Abstract
PURPOSE
To present a summary of the recommendations for the treatment and follow-up for metastatic castration-resistant prostate cancer (mCRPC) as acquired through a questionnaire administered to 99 physicians working in the field of prostate cancer in developing countries who attended the Prostate Cancer Consensus Conference for Developing Countries.
METHODS
A total of 106 questions out of more than 300 questions addressed the use of imaging in staging mCRPC, treatment recommendations across availability and response to prior drug treatments, appropriate drug treatments, and follow-up, and those same scenarios when limited resources needed to be considered. Responses were compiled and the percentages were presented by clinicians to support each response. Most questions had five to seven relevant options for response including abstain and/or unqualified to answer, or in the case of yes or no questions, the option to abstain was offered.
RESULTS
Most of the recommendations from this panel were in line with prior consensus, including the preference of a new antiandrogen for first-line therapy of mCRPC. Important aspects highlighted in the scenario of limited resources included the option of docetaxel as treatment preference as first-line treatment in several scenarios, docetaxel retreatment, consideration for reduced doses of abiraterone, and alternative schedules of an osteoclast-targeted therapy.
CONCLUSION
There was wide-ranging consensus in the treatment for men with mCRPC in both optimal and limited resource settings.
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer in men,1 with 15% of men worldwide being diagnosed with it at some point during their life. Of those diagnosed, 80% will have a localized form and have a nearly 100% 5-year survival rate. The 20% patients remaining will have an advanced or metastatic form of the disease with only 26%-30% of them surviving 5 years.2 The initial management of PCa has for decades been based on androgen deprivation therapy (ADT) as its cancer cells are sensitive to the manipulation of testosterone and its metabolites.3,4 Reducing circulating testosterone to castrate levels (< 50 ng/dL) deprive the cells of their primary stimulus for growth5 and can induce PCa cell death.6 Unfortunately, PCa cells eventually become resistant to ADT because of different resistance mechanisms thereby becoming castration-resistant PCa. This form of the disease typically progresses rapidly, with patients dying within 2-4 years.7,8 Castration-resistant prostate cancer (CRPC) is defined by disease progression despite castrate levels of testosterone, and may present as either a continuous rise in serum prostate-specific antigen (PSA) levels, the progression of pre-existing disease, and/or the appearance of new metastases.9
CONTEXT
Key Objective
Generate a consensus on critical issues relevant to treatment of metastatic castration-resistant prostate cancer (PCa) focused in developing countries.
Knowledge Generated
Consensus was reached for metastatic castration-resistant prostate cancer of treatment-naive patients with docetaxel. For docetaxel-refractory cases, abiraterone reached consensus. Multiple other options were considered and reached consensus in cases of docetaxel-refractory disease in areas of limited resources including low-dose abiraterone, corticosteroids, ketoconazole, bicalutamide, mitoxantrone, and diethylstilbestrol.
Relevance
The voting results presented in this document can be used to support the treatment of metastatic castration-resistant PCa in areas of limited resources lacking specific guidelines.
In patients with CRPC who fail the initial therapy with curative intent (ie, radical prostatectomy, external beam radiotherapy, and brachytherapy), treatment options were once limited. Since 2004, chemotherapy is often used in patients with metastatic CRPC. Some landmark trials demonstrated an overall survival advantage of docetaxel compared with mitoxantrone, despite the marginal clinical benefit (2.4 months in TAX 327 and 1.9 months in SWOG 99-16; add P value).10,11 Currently, in the castration-resistant setting, since 2010, several key randomized controlled trials have demonstrated survival benefit with new therapies before and after docetaxel chemotherapy. Multiple new agents were approved by the US Food and Drug Administration (FDA) for the management of mCRPC, which all have varying mechanisms of action, namely sipuleucel-T,12 abiraterone acetate,13,14 enzalutamide,15,16 cabazitaxel,17 and radium-223.18 All these agents increased overall survival by some months, compared with the control group. New hormonal agents (abiraterone and enzalutamide) prolonged median survival by up to 3.9 and 4.8 months, respectively,19 chemotherapy treatment with docetaxel and cabazitaxel, often associated with significant side effects, prolonged overall survival by a few months,11,15,20 and treatment for diffused or painful bone metastases with radium-223 improved median overall survival by 3.6 months.21 Sipuleucel-T was only approved by the FDA and is not available elsewhere. Currently, FDA approved in 2020 olaparib and rucaparib, two poly ADP ribose polymerase inhibitors for adult patients with deleterious or suspected deleterious germline or somatic homologous recombination repair gene-mutated metastatic castration-resistant prostate cancer (mCRPC), which have progressed following prior treatment.22,23
With the incidence of PCa and burden of disease steadily increasing globally, healthcare systems, especially in regions of limited resources, will struggle with its management when balancing the cost-effectiveness of all innovative technologies for the overall healthcare system.24
The following manuscript will summarize the recommendations of a large panel of physicians from developing countries, specializing in PCa, regarding the treatment and follow-up of patients presenting with mCRPC both with and without contemplating the restrictions of limited resources in the decision-making process, with the objective of providing guidance in clinical practice and policy development and modification. The complete methodology of Prostate Cancer Consensus Conference for Developing Countries including the elaboration process of the questionnaires to guide the panelists, the design of voting sessions, and consensus criteria were presented in the editorial and are valid for all the manuscripts.
STAGING
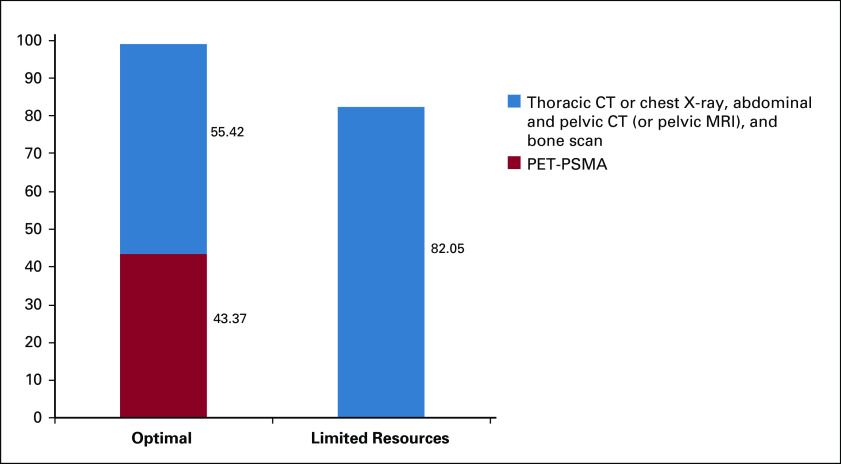
The majority (55.42%) of experts recommended using a combination of thoracic computed tomography (CT) or chest X-ray, abdominal and pelvic CT (or pelvic MRI), and a bone scan to stage patients with mCRPC. Findings are summarized in Figure 1. As seen, in areas of limited resources, that number rises to more than 80% of experts recommending the use of multiple imaging, therefore reaching a consensus. By contrast, in best practices, 43% of experts recommended the use of positron emission tomography (PET)-CT with prostate-specific membrane antigen (PSMA) or PET-MRI. It is important to point out that PET-CT PSMA or PET-MRI is far more expensive than conventional imaging. Also, a recent study has not shown any treatment changes using next-generation image compared with conventional images in 35 patients with metastatic disease.25
FIG 1.
Recommendations for imaging for patients with metastatic castration-resistant prostate cancer (without and with limited resources). CT, computed tomography; PET-PSMA, positron emission tomography-prostate-specific membrane antigen.
TREATMENT RECOMMENDATIONS
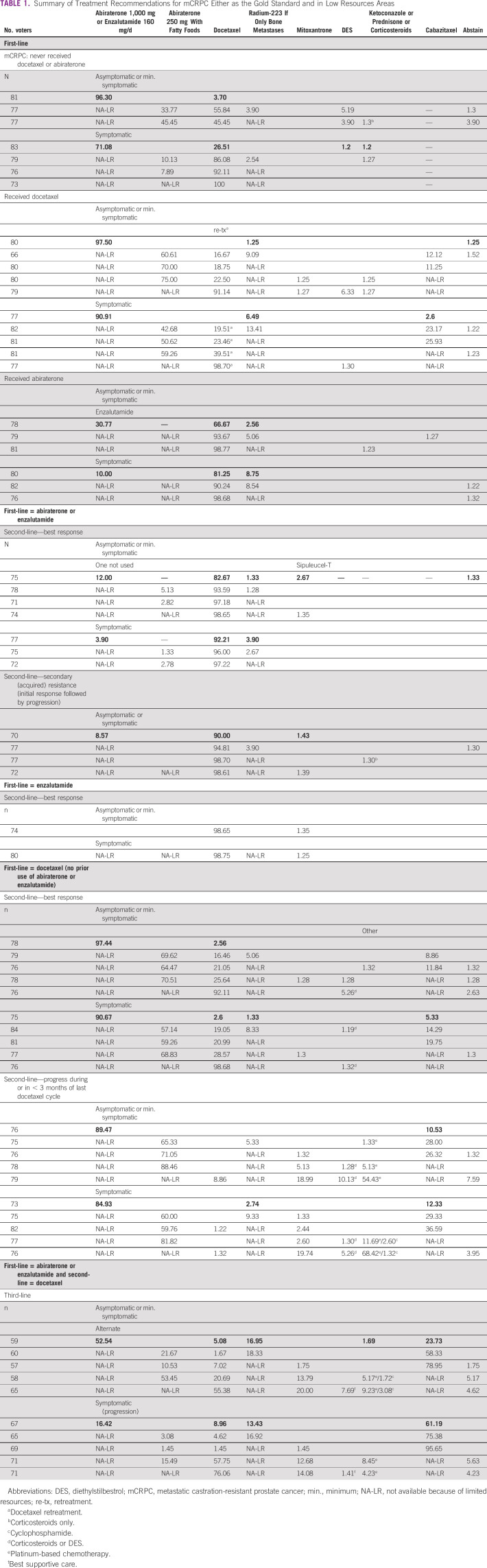
Table 1 summarizes physician responses for treatment recommendations and different scenarios when management options are limited because of resources. The best practice option is bolded, a dash (—) indicates that the treatment option was not offered as a response, a blank cell in the column had the treatment option offered though had zero physician respondents choosing that treatment, and the abbreviated NA-LR (not available because of limited resources) is an option eliminated to explore scenarios of limited resources.
TABLE 1.
Summary of Treatment Recommendations for mCRPC Either as the Gold Standard and in Low Resources Areas
In cases when chemotherapy is not recommended, 86.6% of the panelists would recommend a first-generation AR antagonist—although not presented in Table 1, all questions are provided in the Data Supplement.
The preferred treatment option for patients who have never received docetaxel or abiraterone, or who had already received docetaxel, was abiraterone 1,000 mg/daily or enzalutamide 160 mg/daily. In cases when full doses were not available because of limited resources, reduced doses of abiraterone 250 mg/daily accompanied by a diet of fatty foods was a viable option for a substantial portion of physicians in all limited resource scenarios.
Many guidelines such as EAU-ESTRO-SIOG20 support the use of one of the following agents for the treatment of mCRPC (level 1 of evidence): abiraterone acetate plus prednisone, enzalutamide, radium-223, docetaxel at 75 mg/m2 every 3 weeks, and sipuleucel-T. By contrast, cabazitaxel, abiraterone acetate plus prednisone, enzalutamide, and radium are approved for second-line treatment of CRPC following docetaxel. A possible explanation for the lack of consensus in recommending abiraterone or enzalutamide as first systemic treatment for mCRPC for asymptomatic or minimally symptomatic men who did and did not receive docetaxel or abiraterone in the castration-sensitive or castration-naive setting may have to do with physicians' perception that both agents have a more favorable toxicity profile. By contrast, 55.8% of the panelists voted for docetaxel (no prior docetaxel exposure) in the same scenario if full doses of abiraterone and enzalutamide are not available. This can be explained by the fact that docetaxel is significantly less expensive than new hormonal therapy agents.26,27
The experts were asked to classify appropriate drugs for healthcare settings with limited resources that were on the WHO essential medicines list and/or could be sourced at an affordable price from a generic manufacturer. Overall, there was an agreement by the group, as Table 2 shows, with 10 of 12 drugs reaching a consensus, one with a near consensus, and the other with a 64% majority.
TABLE 2.
Considered Appropriate in Healthcare Settings With Limited Resources for Men With mCRPC Who Are Progressing on or After Docetaxel
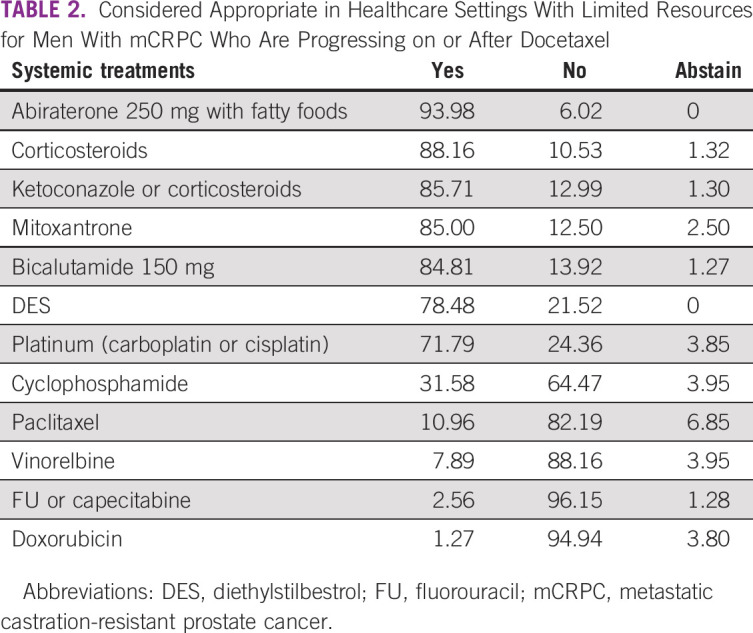
A consensus was reached favoring low-dose abiraterone with fatty diet if full doses of abiraterone and enzalutamide as well as radium-223 and cabazitaxel were not available. The most robust data in this regard come from initial studies showing that in patients with mCRPC, abiraterone AUC was ∼2-fold higher with a high-fat meal and similar with a low-fat meal versus modified fasting state.27 These data were followed by a randomized phase II study including 72 patients with progressive CRPC, which compared low-dose abiraterone (250 mg qd) given with a low-fat meal versus standard-dose abiraterone (1,000 mg qd) under fasting conditions. Both arms received prednisone 5 mg twice daily. At 12 weeks, there was a greater effect on PSA in the low abiraterone arm (mean log change, −1.59) compared with standard dose (−1.19), and noninferiority of low abiraterone was established according to predefined criteria. The PSA response rate was 58% in low abiraterone and 50% in standard abiraterone arm, and the median progression-free survival was around 9 months in both groups.28,29
Bicalutamide or flutamide was preferred by 86.59% of the panelists as the second-line endocrine manipulation in conditions where neither abiraterone nor enzalutamide was available, and the patient is not a candidate for chemotherapy. This consensus may be explained by the popularity of these agents for many years associated with their low cost and side effects.30
In a different context, the panelists recommended as a consensus docetaxel retreatment for the majority of asymptomatic or minimally symptomatic men who did receive docetaxel in the castration-sensitive or castration-naive setting if abiraterone and enzalutamide as well as radium-223 and cabazitaxel are not available. Similarly, a consensus was reached in favor of docetaxel retreatment in either asymptomatic or minimally symptomatic or symptomatic patients who did receive abiraterone in the castration-sensitive or castration-naive setting in an area of limited resources if enzalutamide is unavailable. In a retrospective analysis from a trial that compared castration with or without docetaxel in the castration-sensitive setting showed that docetaxel retreatment was associated with limited activity with no predictive factors of response. In this retrospective analysis including 245 patients, the response rate to docetaxel retreatment in the CRPC scenario was only 20%. The median biochemical progression-free survival was only 4.1 months.31 Consequently, the panelists favored low-dose abiraterone (42.68%) over docetaxel retreatment (19.51%) in symptomatic patients when available.
For most symptomatic patients with mCRPC who did not receive docetaxel or abiraterone in the castration-sensitive or castration-naive setting, 71.08% of the panelists recommended abiraterone or enzalutamide. It is important to mention that both COU-AA-30232 and Prevail17 did not include truly symptomatic patients because the control arm was a placebo. However, there is no reason aside from trial design that would preclude the activity of both agents in this setting. By contrast, in limited resource areas where full doses of abiraterone and enzalutamide are not available, the panelists reached consensus in recommending docetaxel.14 When evaluating different drugs for the management of PCa that have no level 1 evidence regarding an overall survival benefit, the panelists reached a consensus favoring mitoxantrone or prednisone.33 Similarly, low-dose abiraterone with fatty diet was determined by consensus to be an adequate option to be used in this limited resource setting and is supported by the phase II randomized data.29
Other forms of hormonal therapy that reached consensus for their use in circumstances of limited resources were diethylstilbestrol, high-dose bicalutamide, ketoconazole, and corticosteroids alone. A phase II trial evaluated the activity of bicalutamide in doses ranging from 50 to 150 mg/d in 61 patients treated with combined androgen blockade. High-dose bicalutamide was associated with a PSA decline by 50% or more in 22% of patients. The median duration of response was 3.7 months and toxicity was mild.34 A recent review of the role of ketoconazole for the treatment of mCRPC showed that this agent is associated with low cost, a relatively favorable toxicity profile compared with chemotherapy, and its improved efficacy, both before and after chemotherapy, despite not showing a survival benefit.35 A recent review of the role of corticosteroids has indicated the relevance of this class of agents in their ability to manage adverse effects, reduce symptoms, and improve patients' quality of life.36 These options are selected based on their easy access and low costs, favorable toxicity profile, and will continue to be used as substitutes for newer agents not available in areas of limited resources.37
A total of 71% of the panelists recommended platinum compounds as appropriate treatment options in the setting of limited healthcare resources and in men with mCRPC who progressed on or after docetaxel. A systematic search on electronic databases evaluated the role of platinum compounds for mCRPC and suggested a statistically significant increase in both clinical as well biochemical response when adding platinum compounds to chemotherapy.38 Similarly, oral cyclophosphamide was favored by 64.47% of the panelists. A comprehensive literature search was performed and concluded that it is active in the treatment for CRPC even among patients previously treated with chemotherapy, including docetaxel, yielding symptomatic and objective responses.39
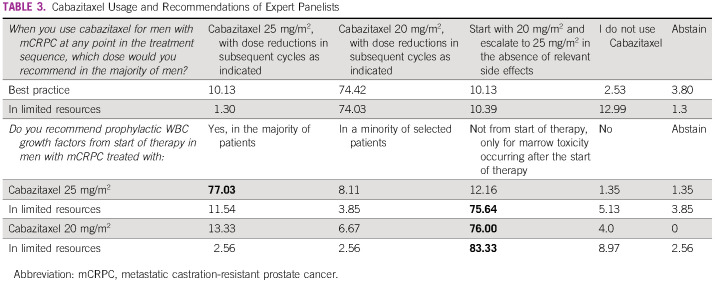
As second-line treatment, after prior docetaxel exposure, a near consensus (74.07%) was reached favoring the use of cabazitaxel at the dose of 20 mg/m2 intravenous every 3 weeks, with dose reductions in subsequent cycles as indicated in areas of limited resources. This recommendation is supported by a phase III study that assessed the noninferiority of cabazitaxel 20 mg/m2 versus 25 mg/m2. This trial showed that cabazitaxel 20 mg/m2 every 3 weeks was not inferior to higher dose in terms of overall survival. Health-related quality of life did not differ between cohorts.40
For second-line therapy, a consensus was reached favoring docetaxel in patients with metastatic asymptomatic or symptomatic CRPC who had progressive disease to first-line abiraterone or enzalutamide either when the resources were available or in areas with limited resources. Although there is no randomized trial comparing docetaxel versus abiraterone or enzalutamide in patients with mCRPC who failed prior enzalutamide or abiraterone, respectively, a recent review suggested a high degree of cross-resistance when abiraterone and enzalutamide are sequentially administered.41
Recently, the investigators of the COU-AA-302 trial described the activity of post-progression therapies in patients treated in the abiraterone experimental arm. The PFS of the patients who were treated with docetaxel as a first post-progression treatment was 7.6 months,42 whereas in those who received enzalutamide as a first post-progression treatment, it was 2.8 months.43 A retrospective report described the outcomes of 546 patients who progressed on first-line treatment with abiraterone or enzalutamide and subsequently received docetaxel or other hormone agents not previously administered as first-line. The authors reported that clinical and PSA response rates at both 3 and 6 months clearly favored docetaxel.44 Also, docetaxel is significantly less expensive than new hormonal therapy agents, which facilitates patient access.26
If low-dose abiraterone, cabazitaxel, and radium-223 were not available, there was a consensus favoring docetaxel retreatment in asymptomatic or minimally symptomatic men, who had response to docetaxel for mCRPC (without prior abiraterone or enzalutamide). It is important to mention that this option must be pursued if there are limitations related to access to other life-prolonging systemic agents as there are no randomized studies to support this strategy. A prospective phase II including 45 patients with mCRPC initially responding to docetaxel who then experienced disease progression after a period of biochemical remission of at least 5 months were enrolled and retreated with docetaxel. A total of 24.5% had a PSA response.45
Regarding third-line therapy, there was a consensus favoring cabazitaxel in symptomatic patients with mCRPC who had been treated with first-line abiraterone or enzalutamide and responded to docetaxel followed by progression if full doses of abiraterone and enzalutamide were not available or if full doses of abiraterone and enzalutamide as well as radium-223 were not available. A randomized phase III trial in patients with mCRPC who were previously treated with docetaxel and had progression within 12 months on abiraterone or enzalutamide compared cabazitaxel versus the alternative inhibitor (abiraterone or enzalutamide). The median overall survival was 13.6 months with cabazitaxel and 11.0 months with the androgen-signaling-targeted inhibitor (P = .008).46
By contrast, in limited resource situations, the preferred third-line mCRPC treatment option was docetaxel retreatment (consensus) in symptomatic patients with mCRPC who had been treated with first-line abiraterone or enzalutamide and responded to docetaxel, followed by progression if abiraterone and enzalutamide as well as radium-223 and cabazitaxel were not available. As seen in Table 3, most physicians recommend the use of cabazitaxel after abiraterone or enzalutamide and docetaxel treatments have been exhausted. There was a near consensus with 74% of physicians recommending a 20 versus 25 mg/m2 dose.
TABLE 3.
Cabazitaxel Usage and Recommendations of Expert Panelists
OSTEOCLAST-TARGETED THERAPY
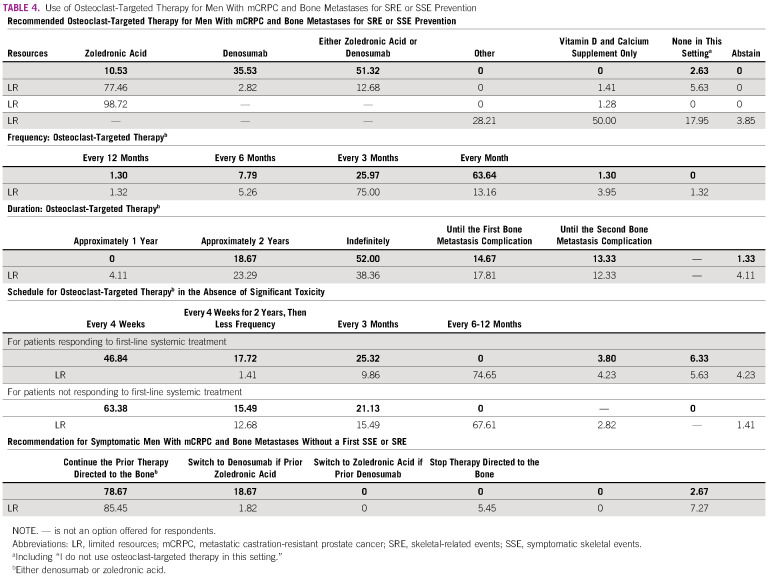
The last topic of the consensus referred to bone direct therapy to prevent bone-related complications. In areas of limited resources, a consensus was reached for the use of zoledronic acid over denosumab in patients with mCRPC and bone metastases for prevention of skeletal-related events (SRE) or symptomatic skeletal events (SSE), the cost of denosumab overcomes potential benefit.47-51 Table 4 summarizes the physicians' recommendations on this topic.
TABLE 4.
Use of Osteoclast-Targeted Therapy for Men With mCRPC and Bone Metastases for SRE or SSE Prevention
There was a consensus favoring the administration of zoledronic acid every 3 months instead of on monthly basis in areas of limited resources. This is supported by a randomized phase III trial including patients with metastatic breast cancer, metastatic PCa, or multiple myeloma who had at least one site of bone involvement. This study compared zoledronic acid administered intravenously every 4 weeks versus every 12 weeks for 2 years. The proportions of SREs did not differ significantly in both cohorts.51
FOLLOW-UP
Regarding follow-up of patients with metastatic CRPC in areas of limited resources, most of the panelists (69.51%) would recommend only PSA every 1-2 months and image studies only in case of PSA elevation and/or symptoms suggesting clinical progression. This differs from the original recommendations as PSA is not a reliable surrogate marker in patients treated with some of the new agents such as abiraterone or enzalutamide.52 One explanation reflects the costs and access to frequent image tests.
In conclusion, the current study illustrates the consensus of physicians in the field of mCRPC treatment. With consensus levels of over 90% in many cases, including limited resource settings, the panelists made determinations that may elucidate treatment decisions and provide solutions for areas that face resource limitations. The use of docetaxel as first-line treatment for mCRPC or as retreatment is one of the most important results of this consensus, given that it entails a less expensive and popular agent. The utility of older agents in areas of resource limitations was not underestimated in the consensus and represents an important finding reflecting a trend toward accepting clinical benefit. Equally, prolonging the treatment interval of zoledronic acid was considered another reasonable solution when financial limitations were an issue. This consensus was developed with the aim of assisting developing countries in the treatment of mCRPC. This consensus concentrated a large group of experts located in South America. It is important to consider the socioeconomic differences of these countries in the incorporation of the results and consensus obtained.
Fernando Cotait Maluf
Honoraria: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Sanofi/Aventis, Merck Serono, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Merck Serono, AstraZeneca
Research Funding: Janssen-Cilag, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer Schering Pharma
Felipe Moraes
Honoraria: Janssen, Zodiac Pharma, MSD, Libbs, Bayer, Amgen
Consulting or Advisory Role: Janssen
Adriano Gonçalves Silva
Honoraria: MSD Oncology, Janssen Oncology, Astellas Pharma, Roche, Pfizer/EMD Serono, Bayer, BMS Brazil
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Pfizer/EMD Serono, MSD Oncology
Research Funding: MSD Oncology, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, MSD Oncology, Roche
Aldo Lourenço Abbade Dettino
Honoraria: Janssen-Cilag, Bayer, Astellas Pharma, Roche
Consulting or Advisory Role: Novartis
Research Funding: Janssen, Roche
Travel, Accommodations, Expenses: MSD Oncology
Ana Paula Garcia Cardoso
Consulting or Advisory Role: Janssen Oncology, Pfizer, Astellas Pharma
Speakers' Bureau: Janssen Oncology, Novartis, MSD Oncology, Astellas Pharma, AstraZeneca, Bayer
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, Bristol-Myers Squibb
André Seeke Sasse
Honoraria: Roche, Astellas Pharma, Janssen-Cilag, Bristol-Myers Squibb, Merck KGaA, MSD Oncology, Novartis
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Merck KGaA, Novartis, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: MSD Oncology, Janssen-Cilag
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol-Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
Ariel Galapo Kann
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, GlaxoSmithKline, Roche
Daniel Herchenhorn
Consulting or Advisory Role: Janssen-Cilag
Denis Leonardo Fontes Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, BMS Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, BMS Brazil, Janssen-Cilag
Diego Emilio Lopera Cortés
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis
Speakers' Bureau: Dr Reddy's Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: MSD Oncology
Fábio Roberto Kater
Consulting or Advisory Role: Janssen-Cilag, Pfizer
Travel, Accommodations, Expenses: Janssen-Cilag
Igor A. Protzner Morbeck
Honoraria: Janssen-Cilag, BMS Brazil, AstraZeneca, MSD Oncology, Astellas Pharma
Consulting or Advisory Role: BMS Brazil, Janssen-Cilag, Takeda
Travel, Accommodations, Expenses: Astellas Pharma, BMS Brazil
Juan Jose Zarbá
Consulting or Advisory Role: Pfizer/EMD Serono, Astellas Pharma
Research Funding: MSD Oncology, Lilly, Exelixis, Astellas Pharma
Expert Testimony: Lilly
Travel, Accommodations, Expenses: Roche, Pfizer
Karine Martins da Trindade
Honoraria: BMS Brazil, Janssen-Cilag, MSD Oncology
Consulting or Advisory Role: MSD Oncology, Janssen-Cilag, Astellas Pharma
Research Funding: BMS Brazil, Roche/Genentech, MSD Oncology, Janssen-Cilag
Travel, Accommodations, Expenses: Janssen-Cilag, BMS Brazil, Ipsen
Leonardo Atem G. A. Costa
Honoraria: Janssen Oncology, Astellas Pharma, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Boehringer Ingelheim, Astellas Pharma, AstraZeneca
Lucas V. dos Santos
Stock and Other Ownership Interests: Merck Sharp & Dohme, Eisai, Fleury Group
Honoraria: BMS Brazil, United Medical, Roche/Genentech
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, MSD, Roche/Genentech
Speakers' Bureau: BMS Brazil, United Medical
Research Funding: Roche/Genentech, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, Boston Scientific, Takeda, BMS Brazil, MSD, Exelixis
Manuel Caitano Maia
Consulting or Advisory Role: AstraZeneca, Janssen Oncology
Speakers' Bureau: Janssen Oncology, AstraZeneca, Bayer, MSD Oncology, Pfizer, Astellas Pharma
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Mariana Bruno Siqueira
Consulting or Advisory Role: Novartis, Janssen-Cilag
Speakers' Bureau: Novartis, MSD Oncology, Bayer, Janssen-Cilag
Travel, Accommodations, Expenses: Roche, Pfizer, Ipsen
Silke Gillessen
Consulting or Advisory Role: Astellas Pharma, Advanced Accelerator Applications, Roche, Janssen, Bayer, Orion Pharma GmbH, Menarini Silicon Biosystems, Tolero Pharmaceuticals, MSD Oncology, Amgen, Pfizer
Speakers' Bureau: Janssen-Cilag
Patents, Royalties, Other Intellectual Property: Method for biomarker (WO 3752009138392 A1)
Travel, Accommodations, Expenses: ProteoMedix
Other Relationship: ProteoMediX, Aranda Pharma
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Fernando Cotait Maluf, Felipe Moraes, Andrey Soares, Ariel Galapo Kann, Denis Leonardo Fontes Jardim, Diego Emilio Lopera Cortés, Igor A. Protzner Morbeck, Juan Pablo Sade, Leonardo Atem G. A. Costa, Mariana Bruno Siqueira, Silke Gillessen
Administrative support: Fernando Cotait Maluf, Juan Jose Zarbá, Juan Pablo Sade
Provision of study materials or patients: Adriano Gonçalves Silva, Aldo Lourenço Abbade Dettino, André Seeke Sasse, Diego Emilio Lopera Cortés, Juan Pablo Sade
Collection and assembly of data: Fernando Cotait Maluf, Felipe Moraes, Adriano Gonçalves Silva, Aldo Lourenço Abbade Dettino, Ana Paula Garcia Cardoso, André Seeke Sasse, Ariel Galapo Kann, Diego Emilio Lopera Cortés, Fábio Roberto Kater, Igor A. Protzner Morbeck, João Francisco Navarro Reolon, José Augusto Rinck Junior, Juan Jose Zarbá, Karine Martins da Trindade, Leonardo Atem G. A. Costa, Lucas V. dos Santos, Manuel Caitano Maia, Mariana Bruno Siqueira
Data analysis and interpretation: Fernando Cotait Maluf, Felipe Moraes, Aldo Lourenço Abbade Dettino, Ana Paula Garcia Cardoso, André Seeke Sasse, Ariel Galapo Kann, Daniel Herchenhorn, Diego Emilio Lopera Cortés, José Augusto Rinck Junior, Lucas V. dos Santos, Manuel Caitano Maia, Silke Gillessen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Fernando Cotait Maluf
Honoraria: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Sanofi/Aventis, Merck Serono, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Bayer Schering Pharma, Merck Sharp & Dohme, Roche/Genentech, BMS Brazil, Ipsen, Merck Serono, AstraZeneca
Research Funding: Janssen-Cilag, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer Schering Pharma
Felipe Moraes
Honoraria: Janssen, Zodiac Pharma, MSD, Libbs, Bayer, Amgen
Consulting or Advisory Role: Janssen
Adriano Gonçalves Silva
Honoraria: MSD Oncology, Janssen Oncology, Astellas Pharma, Roche, Pfizer/EMD Serono, Bayer, BMS Brazil
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Pfizer/EMD Serono, MSD Oncology
Research Funding: MSD Oncology, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, MSD Oncology, Roche
Aldo Lourenço Abbade Dettino
Honoraria: Janssen-Cilag, Bayer, Astellas Pharma, Roche
Consulting or Advisory Role: Novartis
Research Funding: Janssen, Roche
Travel, Accommodations, Expenses: MSD Oncology
Ana Paula Garcia Cardoso
Consulting or Advisory Role: Janssen Oncology, Pfizer, Astellas Pharma
Speakers' Bureau: Janssen Oncology, Novartis, MSD Oncology, Astellas Pharma, AstraZeneca, Bayer
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, Bristol-Myers Squibb
André Seeke Sasse
Honoraria: Roche, Astellas Pharma, Janssen-Cilag, Bristol-Myers Squibb, Merck KGaA, MSD Oncology, Novartis
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Merck KGaA, Novartis, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: MSD Oncology, Janssen-Cilag
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol-Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
Ariel Galapo Kann
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, GlaxoSmithKline, Roche
Daniel Herchenhorn
Consulting or Advisory Role: Janssen-Cilag
Denis Leonardo Fontes Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, BMS Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, BMS Brazil, Janssen-Cilag
Diego Emilio Lopera Cortés
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis
Speakers' Bureau: Dr Reddy's Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: MSD Oncology
Fábio Roberto Kater
Consulting or Advisory Role: Janssen-Cilag, Pfizer
Travel, Accommodations, Expenses: Janssen-Cilag
Igor A. Protzner Morbeck
Honoraria: Janssen-Cilag, BMS Brazil, AstraZeneca, MSD Oncology, Astellas Pharma
Consulting or Advisory Role: BMS Brazil, Janssen-Cilag, Takeda
Travel, Accommodations, Expenses: Astellas Pharma, BMS Brazil
Juan Jose Zarbá
Consulting or Advisory Role: Pfizer/EMD Serono, Astellas Pharma
Research Funding: MSD Oncology, Lilly, Exelixis, Astellas Pharma
Expert Testimony: Lilly
Travel, Accommodations, Expenses: Roche, Pfizer
Karine Martins da Trindade
Honoraria: BMS Brazil, Janssen-Cilag, MSD Oncology
Consulting or Advisory Role: MSD Oncology, Janssen-Cilag, Astellas Pharma
Research Funding: BMS Brazil, Roche/Genentech, MSD Oncology, Janssen-Cilag
Travel, Accommodations, Expenses: Janssen-Cilag, BMS Brazil, Ipsen
Leonardo Atem G. A. Costa
Honoraria: Janssen Oncology, Astellas Pharma, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, AstraZeneca
Travel, Accommodations, Expenses: Janssen Oncology, Boehringer Ingelheim, Astellas Pharma, AstraZeneca
Lucas V. dos Santos
Stock and Other Ownership Interests: Merck Sharp & Dohme, Eisai, Fleury Group
Honoraria: BMS Brazil, United Medical, Roche/Genentech
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, MSD, Roche/Genentech
Speakers' Bureau: BMS Brazil, United Medical
Research Funding: Roche/Genentech, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, Boston Scientific, Takeda, BMS Brazil, MSD, Exelixis
Manuel Caitano Maia
Consulting or Advisory Role: AstraZeneca, Janssen Oncology
Speakers' Bureau: Janssen Oncology, AstraZeneca, Bayer, MSD Oncology, Pfizer, Astellas Pharma
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Mariana Bruno Siqueira
Consulting or Advisory Role: Novartis, Janssen-Cilag
Speakers' Bureau: Novartis, MSD Oncology, Bayer, Janssen-Cilag
Travel, Accommodations, Expenses: Roche, Pfizer, Ipsen
Silke Gillessen
Consulting or Advisory Role: Astellas Pharma, Advanced Accelerator Applications, Roche, Janssen, Bayer, Orion Pharma GmbH, Menarini Silicon Biosystems, Tolero Pharmaceuticals, MSD Oncology, Amgen, Pfizer
Speakers' Bureau: Janssen-Cilag
Patents, Royalties, Other Intellectual Property: Method for biomarker (WO 3752009138392 A1)
Travel, Accommodations, Expenses: ProteoMedix
Other Relationship: ProteoMediX, Aranda Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Steele CB, Li J, Huang B, et al. Prostate cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study Cancer 1235160–51772017suppl 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marques RB, Dits NF, Erkens-Schulze S, et al. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PLoS One. 2010;5:e13500. doi: 10.1371/journal.pone.0013500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris WP, Mostaghel EA, Nelson PS, et al. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion Nat Clin Pract Urol 676–852009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ritch C, Cookson M. Recent trends in the management of advanced prostate cancer. F1000Res. 2018;7:1513. doi: 10.12688/f1000research.15382.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huggins C, Hodges CV.Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate CA Cancer J Clin 22232–2401972 [DOI] [PubMed] [Google Scholar]
- 7.Patrikidou A, Loriot Y, Eymard JC, et al. Who dies from prostate cancer? Prostate Cancer Prostatic Dis 17348–3522014 [DOI] [PubMed] [Google Scholar]
- 8.Omlin A, Pezaro C, Mukherji D, et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms Eur Urol 64300–3062013 [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Chi KN, Finelli A, et al. The 2015 CUA-CUOG guidelines for the management of castration-resistant prostate cancer (CRPC) Can Urol Assoc J 990–962015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer N Engl J Med 3511502–15122004 [DOI] [PubMed] [Google Scholar]
- 11.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer N Engl J Med 3511513–15202004 [DOI] [PubMed] [Google Scholar]
- 12.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer N Engl J Med 363411–4222010 [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer N Engl J Med 3641995–20052011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study Lancet Oncol 16152–1602015 [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy N Engl J Med 3671187–11972012 [DOI] [PubMed] [Google Scholar]
- 16.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy N Engl J Med 371424–4332014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial Lancet 3761147–11542010 [DOI] [PubMed] [Google Scholar]
- 18.Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer N Engl J Med 369213–2232013 [DOI] [PubMed] [Google Scholar]
- 19.Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel Ann Oncol 27454–4602016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer Eur Urol 71630–6422017 [DOI] [PubMed] [Google Scholar]
- 21.Parker C, Sartor O.Radium-223 in prostate cancer N Engl J Med 3691659–16602013 [DOI] [PubMed] [Google Scholar]
- 22.de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer N Engl J Med 3822091–21022020 [DOI] [PubMed] [Google Scholar]
- 23.Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients (pts) with DNA damage repair (DDR)-deficient metastatic castration-resistant prostate cancer (mCRPC): Updated analyses Ann Oncol 30v327–v3282019 [Google Scholar]
- 24.Pishgar F, Ebrahimi H, Saeedi Moghaddam S, et al. Global, regional and national burden of prostate cancer, 1990 to 2015: Results from the global burden of disease study 2015 J Urol 1991224–12322018 [DOI] [PubMed] [Google Scholar]
- 25.Davidson T, Amit U, Saad A, et al. Gallium-68 prostate-specific membrane antigen PET-CT and the clinical management of prostate cancer Nucl Med Comm 40913–9192019 [DOI] [PubMed] [Google Scholar]
- 26.Aguiar P, Tan P, Simko S, et al. Cost-effectiveness analysis of abiraterone, docetaxel or placebo plus androgen deprivation therapy for hormone-sensitive advanced prostate cancer. Einstein (Sao Paulo) 2019;17:eGS4414. doi: 10.31744/einstein_journal/2019GS4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guirgis H. Outcome, costs and dollar value of docetaxel and abiraterone in prostate cancer. J Clin Oncol. 2019;37 suppl; abstr 155. [Google Scholar]
- 28.Chi K, Spratlin J, Kollmannsberger C, et al. Food effects on abiraterone pharmacokinetics in healthy subjects and patients with metastatic castration-resistant prostate cancer J Clin Pharm 551406–14142015 [DOI] [PubMed] [Google Scholar]
- 29.Szmulewitz R, Peer C, Ibraheem A, et al. Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer J Clin Oncol 361389–13952018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford E, Schellhammer P, McLeod D, et al. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens J Urol 200956–9662018 [DOI] [PubMed] [Google Scholar]
- 31.Lavaud P, Gravis G, Foulon S, et al. Anticancer activity and tolerance of treatments received beyond progression in men treated upfront with androgen deprivation therapy with or without docetaxel for metastatic castration-naïve prostate cancer in the GETUG-AFU 15 phase 3 trial Eur Urol 73696–7032018 [DOI] [PubMed] [Google Scholar]
- 32.Ryan C, Smith M, de Bono J, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy N Engl J Med 368138–1482013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannock I, Osoba D, Stockler M, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: A Canadian randomized trial with palliative end points J Clin Oncol 141756–17641996 [DOI] [PubMed] [Google Scholar]
- 34.Klotz L, Drachenberg D, Singal R, et al. An open-label, phase 2 trial of bicalutamide dose escalation from 50 mg to 150 mg in men with CAB and castration resistance. A Canadian Urology Research Consortium Study Prostate Cancer Prostatic Dis 17320–3242014 [DOI] [PubMed] [Google Scholar]
- 35.Patel V, Liaw B, Oh W.The role of ketoconazole in current prostate cancer care Nat Rev Urol 15643–6512018 [DOI] [PubMed] [Google Scholar]
- 36.De Santis M, Saad F.Practical guidance on the role of corticosteroids in the treatment of metastatic castration-resistant prostate cancer Urology 96156–1642016 [DOI] [PubMed] [Google Scholar]
- 37.Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: The report of the Advanced Prostate Cancer Consensus Conference APCCC 2017 Eur Urol 73178–2112018 [DOI] [PubMed] [Google Scholar]
- 38.Leal F, García-Perdomo HA.Effectiveness of platinum-based chemotherapy in patients with metastatic prostate cancer: Systematic review and meta-analysis Clin Genitourin Cancer 17e627–e6442019 [DOI] [PubMed] [Google Scholar]
- 39.Nelius T, Rinard K, Filleur S.Oral/metronomic cyclophosphamide-based chemotherapy as option for patients with castration-refractory prostate cancer: Review of the literature Cancer Treat Rev 37444–4552011 [DOI] [PubMed] [Google Scholar]
- 40.Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m) and the currently approved dose (25 mg/m) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA J Clin Oncol 353198–32062017 [DOI] [PubMed] [Google Scholar]
- 41.Tucci M, Caffo O, Buttigliero C, et al. Therapeutic options for first-line metastatic castration-resistant prostate cancer: Suggestions for clinical practise in the CHAARTED and LATITUDE era Cancer Treat Rev 7435–422019 [DOI] [PubMed] [Google Scholar]
- 42.De Bono J. Reply to Robert J. van Soest and Ronald de Wit's Letter to the Editor re: Johann S. de Bono, Matthew R. Smith, Fred Saad, et al. Subsequent chemotherapy and treatment patterns after abiraterone acetate in patients with metastatic castration-resistant prostate cancer: Post hoc analysis of COU-AA-302. Eur Urol. 2017;71:e11. doi: 10.1016/j.eururo.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith MR, Saad F, Rathkopf DE, et al. Clinical outcomes from androgen signaling-directed therapy after treatment with abiraterone acetate and prednisone in patients with metastatic castration-resistant prostate cancer: Post hoc analysis of COU-AA-302 Eur Urol 7210–132017 [DOI] [PubMed] [Google Scholar]
- 44.Oh WK, Miao R, Vekeman F, et al. Real-world characteristics and outcomes of patients with metastatic castration-resistant prostate cancer receiving chemotherapy versus androgen receptor-targeted therapy after failure of first-line androgen receptor-targeted therapy in the community setting Clin Genitourin Cancer 16P50–P572018 [DOI] [PubMed] [Google Scholar]
- 45.Di Lorenzo G, Buonerba C, Faiella A, et al. Phase II study of docetaxel re-treatment in docetaxel-pretreated castration-resistant prostate cancer BJU Int 107234–2392011 [DOI] [PubMed] [Google Scholar]
- 46.De Wit R, De Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer N Engl J Med 3812506–25182019 [DOI] [PubMed] [Google Scholar]
- 47.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma J Natl Cancer Inst 941458–14682002 [DOI] [PubMed] [Google Scholar]
- 48.Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer J Natl Cancer Inst 96879–8822004 [DOI] [PubMed] [Google Scholar]
- 49.Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study Lancet 377813–8222011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie J, Namjoshi M, Wu EQ, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases J Manag Care Pharm 17621–6432011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial JAMA 31748–582017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford ED, Koo PJ, Shore N, et al. A clinician's guide to next generation imaging in patients with advanced prostate cancer (RADAR III) J Urol 201682–6922019 [DOI] [PubMed] [Google Scholar]