Abstract
Purpose:
To investigate whether T2-weighted magnetic resonance imaging (MRI) findings could improve upon established prognostic indicators of metastatic disease and cancer-specific survival.
Materials and Methods:
For a cohort of 3,406 consecutive men who underwent prostate MRI before definitive prostatectomy (n=2,160) or radiotherapy (n=1,246) between 2001 and 2006, T2-weighted MRI exams were retrospectively interpreted and categorized as (I) No focal suspicious lesion; (II) organ-confined focal lesion; (III) focal lesion with extraprostatic extension; or (IV) focal lesion with seminal vesicle invasion. Clinical risk was recorded based on European Association of Urology (EAU) guidelines and the Cancer of the Prostate Risk Assessment (CAPRA) scoring system. Survival probabilities and c-indices were estimated using Cox models and inverse probability censoring weights, respectively.
Results:
The median follow-up was 10.8 years (IQR: 8.6-13.0 years). Higher MRI categories were associated with a higher likelihood of developing metastases (hazard ratios: 3.5-18.1, p<0.001 for all MRI categories) and prostate cancer death (hazard ratios: 3.1-29.7, p<0.001-0.025); these associations were statistically independent of EAU risk categories, CAPRA scores, and treatment type (surgery vs. radiation). Combining EAU risk or CAPRA scores with MRI categories significantly improved prognostication of metastases (c-indices: EAU: 0.798, EAU+MRI: 0.872; CAPRA: 0.808, CAPRA+MRI: 0.877) and prostate cancer death (c-indices: EAU: 0.813, EAU+MRI: 0.889; CAPRA: 0.814, CAPRA+MRI: 0.892) (p<0.001 for all).
Conclusion:
MRI findings of localized prostate cancer are associated with clinically relevant long-term oncologic outcomes. Combining MRI and clinicopathologic data results in more accurate prognostication, which could facilitate individualized patient management.
Keywords: Prostate Cancer, Magnetic Resonance Imaging, Prognosis, Risk Assessment, Disease-Free Survival
Introduction
With more than 1.2 million new cases diagnosed each year worldwide 1 prostate cancer poses a genuine threat to men’s health and an economic challenge for health care systems across the world. Growing understanding of the biological heterogeneity of this disease points to the importance of accurate risk assessment for individualized management decision-making at the time of diagnosis. Most men with prostate cancer are unlikely to die of the disease and tools that can accurately predict who will develop metastatic or lethal prostate cancer are highly sought after. Traditionally, such prognostication is done using a combination of digital rectal examination, prostate biopsy Gleason grades, and measurement of blood levels of prostate-specific antigen (PSA) 2. Comprehensive statistical models have corroborated the prognostic capabilities of these three biomarkers 3, and in current clinical practice, they are used to stratify patients into low-, intermediate- or high-risk categories. The prognostic precision of these risk stratification algorithms, however, is not optimal 4. A systematic literature review on more than 50,000 patients, for example, showed that the reported recurrence-free survival rates for patients with ‘high-risk’ disease ranged between less than 30% and more than 90% 5.
Magnetic resonance imaging (MRI) is widely considered the most accurate imaging modality for guiding “targeted” prostate biopsies 6. The literature contains thousands of articles focusing on prostate MRI, almost all reporting on its ability to detect tumors of different sizes, histologic patterns, and other surrogates of cancer aggressiveness, using histopathologic findings as reference standards. However, little is known about the relationship between MRI findings and long-term oncologic outcomes and whether MRI could also be used as an adjunct to established risk-stratification tools. In this study, we report associations between pre-treatment prostate MRI findings and the development of metastatic disease and cancer-specific survival after radical treatment of clinically localized prostate cancer.
Patients and Methods
We conducted a HIPAA-compliant and IRB-approved retrospective, single-center cohort study of consecutive men with clinically localized adenocarcinoma of the prostate who underwent prostate MRI within 180 days before the initiation of cancer therapy with curative intent by radical prostatectomy or radiation therapy at a tertiary care academic cancer center between 2001 and 2006. Patients with locally advanced or metastatic disease on clinical staging (i.e. cT4, cN1, or cM1) were not included in our database query. The need for written informed consent was waived by the IRB. We excluded all patients whose imaging and/or therapeutic protocol deviated from what was considered standard of care during the study period, including any neoadjuvant systemic therapy before prostatectomy (n=73), neoadjuvant cytotoxic chemotherapy before radiation therapy (n=10), and the lack of an endorectal coil on the MRI (n=67). We also excluded 222 patients in whom hormonal therapy was initiated before the MRI examination, as it is documented that this substantially alters the MRI appearance of normal as well as cancerous prostate tissue 7. In addition, 47 individuals with no post-therapeutic follow-up data were excluded. The final study cohort consisted of 3,406 men. A flow chart detailing patient selection is provided in Supplementary Figure 1.
MRI technique and interpretation
MRI examinations were performed on a 1.5-Tesla magnet with a body coil for excitation and an endorectal coil for signal reception and included prostate-focused T2-weighted images in the transverse and coronal and/or sagittal planes. Detailed MRI acquisition parameters are provided in Supplementary Table 1. For the purpose of this study, each MRI scan was re-interpreted by one of seven sub-specialized genitourinary radiologists according to the Prostate Imaging Reporting and Data System (PI-RADS) version 2 8. PI-RADS provides standardized consensus diagnostic criteria for the interpretation of T2-weighted prostate MRI sequences rendering a 5-point score that indicates the likelihood of clinically significant prostate cancer. For cases with a focal suspicious lesion on T2-weighted MRI (i.e. score of 4 or 5), the likelihood of extraprostatic tumor extension and seminal vesicle invasion was assessed on 5-point Likert scales adherent to previously described diagnostic criteria 9. Study radiologists were blinded to all clinical, histopathologic, and outcome data. For our analyses, patients were classified into one of four MRI categories broadly following the prostate cancer staging guidelines of the American Joint Committee on Cancer 10, as detailed in Table 1.
Table 1:
Description of the four MRI categories.
| MRI Category |
Description | PI-RADS T2w-score |
Extraprostatic Extension |
Seminal Vesicle Invasion |
|---|---|---|---|---|
| I | No focal suspicious lesion | 1, 2, or 3 | Absent* | Absent* |
| II | Organ-confined suspicious lesion | 4 or 5 | Absent* | Absent* |
| III | Suspicious lesion with EPE, no SVI | 4 or 5 | Present# | Absent* |
| IV | Suspicious lesion with EPE and SVI | 4 or 5 | Present# | Present# |
EPE: Extraprostatic Extension, SVI: Seminal Vesicle Invasion
Level of Suspicion ≤3 out of 5
Level of Suspicion ≥4 out of 5
Biopsy, clinical risk categories, and therapy
The median number of transrectal ultrasound-guided biopsy cores was 12 (range: 2-28), and less than 6 in 70 patients (2.1%). All biopsy samples taken at our institution (n=408/3,406, 12.0%) and 99.5% of samples taken at an outside institution (n=2,984/2,998) were interpreted by pathologists at our institution; while these biopsy interpretations largely took place before the changes in Gleason grading advanced by the International Society of Urologic Pathology in 2005, some of the central modifications–including assigning pattern 4 to cribriform glands–were in routine use at our institution during the period studied. Clinical tumor stage was assessed on digital rectal examination and recorded as documented in the medical record. Disease was stratified clinically based on the European Association of Urology (EAU) guidelines (i.e. low, intermediate, or high risk) 11, the Cancer of the Prostate Risk Assessment (CAPRA) score 3, and National Comprehensive Cancer Network (NCCN) guidelines 12; these risk stratification algorithms are detailed in supplementary Table 2. All patients were treated with curative intent by radical prostatectomy (n=2,160, 63.4%) or radiotherapy with external beam radiation (n=800, 23.5%), brachytherapy (n=244, 7.2%), or a combination of the two (n=202, 5.9%). Four-hundred-fifty-four patients in the radiotherapy cohort (n=454/1,246; 36.4%) received androgen-deprivation therapy after prostate MRI was performed.
Follow-up and endpoints
Follow-up appointments were scheduled as per standard of care, 3 months after completion of therapy, then every 6 months until year 5, and annually thereafter. Endpoints were development of metastatic disease, chosen as recommended by the Intermediate Clinical Endpoints of Cancer of the Prostate working group for studying localized prostate cancer 13, and prostate cancer-specific survival, as documented in the patient’s medical record.
Statistical considerations
Cox proportional hazards regression was used to estimate hazard ratios corresponding to EAU categories/CAPRA scores and MRI risk categories, separately, as well as in a model with both and the initial treatment. P-values for each variable in these models were generated from a score test. Predictive accuracy of the models was assessed by the concordance index (c-index), which was estimated using inverse probability censoring weights 14 to minimize censoring bias 15. C-indices were compared using 1000 bootstrap samples and Efron’s asymptotic significance level 16.
Results
Study Cohort
The median patient age at the time of treatment was 62.2 years (IQR: 56.5-68.0 years). According to the EAU criteria, 1,471 patients (43.2 %) had low-risk, 1,347 (39.6 %) had intermediate-risk, and 588 (17.3 %) had high-risk disease. The median CAPRA score was 2 (IQR:2-4). On prostate MRI no focal suspicious lesion was detected in 1,885 (55.3%) cases (MRI category I); of the 1,521 (44.7%) patients with focal suspicious lesion(s), 838 (24.6%) had no evidence of extraprostatic tumor spread on MRI (MRI category II). In 568 (16.7%) cases, extraprostatic tumor spread was present (MRI category III), and 115 (3.4%) cases had tumor extension into the seminal vesicles (MRI category IV). Table 2 provides more detailed descriptive statistics. The median follow-up after therapy start was 10.8 years (IQR: 8.6-13.0 years).
Table 2:
Study cohort descriptors.
| Parameter | Value |
|---|---|
|
Age - years Median [IQR] (range) |
62.2 [56.5, 68.0] (32.9, 89.2) |
| Clinical Stage on Digital Rectal Examination (%) | |
| cT1 | 2,153 (63.2) |
| cT2 | 1,133 (33.3) |
| cT3 | 119 (3.5) |
| Not available | 1 (0.03) |
|
Prostate-Specific Antigen (ng/ml) Median [IQR] (range) |
5.4 [4.3, 7.9] (0.2, 188.0) |
| Biopsy Gleason Score (%) | |
| <=6 | 1,808 (53.1) |
| 7 | 1,263 (37.1) |
| 3+4 | 859 (25.2) |
| 4+3 | 404 (11.9) |
| 8 | 215 (6.3) |
| >=9 | 120 (3.5) |
| EAU risk (%) | |
| Low | 1,471 (43.2) |
| Intermediate | 1,347 (39.6) |
| High | 588 (17.3) |
|
CAPRA score Median [IQR] (range) |
2 [1, 4] (0, 10) |
|
Time between MRI and Treatment - days Median [IQR] (range) |
13 [30, 60] (0, 180) |
| MRI category (%) | |
| I: No focal suspicious lesion | 1,885 (55.3) |
| II: Lesion present, no EPE, no SVI | 838 (24.6) |
| III: Lesion present, with EPE, no SVI | 568 (16.7) |
| IV: Lesion present, with EPE, with SVI | 115 (3.4) |
CAPRA: Cancer of the Prostate Risk Assessment, EAU: European Association of Urology, EPE: Extraprostatic Extension, IQR: Inter-Quartile Range, MRI: Magnetic Resonance Imaging, SVI: Seminal Vesicle Invasion
Metastatic Disease
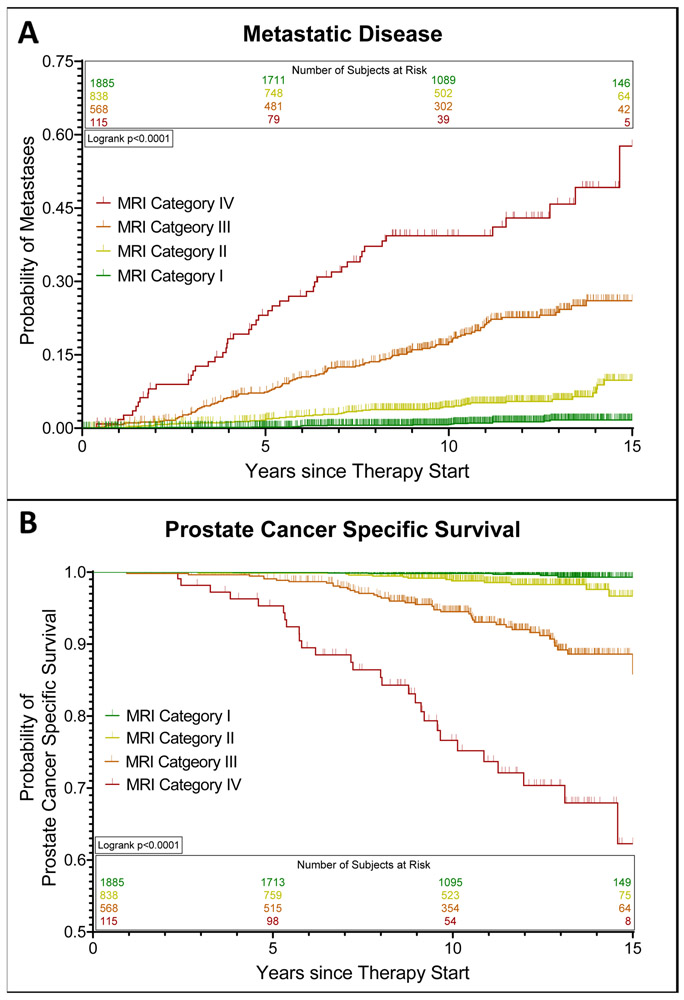
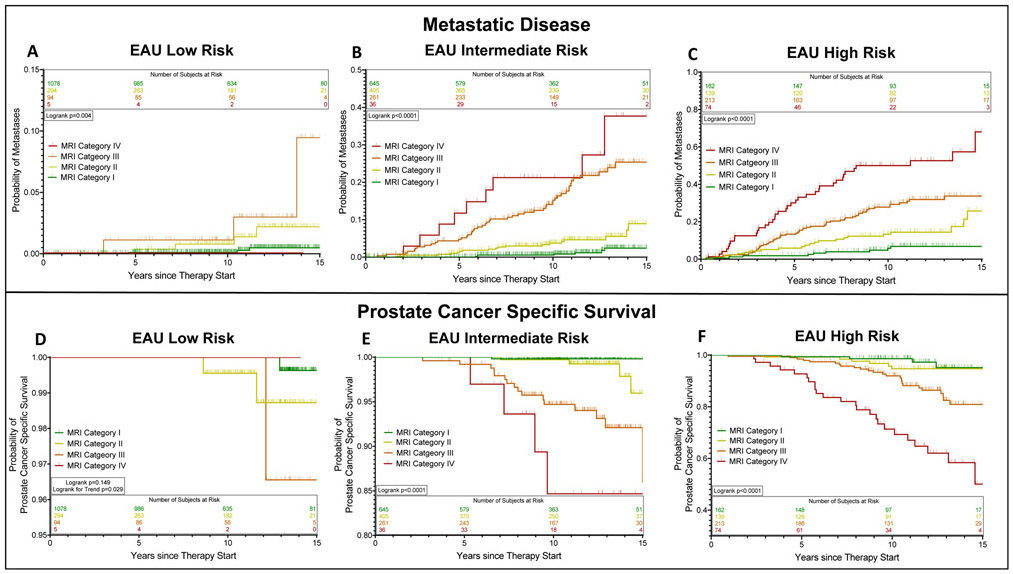
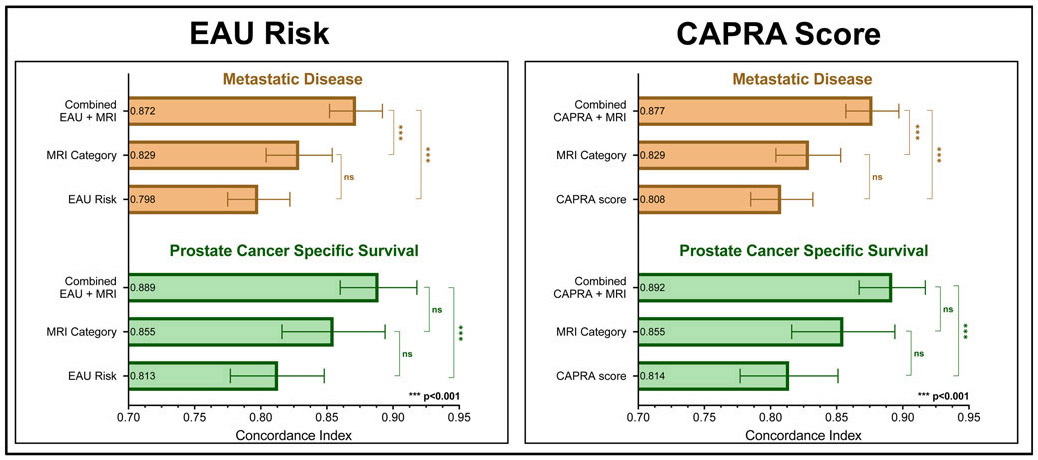
The overall 5-, 10-, and 15-year probabilities of developing metastatic disease were 2.5±0.3%, 5.7±0.4%, and 10.0±0.8%, respectively. A higher MRI category was associated with a higher likelihood of metastatic disease at 15 years (MRI category I: 1.7%±0.4%, MRI category II: 9.8±1.7%, MRI category III: 26.0±2.5%, MRI category IV: 57.7±9.3%; p<0.0001; Figure 1a). The association between the MRI category and development of metastases was present in all EAU risk categories, and strongest in intermediate- and high-risk patients (Figure 2a-c). On the multivariate analyses, this association was independent of EAU risk categories and CAPRA scores, respectively, and also independent of the type of cancer therapy (surgery vs. radiotherapy) (Table 3, Figure 3). Statistical models combining the MRI categories with EAU risk and CAPRA scores, respectively, yielded a more precise estimate of developing metastatic disease than did either model alone (c-indices: EAU: 0.798, EAU+MRI: 0.872, p<0.001; CAPRA: 0.808, CAPRA+MRI: 0.877, p<0.001) (Figure 4).
Figure 1:
Estimated probabilities of developing metastatic disease (A) and prostate cancer-specific survival (B) after definitive treatment of localized prostate cancer stratified by MRI category.
Figure 2:
Estimated probabilities of developing metastatic disease (A-C) and prostate cancer-specific survival (D-F) after radical treatment of localized prostate cancer stratified by MRI category, separately for EAU low-risk (A, D), intermediate-risk (B, E), and high-risk (C, F) patients.
Table 3:
Multivariate Cox regression models of metastasis-free and cancer-specific survival with EAU risk categories (A), and the CAPRA scores (B) as benchmark prognosticators. Numbers are presented graphically in Figure 3.
| Metastasis-free Survival | Cancer-Specific Survival | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio [95% CL] |
p-value | Hazard Ratio [95% CL] |
p-value | |||
| A | EAU Risk | Low | Reference | Reference | ||
| Intermediate | 5.59 [3.27 – 9.58] |
<.0001 | 3.31 [1.13 – 9.71] |
0.029 | ||
| High | 11.63 [6.65-20.31] |
<.0001 | 8.85 [3.05-25.70] |
<.0001 | ||
| MRI Category | I | Reference | Reference | |||
| II | 3.53 [2.06 – 6.13] |
<.0001 | 3.10 [1.51 – 8.35] |
0.025 | ||
| III | 9.53 [6.14 – 16.74] |
<.0001 | 10.93 [4.50 – 26.54] |
<.0001 | ||
| IV | 18.05 [11.30 – 34.59] |
<.0001 | 29.68 [11.68 – 75.38] |
<.0001 | ||
| Treatment | Surgery | Reference | Reference | |||
| Radiotherapy | 1.15 [0.88 – 1.51] |
0.311 | 1.64 [1.07 – 2.49] |
0.022 | ||
| B | CAPRA Score | 1.41 [1.32 – 1.51] |
<0.001 | 1.32 [1.19 – 1.46] |
<0.001 | |
| MRI Category | I | Reference | Reference | |||
| II | 3.72 [2.16 – 6.42] |
<0.001 | 3.40 [1.27 – 9.13] |
0.015 | ||
| III | 9.90 [5.94 – 16.47] |
<0.001 | 12.01 [4.93 – 29.24] |
<0.001 | ||
| IV | 16.70 [9.35 – 29.83] |
<0.001 | 29.66 [11.37 – 77.38] |
<0.001 | ||
| Treatment | Surgery | Reference | Reference | |||
| Radiotherapy | 0.95 [0.72 – 1.26] |
0.723 | 1.46 [0.95 – 2.25] |
0.084 | ||
CAPRA: Cancer of the Prostate Risk Assessment CL: Confidence Limits, EAU: European Association of Urology, MRI: Magnetic Resonance Imaging
Figure 3:
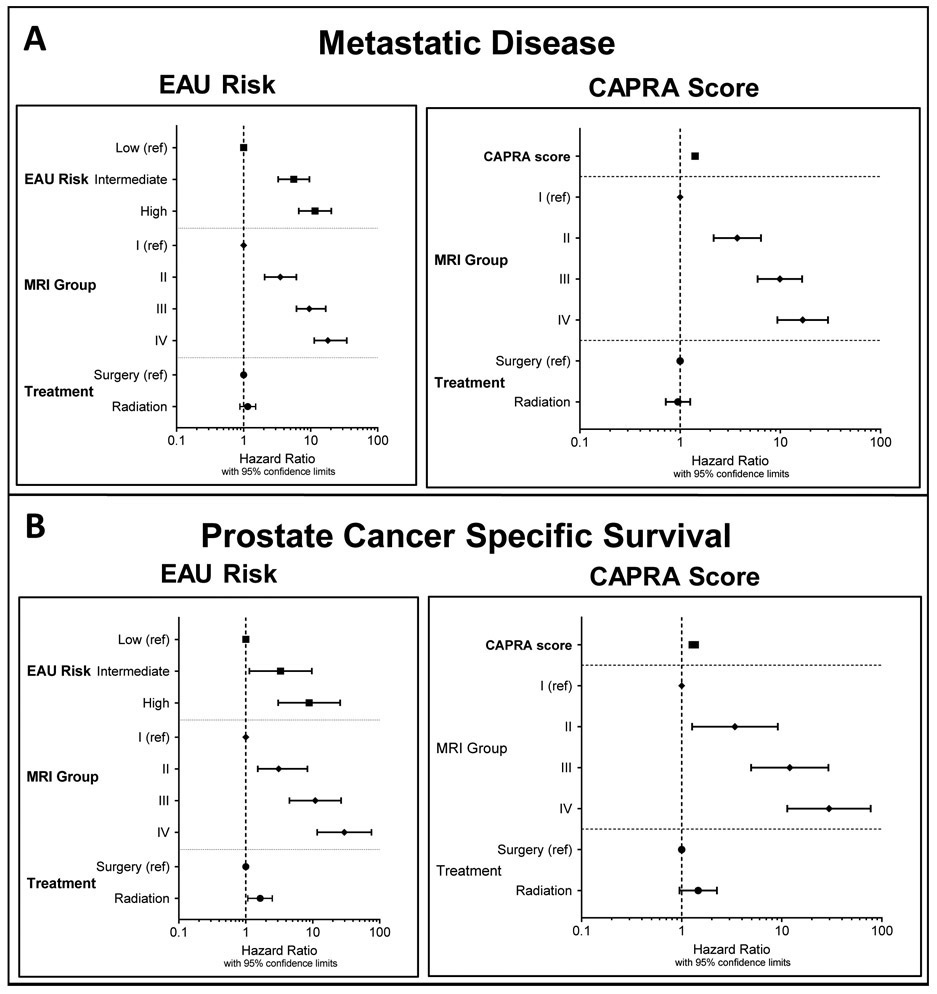
Hazard ratios (with 95% confidence limits) from multivariate Cox models estimating the risk of developing metastatic disease (A) and prostate cancer-specific survival (B), using EAU risk categories and the CAPRA score as benchmark prognosticators, respectively. Detailed numbers are provided in Table 3.
Figure 4:
Comparison of concordance indices describing the prognostic precision of Cox models of metastasis-free (brown) and prostate cancer-specific (green) survival. Detailed numbers are given in Supplementary Table 3.
Prostate Cancer-Specific Survival
The overall 5-, 10-, and 15-year probabilities of prostate cancer-specific survival were 99.6±0.1%, 97.9±0.3%, and 95.3±0.6%, respectively. Patients in higher MRI categories were more likely to die from prostate cancer: The 15-year cancer-specific survival probabilities for patients with an MRI category of I, II, III, and IV were 99.3±0.3%, 96.7±1.3%, 85.8±2.6%, and 62.3±7.4%, respectively (p<0.0001) (Figure 1b). This association between MRI category and cancer-specific survival was statistically significant in patients with EAU intermediate- and high-risk disease (Figure 2e-f) and showed a significant trend in those with low-risk disease (Figure 2d). In the multivariate survival analyses, MRI categories were associated with the risk of prostate cancer death, independently of EAU risk and CAPRA score, respectively, and independently of type of cancer therapy (Table 3, Figure 3). Combined statistical models were more accurate in estimating the probability of prostate cancer death than any of the models alone (c-indices: EAU: 0.813, EAU+MRI: 0.889, p<0.001; CAPRA: 0.814, CAPRA+MRI: 0.892, p<0.001) (Figure 4). We observed similar results when using the risk stratification algorithm of the NCCN as benchmark prognostication model (Supplementary Tables 4 and 5).
Discussion
This study corroborates the well-documented value of combining biopsy-based Gleason grades, serum PSA levels, and digital rectal examination in patients with clinically localized prostate cancer for the prognostication of key long-term oncologic outcomes—namely, metastasis-free and cancer-specific survival. It also confirms that the oncologic outcomes of intermediate- and high-risk prostate cancers are more diverse and harder to predict than those of low-risk prostate cancers 5. Furthermore, our study adds to the literature showing that the imaging characteristics of prostate cancer on MRI hold independent prognostic value and that the integration of established risk-stratifications tools with MRI-derived information improves overall prognostic precision.
It is widely accepted that many cancerous lesions identified on histopathologic assessment are “missed” on prostate MRI 17, 18. Our study corroborates this observation as no suspicious lesion was identified on T2-weighted MRI in over 50% of our patients. However, the 15-year probabilities for metastases or prostate cancer death in patients with no MRI-detectable cancer were <2% and <1%, respectively, while patients with an MRI-visible lesion (i.e. PI-RADS score ≥4) had an almost 20% chance of developing metastases and a 10% probability of cancer-specific death. Of note, these increased hazards were independent of the clinical risk, and we therefore hypothesize that the mere visibility of prostate cancer on MRI signifies a more aggressive phenotype of the disease. Similarly, although the literature indicates that MRI performs only modestly in detecting extraprostatic cancer extension compared to pathology 19,20, in our study, the presence of extraprostatic tumor spread on MRI was associated with significantly higher risks of metastases and prostate cancer death. Given these findings, we posit that the presence of extraprostatic tumor spread on MRI–despite its imperfect correlation with the cancer’s pathologic T stage–is another prognostically relevant phenotypic trait of prostate cancer. Another notable finding of our analysis is that prostate MRI provides information about clinically relevant outcomes both in patients treated surgically and those treated with radiation therapy, thus potentially meeting the definition of a prognostic biomarker. Finally, the added prognostic value of MRI seems to be most pronounced in patients with clinically intermediate- and high-risk disease. This might be partially attributable to the very low incidence of metastases or prostate cancer-related deaths in the low-risk group, an observation that aligns with recent trends towards active surveillance in this patient population 21. As all patients in the current study underwent definitive treatment, our data does not inform as to whether MRI can prognosticate the natural history of clinically low-risk prostate cancer managed with active surveillance.
The results of this study must be carefully interpreted in view of its methodologic and technical limitations. First, the study design was retrospective and selection bias might be present because clinicians were aware of the MRI findings. As the diagnostic accuracy and clinical utility of prostate MRI were being explored during the study period, it is uncertain to which extent the MRI influenced clinical staging and therapeutic strategies. It needs to be considered, however, that due to the rare and generally late occurrence of metastases and cancer death from initially localized prostate cancer, studies like ours require sizable patient cohorts to observe a sufficient number of events for meaningful statistical analyses. Given that prostate MRI was limited to few academic centers during the early 2000s, data on MRI and long-term outcomes is generally scarce. While an ongoing prospective study (i.e. ECOG-ACRIN Cancer Research Group EA8171, NCT03697148) aims to develop an MRI-inclusive risk prediction model, it will take at least another decade to analyze this data regarding metastasis-free and cancer-specific survival. The prostate MRIs in this study were performed when MRI technology was in an earlier stage of development, and routine clinical imaging protocols were limited to ‘anatomical sequences’ (i.e., T1- and T2-weighted images). Although these sequences continue to be an integral part of prostate MRI protocols, modern prostate MRI is ‘multiparametric’ and encompasses at least one ‘functional sequence’ (i.e., diffusion-weighted and/or dynamic contrast-enhanced imaging). Multiple studies have documented the incremental diagnostic precision offered by multiparametric MRI 22, and it is uncertain how the availability of those modern sequences would have affected the results of our study. It seems plausible, however, that the superior diagnostic precision of modern MRI protocols would translate into more accurate prognostication.
The accrual period of our study also falls into an era when radical treatment was considered the standard of care for localized prostate cancer, and alternative management options, such as active surveillance and focal treatments, were experimental. We also did not evaluate the impact of different surgical (e.g. open vs laparoscopic) or radiation therapy approaches (e.g. external beam vs brachytherapy, with or without concurrent androgen deprivation). Consequently, we were unable to study the prognostic implications of prostate MRI as a component of these specific management strategies or compare the effectiveness of different treatments, which was beyond the aim of this study. Similarly, the early accrual period of this study precludes comparison with modern prognostication tools, such as blood-, urine-, or biopsy-derived molecular biomarkers, several of which have been demonstrated to independently prognosticate long-term outcome and are variably used in clinic to assist decision making 23.
As with any study involving multiple readers, in this study there is a potential for inter-observer variability among radiologists 24. It must be noted, however, that inter-reader variability has also been documented among pathologists in the assignment of Gleason scores 25, among urologists in assessment of digital rectal examination findings 26, and commercially available PSA assays 27. However, none of these has precluded the successful clinical application of these tests as prognostic biomarkers. The single-center nature of our study is another limitation, as all study radiologists practiced at this one institution, and we were not able to assess whether extramural/general radiologists would have achieved comparable results. We attempted to mitigate this limitation by re-interpreting all scans in accordance with the standardized PI-RADS diagnostic criteria. However, this bias probably remains unresolved, given the strong influence of dedicated training 28 and sub-specialization 29 on a radiologist’s ability to accurately interpret prostate MRIs. It should be considered, however, that the benefits of dedicated training and sub-specialization are also documented for pathologists interpreting prostate biopsy samples 30.
Conclusions
Prostate MRI findings are strongly associated with long-term oncologic outcomes following radical treatment for localized prostate cancer. Combining MRI information with clinical and biopsy data results in more accurate prognostication and could facilitate individualized patient management.
Supplementary Material
Supplementary Figure 1: Flow chart of study cohort selection.
Acknowledgments
The authors would like to thank Ada Muellner, MS for editing the manuscript. This work was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, NIH/NCI Research Project (R01) CA076423-01A2, as well as through the Peter Michael Foundation.
Key of Definitions for Abbreviations
- MRI
Magnetic Resonance Imaging
- EAU
European Association of Urology
- CAPRA
Cancer of the Prostate Risk Assessment
- NCCN
National Comprehensive Cancer Network
- PSA
Prostate Specific Antigen
- PI-RADS
Prostate Imaging Reporting and Data System
References
- 1.Ferlay J, Colombet M, Soerjomataram I et al. : Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer, 144: 1941, 2019 [DOI] [PubMed] [Google Scholar]
- 2.D'Amico AV, Whittington R, Malkowicz SB et al. : Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama, 280: 969, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Pasta DJ, Elkin EP et al. : The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol, 173: 1938, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurtle D, Rossi SH, Berry B et al. : Models predicting survival to guide treatment decision-making in newly diagnosed primary non-metastatic prostate cancer: a systematic review. BMJ Open, 9: e029149, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimm P, Billiet I, Bostwick D et al. : Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int, 109 Suppl 1: 22, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Park KJ, Choi SH, Lee JS et al. : Risk Stratification of Prostate Cancer According to PI-RADS Version 2 Categories: Meta-Analysis for Prospective Studies. J Urol: 101097ju0000000000001306, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Hricak H, Kalbhen CL et al. : Hormonal ablation of prostatic cancer: effects on prostate morphology, tumor detection, and staging by endorectal coil MR imaging. AJR Am J Roentgenol, 166: 1157, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Weinreb JC, Barentsz JO, Choyke PL et al. : PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol, 69: 16, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barentsz JO, Richenberg J, Clements R et al. : ESUR prostate MR guidelines 2012. Eur Radiol, 22: 746, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB, American Joint Committee on Cancer., American Cancer Society.: AJCC cancer staging manual, Eight edition / editor-in-chief, Amin Mahul B. ; editors, Edge Stephen B. and 16 others ; Gress Donna M. - Technical editor ; Meyer Laura R.- Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer, pp. xvii, 1024 pages, 2017 [Google Scholar]
- 11.European Association of Urology (EAU) guidelines - Prostate Cancer, 2020 [Google Scholar]
- 12.Network NCC: NCCN Guidelines Version 2.2019 Prostate Cancer, 2019 [Google Scholar]
- 13.Xie W, Regan MM, Buyse M et al. : Metastasis-Free Survival Is a Strong Surrogate of Overall Survival in Localized Prostate Cancer. J Clin Oncol, 35: 3097, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uno H, Cai T, Pencina MJ et al. : On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med, 30: 1105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonen M, Heller G: Concordance probability and discriminatory power in proportional hazards regression. Biometrika, 92: 965, 2005 [Google Scholar]
- 16.Efron B, Tibshirani R: An introduction to the bootstrap. New York: Chapman & Hall, pp. xvi, 436 p., 1993 [Google Scholar]
- 17.Liss MA, Newcomb LF, Zheng Y et al. : Magnetic Resonance Imaging for the Detection of High Grade Cancer in the Canary Prostate Active Surveillance Study. J Urol: 101097ju0000000000001088, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg H, Ahmad AE, Chandrasekar T et al. : Comparison of Magnetic Resonance Imaging and Transrectal Ultrasound Informed Prostate Biopsy for Prostate Cancer Diagnosis in Biopsy Naïve Men: A Systematic Review and Meta-Analysis. J Urol, 203: 1085, 2020 [DOI] [PubMed] [Google Scholar]
- 19.de Rooij M, Hamoen EH, Witjes JA et al. : Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol, 70: 233, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Falagario UG, Ratnani P, Lantz A et al. : Staging Accuracy of Multiparametric Magnetic Resonance Imaging in Caucasian and African American Men Undergoing Radical Prostatectomy. J Urol, 204: 82, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Carlsson S, Benfante N, Alvim R et al. : Long-Term Outcomes of Active Surveillance for Prostate Cancer: The Memorial Sloan Kettering Cancer Center Experience. J Urol, 203: 1122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CH, Hobbs BP, Wei W et al. : Dynamic contrast-enhanced MRI for the detection of prostate cancer: meta-analysis. AJR Am J Roentgenol, 204: W439, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alford AV, Brito JM, Yadav KK et al. : The Use of Biomarkers in Prostate Cancer Screening and Treatment. Rev Urol, 19: 221, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park KJ, Choi SH, Lee JS et al. : Interreader Agreement with Prostate Imaging Reporting and Data System Version 2 for Prostate Cancer Detection: A Systematic Review and Meta-Analysis. J Urol: 101097ju0000000000001200, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Allsbrook WC Jr., Mangold KA, Johnson MH et al. : Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum Pathol, 32: 81, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Gosselaar C, Kranse R, Roobol MJ et al. : The interobserver variability of digital rectal examination in a large randomized trial for the screening of prostate cancer. Prostate, 68: 985, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Murthy V, Rishi A, Gupta S et al. : Clinical impact of prostate specific antigen (PSA) inter-assay variability on management of prostate cancer. Clin Biochem, 49: 79, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Akin O, Riedl CC, Ishill NM et al. : Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol, 20: 995, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wibmer A, Vargas HA, Donahue TF et al. : Diagnosis of Extracapsular Extension of Prostate Cancer on Prostate MRI: Impact of Second-Opinion Readings by Subspecialized Genitourinary Oncologic Radiologists. AJR Am J Roentgenol, 205: W73, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Brimo F, Schultz L, Epstein JI: The value of mandatory second opinion pathology review of prostate needle biopsy interpretation before radical prostatectomy. J Urol, 184: 126, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Flow chart of study cohort selection.