Abstract
Background:
Findings from epidemiological studies of prenatal phthalate exposure and child cognitive development are inconsistent. Methods for evaluating mixtures of phthalates, such as weighted quantile sum (WQS) regression, have rarely been applied. We developed a new extension of the WQS method to improve specificity of full-sample analyses and applied it to estimate associations between prenatal phthalate mixtures and cognitive and language outcomes in a diverse pregnancy cohort.
Methods:
We measured 22 phthalate metabolites in third trimester urine from mother-child dyads who completed early childhood visits in the Conditions Affecting Neurodevelopment and Learning in Early childhood (CANDLE) study. Language and cognitive ability were assessed using the Bayley Scales of Infant Development (age 3) and the Stanford Binet-5 (age 4–6), respectively. We used multivariable WQS regression to identify phthalate mixtures that were negatively and positively associated with language score and full-scale IQ, in separate models, adjusted for maternal IQ, race, marital status, smoking, BMI, socioeconomic status (SES), child age, sex, and breastfeeding. We evaluated effect modification by sex and SES. If full sample 95% WQS confidence intervals (which are known to be anti-conservative) excluded the null, we calculated a p-value using a permutation test (ppermutation). The performance of this new approach to WQS regression was evaluated in simulated data. We compared the power and type I error rate of WQS regression conducted within datasets split into training and validation samples (WQSSplit) and in the full sample (WQSNosplit) to WQS regression with a permutation test (WQSpermutation). Individual metabolite associations were explored in secondary analyses.
Results:
The analytic sample (N = 1015) was 62.1% Black/31.5% White, and the majority of mothers had a high school education or less (56.7%) at enrollment. Associations between phthalate mixtures and primary outcomes (language score and full-scale IQ) in the full sample were null. Individual metabolites were not associated with IQ, and only one metabolite (mono-benzyl phthalate, MBzP) was associated with Bayley language score (β = −0.68, 95% CI: −1.37, 0.00). In analyses stratified by sex or SES, mixtures were positively and negatively associated with outcomes, but the precision of full-sample WQS regression results were not supported by permutation tests, with one exception. In the lowest SES category, a phthalate mixture dominated by mono-methyl phthalate (MMP) and mono-carboxy-isooctyl phthalate (MCOP) was associated with higher language scores (βlow SES = 2.41, full-sample 95%CI: 0.58, 4.24; ppermutation = 0.04). Performance testing in simulated data showed that WQSpermutation had improved power over WQSSplit (90% versus 56%) and a lower type I error rate than WQSNosplit (7% versus 47%).
Conclusions:
In the largest study of these relationships to date, we observed predominantly null associations between mixtures of prenatal phthalates and both language and IQ. Our novel extension of WQS regression improved sensitivity to detect true associations by obviating the need to split the data into training and test sets and should be considered for future analyses of exposure mixtures.
Keywords: Prenatal exposures, Phthalates, Exposure mixtures, Neurodevelopment
1. Introduction
Phthalates are a group of synthetic chemicals found in personal care products, food packaging, medical supplies, and processed foods (Hauser and Calafat, 2005). Nearly ubiquitous in our environment, phthalates and their metabolites have been detected in almost all pregnant women tested, including in amniotic fluid (Silva et al., 2004). Phthalates have well-described endocrine disrupting properties. Animal models suggest that prenatal exposure may have lasting effects on child neurodevelopment and that some effects are sex-specific (Barakat et al., 2018; Hatcher et al., 2019; Lin et al., 2015; Miodovnik et al., 2014).
However, as reviewed by Lee et al. (2018) and Radke et al. (2020), epidemiological support for the hypothesis that prenatal phthalate exposure impairs cognitive development is weak and inconsistent. Adverse impacts on early childhood language skills have been suggested in a small number of studies but with inconsistency as to which metabolites are linked to language impairment (Bornehag et al., 2018; Olesen et al., 2018). The heterogeneity of findings across epidemiological studies may be due to methodological limitations, including lack of consideration of phthalate mixtures. With very few exceptions (Daniel et al., 2020), previous studies of phthalates and pediatric neurodevelopment have not utilized statistical methods developed to estimate effects of mixtures. Analyzing metabolites separately typically creates a multiple comparison problem and could yield spurious findings. In addition, analyses of individual metabolites may be underpowered to detect any true effects of phthalate mixtures, if they exist (Lazarevic et al., 2019). Several methods for analyzing effects of exposure mixtures are emerging, including weighted quantile sum (WQS)1 regression (Carrico et al., 2015; Czarnota et al., 2015). One limitation of WQS regression is that estimation of accurate 95% confidence intervals requires splitting the sample into training and validation datasets, which reduces statistical power (Carrico et al., 2015). In many studies, WQS regression has been applied to a full sample without training and validation, potentially yielding false positive findings (Brunst et al., 2017; Horton et al., 2015; Malin et al., 2018; Nieves et al., 2016; Romano et al., 2018; Wu et al., 2018).
To address limitations in previous studies, we examined the links between prenatal phthalates and early childhood cognition and language in a longitudinal, well-characterized pregnancy cohort of over 1500 mother–child dyads, the Conditions Affecting Neurodevelopment and Learning in Early childhood (CANDLE) study. To avoid bias associated with applying WQS regression in a full sample without training and validation, we developed a permutation test extension of the existing WQS algorithm. We estimated associations between mixtures of phthalate metabolites measured in third trimester urine and language development (age 3 years) and full-scale IQ (age 4–6 years) and rigorously adjusted for confounders given the strong and complex relationships between phthalate exposure and sociodemographics (James-Todd et al., 2017; Wenzel et al., 2018). Due to biological plausibility that phthalates may have sex-specific effects on neurodevelopment, effect modification by sex was of primary interest. We also investigated whether SES modified associations given prior evidence that low SES children may be more vulnerable to poor neurodevelopmental outcomes associated with chemical exposures (Tong et al., 2000).
2. Methods
2.1. Study population
The CANDLE study is a community pregnancy cohort established to describe pre- and postnatal factors that impact child development and learning. The design and methods have been described previously (LeWinn et al., 2020; Sontag-Padilla et al., 2015). Between 2008 and 2011, CANDLE recruited 1,503 women in their 2nd trimester of pregnancy and residing in the Memphis, TN, region. Specifically, women were considered eligible if they were Shelby County residents between 16 and 40 years of age, had low-risk singleton pregnancies, and planned to deliver at a participating study hospital. The University of Tennessee Health Sciences Center (UTHSC) Institutional Review Board (IRB) approved CANDLE research activities, and women provided informed consent before enrolling. Data were collected at several points across pregnancy and early childhood, including clinic visits, a hospital visit, home visits, and numerous phone-based assessments. In the current study, we included 1,015 mother–child dyads with gestational age at birth of at least 34 weeks, measurements of phthalate metabolites in third trimester urine, and either the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III) at age 3 visit or the Stanford-Binet intelligence test at age 4–6 visit. The current analysis was conducted as part of the ECHO PATHWAYS Consortium and was approved by the University of Washington IRB.
2.2. Maternal urinary phthalate metabolites
Individual metabolites of parent phthalates were measured in spot urine samples collected in the 3rd trimester. Samples were collected in sterile, phthalate-free specimen cups, transferred to cryovials, and stored at − 80 ◦C in the study repository (UTHSC Department of Pathology) until shipment for analysis. Specific gravity (SG) was measured with a handheld refractometer at the time of urine collection. Samples were analyzed for 22 metabolites at Wadsworth Laboratory of the New York State Department of Health. This process involved enzymatic deconjugation of phthalate metabolites from glucuronidated form, automated online solid phase extraction, separation with high performance liquid chromatography (HPLC), and detection by isotope-dilution tandem mass spectrometry. Detailed methods have been previously described (Asimakopoulos et al., 2016; Guo et al., 2014; Rocha et al., 2017). Process and instrument blanks were included for quality assurance. The limit of detections (LODs) were between 0.012 and 0.304 ng/mL.
The 13 individual phthalate metabolites that were detected in >80% of the study population were included in this analysis: mono-isobutyl phthalate (MiBP), monoethyl phthalate (MEP), mono-methyl phthalate (MMP), mono-n-butyl phthalate (MnBP), mono-benzyl phthalate (MBzP), mono-carboxy-isononyl phthalate (MCNP), mono-carboxy-isooctyl phthalate (MCOP), mono-(3-carboxypropyl) phthalate (MCPP), mono-(2-ethylhexyl) phthalate (MEHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP) and mono(2-carboxymethylhexyl) phthalate (MCMHP). We imputed values lower than the limit of detection (LOD) as LOD divided by the square root of 2. Concentrations of metabolites were corrected for dilution using the following formula (Boeniger et al., 1993):
where P is the measured urinary phthalate concentration, SG is the sample specific gravity, and SGmedian is the median SG over all samples. To calculate the summed molar concentration of DEHP metabolites (∑DEHP), the mass concentrations of each of the five individual DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP and MCMHP) were divided by molecular weight and summed. Metabolites were natural log-transformed (loge) for all analyses.
2.3. Outcomes
Child language development was assessed at the age 3 visit using the Bayley-III administered by a licensed clinical psychologist. The Bayley-III is a standardized tool for evaluating developmental function of infants and young children up to 42 months of age. Age-standardized composite scores and subscale scores were obtained for the domains of cognitive, language and motor development. The language composite score was utilized as our primary outcome measure of language development (Bayley, 2006).
Standardized full scale child IQ (mean = 100; SD = 15) was assessed at the age 4–6 visit by trained psychologists using the Stanford-Binet Intelligence Scales (SB-5), which has been validated and normed in large, diverse populations and includes tasks assessing working memory, processing speed, visual-spatial skills, vocabulary and language comprehension (Roid and Pomplun, 2012). The full-scale age-standardized score was our primary outcome measure of cognitive development.
For both outcome metrics, higher scores indicate more favorable neurocognitive outcomes.
2.4. Covariates
Several characteristics were ascertained by maternal report during pregnancy, including: maternal age, race, education, marital status, insurance status, pre-pregnancy body mass index (BMI), prenatal smoking, parity and income. We adjusted household income for number of adults and children supported by the income (Burniaux et al., 1998). Maternal psychopathology was characterized by Brief Symptom Inventory (BSI), administered during pregnancy (Derogatis and Melisaratos, 1983), and maternal cognition was assessed using the Weschler Abbreviated Scale Intelligence (WASI) (Axelrod, 2002). The Knowledge of Infant Development Inventory (KIDI) was used as a measure of maternal knowledge of child caretaking (MacPhee, 1981). Child birth weight and gestational age at birth were abstracted from the birth record. During a home visit, trained staff conducted a Home Observation Measurement of the Environment (HOME) assessment, to characterize the quality of the care-taking environment (Elardo and Bradley, 1981). Breastfeeding history of the enrolled child was reported at the age 4–6 visit. Maternal addresses were collected at enrollment and updated across pregnancy. Each residence was geocoded and linked by census tract to values of the Childhood Opportunity Index (COI), a measure of neighborhood resources and opportunities for healthy childhood development (Acevedo-Garcia et al., 2014), and a pregnancy average was calculated for each mother–child dyad by time-weighting across all reported prenatal residences.
2.5. Statistical analysis
All analyses were performed using STATA 15 or R studio 3.5.2. We conducted descriptive analyses to summarize distributions of outcome measures, phthalate exposure, and covariates in the analytic population. Distributions of phthalate exposure within strata of effect modifiers were also calculated. We summarized pairwise correlations among specific-gravity adjusted metabolites using Spearman correlation coefficients.
For the primary analyses, we utilized weighted quantile sum (WQS) regression to characterize the association between phthalate metabolite mixtures and child neurodevelopment (Bayley language score and full-scale IQ, in separate models). We used the R package (“gWQS”) to estimate WQS scores comprised of weighted sums of individual phthalate concentrations (each normalized by converting to quintiles). Weights were selected using bootstrap resampling methods (1000 bootstrap runs for each analysis) to optimize the association between the WQS score and outcome in a multivariate linear regression model, adjusted for covariates as described below. Standard errors and statistical significance were evaluated using robust sandwich standard errors. WQS regression estimates sum mixture effects in either the positive or negative direction separately, and we chose to evaluate both directions in separate models to allow for the possibility of associations in both the hypothesized (negative) and non-hypothesized (positive) directions. In the primary analyses we included ∑DEHP as one component of the mixture, rather than the individual DEHP metabolites.
Because we did not split the sample into separate datasets for training and validation in WQS regression analyses, the observed 95% confidence intervals (CIs) for coefficients of WQS indices in the full sample (i.e., full-sample 95% CIs) are likely anti-conservative estimates of true precision (Borovicka et al., 2012). We therefore developed an extension of the WQS method that applies a permutation test to estimate p-values that more accurately represent the uncertainty in the WQS coefficient. See Supplement A1 for expanded background and rationale for the permutation test approach and Supplement A2 for a detailed description of the methodology. In brief, a permutation test is a widely used statistical method for calculating a p-value based on simulations of the null distribution upon which the p-value is based. To our knowledge, we are the first to apply this approach to WQS regression. For linear regression coefficients, the p-value indicates the probability of randomly observing a coefficient as great or greater in value given that the true coefficient is zero. For a linear regression with a single predictor variable, the permutation test simulates the condition in which the true coefficient is zero by randomly permuting outcome values and running linear regressions with those new outcome variables each time, thereby generating a distribution of coefficient values given a true null hypothesis (Manly, 1991). The permutation test p-value in this case is the number of times the absolute value of the permuted coefficient is as great or greater than the absolute value of the initially observed coefficient. For regression with multiple predictor variables, the permutations are performed on the residuals of predicted outcome values from a regression omitting the predictor variable of interest (Freedman and Lane, 1983). We utilize this latter method for permutation tests with WQS regressions. It should be noted that permutation tests only provide a p-value and not valid confidence intervals. Also, permutation tests, while improving the accuracy of p-values, do no change point estimates.
To verify that the permutation test does restore the nominal false positive rate of 5% while still improving statistical power, we performed our own repeated simulations comparing WQS regressions within a training and validation dataset using a 40:60 split (WQSSplit), within the full sample (WQSNosplit), and within the full sample but also including a permutation test (WQSPermutation). For the WQS coefficient, we tested statistical power (i.e., the true positive rate, also called sensitivity), and the Type I error rate (i.e., the false positive rate, equal to one minus the specificity). More details about the WQS regression performance testing are provided in Supplement A3.
For every full-sample WQS analysis that resulted in 95% confidence intervals that did not include the null, we repeated WQS regression with the permutation test and estimated a permutation test p-value (ppermutation), which is a more conservative measure of the strength of evidence to reject the null hypothesis than the full-sample 95% CIs. We loosely distinguish three levels of evidence against the null: 1) full-sample 95% CIs overlap the null (weakest evidence); 2) full-sample 95% CIs do not overlap the null but ppermutation > 0.05 (moderate evidence); and 3) full-sample 95% CIs do not overlap the null and ppermutation < 0.05 (strongest evidence).
We chose covariates for adjusted regression models a priori by identifying established risk factors for pediatric language and cognitive development that could be directly or indirectly associated with phthalate exposure but not on the causal pathway. See Fig. S1 for a directed acyclic graph (DAG) presenting our conceptual model. We implemented a staged approach to modeling with varying levels of adjustment: Model 1 was adjusted for child sex and age at visits. Model 2 (main adjustment model) was additionally adjusted for maternal education, income adjusted for household size, maternal race, mother’s IQ, maternal age at delivery, marital status (married vs. living with partners vs. single), prenatal smoking, child birth order, recruitment site, child year of birth, pre-pregnancy BMI class, breastfeeding, maternal prenatal psychopathology (BSI global severity index t-score), COI indices (education, health and environment, and social and economic), Knowledge of Infant Development Inventory score (KIDI), and insurance coverage. Model 3 was an extensive model further controlled for HOME subscale scores (learning materials, parental involvement and variety of experiences), which were not included in main models due to substantial missingness. Model 4 included additional adjustment for birth outcomes (birth weight and gestational age at birth) which we did not include in the main adjustment model (Model 2) because they may lie on the causal pathway if phthalates are adversely associated with child neurodevelopment in this study sample. All analyses were conducted using complete case analysis.
In secondary analyses, we explored effect modification in associations between phthalate mixtures and primary outcome measures by socioeconomic status (SES) and child sex. We calculated a composite SES index from maternal education and adjusted household income by averaging z-scores of each separate measure and categorized the composite into tertiles. Effect modification was assessed in main adjustment models only. If WQS analysis suggested evidence of an association between a phthalate mixture and either outcome measure in the full population, we assessed interaction by SES and child sex by adding an interaction term to the WQS regression model. Finally, we conducted fully stratified WQS analyses of phthalate mixtures and both primary outcomes, deriving stratum-specific weights and WQS scores in each stratum of SES and child sex.
In sensitivity analyses, we repeated the main WQS analyses but included the individual DEHP metabolite constituents (MEHP, MEHHP, MEOHP, MECPP and MCMHP) instead of ∑DEHP. For these sensitivity studies, we report full-sample 95% CIs but did not calculate permutation test p-values for the sensitivity analyses due to the computational burden of these calculations.
3. Results
3.1. Characteristics of the study population
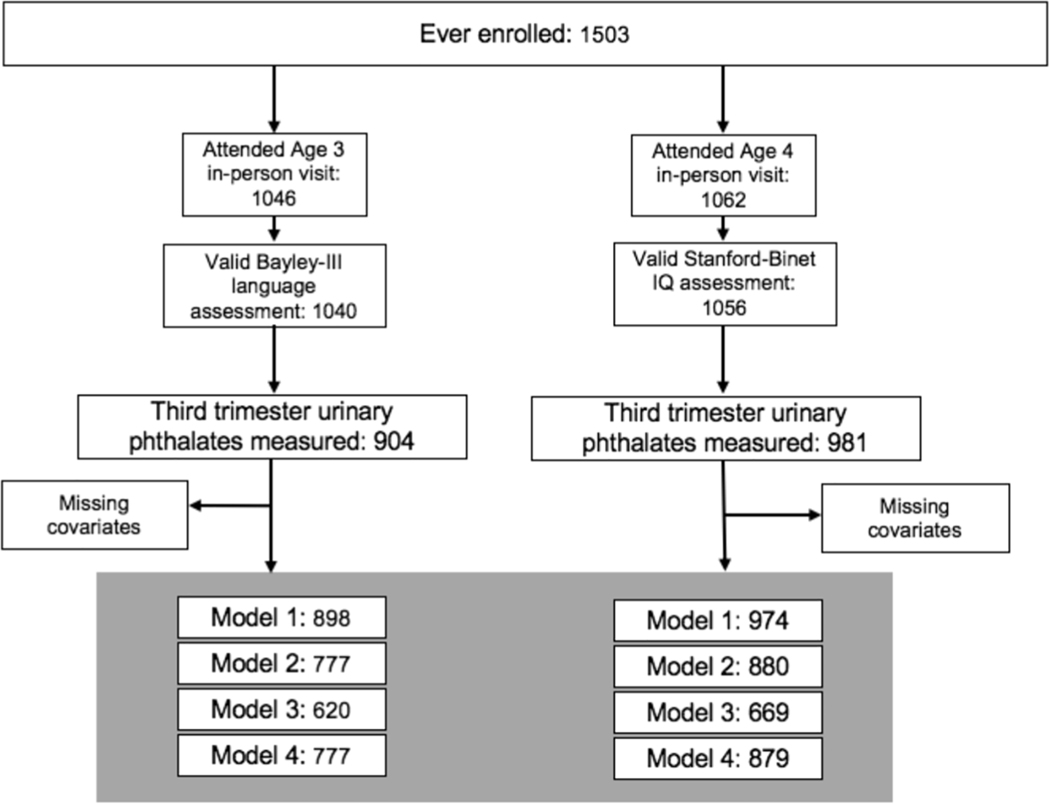
Fig. 1 illustrates CANDLE cohort retention between enrollment and the time of outcome assessments and sample sizes for all primary models. Of 1503 pregnancies enrolled in CANDLE, 1015 mother–child pairs completed an assessment for at least one primary outcome and had prenatal phthalate data. Mothers included in analyses were racially diverse, with 62% identifying as Black and 32% identifying as White (Table 1). At enrollment, more than half of them reported a high school education or less, and about 40% reported never being married. One third of the participating families had a baseline household income less than $20,000 per year, and more than half were covered by Medicaid or Medicare only. Few mothers reported smoking (8.8%) during pregnancy, and 66% breastfed their newborn. Characteristics of women and children of the each of the analytic samples for the two outcomes were similar, and did not differ meaningfully from those of the 1503 who enrolled in CANDLE (Table 1).
Fig. 1. Inclusion Flowchart.
N = 1503 mother–child dyads were enrolled in the CANDLE Study. We included those with analysis of phthalates in a third trimester urine sample and at least one valid outcome measure. We conducted complete case analyses, and the sample size in each adjustment model varied by covariate missingness.
Table 1.
Description of CANDLE participants.
| Characteristic | Valid IQ assessment at Age 4–6 (N = 974) | Valid Bayley-III language assessment at Age 3 (N =898) | At least one assessment (N = enrolled (N = 1015) | CANDLE mothers ever enrolled (N = 1503) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | %/mean (SD) | N | %/mean (SD) | N | %/mean (SD) | N | %/mean (SD) | |
|
| ||||||||
| Child characteristics | ||||||||
| Child age at visit (yrs) 1 | 974 | 4.4 (0.5) | 898 | 3.1 (0.1) | NA | NA | NA | NA |
| Child sex 1 | ||||||||
| Male | 482 | 49.5% | 437 | 48.70% | 501 | 49.4% | 736 | 49.0% |
| Female | 492 | 50.5% | 461 | 51.30% | 514 | 50.6% | 726 | 48.3% |
| Missing | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 41 | 2.7% |
| Child’s birth order 1 | ||||||||
| Not first-born | 584 | 60.0% | 547 | 60.90% | 609 | 60.0% | 881 | 58.6% |
| First-born | 390 | 40.0% | 351 | 39.10% | 406 | 40.0% | 622 | 41.4% |
| Missing | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Birth weight (kg) | 973 | 3.3 (0.5) | 898 | 3.3 (0.5) | 1014 | 3.3 (0.5) | 1454 | 3.2 (0.6) |
| Pre-term delivery (<37 wks) | ||||||||
| No | 906 | 93% | 837 | 93.20% | 945 | 93.1% | 1323 | 88.0% |
| Yes | 68 | 7% | 61 | 6.80% | 70 | 6.9% | 133 | 8.9% |
| Missing | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 47 | 3.1% |
| Maternal characteristics | ||||||||
| Maternal age at birth (years) 1 | 974 | 26.8 (5.6) | 898 | 27 (5.5) | 1015 | 26.7 (5.5) | 1462 | 26.4 (5.5) |
| Maternal race 1 | ||||||||
| Black | 608 | 62.4% | 548 | 61.0% | 630 | 62.1% | 936 | 62.3% |
| White | 301 | 30.9% | 296 | 33.0% | 320 | 31.5% | 467 | 31.1% |
| Asian | 9 | 0.9% | 9 | 1.0% | 9 | 0.9% | 13 | 0.9% |
| American Indian | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0.1% |
| Native Hawaiian/Pacific Islander | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0.1% |
| Other | 3 | 0.3% | 3 | 0.3% | 3 | 0.3% | 6 | 0.4% |
| Multiple race | 53 | 5.4% | 42 | 4.7% | 53 | 5.2% | 77 | 5.1% |
| Missing | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 2 | 0.1% |
| Maternal education at enrollment 1 | ||||||||
| High School/GED or less | 556 | 57.1% | 487 | 54.2% | 575 | 56.7% | 893 | 59.4% |
| College Degree/Technical School | 297 | 30.5% | 293 | 32.6% | 317 | 31.2% | 437 | 29.1% |
| Grad/Professional Degree | 120 | 12.3% | 117 | 13.0% | 122 | 12.0% | 171 | 11.4% |
| Missing | 1 | 0.1% | 1 | 0.1% | 1 | 0.1% | 2 | 0.1% |
| Maternal marital status at enrollment 1 | ||||||||
| Married | 385 | 39.5% | 379 | 42.2% | 403 | 39.7% | 563 | 37.5% |
| Living with partner | 164 | 16.8% | 151 | 16.8% | 170 | 16.8% | 285 | 19.0% |
| Widowed/Divorced/Separated/Never Married | 424 | 43.5% | 367 | 40.9% | 441 | 43.5% | 654 | 43.5% |
| Missing | 1 | 0.1% | 1 | 0.1% | 1 | 0.1% | 1 | 0.1% |
| Insurance status at enrollment 1 | ||||||||
| No insurance | 2 | 0.2% | 1 | 0.1% | 2 | 0.2% | 2 | 0.1% |
| Medicaid or Medicare only | 537 | 55.1% | 481 | 53.6% | 559 | 55.1% | 859 | 57.2% |
| Medicaid/Medicare and private insurance | 32 | 3.3% | 24 | 2.7% | 34 | 3.4% | 42 | 2.8% |
| Private insurance only | 403 | 41.4% | 392 | 43.7% | 420 | 41.4% | 600 | 39.9% |
| Missing | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Enrollment household income | ||||||||
| $0–$19,999 | 320 | 32.9% | 279 | 31.1% | 331 | 32.6% | 499 | 33.2% |
| $20,000–$44,999 | 238 | 24.4% | 225 | 25.1% | 251 | 24.7% | 361 | 24.0% |
| $45,000–$74,999 | 188 | 19.3% | 183 | 20.4% | 198 | 19.5% | 274 | 18.2% |
| $75,000 or over | 156 | 16.0% | 154 | 17.2% | 162 | 16.0% | 234 | 15.6% |
| Missing | 72 | 7.4% | 57 | 6.4% | 73 | 7.2% | 135 | 9.0% |
| Adjusted household income 1 | 969 | 18717.7 (17101.0) | 894 | 19650.8 (17258.7) | 1010 | 18794.6 (17060.3) | 1365 | 18864.5 (17229.4) |
| Prenatal maternal smoking 1 | ||||||||
| No | 887 | 91.1% | 820 | 91.3% | 925 | 91.1% | 1351 | 89.9% |
| Yes | 86 | 8.8% | 77 | 8.6% | 89 | 8.8% | 151 | 10.1% |
| Missing | 1 | 0.1% | 1 | 0.1% | 1 | 0.1% | 1 | 0.1% |
| Pre-pregnancy BMI class 1 | ||||||||
| Underweight | 42 | 4.3% | 38 | 4.2% | 45 | 4.4% | 66 | 4.4% |
| Normal | 378 | 38.8% | 352 | 39.2% | 399 | 39.3% | 633 | 42.1% |
| Overweight | 231 | 23.7% | 211 | 23.5% | 241 | 23.7% | 354 | 23.6% |
| Obese | 320 | 32.9% | 294 | 32.7% | 327 | 32.2% | 445 | 29.6% |
| Missing | 3 | 0.3% | 3 | 0.3% | 3 | 0.3% | 5 | 0.3% |
| Breastfeeding 1 | ||||||||
| No | 337 | 34.6% | 281 | 31.3% | 344 | 33.9% | 401 | 26.7% |
| Yes | 631 | 64.8% | 611 | 68.0% | 665 | 65.5% | 747 | 49.7% |
| Missing | 6 | 0.6% | 6 | 0.7% | 6 | 0.6% | 355 | 23.6% |
| Maternal IQ 1 | 967 | 95.2 (16.4) | 896 | 96.2 (16.4) | 1008 | 95.4 (16.4) | 1281 | 95.4 (16.3) |
| BSI global symptom t-score 1 | 968 | 46.9 (10.6) | 852 | 46.6 (10.4) | 968 | 46.9 (10.6) | 1055 | 46.6 (10.6) |
| Knowledge of Infant Development total score 1 | 973 | 0.7 (0.1) | 897 | 0.7 (0.1) | 1014 | 0.7 (0.1) | 1362 | 0.7 (0.2) |
| Other characteristics | ||||||||
| HOME subscale scores | ||||||||
| Learning materials | 737 | 8.0 (1.3) | 713 | 8.0 (1.3) | 766 | 8.0 (1.3) | 921 | 8.0 (1.3) |
| Variety of experience | 761 | 4.1 (1.0) | 736 | 4.1 (1.0) | 792 | 4.1 (1.0) | 925 | 4.1 (1.0) |
| Parental involvement | 738 | 5.3 (1.0) | 712 | 5.3 (1.0) | 767 | 5.3 (1.0) | 951 | 5.3 (1.0) |
| Childhood Opportunity Index in census tract | ||||||||
| Educational index1 | 910 | 0.01 (0.4) | 828 | 0.03 (0.4) | 938 | 0.01 (0.4) | 1046 | 0.02 (0.5) |
| Health index1 | 910 | 0.03 (0.6) | 828 | 0.05 (0.6) | 938 | 0.03 (0.6) | 1046 | 0.01 (0.4) |
| Economic index1 | 910 | 0.02 (0.4) | 828 | 0.04 (0.4) | 938 | 0.02 (0.4) | 1046 | −0.05 (0.6) |
BMI – Body Mass Index; BSI – Brief Symptom Index; CANDLE – Conditions Affecting Neurocognitive Development and Learning in Early Childhood; GED – General Education Development; HOME – Home Observational Measure of Environment; IQ – Intelligence Quotient; SD – Standard Deviation.
Included as covariates in main adjustment model (Model 2).
The Age 3 clinic visit was attended by 1046 mother–child dyads, of which 1040 had a valid Bayley language assessment (average age 3.1 years; SD = 0.1 years), and the average (SD) language composite score was 101.4 (12.3). The age 4 visit was attended by 1062 dyads; of these, 1056 children completed a valid IQ assessment. The mean (SD) age of children at this visit was 4.4 (0.6) years, and the mean FSIQ score was 100.3 (14.9). A total of 857 dyads attended both visits.
3.2. Prenatal phthalate exposure
The 13 individual phthalate metabolites that were detectable in at least 80% of the samples were included in this analysis (Table 2). MCNP had the lowest concentrations (geometric mean = 0.51 ng/mL, adjusted for specific gravity) while the phthalate present at the highest concentrations was MEP (geometric mean = 126.3 ng/mL, adjusted for specific gravity). Metabolite pairwise correlations varied from weak to strong (Table S1). Compared to geometric means reported in the US National Health and Nutrition Examination Survey (NHANES) in 2009–2010 (Zota et al., 2014), those in our analytic population were similar, with some exceptions. The geometric mean concentration of MEP in CANDLE women was almost twice as high (110.86 versus 64.4 ng/mL) while MBzP and MEHP were somewhat higher (10.29 versus 6.46 and 2.26 versus 1.59 ng/mL, respectively). The geometric mean of MCPP was in CANDLE less than that observed in NHANES (1.40 versus 3.02 ng/mL) while that of MECPP and MEHHP were also lower (11.75 versus 20.7 and 8.13 versus 12.9 ng/mL, respectively).
Table 2.
Distributions of specific gravity adjusted urinary phthalate concentrations in CANDLE.
| Metabolites | LOD (ng/mL)1 | Percent detected | 25th percentile (ng/mL) | Median (ng/mL) | 75th percentile (ng/mL) | Geometric Mean (SD) (ng/mL) |
|---|---|---|---|---|---|---|
|
| ||||||
| MnBP | 0.101 | 100.0% | 9.49 | 15.88 | 25.45 | 15.80 (2.10) |
| MiBP | 0.056 | 100.0% | 4.31 | 7.66 | 12.37 | 7.54 (2.19) |
| MECPP | 0.044 | 100.0% | 7.49 | 11.62 | 19.27 | 13.05 (2.38) |
| MEP | 0.137 | 100.0% | 47.18 | 110.87 | 312.61 | 126.27 (3.86) |
| MCPP | 0.023 | 99.9% | 0.89 | 1.39 | 2.31 | 1.51 (2.34) |
| MEOHP | 0.092 | 99.9% | 3.74 | 6.13 | 10.07 | 6.67 (2.51) |
| MCOP | 0.012 | 99.8% | 1.15 | 2.17 | 4.87 | 2.49 (3.02) |
| MCNP | 0.016 | 99.6% | 0.28 | 0.47 | 0.85 | 0.51 (2.45) |
| MEHHP | 0.019 | 99.2% | 4.96 | 8.02 | 14.08 | 8.44 (2.95) |
| MCMHP | 0.039 | 98.6% | 3.85 | 6.11 | 10.67 | 6.60 (2.89) |
| MBzP | 0.092 | 98.6% | 5.42 | 10.23 | 19.06 | 10.04 (3.04) |
| MMP | 0.115 | 89.6% | 1.40 | 2.36 | 3.87 | 1.99 (3.46) |
| MEHP | 0.304 | 80.6% | 0.73 | 2.26 | 5.25 | 2.14 (4.06) |
| Sum DEHP1 | NA | NA | 0.07 | 0.12 | 0.20 | 0.13 (2.39) |
MIBP - mono-isobutyl phthalate; MEP - monoethyl phthalate; MMP - mono-methyl phthalate; MnBP - mono-n-butyl phthalate; MBzP - mono-benzyl phthalate; MCNP - mono-carboxy-isononyl phthalate; MCOP - mono-carboxy-isooctyl phthalate; MCPP - mono-(3-carboxypropyl) phthalate; MEHP - mono-(2-ethylhexyl) phthalate; MEHHP - mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP - mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - mono-(2-ethyl-5-carboxypentyl) phthalate; MCMHP - mono(2-carboxymethylhexyl) phthalate; Sum DEHP - sum of di(2-ethylhexyl)phthalate metabolites.
Sum DEHP was calculated as the molar sum of DEHP metabolites, and the units are nmol/mL.
The phthalate distributions were comparable between male and female children (Table S2). Participants in the lowest SES category were more likely to have higher MnBP, MEP, MiBP, MBzP and MEHP (Table S3). The variability of each individual phthalate (geometric SD) was relatively low in the overall study population, and variability of each metabolite was similar across strata by child sex and SES category as well.
3.3. Performance of the permutation test
To assess performance of WQS regression with the permutation test extension, we calculated the power (i.e., sensitivity) and type I error rate of the WQS coefficient estimated in various implementations of WQS regression (Table 3). When WQS regression was performed with the sample split into training and validation datasets (WQSSplit), the type I error rate was low (6%) but statistical power was also low (56%). With full-sample WQS (WQSNosplit), conducted without splitting the sample, power was maximized but the type I error rate was 47%. With calculation of permutation test p-values in full-sample WQS (WQSPermutation), we observed that statistical power was high (90%) and the type I error rate was approximately equal to that achieved by splitting the sample into training and validation datasets (7%). See Supplement A3 for additional details.
Table 3.
Performance of WQS regression models in simulated datasets.
| Model | Power | Type I Error Rate |
|---|---|---|
|
| ||
| WQSSplit | 56% | 6% |
| WQSNosplit | 100% | 47% |
| WQSPermutation | 90% | 7% |
WQS – weighted quantile sum; WQSSplit – WQS regression model with 40:60 sample splitting into training and validation datasets; WQSNosplit – WQS regression model using the full sample, with no splitting; WQSPermutation – WQS regression using the full sample, and p-values estimated using the permutation t.
3.4. Associations between phthalates and language measures
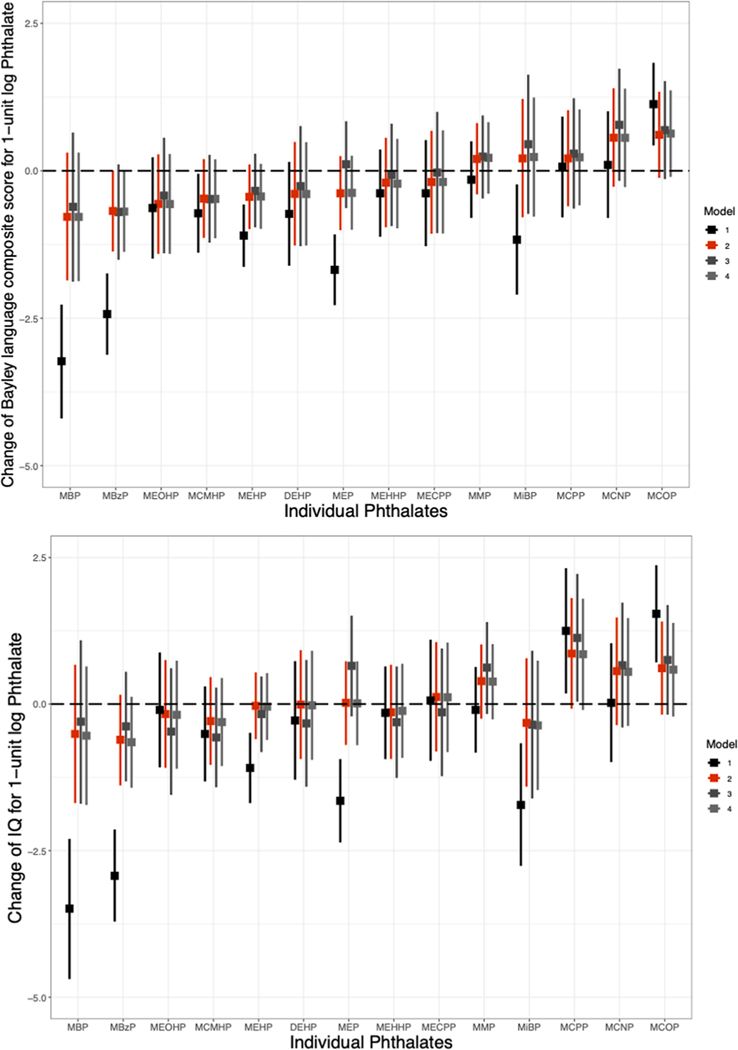
We found little evidence for associations between individual metabolites and Bayley language composite scores (Fig. 2a). While several metabolites were associated with lower language score in minimally adjusted models (MnBP, MBzP, MEP, MiBP, MCMHP, and MEHP), the associations did not persist with further adjustment. In the main adjustment models (Model 2), only MBzP was associated with lower language scores (β = −0.68, 95% CI: −1.37, 0.00). MCOP and MCNP were suggestively associated with higher language scores in Model 2. Model 3 results were similar, and adjustment for possible mediators did not alter associations (Model 4).
Fig. 2. Associations between individual phthalate metabolites and a) Bayley-III language composite score and b) full-scale IQ.
Phthalates were adjusted for specific gravity and log-transformed. Robust standard errors were used. Model 1: Adjusted for child age and sex; Model 2 (Full model): Additionally adjusted for maternal education, log transformed income adjusted for household size, maternal race, maternal IQ, maternal age, marital status (married vs. living with partners vs. single), insurance status, prenatal smoking, child birth order, recruitment site, child year of birth, pre-pregnancy BMI class, breastfeeding, prenatal psychopathology (the BSI global severity t-score), the Childhood Opportunity Index subscale scores (all 3 subscales, separately) and the KIDI score (total score); Model 3: Full model, additionally adjusted for the HOME subscale scores (learning materials, variety in experience and parental involvement); Model 4: Full model, additionally adjusted for birthweight and preterm birth.
We also observed little evidence of associations with language score when phthalates were analyzed as mixtures using WQS (Table 4 and Fig. 3). When analyzing negative relationships between phthalates and language (adverse effects) in the main adjustment models, a 1-unit increase in WQS index was associated with −0.76 language composite score points (full-sample 95% CIs: −1.73, 0.21), and MEHP and MBzP had the largest weights (i.e., weights in the highest sextile across metabolites; Fig. S3). For positive associations, an increase in WQS index of one unit was associated with 0.75 higher points (full-sample 95% CIs: −0.11, 1.6). MCOP and MCNP were the metabolites with the highest weights in the positive WQS index (Fig. S3). Since the full-sample 95% CIs included the null in main adjustment models, we did not calculate permutation test p-values.
Table 4.
Associations between prenatal phthalate mixtures and primary outcomes in WQS regression.
| Direction | Models1 | WQS estimate4 | Full sample 95% CI | ppermutation2 |
|---|---|---|---|---|
|
| ||||
| Bayley-III language composite score | ||||
| Positive | Model 13 | NA | NA | NA |
| Model 2 | 0.75 | (−0.11, 1.60) | NA | |
| Model 3 | 1.04 | (0.00, 2.09) | NA | |
| Model 4 | 1.05 | (−0.09, 2.19) | NA | |
| Negative | Model 1 | −3.57 | (−4.37, −2.77) | <0.01 |
| Model 2 | −0.76 | (−1.73, 0.21) | NA | |
| Model 3 | −0.52 | (−1.57, 0.53) | NA | |
| Model 4 | −0.63 | (−1.73, 0.47) | NA | |
| Full scale IQ | ||||
| Positive | Model 13 | NA | NA | NA |
| Model 2 | 1.01 | (−0.03, 2.05) | NA | |
| Model 3 | 1.58 | (−0.37, 2.79) | 0.075 | |
| Model 4 | 0.99 | (−0.03, 2.02) | NA | |
| Negative | Model 1 | −3.88 | (−4.80, −2.97) | <0.01 |
| Model 2 | −0.42 | (−1.43, 0.58) | NA | |
| Model 3 | −0.58 | (−1.65, 0.49) | NA | |
| Model 4 | −0.49 | (−1.49, 0.52) | NA | |
IQ – Intelligence Quotient; WQS – Weighted Quantile Sum.
Model 1: Adjusted for child age and sex; Model 2 (Full model): Additionally adjusted for maternal education, log transformed income adjusted for household size, maternal race, maternal IQ, maternal age, marital status (married vs. living with partners vs. single), insurance status, prenatal smoking, child birth order, recruitment site, child year of birth, pre-pregnancy BMI class, breastfeeding, prenatal psychopathology (the BSI global severity t-score), the Childhood Opportunity Index subscale scores (all 3 subscales, separately) and the KIDI score (total score); Model 3: Full model, additionally adjusted for the HOME subscale scores (learning materials, variety of experiences, and parental involvement); Model 4: Full model, additionally adjusted for birthweight and preterm birth.
A p-value was calculated using the permutation test when the full sample 95% CIs excluded the null.
There was no positive association in any bootstrap runs.
The WQS estimate is the average change in outcome associated with a one-unit increase in WQS index.
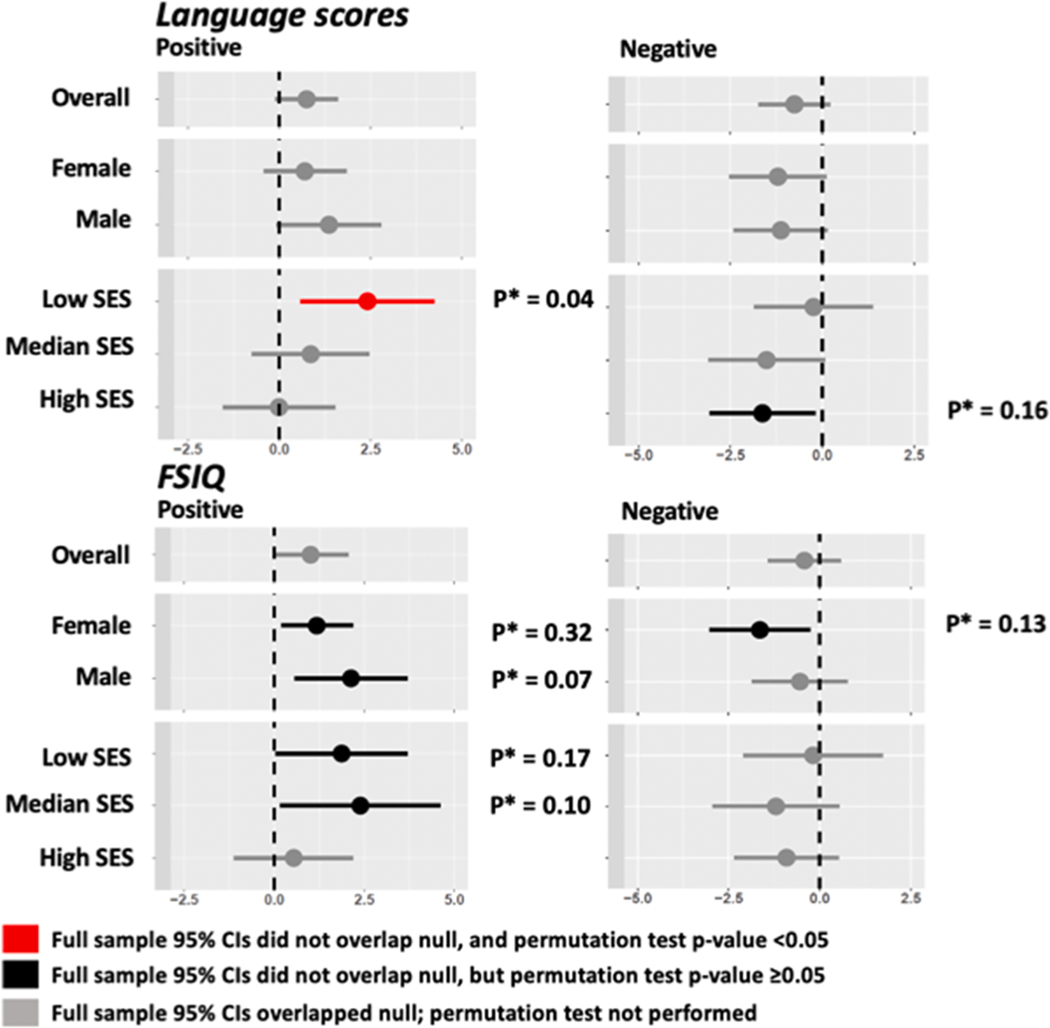
Fig. 3. Associations between phthalate mixtures and Bayley-III language composite score and full-scale IQ phthalates estimated using WQS in the overall population and by the strata of child sex and SES tertiles.
When the 95% Cis estimated in full sample WQS excluded the null, we calculated a confirmatory p-value using a permutation test (p*). All associations calculated using the main adjustment model (adjusted for child sex, child age, maternal education, log transformed income adjusted for household size, maternal race, maternal IQ, maternal age, marital status, insurance status, prenatal smoking, child birth order, recruitment site, child year of birth, pre-pregnancy BMI class, breastfeeding, prenatal psychopathology [the BSI global severity t-score], the Childhood Opportunity Index subscale scores [all 3 subscales, separately] and the KIDI score [total score]). SES categories were calculated using a composite of enrollment income and maternal education.
We used WQS regression to evaluate both negative and positive associations between phthalate mixtures and language scores for females and males separately (Fig. 3). Positive associations were somewhat higher in males than females (βmale = 1.36, full sample 95%CI: −0.07, 2.79; βfemale=0.7, full sample 95%CI: −0.43, 1.83). Weights in positive WQS indices were largest for MMP and MCOP for males, and MECPP, MCPP and MCNP for females (Fig. S3). Adverse associations were similar for males and females (βmale = −1.13, full sample 95%CI: −2.41, 0.15; βfemale= −1.21, full sample 95%CI: −2.54, 0.11). The adverse mixture determined by WQS regression in males was driven by MCMHP and MBzP whereas for females it was MEP, MEHP and MnBP (Fig. S3).
When stratified by SES in WQS analysis, we observed a positive association between phthalate mixtures and language score only in the lowest SES tertile (βlow = 2.41, full sample 95%CI: 0.58, 4.24, ppermutation = 0.04), with the highest weights assigned to MMP and MCOP (Fig. S3). For adverse associations between mixtures and language scores, the largest effects were observed in the higher two tertiles (βmiddle = −1.52, full sample 95%CI: −3.11, 0.08; βhigh = −1.63, full sample 95%CI: −3.07, −0.19, ppermutation = 0.17). For the middle SES group, MCMHP, MEHP and MEP had the largest weights in the WQS index; for the highest SES group, it was MEOHP. The sensitivity analysis replacing the five DEHP metabolites with sum DEHP yielded the same conclusions as the primary analysis (Table S4, Fig. S2 and Fig. S5).
3.5. Associations between phthalates and FSIQ
When phthalate metabolites were evaluated individually, we observed little evidence supporting adverse effects of phthalates on FSIQ (Fig. 2b). Five metabolites were significantly associated with lower FSIQ in minimally adjusted models (MBzP, MnBP, MiBP, MEHP and MEP), but these associations were strongly attenuated or reversed after further adjustment, including in our main adjustment model (Model 2). MMP, MCNP, MCOP and MCPP exhibited suggestive associations with higher FSIQ in Model 2, though 95% confidence intervals included the null (Fig. 2b). Adjustment for possible mediators had no effect on associations (Model 4).
We used WQS regression to estimate adverse and positive associations between phthalate mixtures and FSIQ using the main adjustment model (Table 4 and Fig. 3). In analyses of negative (adverse) relationships between phthalate mixtures and FSIQ, no association was observed. When positive relationships were analyzed, the WQS effect size was 1.01 points (full sample 95%CI: −0.03, 2.05) per WQS unit in the main adjustment model (Model 2), and MCNP had the highest weight (Fig. S4). With adjustment for additional confounders in the expanded model (Model 3), this positive effect was stronger and more precise (β = 1.58, full sample 95%CI: 0.37, 2.79; ppermutation = 0.075). Since the full sample CIs overlapped the null in all cases, we did not use the permutation test to calculate p-values, and we did not evaluate effect modification by child sex or SES using the mixture weights derived for the overall population.
We estimated child sex- and SES-specific positive and negative associations between prenatal phthalate mixtures and FSIQ in stratified WQS analyses (Fig. 3). In both sexes, the positive WQS index was associated with higher FSIQ, but p-values calculated with a permutation test indicated that the strength of the evidence to reject the null was not strong (βmale = 2.13, full sample 95%CI: 0.56, 3.69; ppermutation = 0.07; βfemale=1.18, full sample 95%CI: 0.19, 2.18; ppermutation = 0.32). In analyses of adverse relationships, phthalate mixtures were associated with lower IQ in females, with MIBP, MBzP, MMP and MCMHP assigned the highest weights (Fig. S4), though the p-value calculated with a permutation test did not confirm the strength of the evidence suggested by full sample 95% CIs (βnegative = −1.64, full sample 95%CI: −3.03, −0.25; ppermutation = 0.13). When stratified by SES, we observed positive associations in the two lowest SES categories, but p-values calculated with a permutation test did not support the strength of the evidence indicated by full sample 95% CIs (βlowSES = 1.87, full sample 95%CI: 0.04, 3.70, ppermutation = 0.17; βmiddleSES=2.39, full sample 95%CI: 0.16, 4.61, ppermutation = 0.10) (Fig. 3). We did not observe evidence of SES strata-specific adverse associations between phthalate mixtures and FSIQ (Fig. 3).
The sensitivity analysis replacing the five DEHP metabolites with sum DEHP yielded the same conclusions as the primary analysis (Table S4, Fig. S2, and Fig. S6).
4. Discussion
We conducted an analysis of prenatal phthalate exposure and child cognitive and language development using the CANDLE Study, a diverse and well-characterized birth cohort representative of Shelby County, TN. In primary analyses, we estimated the effect of phthalate mixtures using WQS regression with a permutation test, a new extension of the WQS method that we developed to improve the specificity of full-sample analyses. We observed little evidence that prenatal phthalate mixtures had adverse impacts on IQ or language in early childhood, either in the overall population or in subgroups. Although some evidence of positive effects of phthalate mixtures on both IQ and language outcomes was observed, in most cases the strength of the evidence was not supported by a permutation test. An exception was a positive association between a mixture dominated by MMP, MCOP, MCNP and MCPP and language scores in the lowest SES stratum that was confirmed to be significant at a traditional alpha of 0.05 by the permutation test. Given the number of comparisons evaluated, this could be a spurious finding. In exploratory analyses, we additionally analyzed all metabolites individually in the overall sample. Most of the adjusted associations were null, though MBzP was linked to lower language scores.
Our study adds a set of largely null findings to a generally inconsistent body of epidemiological evidence on prenatal phthalates and cognition. A recent systematic review on this topic concluded that the weight of epidemiological evidence for adverse effects of phthalates on cognition was “slight” (Radke et al., 2020). Included in this review were 11 birth cohort studies set in Asia, Europe or North America, ranging in size from N = 64 to 802. Overall, there was little between-study consistency in the magnitude or direction of effects. While most studies reported at least one adverse association (Factor-Litvak et al., 2014; Gascon et al., 2015; Huang et al., 2015; Kim Yeni et al., 2011; Polanska et al., 2014; Téllez-Rojo et al., 2013; Whyatt et al., 2012), in general the associations with individual phthalates were null. Among the studies reviewed by Radke et al. (2020), no clear patterns emerged as to which metabolites might have the strongest adverse or protective effects. We identified three recent studies of cognition not included in the Radke et al. review. Hyland et al. (2019) analyzed prenatal phthalates and a wide range of neurodevelopmental outcomes assessed at adolescence in the CHAMACOS cohort and observed no associations with IQ overall but found sex-specific effects, with positive effects in girls and adverse effects in boys. Prenatal and postnatal phthalate metabolites were measured in cohort of N = 134 Polish mothers by Jankowska et al. (2019) and examined in relation to cognitive outcomes at 7 years. Associations with prenatal metabolites were null, with the exception of MEHP, which was linked to higher cognition. Qian et al. (2019) found no associations between urinary phthalate metabolites measured in three trimesters of N = 476 pregnancies in China and the Bayley Scales of Infant Development mental development index at age 2. Taken together, findings of these newer studies and ours likely do not shift the collective evidence for potential causal effects above the “slight” determination of Radke et al. (2020).
Fewer studies have explored relationships between prenatal phthalate exposure and early childhood language skills. In the Danish Odense Cohort (N = 518 pregnancies), Olesen et al. (2018) observed that higher prenatal MEP and ∑DEHP was associated with poorer language outcomes at age 20–36 months, but only in boys. Bornehag et al. (2018) explored prenatal phthalate exposure in relation to reported number of words understood around 3 years in separate analyses of cohorts in the US (TIDES; n = 309) and Sweden (SELMA; n = 963). Results in each cohort suggested a higher risk of language delay with higher MnBP and MBzP exposure, while risk of delay with MEP exposure were observed in SELMA only. Like Bornehag et al. (2018), we observed possible adverse effects of MBzP, MnBP and MEP on language outcomes, but those associations were sensitive to covariate inclusion in our analyses and effectively disappeared after adjustment for confounders. Both Bornehag et al. (2018) and Olesen et al. (2018) included minimal covariates describing SES or related factors in regression models. Another methodological difference is that language outcomes analyzed by Olesen et al. (2018) and Bornehag et al. (2018) were parental report of words spoken, while the language assessment in our analysis was a rigorous direct assessment by a trained examiner. Some studies of phthalates and IQ additionally explored relationships with IQ subtests, including the WPPSI verbal IQ factor (Huang et al., 2017; Nakiwala et al., 2018) and the verbal comprehensive index of the WISC-IV (Factor-Litvak et al., 2014; Huang et al., 2017; Hyland et al., 2019). These involved direct assessments of language but were all smaller studies conducted on older children, providing limited insight into early life language development.
A limitation of previous studies of phthalates and cognition or language is a missing perspective on possible mixture effects. Studies in pregnant women have detected widespread exposure to complex mixtures of phthalates (Woodruff et al., 2011). Analyzing metabolites independently may fail to uncover true effects attributable to co-exposure to several phthalates (Kortenkamp Andreas, 2007). In addition, because metabolites are highly correlated, true associations with one metabolite may appear as a spurious association with another phthalate metabolite when they are analyzed separately (Braun et al., 2016). We addressed this shortcoming in the phthalate-neurodevelopment literature by applying WQS regression and, further, advancing the existing WQS algorithm with the addition of a permutation test. In our simulation models, WQS with the permutation test had improved power to detect true associations in comparison to WQS performed on a split sample (90% vs 56% power, respectively) and substantial improvement in specificity compared to WQS performed without splitting the sample (a 7% vs 47% false positive rate, respectively.) It is important to note that in our study sample, all but one of the associations that appeared significant in full sample WQS had permutation test p-values > 0.05, indicating that applications of full-sample WQS in the literature may include false positive findings. We recommend that future applications of WQS in a full sample use the permutation test.
In addition to applying our enhanced WQS methodology, which improved sensitivity to detect true associations by obviating the need to split the data into training and test sets, this analysis involved a larger sample size than previous studies. Despite these advantages, our findings were predominantly null. One possible explanation is that prenatal phthalates at environmentally-relevant levels do not have a measurable impact on childhood cognitive or language development. In this case, adverse associations observed in other studies may have been caused by chance. Analyzing multiple metabolites independently, a common approach, increases the possibility of spurious findings. Another feasible explanation for observed associations in other studies is residual confounding. Phthalate exposure is associated with SES, but this relationship varies by metabolite and by population (James-Todd et al., 2017; Wenzel et al., 2018). Since pediatric neurodevelopmental outcomes are strongly related to SES, incomplete adjustment for SES and/or downstream factors could bias observed associations away from the null. Most studies reporting adverse associations adjusted for only a single SES variable (Bornehag et al., 2018; Factor-Litvak et al., 2014; Gascon et al., 2015; Huang et al., 2017; Olesen et al., 2018; Téllez-Rojo et al., 2013; Whyatt et al., 2012). At the same time, we cannot rule out the possibility that different findings between studies are due to differences in the magnitude or variability of phthalate exposure. We may have failed to detect true associations with some metabolites if exposure variability was limited in CANDLE or in the case of nonlinear exposure–response relationships and between-study differences in exposure magnitude.
Despite null findings in many observational studies, including ours, there is ample toxicological support for neurotoxic effects of phthalates. Animal models suggest a variety of mechanisms by which prenatal or postnatal exposure to phthalates can influence neurodevelopment, including the disruption of dopamine production, lipid metabolism, and signaling induced by calcium, thyroid hormones, and sex hormones (Miodovnik et al., 2014; Tanida et al., 2009; Xu et al., 2015). Specific to cognitive development, in rodents, postnatal phthalate administration reduced hippocampal neuronal density and plasticity when administered at postnatal stages largely corresponding to postnatal neurodevelopmental stages in humans (Li et al., 2013; Semple et al., 2013; Smith et al., 2011). Postnatal BBzP administration in rats decreased the expression of amygdalar proteins involved in synaptic plasticity, a mechanism potentially underlying our observed association between MBzP and language scores (Betz et al., 2013). It is unclear what mechanisms could underlie sporadic positive associations between phthalates and neurocognitive outcomes observed in our and other studies. We observed relatively strong evidence for positive associations between a mixture of metabolites dominated by MMP, MCOP, MCNP and MCPP in the lowest SES category. By contrast, other studies of prenatal exposure and child cognition observed mostly null or negative associations for these metabolites (Huang et al., 2015; Dong et al., 2019; Hyland et al., 2019). These sporadic positive associations observed in our study and others may be spurious or due to residual confounding.
Important limitations of our work include analyzing phthalate metabolites in only one urine sample per pregnancy. There is a high degree of within-individual temporal variability in urinary metabolite concentrations; the exposure measurement error associated with using a single time point could have biased observed associations towards the null. Related, if susceptibility to phthalate exposure varies across pregnancy, we could have missed a critical window by using only one measure (Li et al., 2019). Most other epidemiological studies of phthalates and neurodevelopment also relied upon one urine sample. A limitation specific to WQS regression is that the algorithm does not account for the relative toxicity of mixture components or any differences in magnitude of exposure between metabolites, as quantiles are calculated for each component based on the distribution in the analytic sample. Another potential limitation to our overall approach is that we only analyzed phthalates, and it is plausible that phthalate health effects are synergistic with exposure to other common endocrine disrupting compounds (EDCs), as suggested by findings of Tanner et al. (2020). A broader perspective on EDC exposure may give better insight into health effects of cumulative exposure to mixtures of EDCs, including phthalates. Finally, it is uncertain whether the findings of our study, set in a sociodemographically diverse region of the southern US, are generalizable to other study populations. However, our participants do share characteristics with other, majority Black communities that face high levels of social disadvantage in the urban South.
There are also a number of strengths. The CANDLE study is a relatively large, pregnancy cohort study set in a diverse, high-adversity US population. Enrollment was representative of the underlying population, and attrition throughout childhood was low. Rich longitudinal data collection provided ample data on important neurodevelopmental predictors and risk factors, including maternal IQ, multiple levels and domains of SES, and HOME score, a coded rating of the home environmental quality. Another strength is that both cognition and language ability were directly assessed by trained examiners. Finally, the novel application of the permutation test to WQS regression reduced the likelihood of false positive findings associated with conducting WQS in a full sample without splitting the sample into a training and validation dataset. This is significant because sample sizes in investigations of environmental chemical exposures and child health outcomes are often limited, and the permutation test extension of WQS regression yields accurate precision estimates without compromising statistical power.
Overall, this study contributes compelling evidence for null relationships between prenatal phthalate exposure and early childhood cognition and language development. We also introduce a new extension of the WQS method that improves the accuracy of precision estimates without the loss of power associated with splitting samples into training and validation samples. Further research of the ECHO PATHWAYS Consortium will include assessments of multiple exposure windows in pregnancy, longitudinal neurodevelopment through middle childhood, and possible modification of associations by prenatal nutrition.
Supplementary Material
Acknowledgments
ECHO PATHWAYS is funded by NIH (1UG3OD023271–01, 4UH3OD023271–03). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute and NIH (R01 HL109977). We are grateful for the participation of families enrolled in the CANDLE cohort, as well as the dedication of CANDLE research staff and investigators.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106409.
BBZP- Benzyl butyl phthalate; BMI - Body Mass Index; BSI - Brief Symptom Index; CANDLE - Conditions Affecting Neurocognitive Development and Learning in Early Childhood; CI - Confidence Intervals; COI - Childhood Opportunity Index; ECHO - Environmental Influences on Child Health Outcomes; EDC - Endocrine Disrupting Compound; HOME - Home Observation Measurement of the Environment; HPLC - High-Performance Liquid Chromatography; IRB - Institutional Review Board; IQ - Intelligence Quotient; KIDI - Knowledge of Infant Development Inventory; LOD - Limit of Detection; MIBP - mono-isobutyl phthalate; MEP - monoethyl phthalate; MMP - mono-methyl phthalate; MnBP - mono-n-butyl phthalate; MBzP - mono-benzyl phthalate; MCNP - mono-carboxy-isononyl phthalate; MCOP - mono-carboxy-isooctyl phthalate; MCPP - mono-(3-carboxypropyl) phthalate; MEHP - mono-(2-ethylhexyl) phthalate; MEHHP - mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP - mono-(2-ethyl-5-oxohexyl) phthalate; MECPP - mono-(2-ethyl-5-carboxypentyl) phthalate; MCMHP - mono(2-carboxymethylhexyl) phthalate; SB-5 - Stanford-Binet 5; SD - Standard Deviation; SES - Socioeconomic Status; SG - Specific Gravity; UTHSC - University of Tennessee Health Science Center; WASI - Weschler Abbreviated Scale Intelligence; WISC-V - Weschler Intelligence Scale of Intelligence V; WPPSI - Weschler Preschool and Primary Scale of Intelligence; WQS - Weighted Quantile Sum; ∑DEHP - sum of di(2-ethylhexyl) phthalate metabolites
References
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, et al. , 2014. The child opportunity index: improving collaboration between community development and public health. Health Aff. 33, 1948–1957. 10.1377/hlthaff.2014.0679. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Xue J, De Carvalho BP, Iyer A, Abualnaja KO, Yaghmoor SS, et al. , 2016. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res 150, 573–581. 10.1016/j.envres.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Axelrod BN, 2002. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment 9, 17–23. 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- Barakat R, Lin P-C, Park CJ, Best-Popescu C, Bakry HH, Abosalem ME, et al. , 2018. Prenatal Exposure to DEHP Induces Neuronal Degeneration and Neurobehavioral Abnormalities in Adult Male Mice. Toxicol. Sci 164, 439–452. 10.1093/toxsci/kfy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N, 2006. Bayley Scales of Infant and Toddler Development: Administration Manual. Harcourt Assessment, San Antonio, TX. [Google Scholar]
- Betz A, Jayatilaka S, Joshi J, Ramanan S, Debartolo D, Pylypiw H, et al. , 2013. Chronic exposure to benzyl butyl phthalate (BBP) alters social interaction and fear conditioning in male adult rats: alterations in amygdalar MeCP2, ERK1/2 and ERα. Neuro Endocrinol. Lett 34, 347–358. [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am. Ind. Hyg. Assoc. J 54, 615–627. 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bornehag C-G, Lindh C, Reichenberg A, Wikström S, Unenge Hallerback M, Evans SF, et al. , 2018. Association of prenatal phthalate exposure with language development in early childhood. JAMA Pediatr. 172, 1169–1176. 10.1001/jamapediatrics.2018.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovicka T Jr. M J, Kordik P, Jirina M,. 2012. Selecting representative data sets. Adv. Data Mining Knowledge Disc. Appl doi: 10.5772/50787. [DOI] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF, 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 124, A6–A9. 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Sanchez Guerra M, Gennings C, Hacker M, Jara C, Bosquet Enlow M, et al. , 2017. Maternal lifetime stress and prenatal psychological functioning and decreased placental mitochondrial DNA copy number in the PRISM study. Am. J. Epidemiol 186, 1227–1236. 10.1093/aje/kwx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burniaux J-M, Dang T-T, Fore D, Förster M, Ercole MM, Oxley H, 1998. Income Distribution and Poverty in Selected OECD Countries. OECD Economic Department Working Paper No. 189, Paris. [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat 20, 100–120. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Wheeler DC, 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform. 14, 159–171. 10.4137/CIN.S17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Balalian AA, Whyatt RM, Liu X, Rauh V, Herbstman J, et al. , 2020. Perinatal phthalates exposure decreases fine-motor functions in 11-year-old girls: Results from weighted Quantile sum regression. Environ. Int 136 (105424) 10.1016/j.envint.2019.105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N, 1983. The Brief Symptom Inventory: an introductory report. Psychol. Med 13, 595–605. 10.1017/S0033291700048017. [DOI] [PubMed] [Google Scholar]
- Dong R, Yu W, Chen J, Wu M, Li S, Chen B, 2019. Lactational exposure to phthalates impaired the neurodevelopmental function of infants at 9 months in a pilot prospective study. Chemosphere. 10.1016/j.chemosphere.2019.03.159. [DOI] [PubMed] [Google Scholar]
- Elardo R, Bradley RH, 1981. The home observation for measurement of the environment (HOME) scale: A review of research. Dev. Rev 1, 113–145. 10.1016/0273-2297(81)90012-5. [DOI] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. , 2014. Persistent associations between maternal prenatal exposure to phthalates on child IQ at Age 7 Years. PLoS One 9, e114003. 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D, Lane D, 1983. A nonstochastic interpretation of reported significance levels. J. Bus. Econ. Stat 1 (4), 292–298. [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martínez D, Júlvez J, et al. , 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int. J. Hyg. Environ. Health 218, 550–558. 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K, 2014. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: longitudinal investigation of fertility and the environment study. Environ. Sci. Technol 48, 9804–9811. 10.1021/es5024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher KM, Willing J, Chiang C, Rattan S, Flaws JA, Mahoney MM, 2019. Exposure to di-(2-ethylhexyl) phthalate transgenerationally alters anxiety-like behavior and amygdala gene expression in adult male and female mice. Physiol. Behav 207, 7–14. 10.1016/j.physbeh.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM, 2005. Phthalates and human health. Occup. Environ. Med 62, 806–818. 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Blount BC, Valentin-Blasini L, Wapner R, Whyatt R, Gennings C, et al. , 2015. CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ. Res 143, 1–9. 10.1016/j.envres.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-B, Chen H-Y, Su P-H, Huang P-C, Sun C-W, Wang C-J, et al. , 2015. Fetal and childhood exposure to phthalate diesters and cognitive function in children up to 12 years of age: Taiwanese maternal and infant cohort study. PLoS One 10. 10.1371/journal.pone.0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P-C, Tsai C-H, Chen C-C, Wu M-T, Chen M-L, Wang S-L, et al. , 2017. Intellectual evaluation of children exposed to phthalate-tainted products after the 2011 Taiwan phthalate episode. Environ. Res 156, 158–166. 10.1016/j.envres.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Hyland C, Mora AM, Kogut K, Calafat AM, Harley K, Deardorff J, et al. , 2019. Prenatal Exposure to Phthalates and Neurodevelopment in the CHAMACOS Cohort. Environ. Health Perspect 127 10.1289/EHP5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Seely EW, Ferguson KK, et al. , 2017. Racial and ethnic variations in phthalate metabolite concentration changes across full-term pregnancies. J. Expo. Sci. Environ. Epidemiol 27, 160–166. 10.1038/jes.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska A, Polańska K, Hanke W, Wesołowska E, Ligocka D, Waszkowska M, et al. , 2019. Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years - Polish Mother and Child Cohort. Environ. Res 177 (108626) 10.1016/j.envres.2019.108626. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. , 2011. Prenatal exposure to phthalates and infant development at 6 months: prospective mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect 119, 1495–1500. 10.1289/ehp.1003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A, 2007. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect 115, 98–105. 10.1289/ehp.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic BAG, Sly PD, Knibbs LD, 2019. Statistical methodology in studies of prenatal exposure to mixtures of endocrine-disrupting chemicals: A review of existing approaches and new alternatives. Environ. Health Perspect 127 10.1289/EHP2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Sep 21. Identification of modifiable social and behavioral factors associated with childhood cognitive performance. JAMA Pediatr. 174 (11), 1–11. 10.1001/jamapediatrics.2020.2904. Epub ahead of print. PMID: 32955555; PMCID: PMC7506587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-W, Kim M-S, Lim Y-H, Lee N, Hong Y-C, 2018. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res 167, 558–566. 10.1016/j.envres.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. , 2019. Identifying periods of susceptibility to the impact of phthalates on children’s cognitive abilities. Environ. Res 172, 604–614. 10.1016/j.envres.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Jiang L, Chen L, Chen H-S, Li X, 2013. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: a study in rats. Environ. Toxicol. Pharmacol 36, 392–402. 10.1016/j.etap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Lin H, Yuan K, Li L, Liu S, Li S, Hu G, et al. , 2015. In Utero Exposure to Diethylhexyl Phthalate Affects Rat Brain Development: A Behavioral and Genomic Approach. Int. J. Environ. Res. Public Health 12, 13696–13710. 10.3390/ijerph121113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D, 1981. Manual for the knowledge of infant development inventory. niversity of North Carolina. Unpublished manuscript. [Google Scholar]
- Malin AJ, Busgang SA, Cantoral AJ, Svensson K, Orjuela MA, Pantic I, et al. , 2018. Quality of prenatal and childhood diet predicts neurodevelopmental outcomes among children in Mexico City. Nutrients 10 (1093). 10.3390/nu10081093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly B, 1991. Randomization and Monte Carlo Methods in Biology London. Chapman and Hall, UK. [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R, 2014. Developmental neurotoxicity of ortho-phthalate diesters: Review of human and experimental evidence. NeuroToxicology 41, 112–122. 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Nakiwala D, Peyre H, Heude B, Bernard JY, Béranger R, Slama R, et al. , 2018. In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environ. Health 17 (17). 10.1186/s12940-018-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves JW, Gennings C, Factor-Litvak P, Hupf J, Singleton J, Sharf V, et al. , 2016. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol 73, 1425–1432. 10.1001/jamaneurol.2016.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen TS, Bleses D, Andersen HR, Grandjean P, Frederiksen H, Trecca F, et al. , 2018. Prenatal phthalate exposure and language development in toddlers from the Odense Child Cohort. Neurotoxicol. Teratol 65, 34–41. 10.1016/j.ntt.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W, 2014. Phthalate exposure and child development: The Polish Mother and Child Cohort Study. Early Human Dev. 90, 477–485. 10.1016/j.earlhumdev.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Qian X, Li J, Xu S, Wan Y, Li Y, Jiang Y, et al. , 2019. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ. Int 131, 105023. 10.1016/j.envint.2019.105023. [DOI] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, Cooper GS, 2020. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int 137, 105408. 10.1016/j.envint.2019.105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Barbosa F, Kannan K, 2017. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ 586, 152–162. 10.1016/j.scitotenv.2017.01.193. [DOI] [PubMed] [Google Scholar]
- Roid GH, Pomplun M, 2012. The Stanford-Binet Intelligence Scales, Fifth Edition. In: Contemporary Intellectual Assessment: Theories, Tests, and issues, 3rd ed., The Guilford Press, New York, NY, US. pp. 249–268. [Google Scholar]
- Romano ME, Eliot MN, Zoeller RT, Hoofnagle AN, Calafat AM, Karagas MR, et al. , 2018. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int. J. Hyg. Environ. Health 221, 623–631. 10.1016/j.ijheh.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol 1–16. 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL, Needham LL, Calafat AM, 2004. Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol 72, 1226–1231. 10.1007/s00128-004-0374-4. [DOI] [PubMed] [Google Scholar]
- Smith CA, Macdonald A, Holahan MR, 2011. Acute postnatal exposure to di(2-ethylhexyl) phthalate adversely impacts hippocampal development in the male rat. Neuroscience 193, 100–108. 10.1016/j.neuroscience.2011.06.082. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. , 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description. Available: https://www.rand.org/pubs/research_reports/RR1336.html [accessed 31 May 2020]. [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, et al. , 2009. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol. Lett 189, 40–47. 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Tanner EM, Hallerbäck MU, Wikström S, Lindh C, Kiviranta H, Gennings C, et al. , 2020. Early prenatal exposure to suspected endocrine disruptor mixtures is associated with lower IQ at age seven. Environ. Int 134 (105185) 10.1016/j.envint.2019.105185. [DOI] [PubMed] [Google Scholar]
- Téllez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. , 2013. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ 461–462, 386–390. 10.1016/j.scitotenv.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, McMichael AJ, Baghurst PA, 2000. Interactions between environmental lead exposure and sociodemographic factors on cognitive development. Arch. Environ. Health 55, 330–335. 10.1080/00039890009604025. [DOI] [PubMed] [Google Scholar]
- Wenzel AG, Brock JW, Cruze L, Newman RB, Unal ER, Wolf BJ, et al. , 2018. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 193, 394–402. 10.1016/j.chemosphere.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. , 2012. Maternal Prenatal Urinary Phthalate Metabolite Concentrations and Child Mental, Psychomotor, and Behavioral Development at 3 Years of Age. Environ. Health Perspect 120, 290–295. 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, A R, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 119, 878–885; doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Gennings C, Wright RJ, Wilson A, Burris HH, Just AC, et al. , 2018. Prenatal Stress, Methylation in Inflammation-Related Genes, and Adiposity Measures in Early Childhood: the Programming Research in Obesity, Growth Environment and Social Stress Cohort Study. Psychosom. Med 80, 34–41. 10.1097/PSY.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y, 2015. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere 124, 22–31. 10.1016/j.chemosphere.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the national health and nutrition examination survey, 2001–2010. Environ. Health Perspect 122, 235–241. 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.