In May 2016, the White House Office of Science and Technology, in collaboration with private-sector stakeholders and federal agencies, posted the Fact Sheet for the National Microbiome Initiative (NMI) for immediate release. The NMI was designed to meet 3 specific goals that collectively foster an understanding of the human symbiotic relationship with its microbial flora: (1) to support interdisciplinary research to answer fundamental questions about the microbiome in diverse ecosystems; (2) to develop platform technologies for enhancing data sharing on microbiomes; and (3) to expand the microbiome workforce. The NMI, which was funded at more than $121 million for fiscal years 2016 and 2017, is comprised of investments from multiple departments of the federal government, including the Departments of Energy, Justice, and Veteran’s Affairs; the Office of Research and Development; the Department of Agriculture; the National Science Foundation; the National Institutes of Health; the Aeronautics and Space Administration; private stakeholders, such as the Bill and Melinda Gates Foundation and the Juvenile Diabetes Research Foundation, and a number of academic institutions.
Early scientific publications on the manipulation of the microbiome to understand and intervene in human disease came as reports of self-infection by Dr J. A. Turton with the hookworm Necator americanus larvae. In 1976, Dr Turton reported that hookworm infection decreased his allergic rhinitis symptoms during the pollen seasons of 1975 and 1976and augmented his production of specific IgE.1 The case report was met with substantial controversy, skepticism, and generation of alternate hypotheses to explain the findings. For example, it was suggested that alterations in IgE antibody levels were not due to infection but rather to the use of levamisole to eradicate the initial infection before self-reinfection. Although current randomized controlled trials in patients with Crohn disease and asthma have not borne out that hookworm infection is superior to placebo for controlling disease2,3 and popular media have run stories on the side effects and loss of effectiveness of infection, the observations of Turton,1 along with the hypotheses of his skeptics, were based on the immunologic reality that interventions that induce dysbiosis can have substantive effects on systemic diseases.
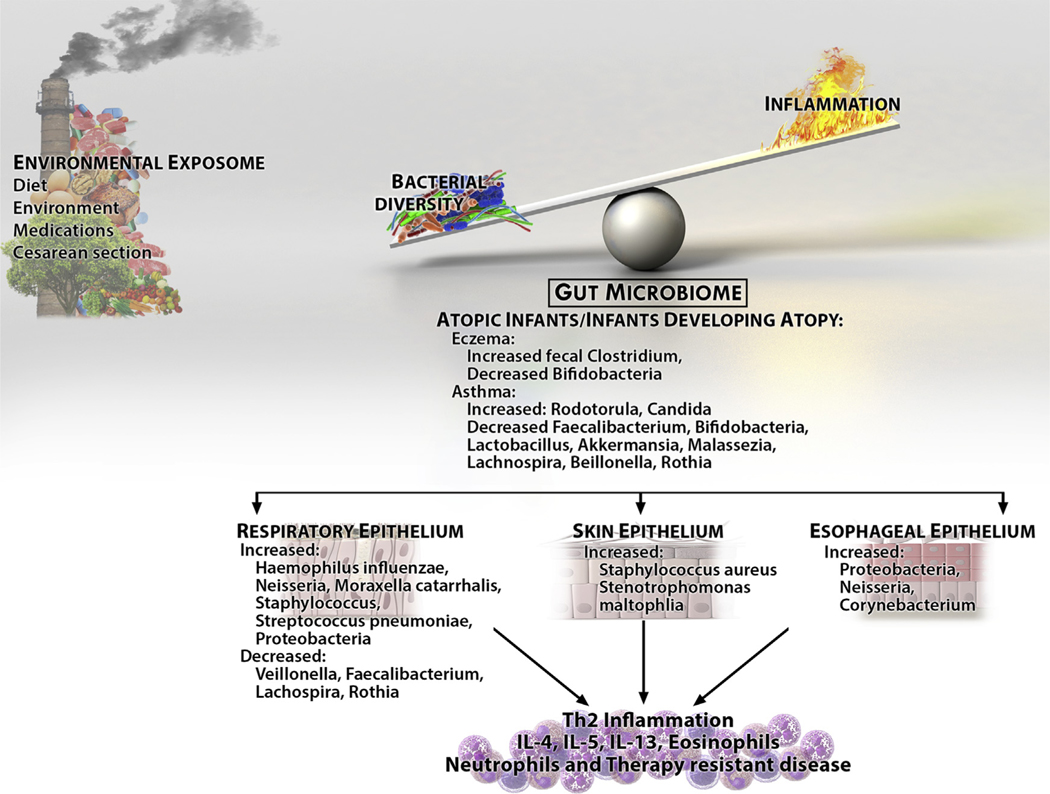
Shifts in the human microbiome are affected by developmental age, diet, and medication use (Fig 1). Underscoring the importance of the microbiome’s development within its host is the fact that the infant microbiome is highly volatile and prone to changes that can be irreversible. Late developmental replacement of the microbiome in germ-free infant mice does not promote proper development of invariant natural killer T (iNKT) cell function and predisposes to development of asthmatic and colitic phenotypes.4 The presence of the commensal microbiome during the neonatal period increases regulatory T-cell numbers and decreases preferential B-cell class-switching to IgE.4 New data demonstrate that infants at risk for asthma have a microbiome depleted of a number of species, including Lactobacillus and Akkermansia species, and that the metabolome of the asthma-prone infants decreases regulatory T-cell numbers while increasing CD4+IL-4+ cell numbers in vitro (Table I).5 Indeed, the gastrointestinal mucosal immune system is dependent on the microbiome for its genesis, and gut-associated lymphoid tissue does not develop in the absence of the intestinal flora. Diets can also induce dysbiosis and affect systemic immune responses. Foods found in Western diets that are high in salt and lipids can promote low-grade systemic inflammation with increased levels of peripheral IL-1β, adiponectin, TNF-α, and TH17 cell accumulation.6
FIG 1.
The microbiota of allergic diseases. Environmental exposures alter bacterial diversity and increase inflammation. The result is tissue and gut dysbiosis with decreased bacterial diversity and changes in the microbiome of target organs. The alterations in normal flora likely interact with increased inflammation to alter patient phenotypes, such as disease response.
TABLE I.
Associations of microbiota with allergic diseases
| Article | Topic | Associations with microbiota |
|---|---|---|
| Chen et al, Arch Intern Med, 2007 | Association of Helicobacter pylori with allergic diseases | H pylori acquisition associates with reduced allergy and asthma risk in children. |
| Dellon et al, Gastroenterology, 2011 | EoE associates with decreased odds of H pylori infection. | |
| Marri et al, J Allergy Clin Immunol, 2013 | Association of microbiome with asthma severity | Asthmatic patients had an increase in Proteobacteria. The microbiome of patients with mild asthma is more similar to that of patients with severe asthma than that of healthy control subjects. |
| Prince et al, Pediatr Clin North Am, 2015 | Review article on the species of bacteria and risk of allergy | Children who had allergy had lower loads of Bacteroides, Bifidobacteria, Enterococci, and more Clostridia. Cesarean sections are associated with less microbial diversity and lower Bacteroides, Bifidobacteria, and Escherichia coli and increased Klebsiella, Enterobacter, and Enterococcus species and Clostridia. Similar effects were seen with birth order and antibiotic use. Food allergy data rely largely on animal models. One study with proved egg allergy showed decreased risk with a dog or older siblings in the home. Patients with cow’s milk allergy had more Clostridium and Atopobium species. |
| Benitez et al, Microbiome, 2015 | Study of microbiome in patients with EoE and control subjects | Patients with EoE had increased Proteobacteria, including Neisseria and Corynebacterium species, with increased Granulicatella and Campylobacter species during food-induced active inflammation. |
| Arrieta et al, Sci Transl Med, 2015 | Influence of microbiome on asthma risk | Decrease in Faecalibacterium, Lachnospira, Veillonella, and Rothia species was seen in the gut microbiome of children at 3 mo of age who were at risk for asthma. |
| Durack et al, J Allergy Clin Immunol, 2016 | Atopy, asthma, and inhaled corticosteroid response | Asthmatic patients had an increase in Haemophilus, Neisseria, Fusobacterium, and Porphyromonas species and Sphingomonadaceae and a decrease in Mogibacteriaceae and Lactobacillales |
| Fujimura et al, Nat Med, 2016 | Association and mechanistic evidence that the gut microbiota at 3 mo influences the risk of atopy and asthma | Infants at risk for asthma had a gut microbiome depleted of Bifidobacteria, Lactobacillus, Faecalibacterium, Akkermansia, and Malassezia species (mycobiome) and increased Rhodotorula and Candida species. The predicted metabolome was proinflammatory, and there were decreased numbers of regulatory T cells and ratios of CD4+IFN- γ+/CD4+IL-4+ cells in vitro. |
| Marrs and Flohr, Pediatr Infect Dis J, 2016 | Excellent review of literature on microbiome and food allergy/eczema | Increased gut microbiotic diversity at 3 mo is generally protective for eczema, but specific constituents of the microbiome vary between studies. Increased cutaneous Staphylococcus aureus colonization associates with eczema severity. It is possible that gut diversity promotes greater oral tolerance to foods. |
| Forsberg et al, Clin Exp Allergy, 2016 | Review on the use of prebotics and probiotics for allergy prevention | Meta-analyses conclude that probiotics can prevent eczema but not other allergic disorders. |
| Zheng et al, PLoS One, 2016 | Case-control study of infants with eczema versus control subjects | Patients with eczema were colonized with bacteria known to be associated with atopy (Faecalibacterium prausnitzii and Ruminococcus gnavus) and decreased intestinal barrier (Akkermansia muciniphila). Anti-inflammatory bacteria (Bacteroides fragilis and Streptococcus salivarius) were reduced. |
| Harris et al, PLoS One, 2016 | Microbiome in adult and pediatric patients with EoE compared with GERD and healthy control subjects | Increased but not distinct bacterial load was found in patients with EoE, with predominance of Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria. |
| Stein et al, N Engl J Med, 2016 | Study of Amish versus Hutterite children in relation to endotoxin levels, microbial composition of house dust | Both populations were genetically similar, but Hutterite children had higher serum IgE levels; more asthma; more exposure to cockroach, dust mites, cats, and dogs; and lower levels of endotoxin. Dust from Amish homes was protective for allergen-induced asthma in mice. |
| Birzele et al, Allergy, 2017 | Study of microbiotic diversity in farm-exposed children in relation to nasal sample-derived microbiota | Farm exposure associated with increased bacterial diversity in mattresses and was greater with exposure to straw and cows. Asthma was related to bacterial diversity, although to a lower extent with mucosal samples, suggesting that not just the type of bacteria but also potentially bacterial products are important. Clostridium and Facklamia were abundant in mattresses associated with farming. |
| Depner et al, J Allergy Clin Immunol, 2017, in press | Association of nasal versus throat microbiota with asthma in rural children | Asthmatic children had decreased bacterial diversity and increased Moraxella species overgrowth that was asthma related only in nonfarm children. The nasal but not the throat microbiome associated with asthma. |
| Dzidic et al, J Allergy Clin Immunol, 2017, in press | Gut IgA coated-bacteria at 1 and 12 mo in pediatric patients with and without allergy/asthma by 7 y of age | Children with asthma and allergy had lower proportions of IgA-coated bacteria compared with healthy control subjects, and the coated bacteria were different between the 2 groups, with asthmatic patients having higher diversity of IgA-coated Bacteroidetes and Proteobacteria than control subjects. |
This table is representative of the literature published close to 2016. A complete comprehensive review is out of the scope of this article, but many excellent reviews on the microbiome and allergy are available.
EoE, Eosinophilic esophagitis; GERD, gastroesophageal reflux disease.
Just as the composition of the microbiome can be modified by the use of medications, such as antibiotics, differences in the intestinal flora can alter how the host metabolizes drugs, leading to complex interactions between the host, its flora, and its environment. The microbiome alters the metabolism of both acetaminophen and irinotecan (used in colon cancer chemotherapy), increasing drug side effects of liver toxicity and diarrhea, respectively.7
The adaptive and innate immune systems can be influenced by the microbiome during health and disease (Table I). For instance, Enterobaceriaceae IgA-coated bacteria can contribute to the development of colitis, whereas Akkermansia muciniphila might offer protection from colitis. In human subjects IgA deficiency has been associated with a more inflammatory microbiome, but mice that lack IgA affinity maturation have more dysbiosis.4 One unifying theme relates to the loss of bacterial diversity and commensal bacteria during an active disease state. In patients with active asthma, there is increased Proteobacteria; neutrophilic therapy-resistant asthma associates with increased sputum concentrations of Haemophilus species, Streptococcus species, and Moraxella catarrhalis; and mechanistic studies suggest that the microbiome in patients with active disease alters the response to corticosteroids.8 Although children with atopic dermatitis have more bacterial diversity than adults, both children and adults have increased Staphylococcus aureus and decreased commensal bacterial load compared with those who do not have atopic dermatitis.9 Finally, the microbiome of the esophagi of patients with active eosinophilic esophagitis has decreased bacterial diversity and less Streptococcus species, a bacterium also seen in normal skin.10
As the current data show, putting the microbiome and its complexities into context can be astounding. The human microbiome has been estimated to have at least 100 genes for every 1 human gene. Although the human has 10 trillion cells, the microbiome has 100 trillion cells. Because the microbiome itself is diverse in its bacterial composition, alterations in the type of microbiotic load changes the range of genomic diversity. As such, microbial genome-wide association studies have to account not only for higher numbers and diversity of genes but also for the relative abundance of any given taxa.7 Given the complexities of the immune response to bacterial products and metabolites, the need for understanding the microbiome, its interaction with the host and its constituents, its temporal and developmental fluctuations, and its metabolomics become daunting tasks requiring interactions between clinical and basic specialists and systems biologists. To this end, combining the NMI with other initiatives, such as the international initiative Developmental Origins of Health and Disease, the Metabolomics Standards Initiative, and the Human Exposome Project could push our understanding of the human microbiome and its mechanisms of interaction with the immune system, and help us determine how it is influenced by the environment.
A vast number of questions remain regarding the effects, importance, and consequences of the microbial exposome, perhaps more than can be answered even by the NMI. These outstanding inquiries include how the microbiome interacts with its own members, such as how viruses, bacteria, and fungi could alter each another’s functional differences; which changes in the microbiome are causal to versus an effect of the disease state or therapeutic intervention; and the mechanisms by which the microbiome alters structural and/or inflammatory cell function and vice versa. In addition, it is not always clear whether live bacteria, their metabolome, or just bacterial components are required to induce immunologic alterations. Through a herculean and integrated effort, our improved understanding of the microbiome and its influences on the human immune system could lead to insights that are pivotal for understanding how to improve disease states.
Acknowledgments
Supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant AI 092135 and Office of Rare Disease Research (ORDR)/National Center for Advancing Translational Sciences (NCATS)/NIAID/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) CEGIR grant U54 AI117804 (G.T.F. and S.S.A.). CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC.
Footnotes
Disclosure of potential conflict of interest: G. T. Furuta has consultant arrangements with Shire, has received a grant from the National Institutes of Health, and has received royalties from UpToDate. S. S. Aceves has received a grant and travel support from the National Institutes of Health/National Institute of Allergy and Infectious Diseases, is a member of the Medical Advisory Board for the American Partnership for Eosinophilic Disorders, has a patent for oral viscous budesonide.
REFERENCES
- 1.Turton JA. Letter: IgE, parasites, and allergy. Lancet 1976;2:686. [DOI] [PubMed] [Google Scholar]
- 2.Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy 2010;40:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg SK, Croft AM, Bager P. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst Rev 2014;(1): CD009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016;535:75–84. [DOI] [PubMed] [Google Scholar]
- 5.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med 2016;22:1187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol 2013;131:23–30. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016;535:94–103. [DOI] [PubMed] [Google Scholar]
- 8.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 2015;136:874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi B, Bangayan NJ, Curd E, Taylor PA, Gallo RL, Leung DY, et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol 2016;138:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dellon ES. The esophageal microbiome in eosinophilic esophagitis. Gastroenterology 2016;151:364–5. [DOI] [PubMed] [Google Scholar]