inflammatory conditions ( Table 1). As patents have expired on some of the originator products, numerous biosimilars of key block-buster drugs adalimumab, infliximab and etanercept have become available. As uptake of biosimilar products increases globally, maintaining standards for these products is paramount for their effectiveness, safety and sustainability. Undoubtedly, the availability of WHO international standards (IS) [9], [10], [11] which have an important role in assessment of bioactivity [12] will help towards achieving this objective (Table 1).
Table 1.
| Infliximab | Adalimumab | Golimumab | Certolizumab | Etanercept | |
|---|---|---|---|---|---|
| Structure | Chimeric | Human | Human | PEGylated humanised Fab fragment | P75TNFR/Fc fusion protein |
| Ligand | sTNF, tmTNF | sTNF, tmTNF | sTNF, tmTNF | sTNF, tmTNF | sTNF, tmTNF, LTα3 |
| Molecular weight (kDa) | 150 | 150 | 150 | 95 | 150 |
| Fully human | No | Yes | Yes | No | Yes |
| Half-life (days) | 8–10 | 10–14 | 12 ± 3 | 3 | 14 |
| Dosing route and frequencya | Intravenousb | Subcutaneous | Subcutaneous | Subcutaneous | Subcutaneous |
| Every 8 weeks following loading at 0, 2 and 6 weeks | Every 2 weeks following initial loading | Monthly following initial loading | Every 2 weeks following initial loading | Weekly/twice Weeklyc | |
| Indications | Crohn’s, ulcerative colitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis | Crohn’s, ulcerative colitis, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, plaque psoriasis, hidradenitis suppurativad, uveitisd | Rheumatoid arthritis, Psoriatic arthritis, Ankylosing spondylitis, Ulcerative colitis, juvenile idiopathic arthritis | Rheumatoid arthritis, Psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, Crohn’s disease* | Rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis |
| Biosimilars | Yes | Yes | No | No | Yes |
| WHO ISe | Yes 16/170 | Yes 17/236 | No | No | Yes 13/204 |
| Product Code |
Frequency of administration can vary based on the indication.
Subcutaneous versions of biosimilars now emerging.
Dose dependent.
Region/country dependent.
WHO International standards with defined international units are an important tool for maintaining product bioactivity and availability of safe and effective medicines worldwide as per regulatory guidance. These ISs are available from NIBSC on the link: https://www.nibsc.org/
With the current pandemic causing devastation throughout the world, there is an urgent need for effective therapies that reduce mortality and limit COVID-19-related damage. Consequently, there is an unprecedented global effort in development of vaccines and other therapeutic interventions including specific anti-SARS-CoV-2 anti-viral drugs, monoclonal antibodies and other novel inhibitors. However, the lengthy process associated with new drug development has made repurposing of current therapeutics (e.g., interferon-alpha/beta, IFN-α/β, monoclonal antibodies, signalling cascade inhibitors, protease inhibitors, others) an excellent choice for the management of COVID-19 [13], [14], [15], [16] with numerous clinical trials underway. Furthermore, despite the recent approval and ongoing development of vaccines for COVID-19, the need for therapeutic agents remains, particularly for patients who cannot be vaccinated for clinical reasons (e.g., immunosuppressed). The potential for repurposing biological medicines for COVID-19 has already been highlighted with recent results from the REM-CAP trial which targeted severely ill patients showing that the IL-6 receptor antagonists tocilizumab and sarilumab reduced mortality to 27.3% and 22.2% respectively compared with 35.8% seen with standard care in intensive care units [17]. The more recent and larger RECOVERY trial, found tocilizumab significantly reduced deaths (29% vs 33%) by 28 days [18]. In this context, we aim to review our understanding of TNF biology and signalling and its blockage in conditions such as rheumatoid arthritis and inflammatory bowel disease and examine the potential role for TNF inhibitors in COVID-19 therapy.
2. TNF biology and signalling
Initially synthesised as a transmembrane protein of 26 kDa, which undergoes proteolytic cleavage by TNF-α-converting enzyme (TACE, also called ADAM17) to release the soluble TNF protein of 17 kDa, TNF exerts its action by binding to and activating two different receptors; TNFR1 and TNFR2 [19]. TNFR1 signalling is activated by both soluble (sTNF) and transmembrane TNF (tmTNF) whereas TNFR2 signalling is activated primarily by tmTNF. Soluble or tmTNF binding to TNFR1 leads to recruitment of TNFR1-associated death domain protein (TRADD) to form complex TNFR1-complex I. Downstream signalling induces inflammation, tissue degeneration, cell survival and is responsible for immune defence against pathogens. Alternative signalling via TNFR1 through complex IIa and IIb leads to apoptosis while signalling via IIc induces necroptosis [20], [21], [22]. TNFR2 recruitment of TNFR-associated factor 2 (TRAF 2) also triggers formation of complex I butTNFR2 engagement primarily mediates homeostatic activities of tissue regeneration, cell survival and proliferation but can also initiate inflammation and immune defence.
Signalling via TNFR1 signalling complex I: Upon engagement of TNF with TNFR1, TNFR1 binds to intracellular TNFR1-associated death domain protein (TRADD) resulting in recruitment of receptor-interacting serine/ threonine-protein kinase 1 (RIPK1), TNFR-associated factor 2 or 5 (TRAF2 or TRAF 5) and cellular inhibitor of apoptosis protein 1 or 2 (cIAP2) to form TNFR1 signalling complex I. cIAP1 and ciAP2 add K63-linked polyubiquitin chains to RIPK1 allowing recruitment of the linear ubiquitin chain assembly complex (LUBAC). This adds M1-linked linear polyubiquitin chains to RIPK1 [20]. The K63-M1-polyubiquitinated RIPK1 then recruits transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) and Mitogen-activated protein kinase kinase 7 (MAP3K7)-binding protein 2 and 3 (TAB2 and TAB3) and TAK1, which trigger downstream signalling through JUN N-terminal kinase, P38 and IKK (inhibitor of nuclear factor-κB, NF-κB kinase) complex [21]. Subsequently, the IKK complex activates NF-κB signalling, allowing transcription of downstream target genes significant in cell proliferation, inflammation, host defence and survival [21]. Indeed, over-activation of such signalling cascades can lead to development of deleterious excessive or chronic inflammation as seen in septic shock.
Signalling via TNFR signalling complex IIa, IIb and IIc: Signalling via these complexes results in distinct signalling and functional outcomes [23], [24], [25]. Complex IIa and IIb mediate apoptosis through interactions with pro-caspase 8 and other proteins. The procaspase 8 homodimer generates active caspase 8, which on release from complex IIa and IIb into the cytoplasm, results in cleavage reactions that activate further caspases causing apoptosis [26]. Apoptosis is fundamental in epithelial homeostasis, organogenesis, inflammation, immunity and disease pathogenesis. Furthermore, macrophages phagocytose apoptotic cells thus diminishing inflammatory cytokine production [21].
In contrast to complexes IIa and IIb, complex IIc stimulates TNF-induced necroptosis by activating the effector, mixed lineage kinase domain-like protein (MLKL), in a RIPK3-dependent mechanism. Necroptosis results in rupture of plasma membranes and release of intracellular contents triggering inflammation [24]. When this occurs at barriers such as skin and intestinal mucosa, it leads to inflammation that can compromise barrier function. Thus, this pathway is under extensive investigation with RIP kinases considered as potential therapeutic targets.
3. TNF inhibitors and mechanisms of action
Five TNF specific inhibitors were originally approved for clinical practice. Infliximab (Remicade®), adalimumab (Humira®) and golimumab (Simponi®) are full-length bivalent IgG1 monoclonal antibodies targeting TNF. Infliximab is a chimeric antibody while adalimumab and golimumab are fully human monoclonal antibodies. Certolizumab pegol (Cimzia®) is a PEGylated Fab’ antibody fragment in which the Fab’ fragment was engineered with a single hinge region free-cysteine residue enabling attachment of a 40 kDa PEG moiety. Consequently, the antibodies have antigen binding capability via their Fab arms and varying effector functions related to their structure. Infliximab, adalimumab and golimumab are capable of Fc-receptor binding and thus cause complement fixation and antibody-dependent cellular cytotoxicity, however certolizumab is a Fab fragment lacking effector functions. Etanercept (Enbrel®) is a fusion protein of two TNFR2 receptor extracellular domains and the Fc portion of portion of human IgG [27]. Etanercept is unique amongst the TNF antagonists in binding members of the lymphotoxin (LT) family, specifically soluble LTα3 and cell surface LTα2β. LTα3 exerts its biological effects through TNFR1 and TNFR2 and thus etanercept neutralises LTα3 and sTNF with a similar potency [28]. Further details of these TNF antagonists are summarised in Table 1.
The mechanisms by which these inhibitors exert their actions have been extensively studied. Direct blockage of TNF-R mediated activity by binding of TNF antagonists to their cognate ligands (sTNF or tm TNF and additionally LTα3 and LTα2β for etanercept) blocks ligand binding to TNFR1 and TNFR2 and prevents the downstream signalling as detailed above. All five TNF antagonists block interaction between tm TNF and TNFR1 and TNFR2 as expressed on cells with a study by Nesbitt and coworkers suggesting comparable abilities of certolizumab, infliximab and adalimumab in neutralising tmTNF mediated signalling, with etanercept being two-fold less potent [29]. Through direct blockage of TNFR mediated activity, TNF antagonists significantly diminish the inflammatory response as shown in numerous studies of infliximab and adalimumab therapy in different diseases. Infliximab therapy has been shown to decrease RA synovial tissue expression of IL-6, IL-8, granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage chemoattractant protein-1 (MCP-1), IL-1β and Interleukin-18(IL-18) [28], [30]. Further studies have shown a reduction in acute-phase reactants e.g., C-reactive protein (CRP), serum amyloid A and fibrinogen in infliximab-treated and adalimumab-treated RA patients [31], [32], [33]. Similar reductions in CRP levels are observed with infliximab, adalimumab and certolizumab in studies with Crohn’s disease [34], [35], [36]. These studies provide evidence of a reduction in production of pro-inflammatory cytokines with use of TNF antagonists.
A further feature of the TNF antagonists used in clinical practice is their capability to reduce cellularity of inflamed tissue. Significant decreases in plasma and T cells were observed following infliximab infusion in patients with RA [37] and psoriatic arthritis [38]. Additionally, in spondyloarthropathy patients, etanercept reduced cellular infiltration of macrophages and T cells in peripheral joint synovitis [39]. The reduced cellularity is most likely explained by reduced recruitment of inflammatory cells, a process that is dependent upon endothelial cell expression of adhesion molecules and chemokine-mediated leukocyte migration. Supporting this, infliximab treatment in RA was shown to decrease synovial tissue expression of key adhesion molecules, vascular cell adhesion protein-1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1) and E-selectin [40] as well as serum concentrations of E-selectin and ICAM-1 [41]. Similar observations were seen with infliximab infusion in patients with psoriatic skin lesions [42]. Likewise, a reduction in chemokines which recruit macrophages and T cells that form granulomas is seen in the gut mucosa of Crohn’s patients following infliximab therapy [36].
TNF antagonist binding to tmTNF can also induce a phenomenon known as reverse signalling, which leads to apoptosis, cell activation or cytokine suppression. It is understood that binding to tmTNF causes phosphorylation of specific serine residues in the cytoplasmic tail of tmTNF resulting in downstream signal transduction. The full significance and mechanisms of reverse signalling are not known, however, evidence has shown reverse signalling through tmTNF can induce cytokine suppression and endotoxin resistance. Activation of macrophages and monocytes by endotoxin/lipopolysaccharide (LPS) through Toll-like receptor 4 (TLR4) induces cytokines TNF, IL-1β, IL-10 and IL-12, which are also produced at sites of inflammation by tmTNF-bearing macrophages. Initiation of reverse-signalling pathways that intersect with LPS-activated pathways renders these downstream signalling pathways refractory to subsequent activation by LPS, a phenomenon described as ‘exhaustion’ [43], and leads to suppression of cytokine production. The extent of cytokine suppression through reverse signalling by TNF antagonists is variable. Infliximab inhibited LPS-induced TNF and IL-1β production, whereas etanercept did not, in a study with human monocyte cell lines [44]. Similarly, adalimumab and infliximab, but not etanercept, suppressed LPS-induced Interleukin-10 (IL-10) and Interleukin-12 (IL-12) production by human monocytes [45]. Both infliximab and etanercept were observed to inhibit release of LPS-induced endothelial cell apoptotic factor (Death Factor X) [46].
A further noted consequence of reverse signalling by infliximab and etanercept through tmTNF in activated T cells is induction of E-selectin [47]. T cell expression of E-selectin allows cell adhesion at sites of inflammation. Furthermore, infliximab, but not etanercept suppresses proliferation of T cells by causing GO/G1 cell cycle arrest through tmTNF reverse signalling. Being a bivalent monoclonal antibody, Infliximab can crosslink monomeric TNF subunits and induce cell cycle arrest whereas etanercept alone (in the absence of rheumatoid factor) cannot, but interestingly is capable of inducing cell cycle arrest in the presence of rheumatoid factor [47]. Indeed, these studies suggest that reverse signalling through tmTNF is an important mechanism for TNF-agonist mediated apoptosis and cytokine suppression.
Through Fc-dependent complement dependent cytotoxicity (CDC) or antibody dependent cell mediated cytotoxicity (ADCC), TNF antagonists may cause cytotoxicity of tmTNF-bearing cells [28]. In CDC, binding of complement component 1q (C1q) to the CH2 domain in the Fc region of cell-bound antibodies or Fc-fusion proteins initiates complement activation by the classical pathway triggering the complement cascade which results in formation of the membrane attack complex, subsequent pore formation and cellular lysis. In ADCC, engagement of target cells causes Fc receptor mediated binding of leukocytes – typically natural killer (NK) cells which produce cell-lysing proteins such as granzymes to kill target cells expressing tmTNF. Studies are inconsistent and conflicting in showing the ability of infliximab in mediating CDC or ADCC when binding tmTNF [28], [47] but there is increasing evidence for these mechanisms in studies from biosimilars; this is also the case with adalimumab [49], [50], [51]. A study of adalimumab, infliximab, etanercept and certolizumab found that all four inhibitors bound to tmTNF, but CDC and ADCC was mediated only by adalimumab, etanercept and infliximab, all of which have IgG1 Fc [52] unlike Certolizumab which is solely a PEGylated Fab’ fragment (lacks the Fc portion). Based on these in vitro results, it is likely these agents are capable of inducing destruction of TNF-producing cells in vivo. However, a further study showed that etanercept mediated only ADCC activity and lacked both CDC activity and reverse-signalling through tmTNF, indicating that etanercept may be less effective than infliximab and adalimumab in elimination of TNF-producing cells. Interestingly, since all three agents neutralise soluble TNF-α and are effective in treatment of RA, it is likely that pathogenesis of RA primarily involves soluble TNF rather than tmTNF. In contrast, in granulomatous conditions such as Crohn’s and Wegener’s granulomatosis, tmTNF may have a significant role as etanercept which has limited effect on tmTNF-α is not clinically effective for these diseases[44].
4. Role of TNF inhibitors in Rheumatoid Arthritis
RA is a chronic inflammatory autoimmune disease that causes joint pain, swelling and stiffness as a result of progressive joint destruction. It is characterised by chronic inflammation and infiltration of synovial joints by haematopoietic cells including memory T cells, macrophages and plasma cells [53]. Activation of these cells leads to release of cytokines predominantly in the hyperplastic synovium resulting in cartilage destruction triggered by cytokine induction of destructive enzymes (matrix metalloproteinases) [53], osteoclast differentiation and invasion of the periosteal surface with associated bone erosion at the joint surface. TNF, in particular, has been identified as playing a fundamental role in driving inflammation and bone degradation in the disease through activation of cytokine and chemokine expression – as an autocrine stimulator and paracrine inducer of pro-inflammatory cytokines including IL-1, IL-6, IL-8 and GM-CSF - expression of endothelial cell adhesion molecules, protection of synovium fibroblasts, promotion of angiogenesis, suppression of regulatory T cell function and induction of pain [54].
The introduction of biological medicines, particularly TNF inhibitors, has revolutionised treatment for RA, beyond the initial disease modifying anti rheumatoid drugs (DMARDs) that were available. As mentioned previously, TNF antagonists bind to sTNF limiting its ability to bind to membrane bound TNF receptors and activate downstream inflammatory pathways, thus limiting the inflammation that drives the pathogenesis of rheumatoid arthritis. Initial evidence for the role of TNF antagonists came from laboratory studies showing that addition of anti-TNF antibodies to synovial cultures from patients reduced synovial expression of pro-inflammatory cytokines including GM-CSF, IL-1, IL-6 and IL-8 [55]. It was subsequently found that virtually all animal models of arthritis were ameliorated by anti-TNF [53]. Infliximab in clinical trials in RA patients demonstrated reduced radiographic progression, clinical amelioration and improved disease scoring (as measured by the Health Assessment Questionnaire for rheumatoid arthritis)[56], [57], [58] leading to its approval. Since then, several trials have shown adalimumab, golimumab, certolizumab and etanercept to be efficacious in improving signs and symptoms of RA and functional status of patients with the disease [59] such that TNF antagonists are now routinely used world-wide to treat RA in clinical practice. In the UK, NICE guidelines recommend these inhibitors as options for treatment of severe RA that has not responded to conventional DMARDs[60].
5. Role of TNF-alpha inhibitors in Inflammatory Bowel Disease
IBD describes chronic inflammatory disorders of the digestive tract and refers mainly to two conditions: ulcerative colitis (UC) and Crohn’s disease (CD). Ulcerative colitis is characterised by continuous chronic inflammation throughout the colon and rectum whereas Crohn’s disease is characterised by transmural, patchy granulomatous inflammation anywhere in the digestive tract. Although the exact aetiology of the two diseases remains poorly understood, their pathogenesis is thought to be due to complex interplay between genetic and environmental factors. Just like the synovial infiltration seen in RA, IBD patients have significant immune cell infiltration within the gut mucosa contributing to inflammation and extensive tissue damage. Additionally, several cytokines – IL-1, IL-6, IL-8, IL-12, IL-18 and TNF are found within inflamed intestinal mucosa and these recruit blood-borne effectors in the mucosa through adhesion molecules and chemokines, leading to further secretion of inflammatory agents and destructive enzymes which contribute to tissue injury, mucosal permeability and fibrosis [61].
Early evidence for TNF in IBD pathology came from detection of increased levels of TNF protein and mRNA levels in mucosal biopsies from patients with CD [62]. Further to this, in vitro experiments using specimens from patients participating in clinical trials of TNF antagonists found that downregulation of TNF and IFN-γ production was associated with reduced functional activity of Th1 T cells in the mucosa of treated patients suggesting a potential mechanism of action of these molecules in IBD [61]. A further proposed mechanism is TNF-mediated leukocyte and T cell apoptosis that was identified to occur by 24 h after administration of infliximab. It has been suggested that this may be induced directly by binding of TNF antagonists to tmTNF on activated T cells or alternatively by interrupting anti-apoptotic signalling through TNFR2 expressed on lamina propria T cells [63]. It has additionally been observed that TNF antagonists with an Fc region are capable of inducing M2-type wound-healing macrophages both in vitro and in vivo that may contribute to mucosal healing. This is supported by greater efficacy of infliximab and adalimumab in mucosal healing in IBD as compared with certolizumab. Additionally, it is proposed that TNF antagonist-mediated induction of CDC and ADCC contributes to their therapeutic benefit in IBD, however this remains to be proven in vivo [49], [62].
Just like for RA, infliximab was the first biological medicine to be used in IBD treatment, first in CD and subsequently in UC. Several trials have demonstrated the efficacy of infliximab in induction and maintenance of remission of CD and UC in patients and subsequently further trials showed adalimumab has a similar therapeutic benefit in inducing and maintaining remission of disease leading to their use for UC and CD in clinical practice. Golimumab has since been shown to have efficacy in UC and certolizumab in CD; the latter is approved for CD in US but not in EU [64], [65].
6. Potential role for TNF inhibitors in COVID-19 cytokine storm
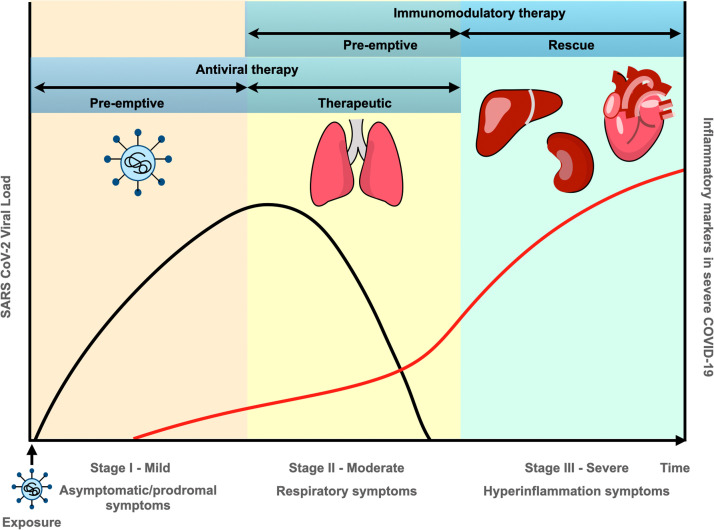
The pathogenesis of COVID-19 has been found to have three distinct stages ( Fig. 1). Stage I (mild) involves infection and early establishment of the disease. SARS-CoV-2 enters host cells by binding to the cell surface receptor of ACE-2 via a trimeric spike glycoprotein [66]. Following entry into cells, the virus is able to replicate and spread throughout the body, infecting cells expressing ACE-2 and other receptors (e.g. neuropilin-1). Entry of virus into the body, triggers the innate and adaptive immune responses to aid the clearance of the virus, and in a majority of people, the immune response clears the virus with mild to moderate symptoms of malaise, fever and dry cough. Treatment at this stage is primarily targeted towards symptomatic relief. Based on evidence suggesting that the anti-viral agent, remdesivir could be beneficial in reducing the duration of symptoms, minimising infectiousness and preventing severe disease progression, remdesivir is now approved for COVID-19 therapy (emergency authorisation by FDA/ conditional authorisation by EMA) [67].
Fig. 1.
Stages of COVID-19 pathogenesis and therapeutic intervention.
Stage II (moderate) involves established pulmonary disease characterised by viral multiplication and localised inflammation – acute lung injury (ALI). During this stage, patients develop a characteristic viral pneumonia with symptoms of cough, fever and hypoxia. Chest imaging with X-rays and CT scans shows bilateral pulmonary infiltrates and inflammatory markers begin to become elevated. In a significant number of COVID-19 infected patients, viral infection leading to acute lung injury may further progress to acute respiratory distress syndrome (ARDS), accounting for their deaths. Consequently, increasing respiratory support ranging from supplemental oxygen to invasive mechanical ventilation is required. Since the RECOVERY trial showed that dexamethasone reduces 28-day mortality in patients receiving respiratory support, this treatment is being used widely [68]. The benefit of dexamethasone became clear in patients treated more than seven days after symptom onset, when inflammatory lung changes are evident, confirming that the disease at this stage is dominated by immunopathological elements rather than active viral replication.
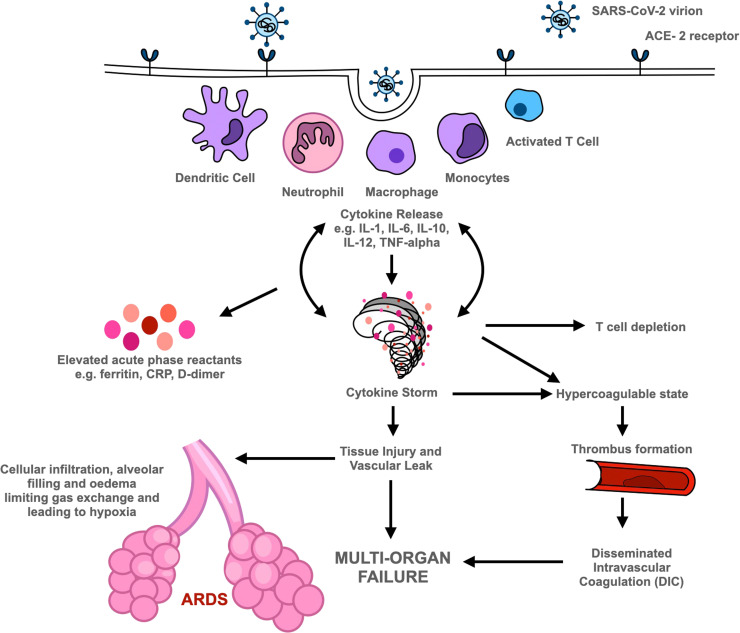
Stage III (severe) is characterised by systemic inflammation. Viral replication within the lungs leads to increased levels of pro-inflammatory cytokines and chemokines (e.g. IFN-α/β, interferon-gamma, IFN-γ and TNF) due to activation of the host immune response. Indeed, ARDS could be considered as the hallmark immune-mediated clinical consequence of SARS-CoV-2 [69]. Huang et al. found that in SARS-CoV-2 patients in intensive care units (ICU), plasma concentrations of several interleukins e.g., IL-2, IL-7, IL-10, granulocyte-colony stimulating factor (G-CSF), Interferon gamma inducible protein-10 (IP-10), MCP-1, macrophage inflammatory protein 1-alpha (MIP-1α), and TNF were significantly raised compared with patients with less severe disease who did not require ICU admission [70]. Indeed, elevated levels of these cytokines were seen previously with SARS-CoV and MERS-CoV infections and linked to development of ARDS. High levels of circulating pro-inflammatory cytokines result in neutrophil recruitment to areas of inflamed lung. Activated neutrophils release chemokines which increase leukocyte recruitment and further exacerbate the inflammatory response. Release of reactive oxygen species, granule contents and neutrophil extracellular traps (NETs) results in host epithelial and endothelial cell damage [71], whilst increasing levels of cytokines also induce cellular apoptosis. This disrupts the alveolar epithelial and endothelial barrier leading to increased vascular leakage and protein-rich fluid collecting in alveoli leading to respiratory insufficiency and hypoxia [69], [70], [72], [73]. A schematic diagram is shown in Fig. 2. A contributory factor in the exacerbation of viral replication and rapid progression of the immunopathological response is the deficient synthesis of type I IFNs – possibly related to genetic mutations or the existence of autoantibodies against IFN-α subtypes impacting IFN function [74], [75]. Reduced levels of type I IFNs are also associated with increased susceptibility to superimposed bacterial infections, which may lead to sepsis causing death [76].
Fig. 2.
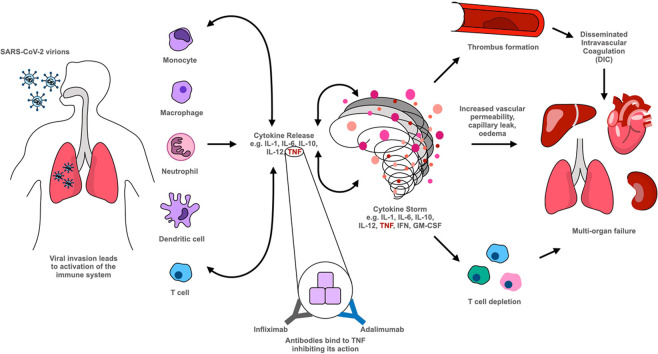
Pathogenesis of COVID-19. SARS-CoV-2 virion binds to ACE-2 receptor gaining entry into cells. This triggers the innate and adaptive immune responses, with cytokine release and elevated levels of acute phase reactants. Increased cytokine release contributes to T cell depletion and cytokine storm. Cytokine storm has multiple effects including widespread activation of the coagulation cascade contributing to thrombus formation and can further lead to disseminated intravascular coagulation; tissue injury within the lung that can progress to acute respiratory distress syndrome (ARDS); systemically increased vascular permeability and tissue injury that can cause multi-organ failure and subsequent death. Footnote (to appear belowFig. 2): WHO international standards are available for a wide range of substances from NIBSC, https://www.nibsc.org/. These include systemic markers of inflammation (cytokines, chemokines, other biomarkers e.g., C-reactive protein, ferritin etc) for use in assays used in measurement of these analytes. Recently available international standards and other reagents for SARS-CoV-2 and COVID-19 research include 1stWHO IS for SARS-CoV-2 RNA for nucleic-acid amplification assays and the WHO IS for anti-SARS-COV-2 for serology assays.
Clearance of SARS-CoV-2, similar to other respiratory viral infections, depends greatly on the adaptive immune response, and particularly that of T-cells [77], [78], [79], [80]. Lymphopenia has been identified as a significant feature of SARS-CoV-2, which is noted to be reversed when patients recover. Some data suggests lymphopenia affects CD4+ T cells, CD8+ T cells, B cells and natural killer cells, however other data suggests SARS-CoV-2 infection preferentially impacts CD8+ T cells [81]. In severe disease, high levels of IL-6, IL-10 and TNF are thought to be related to T cell depletion [77], [80], [82] with T cell numbers negatively associated with serum IL-6, IL-10 and TNF concentration, with restored T cell counts and decreased levels of IL-6, IL-10 and TNF seen with disease resolution. It has been postulated that T cell depletion may be the critical step in causing COVID pneumonia. Interestingly, T cell responses also dampen the innate immune response [83], [84], thus T cell death can contribute to amplification of the inflammatory response.
Data thus indicates that release of pro-inflammatory cytokines (IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-18, IL-33, TNF, TGF-β) and chemokines (CXCL10, CXCL8, CXCL9, CCL2, CCL3, CCL5) leads to an excessive systemic inflammatory response and immune dysregulation contributing to cardiopulmonary collapse that manifests clinically as shock and leads to multi-organ failure and death in the most severe of cases [70], [71], [72]. A further cause of death in COVID-19 patients is the significant incidence of both pulmonary and extrapulmonary venous and arterial thromboembolisms that are associated with the disease. The exact pathogenesis is yet uncertain however thromboembolism is associated with a 74% higher odds of mortality in COVID-19 patients [85]. Furthermore, systemic inflammation can even lead to widespread activation of the clotting cascade resulting in disseminated intravascular coagulation (DIC). To reduce the risk of thromboembolism, patients admitted with COVID-19 are now commenced on prophylactic heparin [86].
Based on the above, effective therapy would hinge on using immunomodulators to reduce systemic inflammation before it results in highly severe disease and multi-organ dysfunction. Utilisation of cytokine inhibitors such as tocilizumab, an IL-6 receptor antibody, anti-TNF alpha agents and anakinra, an IL-1 receptor antagonist between stages II and III may have considerable role in slowing disease progression [87] (Fig. 1). Indeed, a small cohort study has recently shown IL-1 inhibition with anakinra to be associated with a significant reduction of mortality in hospitalized patients with COVID-19 respiratory insufficiency and hyperinflammation [88].
Feldman and colleagues [89] have already highlighted that cytokines, especially TNF, are a promising therapeutic target for COVID-19 patients since TNF is pivotal in initiating the inflammatory cascade by coordinating cellular recruitment through chemokines and cell adhesion molecules [90]. Indeed, evidence from animal models of sepsis and RA patients shows blocking TNF leads to decreased levels of circulating pro-inflammatory cytokines, IL-1, TNF and IL-6 [91], [92] and more importantly also of vascular endothelial growth factor (also known as vascular permeability factor) suggesting a further potential benefit of anti-TNF drugs in COVID-19 therapy particularly as these cytokines are of relevance in capillary leakage associated with deteriorating lung function in patients with COVID-19 [93]. Furthermore, acute phase proteins are reduced, many within 24 h of anti-TNF treatment with down-regulation of CRP, serum amyloid A and haptoglobin as previously mentioned. Anti-TNF administration also appears to have a significant effect on the clotting cascade, with rapid and significant reduction in plasma biomarkers of coagulation such as D-dimer and pro-thrombin fragments [94], suggesting anti-TNF therapy may also reduce COVID-19 related thromboembolic events. Such dampening of acute phase response and clotting cascade seems unique for anti-TNFs and is not documented for anti-IL-6 or anti-IL-1 therapies [93], [95]. Furthermore, TNF antagonists are capable of reducing the cellularity of inflamed tissue due to reduced endothelial cell expression of adhesion molecules [40], [41]and chemokine-mediated leukocyte migration and recruitment of inflammatory cells [41], [89] that is evident in the lung pathology in COVID-19. This is supported by studies in SARS-COV and SARS-COV-2 infected animals and also in models of sepsis, hemophagocytic lymphohistiocytosis (HLH), and cytokine shock [96], [97]. Data from SARS-COV infected mice showed that severe disease contributing to lung immunopathology caused by accumulation of inflammatory monocyte macrophages and associated increase in IL-1β, TNF and IL-6 is ameliorated by depletion of inflammatory cells which, also reduce vascular leakage or by administration of a neutralising TNF antibody; the latter provided partial but significant protection in comparison with control mice [96]. Karki and coworkers elegantly showed that a combination of neutralising antibodies against both TNF and IFN-γ (rather than alone) protected mice from mortality caused by the synergistic effect of TNF and IFN-γ on inflammatory cell death, tissue damage in SARS-COV-2 infected mice [97]. A similar response was also seen in sepsis, HLH and cytokine shock reinforcing the notion that inhibiting cytokine-mediated inflammatory cell death signalling pathway may prove beneficial in patients with COVID-19 or other infectious and auto-inflammatory diseases by limiting tissue damage/inflammation [97]. Additionally, anti-TNF therapy can target NETosis or NET release, a mechanism whereby neutrophils release sticky extracellular traps to limit the spread of infection. Patients with COVID-19 demonstrate higher levels of NETs than healthy patients and SARS-CoV-2 has been shown to directly enhance the release of NETs [95], [98]. NETs are prothrombotic and activate platelets within the clotting cascade, and NET-containing microthrombi have been found in the lungs of patients with COVID-19. Anti-TNF therapy has been found to reduce NET formation in vitro, in animal models and in patients with immune mediated disease, suggesting another putative role in targeting NETosis in COVID-19 [95]. Consequently, it is likely that anti-TNF therapy can rein in some of the processes that occur in the inflammation-driven cascade and have a major impact on lung pathology and patient mortality.
Observational data has indicated the potential benefit of TNF inhibitors in COVID-19 patients. The COVID-19 global rheumatology alliance registry examined the relationship between drug treatments and COVID-19 infection in patients with rheumatic disease [99]. Data showed TNF inhibitor use reduced the risk of hospitalisation in RA patients diagnosed with COVID-19 infection. Additionally, the use of biologics such as IL-6, IL-17, IL-23 and TNF inhibitors was not associated with COVID-19 related deaths as compared with methotrexate monotherapy in patients with RA. In contrast, high doses of glucocorticoids, sulfasalazine and rituximab were associated with higher COVID-19 related deaths in patients with rheumatic disease [100]. Similarly, an analysis of outcomes, therapy and epidemiology of COVID-19 infection in the global psoriasis patient registry revealed that use of biologics – including TNF inhibitors, IL-17 inhibitors, IL-12/IL-23p40 or IL-23p19 inhibitors – reduced the risk of COVID-19-related hospitalisation [101]. Further support comes from studying outcomes of patients with IBD and confirmed COVID-19 in the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry. Notably, TNF antagonist use was not significantly associated with severe COVID-19 as defined by the composite of ICU admission, ventilator use, and/or death, and was in fact inversely associated with the outcome of hospitalisation of death in this registry [102]. Despite the limitations of observational data – namely selection bias, channelling bias and difficulties adjusting for all confounding variables – these observational studies lend promise to the suggestion of using TNF inhibitors for COVID-19.
Early feasibility data from a case series involving use of infliximab as an experimental therapy in critically ill patients with confirmed COVID-19 in the absence of chronic inflammatory disease also showed a reduction in pro-inflammatory cytokines over the first ten days following administration of a single dose of infliximab 5 mg/kg between day 0 and day 3 after admission [95]. Although a small study that needs to be treated with caution, it is nevertheless indicative of the potential therapeutic benefit of TNF antagonists in COVID-19.
7. Trials of TNF inhibitors in COVID-19
Table 2 highlights the different clinical trials currently underway. The CATALYST phase 2 randomised platform trial (ISRCTN40580903) is currently recruiting hospitalised adult patients (≥ 16 years) with confirmed diagnosis of COVID-19 with reverse transcription polymerase chain reaction (RT-PCR) in the United Kingdom. Following diagnosis, patients are randomised into three active treatment groups; Mylotarg (anti-CD33), namilumab (anti-GM-CSF) and infliximab (anti-TNF) or the standard of care treatment group. Those patients randomised into the infliximab group are given usual care plus a single dose of infliximab 5 mg/kg intravenously and actively followed up for 28 days from this dose. The primary outcome measure is the ratio of the oxygen saturation to fractional inspired oxygen concentration (SpO2/FiO2), and secondary outcomes include efficacy as measured by time to improvement to day 7, 14 and 28, clinical observations including CRP, full blood count (FBC), ferritin, D-dimer, lactate dehydrogenase (LDH) and triglyceride levels to day 14, hospital discharge/survival status at day 28 and incidence of adverse events. The trial completed in May 2021 and it is expected that preliminary data will be released in the next few months.
Table 2.
Trials of TNF inhibitors in COVID-19.
| Trial (Country) | Design | Intervention | Patient Cohort | Cases/Controls | Status | Trial Number |
|---|---|---|---|---|---|---|
| CATALYST (UK) | Randomised controlled platform study - prospective | Infliximab vs Nalimumab vs Mylotarg vs Standard | Hospitalized | 60 patients per intervention arm 1:1 | Completed Awaiting results | ISRCTN40580903 |
| Tufts (USA) | Uncontrolled single arm study | Infliximab | Hospitalized | 17 cases and 0 controls | Awaiting results | NCT04425538 |
| ACTIV-1 (USA) | Randomised control platform study | Remdesivir + Infliximab vs Remdesivir + Abatacept vs Remdesivir +Cenicriviroc vs Standard | Hospitalized | 2160 patients across 3 interventions and 1 control arm | Recruiting | NCT04593940 |
| AVID-CC (UK) | Randomised controlled study | Adalimumab vs standard | Community | 375 patients per arm 1:1 | Recruiting | ISRCTN33260034 |
| COMBAAT (USA) | Randomised controlled study | Adalimumab vs standard | Community | 1444 patients across 2 arms 1:1 | Pre-recruitment | NCT04705844 |
| Xu (China) | Randomised controlled study | Adalimumab vs standard | Severe or critically ill | 30 patients per arm 1:1 | Suspended | ChiCTR2000030089 |
Tufts Medical Center is currently running a small uncontrolled Phase 2 Trial of infliximab in COVID-19 (NCT04425538). 17 adult patients (≥ 18 years) with either laboratory confirmed infection by RT-PCR assay or strongly suspected to be infected with SARS-CoV-2 pending confirmation studies and either respiratory rate of ≥ 30/min, blood oxygen saturation ≤ 93% on room air, partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2/FiO2)< 300 or deteriorating lung involvement have been included. These patients have been treated with infliximab or the biosimilar infliximab-abda 5 mg/kg intravenously.
Retreatment with infliximab is permitted at the discretion of the physician at 1–3 weeks following primary therapy and based on initial response; the usual treatment schedule is every 2 weeks although given the uncertainty of outcomes with primary therapy, this interval is not strict. The primary outcome is time to improvement in oxygenation sustained for at least 48 h, with various secondary outcome measures including mortality at 28 days, assessment of cytokine and inflammatory profile to 48 h, incidence and duration of: supplemental oxygen administration, mechanical ventilation, vasopressor support, extracorporeal membrane oxygenation, fever, hospitalisation and secondary infections to 28 days. The study was expected to be completed in February 2021 with results expected in due course.
The ACTIV-1 phase 3 randomised control trial (NCT04593940) aims to evaluate the effectiveness of either infliximab or abatacept (a T cell co-stimulatory blocker) or cenicriviroc (a CCR2-CCR5 dual antagonist) in combination with remdesivir. The trial aims to recruit 2160 hospitalised or emergency department adult patients (≥ 18 years) with a confirmed SARS-CoV-2 diagnosis and ongoing illness as defined as radiographic infiltrates on imaging, blood oxygen saturation ≤ 94% on room air, requiring oxygen or ventilator support. Those randomised to the infliximab arm will be given remdesivir plus a single dose of infliximab 5 mg/kg intravenously. The primary outcome measure is the number of patients that recover from COVID-19 by day 29 with secondary outcomes including number of patients that improved clinically to 60 days, number of patient deaths by day 14, change in number of patients hospitalised on invasive mechanical ventilation by day 29 and number of days in hospital. The study is due to be completed in September 2021.
The AVID-CC trial is a phase 2 randomised control trial (ISRCTN33260034) that aims to trial adalimumab in the community for preventing progression from community infection to severe disease as defined by severe illness, critical illness or death. The trial will recruit 750 adults (≥18 years) with confirmed SARS-CoV-2 infection, CRP >50 mg/L or lymphopenia (<1.5 × 109/L) or neutrophilia (>7.5 × 109/L) and oxygen saturation >93% on air, across the two arms of the study – adalimumab and standard of care. Those randomised to the adalimumab arm will either receive a loading dose of 80 mg or 160 mg subcutaneously and those with persistent symptoms will receive a second adalimumab dose of 40 mg or 80 mg respectively, after 14 days. Patient follow up will continue until 120 days. The primary outcome is the rate of progression to severe disease as defined by severe illness, critical illness, or death from any cause in COVID-19 community patients at 28 days. Several secondary outcome measures such as adverse events including serious events, clinical status, admission to secondary care, incidence of venous thromboembolism and acute kidney injury are additionally recorded.
The COMBAAT phase 3 randomised double blind placebo controlled trial (NCT04705844) aims to establish whether treatment with adalimumab is associated with a lower rate of progression to severe disease or death in outpatients with mild-moderate COVID-19. The trial will recruit 1444 patients aged between 40 and 80 years with confirmed SARS-CoV-2 infection, COVID-related symptoms, CRP >50 mg/L or lymphopenia (<1.5 × 109/L) or neutrophilia (>7.5 × 109/L) and oxygen saturation >93% on air, across the two arms of the study – adalimumab and placebo. The experimental arm will receive adalimumab 160 mg subcutaneously. The primary outcome measures are the rate of progression to severe disease as defined by severe illness or critical illness, or death in outpatient subjects with COVID-19 to day 28 and the incidence of adverse events to day 28. Secondary outcomes include clinical status (using WHO COVID-19 ordinal scale) from first dose to day 120. The study is expected to start in May 2021 and continue until November 2021.
Another adalimumab trial for severe COVID-19 (ChiCTR2000030089), due to recruit 30 patients in each arm and commence in February 2020 was suspended due to the pandemic being controlled in China.
8. Safety considerations in anti-TNF therapy
Use of anti-TNFs for the aforementioned conditions has highlighted the potential for secondary infections due to the role of TNF in host defence [95]. Long-term use of antagonists can result in greater risk of infection due to increased exposure as noted in some patients with chronic inflammatory conditions, and such risk is compounded with concomitant chronic glucocorticoid use. Further safety consideration arises with the possibility of reactivation of latent tuberculosis, which is again related to exposure. In the case of anti-TNF use for COVID-19, however, the risk of secondary infection is likely to be much lower as inhibitors are only likely to be used in the short-term for a single dose or at most two doses which also potentially eliminates any concerns around immunogenicity that are now well recognised in recipients of these products [103], [104], [105].
9. Conclusion
The need for effective therapies to reduce disease mortality and COVID-19 related damage in patients is increasingly evident, despite the development of vaccination strategies. Based on their mechanism of action and their demonstrated utility in chronic inflammatory conditions and early feasibility studies in COVID-19, there is a strong case for TNF inhibitor use in COVID-19. Ongoing randomised trials with different TNF antagonists will provide information on the effectiveness of these therapeutic interventions along with the risks of these measures in COVID-19 therapy. However, given that the disease varies considerably among individual patients, it is likely that tailored approaches which include combination therapies may be necessary to achieve an optimal outcome.
Conflict of interest statement
This manuscript, based on independent research, has been conceptually prepared and written by the authors. The authors report no declarations of interest and the views expressed in this manuscript are solely those of the authors. This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheuma. 2020:1–10. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 4.Bradley J.R. TNF-mediated inflammatory disease. J. Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 5.Radner H., Aletaha D. Anti-TNF in rheumatoid arthritis: an overview. Wien. Med. Woche. 1946;165(2015):3–9. doi: 10.1007/s10354-015-0344-y. [DOI] [PubMed] [Google Scholar]
- 6.Pugliese D., Felice C., Papa A., Gasbarrini A., Rapaccini G.L., Guidi L., Armuzzi A. Anti TNF-α therapy for ulcerative colitis: current status and prospects for the future. Expert Rev. Clin. Immunol. 2017;13:223–233. doi: 10.1080/1744666X.2017.1243468. [DOI] [PubMed] [Google Scholar]
- 7.Campanati A., Paolinelli M., Diotallevi F., Martina E., Molinelli E., Offidani A. Pharmacodynamics OF TNF α inhibitors for the treatment of psoriasis. Expert Opin. Drug Metab. Toxicol. 2019;15:913–925. doi: 10.1080/17425255.2019.1681969. [DOI] [PubMed] [Google Scholar]
- 8.Monaco C., Nanchahal J., Taylor P., Feldmann M. Anti-TNF therapy: past, present and future. Int. Immunol. 2015;27:55–62. doi: 10.1093/intimm/dxu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadhwa M., Bird C., Dilger P., Rigsby P., Jia H., Gross M.E.B., participants of the study Establishment of the first WHO International Standard for etanercept, a TNF receptor II Fc fusion protein: report of an international collaborative study. J. Immunol. Methods. 2017;447:14–22. doi: 10.1016/j.jim.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe C., Dougall T., Bird C., Rigsby P., Behr-Gross M.-E., Wadhwa M., participants of the study The first World Health Organization International Standard for infliximab products: a step towards maintaining harmonized biological activity. MAbs. 2018;11:13–25. doi: 10.1080/19420862.2018.1532766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wadhwa M., Bird C., Atkinson E., Cludts I., Rigsby P. The first WHO international standard for adalimumab: dual role in bioactivity and therapeutic drug monitoring. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.636420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prior S., Metcalfe C., Hufton S.E., Wadhwa M., Schneider C.K., Burns C. Maintaining ‘standards’ for biosimilar monoclonal antibodies. Nat. Biotechnol. 2021:1–5. doi: 10.1038/s41587-021-00848-0. [DOI] [PubMed] [Google Scholar]
- 13.Shamsi A., Mohammad T., Anwar S., AlAjmi M.F., Hussain A., Rehman Md.T., Islam A., Hassan Md.I. Glecaprevir and Maraviroc are high-affinity inhibitors of SARS-CoV-2 main protease: possible implication in COVID-19 therapy. Biosci. Rep. 2020;40 doi: 10.1042/BSR20201256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamsi A., Mohammad T., Anwar S., Amani S., Khan M.S., Husain F.M., Rehman Md.T., Islam A., Hassan M.I. Potential drug targets of SARS-CoV-2: From genomics to therapeutics. Int. J. Biol. Macromol. 2021;177:1–9. doi: 10.1016/j.ijbiomac.2021.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monk P.D., Marsden R.J., Tear V.J., Brookes J., Batten T.N., Mankowski M., Gabbay F.J., Davies D.E., Holgate S.T., Ho L.-P., Clark T., Djukanovic R., Wilkinson T.M.A., Crooks M.G., Dosanjh D.P., Siddiqui S., Rahman N.M., Smith J.A., Horsley A., Harrison T.W., Saralaya D., McGarvey L., Watson A., Foster E., Fleet A., Singh D., Hemmings S., Aitken S., Dudley S., Beegan R., Thompson A., Rodrigues P.M. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial, Lancet. Respir. Med. 2021;9:196–206. doi: 10.1016/S2213-2600(20)30511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nissen C.B., Sciascia S., de Andrade D., Atsumi T., Bruce I.N., Cron R.Q., Hendricks O., Roccatello D., Stach K., Trunfio M., Vinet É., Schreiber K. The role of antirheumatics in patients with COVID-19. Lancet Rheumatol. 2021;0 doi: 10.1016/S2665-9913(21)00062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise J. Covid-19: Arthritis drugs improve survival in intensive care patients, shows study. BMJ. 2021;372:61. doi: 10.1136/bmj.n61. [DOI] [PubMed] [Google Scholar]
- 18.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. The Lancet. 2021;397(10285):1637–1645. doi: 10.1016/S0140-6736(21)00676-0. https://www.sciencedirect.com/science/article/pii/S0140673621006760?via%3Dihub#fn1 accessed May 29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalliolias G.D., Ivashkiv L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016;12:49–62. doi: 10.1038/nrrheum.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi K., Meier P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol. Cell. 2009;36:736–742. doi: 10.1016/j.molcel.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 21.Brenner D., Blaser H., Mak T.W. Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol. 2015;15:362–374. doi: 10.1038/nri3834. [DOI] [PubMed] [Google Scholar]
- 22.Silke J., Rickard J.A., Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat. Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 23.Linkermann A., Green D.R. Necroptosis. N. Engl. J. Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 25.Chan F.K.-M., Luz N.F., Moriwaki K. Programmed necrosis in the cross talk of cell death and inflammation. Annu. Rev. Immunol. 2015;33:79–106. doi: 10.1146/annurev-immunol-032414-112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L., Du F., Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Taylor P.C. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr. Opin. Pharmacol. 2010;10:308–315. doi: 10.1016/j.coph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Tracey D., Klareskog L., Sasso E.H., Salfeld J.G., Tak P.P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Nesbitt A., Fossati G., Bergin M., Stephens P., Stephens S., Foulkes R., Brown D., Robinson M., Bourne T. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor α agents. Inflamm. Bowel Dis. 2007;13:1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 30.Maini R.N., Feldmann M. How does infliximab work in rheumatoid arthritis? Arthritis Res. 2002;4(Suppl 2):22–28. doi: 10.1186/ar549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.den Broeder A.A., Joosten L.A.B., Saxne T., Heinegård D., Fenner H., Miltenburg A.M.M., Frasa W.L.H., van Tits L.J., Buurman W.A., van Riel P.L.C.M., van de Putte L.B.A., Barrera P. Long term anti-tumour necrosis factor α monotherapy in rheumatoid arthritis: effect on radiological course and prognostic value of markers of cartilage turnover and endothelial activation. Ann. Rheum. Dis. 2002;61:311–318. doi: 10.1136/ard.61.4.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrera P., Joosten L.A.B., Den Broeder A.A., Van de Putte L.B.A., Van Riel P.L.C.M., Van den Berg W.B. Effects of treatment with a fully human anti-tumour necrosis factor α monoclonal antibody on the local and systemic homeostasis of interleukin 1 and TNFα in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2001;60:660–669. doi: 10.1136/ard.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elliott M.J., Maini R.N., Feldmann M., Long‐Fox A., Charles P., Katsikis P., Brennan F.M., Walker J., Bijl H., Ghrayeb J., Woody J.N. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber S., Rutgeerts P., Fedorak R.N., Khaliq–Kareemi M., Kamm M.A., Boivin M., Bernstein C.N., Staun M., Thomsen O.Ø., Innes A., Randomized A. Placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 35.Hanauer S.B., Sandborn W.J., Rutgeerts P., Fedorak R.N., Lukas M., MacIntosh D., Panaccione R., Wolf D., Pollack P. Human anti–tumor necrosis factor monoclonal antibody (Adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 36.Deventer S.J.V. Tumour necrosis factor and Crohn’s disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smeets T.J.M., Kraan M.C., Van Loon M.E., Tak P.-P. Tumor necrosis factor α blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 2003;48:2155–2162. doi: 10.1002/art.11098. [DOI] [PubMed] [Google Scholar]
- 38.Goedkoop A.Y., Kraan M.C., Picavet D.I., de Rie M.A., Teunissen M.B.M., Bos J.D., Tak P.P. Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study. Arthritis Res. Ther. 2004;6:R326–R334. doi: 10.1186/ar1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruithof E., De Rycke L., Roth J., Mielants H., Van den Bosch F., De Keyser F., Veys E.M., Baeten D. Immunomodulatory effects of etanercept on peripheral joint synovitis in the spondylarthropathies. Arthritis Rheum. 2005;52:3898–3909. doi: 10.1002/art.21426. [DOI] [PubMed] [Google Scholar]
- 40.Tak P.P., Taylor P.C., Breedveld F.C., Smeets T.J.M., Daha M.R., Kluin P.M., Meinders A.E., Maini R.N. Decrease in cellularity and expression of adhesion molecules by anti–tumor necrosis factor α monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- 41.Paleolog E.M., Hunt M., Elliott M.J., Feldmann M., Maini R.N., Woody J.N. Deactivation of vascular endothelium by monoclonal anti–tumor necrosis factor α antibody in rheumatoid arthritis. Arthritis Rheum. 1996;39:1082–1091. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- 42.Goedkoop A.Y., Kraan M.C., Teunissen M.B.M., Picavet D.I., De Rie M.A., Bos J.D., Tak P.P. Early effects of tumour necrosis factor α blockade on skin and synovial tissue in patients with active psoriasis and psoriatic arthritis. Ann. Rheum. Dis. 2004;63:769–773. doi: 10.1136/ard.2003.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eissner G., Kolch W., Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15:353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Mitoma H., Horiuchi T., Tsukamoto H., Tamimoto Y., Kimoto Y., Uchino A., To K., Harashima S., Hatta N., Harada M. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58:1248–1257. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 45.Shen C., Assche G.V., Colpaert S., Maerten P., Geboes K., Rutgeerts P., Ceuppens J.L. Adalimumab induces apoptosis of human monocytes: a comparative study with infliximab and etanercept. Aliment. Pharmacol. Ther. 2005;21:251–258. doi: 10.1111/j.1365-2036.2005.02309.x. [DOI] [PubMed] [Google Scholar]
- 46.Kirchner S., Holler E., Haffner S., Andreesen R., Eissner G. Effect of different tumor necrosis factor (TNF) reactive agents on reverse signaling of membrane integrated TNF in monocytes. Cytokine. 2004;28:67–74. doi: 10.1016/j.cyto.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Mitoma H., Horiuchi T., Hatta N., Tsukamoto H., Harashima S.-I., Kikuchi Y., Otsuka J., Okamura S., Fujita S., Harada M. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-α. Gastroenterology. 2005;128:376–392. doi: 10.1053/j.gastro.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 48.Anti-TNF therapy, Best Pract. Res. Clin. Rheumatol. 25, 2011: 549–567. https://doi.org/10.1016/j.berh.2011.10.004. [DOI] [PubMed]
- 49.Lee C., Jeong M., Lee J.J., Seo S., Cho S.C., Zhang W., Jaquez O. Glycosylation profile and biological activity of Remicade® compared with Flixabi® and Remsima®. MAbs. 2017;9:968–977. doi: 10.1080/19420862.2017.1337620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim K.J., Lee S.J., Kim S., Lee S.Y., Lee M.S., Park Y.A., Choi E.J., Lee E.B., Jun H.K., Cho J.M., Lee S., Kwon K.S., Lim B.P., Jeon M.-S., Shin E.C., Choi Y.S., Fudim E., Picard O., Yavzori M., Ben-Horin S., Chang S.J. Comparable immune function inhibition by the infliximab biosimilar CT-P13: implications for treatment of inflammatory bowel disease. J. Crohns Colitis. 2017;11:593–602. doi: 10.1093/ecco-jcc/jjw183. [DOI] [PubMed] [Google Scholar]
- 51.Kronthaler U., Fritsch C., Hainzl O., Seidl A., da Silva A. Comparative functional and pharmacological characterization of Sandoz proposed biosimilar adalimumab (GP2017): rationale for extrapolation across indications. Expert Opin. Biol. Ther. 2018;18:921–930. doi: 10.1080/14712598.2018.1495193. [DOI] [PubMed] [Google Scholar]
- 52.Fossati G., Nesbitt A.M. In vitro complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity by the anti-TNF agents adalimumab, etanercept, infliximab, and certolizumab pegol (CDP870): 807. Am. J. Gastroenterol. 2005;100:S299. [Google Scholar]
- 53.Feldmann M., Brennan F.M., Maini R.N. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/S0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 54.Farrugia M., Baron B. The role of TNF-α in rheumatoid arthritis: a focus on regulatory T cells. J. Clin. Transl. Res. 2016;2:84–90. [PMC free article] [PubMed] [Google Scholar]
- 55.Brennan F., Jackson A., Chantry D., Maini R., Feldmann M. Inhibitory effect of TNF-α antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;334:244–247. doi: 10.1016/S0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 56.St Clair E.W., van der Heijde D.M.F.M., Smolen J.S., Maini R.N., Bathon J.M., Emery P., Keystone E., Schiff M., Kalden J.R., Wang B., Dewoody K., Weiss R., Baker D. Active-controlled study of patients receiving infliximab for the treatment of rheumatoid arthritis of early onset study group, combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–3443. doi: 10.1002/art.20568. [DOI] [PubMed] [Google Scholar]
- 57.Maini R.N., Breedveld F.C., Kalden J.R., Smolen J.S., Furst D., Weisman M.H., St Clair E.W., Keenan G.F., van der Heijde D., Marsters P.A., Lipsky P.E. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group, sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–1065. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi T., Miyasaka N., Inoue K., Abe T., Koike T. Impact of trough serum level on radiographic and clinical response to infliximab plus methotrexate in patients with rheumatoid arthritis: results from the RISING study. Mod. Rheumatol. 2009;19:478–487. doi: 10.1007/s10165-009-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma X., Xu S. TNF inhibitor therapy for rheumatoid arthritis. Biomed. Rep. 2013;1:177–184. doi: 10.3892/br.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drug treatment for rheumatoid arthritis - NICE Pathways, n.d. 〈https://pathways.nice.org.uk/pathways/rheumatoid-arthritis/drug-treatment-for-rheumatoid-arthritis#content=view-node%3Anodes-initial-treatment〉 (accessed 23 January 2021).
- 61.Papadakis K.A., Targan S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 62.Reimund J.-M., Wittersheim C., Dumont S., Muller C.D., Baumann R., Poindron P., Duclos B. Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn’s disease. J. Clin. Immunol. 1996;16:144–150. doi: 10.1007/BF01540912. [DOI] [PubMed] [Google Scholar]
- 63.Levin A.D., Wildenberg M.E., van den Brink G.R. Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J. Crohns Colitis. 2016;10:989–997. doi: 10.1093/ecco-jcc/jjw053. [DOI] [PubMed] [Google Scholar]
- 64.Vulliemoz M., Brand S., Juillerat P., Mottet C., Ben-Horin S., Michetti P. an official working group of the S.S. of G. on behalf of Swiss IBDnet, TNF-alpha blockers in inflammatory bowel diseases: practical recommendations and a user’s guide: an update. Digestion. 2020;101:16–26. doi: 10.1159/000506898. [DOI] [PubMed] [Google Scholar]
- 65.Adegbola S.O., Sahnan K., Warusavitarne J., Hart A., Tozer P. Anti-TNF therapy in Crohn’s disease. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dal-Ré R., Banzi R., Georgin-Lavialle S., Porcher R., Sofat R., Zeitlinger M., Rosendaal F.R. Remdesivir for COVID-19 in Europe: will it provide value for money?, Lancet. Respir. Med. 2021;9:127–128. doi: 10.1016/S2213-2600(20)30568-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.RECOVERY Collaborative Group, Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., Jeffery K., Montgomery A., Rowan K., Juszczak E., Baillie J.K., Haynes R., Landray M.J. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams A.E., Chambers R.C. The mercurial nature of neutrophils: still an enigma in ARDS? Am. J. Physiol. - Lung Cell. Mol. Physiol. 2014;306:L217–L230. doi: 10.1152/ajplung.00311.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatia M., Zemans R.L., Jeyaseelan S. Role of chemokines in the pathogenesis of acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012;46:566–572. doi: 10.1165/rcmb.2011-0392TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A.A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Pen J.L., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A.N., Delmonte O.M., Abers M.S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R.P., Vasse M., Smadja D.M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D.C., Tangye S.G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M.C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T.H., Oler A.J., Gu J., Burbelo P.D., Cohen J.I., Biondi A., Bettini L.R., D’Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E.S., Fusco F., Ursini M.V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Duval X., Ghosn J., Lab§ H., N.-U.I.R. to C. Group§, Clinicians§ C., Clinicians§ C.-S., Group§ I.C., Group§ F.C.C.S., Consortium§ T.M.I., Cohort§ C.-C., A.U.C.-19 Biobank§, Effort§ C.H.G., Tsang J.S., Goldbach-Mansky R., Kisand K., Lionakis M.S., Puel A., Zhang S.-Y., Holland S.M., Gorochov G., Jouanguy E., Rice C.M., Cobat A., Notarangelo L.D., Abel L., Su H.C., Casanova J.-L. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Q., Bastard P., Liu Z., Pen J. Le, Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., Rosain J., Bilguvar K., Ye J., Bolze A., Bigio B., Yang R., Arias A.A., Zhou Q., Zhang Y., Onodi F., Korniotis S., Karpf L., Philippot Q., Chbihi M., Bonnet-Madin L., Dorgham K., Smith N., Schneider W.M., Razooky B.S., Hoffmann H.-H., Michailidis E., Moens L., Han J.E., Lorenzo L., Bizien L., Meade P., Neehus A.-L., Ugurbil A.C., Corneau A., Kerner G., Zhang P., Rapaport F., Seeleuthner Y., Manry J., Masson C., Schmitt Y., Schlüter A., Voyer T. Le, Khan T., Li J., Fellay J., Roussel L., Shahrooei M., Alosaimi M.F., Mansouri D., Al-Saud H., Al-Mulla F., Almourfi F., Al-Muhsen S.Z., Alsohime F., Turki S. Al, Hasanato R., van de Beek D., Biondi A., Bettini L.R., D’Angio’ M., Bonfanti P., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Oler A.J., Tompkins M.F., Alba C., Vandernoot I., Goffard J.-C., Smits G., Migeotte I., Haerynck F., Soler-Palacin P., Martin-Nalda A., Colobran R., Morange P.-E., Keles S., Çölkesen F., Ozcelik T., Yasar K.K., Senoglu S., Karabela Ş.N., Rodríguez-Gallego C., Novelli G., Hraiech S., Tandjaoui-Lambiotte Y., Duval X., Laouénan C., COVID-STORM Clinicians, COVID Clinicians, Imagine COVID Group, French COVID Cohort Study Group, CoV-Contact Cohort, Amsterdam UMC Covid-19 Biobank, COVID Human Genetic Effort, NIAID-USUHS/TAGC COVID Immunity Group, Snow A.L., Dalgard C.L., Milner J.D., Vinh D.C., Mogensen T.H., Marr N., Spaan A.N., Boisson B., Boisson-Dupuis S., Bustamante J., Puel A., Ciancanelli M.J., Meyts I., Maniatis T., Soumelis V., Amara A., Nussenzweig M., García-Sastre A., Krammer F., Pujol A., Duffy D., Lifton R.P., Zhang S.-Y., Gorochov G., Béziat V., Jouanguy E., Sancho-Shimizu V., Rice C.M., Abel L., Notarangelo L.D., Cobat A., Su H.C., Casanova J.-L. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bengoechea J.A., Bamford C.G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol. Med. 2020;12:12560. doi: 10.15252/emmm.202012560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., Kuthuru O., Apostolidis S.A., Bershaw L., Dougherty J., Greenplate A.R., Pattekar A., Kim J., Han N., Gouma S., Weirick M.E., Arevalo C.P., Bolton M.J., Goodwin E.C., Anderson E.M., Hensley S.E., Jones T.K., Mangalmurti N.S., Luning Prak E.T., Wherry E.J., Meyer N.J., Betts M.R. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., Simpson L.J., Grant P., Subramanian A., Rogers A.J., Blish C.A. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;26:1070–1076. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Z., Wherry E. John. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazzoni A., Salvati L., Maggi L., Capone M., Vanni A., Spinicci M., Mencarini J., Caporale R., Peruzzi B., Antonelli A., Trotta M., Zammarchi L., Ciani L., Gori L., Lazzeri C., Matucci A., Vultaggio A., Rossi O., Almerigogna F., Parronchi P., Fontanari P., Lavorini F., Peris A., Rossolini G.M., Bartoloni A., Romagnani S., Liotta F., Annunziato F., Cosmi L. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 2020;130:4694–4703. doi: 10.1172/JCI138554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim K.D., Zhao J., Auh S., Yang X., Du P., Tang H., Fu Y.-X. Adaptive immune cells temper initial innate responses. Nat. Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palm N.W., Medzhitov R. Not so fast: adaptive suppression of innate immunity. Nat. Med. 2007;13:1142–1144. doi: 10.1038/nm1007-1142b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hippensteel J.A., LaRiviere W.B., Colbert J.F., Langouët-Astrié C.J., Schmidt E.P. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2020;319:L211–L217. doi: 10.1152/ajplung.00199.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cavalli G., Larcher A., Tomelleri A., Campochiaro C. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. The Lancet Rheumatology. 2021 doi: 10.1016/S2665-9913(21)00012-6. (accessed 5 February 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldmann M., Brennan F.M., Maini R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 91.Fong Y., Tracey K.J., Moldawer L.L., Hesse D.G., Manogue K.B., Kenney J.S., Lee A.T., Kuo G.C., Allison A.C., Lowry S.F. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 1989;170:1627–1633. doi: 10.1084/jem.170.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charles P., Elliott M.J., Davis D., Potter A., Kalden J.R., Antoni C., Breedveld F.C., Smolen J.S., Eberl G., deWoody K., Feldmann M., Maini R.N. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J. Immunol. Baltim. Md 1950. 1999;163:1521–1528. [PubMed] [Google Scholar]
- 93.Robinson P.C., Richards D., Tanner H.L., Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2:e653–e655. doi: 10.1016/S2665-9913(20)30309-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ingegnoli F., Fantini F., Favalli E.G., Soldi A., Griffini S., Galbiati V., Meroni P.L., Cugno M. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J. Autoimmun. 2008;31:175–179. doi: 10.1016/j.jaut.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 95.Robinson P.C., Liew D.F.L., Liew J.W., Monaco C., Richards D., Shivakumar S., Tanner H.L., Feldmann M. The potential for repurposing anti-TNF as a therapy for the treatment of COVID-19. Med. 2020;1:90–102. doi: 10.1016/j.medj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., Schreiner P., Neale G., Vogel P., Webby R., Jonsson C.B., Kanneganti T.-D. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Woodard W., Lezak S.P., Lugogo N.L., Knight J.S., Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. Journal of Thrombosis and Thrombolysis. 2021;51:446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., Izadi Z., Jacobsohn L., Katz P., Lawson-Tovey S., Mateus E.F., Rush S., Schmajuk G., Simard J., Strangfeld A., Trupin L., Wysham K.D., Bhana S., Costello W., Grainger R., Hausmann J.S., Liew J.W., Sirotich E., Sufka P., Wallace Z.S., Yazdany J., Machado P.M., Robinson P.C. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Strangfeld A., Schäfer M., Gianfrancesco M.A., Lawson-Tovey S., Liew J.W., Ljung L., Mateus E.F., Richez C., Santos M.J., Schmajuk G., Scirè C.A., Sirotich E., Sparks J.A., Sufka P., Thomas T., Trupin L., Wallace Z.S., Al-Adely S., Bachiller-Corral J., Bhana S., Cacoub P., Carmona L., Costello R., Costello W., Gossec L., Grainger R., Hachulla E., Hasseli R., Hausmann J.S., Hyrich K.L., Izadi Z., Jacobsohn L., Katz P., Kearsley-Fleet L., Robinson P.C., Yazdany J., Machado P.M. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahil S.K., Dand N., Mason K.J., Yiu Z.Z.N., Tsakok T., Meynell F., Coker B., McAteer H., Moorhead L., Mackenzie T., Rossi M.T., Rivera R., Mahe E., Carugno A., Magnano M., Rech G., Balogh E.A., Feldman S.R., Cruz C.D.L., Choon S.E., Naldi L., Lambert J., Spuls P., Jullien D., Bachelez H., McMahon D.E., Freeman E.E., Gisondi P., Puig L., Warren R.B., Meglio P.D., Langan S.M., Capon F., Griffiths C.E.M., Barker J.N., Smith C.H., Shah A., Barea A., Romero-Maté A., Singapore A., Paolino A., Mwale A., Callaghan A.M.M., Martinez A., DeCrescenzo A., Pink A.E., Jones A., Sergeant A., Essex A., Bewley A., Makrygeorgou A., van Huizen A., Pérez-Suárez B., Farida B., Claréus B.W., Prims C.T., Davis C., Quinlan C., Maybury C., Cesar G.A., Barclay C., Greco C., Brassard D., Cummings D., Kolli D., Descamps V., Genao D.R., Carras E., Hawryluk E., Martínez-García E., Klujszo E., Dwyer E., Toni E., Sonkoly E., Loayza E., Daudén E., Valenzuela F., Popov G., King G., Celine G., Aparicio G., Johnston G.A., Cardozo G.A., Pearson I., Yanguas I., Weisman J., Carolan J.E., Hughes J., Ortiz-Salvador J.-M., Carrascosa J.-M., Schwartz J.J., Jackson K., Kerisit K.G., Wu K., Asfour L., de Graaf L., Lesort C., Meuleman L., Eidsmo L., Skov L., Gribben L., Rustin M., Velasco M., Panchal M., Lakhan M., Franco M.D., Svensson M.-L., Vandaele M., Marovt M., Zargari O., Caso P.D., Varela P., Jenkin P., Phan C., Hampton P., Goldsmith P., Bak R., Speeckaert R., Romiti R., Woolf R., Mercado-Seda R., Khatun R., Ceovic R., Taberner R., Cohen R.W., Stefanescu S., Kirk S., Reeken S., Ayob S., Pérez-Barrio S., Piaserico S., Hoey S., Torres T., Talme T., Desai T.V., van Geest A.J., King V., Lernia V.D., Koreja Z., Hasab V.Z. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—insights from a global registry–based study. J. Allergy Clin. Immunol. 2021;147:60–71. doi: 10.1016/j.jaci.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenner E.J., Ungaro R.C., Gearry R.B., Kaplan G.G., Kissous-Hunt M., Lewis J.D., Ng S.C., Rahier J.-F., Reinisch W., Ruemmele F.M., Steinwurz F., Underwood F.E., Zhang X., Colombel J.-F., Kappelman M.D. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cludts I., Spinelli F.R., Morello F., Hockley J., Valesini G., Wadhwa M. Anti-therapeutic antibodies and their clinical impact in patients treated with the TNF antagonist adalimumab. Cytokine. 2017;96:16–23. doi: 10.1016/j.cyto.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kalden J.R., Schulze-Koops H. Immunogenicity and loss of response to TNF inhibitors: implications for rheumatoid arthritis treatment. Nat. Rev. Rheumatol. 2017;13:707–718. doi: 10.1038/nrrheum.2017.187. [DOI] [PubMed] [Google Scholar]
- 105.Bartelds G.M., Krieckaert C.L.M., Nurmohamed M.T., van Schouwenburg P.A., Lems W.F., Twisk J.W.R., Dijkmans B.A.C., Aarden L., Wolbink G.J. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]