Abstract
Sound-induced mechanical stimuli are detected by elaborate mechanosensory transduction (MT) machinery in highly specialized hair cells of the inner ear. Genetic studies of inherited deafness in the past decades have uncovered several molecular constituents of the MT complex, and intense debate has surrounded the molecular identity of the pore-forming subunits. How the MT components function in concert in response to physical stimulation is not fully understood. In this review, we summarize and discuss multiple lines of evidence supporting the hypothesis that transmembrane channel-like 1 is a long-sought MT channel subunit. We also review specific roles of other components of the MT complex, including protocadherin 15, cadherin 23, lipoma HMGIC fusion partner-like 5, transmembrane inner ear, calcium and integrin-binding family member 2, and ankyrins. Based on these recent advances, we propose a unifying theory of hair cell MT that may reconcile most of the functional discoveries obtained to date. Finally, we discuss key questions that need to be addressed for a comprehensive understanding of hair cell MT at molecular and atomic levels.
Keywords: hair cells, mechanotransduction, MET, TMC1, ion channel, hearing, deafness
INTRODUCTION
The sense of hearing in mammals depends on the ability of hair cells in the inner ear to convert sound-evoked mechanical stimuli into electrochemical signals. This mechanosensory transduction (MT) process is initiated by deflection of the hair bundle on the apical surface of hair cells, which induces rapid opening of sensory transduction channels. Inward current, carried mainly by K+ ions, through the channel pores causes depolarization of hair cells, triggering neurotransmitter release onto the afferent nerve fibers, which in turn carry auditory information to the central nervous system.
Over 40 years ago, electrophysiological evidence from vertebrate hair cells first demonstrated the existence of mechanosensitive ion channels (25, 26). Since then, intense effort has focused on the search for the molecular identity of the channels carrying these mechanically activated currents, but with slow progress. During the past 30 years, with the application of genetic studies of deafness in both mice and humans, several protein molecules have been identified that are implicated in hair cell MT (37). These include cadherin 23 (CDH23), protocadherin 15 (PCDH15), lipoma HMGIC fusion partner-like 5 (LHFPL5), transmembrane inner ear (TMIE), transmembrane channel-like 1/2 (TMC1/2), and calcium and integrin-binding family member 2 (CIB2), which together are thought to constitute the MT machinery. In particular, within the past 10 years, TMC1 and TMC2 have emerged as pore-forming subunits of the transduction channel. Recently, definitive evidence has shown that TMC1 and TMC2 are indeed mechanosensitive ion channels. Despite substantial progress and new insight into the mechanism of MT, fundamental questions remain, e.g., what the specific function of the individual components is, how they assemble to ensure proper functioning of the channel subunits TMC1/2, and how mechanical forces induce channel opening. We anticipate that, in the next decade, a clear and complete understanding of MT at both molecular and biophysical levels will be reached.
In this review, we first introduce hair bundles, the mechanosensitive organelles of hair cells, and MT. We then focus on TMC1/2, the core proteins of MT machinery, and summarize research progress leading to the conclusion that TMC1/2 are the long-sought vertebrate MT channel subunits. We also highlight progress made in elucidating invertebrate TMC orthologs in Drosophila and Caenorhabditis elegans as mechanosensors. We then discuss other integrated components of the MT complex. Taking advantage of the recent advances in understanding TMC1/2 function, we present a unifying MT theory.
HAIR BUNDLES AND MECHANOSENSORY TRANSDUCTION
Located in the cochlea of the mammalian inner ear, hair cells functionally evolved into two groups: outer hair cells (OHCs) that amplify and tune the input sound signals and inner hair cells (IHCs) that contact afferent neuron fibers and convey sound information to the brain (91) (Figure 1a). Although they have distinct functions, both OHCs and IHCs depend on their ability to convert mechanical information into electrical signals. This MT relies on highly specialized organelles, called hair bundles, located at the apical surface of hair cells (Figure 1b). Each hair bundle is composed of rows of F-actin-filled microvilli (known as stereocilia) arranged in a staircase pattern, with the height of each row increasing toward the tall edge. A true cilium, filled with microtubules and termed the kinocilium, can be found in developing hair cells but not in mature cochlear hair cells.
Figure 1.
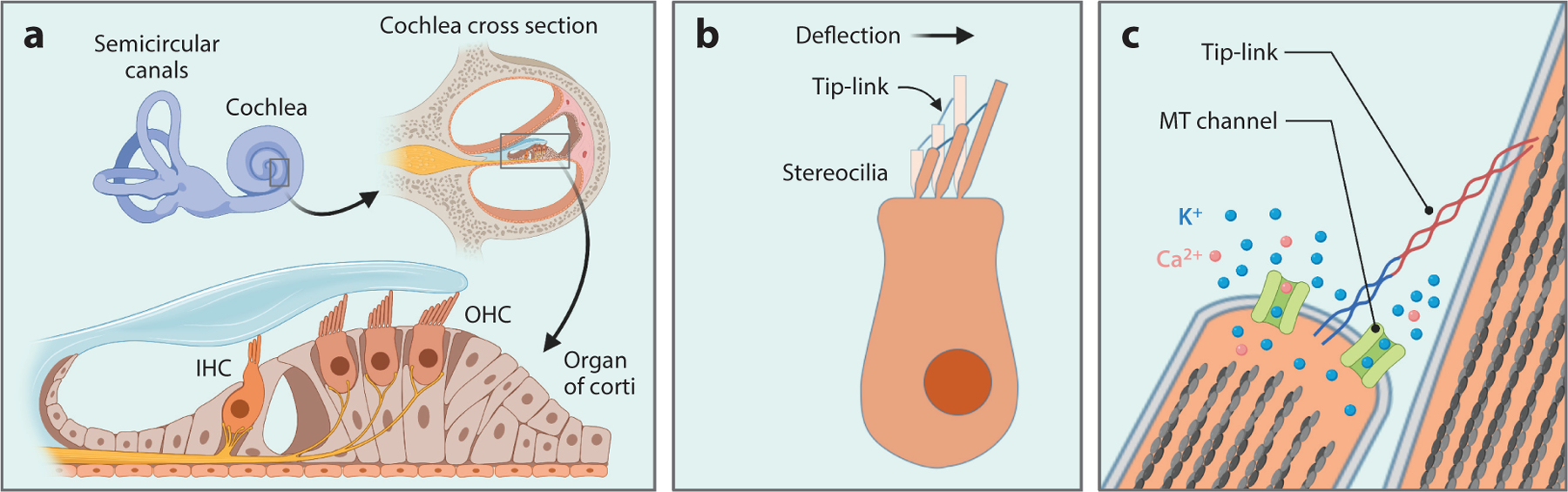
Schematic diagram illustrating the anatomy of the mammalian cochlea, hair cells, and mechanosensory transduction (MT) apparatus. (a) Illustration of three rows of outer hair cells (OHCs) and one row of inner hair cells (IHCs) in the organ of Corti in the cochlea of the inner ear. OHCs amplify input sound signals and connect to efferent neurons, while IHCs are innervated by afferent neuron fibers that carry sound information to the central nervous system. (b) Diagram of a hair cell showing the deflection of the hair bundle toward the tallest stereocilia in response to sound-induced vibration. The tops of shorter stereocilia are connected to the side walls of the next taller ones. (c) The opening of MT channels by tension in the tip-link during hair bundle deflection. MT channels are localized at the tips of shorter stereocilia near the lower end of the tip-link. Once activated, the channel pore carries K+ and Ca2+ ions from outside to inside the stereocilia, leading to depolarization of hair cells.
Sound-induced vibration evokes deflection of hair bundles toward the tallest stereocilia, which exerts tension onto a fine extracellular filament, called the tip-link, that connects the top of the shorter stereocilia to the side of the adjacent taller stereocilia (84) (Figure 1b). MT channels are localized at the tips of shorter stereocilia near the lower end of the tip-link and respond to mechanical force transmitted through tip-links (14) (Figure 1c). Supporting the idea that tip-links are required for MT, the loss and recovery of MT current are highly correlated with the disruption and regeneration of tip-links, respectively (6, 65, 112), and the MT current can be elicited by directly pulling tip-links (9). Hair bundle deflection toward the tallest stereocilia opens MT channels (increases open probability), while deflection in the opposite direction closes channels (decreases open probability) (49). Thus, MT channels in the resting state are in a region of greatest sensitivity to the force, i.e., a typical 0.5-μm deflection of the hair bundle is able to evoke a maximal response. Electrophysiological characterization revealed that MT channels are nonselective cation channels with high permeability to Ca2+ (79). Since the endolymph solution bathing the hair bundle contains high K+ and low Ca2+ (approximately 140 mM K+ and 20–40 μM Ca2+), the predominant MT current in vivo is carried by K+. MT channels have a minimal pore diameter of 1.2 nm and are permeable to large cations such as FM1–43 and aminoglycosides (38).
THE LONG-SOUGHT MECHANOSENSORY TRANSDUCTION CHANNEL: TMC1/2
The molecular identity of hair cell MT channels has been a major research interest for the fields of hearing and deafness since these channels were first described approximately 40 years ago. Various possible types of ion channels have been proposed, but most of them were rejected by subsequent studies. The hypothesis that TMC1 and TMC2 form bona fide MT channels has been under consideration for nearly 10 years and is now widely accepted, as these proteins have satisfied numerous stringent criteria.
Identification of TMC1 as an Essential Component for Hearing
Like many genes involved in hair cell MT, TMC1 was identified by genetic studies of deafness in both mice and humans, but this process took over half a century. In 1958, Deol & Kocher (35) reported one of the first mouse lines of deafness (dn) with recessive inheritance and no other complications. They observed normal hair cell morphology during the second postnatal week, suggesting that the deafness in these mice was not caused by degeneration of hair cells. More than 20 years later, in 1980, Steel & Bock (96) characterized the dn mice in more detail with electrophysiological approaches and found a complete lack of cochlear microphonics, which indicated defective hair cell function. Another 15 years later, Jain et al. (51) identified nonsyndromic human deafness cases in an Indian family with recessive inheritance (DFNB7/11) and mapped the causative gene to locus 9q11–q21, which, interestingly, is syntenic to the mouse dn locus. This raised the intriguing possibility that the dn mouse and human DFNB7/11 could be attributed to a common gene. In 2002, Kurima et al. (61) described dominant, progressive human hearing loss (DFNA36) with a locus (9q13–q21) in the same region as DFNB7/11. Through positional cloning, Kurima and colleagues identified a new gene in the region that is mutated in the DFNB7/11 and DFNA36 families. They gave it the name transmembrane cochlear-expressed gene 1, but it was subsequently renamed Tmc1. They also found a 1.6-kb deletion in the Tmc1 gene of dn mice. By searching for similar sequences in the genome, they identified Tmc2, a close homolog to Tmc1. At the same time, Vreugde et al. (101) reported a mouse, named Beethoven (Bth), that is a model of dominant, progressive hearing loss analogous to human DFNA36 and identified a Tmc1 missense mutation (M412K) in these mice. Together, these genetic studies demonstrated an essential role for TMC1 in the mammalian auditory system, but its precise function remained elusive.
Evidence Supporting TMC1 and TMC2 as Bona Fide Mechanosensory Transduction Channels
Based on the measured biophysical properties of MT currents, many existing ion channels, including transient receptor potential (TRP) channels, have been suggested as MT channel candidates, but most of them were later ruled out for various reasons, including lack of expression in hair cells and lack of hearing loss in knockout mice. Since TMCs were first cloned from deaf humans and mice in 2002 (61), support for the TMC1/2 channel hypothesis has waxed and waned, but these candidates have now satisfied stringent criteria for defining bona fide hair cell MT channels. Below, we discuss the accumulated evidence that demonstrates that TMC1 and TMC2 are indeed the MT channels in auditory and vestibular hair cells.
TMC1 and TMC2 are expressed at the right time in the right place.
Both Tmc1 and Tmc2 mRNA were detected in mouse inner ear hair cells at early postnatal stages using in situ hybridization assays (56, 61). Further analysis with quantitative reverse transcriptase–polymerase chain reaction revealed a dynamic temporal expression profile for these two genes (56). Tmc2 mRNA is only transiently expressed in the early postnatal mouse cochlea, beginning to appear around birth, reaching a peak during the first postnatal week, and then declining to an undetectable level by P10. The rise of Tmc2 mRNA is tightly correlated with the onset of MT (64). In comparison, the onset of Tmc1 mRNA expression, around P3, is delayed a few days relative to Tmc2. Tmc1 expression rises to a plateau before the acquisition of hearing at P12 and is sustained into adulthood. While Tmc1 expression rises, Tmc2 mRNA declines to near zero. Although the functional significance of the expression switch from Tmc2 to Tmc1 in mouse cochlear hair cells during the first postnatal week is unclear, it is clear that Tmc2 expression cannot substitute for Tmc1 in mature cochlear hair cells (5, 77). It is also unclear whether a similar switch occurs in human cochlear hair cells.
To detect TMC1 and TMC2 protein expression and subcellular localization in hair cells, Kurima et al. (60) generated TMC1-mCherry and TMC2-AcGFP transgenic mice with bacterial artificial chromosomes that included the full gene and promoter structure, attempting to mimic native expression. They showed temporal expression patterns of fluorescently tagged TMC1 and TMC2 proteins that were similar to the expression pattern of the mRNA. In developing hair cells, TMC1-mCherry and TMC2-AcGFP were detected along the length of the stereocilia in both IHCs and OHCs. However, as hair cells developed, the two proteins localized predominantly to the tips of the shorter-row stereocilia, where the MT channels reside (14). Stereocilia tip localization of TMC1 and TMC2 was also confirmed by the immunofluorescence detection of TMC1-HA and TMC2-HA, in which the sequence of a small HA tag was introduced before the stop codon of native Tmc1 or Tmc2 gene using the CRISPR/Cas9 technique (33). Finally, using antibodies reported to be specific, native TMC1 and TMC2 were also found to be localized at the tips of shorter, but not the tallest, stereocilia using either immunolabeling or immunogold electron microscopy (60, 71). Taken together, these studies provided conclusive evidence that TMC1 and TMC2 are expressed at the right time and in the right place for hair cell MT, which satisfies important criteria for components of the MT complex.
TMC1 and TMC2 are required for mechanosensitivity of hair cells.
If TMC1 is a component of MT channels, then knockout of Tmc1 should lead to loss of MT current. Initial testing of TMC1 for mechanosensitivity in cochlear hair cells was confounded by the coexpression of TMC2, which provides similar MT currents, at early postnatal stages, and also by expression of Piezo2, which carries distinct mechanosensitive currents. Four years after Tmc1 and Tmc2 were cloned, Marcotti and colleagues (72) first described electrophysiological recordings of cochlear hair cells from the Tmc1 mutant mice dn and Bth and reported intact MT current in OHCs of homozygous mice between P6 and P8. They observed developmental defects and degeneration of hair cells at later stages, i.e., after the second postnatal week, time points that were not examined in the earlier Deol & Kocher (35) study. Based on these observations, they concluded that TMC1 is necessary for hair cell maturation and survival but is not required for MT current. However, later studies revealed transient expression of TMC2 in cochlear hair cells during the first postnatal week, overlapping with that of TMC1 and suggesting that the presence of TMC2 may have accounted for the seemingly normal MT currents recorded from mice during the first postnatal week in the Marcotti et al. (25) study. Indeed, when Tmc2 was knocked out from dn homozygous mice, the MT currents were completely lost (56). Further supporting this notion, MT current was reduced to undetectable levels in OHCs of dn mice in the second postnatal week (59), when TMC2 expression declined to near zero. An earlier, more comprehensive study from Kawashima et al. (56) showed that, although the MT current was little affected in Tmc1 or Tmc2 single-knockout mice, it was fully abolished in the double-knockout mice Tmc1−/−;Tmc2−/−, regardless of the age of the mice. Furthermore, exogenous expression of either TMC1 or TMC2 using viral vectors in hair cells of Tmc1−/−;Tmc2−/− mice was sufficient to rescue the MT current (54). These data together strongly suggest that TMC1 and TMC2 are required for MT in hair cells. However, in the Tmc1−/−;Tmc2−/− mice, Kim and colleagues (58) reported a form of nonconventional or anomalous mechanically activated current, which was revealed by vigorous mechanical stimulation of apical or basolateral membranes of hair cells. These anomalous currents called into question whether TMC1 and TMC2 were required for hair cell mechanosensitivity. In 2017, Wu et al. (105) demonstrated that the anomalous mechanocurrents were actually mediated by the mechanosensitive ion channel Piezo2, which is localized in the apical surface of hair cells and activated by membrane stretch. Piezo2 is highly expressed in Merkel cells and a sub-group of dorsal root ganglia and mediates gentle touch sensation in mammalian skin (87), but its function in hair cells is currently unclear, since Piezo2-knockout mice showed little hearing defect. Nevertheless, by eliminating a confounding factor, the Piezo2 studies (105) helped strengthen the conclusion that TMC1 and TMC2 are required for normal MT current.
Mutations and modifications in TMC1 alter channel properties.
One of the strongest lines of evidence indicating that a protein is a pore-forming subunit of the MT channel is that mutations in the protein result in changes in the pore properties, including single-channel conductance and ion selectivity, which are intrinsic to ion channel biophysics and independent of channel expression level or localization. Due to the extremely small size of single stereocilia (submicron range), it has not been feasible to estimate single-channel conductance of hair cell MT channels using conventional cell-attached or excised patch recordings. Instead, whole-cell recordings have been used to detect single-channel events, with two approaches developed to stimulate just one channel. The first, developed by the Fettiplace group, briefly treats the hair bundle with a Ca2+ chelator, EGTA or BAPTA, to destroy all tip-links except one. The remaining intact tip-link and channel are then stimulated with a fluid jet (31). The other technique, developed by Pan et al. (82), directly deflects a single stereocilium in an intact hair bundle with a fine-tipped stiff glass probe. Recordings from the two methods showed predominant unitary currents and led to agreement that MT channels have a large unitary conductance of approximately 100–300 pS, depending on species, hair cell type and location along the cochlea, expression of TMC1 or TMC2, and Ca2+ concentration in the bath solution (13, 16, 31, 58, 82, 89, 106) (Table 1). Nonstationary noise analysis of whole-cell macroscopic currents also suggested a large unitary conductance in a similar range (81). However, a recent report (12) found multiple conductance states in mouse OHCs and IHCs with approximately 50 pS increments, which lead the authors of that study to propose that the previously published large unitary currents are the sum of simultaneous opening of multiple channels, gated by tip-link tension with high cooperativity. Consistent with this concept, a study on purified sea turtle TMC1 and budgerigar TMC2 reconstituted in liposomes reported a single-channel conductance of approximately 40 pS and 35 pS, respectively (see below) (52). To clarify the apparent discrepancy of the single-channel conductance data, direct recording in cell-attached or excised configuration will be needed to detect single-channel events in either hair cells or reconstituted artificial lipid bilayers.
Table 1.
Unitary conductance measurements from hair cells
| [Ca2+]bath | Method | Conductance (pS) |
|---|---|---|
| 2.8 mM | BAPTA + fluid jet | 106 |
| 2.8 mM | BAPTA + fluid jet | 80–163 |
| 0.05 mM | BAPTA + fluid jet | 149–300 |
| 1.5 mM | BAPTA + fluid jet | 95, 139 |
| 1.5 mM | BAPTA + fluid jet | 170 |
| 1.3 mM | Fluid jet | 112 |
| 2.8 mM | EGTA + fluid jet | 87 |
| 0.05 mM | BAPTA + fluid jet | 63–102 |
| 0.05 mM | Fine-tipped probe | 90–320 |
| 1.3 mM | Noise analysis | 154 |
| 0.04 mM | BAPTA + fluid jet | 50 |
| Ca2+-free | Liposome patch | 40, 35 |
Abbreviations: IHC, inner hair cell; OHC, outer hair cell; TMC1/2, transmembrane channel-like 1/2.
The MT channel exhibits distinct pore properties depending on expression of TMC1 or TMC2. Hair cells expressing only TMC1 (Tmc2-deficient) exhibited smaller single-channel conductance and lower Ca2+ permeability than those expressing only TMC2 (Tmc1-deficient) (59, 82). Disease-causing missense mutations in TMC1 have been found to result in alterations of MT channel pore properties in mouse cochlear hair cells (11, 15, 27, 43, 82). Pan et al. (82) first characterized the Bth mutation (M412K), which adds a positive charge in a predicted pore region. In the Tmc2-deficient background, they found that the M412K mutation in TMC1 caused a significant decrease of single-channel conductance and Ca2+ permeability in MT channel in mouse IHCs (82). These changes have also been observed in mouse OHCs (27). Although another study found a similar decrease in Ca2+ permeability with M412K mutation, it also reported unaltered single-channel conductance (11), perhaps because the measurements were carried out in the presence of native TMC2. More recently, another TMC1 mutation, D569N, which causes human deafness DFNA36, was shown to result in a dramatic reduction in Ca2+ permeability of the MT channel in mice, even though the single-channel conductance remained intact (11, 43); however, Jia et al. (52) showed a reduction in single-channel conductance in sea turtle TMC1 bearing the same substitution in the orthologous position. Notably, although this mutational data was exciting and in strong agreement with the idea that TMC1 plays a functional role as a pore-forming subunit of the MT channel, the possibility remained that TMC1 may be an auxiliary protein. This notion has precedent: The pore properties of the store-operated Ca2+ channel ORAI1 have been reported to be altered by a mutation in the accessory protein STIM (73), which does not line the channel pore.
To explore the contribution of TMC1 to the MT channel pore, Pan and colleagues (81) employed a cysteine modification assay to examine whether TMC1 residues directly line the MT channel pore. The idea was to introduce a cysteine substitution at positions hypothesized to line the pore. Addition of a cysteine modification reagent, e.g., MTSET, was expected to cause a reaction, forming a disulfide bond and perhaps altering ion permeation due to steric hindrance or charge repulsion. To avoid confounding factors, Pan et al. expressed TMC1 wild-type or cysteine mutants with viral vector in mouse hair cells that lacked native TMCs (Tmc1−/−;Tmc2−/−). Indeed, among the 17 cysteine substitutions tested, 13 of them caused changes in ion permeation of MT channels with or without application of MTSET. Specifically, 11 cysteine mutants showed reduced Ca2+ permeability due to either the mutation itself or exposure to the MTSET, and 5 mutants showed reduced macroscopic whole-cell currents. Noise analysis of three selected mutants revealed that they all had single-channel currents that were reduced by up to 70% following MTSET application. The reduced currents could not be reversed by washing out the MTSET but were recovered by exposure to the reducing reagent DTT, suggesting that the effect of MTSET is through covalent modification on the cysteine residue. More importantly, when the accessibility of the channel pore was inhibited by either the open-channel blockers or pore closure through deflection of the hair bundle toward short stereocilia, the cysteine modification by MTSET was largely prevented, which strongly suggests that the modifiable cysteine residues are indeed located inside the channel pore. These experiments provided the first unequivocal evidence demonstrating that TMC1 forms at least part of the pore of the MT channel. However, questions still remain as to whether TMC1 itself is sufficient to form an ion channel on its own, or whether it interacts with other subunits to form the MT channel.
Purified TMC1 and TMC2 confer mechanosensitive channel activity in liposomes.
A standard way to show whether a protein can form an ion channel by itself is to measure channel activity in a reduced system, such as heterologous cells or artificial lipid bilayers. This approach has been successfully applied to other mechanosensitive ion channels, including NOMPC and Piezo1/2 (28, 29, 107). However, TMC1 fails to traffic to the plasma membrane in various heterologous cells tested to date (41, 62), which has precluded electrophysiological examination. Instead, Jia et al. (52) turned to a liposome approach with purified TMC1 and TMC2 proteins, which allowed them to record both spontaneous and mechanically activated channel activities. With fluorescence detection size-exclusion chromatography assay, Jia and colleagues first screened 21 TMC1 and TMC2 orthologs from various species for appropriate candidates to be used in protein expression and purification. They identified TMC1 from the sea turtle Chelonia mydas (CmTMC1) and TMC2 from the budgerigar Melopsittacus undulates (MuTMC2) as having high expression levels in heterologous cells. To increase protein stability and yield, they truncated the predicted cytoplasmic N and C termini. The proteins were expressed and purified from insect Sf9 cells and reconstituted in liposomes for functional studies. With the patch-clamp technique, the investigators recorded robust spontaneous single-channel currents for both CmTMC1 and MuTMC2, which were validated with various MT channel blockers, including DHS, FM1–43, and neomycin. From these recordings, they calculated that CmTMC1 and MuTMC2 had single-channel conductance of approximately 40pS and 35 pS, respectively, values that are smaller than previous measurements from hair cell MT channels (approximately 100–300 pS) (13, 41, 59, 82). The reason for the discrepancy is unclear but might be related to the different environments surrounding the channels, such as lipid and protein binding partners and species of origin. By applying negative pressure on the liposome membrane, Jia et al. were able to record stretch-activated currents with high thresholds. They also introduced disease-causing point mutations into CmTMC1 and observed either reduced spontaneous and activated channel activity or no channel activity. In particular, the reduced single-channel conductance associated with the Bth mutation was consistent with previous reports (27, 82). CmTMC1 and MuTMC2 showed high sequence similarities with their mammalian counterparts; however, future studies are needed to examine mammalian TMC1 and TMC2 with similar methodology.
Functional studies of TMC orthologs in invertebrates.
TMC proteins are evolutionarily conserved from worm to human. Recent research progress on TMC protein functions in invertebrate species, especially Drosophila and C. elegans, have provided important clues regarding the function of their mammalian counterparts. In both widely used animal models, TMC proteins have been shown to be implicated in various physiological processes involving mechanotransduction, presumably as a mechanosensitive ion channel. While eight Tmc genes (Tmc1–8) were identified in mammals, the Drosophila genome encodes only one TMC protein. In 2016, Zhang et al. (110) reported that Drosophila tmc is expressed in a group of uncharacterized multidendritic (md-L) neurons located in the base of the sensilla in the belallum (the fly tongue) and is essential for md-L neuron–mediated food texture discrimination. The TMC protein is present in the dendritic arbors of the md-L neuron, where it is thought to act as a mechanosensitive ion channel, responding to deflection of sensilla hair caused by contact with the food surface. In support of this idea, genetic disruption of tmc eliminated mechanically activated action potentials in md-L neurons induced by sensilla deflection. At the same time, Guo et al. (44) showed that TMC is also expressed in Drosophila larval proprioceptors, the class I and II dendritic arborization (da) and bipolar dentrite (bd) neurons, and is required for proprioception, which is the sense of position and movement of body parts and provides sensory feedback for larval locomotion. Similar to md-L neurons, TMC proteins localize to the dendrites of da and bd neurons. Genetic deletion of tmc lead to defective locomotion in larva and diminished mechanosensitive Ca2+ influx in da and bd neurons. Interestingly, using high-speed confocal microscopy, two studies recently reported a high correlation between the Ca2+ responses in da and bd neurons and displacements of the sensory dendrites induced by bending of the larval body during movement (46, 100). These studies further support the idea that Drosophila TMC is an ion channel that is responsive to mechanical force. Interestingly, the defects in larval locomotion by tmc deletion can be rescued by exogenous expression of either Drosophila TMC or mammalian TMC1 or TMC2 (44), implying that TMC proteins may be functionally conserved in mechanosensation.
In C. elegans, two TMC proteins, TMC-1 and TMC-2, have been identified. The worm TMC-1 was initially described as mediating high salt chemosensation in ASH polymodal sensory neurons, and ectopic expression in heterologous cells conferred sodium-sensitive channel activity (22). However, this result has not been reproduced (103). Instead, Wang et al. (103) later reported that TMC-1 in ASH neurons mediates alkaline sensation and behavioral avoidance of high-pH environments in C. elegans. With whole-cell measurements, Wang and colleagues were able to record alkali-evoked currents in ASH neurons, which were dramatically reduced with tmc-1 deletion. In 2018, Yue et al. (108) showed that TMC-1 and TMC-2 are essential for normal egg laying in C. elegans and proposed a role for TMC proteins in maintaining membrane excitability by conducting Na+ leak currents. Using electrophysiological measurements from ASH neurons, they recorded background Na+ leak currents, which were abolished by tmc-1 deletion. The abolished currents were rescued by reintroduction of either worm TMC-1 or mammalian TMC1. Interestingly, mouse TMC1 has recently been reported to mediate a leak current on the apical surface of cochlear hair cells and modulate excitability of hair cells (66). Furthermore, echoing the role of mammalian TMC1 in hair cell MT currents, worm TMC-1 has been shown to be expressed in the cilia of mechanoreceptor OLQ neurons, where it is required for gentle nose-touch responses and touch-evoked Ca2+ responses (97). Intriguingly, ectopic expression of worm TMC-1 or mammalian TMC1 in mechanoinsensitive ASK neurons conferred mechanosensory activity. Taken together, the studies of TMC in Drosophila and C. elegans indicate ion channel functions in sensory processes, including chemo- and mechanosensation.
Hypothetical Structure of TMC1 by Homology Modeling
To gain insight into the assembly, function, and gating mechanism of TMC1 as an ion channel, atomic structures with high resolution in both open and closed states are needed. To date, no structure of TMC1 or any other TMC member has been published. Combining biochemical characterizations and electron microscopy of purified TMC1 protein from human embryonic kidney cells, Pan et al. (81) showed that mouse or human TMC1 assembles as a dimer, which was confirmed by a later study showing that CmTMC1 or MuTMC2 purified from insect Sf9 cells also forms dimers (52). Based on limited protein sequence similarity, the TMC family was suggested to be related to the TMEM16 family, which contains ion channels and lipid scramblases (45, 74). Using the atomic structures of mouse Ca2+-activated Cl− channel mmTMEM16a (34, 83) and the fungus Nectria hematococca lipid scramblase nhTMEM16 (21), our group and the Swartz group independently generated similar TMC1 structural models (7, 81). Like the TMEM16 structures, the predicted TMC1 model structures showed a dimeric assembly, with each subunit containing 10 transmembrane domains (TMs) and intracellular N and C termini (Figure 2). Within each monomer, an ion conduction pathway was found to be lined by TM4–TM7, which is in agreement with our functional study showing that 13 TMC1 residues located throughout TM4–TM7 face the MT channel pore (81). Interestingly, a large cavity was observed near the pore–lipid interface, which potentially explains the permeation of large molecules of up to 3 kDa, such as dextrans, through MT channels (7). We noticed some conserved acidic residues in the TMC1 extracellular TM3/TM4 loop and TM5/TM6 loop sitting on the top of the pore, which are not present in the anion channel TMEM16a. These residues create an overall negative electrostatic environment at the pore entrance, which may help concentrate cations proximal to the pore and enhance conductance. A similar arrangement was observed in TRP nonselective cation channels (68). Interestingly, the Bth mutation (M412K) added a positive charge to the 412 position, which is located in TM4 of the predicted structure and faces the pore. In agreement with this location, hair cells containing the Bth mutations have smaller single-channel conductance and lower Ca2+ permeability (11, 27, 82). Although the predicted structure of TMC1 can reasonably explain many aspects of MT channel biophysics, a true high-resolution atomic structure is required.
Figure 2.
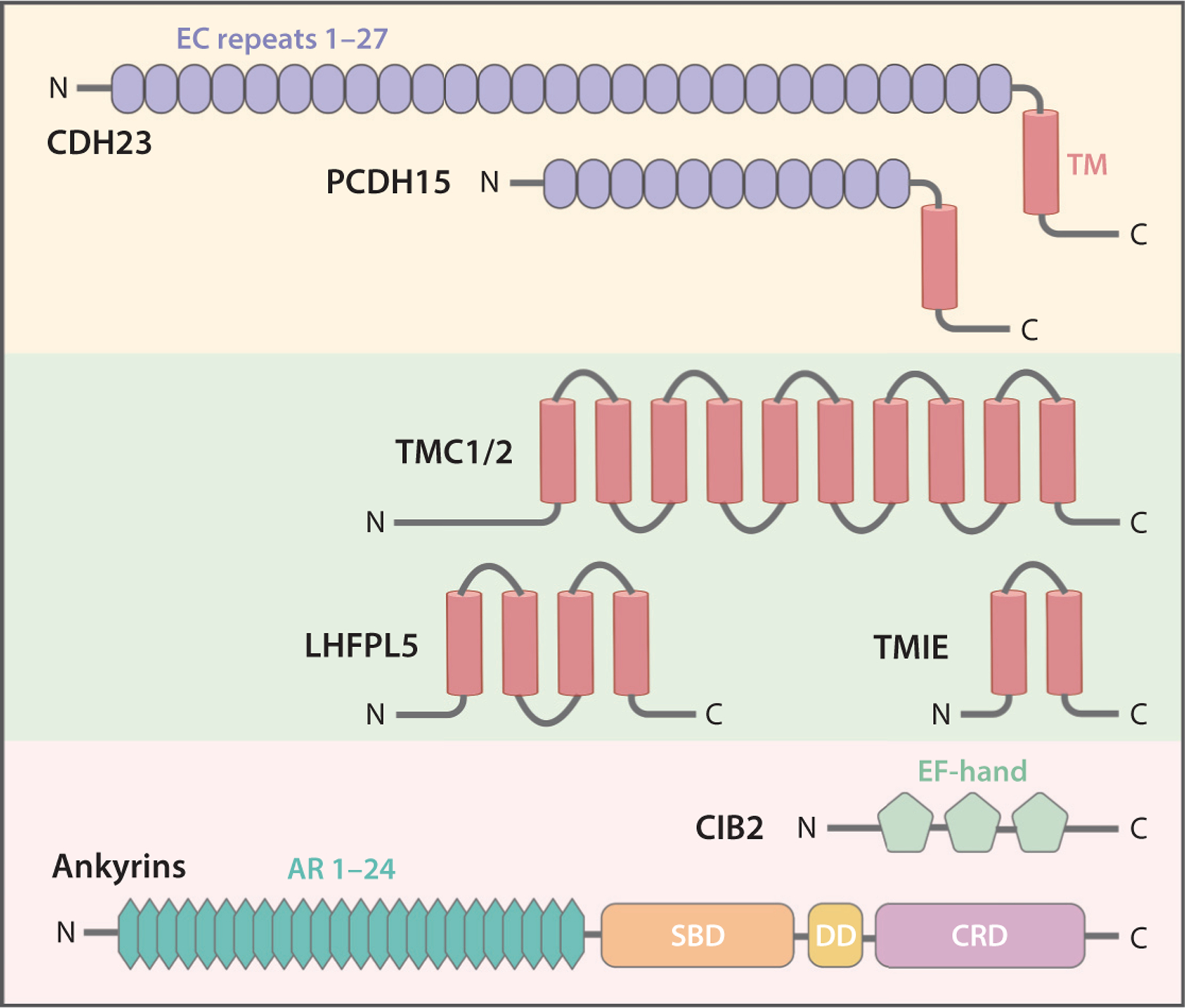
Protein molecules that are integral components of the transduction machinery. Secondary protein domain structures are indicated. Abbreviations: AR, ankyrin repeat; CDH23, cadherin 23; CIB2, calcium and integrin-binding family member 2; CRD, C-terminal regulatory domain; DD, death domain; EC, extracellular cadherin; LHFPL5, lipoma HMGIC fusion partner-like 5; PCDH15, protocadherin 15; SBD, spectrin-binding domain; TM, transmembrane domain; TMC1/2, transmembrane channel-like 1/2; TMIE, transmembrane inner ear.
MOLECULAR COMPONENTS OF MECHANOSENSORY TRANSDUCTION MACHINERY
In addition to TMC1 and TMC2, about a dozen other proteins, identified in genetic studies of deafness, have been shown to be directly involved in hair cell MT. Together with TMC1 and TMC2, these proteins coassemble to form the MT machinery. Studies of these components have allowed further insight into the transduction mechanism of this exquisite molecular apparatus.
CDH23 and PCDH15
Tip-links were discovered in 1984 in ultrastructural studies of hair bundles, which revealed fine extracellular filaments that connect the tips of shorter stereocilia to the side of the next taller row (84). This orientation of tip-links provides directional sensitivity, i.e., bundle deflection toward the tallest edge transmits force to the tip-links, while the opposite deflection reduces force. Later studies with electron microscopy revealed that tip-links are composed of a pair of coiled filaments 8–11 nm in diameter and approximately 150 nm in length (54, 99). Genetic studies of Usher syndrome type 1 (1, 3, 19, 20, 36) revealed that tip-links are comprised of the cell adhesion proteins CDH23 and PCDH15. Both CDH23 and PCDH15 have long extracellular N termini containing multiple cadherin repeats, a transmembrane domain, and short cytoplasmic C termini (Figure 2). Follow-up studies demonstrated that parallel PCDH15 homodimers interact with parallel CDH23 homodimers through their N termini, with their cytoplasmic domains localizing at the lower and upper ends of tip-link, respectively (see, e.g., 57). Structural studies revealed that the linkage between the two cadherin proteins is mediated by trans-interactions through their first two cadherin repeats (94, 95). Since their discovery, tip-links have been proposed to transmit mechanical force to the MT channels (84), localized at the tips of the shorter stereocilia (14). Indeed, MT current is highly dependent on the integrity of tip-links (6, 65, 112) and is disrupted in mice with Pcdh15 or Cdh23 mutations (2). In vitro biochemical assays suggested that the tip-link lower component PCDH15 interacts with TMC1 (15, 69). However, such interaction was not observed in a subsequent structural study (39), which instead found a stable complex formation between PCDH15 and LHFPL5, another transmembrane protein component of MT machinery (see below). An atomic structure of PCDH15/LHFPL5 (39) revealed that the dimeric PCDH15 forms a complex with two LHFPL5 molecules in a twofold symmetry. Extensive interactions among the TMs of the two proteins have been observed to mediate the overall PCDH15/LHFPL5 assembly. The two LHFPL5 proteins act like a clamp to stabilize or immobilize the two TMs of the dimeric PCDH15. Thus, the force in the tip-link may be transmitted to the MT channel through LHFPL5. More structural and functional studies are needed to further clarify force transmission from the tip-links to the MT channels and opening of channel pores.
LHFPL5
Lhfpl5, also known as Tmhs, was first identified in 2005 in a mutant mouse model (Hurry-scurry) with deafness and vestibular dysfunction (67). A year later, mutations in human Lhfpl5 were identified and associated with autosomal recessive nonsyndromic hearing loss (DFNB67) (55, 92). LHFPL5 is a membrane protein predicted to have four TMs and belongs to a superfamily of tetraspan proteins (Figure 2).
To determine the precise function of LHFPL5 in hearing, Xiong et al. (106) first explored the expression and localization of LHFPL5 and found that the LHFPL5 protein can be detected in stereocilia of mouse cochlear hair cells during the first postnatal week, with the emergence of MT currents. A more comprehensive study (70) found that, during the first postnatal week (P3–P6), LHFPL5 reached peak expression and was localized to the tips of stereocilia in all rows. By P12, LHFPL5 expression level declined slightly and became restricted to the tips of shorter stereocilia, where MT occurs (14). LHFPL5, exogenously expressed by electroporation in hair cells in vitro, was also targeted to stereocilia tips (106).
In Lhfpl5-null hair cells, the macroscopic MT currents were found to be reduced by 90% (106), but the number of tip-links was also dramatically reduced (106), and the localization of TMC1 in the stereocilia was impaired (17). Thus, LHFPL5 is important for tip-link assembly and TMC1 subcellular trafficking. Further analysis of residual MT currents in Lhfpl5-deficient hair cells revealed reduced unitary conductance and slower activation (17, 106), which suggested that LHFPL5 is also directly involved in MT. This concept is supported by biochemical data showing that LHFPL5 physically interacts with PCDH15 (17, 106), which is confirmed by the atomic structure of PCDH15/LHFPL5. LHFPL5 might act as an intermediate protein to transmit force from the tip-link to the MT channel, but the link between LHFPL5 and the MT channel is unclear. Whether force is transmitted via a direct protein interaction or via a stretch of local membrane lipid is under investigation. Physical interaction between LHFPL5 and TMC1 was not observed with coimmunoprecipitation (17); thus, further examination is needed, perhaps with more sensitive assays or in the presence of TMIE, another membrane protein component of the MT machinery.
TMIE
Using a positional cloning strategy, Tmie was coincidently identified in 2002 from deaf mice (the spinner mouse) (76) and humans (DFNB6) (78). A subsequent study also identified a frameshift mutation of tmie in zebrafish with deafness and defective balance (42). With no sequence homology to any other protein, TMIE is an integral membrane protein predicted to contain two TMs with cytoplasmic N and C termini (Figure 2). Tmie-deficient hair cells showed complete loss of MT currents; this loss was fully rescued by exogenous expression of wild-type TMIE but only partially recovered by TMIE carrying missense mutations associated with deafness in humans (33, 111). In Tmie-null mice, immunofluorescence showed that TMC1 no longer trafficked to stereocilia but remained in the cell body, while TMC2 localization in stereocilia was only mildly affected (33). Likewise, in zebrafish tmie mutants, GFP-tagged Tmc1 and Tmc2b failed to target to the hair bundle, and overexpression of Tmie rescued localization of Tmc1 and Tmc2b (80). Thus, like LHFPL5, TMIE is required for proper trafficking of TMC1 to stereocilia, which explains, at least in part, the absence of MT currents in Tmie-knockout mice.
Multiple lines of evidence support a direct role for TMIE as an integral component of the MT complex. First, TMIE is expressed in the shorter stereocilia of mouse cochlear hair cells as early as the first postnatal week, when MT currents begin to emerge (33, 76, 111). Second, in the case of acute overexpression of TMC1 in Tmie-deficient hair cells, little MT current was recorded, even when robust TMC1 localization at the tips of stereocilia was observed (33). Third, deafness-associated point mutations within the TMIE C terminus resulted in small changes in unitary conductance and Ca2+ permeability. Although they were modest, the data suggest that TMIE may be closely associated with MT channels (33), perhaps as an accessory subunit in a manner similar to the association between TARP proteins and the pore-forming subunit of AMPA receptors. Finally, with coimmunoprecipitation, TMIE has been shown to be in the same complex with PCDH15 and LHFPL5 (111), and more recently, TMIE was also shown to physically interact with TMC1 and TMC2 (33). Although these interactions need to be confirmed, TMIE could act as a coupling protein to link TMC1 or TMC2 to the LHFPL5/PCDH15 tip-link complex.
CIB2
CIB2 is a small protein that belongs to a family containing four members (CIB1–4), which are characterized by multiple EF-hand domains that bind with Ca2+ (18, 48) (Figure 2). Mutations in CIB2 were first identified as associated with human nonsyndromic deafness DFNB48 and Usher syndrome type 1J (88). Mice harboring either a null mutation or a point mutation linked with human disease also showed early onset profound hearing loss (41, 75, 104).
CIB2 is present in the stereocilia of mouse cochlear hair cells, and deletion of Cib2 completely abolishes MT currents (41, 75, 88, 104). The hair bundle and tip-link were seemingly normal when MT currents were measured during the first postnatal week, even though the regression of the stereocilia and hair cell death have been observed at later stages (41, 75, 104). Localization of TMC1 and TMC2 in stereocilia appears normal in the absence of CIB2 (41). Moreover, CIB2 binds to the cytoplasmic N terminus of TMC1 or TMC2 when expressed in heterologous cells, and this interaction was diminished with a disease-causing missense mutation in CIB2 (41). These data provide consistent and compelling evidence to support the idea that CIB2 contributes to hair cell MT through interaction with TMC1 and TMC2.
A recent study (97) in C. elegans offers important clues to the precise role of CIB2 in TMC-1 mechanotransduction. Using coimmunoprecipitation and proteomic screening, Tang et al. (97) identified UNC-44, the sole C. elegans homolog to mammalian ankyrin proteins, as a binding partner to CALM-1, the CIB homolog in C. elegans. Further in vitro biochemical assays revealed that UNC-44/ankyrin protein physically connects to the N terminus of TMC-1 via CALM-1/CIB, and in vivo functional studies found that this TMC-1/CIB/ankyrin triple complex is required for TMC-1-mediated mechanosensitivity in C. elegans mechanoreceptor OLQ neurons. Since UNC-44/ankyrin protein is known to connect to the actin filament network via spectrin proteins (10), the TMC-1/CIB/ankyrin/spectrin/actin cytoskeleton forms an intriguing model for TMC1 mechanosensitivity in mammalian hair cells.
Ankyrin Proteins
As mentioned above, the UNC-4/ankyrin protein was recently shown to bind to TMC-1 via CALM-1/CIB in C. elegans and was shown to be essential for TMC-1 mechanosensitivity. Whether this mechanism also applies to mammalian TMC1 in hair cells needs to be explored. There are three ankyrin genes in mammals, ANK1, 2, and 3, which encode ankR, ankB, and ankG proteins, respectively. These three isoforms have specific tissue distributions, and it is currently unclear whether these isoforms are expressed in mammalian auditory hair cells. Ankyrins function as scaffold proteins to link integral membrane proteins, including ion channels and signaling receptors, with the β-spectrin/actin cytoskeleton beneath plasma membranes and thereby provide mechanical support for membranes (10, 32). Each ankyrin isoform contains 24 ankyrin repeats in its N terminus, which form a complete superhelical turn with spring properties (23, 102) (Figure 2), similar to that formed by the 29 ankyrin repeats in the mechanosensitive NOMPC channel (53). Interestingly, atomic force microscopy measurements and molecular dynamics simulations of ankyrin-repeat structures (63, 93) indicate that their extensibility and elasticity match those predicted by the gating spring model of MT channels (see below). Thus, ankyrin proteins are potential candidates for the long-sought gating springs that tether MT channels to the cytoskeleton.
MODELS FOR MECHANOSENSORY TRANSDUCTION
With little information about the molecular components of the MT machinery, early hypothetical models of MT were proposed based solely on the kinetics of MT channel activation and mechanical properties of hair bundles. These models have been updated and evolved with the emergence of new information, in particular, that obtained from identification and characterization of individual molecular components. In response to deflection of the hair bundle, MT channels open with time constants in the microsecond range (30, 90), suggesting that channel activation does not occur through a second-messenger cascade, but instead is directly gated by mechanical force. With measurements and analyses of nonlinear relationships among hair bundle mechanics, hair bundle deflection, and channel gating, it was hypothesized that MT channel activation involves an elastic gating spring element, which is in series with the channel protein and is stretched upon hair bundle deflection (26, 47). Combining the model and experimental data, the gating spring was estimated to have a stiffness of approximately 1 mN/m and a gate that moves by approximately 2.5 nm in association with channel conformational change during activation (24, 47). Tip-links were initially proposed to be gating springs (50, 84), but subsequent data from structural studies and molecular dynamics simulations indicated that tip-links are too rigid to be gating springs by themselves (54, 93–95), although more recent evidence revealed that tip-links have some elasticity (4, 8, 98). It is possible that the biophysically defined gating spring may include multiple molecular components, with the tip-link being one of them. Other possible components that contribute to the gating spring include the stereocilia membrane, intracellular tether proteins, and MT channel domains. Two broad models for MT channel activation have been proposed, the tethered-channel model and the lateral-tension model. Based on recent breakthroughs, we propose a grand unifying theory for hair cell MT.
The Tethered-Channel Model
In the tethered-channel model, MT channels are physically anchored to extracellular tip-links and the intracellular cytoskeleton. The forces that tip-links exert on channels through protein–protein interactions open channels directly. This model predicts that channels are connected to the intracellular cytoskeleton directly via tether proteins. The recently identified TMC-1/CIB/ankyrin functional triple complex in C. elegans (97) provided strong support for the tethered-channel model and raised an intriguing hypothesis that ankyrin proteins may serve as the long-sought gating spring. Interestingly, an intracellular filament has been previously proposed to exist between tip-links and the cytoskeleton (85). Other supporting evidence for this model came from reported extensive protein–protein interactions among components of MT machinery. However, the physical link between TMC1 and tip-links has not been firmly established, which casts doubt on the idea that force is transmitted from the tip-links to TMC1 via protein–protein interactions. In addition, although previous studies estimated only 1–2 MT channels for each stereocilia (see, e.g., 89), a recent study with single-molecule photobleaching and analyses of single-channel recordings suggested that there may be 8–20 MT channels in each stereocilium depending on location along the tonotopic axis of the cochlea (12). However, this may be an overestimate, since the study examined transgenic mice with multiple copies of the transgene and potential overexpression of fluorescently tagged TMCs. If the result is accurate, then it may be challenging to reconcile a larger and variable number of MT channels with the dimeric PCDH15 structure and the tethered-channel model.
The Lateral-Tension Model
In the lateral-tension model, MT channels sit freely, untethered in the membrane at stereocilia tips, and the membrane tension, induced by the pulling force in tip-links, is sufficient to open channels. There is no direct physical connection of MT channels to either tip-links or the intracellular cytoskeleton. In this scenario, the membrane tension itself may function to gate the channels. This model can easily explain the existence of multiple MT channels in each stereocilium (12) and is supported by the observation of tenting, in which the membrane in the tip of shorter stereocilia is pulled away from the underlying cytoskeleton by tip-link tension (6). Recently, purified CmTMC1 and MuTMC2, reconstituted in liposomes, were shown to be activated by negative pressure applied on the membranes (52), supporting the idea that membrane tension is sufficient to activate TMC1 and TMC2. However, thresholds for these membrane tension–induced activations were very high (52), and thus this theory cannot explain the exquisite sensitivity of MT channels to mechanical forces in hair cells. Computational modeling of membrane deformation by tension in tip-links suggested that the membrane alone is too compliant to be the gating spring, and an intracellular tether is required (86). Given the discovery of the TMC-1/CIB/ankyrin triple complex in C. elegans, it is intriguing that TMC1 or TMC2 mechanosensitivity may be tuned by CIB/ankyrin to be high enough to respond to small hair bundle deflections in mammalian cochleas (97). The lateral-tension model is also at odds with the observation of gating compliance, a measure of the tight coupling between MT channel opening and hair bundle relaxation (47). It seems less likely that the rapid and reproducible compliance could be transmitted by membrane lipids from untethered MT channels.
Grand Unifying Theory for Hair Cell Mechanosensory Transduction
As discussed above, each of the two models has both supporting and opposing evidence. The two models are not mutually exclusive. With recent data, we can reconcile them and propose a unifying model (Figure 3). In this new model, MT channels (TMC1/2) are connected to the cytoskeleton via CIB/ankyrin (gating spring) but not physically attached to tip-links. During hair bundle deflection, the force in tip-links pulls the shorter stereocilia tip membrane away from the cytoskeleton beneath. The induced membrane stretch and spring force in the CIB/ankyrin cytoskeletal anchor synergistically open the TMC1/2 pore. In this case, tip-links function to transmit membrane tension locally in the vicinity of the TMC/CIB/ankyrin complex but are not directly connected to the complex. The TMC/CIB/ankyrin complex may function in a manner similar to mechanosensitive NOMPC channels in Drosophila (109). The two LHFPL5 proteins (each with four TMs) form a complex with one PCDH15 dimer (each with one TM), with extensive interaction between their TMs (39) and the cell membrane, which may help anchor the complex in the membrane and facilitate local delivery of tension to the membrane. TMIE is likely part of the complex with TMC1/2 (33) and may either act as an accessory protein to modulate channel gating and pore properties or directly contribute to pore formation. Since TMIE has been observed to interact with PCDH15/LHPFL5 (111), it might also function to hold TMC1/2 in the vicinity of the lower tip-link complex and facilitate proper responses to membrane tension. This new model is compatible with the majority of experimental evidence obtained to date and awaits validation and revision as further structural and functional data emerge.
Figure 3.
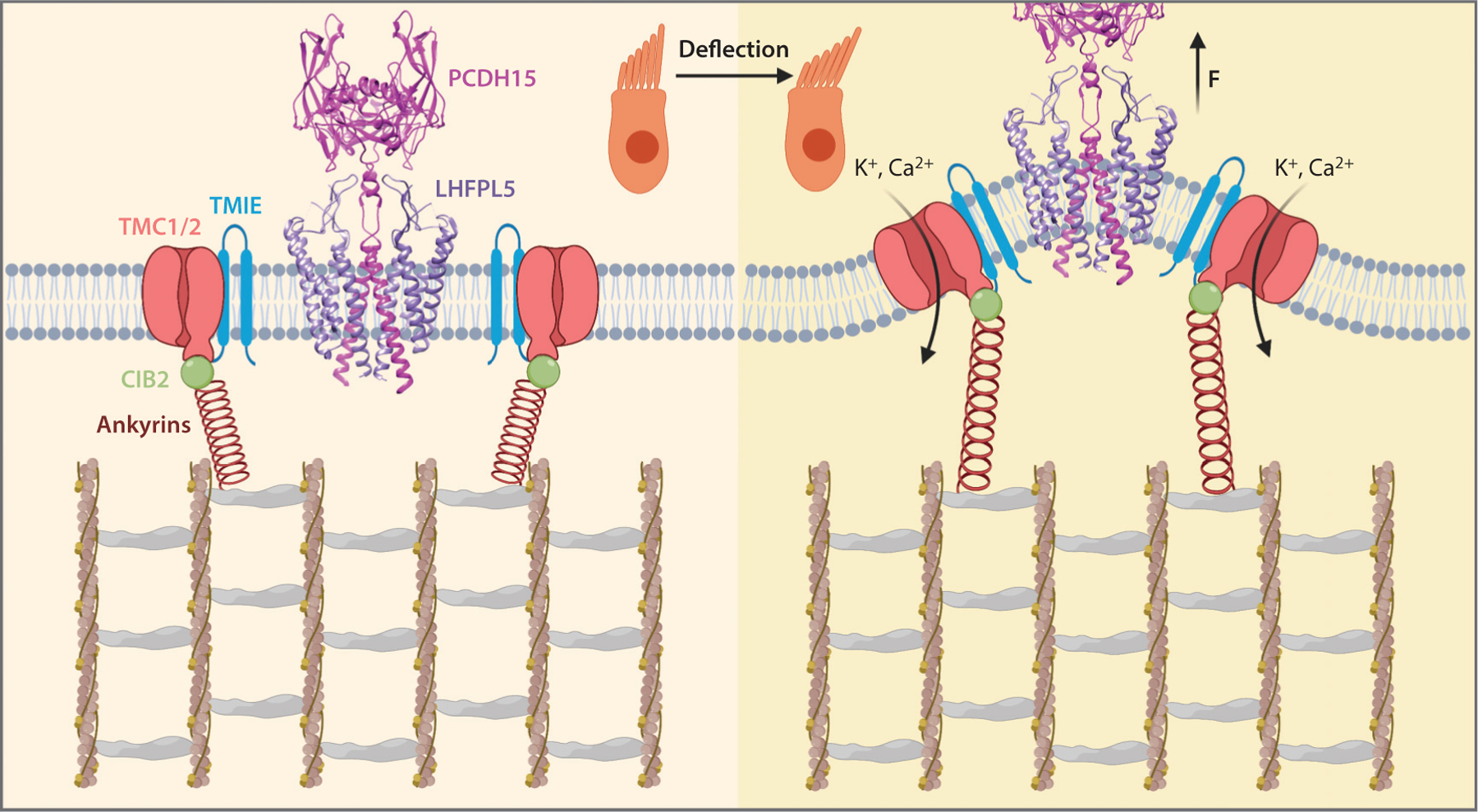
A grand unifying model of hair cell MT. The MT channel TMC1 is in complex with TMIE and is attached to the actin filament cytoskeleton via CIB2/ankyrin tether. The TMC1/TMIE complex sits in the membrane area surrounding the PCDH15/LHFPL5 complex, without physical connection. Upon hair bundle deflection, the tip membrane of shorter stereocilia is pulled away from the underlying cytoskeleton by the tip-link. TMC1 is then activated by tension in the membrane and ankyrin proteins, i.e., the gating spring. Abbreviations: CIB2, calcium and integrin-binding family member 2; LHFPL5, lipoma HMGIC fusion partner-like 5; MT, mechanosensory transduction; PCDH15, protocadherin 15; TMC1, transmembrane channel-like 1; TMIE, transmembrane inner ear.
CONCLUDING REMARKS
The past several decades have witnessed extraordinary progress in the field of hair cell MT. With the identification of multiple components of the MT machinery and elucidation of TMC1 as the MT channel, we have gained enormous insight into the mechanisms of MT at a molecular level. With the development of cryo-electron microscopy, there is opportunity to study individual components of the MT complex and potential cooperation among them at the atomic level. These efforts may finally allow us to obtain a complete understanding of how mechanical force is transmitted to MT channels to open the pore and initiate the sense of hearing. Several key issues remain to be resolved. The most prominent task is to reconstitute the MT machinery in a reduced system, either heterologous cells or artificial lipid bilayer. This will not only deepen our understanding of the molecular composition of the MT complex, but also offer a valuable tool to study force-mediated gating mechanisms in greater detail. Next, we need to determine the atomic structure of each individual component of the MT machinery, especially the MT channels TMC1 and TMC2, with the final goal of resolving the structure of the MT complex as a whole. In addition, mammalian TMC1 and TMC2 need to be tested for ion channel activity in the same way as CmTMC1 and MuTMC2 have been. Moreover, the involvement and functional role of ankyrin proteins in hair cell MT merit further exploration. Finally, more efforts are needed to elucidate mechanisms underlying the pathology of deafness-causing mutations in MT machinery components. We envision that the next decade could be the most exciting era for the study of MT and hearing, and future discoveries will not only shape our fundamental understanding of the transduction process, but also help translate these discoveries into novel therapeutics for human hearing loss.
ACKNOWLEDGMENTS
We thank members of the Holt/Géléoc Lab for helpful discussions and critical review of an earlier version of this manuscript. This work was supported by a National Institutes of Health/National Institute on Deafness and Other Communication Disorders grant to J.R.H. (RO1 DC013521) and by the Fondation Pour L’Audition.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, et al. 2001. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am. J. Hum. Genet 69:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, et al. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLOS ONE 6:e19183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, et al. 2001. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum. Mol. Genet 10:1709–18 [DOI] [PubMed] [Google Scholar]
- 4.Araya-Secchi R, Neel BL, Sotomayor M. 2016. An elastic element in the protocadherin-15 tip link of the inner ear. Nat. Commun 7:13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asai Y, Pan B, Nist-Lund C, Galvin A, Lukashkin AN, et al. 2018. Transgenic Tmc2 expression preserves inner ear hair cells and vestibular function in mice lacking Tmc1. Sci. Rep 8:12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Assad JA, Shepherd GM, Corey DP. 1991. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7:985–94 [DOI] [PubMed] [Google Scholar]
- 7.Ballesteros A, Fenollar-Ferrer C, Swartz KJ. 2018. Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. eLife 7:e38433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartsch TF, Hengel FE, Oswald A, Dionne G, Chipendo IV, et al. 2019. Elasticity of individual protocadherin 15 molecules implicates tip links as the gating springs for hearing. PNAS 116:11048–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu A, Lagier S, Vologodskaia M, Fabella BA, Hudspeth AJ. 2016. Direct mechanical stimulation of tip links in hair cells through DNA tethers. eLife 5:e16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett V, Lorenzo DN. 2016. An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr. Top. Membr 77:143–84 [DOI] [PubMed] [Google Scholar]
- 11.Beurg M, Barlow A, Furness DN, Fettiplace R. 2019. A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. PNAS 116:20743–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beurg M, Cui R, Goldring AC, Ebrahim S, Fettiplace R, Kachar B. 2018. Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat. Commun 9:2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurg M, Evans MG, Hackney CM, Fettiplace R. 2006. A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J. Neurosci 26:10992–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beurg M, Fettiplace R, Nam JH, Ricci AJ. 2009. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci 12:553–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beurg M, Goldring AC, Fettiplace R. 2015. The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J. Gen. Physiol 146:233–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beurg M, Kim KX, Fettiplace R. 2014. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J. Gen. Physiol 144:55–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beurg M, Xiong W, Zhao B, Muller U, Fettiplace R. 2015. Subunit determination of the conductance of hair-cell mechanotransducer channels. PNAS 112:1589–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blazejczyk M, Sobczak A, Debowska K, Wisniewska MB, Kirilenko A, et al. 2009. Biochemical characterization and expression analysis of a novel EF-hand Ca2+ binding protein calmyrin2 (Cib2) in brain indicates its function in NMDA receptor mediated Ca2+ signaling. Arch. Biochem. Biophys 487:66–78 [DOI] [PubMed] [Google Scholar]
- 19.Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, et al. 2001. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat. Genet 27:108–12 [DOI] [PubMed] [Google Scholar]
- 20.Bork JM, Peters LM, Riazuddin S, Bernstein SL, Ahmed ZM, et al. 2001. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am. J. Hum. Genet 68:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunner JD, Lim NK, Schenck S, Duerst A, Dutzler R. 2014. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 516:207–12 [DOI] [PubMed] [Google Scholar]
- 22.Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. 2013. Tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 494:95–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Li J, Wang C, Wei Z, Zhang M. 2017. Autoinhibition of ankyrin-B/G membrane target bindings by intrinsically disordered segments from the tail regions. eLife 6:e29150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung EL, Corey DP. 2006. Ca2+ changes the force sensitivity of the hair-cell transduction channel. Biophys. J 90:124–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corey DP, Hudspeth AJ. 1979. Ionic basis of the receptor potential in a vertebrate hair cell. Nature 281:675–77 [DOI] [PubMed] [Google Scholar]
- 26.Corey DP, Hudspeth AJ. 1983. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci 3:962–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corns LF, Johnson SL, Kros CJ, Marcotti W. 2016. Tmc1 point mutation affects Ca2+ sensitivity and block by dihydrostreptomycin of the mechanoelectrical transducer current of mouse outer hair cells. J. Neurosci 36:336–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, et al. 2010. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, et al. 2012. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483:176–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford AC, Evans MG, Fettiplace R. 1989. Activation and adaptation of transducer currents in turtle hair cells. J. Physiol 419:405–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crawford AC, Evans MG, Fettiplace R. 1991. The actions of calcium on the mechano-electrical transducer current of turtle hair cells. J. Physiol 434:369–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunha SR, Mohler PJ. 2009. Ankyrin protein networks in membrane formation and stabilization. J. Cell Mol. Med 13:4364–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, et al. 2020. TMIE defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dang S, Feng S, Tien J, Peters CJ, Bulkley D, et al. 2017. Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552:426–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deol MS, Kocher W. 1958. A new gene for deafness in the mouse. Heredity 12:463–66 [Google Scholar]
- 36.Di PF, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, et al. 2001. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in Waltzer, the mouse model for Usher syndrome type 1D. Nat. Genet 27:103–7 [DOI] [PubMed] [Google Scholar]
- 37.Dror AA, Avraham KB. 2009. Hearing loss: mechanisms revealed by genetics and cell biology. Annu. Rev. Genet 43:411–37 [DOI] [PubMed] [Google Scholar]
- 38.Farris HE, LeBlanc CL, Goswami J, Ricci AJ. 2004. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J. Physiol 558:769–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ge J, Elferich J, Goehring A, Zhao H, Schuck P, Gouaux E. 2018. Structure of mouse protocadherin 15 of the stereocilia tip link in complex with LHFPL5. eLife 7:e38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geleoc GS, Lennan GW, Richardson GP, Kros CJ. 1997. A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc. Biol. Sci 264:611–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giese APJ, Tang YQ, Sinha GP, Bowl MR, Goldring AC, et al. 2017. CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat. Commun 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, Hudspeth AJ. 2009. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. PNAS 106:21347–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldring AC, Beurg M, Fettiplace R. 2019. The contribution of TMC1 to adaptation of mechanoelectrical transduction channels in cochlear outer hair cells. J. Physiol 597:5949–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y, Wang Y, Zhang W, Meltzer S, Zanini D, et al. 2016. Transmembrane channel-like (tmc) gene regulates Drosophila larval locomotion. PNAS 113:7243–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hahn Y, Kim DS, Pastan IH, Lee B. 2009. Anoctamin and transmembrane channel-like proteins are evolutionarily related. Int. J. Mol. Med 24:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He L, Gulyanon S, Mihovilovic SM, Karagyozov D, Heckscher ES, et al. 2019. Direction selectivity in Drosophila proprioceptors requires the mechanosensory channel Tmc. Curr. Biol 29:945–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howard J, Hudspeth AJ. 1988. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1:189–99 [DOI] [PubMed] [Google Scholar]
- 48.Huang H, Ishida H, Yamniuk AP, Vogel HJ. 2011. Solution structures of Ca2+−CIB1 and Mg2+−CIB1 and their interactions with the platelet integrin alphaIIb cytoplasmic domain. J. Biol. Chem 286:17181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudspeth AJ. 1985. The cellular basis of hearing: the biophysics of hair cells. Science 230:745–52 [DOI] [PubMed] [Google Scholar]
- 50.Hudspeth AJ. 1992. Hair-bundle mechanics and a model for mechanoelectrical transduction by hair cells. Soc. Gen. Physiol. Ser 47:357–70 [PubMed] [Google Scholar]
- 51.Jain PK, Fukushima K, Deshmukh D, Ramesh A, Thomas E, et al. 1995. A human recessive neurosensory nonsyndromic hearing impairment locus is potential homologue of murine deafness (dn) locus. Hum. Mol. Genet 4:2391–94 [DOI] [PubMed] [Google Scholar]
- 52.Jia Y, Zhao Y, Kusakizako T, Wang Y, Pan C, et al. 2020. TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron 105:310–21 [DOI] [PubMed] [Google Scholar]
- 53.Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, et al. 2017. Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547:118–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. 2000. High-resolution structure of hair-cell tip links. PNAS 97:13336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalay E, Li Y, Uzumcu A, Uyguner O, Collin RW, et al. 2006. Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum. Mutat 27:633–39 [DOI] [PubMed] [Google Scholar]
- 56.Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, et al. 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Investig 121:4796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, et al. 2007. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449:87–91 [DOI] [PubMed] [Google Scholar]
- 58.Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R. 2013. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J. Gen. Physiol 142:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim KX, Fettiplace R. 2013. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J. Gen. Physiol 141:141–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, et al. 2015. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep. 12:1606–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, et al. 2002. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet 30:277–84 [DOI] [PubMed] [Google Scholar]
- 62.Labay V, Weichert RM, Makishima T, Griffith AJ. 2010. Topology of transmembrane channel-like gene 1 protein. Biochemistry 49:8592–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. 2006. Nanospring behaviour of ankyrin repeats. Nature 440:246–49 [DOI] [PubMed] [Google Scholar]
- 64.Lelli A, Asai Y, Forge A, Holt JR, Geleoc GS. 2009. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J. Neurophysiol 101:2961–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lelli A, Kazmierczak P, Kawashima Y, Muller U, Holt JR. 2010. Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15. J. Neurosci 30:11259–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu S, Wang S, Zou L, Li J, Song C, et al. 2019. TMC1 is an essential component of a leak channel that modulates tonotopy and excitability of auditory hair cells in mice. eLife 8:e47441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR. 2005. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. PNAS 102:7894–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.López-Romero AE, Hernández-Araiza I, Torres-Quiroz F, Tovar YRL, Islas LD, Rosenbaum T. 2019. TRP ion channels: proteins with conformational flexibility. Channels 13:207–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, et al. 2014. Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. PNAS 111:12907–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahendrasingam S, Fettiplace R, Alagramam KN, Cross E, Furness DN. 2017. Spatiotemporal changes in the distribution of LHFPL5 in mice cochlear hair bundles during development and in the absence of PCDH15. PLOS ONE 12:e0185285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahendrasingam S, Furness DN. 2019. Ultrastructural localization of the likely mechanoelectrical transduction channel protein, transmembrane-like channel 1 (TMC1) during development of cochlear hair cells. Sci. Rep 9:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ. 2006. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J. Physiol 574:677–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNally BA, Somasundaram A, Yamashita M, Prakriya M. 2012. Gated regulation of CRAC channel ion selectivity by STIM1. Nature 482:241–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medrano-Soto A, Moreno-Hagelsieb G, McLaughlin D, Ye ZS, Hendargo KJ, Saier MH Jr. 2018. Bioinformatic characterization of the Anoctamin Superfamily of Ca2+-activated ion channels and lipid scramblases. PLOS ONE 13:e0192851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michel V, Booth KT, Patni P, Cortese M, Azaiez H, et al. 2017. CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol. Med 9:1711–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, et al. 2002. Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum. Mol. Genet 11:1887–98 [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi H, Kurima K, Pan B, Wangemann P, Fitzgerald TS, et al. 2018. Tmc2 expression partially restores auditory function in a mouse model of DFNB7/B11 deafness caused by loss of Tmc1 function. Sci. Rep 8:12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, et al. 2002. Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am. J. Hum. Genet 71:632–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ohmori H 1985. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J. Physiol 359:189–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pacentine IV, Nicolson T. 2019. Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells. PLOS Genet. 15:e1007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pan B, Akyuz N, Liu XP, Asai Y, Nist-Lund C, et al. 2018. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron 99:736–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, et al. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paulino C, Kalienkova V, Lam AKM, Neldner Y, Dutzler R. 2017. Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552:421–25 [DOI] [PubMed] [Google Scholar]
- 84.Pickles JO, Comis SD, Osborne MP. 1984. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res 15:103–12 [DOI] [PubMed] [Google Scholar]
- 85.Pickles JO, Rouse GW, von Perger M. 1991. Morphological correlates of mechanotransduction in acousticolateral hair cells. Scanning Microsc. 5:1115–24 [PubMed] [Google Scholar]
- 86.Powers RJ, Roy S, Atilgan E, Brownell WE, Sun SX, et al. 2012. Stereocilia membrane deformation: implications for the gating spring and mechanotransduction channel. Biophys. J 102:201–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, et al. 2014. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516:121–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, et al. 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet 44:1265–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricci AJ, Crawford AC, Fettiplace R. 2003. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40:983–90 [DOI] [PubMed] [Google Scholar]
- 90.Ricci AJ, Kennedy HJ, Crawford AC, Fettiplace R. 2005. The transduction channel filter in auditory hair cells. J. Neurosci 25:7831–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwander M, Kachar B, Muller U. 2010. The cell biology of hearing. J. Cell Biol 190:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, et al. 2006. Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J. Med. Genet 43:634–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sotomayor M, Corey DP, Schulten K. 2005. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 13:669–82 [DOI] [PubMed] [Google Scholar]
- 94.Sotomayor M, Weihofen WA, Gaudet R, Corey DP. 2010. Structural determinants of cadherin-23 function in hearing and deafness. Neuron 66:85–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sotomayor M, Weihofen WA, Gaudet R, Corey DP. 2012. Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492:128–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steel KP, Bock GR. 1980. The nature of inherited deafness in deafness mice. Nature 288:159–61 [DOI] [PubMed] [Google Scholar]
- 97.Tang YQ, Lee SA, Rahman M, Vanapalli SA, Lu H, Schafer WR. 2020. Ankyrin is an intracellular tether for TMC mechanotransduction channels. Neuron. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tobin M, Chaiyasitdhi A, Michel V, Michalski N, Martin P. 2019. Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea. eLife 8:e43473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsuprun V, Goodyear RJ, Richardson GP. 2004. The structure of tip links and kinocilial links in avian sensory hair bundles. Biophys. J 87:4106–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaadia RD, Li W, Voleti V, Singhania A, Hillman EMC, Grueber WB. 2019. Characterization of proprioceptive system dynamics in behaving Drosophila larvae using high-speed volumetric microscopy. Curr. Biol 29:935–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, et al. 2002. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat. Genet 30:257–58 [DOI] [PubMed] [Google Scholar]
- 102.Wang C, Wei Z, Chen K, Ye F, Yu C, et al. 2014. Structural basis of diverse membrane target recognitions by ankyrins. eLife 3:e04353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X, Li G, Liu J, Liu J, Xu XZ. 2016. TMC-1 mediates alkaline sensation in C. elegans through nociceptive neurons. Neuron 91:146–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Li J, Yao X, Li W, Du H, et al. 2017. Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Front Mol. Neurosci 10:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, et al. 2017. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat. Neurosci 20:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, et al. 2012. TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151:1283–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, et al. 2013. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493:221–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yue X, Zhao J, Li X, Fan Y, Duan D, et al. 2018. TMC proteins modulate egg laying and membrane excitability through a background leak conductance in C. elegans. Neuron 97:571–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang W, Cheng LE, Kittelmann M, Li J, Petkovic M, et al. 2015. Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162:1391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang YV, Aikin TJ, Li Z, Montell C. 2016. The basis of food texture sensation in Drosophila. Neuron 91:863–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao B, Wu Z, Grillet N, Yan L, Xiong W, et al. 2014. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84:954–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao Y, Yamoah EN, Gillespie PG. 1996. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. PNAS 93:15469–74 [DOI] [PMC free article] [PubMed] [Google Scholar]


