Abstract
PURPOSE
To present a summary of the recommendations for the treatment and follow-up for the biochemical recurrence of castration-resistant prostate cancer (PCa) as acquired through a questionnaire administered at the Prostate Cancer Consensus Conference for Developing Countries.
METHODS
A total of 27 questions were identified as relating to this topic. Responses from the clinician were tallied and are presented in percentage format. Topics included the use of imaging in staging, treatment recommendations across different patient scenarios of life expectancy and prostate-specific antigen (PSA) doubling time, and follow-up for nonmetastatic castration-resistant PCa.
RESULTS
A consensus agreed that in optimal conditions, positron emission tomography-computed tomography with prostate-specific membrane antigen would be used although in limited resource situations the combined use of CT of the abdomen and pelvic (or pelvic MRI), a bone scan, and a CT of the thorax or chest x-ray was recommended. In cases when PSA levels double in < 10 months, more than 90% of clinicians agreed on the use of apalutamide or enzalutamide, regardless of life expectancy. With a doubling time of more than 10 months, > 54% of experts recommended no treatment independent of life expectancy. More than half of the experts, regardless of resources, recommended follow-up with a physical examination and PSA levels every 3-6 months and imaging only in the case of symptoms.
CONCLUSION
The voting results and recommendations presented in this document can be used by physicians to support management for biochemical recurrence of castration-resistant PCa in areas of limited resources. Individual clinical decision making should be supported by available data.
INTRODUCTION
Fifteen percent of all men worldwide will be diagnosed with prostate cancer (PCa). Although it is the second most common cancer for men,1 only around 3% will die from the disease and those that do often after the age of 75.2 As such, there are varying degrees of risk for the disease and several factors that are considered in determining the lifetime therapy of patients. Many cases of PCa are curative; however, approximately 30%-40% of men will develop a rise in serum prostate-specific antigen (PSA) after treatment with definitive local therapies. This phenomenon is defined as a biochemical recurrence of the disease,3,4 and even when there are no signs or symptoms of a locally recurrent or metastatic disease, it does signify a return of the cancer, but it should be noted that it does not necessarily correlate with or affect overall survival (OS).5
CONTEXT
Key Objective
The treatment of prostate cancer (PCa) is challenging in low- and middle-income countries. Limited access to several treatments is one of the mainstay problems.
The Prostate Cancer Consensus Conference for Developing Countries was the first global effort to help physicians in these countries to guide treatment decisions with limited resources.
Knowledge Generated
Effective therapies for patients with nonmetastatic castration-resistant PCa were lacking until recently. New hormonal therapies (abiraterone, apalutamide, and darolutamide) changed this scenario improving survival. Molecular images for nonmetastatic castration-resistant PCa did not show the same good results as in biochemical recurrence.
Relevance
This article provides recommendations to manage nonmetastatic castration-resistant PCa in countries with limited resources.
Clinicians are challenged to prevent or delay the onset of metastatic disease and the resulting increased morbidity and mortality while needing to consider the negative impact on patients' quality of life and avoid overtreating PCa that is at low risk of clinical progression. When PSA levels rise following local treatment, the role of early salvage androgen deprivation therapy (ADT) is still debated6,7; however, if salvage androgen deprivation therapy is used in men with biochemical relapse, the disease nearly always re-emerges despite castrate levels of testosterone, resulting in the biological transformation to what is known as castration-resistant prostate cancer, metastatic or nonmetastatic (CRPC).8 As PCa incidence and burden of disease steadily increase globally,9 healthcare systems especially in regions of limited resources will struggle with its management when balancing the benefits of recent advances and their cost-effectiveness for the overall healthcare system.9 This article summarizes the recommendations of a large panel of physicians working with PCa in developing countries for their recommended treatment and follow-up of patients presenting with nonmetastatic castration-resistant prostate cancer (nmCRPC)—with and without the consideration of limited resources to provide guidance in clinical practice and in the development of nmCRPC treatment policies.
Staging
The use of positron emission tomography-computed tomography (PET-CT) with prostate-specific membrane antigen (PSMA) imaging in patients with nmCRPC (PSA 2 ng/mL and doubling time < 10 months and/or Gleason 8 or higher) using regular images showed that 98% of these patients have spread of disease.10 Despite that the treatment strategies in nmCRPC are based on studies that used regular images, the use of PET-CT with PSMA is not compulsory in a context of limited resources.
Treatment Recommendations
In patients with nmCRPC with a rising PSA level after primary curative treatment, indicating a nonmetastatic recurrence of the disease, PSA doubling time was the stratifying factor in treatment recommendations. Short PSA doubling time showed to be prognostic in patients with nmCRPC.11,12
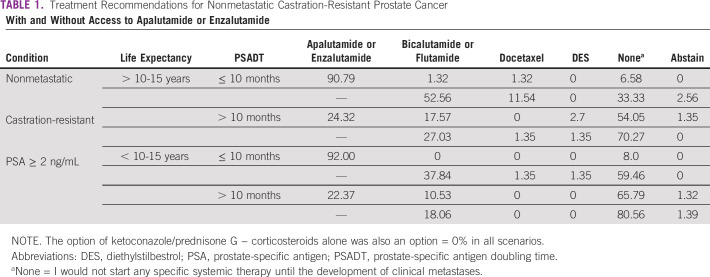
Questions were posed to the panel of experts who examined scenarios with the above profile in patients with a life expectancy of more than 10-15 years and < 10-15 years, with PSA doubling time >10 months and < 10 months (Data Supplement). These results are presented in Table 1. A clear consensus was reached for patients with PSA doubling time < 10 months to use apalutamide or enzalutamide, regardless of life expectancy (for > 10-15 years 91% and for < 10-15 years 92% recommend to start either). Without access to apalutamide or enzalutamide, life expectancy played a role in treatment recommendations with 53% recommending those with > 10-15 years of life expectancy alternative treatment bicalutamide or flutamide and 33% not pursuing treatment, whereas in those with less life expectancy (< 10-15 years), almost 60% did not seek additional treatment and only 38% recommended bicalutamide or flutamide.
TABLE 1.
Treatment Recommendations for Nonmetastatic Castration-Resistant Prostate Cancer
In patients with a PSA doubling time > 10 months, treatment recommendations were seen across life expectancy although with the same trends of a greater proportion of clinicians choosing not to treat. Apalutamide or enzalutamide was only recommended by approximately 25% of the clinicians with 54% (> 10-15 years) and 66% (< 10-15 years) choosing not to treat. In limited resource settings, without the option of apalutamide or enzalutamide, the percentage of clinicians choosing not to treat rose to 70% and 81%, respectively.
The follow-up of nmCRPC was supported by more than half of the panel (56%) with primarily a physical examination and PSA measurement every 3-6 months and imaging in the case of symptoms, and 78.4% would implement in areas with limited resources. Only 30% of clinicians suggested a CT or chest x-ray of thorax, CT of the abdomen and pelvis, and bone scan every 3-6 months, and the percentage decreased to 16% for areas of limited resources. Particularly in areas of limited resources, the panelists favored a more conservative follow-up strategy to avoid an unnecessary increase in costs without a clear benefit in terms of OS.
DISCUSSION
The impact of nonmetastatic recurrence on PCa-specific mortality or OS is not clear. Rising PSA levels correlate with metastasis and PCa mortality but can predate local recurrence or metastasis by typically 7-8 years on average.13,14 CRPC has an added layer of difficulty in treatment as these patients no longer respond to ADT or hormonal therapy. In high-risk cases where PSA levels are doubling in < 10 months, there is clear consensus by clinicians indicating treatment with apalutamide or enzalutamide; however, without access to these innovative treatments, the clinician's recommendations to not treat increased, especially in those with less life expectancy. By the time the questionnaire of this consensus was concluded, darolutamide had not yet been approved in this context.15 In lower risk cases, the decision to not further treat these patients was held by more than 50% of the clinicians participating in the questionnaire independent of limitation of resources—balancing the difficulty of treatment of these patients with the possibility for benefit for the patient. These cases must also consider the challenges for clinicians in developing countries where healthcare systems have cost containment pressures, limited or delayed access to specialists, and access barriers.
These recommendations are in concordance with the results of the trials in the nmCRPC. There is good evidence of benefit in terms of metastasis-free survival and PSA response. It is important to note that metastasis-free survival in the nmCRPC scenario is not an OS surrogate end point. These end points seem to be important to avoid symptoms and patient anxiety, respectively. The absence of OS benefit published until the date of this consensus, no major impact in quality of life, and the increase of costs are very concerning points, mainly in areas of limited resources. After the consensus, recent data showed OS benefits of new androgen blockers in nmCRPC scenario that can lead to a new discussion about cost-effectiveness of this strategy in limited resources countries.16
Felipe Moraes Toledo Pereira
Honoraria: Janssen, Zodiac Pharma, MSD, Libbs, Bayer, Amgen
Consulting or Advisory Role: Janssen
Adriano Gonçalves e Silva
Honoraria: MSD Oncology, Janssen Oncology, Astellas Pharma, Roche, Pfizer/EMD Serono, Bayer, Bristol-Myers Squibb Brazil
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Pfizer/EMD Serono, MSD Oncology
Research Funding: MSD Oncology, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, MSD Oncology, Roche
Aldo Lourenço Abbade Dettino
Honoraria: Janssen-Cilag, Bayer, Astellas Pharma, Roche
Consulting or Advisory Role: Novartis
Research Funding: Janssen, Roche
Travel, Accommodations, Expenses: MSD Oncology
Ana Paula Garcia Cardoso
Consulting or Advisory Role: Janssen Oncology, Pfizer, Astellas Pharma
Speakers' Bureau: Janssen Oncology, Novartis, MSD Oncology, Astellas Pharma, AstraZeneca, Bayer
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, Bristol-Myers Squibb
Andre Deeke Sasse
Honoraria: Roche, Astellas Pharma, Janssen-Cilag, Bristol-Myers Squibb, Merck KGaA, MSD Oncology, Novartis
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Merck KGaA, Novartis, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: MSD Oncology, Janssen-Cilag
Ariel Galapo Kann
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, GlaxoSmithKline, Roche
Carlos Dzik
Consulting or Advisory Role: Janssen-Cilag, IPSEN, Novartis
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Daniel Herchenhorn
Consulting or Advisory Role: Janssen-Cilag
Denis Leonardo Fontes Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, Bristol-Myers Squibb Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, Bristol-Myers Squibb Brazil, Janssen-Cilag
Diego Lopera
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis
Speakers' Bureau: Dr Reddy's Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: MSD Oncology
Pamela Salman
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Merck Serono
Speakers' Bureau: Roche/Genentech, Novartis, Lilly
Ray Antonio Manneh Kopp
Honoraria: Astellas Scientific and Medical Affairs Inc, Janssen-Cilag, Bayer, Merck Sharp & Dohme, Roche, Bristol-Myers Squibb, Sanofi, AstraZeneca, Pfizer
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Roche, Merck Serono, Merck Sharp & Dohme, AstraZeneca, Sanofi, Pfizer
Speakers' Bureau: San Jorge Foundation, ACHO
Research Funding: Pfizer, Merck Sharp & Dohme, Novartis
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Sandro Roberto De Araujo Cavallero
Honoraria: Pfizer, Astellas Pharma, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, MSD Oncology, Astellas Pharma
Speakers' Bureau: Pfizer, Janssen-Cilag, Astellas Pharma
Travel, Accommodations, Expenses: Pfizer, Roche/Genentech, Janssen-Cilag, Astellas Pharma
Vinicius Carrera Souza
Consulting or Advisory Role: Janssen, Astellas Pharma, Bayer, Bristol-Myers Squibb Brazil
Research Funding: Janssen
Travel, Accommodations, Expenses: Janssen
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol-Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Felipe Moraes Toledo Pereira, Ariel Galapo Kann, Denis Leonardo Fontes Jardim, Diego Lopera, Mouna Ayadi, Sandro Roberto De Araujo Cavallero, Sergio Aguiar, Vinicius Carrera Souza, Andrey Soares
Administrative support: Andrey Soares
Provision of study materials or patients: Aldo Lourenço Abbade Dettino, Andre Deeke Sasse, Carlos Dzik, Diego Lopera, Ray Antonio Manneh Kopp
Collection and assembly of data: Felipe Moraes Toledo Pereira, Adriano Gonçalves e Silva, Aldo Lourenço Abbade Dettino, Ana Paula Garcia Cardoso, Ariel Galapo Kann, Carlos Dzik, Daniel Herchenhorn, Diego Lopera, Pamela Salman, Ray Antonio Manneh Kopp, Sandro Roberto De Araujo Cavallero, Vinicius Carrera Souza, Pedro Luiz Serrano Uson Junior, Andrey Soares
Data analysis and interpretation: Felipe Moraes Toledo Pereira, Aldo Lourenço Abbade Dettino, Ana Paula Garcia Cardoso, Andre Deeke Sasse, Ariel Galapo Kann, Diego Lopera, Pamela Salman, Ray Antonio Manneh Kopp, Ricardo Saraiva De Carvalho, Sandro Roberto De Araujo Cavallero, Vinicius Carrera Souza, Pedro Luiz Serrano Uson Junior, Andrey Soares
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Felipe Moraes Toledo Pereira
Honoraria: Janssen, Zodiac Pharma, MSD, Libbs, Bayer, Amgen
Consulting or Advisory Role: Janssen
Adriano Gonçalves e Silva
Honoraria: MSD Oncology, Janssen Oncology, Astellas Pharma, Roche, Pfizer/EMD Serono, Bayer, Bristol-Myers Squibb Brazil
Consulting or Advisory Role: Janssen Oncology, Astellas Pharma, Pfizer/EMD Serono, MSD Oncology
Research Funding: MSD Oncology, Janssen Oncology
Travel, Accommodations, Expenses: Janssen Oncology, Astellas Pharma, MSD Oncology, Roche
Aldo Lourenço Abbade Dettino
Honoraria: Janssen-Cilag, Bayer, Astellas Pharma, Roche
Consulting or Advisory Role: Novartis
Research Funding: Janssen, Roche
Travel, Accommodations, Expenses: MSD Oncology
Ana Paula Garcia Cardoso
Consulting or Advisory Role: Janssen Oncology, Pfizer, Astellas Pharma
Speakers' Bureau: Janssen Oncology, Novartis, MSD Oncology, Astellas Pharma, AstraZeneca, Bayer
Travel, Accommodations, Expenses: Ipsen, Janssen Oncology, Bristol-Myers Squibb
Andre Deeke Sasse
Honoraria: Roche, Astellas Pharma, Janssen-Cilag, Bristol-Myers Squibb, Merck KGaA, MSD Oncology, Novartis
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Merck KGaA, Novartis, Roche
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: MSD Oncology, Janssen-Cilag
Ariel Galapo Kann
Consulting or Advisory Role: Janssen Oncology
Speakers' Bureau: Janssen Oncology, GlaxoSmithKline, Roche
Carlos Dzik
Consulting or Advisory Role: Janssen-Cilag, IPSEN, Novartis
Speakers' Bureau: Janssen Oncology
Travel, Accommodations, Expenses: Astellas Pharma, Janssen Oncology
Daniel Herchenhorn
Consulting or Advisory Role: Janssen-Cilag
Denis Leonardo Fontes Jardim
Honoraria: Janssen-Cilag, Roche/Genentech, Astellas Pharma, MSD Oncology, Bristol-Myers Squibb Brazil, Pfizer, Libbs, Merck
Consulting or Advisory Role: Janssen-Cilag, Pfizer, MSD
Travel, Accommodations, Expenses: MSD, Bristol-Myers Squibb Brazil, Janssen-Cilag
Diego Lopera
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Novartis
Speakers' Bureau: Dr Reddy's Laboratories, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer
Travel, Accommodations, Expenses: MSD Oncology
Pamela Salman
Consulting or Advisory Role: Roche/Genentech, Novartis, Lilly, Merck Serono
Speakers' Bureau: Roche/Genentech, Novartis, Lilly
Ray Antonio Manneh Kopp
Honoraria: Astellas Scientific and Medical Affairs Inc, Janssen-Cilag, Bayer, Merck Sharp & Dohme, Roche, Bristol-Myers Squibb, Sanofi, AstraZeneca, Pfizer
Consulting or Advisory Role: Astellas Pharma, Janssen-Cilag, Roche, Merck Serono, Merck Sharp & Dohme, AstraZeneca, Sanofi, Pfizer
Speakers' Bureau: San Jorge Foundation, ACHO
Research Funding: Pfizer, Merck Sharp & Dohme, Novartis
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Sandro Roberto De Araujo Cavallero
Honoraria: Pfizer, Astellas Pharma, Janssen Oncology, AstraZeneca
Consulting or Advisory Role: Janssen Oncology, MSD Oncology, Astellas Pharma
Speakers' Bureau: Pfizer, Janssen-Cilag, Astellas Pharma
Travel, Accommodations, Expenses: Pfizer, Roche/Genentech, Janssen-Cilag, Astellas Pharma
Vinicius Carrera Souza
Consulting or Advisory Role: Janssen, Astellas Pharma, Bayer, Bristol-Myers Squibb Brazil
Research Funding: Janssen
Travel, Accommodations, Expenses: Janssen
Andrey Soares
Honoraria: Janssen, Pfizer, Bayer, Novartis, AstraZeneca, Astellas Pharma, Pierre Fabre, Merck Serono, Sanofi, Roche, MSD
Consulting or Advisory Role: Astellas Pharma, Janssen, Roche, Bayer, Lilly, AstraZeneca, Novartis, MSD, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Astellas Pharma, Bristol-Myers Squibb, Bayer, Roche, Janssen, Merck Serono, Sanofi, Ipsen, MSD
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Wever EM, Hugosson J, Heijnsdijk EA, et al. To be screened or not to be screened? Modeling the consequences of PSA screening for the individual Br J Cancer 107778–7842012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishan AU, Shaikh T, Wang PC, et al. Clinical outcomes for patients with Gleason Score 9-10 prostate adenocarcinoma treated with radiotherapy or radical prostatectomy: A multi-institutional comparative analysis Eur Urol 71766–7732017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han M, Partin AW, Zahurak M, et al. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer J Urol 169517–5232003 [DOI] [PubMed] [Google Scholar]
- 5.Artibani W, Porcaro AB, De Marco V, et al. Management of biochemical recurrence after primary curative treatment for prostate cancer: A review Urol Int 100251–2622018 [DOI] [PubMed] [Google Scholar]
- 6.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): A randomised, multicentre, non-blinded, phase 3 trial Lancet Oncol 17727–7372016 [DOI] [PubMed] [Google Scholar]
- 7.Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomised, multicentre, open-label phase 3 trial Lancet Oncol 17747–7562016 [DOI] [PubMed] [Google Scholar]
- 8.Mateo J, Fizazi K, Gillessen S, et al. Managing nonmetastatic castration-resistant prostate cancer Eur Urol 75285–2932019 [DOI] [PubMed] [Google Scholar]
- 9.Pishgar F, Ebrahimi H, Saeedi Moghaddam S, et al. Global, regional and national burden of prostate cancer, 1990 to 2015: Results from the Global Burden of Disease Study 2015 J Urol 1991224–12322018 [DOI] [PubMed] [Google Scholar]
- 10.Fendler W, Weber M, Iravani A, et al. Prostate-specific membrane antigen ligand positron emission tomography in men with nonmetastatic castration-resistant prostate cancer Clin Cancer Res 257448–74542019 [DOI] [PubMed] [Google Scholar]
- 11.Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer J Clin Oncol 232918–29252005 [DOI] [PubMed] [Google Scholar]
- 12.Smith M, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial Lancet 37939–462012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy JAMA 2811591–15971999 [DOI] [PubMed] [Google Scholar]
- 14.Zagars GK, Pollack A.The fall and rise of prostate-specific antigen. Kinetics of serum prostate-specific antigen levels after radiation therapy for prostate cancer Cancer 72832–8421993 [DOI] [PubMed] [Google Scholar]
- 15.Fizazi K, Shore N, Tammela T, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer N Engl J Med 3801235–12462019 [DOI] [PubMed] [Google Scholar]
- 16.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer Eur Urol 79150–1582020 [DOI] [PubMed] [Google Scholar]