Abstract
Podocytopathies are kidney diseases in which direct or indirect podocyte injury drives proteinuria or nephrotic syndrome. In children and young adults, genetic variants in >50 podocyte-expressed genes, syndromal non-podocyte-specific genes and phenocopies with other underlying genetic abnormalities cause podocytopathies associated with steroid-resistant nephrotic syndrome or severe proteinuria. A variety of genetic variants likely contribute to disease development. Among genes with non-Mendelian inheritance, variants in APOL1 have the largest effect size. In addition to genetic variants, environmental triggers such as immune-related, infection-related, toxic and haemodynamic factors and obesity are also important causes of podocyte injury and frequently combine to cause various degrees of proteinuria in children and adults. Typical manifestations on kidney biopsy are minimal change lesions and focal segmental glomerulosclerosis lesions. Standard treatment for primary podocytopathies manifesting with focal segmental glomerulosclerosis lesions includes glucocorticoids and other immunosuppressive drugs; individuals not responding with a resolution of proteinuria have a poor renal prognosis. Renin–angiotensin system antagonists help to control proteinuria and slow the progression of fibrosis. Symptomatic management may include the use of diuretics, statins, infection prophylaxis and anticoagulation. This Primer discusses a shift in paradigm from patient stratification based on kidney biopsy findings towards personalized management based on clinical, morphological and genetic data as well as pathophysiological understanding.
The majority of diseases underlying chronic kidney disease (CKD) present with proteinuria, that is, loss of plasma proteins into the urine. Proteinuric kidney diseases can be divided into glomerular or non-glomerular forms, depending on whether protein loss occurs across the glomerular filtration barrier or results from insufficient reabsorption of filtered protein by the proximal tubule1. Glomerular proteinuria is defined by a predominance of albumin whereas, in non-glomerular forms, albumin is only a minor component.
Proteinuria and proteinuria-related symptoms are the only or the main clinical presentation of diseases affecting podocytes, which are ‘octopus-like’ highly specialized cells in the glomerulus that act as part of the filter2-4. Causes of podocyte injury include all forms of immune complex glomerulonephritis that engender distinct histopathological patterns; for example, subepithelial localization of immune complexes in membranous nephropathy causes direct podocyte injury and massive proteinuria. By contrast, podocyte injuries without immune complex deposits produce different histopathological lesion patterns evident on biopsy, of which four types can be distinguished: diffuse mesangial sclerosis (DMS), which presents early in life and is characterized by mesangial matrix expansion and podocyte hypertrophy5; minimal changes (also referred to as minimal change disease), which are predominantly present in children and are so-called owing to a seeming paucity of histopathological abnormalities that can only be visualized by ultrastructural analysis5,6; focal segmental glomerulosclerosis (FSGS) lesions, which involves sclerotic lesions evident in segments of glomeruli4; and collapsing glomerulopathy, which presents as collapse of the glomerular capillaries and hyperplasia of parietal epithelial cells migrating to the tuft to give the appearance of ‘pseudocrescents’4,5. The stratification of patients with these histological lesions has been complemented by clinical criteria, particularly the response to immunosuppressive therapy2,4,6. For example, most patients with minimal changes who respond to steroids have a favourable prognosis6 but, for those resistant to steroids, the information from the kidney biopsy falls short in adequately allowing a personalized prediction of prognosis and the selection of optimal treatments directed to the specific cause of proteinuria.
Increasing knowledge about monogenetic causes of proteinuria or nephrotic syndrome as a molecular diagnosis has revealed that the histomorphological lesions of DMS, minimal changes, FSGS or collapsing glomerulopathy are unspecific lesions and represent different patterns of podocyte injury rather than defining a unique disease cause or diagnosis that would imply a specific therapy. Indeed, all these pathological patterns can be associated with the same genetic disease or the same pathological pattern can be associated with many different genetic diseases or treatment responses2-4. Thus, it has become important to rename this family of diseases as ‘podocytopathies’3,5,7, which accomplishes several objectives. This classification localizes the injury to the podocyte and implies a cellular target for therapy. The classification also helps to overcome the outdated notion that DMS, minimal changes, FSGS or collapsing glomerulopathy are ‘diseases’ or define a diagnosis. Finally, this approach prompts a diagnostic workup to identify the causative trigger or triggers of podocyte injury and to define individualized prognosis and treatment.
In this Primer, we present a conceptual reappraisal of the evolving knowledge concerning the podocytopathies usually referred to as DMS, minimal changes, FSGS and collapsing glomerulopathy in kidney biopsy reports. The literature often refers to these lesions as if they were definite diagnoses, yet they are not. The combination of proteinuria and the presence of any of these lesions on kidney biopsy defines podocyte injury as a unifying underlying mechanism that can result from numerous different causes and risk factors, each of which defines a different diagnosis and, possibly, a specific treatment. As such, the conceptual attempt of this Primer is to move away from the traditional view that considered tissue lesions as diagnoses and uses the term ‘podocytopathies’. This approach requires a diagnostic workup to identify the underlying disease process and/or risk factors that produce the unspecific clinical and histological constellations. Our goal is to facilitate the understanding, clinical assessment and effective management of these disorders. We do not discuss podocyte injury secondary to systemic disorders, such as diabetes, immune complex glomerulonephritis, monoclonal gammopathies, amyloidosis or metabolic storage diseases, in detail as these are defined systemic disease entities that need other disease-specific treatment approaches (BOX 1).
Box 1 ∣. Systemic diseases with podocyte injury.
Diabetic nephropathy
Long-standing, poorly controlled diabetes mellitus can cause diabetic nephropathy with macroproteinuria as a manifestation of podocyte injury. Although hyperglycaemia, impaired insulin receptor signalling, advanced glycation end-product toxicity and glomerular inflammation can directly affect podocyte function, clinical trials have demonstrated that haemodynamic factors promoting single-nephron glomerular hyperfiltration represent the central pathogenetic mechanism of diabetic nephropathy. Dual blockade of the sodium/glucose cotransporter 2 and the renin-angiotensin system and glucose control can control such hyperfiltration.
Immune complex glomerulonephritis
Glomerular immune complex deposits result from adaptive immune responses directed against infectious organisms, circulating autoantigens or autoantigens within the glomerulus. Immune complexes that activate the complement system cause glomerular injury. When immune complexes directed at podocyte antigens (for example, in membranous glomerulonephritis) cause podocyte injury, particularly severe proteinuria, treatment aims to control aberrant adaptive immunity with immunosuppression.
Fibrillary glomerulonephritis
Fibrillary glomerulonephritis involves glomerular deposits of immunoglobulins and fibrils of DNAJB9, a protein of the unfolded protein response pathway. Such deposits cause indirect podocyte injury.
Monoclonal gammopathies and amyloid light-chain amyloidosis
Plasma cell clones producing aberrant immunoglobulins or light chains can affect the kidneys in many different ways. Some of these proteins form amyloid aggregates, which deposit in glomeruli and cause indirect podocyte injury. Others form crystals, fibrils, granules or microtubules that deposit in proximity to or inside podocytes. Treatment aims to suppress the plasma cell clone in the bone marrow responsible for their production.
Amyloid A amyloidosis
Chronic forms of systemic inflammation, for example, in hereditary fever syndromes or long-standing autoimmunity, can cause aggregation and deposition of serum amyloid A in various tissues, including the kidney. Glomerular deposits can cause indirect podocyte injury and nephrotic syndrome.
Metabolic storage diseases and drug-related pigment deposits
Genetic α-galactosidase deficiency in Fabry disease causes intracellular accumulation of globotriaosylceramide. In podocytes, such deposits cause damage associated with proteinuria and glomerulosclerosis. Glycogen storage disease or long-standing exposure to drugs such as hydroxychloroquine can have similar effects.
Epidemiology
Prevalence
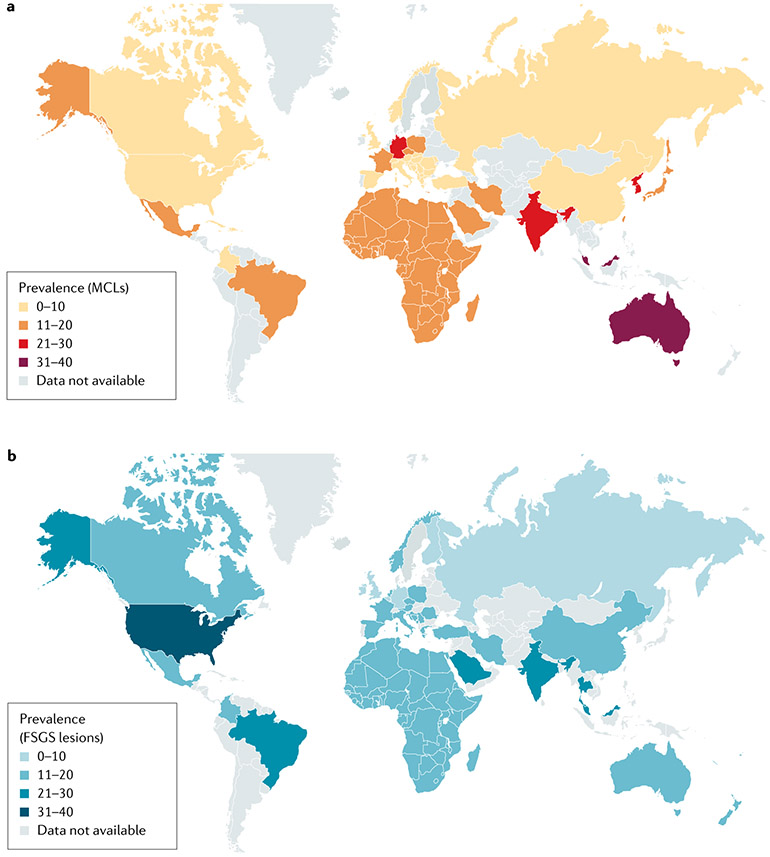
Reliable epidemiological data for podocytopathies are lacking. Pathological diagnosis is based on kidney biopsy and many patients, particularly children and individuals in low-resource settings, do not undergo biopsy. This reality introduces a bias towards steroid-resistant cases and under-reports the incidence of minimal change lesions in children. International biopsy registries report FSGS or minimal change lesions; the two rarer subtypes (DMS and collapsing glomerulopathy) are usually included among FSGS in international registries (FIG. 1). Despite this limitation, the prevalence of podocytopathies, both relative to other glomerular disease entities and in absolute terms, seems to be increasing worldwide and they are major contributors to end-stage kidney disease (ESKD)4. This increased prevalence is partly due to the increased diagnosis given the increased global availability of kidney biopsy and pathological examination4 and partly due to the increased prevalence of risk factors for podocyte injury8. However, the available data may underestimate the prevalence of podocytopathies. Indeed, idiopathic nephrotic syndrome in children (0–18 years of age) has a prevalence of 10–50 cases per 100,000 population globally6 and is most commonly associated with minimal changes, although, in the majority of these cases, the pathological lesion pattern is not established by kidney biopsy6. Idiopathic nephrotic syndrome in children has a male predominance with a ratio of 3:1, for unknown reasons, and is an interesting research question, the answer to which might lead to pathogenetic insights6. Globally and considering all age groups, FSGS is the most common lesion (FIG. 1), representing 10–40% of all the biopsies, except in Asia, where IgA nephropathy is prevalent9.
Fig. 1 ∣. Worldwide prevalence of podocytopathies.
a ∣ Worldwide prevalence of podocytopathy with minimal change lesions (MCLs). b ∣ Worldwide prevalence of podocytopathy with focal segmental glomerulosclerosis (FSGS) lesions. Data are expressed as a percentage of total kidney biopsies and were obtained from international registries8,9,254-262.
Risk factors
Podocytopathies can have a single cause, as frequently in the many monogenetic forms manifesting early in life (see Supplementary Table 1) or in the forms arising from a single environmental risk factor. Alternatively, podocytopathies can have a combination of multiple genetic and/or environmental risk factors causing podocyte injury, acting in concert to reach a threshold effect for the development of proteinuria.
Susceptibility genes.
Genome-wide association studies have identified several susceptibility genes associated with podocytopathies10-14. These genetic variants seemingly cannot cause a podocytopathy per se but represent important risk factors in the presence of a ‘second hit’. The best-studied association is with APOL1 (encoding apolipoprotein L1), which involves protein-changing mutations (G1 and G2 alleles) that have an unusually large effect for common genetic variants. Individuals with sub-Saharan ancestry, and particularly west African ancestry, carry a 3–5-fold higher risk for FSGS lesions and CKD than European populations15, with this disparity being largely explained by these APOL1 genetic variants16,17. The frequency of APOL1 risk alleles (G1 and G2 variants combined) is ~35% among African Americans, 26% in central African populations and ~50% in west African populations18. The enhanced protective effect of these gene variants against African sleeping sickness caused by Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense likely explains their strikingly high allele frequency in these populations16. In areas of Africa with a high frequency of APOL1 risk alleles, CKD prevalence reaches 16%19.
High-risk APOL1 alleles in kidney transplant recipients are linked to shorter allograft survival and lower post-donation estimated glomerular filtration rate (GFR)20. Transgenic animal studies in mice and flies indicate that the expression of either APOL1 risk variant is sufficient to induce FSGS, global glomerulosclerosis and CKD; in these models, disease severity increases with increased APOL1 expression levels21,22. In clinical studies, kidney biopsy manifestations of APOL1-associated kidney disease include FSGS lesions, collapsing glomerulopathy and non-specific focal global glomerulosclerosis (affecting the entire glomerular tuft) with arterionephrosclerosis23, similar to that observed in ageing and so-called hypertensive nephropathy24,25. Indeed, APOL1 podocytopathy is a major cause among African Americans of what was formerly called hypertensive CKD26,27.
Obesity and diabetes.
Conditions of increased single-nephron GFR and hence increased podocyte shear stress confer an increased risk of developing a podocytopathy. The increasing global prevalence of obesity contributes to the increasing prevalence of podocytopathies as well as to serving as a factor that accelerates CKD progression8. Obesity is associated with a substantial increase in body mass causing single-nephron hyperfiltration, which in turn causes podocyte hypertrophy and podocyte shear stress28. Obesity drives foot process effacement (FPE), the earliest morphological pattern of podocyte injury, and subsequent FSGS lesions29-32. The regression of proteinuria after bariatric surgery suggests that the earlier stages of this process are reversible30. Although progression is typically slow, ESKD develops in 10–33% of those with obesity-related CKD30,32. Likewise, diabetes mellitus is associated with glomerular hyperfiltration33. The GFR increase is driven by enhanced proximal tubular glucose and sodium reabsorption, mediated by sodium/glucose cotransporters (SGLTs) that lead to a reduction in afferent arteriolar resistance and an increase in single-nephron GFR through the inhibition of tubuloglomerular feedback34. Increased GFR in single remnant nephrons promotes podocyte stress driving FPE and podocyte detachment, leading to macroalbuminuria that accelerates renal function decline in long-standing and poorly controlled diabetes34. Hence, even recent onset of diabetes can promote proteinuria and podocyte loss, whereas the ‘diabetic nephropathy’ can take several years to develop.
Low nephron mass and nephron loss.
Conditions such as congenital kidney hypoplasia, unilateral agenesis and reflux nephropathy predispose to podocytopathy with proteinuria, hypertension and secondary FSGS lesions at biopsy (so-called secondary, because the podocyte is not primarily affected but is injured by external factors). Surgical studies in rodents and humans suggest that losing >75% of renal mass (nephrons) poses the greatest risk for developing proteinuria, glomerulosclerosis and, in some cases, progressive loss of kidney function35. Living kidney donors or patients who lose 50% of their kidney mass are at increased risk of proteinuria and hypertension but rarely develop progressive CKD, suggesting that, for most healthy individuals, a 50% reduction in renal mass is not sufficient to trigger progressive hyperfiltration injury36. Similarly, low nephron endowment due to low birthweight and pre-term birth are associated with CKD and FSGS lesions at biopsy, suggesting an adaptive podocytopathy37. The same process operates in all forms of CKD as nephron loss increases single-nephron GFR in the remnant nephrons. As ageing is also associated with nephron loss, adaptive FSGS lesions can also occur at older age. Sickle cell disease38, glucose-6-phosphatase deficiency, glycogen storage disease type I, von Gierke disease39, cyanotic heart disease40, familial dysautonomia and extreme muscular hypertrophy (most commonly associated with body building)41 are associated with podocytopathy in conditions in which single-nephron glomerular pressure and filtration rate as well as podocyte shear stress are all increased, ultimately causing nephron loss.
Mechanisms/pathophysiology
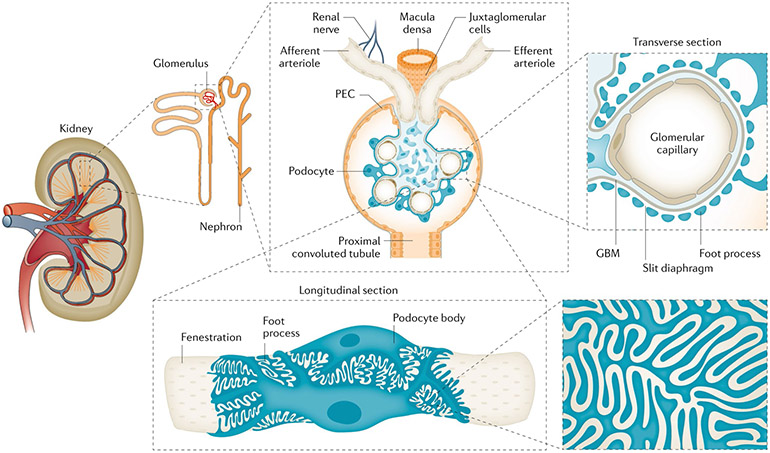
Podocytes are terminally differentiated epithelial cells, the primary and secondary processes of which extend to wrap around the basement membrane of glomerular capillaries in the glomerulus7,25,42 (FIG. 2). Podocytes possess primary, secondary and tertiary foot processes, all of which contain an extensive actin cytoskeleton and interdigitate with the foot processes of adjacent podocytes. The ~200 nm gap between adjacent foot processes is spanned by the tri-laminar slit diaphragm, which serves as an ~60 kDa size-selective filter. The barrier is selective for both molecular size and electric charge, the latter property being conferred by anionic charges that retard the passage of anionic proteins. Even in the healthy glomerulus, podocytes must withstand circumferential stress and shear stress (FIG. 3). Podocyte loss following injury increases mechanical stress on the remaining podocytes.
Fig. 2 ∣. Structure of the nephron, the glomerulus and the filtration barrier.
The kidney is comprised of functional units, nephrons, each of which is made of a glomerulus and a tubule. In healthy humans, the average number of nephrons is ~1 million (range 250,000 to <2.5 million). The glomerulus is composed of a tuft of capillaries covered by visceral epithelial cells — the podocytes — and surrounded by a capsule lined on the inner surface by parietal epithelial cells (PECs). The latter cell population contains podocyte progenitors, which are motile and progressively differentiate into podocytes in the region near the vascular pole of the glomerulus. The vascular pole of the glomerulus includes both the afferent and efferent arterioles (transverse section). The outermost layer is composed of podocytes adhering to the glomerular basement membrane (GBM) and interdigitating (longitudinal section), with the slit diaphragm spanning each gap between pairs of foot processes. The innermost layer is constituted by fenestrated endothelial cells.
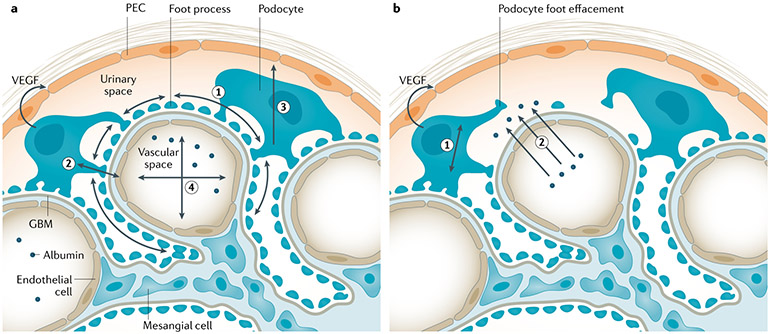
Fig. 3 ∣. Mechanical podocyte stress.
a ∣ Under normal conditions, several kinds of physical stress are present in the glomerulus46,47. The hydrostatic pressure gradient across the glomerular capillary and the Bowman (urinary) space outside the capillary creates circumferential stress on the podocyte foot processes (1). Fluid filtration across the glomerulus generates shear stress on the lateral aspects of the foot processes (2). Filtrate flow laterally across the podocyte cell body in the Bowman space confers shear stress (3). Podocyte-derived vascular endothelial growth factor (VEGF) acts on intravascular endothelial cells and is needed for maintaining the glomerular filtration barrier and keeping serum proteins such as albumin inside the vasculature (4). b ∣ Numerous medical conditions increase the filtration load to the kidneys, which translates into increasing filtration pressure at the level of individual glomeruli. As a trade-off, horizontal podocyte stress (1) increases as the number of podocytes remains constant. Such podocyte stretching ultimately leads to compromise of the filtration barrier, podocyte detachment and loss, and proteinuria. Proteinuria also increases oncotic pressure acting on podocytes (2), as protein in the Bowman space further increases the amount of fluid passing the filtration barrier, which defines single-nephron filtration. This mechanism is common to most forms of progressive chronic kidney disease, as the total effective glomerular filtration surface declines. Hyperfiltration is present early in some forms of chronic glomerular disease, including diabetes mellitus. GBM, glomerular basement membrane; PEC, parietal epithelial cell.
Podocyte effacement, detachment and loss
Foot process simplification and FPE are the earliest morphological patterns of podocyte injury and can be associated with massive proteinuria even without podocyte loss. Incident injuries can induce FPE, which causes the podocytes to resemble immature podocytes of the developing kidney at the ultrastructural level42. The reorganization of the actin cytoskeleton plays a key part in FPE43. A functional imbalance among key regulators of the actin cytoskeleton, such as the Rho family of small GTPases, including RhoA, CDC42 and RAC1, is usually observed and can result in FPE44,45. RAC1 activity promotes the formation of a branched actin network as present in the podocyte lamellipodia43. RhoA activity favours actin polymerization and the formation of actin bundles43. A finely tuned balance between active RAC1 and active RhoA seems to maintain normal foot process morphology and function43.
Although FPE is potentially reversible, podocyte detachment or death implies irreversible podocyte loss46,47. Indeed, live and dead podocytes appear in the urine of patients with glomerular disorders45, which is thought to occur through a substantial increase in the mechanical forces of fluid filtration, leading to glomerular tuft expansion or podocyte fragility. Genetic, metabolic, toxic or inflammatory factors, such as increased expression of NOTCH and WNT/β-catenin by podocytes, promotes podocyte dedifferentiation and protects cells from cell death under injury conditions; however, as cells dedifferentiate into an earlier developmental stage, the concomitant loss of markers such as nephrin and podocin occurs, leading to functional defects48. These defects are also thought to contribute to podocyte detachment and loss49 (FIG. 4).
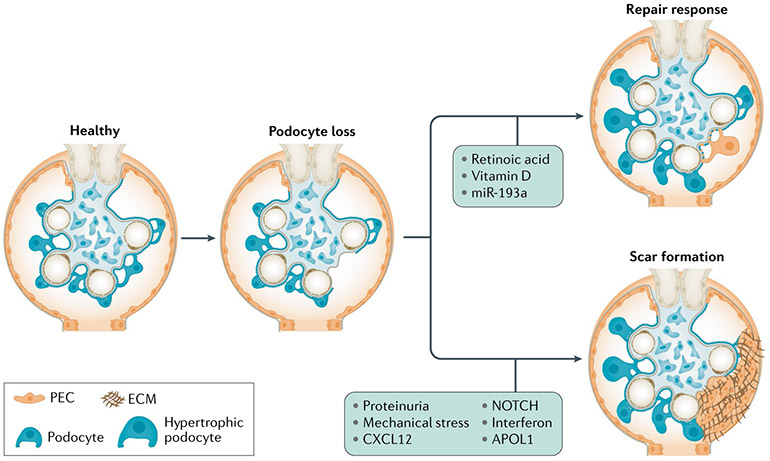
Fig. 4 ∣. Consequences of podocyte loss.
Following injury, podocyte loss can occur that can trigger two responses. First, the remaining podocytes adapt by increasing their size to cover the newly denuded glomerular basement membrane (podocyte hypertrophy). Second, parietal epithelial cells (PECs) along the Bowman capsule, which include a population of resident podocyte progenitors, supply new podocytes after injury and loss. These mechanisms contribute to podocyte functional recovery and reduce proteinuria following injury but can be inefficient or become maladaptive. Indeed, hypertrophic podocytes may be unable to maintain a normal foot process structure, leading to a further increase in local shear stress that triggers further podocyte detachment. In addition, differentiation of PECs into podocytes can be hampered by mechanical stress and proteinuria, leading to inefficient podocyte regeneration and scar formation. ECM, extracellular matrix.
The same mechanical forces that trigger the onset of a podocytopathy also accelerate established glomerular injury46,47. First, the fluid drag of oncotic pressure generated by albumin in the Bowman space increases shear stress on podocytes and hyperfiltration46,47,49 (FIG. 3). Second, nephron loss during CKD progression and normal ageing reduces the total glomerular filtration surface, which increases filtration and the vertical podocyte shear stress at the level of the single nephron46,47. Third, remnant nephrons undergo an increase in size (hypertrophy) to compensate the nephron loss-related decline in filtration surface, which has the potential to lead to maladaptive podocyte hypertrophy. In addition, podocyte loss reduces podocyte density in the glomerular tuft, a process that results in increased horizontal stress forces on the remaining podocytes46,47. The podocyte hypertrophic capacity is limited; hypertrophic podocytes may also be unable to maintain a normal foot process structure, increasing local shear stress50, which triggers further podocyte detachment51.
Podocytes are postmitotic cells and, although they can replicate DNA, they cannot complete cytokinesis25. When podocytes are lost, a subset of parietal epithelial cells along the Bowman capsule, which are resident podocyte progenitors, can supply new podocytes52-56. However, regeneration is frequently inefficient or can drive focal scarring. Indeed, the proliferation, migration and differentiation of parietal epithelial cells towards the podocyte lineage are tightly temporally and spatially regulated. The chemokine CXCL12 is normally constitutively produced by healthy podocytes and serves as a podocyte-to-progenitor feedback mechanism to maintain local podocyte progenitors in a quiescent state and to suppress their intrinsic capacity to generate new podocytes53. After podocyte loss, the reduced expression of CXCL12 promotes NOTCH activation in parietal epithelial cells, which drives their proliferation and migration towards the glomerulus. Podocyte loss also permits the passage of circulating retinol through the damaged glomerular filtration barrier, which is transformed into the Bowman space to retinoic acid; this acts as a powerful inducer of parietal epithelial cell differentiation into podocytes56,57. In addition, activated parietal epithelial cells synthesize retinoic acid to self-promote their differentiation into podocytes56,57. Retinoic acid induces NOTCH gene downregulation, cell cycle arrest and the upregulation of podocyte markers in parietal epithelial cells56,57. However, with high-grade proteinuria, retinoic acid is sequestered by albumin in the Bowman space58 and parietal epithelial cell differentiation into podocytes is impaired. The absence of APOL1 also promotes parietal epithelial cell quiescence; the microRNA miR-193a suppresses APOL1 translation59. Under conditions of mechanical stress60, impaired APOL1-miR-193a axis (for example, in those with the G1 or G2 genotype)59 or persistent NOTCH expression61, activated parietal epithelial cells cannot properly differentiate into podocytes and contribute to the formation of hyperplastic lesions and fibrous lesions, including FSGS58,62, by synthesizing and releasing extracellular matrix63 (FIG. 4). Interestingly, superficial and mid-cortical nephrons retain a potent capacity for podocyte regeneration, which may explain why juxtamedullary nephrons are particularly susceptible to glomerulosclerosis53.
Podocyte injury
In addition to associations with low nephron number and increased body mass, podocyte injury can result from genetic, immunological, infectious (for example, hepatitis C virus (HCV) infection) and toxic (for example, from various drugs and metals) causes. The prevalence of these syndromes differs across the lifespan and different contributing factors (and with different relative contributions) can occur in combination to reach a threshold of podocyte injury and loss.
Genetic causes.
Next-generation sequencing techniques have greatly facilitated the identification of ≥50 causal genes in hereditary podocytopathies (Supplementary Table 1). Moreover, DNA sequencing has revealed mutations in unexpected genes64,65 and has widened the extrarenal phenotypes associated with podocyte gene mutations. For example, these approaches have identified that genes expressed in podocytes as well as genes expressed in other tissues in the context of syndromic disorders are affected. Additionally, many small effect variants, mostly non-coding, conferring susceptibility for podocytopathies have been described in adults.
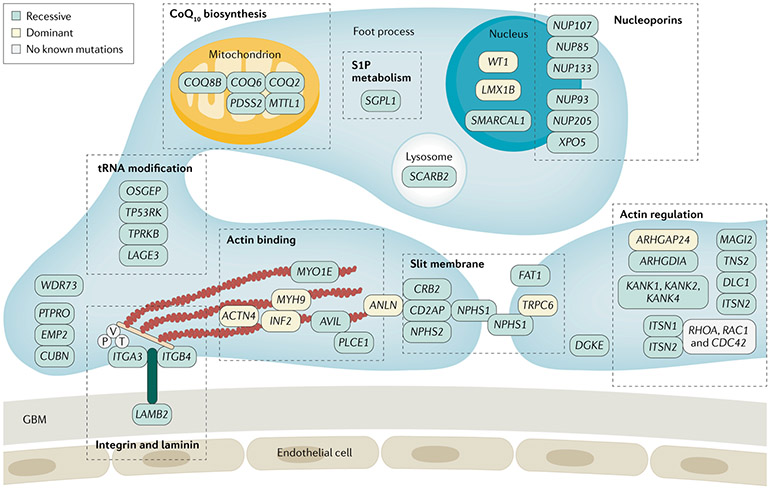
More than 50 genes mutated in hereditary podocytopathies have been identified to date, encompassing genes expressed in podocytes (FIG. 5). The discovery of these genes as monogenetic causes of steroid-resistant nephrotic syndrome (SRNS) has shown that particular proteins are critical for glomerular function. For example, the identification of mutations in NPHS1 (encoding nephrin) and NPHS2 (encoding podocin) demonstrated the central role of the slit diaphragm in glomerular function. Identification of ACTN4 (encoding α-actin 4) and ANLN (encoding anillin) mutations emphasized the importance of the podocyte actin cytoskeleton in kidney physiology and pathophysiology64,66-69. A rare subset of patients carrying mutations in genes encoding Rho-like small GTPases are, at least partially, sensitive to immunosuppression, suggesting that glucocorticoids may also directly affect podocyte function70. Rare cases of steroid-sensitive nephrotic syndrome (SSNS) with apparent Mendelian transmission also exist but the gene or genes involved are unknown71. The discovery of recessive mutations in genes that participate in coenzyme Q10 biosynthesis (namely COQ2, COQ6 and ADCK4) illustrates the opportunity for a ‘personalized medicine approach’ to specific podocytopathies as these patients may respond to oral coenzyme Q10 supplementation72,73. Thus, the availability of effective therapies for several genetic podocyte diseases supports the notion that genetic discovery can enable personalized medicine.
Fig. 5 ∣. Monogenetic diseases and SRNS.
Identification of single-gene causes of steroid-resistant nephrotic syndrome (SRNS) placed the podocyte at the centre of SRNS pathogenesis because most of the implicated genes are expressed in podocytes. Foot processes interdigitate with those from neighbouring podocytes, forming the glomerular slit membrane, which is critical for filtering and retention of protein in the bloodstream. Its integrity is lost in nephrotic syndrome. Proteins encoded by genes that, if mutated, cause monogenic SRNS localize to specific subcellular sites of podocytes depicted here. CoQ10, coenzyme Q10; GBM, glomerular basement membrane; P, paxillin; T, talin; V, vilin. Adapted with permission from REF.263, Oxford University Press.
In the context of a syndromic disorder involving multiple organs, pathogenetic variants in genes expressed in other tissues can also cause podocytopathies74 (Supplementary Table 1). For example, Alport syndrome, Dent disease and Fabry disease can all present as a podocytopathies, sometimes with minimal or absent extrarenal manifestations, and may manifest isolated FSGS lesions as a pattern of injury64. Often, there is an extra-renal phenotype in an organ that expresses the affected gene, for instance, sensorineural deafness in Alport syndrome, due to mutations in genes encoding collagen75. However, SRNS or isolated proteinuria, even in the nephrotic range, can sometimes be the only evident clinical sign of these disorders, at least at the time of presentation65.
Immunological and soluble factors.
The effectiveness of glucocorticoids and other immunosuppressive drugs in many podocytopathies for which a genetic cause can not be determined suggests a central role of the immune system in the pathogenesis of these disorders; the direct effects of these agents on cultured podocytes have also been demonstrated76 and, therefore, a local, non-immune effect is also possible. Several genome-wide association studies have revealed that HLA-DR and HLA-DQ are the strongest susceptibility loci for childhood SSNS in various populations11-14. These features, along with the absence of immune complex deposition and the spontaneous remission of proteinuria after measles infection, originally led to the consideration of these forms as T cell-mediated diseases77. Early studies proposed a 60–160 kDa glomerular permeability factor released from T cells isolated from a patient with nephrotic syndrome and minimal change lesions6. In a rat model, this factor induced proteinuria and tertiary FPE of podocytes but the causative protein has not been conclusively identified6.
Subsequently, alterations in circulating T cell subsets and in cytokine profiles have been described in patients with podocytopathies, suggesting a shift towards a T helper 2 cytokine profile with a possible role for IL-4 or IL-13 as the circulating permeability factors78-80. Indeed, peripheral CD4+ and CD8+ T cells in children with SSNS express IL-13, a cytokine that can, upon experimental overexpression, induce a podocytopathy in rats81. The number of regulatory T cells (those that suppress immune responses) is also reduced in patients with podocytopathies compared with healthy controls and tends to normalize when remission of proteinuria is achieved79,82,83. Consistently, renal disorders including podocytopathy with a minimal changes lesion pattern and SSNS are reported in 20% of individuals with immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, a rare congenital immunodeficiency with severe regulatory T cell defects84.
Evidence of persistent remission of proteinuria after treatment with rituximab or ofatumumab85,86, both of which deplete B cells, sparked renewed interest in the role of B cells in these disorders, However, these agents may have effects independent of their effects on B cells. Rituximab has been reported to stabilize the podocyte actin cytoskeleton through its binding to a cross-reactive epitope of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b)87. Ofatumumab was also effective in rituximab-resistant nephrotic syndrome88; whether it also binds to SMPDL3b remains unknown88. B cell-derived IL-4 promotes proteinuria in mice89; however, human studies have not been reported and so its relevance to proteinuria in humans remains to be established. Selective extracorporeal immunoadsorption induces the remission of nephrotic syndrome relapses, suggesting that the factor responsible for proteinuria could be an immunoglobulin or could bind to immunoglobulins90. B cells can also act as antigen-presenting cells and their depletion may also modify T cell function in patients with steroid-dependent nephrotic syndrome (SDNS)91. In a subset of patients, hyposialylated IgM on the surface of T cells predisposes patients to steroid dependence92; rituximab can reverse this phenomenon and improve outcomes. Furthermore, B cells may contribute to podocyte injury by other mechanisms; activated antigen-specific B cells can induce FPE and proteinuria through local release of IL-4 in vivo89.
Other observations suggest a role for circulating permeability factors in patients with primary podocytopathies. First, some patients experience a relapse of nephrotic syndrome immediately after kidney transplantation but enter remission following plasma exchange93. Second, kidneys with minimal changes from deceased donors can undergo remission of proteinuria after transplantation into a non-proteinuric recipient94. At least three candidates for circulating permeability factors have been put forwards: cardiotrophin-like cytokine 1, soluble urokinase plasminogen activator receptor (suPAR) and anti-CD40 IgG95. Serum suPAR levels are reportedly higher in patients with FSGS than in those with other forms of proteinuric kidney disease such as membranous nephropathy96,97, although numerous subsequent studies have attributed this finding to differences in GFR98. One study reported angiopoietin-related protein 4 (ANGPTL4), a glucocorticoid-sensitive secreted glycoprotein, as a circulating permeability factor in minimal change lesions99 but this was not confirmed by other studies100. Overall, none of the proposed permeability factors elicits consistent proteinuric effects in vitro or in vivo and further testing of these interesting hypotheses is needed, including in large cohorts that encompass patients with FSGS and glomerular and non-glomerular disease controls98.
Infectious agents.
Viral infections are common causes of podocytopathies, especially with a lesion pattern of collapsing glomerulopathy101. HIV infection can cause a characteristic podocytopathy with nephrotic syndrome and rapid disease progression. Histologically, HIV-associated nephropathy presents as the combination of collapsing glomerulopathy and marked tubular-interstitial disease, including microcystic tubular dilatation102. Other patients who are HIV-positive develop FSGS lesions without collapsing features102. Both forms of glomerular disease are associated with APOL1 high-risk status, with 72% of African Americans with these disorders carrying two APOL1 risk alleles102. Additionally, HIV infects podocytes in vivo and elicits cytotoxicity102. The associated type 1 interferon-mediated antiviral immune response promotes APOL1 gene transcription, inducing inflammatory cell death pathways21; in addition, it further promotes podocyte death by mitotic catastrophe103. The estimated global prevalence of HIV-related CKD is of 6–12% of people who are HIV-positive104. HIV-related CKD is particularly prevalent in sub-Saharan Africa, where the high prevalence of both uncontrolled HIV1 infection and APOL1 risk alleles predispose to CKD and to rapid progression to ESKD105.
Chronic HCV infection also causes several immune-mediated glomerular disorders, including podocytopathy with FSGS lesions106,107. Although the pathogenic mechanisms are unclear, it is hypothesized that, similar to HIV, HCV directly injures podocytes, leading to glomerulosclerosis107. Eliminating HCV using antiviral medications can clear the virus from >95% of individuals with chronic HCV infection and improve the outcome by reducing proteinuria and preventing or delaying CKD106.
Podocytopathy with FSGS and collapsing glomerulopathy may also occur as a consequence of the common childhood infection with parvovirus B19 (REFS108,109), with DNA evidence of parvovirus B19 in kidney tissue109. However, only a few individuals who are infected develop FSGS lesions and the predisposing factors to glomerular injury are unknown. Clearance of the virus can be associated with an improvement of proteinuria108. Epstein-Barr virus, cytomegalovirus infection and SARS-CoV-2 (the virus that causes COVID-19)110 also cause podocytopathy101. SARS-CoV-2 can directly infect podocytes or indirectly harm them by promoting cytokine secretion, causing proteinuria with the pathological feature of a collapsing glomerulopathy110. Other pathogens that can trigger podocytopathies include Borrelia spp.111, the plasmodium Schistosoma mansoni and filarial nematodes4.
Pregnancy-related VEGF inhibition.
Renal physiological changes characteristic of pregnancy include increased renal blood flow and increased glomerular filtration. Factors that oppose the actions of podocyte trophic factors may cause podocyte injury and detachment. For example, podocyte-expressed vascular endothelial growth factor (VEGF)112 acts in paracrine and autocrine ways to protect podocytes113. Pre-eclampsia is a disorder of pregnancy characterized by the onset of hypertension and often manifests with high proteinuria. Increased plasma levels of soluble FMS-like tyrosine kinase 1 (sFLT1), produced by the hypoxic placenta, antagonize VEGF action and cause podocyte loss and proteinuria113. In these patients, a podocytopathy can result from the combination of VEGF antagonism and pregnancy-related glomerular hyperfiltration, particularly when associated with excessive weight gain, diabetes or a multiple pregnancy, all of which increase glomerular hyperfiltration113. In other contexts, treatment with anti-VEGF or anti-VEGF receptor agents, which are commonly used to prevent vascular proliferation in tumours and retinal diseases, can cause proteinuria and hypertension114 and are associated with renal thrombotic microangiopathy and with podocytopathies, chiefly minimal change lesions and collapsing glomerulopathy115.
Drugs.
Certain drugs can cause podocytopathies via direct toxic effects4. Interferon therapy can be associated with the development of collapsing glomerulopathy; indeed, IFNβ promotes podocyte death and IFNα inhibits the migration of podocyte progenitors. In addition, interferon inhibits the differentiation of podocyte progenitors into podocytes6,116. Finally, interferon is a potent stimulus to APOL1 gene expression, driving podocyte damage in individuals with two APOL1 risk alleles117.
Bisphosphonate therapy, which is used to treat the loss of bone density, is rarely associated with a toxic podocytopathy and presents histologically as minimal change lesions with FPE, FSGS lesions or collapsing glomerulopathy116; the molecular mechanisms remain obscure. Lithium therapy to treat conditions such as bipolar disorders rarely causes proteinuria and minimal changes or FSGS lesions, which can resolve within weeks after stopping the drug118; however, the mechanism is controversial119,120. Sirolimus, doxorubicin and daunomycin can each cause a podocytopathy with FSGS lesions by directly inducing podocyte death121-123. Doxorubicin, used as a cancer chemotherapy in humans, is a podocyte toxin that is widely used to induce podocyte injury lesions in mice; it promotes mitotic catastrophe in podocytes, with consequent detachment, followed by FSGS lesions124. As already mentioned, VEGF antagonists cause a usually transient podocytopathy114.
Clinical manifestations
Substantial proteinuria, with at least 50% of the protein being albumin, is the defining feature of the podocytopathies. The magnitude of urinary protein excretion defines a spectrum of conditions with increasing degrees of severity: sub-nephrotic proteinuria, nephrotic-range proteinuria and nephrotic syndrome (TABLE 1). In addition, podocyte injury and loss can contribute to a range of other clinical manifestations, including oedema and hyperlipidaemia, and increase the risk of infection.
Table 1 ∣.
Clinical definitions
| Condition | Adults | Children |
|---|---|---|
| Proteinuria | Proteinuria of 300–3,400 mg per day or urinary protein to creatinine ratio <300 mg/g (or<300 mg/mmol) | Urinary protein to creatinine ratio >0.2 or proteinuria >100 mg/m2 per day or ≥4 mg/m2 per hour or positive urine dipstick149 |
| Nephrotic range proteinuria | Proteinuria of ≥3.5 g per day or urinary protein to creatinine ratio ≥3,000 mg/g (or ≥300 mg/mmol) with normal serum albumin | Urinary protein to creatinine ratio ≥200 mg/mmol (2 mg/mg) in first morning void or 24-hour urine sample ≥1,000 mg/m2 per day corresponding to 3+ or 4+ by urine dipstick or ≥40 mg/m2 per hour |
| Nephrotic syndrome | Proteinuria of ≥3.5 g per day or urinary protein to creatinine ratio of ≥3,000 mg/g (or ≥300 mg/mmol) with oedema, serum albumin of <3.0 g/dl and hypercholesterolaemia | Urinary protein to creatinine ratio ≥200 mg/mmol (2 mg/mg) in first morning void or 24-hour urine sample ≥1,000 mg/m2 per day corresponding to 3+ or 4+ by urine dipstick and either hypoalbuminaemia (serum albumin of <30 g/l) or oedema when serum albumin level is not available |
| Partial remission | Proteinuria of 0.3–3.5 g per day or urinary protein to creatinine ratio of 300–3,500 mg/g (or 30–350 mg/mmol) with >50% decrease from baseline and stable renal function | Urinary protein to creatinine ratio >20 mg/mmol but <200 mg/mmol and, if available, serum albumin ≥30 g/l |
| Complete remission | Proteinuria of <0.3 g per day or urinary protein to creatinine ratio of <300 mg/g (or <30 mg/mmol), stable renal function and normal serum albumin | Urinary protein to creatinine ratio ≤20 mg/mmol (0.2 mg/mg) or negative or trace dipstick on 3 or more consecutive occasions |
| Relapse | Proteinuria of >3.5 g per day or urinary protein to creatinine ratio of >3,500 mg/g (or >350 mg/mmol) after achievement of remission | Urinary protein to creatinine ratio of ≥200 mg/mmol (2 mg/mg) on a first morning urine sample or ≥3+ protein on urine dipstick for 3 consecutive days |
| Frequently relapsing nephrotic syndrome | Two or more relapses within 6 months (or >4 relapses within 12 months) | Two or more relapses within 6 months (or >4 relapses within 12 months) |
| Steroid-dependent nephrotic syndrome | Two or more relapses during or within 14 days of completing steroid therapy | Two consecutive relapses during corticosteroid therapy or within 14 days of ceasing therapy |
| Steroid-resistant nephrotic syndrome | Failure to achieve remission after 16 weeks of corticosteroid therapy | Failure to achieve remission after 4–6 weeks of corticosteroid therapya |
Hypoalbuminaemia.
When the amount of albumin lost in the urine exceeds the capacity of the liver to replace the losses, the level of plasma albumin declines. The prevalence of hypoalbuminaemia varies even among patients with the same genetic disorder65 for reasons that are unknown. One factor may be a variability in the capacity of the proximal tubule to catabolize albumin, returning amino acids to the circulation125. Why the liver, which normally produces ~15 g per day of protein in an adult, is unable to compensate for protein loss of 4–6 g per day in some patients but not in others is also unclear. One hypothesis is that TNF and IL-1 expression may suppress hepatic albumin synthesis and contribute to hypoalbuminaemia, at least in podocytopathies associated with inflammatory conditions126.
Oedema.
Two main factors contribute to oedema development. First, sodium reabsorption in the renal tubules is influenced by proteinuria and proteasuria (overfill scenario)127,128; these patients are more likely to show arterial hypertension as a sign of hypervolaemia128. In adults, the onset of proteinuria is typically gradual and causes a parallel drop of oncotic pressure in plasma and in the renal interstitium, such that substantial extra-cellular volume shifts and acute kidney injury (AKI) do not typically occur. In adults, oedema develops from a positive sodium balance via increased sodium retention in the kidney. In children, acute onset of massive proteinuria typically develops together with severe hypoalbuminaemia (often <1 g/dl) without an equivalent drop of albumin in interstitial tissues throughout the body; this leads to a fluid shift from plasma to the relatively hyperoncotic interstitium129.
An extracellular volume shift is the second factor contributing to oedema and is associated with a hypovolaemic state (underfill scenario). In some cases, hypovolaemia may present as shock, with hypotension, tachycardia, peripheral vasoconstriction, oliguria (including AKI), and compensatory elevations of plasma renin and aldosterone130. Intravascular hypovolaemia, despite total body sodium excess, may be sustained by diuretic therapy, sepsis or diarrhoea131. Interstitial oedema, ischaemic tubular injury, and the use of NSAIDs, diuretics and a renin–angiotensin system inhibitor (RASi) may contribute to AKI in such patients127.
Thromboembolism.
Patients with nephrotic syndrome, even if asymptomatic, have a hypercoagulable state132 related to a multitude of factors. These factors include urinary losses of endogenous anticoagulants, such as antithrombin III, plasminogen, protein C and protein S, as well as increased platelet activation, hyperfibrin-ogenaemia, inhibition of plasminogen activation by type 1 plasminogen activator inhibitor (PAI1) and the presence of high-molecular-weight fibrinogen moieties in the circulation133. The exit of saline from the glomerular capillaries into the urinary space, due to reduced plasma oncotic pressure, promotes haemoconcentration in the post-glomerular circulation, which is worsened by diuretic therapy. All of these factors likely contribute to the tendency to form thrombi, particularly in the renal vein134.
Hyperlipidaemia.
Marked elevations in the plasma levels of cholesterol, LDL, triglycerides and lipoprotein A often occur in nephrotic syndrome135. Decreased plasma oncotic pressure probably stimulates hepatic lipoprotein synthesis, resulting in hypercholesterolaemia135. HDL cholesterol levels are usually normal or reduced in nephrotic syndrome and there is often a pronounced decline in the cardioprotective HDL2 fraction135. Nephrotic syndrome may manifest elevated levels of apolipoproteins B, C-II and E, which are associated with VLDL and LDL particles; on the other hand, the levels of the major apolipoproteins associated with HDL (apolipoproteins A-I and A-II) are usually normal136. ANGPTL4 contributes to a feedback loop driven by hypoalbuminaemia and free fatty acid concentrations that promotes hypertriglyceridaemia137. Altogether, these changes in lipid and lipoprotein profiles increase the risk of thromboembolism, premature atherosclerosis and progressive kidney disease135.
Anaemia.
Patients with persistent nephrotic syndrome may develop anaemia due to urinary losses of iron, transferrin, erythropoietin, transcobalamin and other metals such as copper138.
Endocrine disturbances.
Due to thyroglobulin loss in the urine, thyroid hormone (total T4 and T3) levels may be low, with normal serum free T4 and T3 and thyrotropin (thyroid-stimulating hormone) concentrations, and, as a result, patients are usually clinically euthyroid. Serum 25-hydroxyvitamin D and total calcitriol concentrations may also be reduced, but the functionally relevant free calcitriol concentrations are typically normal139. Hypocalcaemia is common owing to hypoalbuminaemia; this does not affect the physiologically important ionized calcium concentration. For these reasons, vitamin D replacement therapy is not routinely recommended to these patients.
Infections.
Patients with nephrotic syndrome, particularly children, are at increased risk of developing serious bacterial infections, including pneumonia, empyema and peritonitis140. Sepsis, meningitis and cellulitis are other serious infections that can occur in children with nephrotic syndrome141. Bacteriuria is common140,141. The increased risk for infection is related to renal losses of IgG leading to hypogammaglobulinaemia, reduced levels of the alternative complement factors B and D lost in urine, and the use of immunosuppressive therapy, all of which promote a state of acquired immunodeficiency. Loss of opsonizing factors may specifically increase the susceptibility to encapsulated bacterial infection, in particular to pneumococcal infections that are potentially lethal142,143.
Diagnosis screening and prevention
The approach to patients with substantial proteinuria or nephrotic syndrome can be different depending on the resources available, which may limit the ability to make a tissue diagnosis as well as management (BOX 2). Importantly, different risk factors and/or causes of podocytopathies can present at certain phases of life or be preferentially associated with a certain sex or ethnicity. Risk factors frequently combine in the same patient and the individual constellation determines the onset of proteinuria and its severity (FIG. 6). Recognizing the likelihood of these causes or risk factors throughout the lifespan can help to improve diagnostic accuracy.
Box 2 ∣. Global variation in diagnosis and management.
Clinical evaluation
Although a urinary dipstick result may be sufficient to diagnose nephrotic syndrome in a patient with massive oedema, the diagnosis may be easily missed in patients with mild or absent oedema that does not prompt urine analysis. Lack of access to HIV testing misses the opportunity to diagnose HIV infection and to institute anti-retroviral therapy. Failure to identify low nephron mass or single-nephron hyperfiltration as a cause for proteinuria and podocyte injury, as it frequently happens when patients are categorized by histopathological lesions only, may expose patients to unnecessary and ineffective immunosuppressive therapies.
Pathology
For children with idiopathic nephrotic syndrome, standard steroid or ciclosporin therapy will be available in most settings. However, in adults, the differential diagnosis is based on kidney biopsy findings; many parts of the world do not routinely provide access to kidney biopsy owing to cost and a lack of well-trained renal pathologists. Sometimes, biopsy samples are shipped from low-income countries to partner institutions abroad but turnaround time remains a problem. If the infrastructure for tissue processing and slide scanning are available, online slide review can be an option to obtain expert pathology reading with an acceptable turnaround time. A lack of access to immunoglobulin staining hinders the diagnosis of IgA nephropathy, membranous nephropathy or systemic immunological diseases that need different management to podocytopathies. A lack of access to electron microscopy compromises the diagnosis of certain glomerular disorders, including membranous nephropathy and Alport syndrome.
Genetic evaluation
A lack of access to genetic testing likely misses the diagnosis of >50 high-penetrance genetic causes of FSGS and may expose patients to unnecessary or ineffective immunosuppressive therapies. Family counselling is, therefore, not available and genetic disorders with systemic manifestations may be missed. Indeed, genetic testing as a key technology to personalized care for patients with podocytopathies is too dynamic to maintain equivalent availability and quality worldwide. Currently, few highly specialized centres regularly define new standards in terms of sequencing hardware and data analysis, which implies that many centres operate with different diagnostic algorithms. Globally, these modern diagnostic tools are not accessible to the majority of patients in low-income and middle-income countries for reasons of local availability and/or costs. Given the contributions of genetic factors to prognosis in certain regions of the world and the risk of progression to end-stage kidney disease, making certain diagnostics available either through raising awareness among local policymakers, through philanthropic initiatives or via research networks is warranted.
Treatment and renal replacement therapies
Cost may limit access to calcineurin inhibitors, mycophenolate mofetil and rituximab as therapies for primary FSGS. Not all patients will have access to dialysis or renal transplantation. A lack of access to renal replacement therapy or kidney transplant will result in premature death.
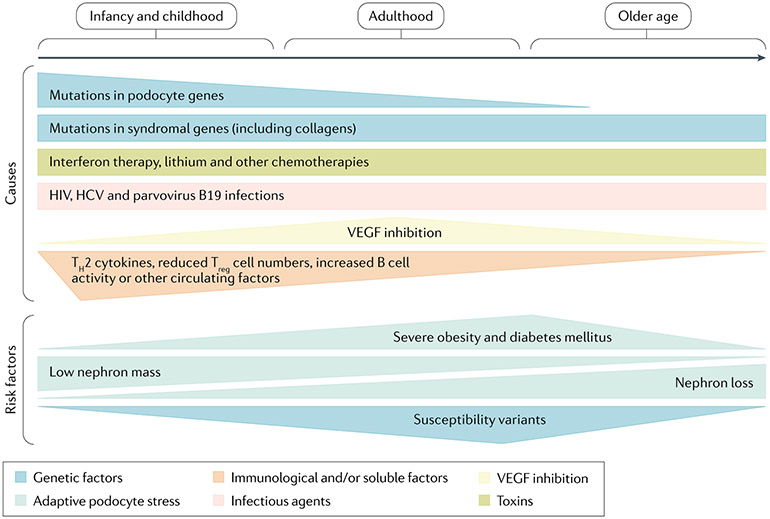
Fig. 6 ∣. Causes and risk factors underlying podocytopathies across the lifespan.
Patient age and sex are associated with an increased probability of different types of podocytopathies related to different causes or risk factors that can frequently even combine in the same patient. For example, genetic causes are more frequent in children and young adults, whereas immunological causes are more frequent in male children. On the other hand, podocytopathies related to inhibition of vascular endothelial growth factor (VEGF) are observed during pre-eclampsia and are, therefore, more prevalent in pregnant women. Major risk factors for the development of a podocytopathy, such as increased single-nephron glomerular filtration rate for obesity or diabetes, are more frequently observed in adult middle-age patients, whereas a low nephron mass endowment can cause a podocytopathy during adolescence or early adulthood. Finally, a susceptibility gene, such as APOL1, is prevalent in Black adult patients. HCV, hepatitis C virus; TH, T helper; Treg cell, regulatory T cell.
Clinical features and initial assessment
In children, podocytopathies frequently present with periorbital and/or peripheral oedema because of hypoalbuminaemia and saline excess. Less commonly, proteinuria is discovered on a routine urinalysis. It must be considered that a positive dipstick for protein on a random urinalysis (defined as ≥1 on dipstick testing) will be positive in 5–10% of normal school-age children and adolescents. However, only 0.1% of such children will have persistent proteinuria144, defined as repeated detection in at least two exams over a period of >3 months, and only these children should be considered as possibly having a podocytopathy. Patients with deafness or with syndromic features should undergo phenotyping for other manifestations of a specific syndrome and, when proteinuria is noted, should be referred to a paediatric nephrologist. A careful patient and family history for kidney diseases or extrarenal manifestations, particularly visual or hearing problems, is important and should prompt evaluation for a syndromic podocytopathy64,65,67,145.
By contrast, in adults, both sub-nephrotic and nephrotic-range proteinuria (TABLE 1) may be incidental findings on a routine urinalysis as adults develop overt nephrotic syndrome less frequently. In these patients, it is important for the initial clinical assessment to investigate possible causes of podocytopathies such as exposure to drugs and toxins, syndromic features, elevated body mass index, signs and symptoms of viral and bacterial infections, autoimmune disorders, and malignancy. Kidney ultrasonography may identify a reduced renal size, renal masses or malformations, or cystic disease, which more typically present with non-nephrotic proteinuria29,50 and are more prevalent in adults than in children. On the other hand, the onset of nephrotic syndrome with marked oedema, and sometimes anasarca, venous thrombosis or infections, should prompt investigation for a podocytopathy of immunological or genetic origin146,147. A positive sodium balance and hypertension are usually present in patients with podocytopathies146.
Kidney biopsy.
In children with persistent non-nephrotic proteinuria, the decision about whether and when to proceed to kidney biopsy is controversial148. Many experts recommend close monitoring of blood pressure, protein excretion and GFR in children with a urinary protein excretion of <500 mg/m2 per day; if any of these parameters shows evidence of progressive disease, a kidney biopsy may be warranted149. Retrospective analyses suggest that children with sub-nephrotic proteinuria and a urinary protein to creatinine ratio of ≥0.5 g/g can have a podocytopathy, typically expressed as FSGS lesions150 (FIG. 7). By contrast, in patients with a urinary protein to creatinine ratio of <0.5 g/g, the risk of FSGS lesions at biopsy is low150,151. DMS shows a histological pattern of small, sclerosed glomeruli that have a reduced number of capillary loops with often prominent podocytes lining the tuft (FIG. 7) and is sometimes diagnosed in small children with genetic podocytopathies5. Biopsy is initially not performed in children with isolated nephrotic syndrome lacking other features because 75–80% of podocytopathies with minimal changes respond to standard steroid therapy with complete remission6,152. In addition, the response to initial steroid therapy is a better predictor of long-term prognosis than the results of kidney renal biopsy6,152. In those 10–20% of children with idiopathic nephrotic syndrome who do not respond to steroids, a kidney biopsy shows minimal change lesions (FIG. 7) in 25% and FSGS lesions in most others6,152.
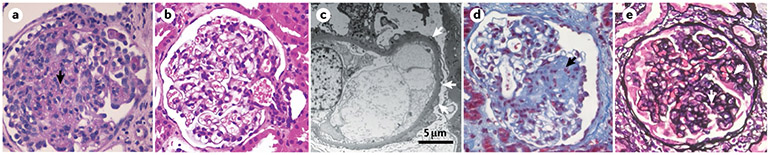
Fig. 7 ∣. Pathology of podocytopathies.
a ∣ The lesion pattern of diffuse mesangial sclerosis occurs only in young children and is usually associated with severe nephrotic syndrome. The glomerulus shows diffuse mesangial sclerosis (arrow) with prominent mesangial consolidation, closure of the capillary loops and overlying prominent immature podocytes. Periodic acid–Schiff staining; magnification ×400. b ∣ The lesion pattern of minimal changes on light microscopy shows a normal glomerulus. Haematoxylin and eosin staining; magnification ×200. c ∣ On electron microscopy, minimal change disease foot process effacement (arrows) is visible. Minimal change lesions are usually associated with steroid-sensitive nephrotic syndrome but can also be associated with isolated proteinuria or, rarely, with steroid-resistant nephrotic syndrome, particularly in the early phases of the disease. d ∣ The lesion pattern of focal segmental glomerulosclerosis (FSGS) is associated with diverse clinical presentations but most frequently with isolated proteinuria or steroid-resistant nephrotic syndrome. FSGS lesions (arrow) have been distinguished into five subtypes but the lack of accuracy to predict a specific cause of disease or outcome limits their clinical relevance264. In addition, particularly in maladaptive FSGS, lesions typically start in juxtamedullary nephrons, which are sensitive to haemodynamic injury and harbour fewer podocyte progenitors, which explains why FSGS starts and is more frequently observed in juxtamedullary glomeruli53. Perihilar and cellular variants share an intermediate prognosis and the former is associated with maladaptive (secondary) causes265. Coarse segmental staining for IgM and C3 can occur with minimal or FSGS lesions. At electron microscopy, limited effacement with narrow foot processes is frequent in maladaptive podocytopathy with secondary FSGS lesions197, whereas diffuse foot process effacement is typical of primary podocytopathies and virus-related or drug-related podocytopathy266-268. Masson’s trichrome staining; magnification ×200. e ∣ The lesion pattern of collapsing glomerulopathy is less common and usually associated with severe steroid-resistant nephrotic syndrome. Traditionally, collapsing glomerulopathy has been associated with people of African descent and a fast rate of progression to end-stage kidney disease, whereas tip lesions (which are the result of proliferation of parietal epithelial cells at the urinary pole) are associated with white ethnicity, a low histological score at presentation and a better response to therapy. The collapse of the tuft is evident by the circular black lines (capillaries) and loss of the urinary space (arrows). Jones methenamine silver staining; magnification ×200.
As the differential diagnosis of proteinuric nephropathies in adults is broad, kidney biopsy is generally performed in all adults presenting with nephrotic-range proteinuria to guide management and provide a prognosis. Common biopsy findings suggesting a podocytopathy include minimal change lesions, FSGS lesions and collapsing glomerulopathy (FIG. 7). Podocytopathies can be distinguished from other glomerular diseases based on the light microscopy appearance and immunoglobulin staining; electron microscopy provides additional information about the extent of FPE and abnormalities of the glomerular basement membrane. A detailed differential diagnosis of the type of podocytopathy may require a family history, serological testing and imaging exams as well as, in some cases, genetic analyses.
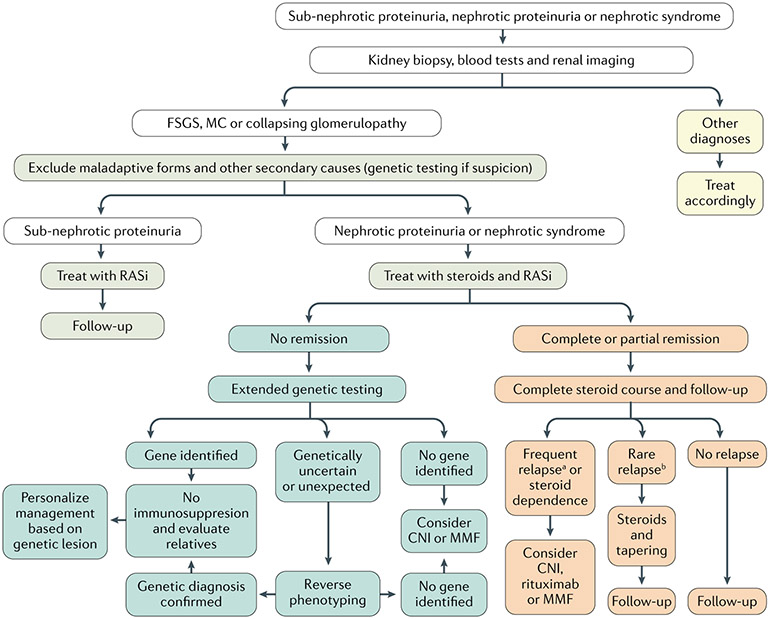
Genetic testing.
Genetic testing is highly advised in all children or young adults (<30 years of age) who do not respond to a course of glucocorticoids (FIG. 8) because, in these individuals, the possibility of making a molecular diagnosis of a genetic disorder can reach 30–60%65,67,68. The proportion of patients newly diagnosed with genetic podocytopathies declines with age at onset. Nevertheless, pathogenetic mutations occur in 14–21% of adults with steroid-resistant glomerular disorders and, therefore, genetic testing is warranted in adults with treatment-resistant proteinuria68,153,154 (FIG. 9). A family history of kidney disease and syndromic features should also trigger genetic testing155. Next-generation sequencing with computational filtering for a panel of all known genes causing podocytopathies plus collagen genes has a diagnostic rate of ~30%64,65,67,68,145.
Fig. 8 ∣. Diagnosis and management of paediatric patients with proteinuria or nephrotic syndrome.
In children with podocytopathies, persistent sub-nephrotic proteinuria of >0.5 g per day is treated with a renin–angiotensin system inhibitor (RASi), maximally titrated. Patients are longitudinally followed up. If the proteinuria is associated with other symptoms, such as hypertension, kidney biopsy is required to exclude other glomerular disorders, which are treated accordingly. The length and frequency of the follow-up is determined for each patient based on renal function and residual proteinuria but, at a minimum, yearly evaluation should be performed even in patients with long-standing complete remission and normal renal function. Paediatric patients with idiopathic nephrotic-range proteinuria or nephrotic syndrome are treated with steroids, the response to which defines further management. Steroid-resistant patients should undergo biopsy and genetic testing; treatment with second-line immunosuppressive agents can be started while awaiting the results of genetic testing and should be continued only if these tests are unrevealing. Uncertain or unexpected genetic diagnosis should be confirmed with re-evaluation of the patient and their family. When a genetic diagnosis is ascertained, management should be personalized on the gene identified. By contrast, paediatric patients with nephrotic-range proteinuria or nephrotic syndrome who achieve complete or partial remission should be followed up and re-treated with steroids in case of relapse. Steroid-sparing immunosuppressive agents should be considered only for frequently relapsing and steroid-dependent patients. Based on guidelines from Kidney Disease Improving Global Outcomes (KDIGO)241 and the International Pediatric Nephrology Association242, updated and modified based on recent literature. ANA, antinuclear antibody; CNI, calcineurin inhibitor; DMS, diffuse glomerulosclerosis; FSGS, focal segmental glomerulosclerosis; Ig, immunoglobulin; LM, light microscopy; MC, minimal changes (with foot process effacement); MMF, mycophenolate mofetil; sCr, serum creatinine. a≥2 relapses in 6 months or ≥4 relapses in 12 months. b<2 relapses in 6 months or <4 relapses in 12 months.
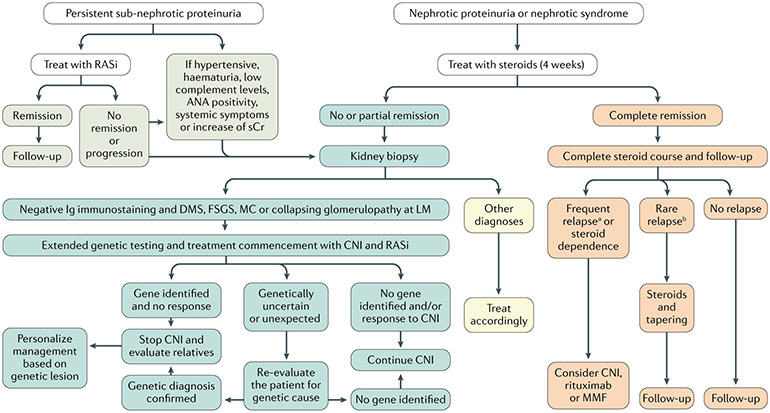
Fig. 9 ∣. Diagnosis and management of adults with proteinuria or nephrotic syndrome.
Renal biopsy, laboratory examinations and renal imaging (and, potentially, genetic testing) exclude other glomerular disorders and rule out secondary aetiologies of podocytopathies, which are treated according to the cause. Sub-nephrotic proteinuria is generally treated with a renin–angiotensin system inhibitor (RASi), maximally titrated; patients are longitudinally followed up based on renal function and residual proteinuria; a yearly evaluation should be performed even in patients with long-standing complete remission and normal renal function. Patients with idiopathic nephrotic-range proteinuria and nephrotic syndrome are treated with a course of steroids and with RASi. Response to steroids defines further management: steroid-resistant patients should undergo genetic testing and treatment with second-line immunosuppressive agents should be considered only if these tests are unrevealing. When a genetic diagnosis is ascertained, management should be targeted to the gene identified. Conversely, patients who achieve complete or partial remission should be followed up and re-treated with steroids in case of relapse. Steroid-sparing immunosuppressive agents should be considered only for frequently relapsing and steroid-dependent patients. Based on Kidney Disease Improving Global Outcomes (KDIGO) guidelines241, updated and modified based on recent literature. CNI, calcineurin inhibitor; FSGS, focal segmental glomerulosclerosis; MC, minimal changes (with foot process effacement); MMF, mycophenolate mofetil. a≥2 relapses in 6 months or ≥4 relapses in 12 months. b<2 relapses in 6 months or <4 relapses in 12 months.
Genetic variants in >50 nuclear and mitochondrial genes have been associated with FSGS and most cases are resistant to steroid therapy65,67,156. Inheritance patterns include autosomal recessive, autosomal dominant and sex-linked. Genetic causes of FSGS can be identified using gene panel testing, which involves resequencing a limited set of genes in which mutations are known to cause a podocytopathy. The gene panels may differ by age of podocytopathy onset. This approach has the advantages of being relatively fast, requiring a fairly simple consent form and having results that can often be conveyed by a nephrologist. Alternatively, whole-exome sequencing yields copious amounts of data, including on novel variants in genes associated in podocytopathies and novel variants in genes not previously associated with podocytopathies. Expanding the analysis for other genetic syndromes reported to occasionally present with isolated SRNS (that is, as phenocopies of podocytopathies) can double the diagnostic rate to 60%65. In these patients, re-evaluation of the patient and their family upon indication of genetic testing is mandatory to establish the correct diagnosis65. Thus, whole-exome sequencing is now the first choice in every centre where this analysis is possible for an isolated case or the first case in a family. However, the correct interpretation of variants of unknown clinical significance or in unexpected genes remains challenging and patients need to be counselled about the possibility of genetic diagnoses unrelated to kidney disease.
A different approach to African Americans with FSGS on kidney biopsy is warranted, as 72% of these individuals have two copies of APOL1 renal risk alleles. For these individuals, it makes sense to proceed directly to APOL1 testing for the G1 and G2 variant alleles.
Management
Progressive CKD is infrequent in children or adults with minimal change lesions6,152 but it is common among those with a podocytopathy, persistent proteinuria and FSGS lesions157. Response to glucocorticoids is critical in defining patient subsets158 and prognosis156, as those who achieve remission (75–92% with minimal change lesions146,159 and 47–66% in those with FSGS lesions160) do not usually develop ESKD161. Conversely, resistance to steroids is the strongest independent predictor of kidney function decline, with a kidney survival of 30% at 10 years from diagnosis. Massive proteinuria, impaired kidney function and interstitial fibrosis with tubular atrophy on kidney biopsy are also associated with progression to ESKD162,163. The prognosis of patients with a genetic podocytopathy is generally poor, with >50% of patients developing ESKD within 5 years of the diagnosis; patients who lack a genetic cause of the disease have a better prognosis, particularly when they are responsive to immunosuppressive treatment65,164. In syndromic podocytopathies, the overall prognosis is also affected by extrarenal manifestations; the prognosis reflects that of the underlying disorder65. Recently, a novel subset of patients has challenged the concept that podocytopathies with a defined genetic cause have a poor prognosis. For example, C-terminal CUBN pathogenetic variants, although associated with FSGS at biopsy, are characterized with albuminuria and normal GFR in large population-based cohorts, even in the absence of response to immunosuppressive treatments and to angiotensin-converting enzyme inhibitors165.
Current guidelines rely on clinical trial evidence and adhere to a ‘one-fits-all’ concept. However, it is becoming evident that, beyond the initial treatment with steroids, a substantial proportion of patients with podocytopathies needs a personalized approach to avoid drug toxicity from unnecessary medications and to apply specific treatments64,65,72,73,164,166. As personalized medicine for podocytopathies has only recently been in development, we will first describe the traditional approach.
Non-nephrotic proteinuria
Children and adults with persistent, non-nephrotic proteinuria are treated with a RASi and salt restriction157, which are frequently effective even in patients with maladaptive podocytopathies with FSGS lesions167. A low-dose thiazide diuretic (such as hydrochlorothiazide) will potentiate the anti-proteinuric effect of these therapies and may be tolerated even in normotensive patients. Such secondary podocytopathies require treatment of the underlying disorder whenever available (FIGS 8,9)
New-onset nephrotic syndrome
Oral steroid therapy for at least 2–3 months is typically initiated with new-onset nephrotic syndrome without histological confirmation by kidney biopsy in children and adolescents, in patients without hypertension, gross haematuria or marked elevation in serum creatinine, in patients with normal complement levels, and in patients with no extrarenal symptoms168. Approximately 80–90% of patients will experience complete remission within the 4 weeks of initiating therapy169 but some centres also administer three intravenous pulses of methylprednisolone every other day at this point169. Patients who do not undergo complete remission (and perhaps those with only a modest partial remission) are categorized as having SRNS and require prompt kidney biopsy and genetic testing170,171. Only 30% of children with SSNS maintain remission, 10–20% will have fewer than four relapses and the remaining will have frequent relapses (frequently relapsing nephrotic syndrome, FRNS) or will relapse while on a steroid taper (SDNS). The doses and length of treatment are personalized for each child as the clinical behaviour and response to steroids are heterogeneous (FIG. 8)
In adults, kidney biopsy at the time of presentation and identification of the underlying cause of the podocytopathy are essential to guide treatment (FIG. 9). In cases in which a specific cause cannot be identified, glucocorticoids represent the first-choice treatment157. Recommended regimens158 derive from paediatric trials as only few adequately powered studies in adults are available172-174. Response to therapy is typically slower in adults than in children, justifying prolonged steroid courses before defining treatment failure. Mycophenolate mofetil (an immunosuppressant) combined with low-dose steroids may induce disease remission in adult podocytopathies at rates comparable with standard therapy175,176, possibly alleviating the risk of steroid-related adverse effects such as diabetes and hypertension in high-risk patients. Slow tapering of immunosuppressive drugs over 6 months is a widely accepted measure to reduce the risk of relapse177.
SDNS and FRNS
Approximately 80% of steroid-responsive children and 70% of adult patients178,179 undergo one or more relapses, which typically remain sensitive to steroids180,181. However, many patients become steroid dependent146 or experience frequent relapses182 (TABLE 1) after treatment discontinuation. The risk of relapse is greatest in children aged <5 years at onset, for reasons unknown. Almost all children with FRNS experience a progressive decrease in the number of relapses over time and many ultimately go into sustained or even permanent remission160,183. Limited long-term outcome data in adults suggests that patients who have frequent relapses or SDNS during childhood are at risk of experiencing relapses during adulthood and adverse drug effects183-185 (TABLE 2). Kidney function remains normal in adulthood as long as patients remain responsive to treatment and long-term sequelae are generally related to medication adverse effects184,185.
Table 2 ∣.
Potential adverse events associated with immunosuppressive treatment
| Treatment | Possible adverse effects | Measures to reduce toxicity |
|---|---|---|
| Steroids | Cataracts233; excessive weight gain, obesity or Cushingoid features243; suppression of the hypothalamic–pituitary–adrenal axis244; behaviour disturbances (such as hyperactivity or depression); hypertension243; osteopenia243; statural growth impairment (in children)232 | Alternate-day therapy or low dose (<1 mg) whenever possible234 |
| Ciclosporin | Nephrotoxicity229,230,245,246; hyperlipidaemia; hypertrichosis; gum hypertrophy | Blood trough level should not exceed 200 ng/ml (REF.247); once remission is achieved, decrease the dose to <5 mg/kg (REF.248) |
| Tacrolimus | Nephrotoxicity249; glucose intolerance; headache; seizures | If possible, target lower trough concentrations (3–5 ng/ml)250 |
| Mycophenolate mofetil | Gastrointestinal disturbances (abdominal pain and diarrhoea); haematological abnormalities and infections; teratogenic effects | Monitoring to target area under the curve (>45 μg·h·ml)251; recommend contraception in women with child-bearing potential252 |
| Rituximab | Infusion-related reactions; leukopenia and/or hypogammaglobulinaemia; neutropenia; hepatitis induced by hepatitis B virus reactivation; progressive multifocal leukoencephalopathy; pulmonary fibrosis | If anaphylaxis is suspected, discontinue treatment; regular monitoring of complete blood count; recommend G-CSF and antibiotics administration if severe neutropenia with infection occurs; discontinue treatment |
| Levamisole | Neutropenia; vasculitis; flu-like symptoms | Regular monitoring of complete blood count; discontinue treatment if neutropenia or vasculitis occur |
| Cyclophosphamide | Neutropenia and infection253; gonadal toxicity; malignancy; alopecia; haemorrhagic cystitis | Discontinue treatment if the WBC count falls <3,000/mm3 (until the count rises); maximum daily dose and cumulative dose should not exceed 2.5 mg/kg and 300 mg/kg, respectively |
G-CSF, granulocyte colony-stimulating factor; WBC, white blood cell.
Various glucocorticoid regimens have been used to treat FRNS and SDNS186,187. Frequent relapses usually require steroid dose adjustment above the individual threshold. Alternate-day steroid dosing (in children) and steroid-sparing agents are commonly used to avoid long-term steroid toxicity. These agents include immunosuppressive drugs such as calcineurin inhibitors (ciclosporin or tacrolimus), rituximab, levamisole and cyclophosphamide. Calcineurin inhibitors are frequently chosen as steroid-sparing agents based on evidence from small trials188 despite relapse rates as high as 75% upon discontinuation. These events often lead to prolonged treatment courses, which pose a considerable risk of calcineurin inhibitor-induced nephrotoxicity (TABLE 2). An increasing number of studies strongly suggests that rituximab can effectively reduce the number of relapses in SDNS and FRNS85,86, minimizing the steroid dose, but relapses can occur after stopping rituximab.
Low-quality evidence supports the use of alkylating agents such as cyclophosphamide in SDNS and FRNS podocytopathies to reduce the cumulative dose of steroids146 but these regimens have been progressively abandoned in developed countries owing to their unfavourable safety profile (TABLE 2). In paediatric patients, mycophenolate mofetil seems equally as effective as levamisole189 but not as effective as ciclosporin in achieving remission190-192, although the evidence is still limited157. In addition, higher doses of mycophenolate mofetil than those used in kidney-transplanted recipients seem to be necessary in children with nephrotic syndrome to achieve remission192. Adrenocorticotropic hormone has been used but its efficacy is controversial because studies yielded conflicting results193,194. Finally, in a very small number of cases, patients who were initially steroid sensitive become steroid resistant but can be usually successfully treated with alternative immunosuppressive therapies195. However, these patients are at increased risk for ESKD195.
SRNS
Steroid resistance is defined as the lack of complete remission despite full-dose steroids for an adequate period of time (FIGS 8, 9). Screening for genetic mutations should be performed in all children as well as in adults with SRNS before considering additional immunosuppressive therapies as these are rarely effective in podocytopathies with a genetic cause. However, as genetic testing results take at least 4–6 weeks to become available, it may be appropriate to start a second-line therapy in selected patients in the interim. In patients in whom a genetic mutation is identified, immunosuppressive treatments can usually be discontinued and anti-proteinuric regimens with a RASi become the mainstay of therapy to attenuate CKD progression.
Approximately 60% of steroid-resistant patients may respond to ciclosporin or tacrolimus at moderate doses with reduced proteinuria and slower or halted CKD progression196. A genetic cause is usually not found in these patients65,67,156. As relapse is frequent after the withdrawal of these drugs196, prolonging treatment for 1–3 years after remission is achieved is advisable157. Mycophenolate mofetil alone achieves remission in fewer than half of patients197,198 but its use as maintenance treatment might reduce the calcineurin inhibitor dose and limit nephrotoxicity199. Thus, mycophenolate mofetil in combination with calcineurin inhibitors may be a rescue approach. The initial report of abatacept efficacy in treating SRNS182 was challenged by subsequent studies200,201 and the effect of other therapies such as rituximab202 and adalimumab (targeting TNF)203 seems to be limited, especially in adult patients. Instead of immunosuppression, SRNS requires rigorous control of glomerular hyperfiltration with a RASi and by reducing dietary salt. Recently, sparsentan, a dual endothelin type A (ETA) and angiotensin I type 1 receptor antagonist showed a robust anti-proteinuric effect in a phase II clinical trial compared with monotherapy with the angiotensin receptor blocker irbesartan204. In secondary podocytopathies, management is focused on treating the underlying disorder.
Personalized treatment
Genetic testing identifies the diagnosis underlying SRNS in up to 30–60% of children and young adults, and some of these patients may benefit from avoiding unnecessary immunosuppressant therapies and from receiving specific treatments65. For example, patients with pathogenetic mutations in coenzyme Q10 biosynthesis (COQ2, COQ6 and ADCK4) may respond to oral coenzyme Q10 supplementation72,73,166. Patients with pathogenetic mutations in collagen genes benefit from early diagnosis and administration of a RASi, while avoiding calcineurin inhibitors to prevent CKD progression154,205. Patients with Dent disease should be treated to avoid failure to thrive and kidney stones64,65,206. Similarly, patients with Fabry disease or cystinosis will likely benefit from early treatment, with enzyme replacement therapy or cysteamine depleting therapy, respectively65,207. Patients with pathogenetic mutations in exon 8 or 9 of WT1 will benefit from regular screening for Wilms tumour and some may undergo prophylactic nephrectomy208. In addition, some patients with moderate proteinuria may benefit from genetic testing. For example, early recognition of patients with C-terminal CUBN pathogenetic variants is paramount as they tend to have a good prognosis without any treatment165. This underscores the need to personalize the diagnosis of distinct types of podocytopathies owing to the important implications for prognosis and treatment.
Complications
Nephrotic syndrome requires additional treatment for symptoms and to prevent complications.
Oedema.
Patients are initially treated with loop diuretics and dietary sodium restriction to ~2 g per day and are monitored closely for clinical signs of hypovolaemia209. Most patients require high doses of diuretics owing to hypoalbuminaemia, expanded extracellular volume (larger volume of drug distribution) and a slower rate of delivery to the target cells within the kidney210. Thiazide diuretics or, alternatively, triamterene 211 or acetazolamide212 can be combined with loop diuretics in patients with refractory oedema. As proteasuria activates the amiloride-sensitive epithelial sodium channel, amiloride can also be useful213,214.
Dyslipidaemia.
Hyperlipidaemia is problematic in patients with nephrotic proteinuria as it increases the risk for progressive loss of renal function and cardiovascular disease135. Statins are the treatment of choice if hyperlipidaemia persists after treatment of the underlying kidney disorder with immunosuppressive therapy and/or a RASi135,215. Drug interactions, for example, with cytochrome P3A4 inhibitors such as cyclosporin, increase plasma levels of statins and require dose adaptations of the statins. Pro-protein convertase subtilisin/ kexin type 9 (PCSK9) inhibitors may have a role when statins are insufficient or are not tolerated but these agents are currently very expensive135.
Thromboembolism.
Adults with nephrotic syndrome have a high incidence (10–40%) of arterial and venous thrombosis, particularly deep vein and renal vein thrombosis, in some cases leading to pulmonary embolism216. By contrast, venous and arterial thromboses are reported in only 2–3% of children with nephrotic syndrome, although this may be an underestimate because many episodes are asymptomatic217. Venous thrombosis, particularly of the renal vein, deep leg veins, inferior vena cava and cerebral vein, account for most cases217. Thromboses of the pulmonary, femoral, iliac, cerebral and meningeal arteries are less common218. Adults and infants with congenital nephrotic syndrome are at increased risk for renal vein thrombosis but this complication is rare in children218. Pulmonary embolism should be suspected in patients with pulmonary or cardiovascular symptoms and can be confirmed by radioisotope scanning219. Prophylactic anticoagulation with oral anticoagulants is recommended only after the first thromboembolic episode or when albumin concentration is <2 g/dl, fibrinogen is >6 g/l or antithrombin III is <70% of normal, and is continued for as long as these alterations persist220.
Infections.
To prevent pneumococcal infections, children with nephrotic syndrome should receive 1–2 doses of conjugate 13-valent pneumococcal vaccine (PCV13) preceding the 23-valent polysaccharide (PPSV23) pneumococcal vaccine, if not previously immunized. Varicella can also cause major morbidity and mortality in these patients221. Adults with nephrotic proteinuria who have not been previously immunized should receive both pneumococcal vaccines, with PCV13 followed 8 weeks later by PPSV23.
Although the use of live attenuated viral vaccines has been discouraged in the past for children receiving immunosuppressive therapy, a recent prospective study suggested that two doses of varicella vaccine can be safely administered to children with a CD4+ T cell count of >500/mm3, a normal lymphocyte blast transformation in response phytohemagglutinin (to assess cell-mediated immune responses), and serum IgG levels of >300 mg/dl (REF.222).
Centre-specific guidelines on influenza vaccination should be followed. In general, vaccinations pose a minimal risk for relapse of nephrotic syndrome; further, the protection gained greatly outweighs this risk222.
Relapse after transplantation
Up to one-third of patients with FSGS lesions at biopsy who undergo kidney transplantation show recurrent nephrotic syndrome in the allograft223. The strongest predictive clinical feature of relapse for patients who undergo kidney transplantation is a previous response to steroids or immunosuppressive drugs for the nephrotic syndrome that caused ESKD223. This finding suggests that the podocytopathy is related to one or several immunological or circulating factors. A younger age at diagnosis, severe hypoalbuminaemia and rapid progression to ESKD (for example, within 3 years of diagnosis) are also more common in patients who experience recurrence after transplantation. Apolipoprotein A-Ib, a high-molecular-weight form of apolipoprotein A-I, was recently proposed as a possible biomarker for recurrent forms of podocytopathies after kidney transplantation224. Typically, relapses occur in the first 2 weeks after kidney transplantation and often respond to combined treatment with plasma exchange, rituximab and intensified immunosuppression, although the response may be transient225,226. Post-transplantation relapses are exceptional in those with a definite genetic diagnosis or with forms of podocytopathies related to increased single-nephron GFR or a low nephron mass226,227.
Quality of life
The symptoms of nephrotic syndrome and the relapsing nature of most podocytopathies greatly compromise the quality of life of patients and their families. In general, patient-reported questionnaires identify oedema as the symptom that most negatively affects the quality of life228. Certain patients are at higher risk of developing drug-related adverse events linked to dose and length of exposure229,230. In steroid-dependent patients, the adverse effects of steroids frequently occur with long-term exposure (that is, prednisone doses of >5 mg per day for ≥3 months)231. These adverse events include cataracts, delayed growth in children, obesity, Cushingoid features, osteoporosis and psychological disturbances232,233. In adolescents, steroid-induced striae rubrae (stretch marks) and ciclosporin-related hypertrichosis (hair growth) can greatly affect self-confidence and mood234. Aesthetic medicine interventions and psychological support can improve these psychosocial impacts. In multidrug-resistant patients, nephrotoxicity from the prolonged use of calcineurin inhibitors may contribute to CKD progression and ESKD229.
For patients with ESKD, dialysis (peritoneal dialysis, centre haemodialysis or home-haemodialysis) negatively affects the quality of life235. However, when patients reach ESKD, oedema and other symptoms of nephrotic syndrome typically improve because urinary protein losses decline and finally cease with progressive oligo-anuria. Kidney transplantation can improve the quality of life and longevity in these patients164,236. However, the recurrence of nephrotic syndrome after transplantation is an important risk factor for allograft loss and the need to return to dialysis223, with a major negative effect on quality of life and increasing the need for psychological support.
Outlook
The concept of ‘podocytopathies’ replacing DMS, FSGS, minimal changes and collapsing glomerulopathy as clinical entities is based on research and will continue to evolve. Indeed, with recent advances in our understanding of molecular and cellular mechanisms, the time is now to move from histologically defined entities of proteinuria to identifying and treating each patient’s podocytopathy. Each podocytopathy arises from some combination of genetic, epigenetic, transcriptional, proteomic, physiological, metabolic, immunological, viral and, possibly, psychological factors — and perhaps other factors that we do not yet appreciate.
Mechanisms
Knowledge about the genetic basis of glomerular disease is rapidly evolving and has generated new opportunities as well as new challenges for clinicians and patients. Close contact between clinicians and geneticists are neither available everywhere nor are genetic evaluations always affordable. These limitations imply that patients with SRNS are not being treated optimally in every region and may have to travel longer distances to reach a specialized centre. Access to geneticists is important, for example, when trying to interpret the clinical relevance of genetic test results, including the many ‘variants of unknown significance’. The rapidly increasing knowledge of human genetics has clearly given rise to the need for genetically oriented nephrologists or nephrogeneticists, similar to nephropathologists, to follow the latest advances in data and interpretation in a highly dynamic field. Initiatives like ClinGen and ClinVar, US NIH-funded initiatives dedicated to building a central resource, can define the clinical relevance of genes and variants for use in precision medicine and research and will make access to genetic information more readily and widely available.
Diagnosis
The promise of personalized diagnosis and management puts into question several aspects of the traditional morphology-based disease classifications and guideline-based patient management. Genetic testing may provide a precise molecular diagnosis and protect the patient from unnecessary immunosuppressive treatments. However, a genetic diagnosis may generate new questions for families, including whether to test other family members. Benefits of genetic testing include the opportunity for early diagnosis through screening and for selecting the optimal therapy. Risks include anxiety about test results, access to health insurance in some countries and the possibility of unexpected information about biological relationships among family members. The universal right to not know, especially for clinically unaffected family members, should be respected.
Big data
We need to better understand the primary nephrotic diseases at the molecular, cellular and clinical levels, addressing the symptoms, signs and effects on physical function and psychosocial well-being in a holistic approach. In the past decade, extensive resources have been brought to bear on these issues, including investigator and patient efforts, to answer questions not yet satisfactorily addressed by single-centre studies. The Nephrotic Syndrome Study Network (NEPTUNE) brings together investigators from throughout North America to enrol children and adults with nephrotic syndrome, currently numbering >500 patients, in a long-term natural history study that encompasses clinical, genetic, transcriptional, proteomic and metabolomic approaches to understand the pathogenesis, to enable testing of novel therapies and to determine the optimal treatment strategies237. The Cure Glomerulopathy (CureGN) consortium brings together investigators from 70 sites, predominantly in the United States as well as in Canada and Europe, with a mandate to enrol 2,400 children and adults with prevalent glomerular diseases, including minimal changes, FSGS, membranous nephropathy and IgA nephropathy, for prospective natural history studies238. Both studies will serve as valuable platforms to develop new knowledge, to bring new investigators into the nephrotic syndrome field and to provide training to the next generation of researchers.
In Europe, the European Rare Kidney Disease Reference Network (ERKNet), a virtual network involving health-care providers across Europe co-funded by the European Commission, was founded with the aim to facilitate discussion on complex or rare renal diseases and conditions that require highly specialized treatment, concentrated knowledge and resources. The ERKnet has facilitated the exchange of information between nephrologists in Europe and permits the review of patients’ diagnoses and treatments by virtual advisory panels of medical specialists across different disciplines, using a dedicated platform and telemedicine tools.
Pharmaceutical and biotechnology companies have shown increasing interest in developing novel therapies for podocyte disorders and will be the major source of the development of innovative therapies. Governmental funding agencies are essential partners. Patient organizations have an important role as they are highly motivated by the unmet medical needs and are strong advocates for the relevance of patient-oriented outcomes; they play essential and formal parts in the NEPTUNE and CureGN studies mentioned above. In nephrology, patient groups, including NephCure, the American Kidney Fund, the National Organization for Rare Disorders, the National Kidney Foundation, the Dutch Kidney Foundation, the Federation for Each Renal Genetic Disease, the Nephrotic Syndrome Association Italy, and La Nuova Speranza non-profit foundation, have served as powerful and compelling ambassadors, sources of research funding, and supporters of scientific meetings that stimulate international exchange and collaborations.
Management
Given the value of rituximab as a treatment in SSNS and immune-mediated podocytopathies, other CD20-targeting or B cell-targeting drugs are being considered. Ofatumumab is a more potent CD20+ B cell depleter and may replace rituximab in patients with drug hypersensitivity88,239. Another agent, belimumab (which targets B cell-activating factor), is an established maintenance therapy in systemic lupus erythematosus and reduces proteinuria in lupus nephritis240 and might control B cell activity also in other forms of nephrotic syndrome.
Many attempts to find new treatments for podocytopathies beyond the traditional immunosuppressive drugs have been unsuccessful. One likely contributing factor is the fact that patients in clinical trials were stratified based on unspecific pathological patterns; in reality, these individuals likely had diverse disorders and potentially required specific therapeutic approaches. Successful trials will possibly require the selection of agents that target specific molecular pathways shown to be altered in the underlying podocytopathy, with enrolment of only those patients who manifest alterations in these pathways. We do not yet have all the tools needed to make such selections but some approaches for selection include genetic studies, kidney transcription profiling and urinary single-cell transcriptional profiling. Encouragingly, several clinical trials for podocytopathies are ongoing (TABLE 3).
Table 3 ∣.
Clinical trials for new drugs in podocytopathies
| NCT number | Drug | Mechanism of action | Status | Phase | Population | Completion |
|---|---|---|---|---|---|---|
| NCT03970122 | GFB-887 | Transient receptor potential channel 5 inhibitor | Completed | Phase I | Healthy volunteers | April 2020 |
| NCT03598036 | Voclosporin | High potency and stability calcineurin inhibitor | Recruiting | Phase II | FSGS | December 2020 |
| NCT03649152 | Propagermanium | CC-chemokine receptor 2 inhibitor | Active, not recruiting | Phase II | FSGS | June 2020 |
| NCT03422510 | CXA-10 | Anti-inflammatory and antifibrotic | Recruiting | Phase II | FSGS | April 2021 |
| NCT03448692 | PF-06730512 | SLIT2 antagonist | Recruiting | Phase II | FSGS | January 2023 |
| NCT02921789 | Bleselumab | Anti-human CD40 antibody | Active, not recruiting | Phase II | FSGS | April 2021 |
| NCT03703908 | CCX140-B | CC-chemokine receptor 2 inhibitor | Recruiting | Phase II | FSGS | March 2021 |
| NCT03536754 | CCX140-B | CC-chemokine receptor 2 inhibitor | Active, not recruiting | Phase II | FSGS | March 2020 |
| NCT04009668 | Adalimumab | Anti-human TNF antibody | Recruiting | Phase II | FSGS, MC | January 2021 |
| NCT02592798 | Abatacept | T cell inhibitor | Completed | Phase II | FSGS, MC | January 2020 |
| NCT03366337 | Bardoxolone | Nuclear factor erythroid 2-related factor activator and NF-κB inhibitor | Completed | Phase II | FSGS | January 2019 |
| NCT03493685 | Sparsentan | Dual endothelin type A and angiotensin II type 1 receptor antagonist | Recruiting | Phase III | FSGS | December 2022 |
| NCT04340362 | VX-147 | APOLl antagonist | Recruiting | Phase II | APOL1-associated FSGS | May 2021 |
| NCT02585804 | Dapagtiflozin | SGLT2 inhibitor | Completed | Phase IV | FSGS | April 2017 |
| NCT02639260 | N-acetyl mannosamine | Sialic acid precursor | Completed | Phase 1 | FSGS | June 2018 |
Trials were selected by using, as key words, FSGS, MC, minimal change disease, DMS and collapsing glomerulopathy. Only trials that have been completed in the past 3 years or that have yet to be completed have been included. Search of clinicaltrials.gov was performed on 12 June 2020. DMS, diffuse mesangial sclerosis; FSGS, focal segmental glomerulosclerosis; MC, minimal changes; SGLT, sodium/glucose cotransporter.
Another high priority is to develop specific podocyte-directed therapies to protect podocytes from injury, detachment and loss. These strategies include stem cell therapy for genetic disorders, the use of genetically modified cells, induced pluripotent stem cells, small interfering RNA therapeutics and other approaches, although none has reached the domain of clinical podocytopathies. Intriguingly, a recent study reported that lowering APOL1 expression using antisense oligonucleotides significantly ameliorated kidney disease in a transgenic animal model of APOL1 podocytopathy117.
Another promising area is the promotion of cell regeneration. The discovery that lost podocytes are potentially replaceable from a pool of local progenitor cells within the parietal epithelial cells is raising new hope to stimulate that system therapeutically, especially in non-genetic podocytopathies53,62. To avoid unwanted effects in other progenitor cell niches, such an appropriate drug target should be specific for podocyte progenitors. Whether such treatments would increase the risk of kidney cancer is currently unknown and will be an important consideration.
In conclusion, this is an exciting era for podocytopathy research and clinical care. New therapies to slow and possibly halt the progression of podocyte injury are in clinical trials and, in the future, therapies to stimulate podocyte regeneration may become available. Renewed energy from patients and patient organizations, academic researchers, pharmaceutical and biotechnology companies, and government agencies is spurring collaboration to develop novel, safe and effective therapies for patients with podocytopathies.
Supplementary Material
Chronic kidney disease.
(CKD). Abnormalities in kidney structure or function (urine composition or impaired excretory function) lasting >3 months; progression is based on the cumulative degeneration of nephrons, the independent functional units of the kidney.
Glomerular filtration barrier.
The parts of the nephron in which the filtration process of the blood takes place and the primary filtrate is formed; podocytes and their interdigitating foot processes, connected by the slit diaphragm, are essential components of the size-selective and charge-selective filtration barrier in the glomerulus
Nephrotic syndrome.
A clinical syndrome defined by symmetrical oedema, hypoalbuminaemia, hyperlipidaemia and proteinuria of >3 g per day, caused by podocyte injury (from any cause) and leading to severe alterations of the glomerular filtration barrier
End-stage kidney disease.
(ESKD) When nephron loss during chronic kidney disease reaches the point that homeostasis can no longer be maintained, presenting as renal failure (uraemia)
Glomerular filtration rate.
(GFR) The central parameter of excretory kidney function that can be accurately measured as the clearance of injected tracers over time or can be estimated from a number of clinical and laboratory parameters, including creatinine and cystatin C
Podocyte shear stress.
The hydrostatic pressure gradient across the glomerular filtration barrier that podocytes must withstand to avoid detachment and loss into the urine
Hyperfiltration.
An elevated total glomerular filtration rate (GFR) is called hyperfiltration and implies hyperfiltration of every nephron; however, reduced total GFR implies compensatory hyperfiltration of the remnant nephrons, as a central mechanism for the progression of chronic kidney disease by promoting podocyte shear stress, podocyte detachment and adaptive focal segmental glomerulosclerosis
Oncotic pressure.
The pressure resulting from the difference within the extracellular fluid between the protein contents of plasma and interstitial fluid
Syndromic disorders.
Genetic diseases with manifestations in different organ systems due to the expression of the mutated gene in diverse tissues
Mitotic catastrophe.
A type of cell death that occurs during mitosis, resulting from DNA damage or deranged spindle formation and linked to checkpoint failure
Overfill scenario.
Nephrotic syndrome associated with oedema secondary to a positive sodium balance, mainly due to sodium retention; patients present with intravascular hypervolaemia (hypertension) and interstitial hypervolaemia (oedema)
Acute kidney injury (AKI).
An abrupt decrease in kidney function, resulting in the retention of urea and other nitrogenous waste products in the blood and in the dysregulation of extracellular volume and electrolytes
Underfill scenario.
Nephrotic syndrome associated with oedema secondary to a rapid fall in blood oncotic pressure with volume shifts into the interstitial compartments; patients present with intravascular hypovolaemia and interstitial oedema
Renin–angiotensin system.
The hormone system that regulates blood pressure, volume and electrolyte balance, and systemic vascular resistance
Anasarca.
General swelling of the whole body that can occur when the tissues of the body retain too much fluid
Cushingoid features.
Weight gain, hypertension, cutaneous striae rubrae and easy bruising
Acknowledgements
J.B.K. was supported by the NIDDK Intramural Research Program, NIH, Bethesda, MD (project ZO1 DK043308). K.S. is supported by NIDDK grants R01DK076077, R01 DK087635 and DP3 DK108220. H.-J.A. was supported by the Deutsche Forschungsgemeinschaft (AN372/24-1). F.H. was supported by the NIH through grant RO1-DK076683. This work was supported by the European Research Council under the Consolidator Grant RENOIR to P.R. (ERC-2014-CoG, grant number 648274). The authors thank A. M. Buccoliero, Meyer Children’s Hospital and Fiammetta Ravaglia, Florence, Italy, for help with histopathological images.
Footnotes
Competing interests
F.H. is a co-founder and member of the scientific advisory board of Goldfinch-Bio. The other authors declare no competing interests.
Peer review information
Nature Reviews Disease Primers thanks A. Bagga, K. Iijima, Moin Saleem and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41572-020-0196-7.
RELATED LINKS
The American Kidney Fund: https://www.kidneyfund.org/
ClinGen: https://clinicalgenome.org
The Dutch Kidney Foundation: https://nierstichting.nl
European Rare Kidney Disease Reference Network: https://www.erknet.org/
The Federation for Each Renal Genetic Disease: http://federg.org
La Nuova Speranza non-profit foundation: http://www.lanuovasperanza.org
The National Kidney Foundation: https://www.kidney.org
The National Organization for Rare Disorders: https://rarediseases.org/
NephCure: https://nephcure.org/
The Nephrotic Syndrome Association, Italy: http://www.asnit.org
References
- 1.Pallet N et al. Proteinuria typing: how, why and for whom? Ann. Biol. Clin 77, 13–25 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Glassock RJ & Fervenza FC Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol. Dial. Transpl 30, 375–384 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Muller-Deile J, Schenk H & Schiffer M Minimal change disease and focal segmental glomerulosclerosis. Internist 60, 450–457 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg AZ & Kopp JB Focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol 12, 502–517 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barisoni L, Schnaper HW & Kopp JB A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin. J. Am. Soc. Nephrol 2, 529–542 (2007).This paper is the first proposal for the reclassification of podocytopathies.
- 6.Vivarelli M, Massella L, Ruggiero B & Emma F Minimal change disease. Clin. J. Am. Soc. Nephrol 12, 332–345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiggins RC The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 71, 1205–1214 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Sim JJ et al. Distribution of biopsy-proven presumed primary glomerulonephropathies in 2000–2011 among a racially and ethnically diverse US population. Am. J. Kidney Dis 68, 533–544 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Pesce F & Schena FP Worldwide distribution of glomerular diseases: the role of renal biopsy registries. Nephrol. Dial. Transpl 25, 334–336 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Qiu C et al. Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med 24, 1721–1731 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gbadegesin RA et al. HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J. Am. Soc. Nephrol 26, 1701–1710 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debiec H et al. Transethnic, genome-wide analysis reveals immune-related risk alleles and phenotypic correlates in pediatric steroid-sensitive nephrotic syndrome. J. Am. Soc. Nephrol 29, 2000–2013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufek S et al. Genetic identification of two novel loci associated with steroid-sensitive nephrotic syndrome. J. Am. Soc. Nephrol 30, 1375–1384 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia X et al. Strong association of the HLA-DR/DQ locus with childhood steroid-sensitive nephrotic syndrome in the Japanese population. J. Am. Soc. Nephrol 29, 2189–2199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsa A et al. APOL1 risk variants, race, and progression of chronic kidney disease. N. Engl. J. Med 369, 2183–2196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genovese G et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329, 841–845 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzur S et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum. Genet 128, 345–350 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siemens TA, Riella MC, Moraes TP & Riella CV APOL1 risk variants and kidney disease: what we know so far. J. Bras. Nefrol 40, 388–402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abd ElHafeez S et al. Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ Open 8, e015069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedla FM & Yap E Apolipoprotein L1 and kidney transplantation. Curr. Opin. Organ. Transpl 24, 97–102 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Beckerman P & Susztak K APOL1: the balance imposed by infection, selection, and kidney disease. Trends Mol. Med 24, 682–695 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beckerman P et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat. Med 23, 429–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogo A et al. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 51, 244–252 (1997). [DOI] [PubMed] [Google Scholar]
- 24.Hodgin JB et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J. Am. Soc. Nephrol 26, 3162–3178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ & Romagnani P Podocyte mitosis - a catastrophe. Curr. Mol. Med 13, 13–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BI & Cohen AH Hypertension-attributed nephropathy: what’s in a name? Nat. Rev. Nephrol 12, 27–36 (2016).An extensive critical reassessment of the role of APOL1 not only in FSGS but also in hypertension-attributed nephropathy.
- 27.Bick AG et al. Association of APOL1 risk alleles with cardiovascular disease in blacks in the Million Veteran Program. Circulation 140, 1031–1040 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton JO et al. Association of anthropometric obesity measures with chronic kidney disease risk in a non-diabetic patient population. Nephrol. Dial. Transpl 27, 1860–1866 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Praga M et al. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol. Dial. Transpl 16, 1790–1798 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Xu T, Sheng Z & Yao L Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front. Med 11, 340–348 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Kriz W & Lemley KV Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 91, 1283–1286 (2017). [DOI] [PubMed] [Google Scholar]
- 32.D’Agati VD et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol 12, 453–471 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Tonneijck L et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol 28, 1023–1039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallon V & Thomson SC The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol 10.1038/s41581-020-0256-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novick AC, Gephardt G, Guz B, Steinmuller D & Tubbs RR Long-term follow-up after partial removal of a solitary kidney. N. Engl. J. Med 325, 1058–1062 (1991). [DOI] [PubMed] [Google Scholar]
- 36.Argueso LR et al. Prognosis of patients with unilateral renal agenesis. Pediatr. Nephrol 6, 412–416 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Luyckx VA et al. A developmental approach to the prevention of hypertension and kidney disease: a report from the Low Birth Weight and Nephron Number Working Group. Lancet 390, 424–428 (2017).A position paper that supports the essential role of nephron mass in determining the risk for FSGS and CKD.
- 38.Aygun B, Mortier NA, Smeltzer MP, Hankins JS & Ware RE Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr. Nephrol 26, 1285–1290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen YT, Coleman RA, Scheinman JI, Kolbeck PC & Sidbury JB Renal disease in type I glycogen storage disease. N. Engl. J. Med 318, 7–11 (1988). [DOI] [PubMed] [Google Scholar]
- 40.Morgan C, Al-Aklabi M & Garcia Guerra G Chronic kidney disease in congenital heart disease patients: a narrative review of evidence. Can. J. Kidney Health Dis 2, 27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwimmer JA et al. Secondary focal segmental glomerulosclerosis in non-obese patients with increased muscle mass. Clin. Nephrol 60, 233–241 (2003). [PubMed] [Google Scholar]
- 42.Mundel P & Shankland SJ Podocyte biology and response to injury. J. Am. Soc. Nephrol 13, 3005–3015 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Falkenberg CV et al. Fragility of foot process morphology in kidney podocytes arises from chaotic spatial propagation of cytoskeletal instability. PLoS Comput. Biol 13, e1005433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin JS & Susztak K Podocytes: the weakest link in diabetic kidney disease? Curr. Diab Rep 16, 45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susztak K, Raff AC, Schiffer M & Bottinger EP Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006). [PubMed] [Google Scholar]
- 46.Kriz W & Lemley KV Mechanical challenges to the glomerular filtration barrier: adaptations and pathway to sclerosis. Pediatr. Nephrol 32, 405–417 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Endlich K, Kliewe F & Endlich N Stressed podocytes-mechanical forces, sensors, signaling and response. Pflug. Arch 469, 937–949 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Sweetwyne MT et al. Notch1 and Notch2 in podocytes play differential roles during diabetic nephropathy development. Diabetes 64, 4099–4111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jefferson JA & Shankland SJ The pathogenesis of focal segmental glomerulosclerosis. Adv. Chronic Kidney Dis 21, 408–416 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vriese AS, Sethi S, Nath KA, Glassock RJ & Fervenza FC Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J. Am. Soc. Nephrol 29, 759–774 (2018).A critical reappraisal of diagnostic criteria for the recognition of many forms of podocytopathies.
- 51.Nishizono R et al. FSGS as an adaptive response to growth-induced podocyte stress. J. Am. Soc. Nephrol 28, 2931–2945 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ronconi E et al. Regeneration of glomerular podocytes by human renal progenitors. J. Am. Soc. Nephrol 20, 322–332 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romoli S et al. CXCL12 blockade preferentially regenerates lost podocytes in cortical nephrons by targeting an intrinsic podocyte-progenitor feedback mechanism. Kidney Int. 94, 1111–1126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaverina NV et al. Dual lineage tracing shows that glomerular parietal epithelial cells can transdifferentiate toward the adult podocyte fate. Kidney Int. 96, 597–611 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eng DG et al. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int. 88, 999–1012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai Y et al. Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor alpha. Kidney Int. 92, 1444–1457 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J et al. Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease. Nephron Exp. Nephrol 121, e23–e37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peired A et al. Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J. Am. Soc. Nephrol 24, 1756–1768 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar V & Singhal PC APOL1 and kidney cell function. Am. J. Physiol. Renal Physiol 317, F463–F477 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melica ME et al. Substrate stiffness modulates renal progenitor cell properties via a ROCK-mediated mechanotransduction mechanism. Cells 8, 1561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niranjan T et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med 14, 290–298 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Lasagni L et al. Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep. 5, 248–263 (2015).A proof-of-concept study that shows podocyte regeneration occurs after damage and can be pharmacologically enhanced.
- 63.Smeets B et al. Renal progenitor cells contribute to hyperplastic lesions of podocytopathies and crescentic glomerulonephritis. J. Am. Soc. Nephrol 20, 2593–2603 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warejko JK et al. Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol 13, 53–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landini S et al. Reverse phenotyping after whole-exome sequencing in steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol (2019).A study reporting the high prevalence of genetic cases caused by unrecognized syndromic disorders in up to 60% of patients with SRNS.
- 66.Assady S, Wanner N, Skorecki KL & Huber TB New insights into podocyte biology in glomerular health and disease. J. Am. Soc. Nephrol 28, 1707–1715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giglio S et al. Heterogeneous genetic alterations in sporadic nephrotic syndrome associate with resistance to immunosuppression. J. Am. Soc. Nephrol 26, 230–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadowski CE et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol 26, 1279–1289 (2015).A large-scale study reporting the presence of primary genetic podocytopathies in 29.5% of cases of SRNS.
- 69.van der Ven AT et al. Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and urinary tract. J. Am. Soc. Nephrol 29, 2348–2361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashraf S et al. Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat. Commun 9, 1960 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dorval G et al. Clinical and genetic heterogeneity in familial steroid-sensitive nephrotic syndrome. Pediatr. Nephrol 33, 473–483 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Doimo M et al. Effect of vanillic acid on COQ6 mutants identified in patients with coenzyme Q10 deficiency. Biochim. Biophys. Acta 1842, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atmaca M et al. Follow-up results of patients with ADCK4 mutations and the efficacy of CoQ10 treatment. Pediatr. Nephrol 32, 1369–1375 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Boyer O et al. INF2 mutations in Charcot-Marie-Tooth disease with glomerulopathy. N. Engl. J. Med 365, 2377–2388 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Nozu K et al. A review of clinical characteristics and genetic backgrounds in Alport syndrome. Clin. Exp. Nephrol 23, 158–168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoo TH & Fornoni A Nonimmunologic targets of immunosuppressive agents in podocytes. Kidney Res. Clin. Pract 34, 69–75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shalhoub RJ Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet 2, 556–560 (1974). [DOI] [PubMed] [Google Scholar]
- 78.Kemper MJ, Zepf K, Klaassen I, Link A & Muller-Wiefel DE Changes of lymphocyte populations in pediatric steroid-sensitive nephrotic syndrome are more pronounced in remission than in relapse. Am. J. Nephrol 25, 132–137 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Shao XS et al. The prevalence of Th17 cells and FOXP3 regulate T cells (Treg) in children with primary nephrotic syndrome. Pediatr. Nephrol 24, 1683–1690 (2009). [DOI] [PubMed] [Google Scholar]
- 80.Araya CE et al. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr. Nephrol 21, 603–610 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Lai KW et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J. Am. Soc. Nephrol 18, 1476–1485 (2007). [DOI] [PubMed] [Google Scholar]
- 82.Liu LL et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin. Immunol 139, 314–320 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Tsuji S et al. Regulatory T cells and CTLA-4 in idiopathic nephrotic syndrome. Pediatr. Int 59, 643–646 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Hashimura Y et al. Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatr. Nephrol 24, 1181–1186 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Iijima K et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384, 1273–1281 (2014). [DOI] [PubMed] [Google Scholar]
- 86.Ruggenenti P et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J. Am. Soc. Nephrol 25, 850–863 (2014).A study establishing the efficacy of rituximab for treatment of FRNS.
- 87.Fornoni A et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl Med 3, 85ra46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonanni A, Rossi R, Murtas C & Ghiggeri GM Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep. 2015, bcr2015210208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim AH et al. B cell-derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight 2, e81836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dantal J et al. Antihuman immunoglobulin affinity immunoadsorption strongly decreases proteinuria in patients with relapsing nephrotic syndrome. J. Am. Soc. Nephrol 9, 1709–1715 (1998). [DOI] [PubMed] [Google Scholar]
- 91.Bhatia D et al. Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr. Res 84, 520–526 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Colucci M et al. Atypical IgM on T cells predict relapse and steroid dependence in idiopathic nephrotic syndrome. Kidney Int. 96, 971–982 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Savin VJ et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med 334, 878–883 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Ali AA et al. Minimal-change glomerular nephritis. Normal kidneys in an abnormal environment? Transplantation 58, 849–852 (1994). [PubMed] [Google Scholar]
- 95.Delville M et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci. Transl Med 6, 256ra136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei C et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med 17, 952–960 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hahm E et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat. Med 23, 100–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maas RJ, Deegens JK & Wetzels JF Permeability factors in idiopathic nephrotic syndrome: historical perspectives and lessons for the future. Nephrol. Dial. Transpl 29, 2207–2216 (2014). [DOI] [PubMed] [Google Scholar]
- 99.Clement LC et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med 17, 117–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cara-Fuentes G et al. Angiopoietin-like-4 and minimal change disease. PLoS ONE 12, e0176198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandra P & Kopp JB Viruses and collapsing glomerulopathy: a brief critical review. Clin. Kidney J 6, 1–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cohen SD, Kopp JB & Kimmel PL Kidney diseases associated with human immunodeficiency virus infection. N. Engl. J. Med 377, 2363–2374 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Migliorini A et al. The antiviral cytokines IFN-α and IFN-β modulate parietal epithelial cells and promote podocyte loss: implications for IFN toxicity, viral glomerulonephritis, and glomerular regeneration. Am. J. Pathol 183, 431–440 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Ekrikpo UE et al. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS ONE 13, e0195443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.An P et al. Impact of APOL1 genetic variants on HIV-1 infection and disease progression. Front. Immunol 10, 53 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Angeletti A, Cantarelli C & Cravedi P HCV-associated nephropathies in the era of direct acting antiviral agents. Front. Med 6, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta A & Quigg RJ Glomerular diseases associated with hepatitis B and C. Adv. Chronic Kidney Dis 22, 343–351 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Sanchez C, Fenves A & Schwartz J Focal segmental glomerulosclerosis and parvovirus B19. Proc. (Bayl. Univ. Med. Cent.) 25, 20–22 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tanawattanacharoen S, Falk RJ, Jennette JC & Kopp JB Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am. J. Kidney Dis 35, 1166–1174 (2000). [DOI] [PubMed] [Google Scholar]
- 110.Nasr SH & Kopp JB COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int. Rep 5, 759–761 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwiatkowska E, Golembiewska E, Ciechanowski K & Kedzierska K Minimal-change disease secondary to Borrelia burgdorferi infection. Case Rep. Nephrol 2012, 294532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bartlett CS, Jeansson M & Quaggin SE Vascular growth factors and glomerular disease. Annu. Rev. Physiol 78, 437–461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Craici IM, Wagner SJ, Weissgerber TL, Grande JP & Garovic VD Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int. 86, 275–285 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ollero M & Sahali D Inhibition of the VEGF signalling pathway and glomerular disorders. Nephrol. Dial. Transpl 30, 1449–1455 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Izzedine H et al. Kidney diseases associated with anti-vascular endothelial growth factor (VEGF): an 8-year observational study at a single center. Medicine 93, 333–339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Markowitz GS et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J. Am. Soc. Nephrol 12, 1164–1172 (2001). [DOI] [PubMed] [Google Scholar]
- 117.Aghajan M et al. Antisense oligonucleotide treatment ameliorates IFN-γ-induced proteinuria in APOL1-transgenic mice. JCI Insight 4, e126124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hurcombe JA et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat. Commun 10, 403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakarcan A, Thomas DB, O’Reilly KP & Richards RW Lithium-induced nephrotic syndrome in a young pediatric patient. Pediatr. Nephrol 17, 290–292 (2002). [DOI] [PubMed] [Google Scholar]
- 120.Xu W, Ge Y, Liu Z & Gong R Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: implications for the protective effect of low-dose lithium in podocytopathy. Am. J. Pathol 184, 2742–2756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Letavernier E et al. High sirolimus levels may induce focal segmental glomerulosclerosis de novo. Clin. J. Am. Soc. Nephrol 2, 326–333 (2007). [DOI] [PubMed] [Google Scholar]
- 122.Puelles VG et al. mTOR-mediated podocyte hypertrophy regulates glomerular integrity in mice and humans. JCI Insight 4, e99271 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muller-Krebs S et al. Cellular effects of everolimus and sirolimus on podocytes. PLoS ONE 8, e80340 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mulay SR et al. Podocyte loss involves MDM2-driven mitotic catastrophe. J. Pathol 230, 322–335 (2013). [DOI] [PubMed] [Google Scholar]
- 125.Kaysen GA, Gambertoglio J, Jimenez I, Jones H & Hutchison FN Effect of dietary protein intake on albumin homeostasis in nephrotic patients. Kidney Int. 29, 572–577 (1986). [DOI] [PubMed] [Google Scholar]
- 126.Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC & Yap SH Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Invest 79, 1635–1641 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hinrichs GR, Jensen BL & Svenningsen P Mechanisms of sodium retention in nephrotic syndrome. Curr. Opin. Nephrol. Hypertens 29, 207–212 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Bockenhauer D Over- or underfill: not all nephrotic states are created equal. Pediatr. Nephrol 28, 1153–1156 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Vande Walle JG, Donckerwolcke RA & Koomans HA Pathophysiology of edema formation in children with nephrotic syndrome not due to minimal change disease. J. Am. Soc. Nephrol 10, 323–331 (1999). [DOI] [PubMed] [Google Scholar]
- 130.Vande Walle JG et al. Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. Lancet 346, 148–152 (1995). [DOI] [PubMed] [Google Scholar]
- 131.Yamauchi H & Hopper J Jr, Hypovolemic shock and hypotension as a complication in the nephrotic syndrome. report of ten cases. Ann. Intern. Med 60, 242–254 (1964). [DOI] [PubMed] [Google Scholar]
- 132.Chen TY, Huang CC & Tsao CJ Hemostatic molecular markers in nephrotic syndrome. Am. J. Hematol 44, 276–279 (1993). [DOI] [PubMed] [Google Scholar]
- 133.Loscalzo J Venous thrombosis in the nephrotic syndrome. N. Engl. J. Med 368, 956–958 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Singhal R & Brimble KS Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb. Res 118, 397–407 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Agrawal S, Zaritsky JJ, Fornoni A & Smoyer WE Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat. Rev. Nephrol 14, 57–70 (2018).An extensive critical reassessment of the pathogenetic mechanisms of dyslipidaemia in nephrotic syndrome.
- 136.Joven J et al. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med 323, 579–584 (1990). [DOI] [PubMed] [Google Scholar]
- 137.Clement LC et al. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat. Med 20, 37–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iorember F & Aviles D Anemia in nephrotic syndrome: approach to evaluation and treatment. Pediatr. Nephrol 32, 1323–1330 (2017). [DOI] [PubMed] [Google Scholar]
- 139.Selewski DT et al. Vitamin D in incident nephrotic syndrome: a Midwest Pediatric Nephrology Consortium study. Pediatr. Nephrol 31, 465–472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alwadhi RK, Mathew JL & Rath B Clinical profile of children with nephrotic syndrome not on glucorticoid therapy, but presenting with infection. J. Paediatr. Child. Health 40, 28–32 (2004). [DOI] [PubMed] [Google Scholar]
- 141.Wilfert CM & Katz SL Etiology of bacterial sepsis in nephrotic children 1963–1967. Pediatrics 42, 840–843 (1968). [PubMed] [Google Scholar]
- 142.Ballow M, Kennedy TL III, Gaudio KM, Siegel NJ & McLean RH Serum hemolytic factor D values in children with steroid-responsive idiopathic nephrotic syndrome. J. Pediatr 100, 192–196 (1982). [DOI] [PubMed] [Google Scholar]
- 143.Anderson DC, York TL, Rose G & Smith CW Assessment of serum factor B, serum opsonins, granulocyte chemotaxis, and infection in nephrotic syndrome of children. J. Infect. Dis 140, 1–11 (1979). [DOI] [PubMed] [Google Scholar]
- 144.Vehaskari VM & Rapola J Isolated proteinuria: analysis of a school-age population. J. Pediatr 101, 661–668 (1982). [DOI] [PubMed] [Google Scholar]
- 145.Trautmann A, Lipska-Zietkiewicz BS & Schaefer F Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet Registry. Front. Pediatr 6, 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Waldman M et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin. J. Am. Soc. Nephrol 2, 445–453 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Korbet SM & Whittier WL Management of adult minimal change disease. Clin. J. Am. Soc. Nephrol 14, 911–913 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hogg RJ et al. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105, 1242–1249 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Leung AK, Wong AH & Barg SS Proteinuria in children: evaluation and differential diagnosis. Am. Fam. Physician 95, 248–254 (2017). [PubMed] [Google Scholar]
- 150.Hama T et al. Renal biopsy criterion in children with asymptomatic constant isolated proteinuria. Nephrol. Dial. Transpl 27, 3186–3190 (2012). [DOI] [PubMed] [Google Scholar]
- 151.Lee YM et al. Analysis of renal biopsies performed in children with abnormal findings in urinary mass screening. Acta Paediatr 95, 849–853 (2006). [DOI] [PubMed] [Google Scholar]
- 152.Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 13, 159–165 (1978). [DOI] [PubMed] [Google Scholar]
- 153.Santin S et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol 6, 1139–1148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gribouval O et al. Identification of genetic causes for sporadic steroid-resistant nephrotic syndrome in adults. Kidney Int. 94, 1013–1022 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Connaughton DM et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 95, 914–928 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mason AE et al. Response to first course of intensified immunosuppression in genetically-stratified steroid resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol 10.2215/CJN.13371019 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Radhakrishnan J & Cattran DC The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines–application to the individual patient. Kidney Int. 82, 840–856 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Zhao J & Liu Z Treatment of nephrotic syndrome: going beyond immunosuppressive therapy. Pediatr. Nephrol 35, 569–579 (2020). [DOI] [PubMed] [Google Scholar]
- 159.Mak SK, Short CD & Mallick NP Long-term outcome of adult-onset minimal-change nephropathy. Nephrol. Dial. Transpl 11, 2192–2201 (1996). [DOI] [PubMed] [Google Scholar]
- 160.Korbet SM Treatment of primary FSGS in adults. J. Am. Soc. Nephrol 23, 1769–1776 (2012). [DOI] [PubMed] [Google Scholar]
- 161.Troyanov S et al. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J. Am. Soc. Nephrol 16, 1061–1068 (2005). [DOI] [PubMed] [Google Scholar]
- 162.Wehrmann M et al. Long-term prognosis of focal sclerosing glomerulonephritis. An analysis of 250 cases with particular regard to tubulointerstitial changes. Clin. Nephrol 33, 115–122 (1990). [PubMed] [Google Scholar]
- 163.Chitalia VC, Wells JE, Robson RA, Searle M & Lynn KL Predicting renal survival in primary focal glomerulosclerosis from the time of presentation. Kidney Int. 56, 2236–2242 (1999). [DOI] [PubMed] [Google Scholar]
- 164.Trautmann A et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J. Am. Soc. Nephrol 28, 3055–3065 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bedin M et al. Human C-terminal CUBN variants associate with chronic proteinuria and normal renal function. J. Clin. Invest 130, 335–344 (2020).This paper identified a genetic form of proteinuria and podocytopathy that does not progress to ESKD.
- 166.Heeringa SF et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Invest 121, 2013–2024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Praga M et al. Nephrotic proteinuria without hypoalbuminemia: clinical characteristics and response to angiotensin-converting enzyme inhibition. Am. J. Kidney Dis 17, 330–338 (1991). [DOI] [PubMed] [Google Scholar]
- 168.Tune BM & Mendoza SA Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults. J. Am. Soc. Nephrol 8, 824–832 (1997). [DOI] [PubMed] [Google Scholar]
- 169.Murnaghan K, Vasmant D & Bensman A Pulse methylprednisolone therapy in severe idiopathic childhood nephrotic syndrome. Acta Paediatr. Scand 73, 733–739 (1984). [DOI] [PubMed] [Google Scholar]
- 170.Hodson EM, Hahn D & Craig JC Corticosteroids for the initial episode of steroid-sensitive nephrotic syndrome. Pediatr. Nephrol 30, 1043–1046 (2015). [DOI] [PubMed] [Google Scholar]
- 171.Larkins N, Kim S, Craig J & Hodson E Steroid-sensitive nephrotic syndrome: an evidence-based update of immunosuppressive treatment in children. Arch. Dis. Child 101, 404–408 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Palmer SC, Nand K & Strippoli GF Interventions for minimal change disease in adults with nephrotic syndrome. Cochrane Database Syst. Rev 2008, CD001537 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Black DA, Rose G & Brewer DB Controlled trial of prednisone in adult patients with the nephrotic syndrome. Br. Med. J 3, 421–426 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Medjeral-Thomas NR et al. Randomized, controlled trial of tacrolimus and prednisolone monotherapy for adults with de novo minimal change disease: a multicenter, randomized, controlled trial. Clin. J. Am. Soc. Nephrol 15, 209–218 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Remy P et al. An open-label randomized controlled trial of low-dose corticosteroid plus enteric-coated mycophenolate sodium versus standard corticosteroid treatment for minimal change nephrotic syndrome in adults (MSN Study). Kidney Int. 94, 1217–1226 (2018). [DOI] [PubMed] [Google Scholar]
- 176.Senthil Nayagam L et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol. Dial. Transpl 23, 1926–1930 (2008). [DOI] [PubMed] [Google Scholar]
- 177.Rovin BH et al. Management and treatment of glomerular diseases (part 2): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 95, 281–295 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Glassock RJ Therapy of relapsing minimal-change disease in adults: a new approach? Kidney Int. 83, 343–345 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Rydel JJ, Korbet SM, Borok RZ & Schwartz MM Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am. J. Kidney Dis 25, 534–542 (1995). [DOI] [PubMed] [Google Scholar]
- 180.Dossier C et al. Five-year outcome of children with idiopathic nephrotic syndrome: the NEPHROVIR population-based cohort study. Pediatr. Nephrol 34, 671–678 (2019). [DOI] [PubMed] [Google Scholar]
- 181.Hodson EM, Willis NS & Craig JC Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst. Rev 4, CD001533 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Yu CC et al. Abatacept in B7–1-positive proteinuric kidney disease. N. Engl. J. Med 369, 2416–2423 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Korsgaard T, Andersen RF, Joshi S, Hagstrom S & Rittig S Childhood onset steroid-sensitive nephrotic syndrome continues into adulthood. Pediatr. Nephrol 34, 641–648 (2019). [DOI] [PubMed] [Google Scholar]
- 184.Ruth EM, Kemper MJ, Leumann EP, Laube GF & Neuhaus TJ Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J. Pediatr 147, 202–207 (2005). [DOI] [PubMed] [Google Scholar]
- 185.Kyrieleis HA et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin. J. Am. Soc. Nephrol 4, 1593–1600 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lombel RM, Gipson DS & Hodson EM, Kidney Disease: Improving Global Outcomes. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr. Nephrol 28, 415–426 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Samuel S et al. Canadian society of nephrology commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis: management of nephrotic syndrome in children. Am. J. Kidney Dis 63, 354–362 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Ponticelli C et al. Cyclosporin versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicentre randomized controlled trial. Nephrol. Dial. Transpl 8, 1326–1332 (1993). [PubMed] [Google Scholar]
- 189.Sinha A et al. Efficacy and safety of mycophenolate mofetil versus levamisole in frequently relapsing nephrotic syndrome: an open-label randomized controlled trial. Kidney Int. 95, 210–218 (2019). [DOI] [PubMed] [Google Scholar]
- 190.Pravitsitthikul N, Willis NS, Hodson EM & Craig JC Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst. Rev 10, CD002290 (2013). [DOI] [PubMed] [Google Scholar]
- 191.Fujinaga S et al. Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab. Eur. J. Pediatr 172, 513–518 (2013). [DOI] [PubMed] [Google Scholar]
- 192.Dorresteijn EM et al. Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr. Nephrol 23, 2013–2020 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hogan J et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin. J. Am. Soc. Nephrol 8, 2072–2081 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kittanamongkolchai W, Cheungpasitporn W & Zand L Efficacy and safety of adrenocorticotropic hormone treatment in glomerular diseases: a systematic review and meta-analysis. Clin. Kidney J 9, 387–396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Straatmann C et al. Treatment outcome of late steroid-resistant nephrotic syndrome: a study by the Midwest Pediatric Nephrology Consortium. Pediatr. Nephrol 28, 1235–1241 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ponticelli C et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 43, 1377–1384 (1993). [DOI] [PubMed] [Google Scholar]
- 197.Segarra A et al. Efficacy and safety of ‘rescue therapy’ with mycophenolate mofetil in resistant primary glomerulonephritis – a multicenter study. Nephrol. Dial. Transpl 22, 1351–1360 (2007). [DOI] [PubMed] [Google Scholar]
- 198.Gipson DS et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 80, 868–878 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gellermann J, Ehrich JH & Querfeld U Sequential maintenance therapy with cyclosporin A and mycophenolate mofetil for sustained remission of childhood steroid-resistant nephrotic syndrome. Nephrol. Dial. Transpl 27, 1970–1978 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Delville M et al. B7–1 blockade does not improve post-transplant nephrotic syndrome caused by recurrent FSGS. J. Am. Soc. Nephrol 27, 2520–2527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Benigni A, Gagliardini E & Remuzzi G Abatacept in B7–1-positive proteinuric kidney disease. N. Engl. J. Med 370, 1261–1263 (2014). [DOI] [PubMed] [Google Scholar]
- 202.Kronbichler A et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am. J. Nephrol 39, 322–330 (2014). [DOI] [PubMed] [Google Scholar]
- 203.Trachtman H et al. Efficacy of galactose and adalimumab in patients with resistant focal segmental glomerulosclerosis: report of the font clinical trial group. BMC Nephrol. 16, 111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Trachtman H et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J. Am. Soc. Nephrol 29, 2745–2754 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gross O, Perin L & Deltas C Alport syndrome from bench to bedside: the potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol. Dial. Transpl 29 (Suppl. 4), iv124–iv130 (2014). [DOI] [PubMed] [Google Scholar]
- 206.Solanki AK et al. A novel CLCN5 mutation associated with focal segmental glomerulosclerosis and podocyte injury. Kidney Int. Rep 3, 1443–1453 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.du Moulin M et al. The mutation p.D313Y is associated with organ manifestation in Fabry disease. Clin. Genet 92, 528–533 (2017). [DOI] [PubMed] [Google Scholar]
- 208.Hu M et al. Prophylactic bilateral nephrectomies in two paediatric patients with missense mutations in the WT1 gene. Nephrol. Dial. Transpl 19, 223–226 (2004). [DOI] [PubMed] [Google Scholar]
- 209.Crew RJ, Radhakrishnan J & Appel G Complications of the nephrotic syndrome and their treatment. Clin. Nephrol 62, 245–259 (2004). [DOI] [PubMed] [Google Scholar]
- 210.Keller E, Hoppe-Seyler G & Schollmeyer P Disposition and diuretic effect of furosemide in the nephrotic syndrome. Clin. Pharmacol. Ther 32, 442–449 (1982). [DOI] [PubMed] [Google Scholar]
- 211.Hoorn EJ & Ellison DH Diuretic resistance. Am. J. Kidney Dis 69, 136–142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fallahzadeh MA et al. Acetazolamide and hydrochlorothiazide followed by furosemide versus furosemide and hydrochlorothiazide followed by furosemide for the treatment of adults with nephrotic edema: a randomized trial. Am. J. Kidney Dis 69, 420–427 (2017). [DOI] [PubMed] [Google Scholar]
- 213.Artunc F, Worn M, Schork A & Bohnert BN Proteasuria – the impact of active urinary proteases on sodium retention in nephrotic syndrome. Acta Physiol. 225, e13249 (2019). [DOI] [PubMed] [Google Scholar]
- 214.Bohnert BN et al. Aprotinin prevents proteolytic epithelial sodium channel (ENaC) activation and volume retention in nephrotic syndrome. Kidney int. 93, 159–172 (2018). [DOI] [PubMed] [Google Scholar]
- 215.Wheeler DC & Bernard DB Lipid abnormalities in the nephrotic syndrome: causes, consequences, and treatment. Am.J. Kidney Dis 23, 331–346 (1994). [DOI] [PubMed] [Google Scholar]
- 216.Llach F Hypercoagulability, renal vein thrombosis, and other thrombotic complications of nephrotic syndrome. Kidney int. 28, 429–439 (1985). [DOI] [PubMed] [Google Scholar]
- 217.Kerlin BA et al. Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: a Midwest Pediatric Nephrology Consortium (MWPNC) study. J. Pediatr 155, 105–110 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Suri D et al. Thromboembolic complications in childhood nephrotic syndrome: a clinical profile. Clin. Exp. Nephrol 18, 803–813 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Rai Mittal B, Singh S, Bhattacharya A, Prasad V & Singh B Lung scintigraphy in the diagnosis and follow-up of pulmonary thromboembolism in children with nephrotic syndrome. Clin. imaging 29, 313–316 (2005). [DOI] [PubMed] [Google Scholar]
- 220.Kelddal S, Nykjaer KM, Gregersen JW & Birn H Prophylactic anticoagulation in nephrotic syndrome prevents thromboembolic complications. BMC Nephrol. 20, 139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Close GC & Houston IB Fatal haemorrhagic chickenpox in a child on long-term steroids. Lancet 2, 480 (1981). [DOI] [PubMed] [Google Scholar]
- 222.Kamei K et al. Prospective study of live attenuated vaccines for patients with nephrotic syndrome receiving immunosuppressive agents. J. Pediatr 196, 217–222.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Bierzynska A & Saleem MA Deriving and understanding the risk of post-transplant recurrence of nephrotic syndrome in the light of current molecular and genetic advances. Pediatr. Nephrol 33, 2027–2035 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jacobs-Cacha C et al. A misprocessed form of apolipoprotein A-I is specifically associated with recurrent focal segmental glomerulosclerosis. Sci. Rep 10, 1159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kienzl-Wagner K et al. Successful management of recurrent focal segmental glomerulosclerosis. Am. J. Transpi 18, 2818–2822 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kashgary A et al. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: a systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 17, 104 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mann N et al. Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. J. Am. Soc. Nephrol 30, 201–215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Canetta PA et al. Health-related quality of life in glomerular disease. Kidney int. 95, 1209–1224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kengne-Wafo S et al. Risk factors for cyclosporin a nephrotoxicity in children with steroid-dependant nephrotic syndrome. Clin. J. Am. Soc. Nephrol 4, 1409–1416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ishikura K et al. Morbidity in children with frequently relapsing nephrosis: 10-year follow-up of a randomized controlled trial. Pediatr. Nephrol 30, 459–468 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Huscher D et al. Dose-related patterns of glucocorticoid-induced side effects. Ann. Rheum. Dis 68, 1119–1124 (2009). [DOI] [PubMed] [Google Scholar]
- 232.Hyams JS & Carey DE Corticosteroids and growth. J. Pediatr 113, 249–254 (1988). [DOI] [PubMed] [Google Scholar]
- 233.Ng JS et al. Ocular complications of paediatric patients with nephrotic syndrome. Clin. Exp. Ophthalmol 29, 239–243 (2001). [DOI] [PubMed] [Google Scholar]
- 234.Polito C, Oporto MR, Totino SF, La Manna A & Di Toro R Normal growth of nephrotic children during long-term alternate-day prednisone therapy. Acta Paediatr. Scand 75, 245–250 (1986). [DOI] [PubMed] [Google Scholar]
- 235.Hogan J et al. Effect of center practices on the choice of the first dialysis modality for children and young adults. Pediatr. Nephrol 32, 659–667 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Morello W et al. Post-transplant recurrence of steroid resistant nephrotic syndrome in children: the Italian experience. J. Nephrol 10.1007/s40620-019-00660-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gadegbeku CA et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 83, 749–756 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mariani LH et al. CureGN study rationale, design, and methods: establishing a large prospective observational study of glomerular disease. Am. J. Kidney Dis 73, 218–229 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang CS et al. Ofatumumab for the treatment of childhood nephrotic syndrome. Pediatr. Nephrol 32, 835–841 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Blair HA & Duggan ST Belimumab: a review in systemic lupus erythematosus. Drugs 78, 355–366 (2018). [DOI] [PubMed] [Google Scholar]
- 241.KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney International Supplements (Elsevier, 2020). [Google Scholar]
- 242.Trautmann A et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr. Nephrol 35, 1529–1561 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fakhouri F et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am. J. Kidney Dis 41, 550–557 (2003). [DOI] [PubMed] [Google Scholar]
- 244.Abeyagunawardena AS, Hindmarsh P & Trompeter RS Adrenocortical suppression increases the risk of relapse in nephrotic syndrome. Arch. Dis. Child. 92, 585–588 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Niaudet P Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. French Society of Pediatric Nephrology. J. Pediatr 125, 981–986 (1994). [DOI] [PubMed] [Google Scholar]
- 246.Ishikura K et al. Two-year follow-up of a prospective clinical trial of cyclosporine for frequently relapsing nephrotic syndrome in children. Clin. J. Am. Soc. Nephrol 7, 1576–1583 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gellermann J et al. Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J. Am. Soc. Nephrol 24, 1689–1697 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Braun N et al. Immunosuppressive treatment for focal segmental glomerulosclerosis in adults. Cochrane Database Syst. Rev 2008, CD003233 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sinha MD, MacLeod R, Rigby E & Clark AG Treatment of severe steroid-dependent nephrotic syndrome (SDNS) in children with tacrolimus. Nephrol. Dial. Transpl 21, 1848–1854 (2006). [DOI] [PubMed] [Google Scholar]
- 250.Bock ME, Cohn RA & Ali FN Treatment of childhood nephrotic syndrome with long-term, low-dose tacrolimus. Clin. Nephrol 79, 432–438 (2013). [DOI] [PubMed] [Google Scholar]
- 251.Sobiak J et al. Monitoring of mycophenolate mofetil metabolites in children with nephrotic syndrome and the proposed novel target values of pharmacokinetic parameters. Eur. J. Pharm. Sci 77, 189–196 (2015). [DOI] [PubMed] [Google Scholar]
- 252.Perez-Aytes A et al. In utero exposure to mycophenolate mofetil: a characteristic phenotype? Am.J. Med. Genet. A 146A, 1–7 (2008). [DOI] [PubMed] [Google Scholar]
- 253.Kamei K et al. Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol. Dial. Transpl 30, 91–96 (2015). [DOI] [PubMed] [Google Scholar]
- 254.McGrogan A, Franssen CF & de Vries CS The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol. Dial. Transpl 26, 414–430 (2011). [DOI] [PubMed] [Google Scholar]
- 255.Cunningham A, Benediktsson H, Muruve DA, Hildebrand AM & Ravani P Trends in biopsy-based diagnosis of kidney disease: a population study. Can. J. Kidney Health Dis 10.1177/2054358118799690 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Woo KT et al. A global evolutionary trend of the frequency of primary glomerulonephritis over the past four decades. Kidney Dis. 5, 247–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.O’Shaughnessy MM et al. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol. Dial. Transpl 33, 661–669 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Polito MG, de Moura LA & Kirsztajn GM An overview on frequency of renal biopsy diagnosis in Brazil: clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol. Dial. Transpl 25, 490–496 (2010). [DOI] [PubMed] [Google Scholar]
- 259.Perkowska-Ptasinska A et al. Clinicopathologic correlations of renal pathology in the adult population of Poland. Nephrol. Dial. Transpl 32, ii209–ii218 (2017). [DOI] [PubMed] [Google Scholar]
- 260.Zink CM et al. Trends of renal diseases in Germany: review of a regional renal biopsy database from 1990 to 2013. Clin. Kidney J 12, 795–800 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ozturk S et al. Demographic and clinical characteristics of primary glomerular diseases in Turkey. Int. Urol. Nephrol 46, 2347–2355 (2014). [DOI] [PubMed] [Google Scholar]
- 262.Rychlik I et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol. Dial. Transpl 19, 3040–3049 (2004). [DOI] [PubMed] [Google Scholar]
- 263.Lovric S, Ashraf S, Tan W & Hildebrandt F Genetic testing in steroid-resistant nephrotic syndrome: when and how? Nephrol. Dial. Transpl 31, 1802–1813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.D’Agati VD, Fogo AB, Bruijn JA & Jennette JC Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am. J. Kidney Dis 43, 368–382 (2004). [DOI] [PubMed] [Google Scholar]
- 265.Fogo AB Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol 11, 76–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.D’Agati VD, Kaskel FJ & Falk RJ Focal segmental glomerulosclerosis. N. Engl. J. Med 365, 2398–2411 (2011). [DOI] [PubMed] [Google Scholar]
- 267.Hommos MS et al. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int. 93, 1175–1182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Deegens JK et al. Podocyte foot process effacement as a diagnostic tool in focal segmental glomerulosclerosis. Kidney Int 74, 1568–1576 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.