Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic is currently threatening the health of individuals worldwide. We compared the clinical characteristics between younger patients (aged <60 years) and older patients (aged ≥60 years) with COVID-19, detected the risk factors associated with a prolonged hospital stay, and examined the treatments commonly used with a particular focus on antiviral therapies.
Methods
This retrospective study was conducted at the West Campus, Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). The sample consisted of 123 patients admitted to the hospital between 9th February, 2020, and 3rd March, 2020. The data related to the demographics, laboratory findings, and treatment were analyzed to identify discrepancies between younger and older patients and those with and without primary diseases. The risk factors that contribute to a prolonged hospital stay were subsequently identified.
Results
Patients aged ≥60 years required longer hospital stay than younger patients (P=0.001). The percentage of lymphocytes was significantly lower in older patients and those with primary diseases (P=0.016 and P=0.042, respectively). The findings revealed that the risk factors that contributed to the length of hospital stay were age, the number of days of illness before hospitalization, white blood cell (WBC) count and albumin levels at admission, a neutrophil fraction at discharge, and antibiotic treatment. Analysis using a model that consisted of the above five risk factors for predicting prolonged hospital stay (>14 days) yielded an area under the ROC (AuROC) curve of 0.716. Antiviral and antibiotic treatments were administered to 97.6% and 39.0% of patients, respectively. The antiviral drugs most commonly administered were traditional Chinese medicine (83.7%) and arbidol (75.6%).
Conclusions
In this study, older patients and those with primary diseases were at a higher risk of worse clinical manifestations. The physicians who treat the patients should pay close attention to the risk factors that contribute to the length of hospital stay, which could be used for predicting prolonged hospital stay. Traditional Chinese medicine and arbidol were the most frequently used antiviral drugs. Nevertheless, the extent to which these medications can effectively treat COVID-19 warrants further investigation.
Keywords: COVID-19, Age, Primary diseases, Risk factors, Treatment
Introduction
In December 2019, a novel coronavirus disease, which termed as coronavirus disease 2019 (COVID-19), was first reported in Wuhan, China. In some cases, COVID-19 can cause progressive lung injury that leads to respiratory failure, followed by circulatory instability, and multiple organ dysfunctions [1]. Previous investigations of population groups who are at risk of mortality and morbidity due to COVID-19 have consistently found that a large proportion of patients who are hospitalized have primary diseases and comorbidities (e.g., hypertension, diabetes, cardiovascular disease, and respiratory diseases) [1,2]. The risk factors associated with in-hospital death include older age, high Sequential Organ Failure Assessment score, history of smoking, markedly elevated body temperature at admission, respiratory failure, albumin, C-reactive protein (CRP), and D-dimer levels >1 µg/L [3], [4], [5]. However, the characteristics of critical COVID-19 infection according to the age of older patients remain unclear. Recently, a study by Liu et al. [6] compared the characteristics of severe COVID-19 infection between young, middle-aged, and older patients. The evidence revealed that older patients who are considered as critical patients showed decreased high-sensitivity CRP and higher pro-brain natriuretic peptide levels. These fluctuations were independent risk factors for admission to the intensive care unit. However, the researchers did not compare the characteristics of patients with and without primary diseases, laboratory findings between admission and discharge, and treatment strategies. Moreover, there is a need to identify the risk factors that contribute to a prolonged hospital stay. This study aimed to further investigate the clinical differences between younger patients (aged <60 years) and older patients (aged ≥60 years) with COVID-19, as well as those with and without primary diseases, at the time of admission and discharge. The objective of this investigation was to detect the risk factors associated with prolonged hospital stay and to examine the commonly used treatments, with a particular focus on antiviral therapies.
Methods
Study design and patient selection
This retrospective study was approved by the Institutional Ethics Board of Xiangya Hospital, Central South University (Changsha, China) (approval number: 202003049). Owing to the retrospective nature of this research, the requirement for informed consent was waived. Patients with confirmed COVID-19 who were admitted to the West Campus, Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, between 9th February, 2020, and 3rd March, 2020, were included. Of note, patients without complete medical records were excluded. COVID-19 was diagnosed according to the interim guidance provided by the World Health Organization [7].
Data collection
Demographic, clinical, laboratory, treatment, and outcome data were extracted from the electronic medical records of confirmed COVID-19 cases. For the purpose of accuracy, two researchers also independently reviewed the data collection forms. Primary diseases (e.g., hypertension, diabetes, coronary heart disease, nervous system disease, chronic kidney disease, malignant tumor, chronic obstructive lung disease, and other diseases) were determined from the past medical history of patients. Routine blood examination data consisted of complete blood count, coagulation profile, renal and liver function, electrolytes, myocardial enzymes, interleukin-6 (IL-6), CRP, and procalcitonin levels were also collected. All inpatients underwent examination through computed tomography (CT). The frequency of examinations was determined by the treating physician. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA was detected by the local Centers for Disease Control and Prevention, local health institutions, Jingyintan Hospital (Wuhan, China), and Wuhan Pulmonary Hospital (Wuhan, China). The criteria for discharge were the absence of fever for ≥3 days, clinical remission of respiratory symptoms, substantial improvement in both lungs shown on chest CT, and two throat-swab samples negative for SARS-CoV-2 RNA obtained every 48 h.
Statistical analysis
Continuous variables were presented as the mean ± standard deviation and compared using Student's independent t-test (for normally distributed data); homogeneity of variance was also detected. For non-normally distributed data, the Mann–Whitney U test was used. Categorical variables were reported as numbers and percentages and compared using the χ² test. A two-tailed P<0.050 considered statistically significant differences.
The risk factors associated with the length of hospital stay were assessed by multivariate logistic regression using a forward-stepwise analysis. The results were summarized by estimating odds ratios (ORs) and respective 95% confidence intervals (CIs). All statistical analyses were performed using the SPSS Version 19.0 for Windows (IBM Corporation, Armonk, NY, USA) software.
Results
During the study, 137 patients were admitted to the West Campus of Union Hospital; of those, 14 patients were excluded due to incomplete medical data. Finally, 123 patients were included in this study [Fig. 1]. The mean age of the patients was 61.9 ± 12.1 years (range: 32–93 years); the majority of patients were males (56.9%; 70/123). The percentage of patients who had primary diseases was 61.8% (76/123); the most common primary diseases were hypertension (n=42, 34.1%), diabetes (n=32, 26.0%), coronary heart disease (n=15, 12.2%), nervous system disease (n=9, 7.3%), chronic kidney disease (n=6, 4.9%), malignant tumor (n=6, 4.9%), chronic obstructive lung disease (n=4, 3.3%), and others (n=286, 15.7%). The mean number of days of illness before hospitalization was 15.1 ± 9.3 days, and the mean length of hospital stay was 23.1 ± 11.0 days. The overall 28-day mortality rate was 4.1% (5/123) [Table 1].
Fig. 1.
Flow diagram of patient enrolment. COVID-19: Coronavirus disease 2019.
Table 1.
Differences between patients aged <60 years and ≥60 years and those with and without primary diseases.
| Variables | Total | Age <60 years | Age ≥60 years | P-value | Without primary diseases | With primary diseases | P-value |
|---|---|---|---|---|---|---|---|
| Demographics and clinical characteristics | |||||||
| Gender (male, %) | 70/123 | 22/46 | 48/77 | 0.097 | 22/47 | 48/76 | 0.075 |
| Age (years) | 61.9 ± 12.1 | 49.2 ± 7.1 | 69.5 ± 6.9 | <0.001 | 57.9 ± 12.4 | 64.4 ± 11.3 | 0.003 |
| Primary diseases (with/without, %) | 76/123 | 25/46 | 51/77 | 0.189 | 26/47 | 51/76 | 0.189 |
| Mortality (%) | 5/123 | 1/46 | 4/77 | 0.412 | 1/47 | 4/76 | 0.392 |
| Days before hospitalization | 15.1 ± 9.3 | 13.5 ± 8.6 | 17.7 ± 10.0 | 0.021 | 17.3 ± 8.5 | 13.9 ± 9.6 | 0.062 |
| Length of hospital stay (days) | 23.1 ± 11.0 | 19.1 ± 8.9 | 25.4 ± 11.4 | 0.001 | 21.0 ± 11.9 | 24.3 ± 10.2 | 0.111 |
| Laboratory findings at admission | |||||||
| WBC (× 109/L) | 6.00 ± 2.11 | 5.75 ± 1.85 | 6.16 ± 2.26 | 0.336 | 5.82 ± 1.84 | 6.13 ± 2.29 | 0.466 |
| Neutrophils (× 109/L) | 4.13 ± 2.06 | 3.75 ± 1.68 | 4.36 ± 2.24 | 0.151 | 3.84 ± 1.82 | 4.32 ± 2.20 | 0.246 |
| N% | 66.27 ± 13.34 | 64.20 ± 11.80 | 67.76 ± 14.14 | 0.219 | 64.07 ± 12.49 | 67.70 ± 13.77 | 0.181 |
| Lymphocytes (× 109/L) | 1.28 (0.84, 1.71) | 1.40 (0.96, 1.91) | 1.10 (0.76, 1.61) | 0.040 | 1.44 (0.93, 1.75) | 1.10 (0.81, 1.70) | 0.217 |
| L% | 23.60 ± 10.50 | 26.76 ± 10.43 | 21.62 ± 10.13 | 0.016 | 26.22 ± 11.24 | 21.89 ± 9.70 | 0.042 |
| PLT (× 109/L) | 242.42 ± 91.87 | 262.38 ± 90.21 | 230.25 ± 91.42 | 0.085 | 249.37 ± 105.30 | 237.82 ± 82.38 | 0.535 |
| Hb (g/L) | 125.23 ± 16.65 | 128.74 ± 16.45 | 123.09 ± 16.54 | 0.095 | 122.34 ± 16.92 | 127.15 ± 16.34 | 0.153 |
| PCT (ng/mL) | 0.06 (0.04, 0.14) | 0.06 (0.38, 0.12) | 0.07 (0.04, 0.14) | 0.513 | 0.06 (0.04, 1.11) | 0.07 (0.05, 0.14) | 0.216 |
| CRP (mg/L) | 3.63 (0.77, 32.04) | 2.44 (0.66, 13.25) | 4.41 (1.12, 55.90) | 0.315 | 3.83 (0.95, 14.27) | 2.60 (0.62, 56.40) | 0.736 |
| IL-6 (pg/mL) | 18.45 ± 43.55 | 3.62 ± 1.69 | 23.78 ± 49.95 | 0.055 | 33.62 ± 67.20 | 10.17 ± 20.29 | 0.261 |
| TnI (ng/L) | 3.50 (1.33, 8.13) | 1.05 (0.80, 2.20) | 5.15 (2.75, 9.88) | 0.001 | 2.25 (0.80, 6.48) | 4.05 (1.83, 9.13) | 0.137 |
| Mb (ng/mL) | 36.90 (24.40, 56.24) | 21.80 (19.00, 35.65) | 50.70 (30.83, 72.93) | 0.019 | 33.55 (18.50, 43.35) | 51.00 (28.20, 59.40) | 0.081 |
| CK (U/L) | 54.50 (39.25, 79.30) | 54.00 (45.00, 108.00) | 60.00 (34.50, 83.00) | 0.704 | 49.50 (38.25, 65.50) | 64.50 (39.00, 112.00) | 0.065 |
| CK-MB (U/L) | 21.71 (5.75, 12.00) | 10.00 (5.00, 12.00) | 9.00 (6.00, 15.00) | 0.795 | 9.00 (5.50, 12.00) | 9.00 (5.00, 15.00) | 0.459 |
| LDH (U/L) | 261.80 ± 134.57 | 254.22 ± 175.83 | 265.86 ± 108.54 | 0.774 | 228.74 ± 120.31 | 291.09 ± 141.30 | 0.060 |
| PT (s) | 13.03 ± 1.20 | 13.09 ± 1.35 | 13.01 ± 1.13 | 0.786 | 12.86 ± 0.97 | 13.13 ± 1.31 | 0.348 |
| APTT (s) | 36.18 ± 4.96 | 32.65 ± 6.14 | 34.76 ± 2.17 | 0.024 | 35.96 ± 5.55 | 36.30 ± 4.64 | 0.773 |
| D-dimer (μg/mL) | 0.62 (0.25, 1.06) | 0.57 (0.16, 0.80) | 0.67 (0.31, 1.56) | 0.098 | 0.57 (0.24, 1.22) | 0.62 (0.27, 1.26) | 0.648 |
| FiB (g/L) | 3.89 ± 1.14 | 3.68 ± 1.16 | 3.99 ± 1.13 | 0.267 | 3.85 ± 1.15 | 3.91 ± 1.15 | 0.840 |
| Albumin (g/L) | 36.62 ± 6.99 | 34.36 ± 6.96 | 36.04 ± 26.91 | 0.213 | 35.17 ± 6.10 | 31.98 ± 6.44 | 0.016 |
| TBil (µmol/L) | 12.19 ± 7.96 | 9.77 ± 4.80 | 13.58 ± 9.05 | 0.008 | 12.21 ± 8.45 | 12.17 ± 7.70 | 0.982 |
| DBil (µmol/L) | 3.30 (2.13, 4.96) | 3.00 (2.00, 4.00) | 3.80 (2.45, 6.50) | 0.098 | 3.05 (1.88, 6.88) | 3.65 (2.40, 5.73) | 0.486 |
| ALT (U/L) | 38.19 ± 24.90 | 40.44 ± 29.22 | 36.80 ± 22.07 | 0.494 | 34.56 ± 26.78 | 40.67 ± 23.46 | 0.240 |
| AST (U/L) | 31.98 ± 26.12 | 33.39 ± 36.59 | 31.10 ± 17.38 | 0.684 | 26.41 ± 10.56 | 35.79 ± 32.33 | 0.045 |
| Cr (µmol/L) | 71.60 (61.00, 77.46) | 66.30 (58.40, 75.75) | 72.50 (62.00, 78.30) | 0.232 | 64.30 (57.80, 77.60) | 73.60 (64.50, 79.05) | 0.035 |
| BUN (mmol/L) | 5.40 (4.00, 5.95) | 4.57 (3.62, 5.81) | 5.50 (4.16, 6.80) | 0.035 | 7.02 ± 9.69 | 5.49 (3.87, 6.44) | 0.808 |
| K (mmol/L) | 3.90 (3.30, 4.09) | 3.96 (3.77, 4.10) | 3.88 (3.43, 4.20) | 0.438 | 3.94 (3.69, 4.11) | 3.90 (3.54, 4.15) | 0.790 |
| Na (mmol/L) | 137.15 ± 15.69 | 139.23 ± 3.15 | 135.98 ± 19.42 | 0.382 | 138.99 ± 2.94 | 136.12 ± 19.46 | 0.441 |
| Laboratory findings at discharge | |||||||
| WBC (× 109/L) | 5.90 ± 2.89 | 5.40 ± 1.65 | 6.20 ± 3.43 | 0.171 | 5.43 ± 1.82 | 6.14 ± 3.31 | 0.236 |
| Neutrophils (× 109/L) | 4.76 ± 7.85 | 3.43 ± 1.20 | 5.73 ± 10.19 | 0.177 | 3.62 ± 1.69 | 5.32 ± 9.49 | 0.342 |
| N% (%) | 60.33 ± 14.09 | 60.12 ± 11.46 | 60.49 ± 15.87 | 0.903 | 59.07 ± 10.62 | 60.93 ± 15.51 | 0.567 |
| Lymphocytes (× 109/L) | 1.60 (1.09, 1.82) | 1.66 (1.23, 2.08) | 1.43 (0.99, 1.82) | 0.249 | 1.60 (1.23, 2.12) | 1.60 (1.00, 1.89) | 0.424 |
| L% (%) | 23.17 ± 13.31 | 26.44 ± 11.43 | 20.99 ± 14.09 | 0.044 | 24.87 ± 14.47 | 22.29 ± 12.69 | 0.361 |
| PLT (× 109/L) | 211.43 ± 110.52 | 231.04 ± 100.18 | 198.47 ± 115.82 | 0.144 | 209.26 ± 117.43 | 212.55 ± 107.67 | 0.887 |
| Hb (g/L) | 130.69 ± 35.19 | 135.05 ± 29.12 | 127.87 ± 38.57 | 0.317 | 132.51 ± 32.10 | 129.73 ± 36.90 | 0.707 |
| PCT (ng/mL) | 0.08 (0.04, 0.14) | 0.08 (0.04, 0.11) | 0.09 (0.04, 0.74) | 0.379 | 0.08 (0.04, 0.98) | 0.08 (0.04, 0.12) | 0.610 |
| CRP (mg/L) | 4.11 (0.10, 7.72) | 3.52 (0.32, 4.58) | 4.84 (0.71, 18.00) | 0.140 | 2.43 (0.21, 16.50) | 4.50 (0.63, 10.60) | 0.325 |
| IL-6 (pg/mL) | 18.80 ± 51.12 | 3.96 ± 5.49 | 26.89 ± 62.42 | 0.102 | 20.62 ± 52.10 | 17.92 ± 51.80 | 0.888 |
| TnI (ng/L) | 2.70 (1.70, 14.44) | 2.10 (1.10, 3.80) | 4.20 (2.20, 26.75) | 0.054 | 2.40 (2.00, 23.50) | 3.80 (1.15, 16.6) | 0.921 |
| CK (U/L) | 40.50 (16.50, 53.00) | 47.00 (17.00, 60.50) | 38.50 (15.50, 60.75) | 0.545 | 39.50 (13.50, 46.50) | 41.50 (21.50, 69.25) | 0.150 |
| CK-MB (U/L) | 8.00 (0.95, 13.40) | 6.00 (0.90, 11.00) | 9.00 (1.08, 49.50) | 0.251 | 7.00 (0.90, 18.00) | 8.50 (1.05, 23.50) | 0.656 |
| LDH (U/L) | 386.27 ± 1334.04 | 169.66 ± 99.83 | 500.95 ± 1654.03 | 0.400 | 183.82 ± 93.71 | 493.45 ± 1646.72 | 0.431 |
| PT (s) | 18.37 ± 14.45 | 18.44 ± 19.43 | 18.33 ± 10.71 | 0.977 | 17.61 ± 9.80 | 18.65 ± 15.89 | 0.801 |
| APTT (s) | 33.22 ± 9.48 | 32.97 ± 6.23 | 33.38 ± 11.06 | 0.851 | 31.05 ± 12.34 | 34.01 ± 8.23 | 0.273 |
| D-dimer (µg/mL) | 0.41 (0.22, 0.90) | 0.23 (0.12, 0.61) | 0.74 (0.31, 1.62) | 0.002 | 0.40 (0.22, 1.11) | 0.43 (0.18, 1.32) | 0.874 |
| FiB (g/L) | 3.01 ± 1.55 | 3.43 ± 1.050 | 2.80 ± 1.72 | 0.067 | 2.72 ± 1.76 | 3.14 ± 1.45 | 0.310 |
| Albumin (g/L) | 84.23 ± 106.39 | 37.86 ± 6.63 | 35.86 ± 7.14 | 0.165 | 37.46 ± 5.38 | 36.20 ± 7.66 | 0.400 |
| TBil (µmol/L) | 11.82 ± 8.51 | 10.22 ± 4.81 | 12.81 ± 10.08 | 0.141 | 11.04 ± 7.50 | 12.19 ± 8.99 | 0.533 |
| DBil (µmol/L) | 0.41 (0.22, 0.90) | 2.75 (1.98, 4.33) | 4.30 (2.50, 22.55) | 0.030 | 2.70 (2.03, 8.55) | 3.70 (2.30, 7.60) | 0.357 |
| ALT (U/L) | 84.56 ± 462.69 | 41.88 ± 43.13 | 111.85 ± 591.71 | 0.464 | 32.06 ± 20.19 | 110.42 ± 564.69 | 0.429 |
| AST (U/L) | 103.68 ± 780.60 | 24.64 ± 12.89 | 154.21 ± 999.32 | 0.421 | 21.19 ± 8.47 | 144.31 ± 953.36 | 0.461 |
| Cr (µmol/L) | 70.7 (57.25, 92.00) | 70.70 (55.70, 98.00) | 70.00 (58.65, 136.43) | 0.654 | 65.95 (56.18, 123.50) | 72.50 (57.70, 112.20) | 0.530 |
| BUN (µmol/L) | 5.70 (4.39, 7.20) | 4.60 (4.18, 6.02) | 6.41 (4.80, 34.49) | 0.012 | 5.69 (4.33, 51.02) | 5.70 (4.43, 8.10) | 0.832 |
| K (mmol/L) | 4.07 (3.77, 4.23) | 4.11 (3.96, 4.26) | 4.00 (3.66, 4.40) | 0.349 | 4.02 (3.91, 4.18) | 4.13 (3.67, 4.40) | 0.776 |
| Na (mmol/L) | 130.83 ± 32.53 | 130.29 ± 33.60 | 131.16 ± 32.20 | 0.908 | 128.84 ± 36.73 | 131.76 ± 30.70 | 0.708 |
| Nucleic acid turn negative | 119/123 | 46/46 | 73/77 | 0.116 | 46/47 | 73/76 | 0.580 |
| Lung imaging changes | 123/123 | 46/46 | 77/77 | 47/47 | 76/76 | ||
| Improvement of lung imaging | 68/76 | 23/24 | 45/52 | 0.220 | 30/31 | 38/45 | 0.085 |
| Treatment(%) | |||||||
| Antiviral treatment | 120/123 | 46/46 | 74/77 | 0.175 | 47/47 | 73/76 | 0.168 |
| Antibiotic treatment | 48/123 | 15/46 | 33/77 | 0.260 | 18/47 | 30/76 | 0.897 |
| Antifungal treatment | 5/123 | 0/46 | 5/77 | 0.078 | 1/47 | 4/76 | 0.392 |
| Glucocorticoid | 17/123 | 2/46 | 15/77 | 0.019 | 6/47 | 11/76 | 0.790 |
| Intravenous immunoglobulin therapy | 2/123 | 0/46 | 2/77 | 0.270 | 1/47 | 1/76 | 0.729 |
| High-flow nasal cannula oxygen therapy | 6/123 | 0/46 | 6/77 | 0.052 | 1/47 | 5/76 | 0.265 |
| Non-invasive mechanical ventilation | 5/123 | 0/46 | 5/77 | 0.078 | 0/47 | 5/76 | 0.073 |
| Invasive mechanical ventilation | 1/123 | 0/46 | 1/77 | 0.438 | 1/47 | 0/76 | 0.202 |
| CRRT | 1/123 | 0/46 | 1/77 | 0.438 | 1/47 | 0/76 | 0.202 |
Laboratory data are expressed as mean ± standard deviation, and median (first quartile, third quartile).
ALT: Alanine transaminase; APTT: Activated partial thromboplastin time; AST: Aspartate aminotransferase; BUN: Blood urea nitrogen; CK: Creatine kinase; CK-MB: Creatine kinase isoenzyme-MB; Cr: Creatinine; CRP: C-reactive protein; CRRT: Continuous renal replacement therapy; DBil: Direct bilirubin; FiB: Fibrinogen; Hb: Hemoglobin; IL: Interleukin; L%: Lymphocyte fraction; LDH: Lactate dehydrogenase; Mb: Myoglobin; N%: Neutrophil fraction; PCT: Procalcitonin; PLT: Platelet count; PT: Prothrombin time; TBil: Total bilirubin; TnI: Cardiac TroponinI; WBC: White blood cell.
Differences between patients aged <60 years and ≥60 years
We compared the differences between the patients aged <60 years and ≥60 years, who were admitted to the hospital with COVID-19. The analysis revealed that the number of days of illness before hospitalization was significantly higher and the length of hospital stay was significantly longer (P=0.021 and P=0.001, respectively) in the latter group than in the former group. At admission, the lymphocyte count was considerably lower in the older patients (P=0.040). In contrast, troponin I and myoglobin levels were significantly higher in older patients (P=0.001 and P=0.019, respectively). In addition, the coagulation index activated partial thromboplastin time (APTT) was significantly longer (P=0.024), and the liver function index total bilirubin and renal function index blood urea nitrogen (BUN) were substantially higher (P=0.008 and P=0.035, respectively) in the older population. At discharge, the percentage of lymphocytes was also lower in older patients (P=0.044), whereas the levels of direct bilirubin and BUN were significantly higher (P=0.030 and P=0.012, respectively) [Table 1].
Differences between patients with and without primary diseases
We also compared COVID-19 patients with and without primary diseases who were admitted to the hospital. The results revealed that the presence or absence of a primary disease did not have a significant influence on mortality and length of hospital stay. The mean age of patients with primary diseases was significantly higher than that of patients without primary diseases (P=0.003). At admission, in patients with primary diseases, the percentage of lymphocytes and albumin levels were significantly lower (P=0.042 and P=0.016, respectively), whereas the levels of aspartate aminotransferase and creatinine were significantly higher (P=0.045 and P=0.035, respectively) [Table 1].
Differences between laboratory findings at admission and discharge
Comparison of the laboratory findings revealed that the neutrophil fraction (N%) was significantly lower at discharge than at admission (P<0.001). The platelet count and fibrinogen levels were also lower at discharge (P=0.030 and P<0.001, respectively). In contrast, procalcitonin was significantly higher at discharge (P=0.028). Prothrombin time was significantly longer (P = 0.013) at discharge. The levels of albumin, direct bilirubin, and BUN were significantly higher at discharge (P<0.001, P<0.001, and P<0.001, respectively) [Table 2].
Table 2.
Differences between laboratory findings at admission and discharge.
| Laboratory findings | At admission (n = 123) | At discharge (n = 118) | P-value |
|---|---|---|---|
| WBC (× 109/L) | 6.00 ± 2.11 | 5.89 ± 2.89 | 0.381 |
| Neutrophils (× 109/L) | 4.13 ± 2.06 | 4.76 ± 7.85 | 0.574 |
| N% | 66.27 ± 13.34 | 60.33 ± 14.09 | <0.001 |
| Lymphocytes (× 109/L) | 1.57 ± 2.78 | 1.94 ± 2.77 | 0.817 |
| L% | 23.60 ± 10.50 | 23.17 ± 13.31 | 0.644 |
| PLT (× 109/L) | 242.42 ± 91.87 | 211.43 ± 110.52 | 0.005 |
| Hb (g/L) | 125.23 ± 16.65 | 130.69 ± 35.19 | 0.105 |
| PCT (ng/mL) | 0.22 ± 0.55 | 1.83 ± 8.29 | 0.028 |
| CRP (mg/L) | 23.49 ± 38.32 | 14.60 ± 28.63 | 0.074 |
| IL-6 (pg/mL) | 18.45 ± 43.55 | 18.80 ± 51.12 | 0.845 |
| TnI (ng/L) | 254.89 ± 1521.68 | 276.40 ± 1136.93 | 0.300 |
| CK (U/L) | 341.52 ± 1613.08 | 47.30 ± 40.89 | 0.097 |
| CK-MB (U/L) | 11.94 ± 14.49 | 43.49 ± 76.80 | 0.053 |
| LDH (U/L) | 261.80 ± 134.57 | 386.27 ± 1334.04 | 0.477 |
| PT (s) | 13.03 ± 1.20 | 18.37 ± 14.45 | 0.013 |
| APTT (s) | 36.18 ± 4.96 | 33.22 ± 9.48 | 0.145 |
| D-dimer (µg/mL) | 1.08 ± 1.40 | 1.00 ± 1.48 | 0.364 |
| FiB (g/L) | 3.89 ± 1.14 | 3.01 ± 1.55 | 0.004 |
| Albumin (g/L) | 33.27 ± 6.47 | 36.62 ± 6.99 | <0.001 |
| TBil (µmol/L) | 12.19 ± 7.96 | 11.82 ± 8.51 | 0.701 |
| DBil (µmol/L) | 4.78 ± 4.73 | 15.37 ± 24.39 | <0.001 |
| ALT (U/L) | 38.19 ± 24.90 | 84.56 ± 462.69 | 0.330 |
| AST (U/L) | 31.98 ± 26.12 | 103.68 ± 780.60 | 0.357 |
| Cr (µmol/L) | 88.84 ± 166.84 | 128.52 ± 140.35 | 0.054 |
| BUN (µmol/L) | 6.51 ± 7.42 | 21.21 ± 31.51 | <0.001 |
| K (mmol/L) | 5.67 ± 15.76 | 4.06 ± 0.46 | 0.104 |
| Na (mmol/L) | 137.15 ± 15.69 | 130.83 ± 32.53 | 0.314 |
Data are expressed as mean ± standard deviation.
ALT: Alanine transaminase; APTT: Activated partial thromboplastin time; AST: Aspartate aminotransferase; BUN: Blood urea nitrogen; CK: Creatine kinase; CK-MB: Creatine kinase isoenzyme-MB; Cr: Creatinine; CRP: C-reactive protein; DBil: Direct bilirubin; FiB: Fibrinogen; Hb: Hemoglobin; IL: Interleukin; L%: Lymphocyte fraction; LDH: Lactate dehydrogenase; N%: Neutrophil fraction; PCT: Procalcitonin; PLT: The platelet count; PT: Prothrombin time; TBil: Total bilirubin; TnI: Cardiac TroponinI; WBC: White blood cell.
Treatments most often used in patients with COVID-19
The treatment most often used in COVID-19 patients was antiviral treatment (97.6%), followed by antibiotic treatment (39.0%), glucocorticoids (13.8%), antifungal treatment (4.1%), and intravenous immunoglobulin therapy (1.6%). The use of glucocorticoids was more common in patients aged >60 years (P=0.019). Among the antiviral treatments administered, the agents most often used were traditional Chinese medicine (83.7%, 103/123), arbidol (75.6%, 93/123), thymosin (62.6%, 77/123), ribavirin (51.2%, 63/123), lopinavir and ritonavir (23.6%, 29/123), and hydroxychloroquine (9.8%, 12/123). The most frequently prescribed glucocorticoids were intravenous methylprednisone (20–80 mg; daily once for 2–8 days) or oral prednisone (15–20 mg; daily once for 4–8 days). All patients were treated with oxygen therapy. The proportions of patients using high-flow nasal cannula oxygen therapy, non-invasive mechanical ventilation, and invasive mechanical ventilation were 4.9%, 4.1%, and 0.8%, respectively. Notably, these treatments were not associated with the length of hospital stay [Table 1].
Risk factors associated with the length of hospital stay
Risk factors associated with the length of hospital stay included age (P<0.001, 95% CI: 0.164–0.459), N% at discharge (P=0.002, 95% CI: −0.179 to −0.043), number of days of illness before hospitalization (P<0.001, 95% CI: −0.558 to −0.168), antibiotic treatment (P<0.001, 95% CI: −11.307 to −3.883), white blood cell (WBC) count at admission (P=0.001, 95% CI: −2.148 to −0.618), and albumin levels at admission (P=0.002, 95% CI: −0.787 to −0.177) [Table 3].
Table 3.
Risk factors associated with the length of hospital stay.
| Items | P-value | OR | 95% CI |
|---|---|---|---|
| Age | <0.001 | 1.365 | 0.164–0.459 |
| N% at discharge | 0.002 | 0.895 | −0.179 to -0.043 |
| Days before hospitalization | <0.001 | 0.696 | −0.558 to -0.168 |
| Antibiotic treatment | <0.001 | 0.001 | −11.307 to -3.883 |
| WBC count at admission | 0.001 | 0.251 | −2.148 to -0.618 |
| Albumin at admission | 0.002 | 0.618 | −0.787 to -0.177 |
CI: Confidence interval; N%: Neutrophil fraction; OR: Odds ratio; WBC: White blood cell.
Predictive ability of a model of risk factors for prolonged hospital-stay (>14 days)
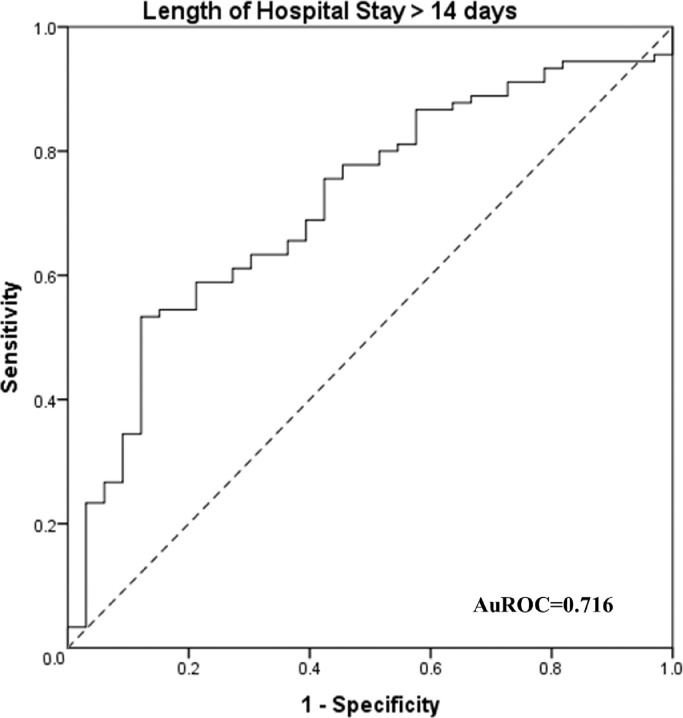
The model consisting of the abovementioned five risk factors for predicting prolonged hospital-stay (>14 days) was as follows: [Model = 0.311 × Age + (−0.111 × N% at discharge) + (−0.363 × Number of days of illness before hospitalization) + (−7.595 × Use of antibiotic treatment) + (−1.383 × WBC count at admission) + (−0.482 × Albumin levels at admission) + 45.433]. The area under the ROC (AuROC) curve was 0.716 (95% CI: 0.618–0.815) [Fig. 2]. Using 27.2 as the cutoff value, the sensitivity and specificity of the model were 53.3% and 87.9%, respectively.
Fig. 2.
ROC curves of the model of risk factors for predicting the length of hospital stay >14 days. AuROC: Area under the ROC curve; ROC: Receiver operating characteristic.
Discussion
This retrospective study involved a cohort of 123 patients with severe laboratory-confirmed COVID-19 who required oxygen therapy. The present investigation focused on the differences in clinical characteristics between younger patients (aged <60 years) and older patients (age ≥60 years) and those with and without primary diseases at the time of admission and discharge. The number of days of illness before hospitalization was higher and the length of hospital stay was longer in the present study than in previous studies [3,4,8]. These differences may be attributed to the relatively older age and more severe disease of patients admitted to the West Campus of Union Hospital. In this study, the in-hospital mortality rate was very low (4.1%). The findings revealed that older patients were ill for more days prior to hospitalization than younger patients, and the length of hospital stay was longer for older patients.
We also analyzed in detail the differences in laboratory examination results between younger and older patients and those with and without primary diseases at the time of admission and discharge. According to a previous study, the age of 60 years was used as the cutoff value for the classification of middle-aged and older patients [6]. A reduction in the total number of lymphocytes was a clinical characteristic of COVID-19 [4,7]. A lymphocyte count <0.8 × 109/L was associated with a higher risk of developing severe COVID-19 [8]. We found that the percentage of lymphocytes was significantly lower in older patients and those with primary diseases, indicating a more severe inhibition of lymphocyte function in these groups. As the patients recovered, their lymphocyte counts increased and a significant decrease of N% at discharge was observed. Coagulation abnormalities (e.g. prothrombin time and APTT prolongation and hypercoagulability) were commonly detected among hospitalized patients with COVID-19 [9]. In the present study, we found that APTT at admission was significantly longer in older patients. Abnormal liver and kidney functions were also more severe in older patients. Acute myocardial injury was common in patients with COVID-19 and associated with severity and adverse prognosis [10,11]. In this study, we also found that the levels of troponin I and myoglobin were higher in older patients, indicating potential acute myocardial injury; however, acute myocardial infarction was not observed in our study.
Previous studies have reported some risk factors associated with in-hospital death, including older age, high Sequential Organ Failure Assessment score, history of smoking, markedly elevated body temperature at admission, respiratory failure, albumin, CRP, and D-dimer levels >1 µg/L [3,5]. Furthermore, patients aged >65 years are at a higher risk of developing symptoms that lead to critical illness or death [2]. In our study, the number of non-survivors was relatively small; hence, we did not investigate the risk factors associated with mortality but instead analyzed those linked to the length of hospital stay. As the mortality rate was very low, confounders such as rapid death of patients would not greatly influence the present results. The findings revealed that age, number of days of illness before hospitalization, WBC count and albumin levels at admission, N% at discharge, and antibiotic treatment were risk factors associated with the length of hospital stay. Previous studies have demonstrated that older age and albumin levels are important risk factors for mortality [3,5]. This study indicated that these factors were also linked to a prolonged hospital stay. Routine blood test results are often the first laboratory results available to physicians who treat patients with COVID-19.
In the early stages of COVID-19, normal or declined WBC counts as well as declined lymphocyte numbers can be observed in routine blood test results [7]. In the progressive stage of illness, WBC and neutrophil counts may increase in response to a secondary bacterial infection. Therefore, these cells are important markers of the severity of inflammation and the function of the immune system. Previous studies have reported that patients with severe COVID-19 had higher WBC and neutrophil counts and fewer lymphocytes [9,12]. The findings of the present study demonstrated that WBC count at admission and N% at discharge were associated with the length of hospital stay. The use of antibiotics is often related to co-infection or secondary bacterial infection, which may lead to a prolonged hospital stay. We constructed a model consisting of five risk factors for predicting prolonged hospital stay (>14 days). The model yielded an AuROC of 0.716, indicating that these five risk factors may be combined to predict prolonged hospitalization. We chose 14 days as the cutoff value for the classification of normal or prolonged length of hospital stay. This selection was based on a systematic review which showed that the median length of hospital stay for patients with COVID-19 in China was 14 days [13].
In this study, antiviral treatment was administered to the vast majority of patients (97.6%). The most frequently used antiviral agents were traditional Chinese medicine (83.7%) and arbidol (75.6%). During the 2003 SARS epidemic, traditional Chinese medicine was found to exert a remarkable therapeutic effect. Therefore, it was also widely used in the fight against COVID-19 in China. The National Medical Products Administration listed COVID-19 as an additional indication for three Chinese patent medicines (e.g., Lianhua Qingwen capsule, Jinhua Qinggan capsule, and Xuebijing injection) and two herbal formulas (e.g., Qingfei Paidu decoction and Huashi Baidu decoction) [14,15]. Studies have shown that some traditional Chinese medicines indirectly inhibited virus growth by improving the immune function of the host or inhibited virus-mediated inflammatory response. Moreover, some traditional Chinese medicines, such as Jin Yin Hua (Flos Lonicerae Japonicae), Huang Qin (Radix Scutellariae), and Da Qing Ye (Folium Isatidis), have exhibited direct broad-spectrum antiviral effects [16]. Studies have also reported that Lianhua Qingwen significantly inhibited the replication of SARS-CoV-2, affected the morphology of the virus, suppressed cytokine release, boosted immunity, and exerted antiviral and anti-inflammatory effects [17]. Previous studies have also reported that, in mild cases of COVID-19 treated with traditional Chinese medicine, the length of clinical symptoms, fever, and hospital stay were reduced by 2 days, 1.7 days, and 2.2 days, respectively [18,19]. Furthermore, the improvement rate shown on CT images and clinical cure rate were increased by 22% and 33%, respectively, while the rate of progression to more severe illness was reduced by 27.4%. In severe cases treated with traditional Chinese medicine, the length of hospital stay and the time required to obtain a negative nucleic acid test were reduced by >2 days [18,19]. These findings indicate that traditional Chinese medicine may be an effective adjuvant therapy for treating COVID-19 alongside Western medicine.
The most frequently prescribed Western medication was arbidol, which has been linked to superior outcomes vs. lopinavir/ritonavir in the treatment of COVID-19 [20]. Thymosin alpha-1 is also frequently prescribed. This agent acts through toll-like receptors in both myeloid and plasmacytoid dendritic cells, leading to the activation and stimulation of the immune response [21]. Therefore, the use of this drug may be beneficial for the treatment of immunosuppression status in the early stages of COVID-19. However, further studies are warranted to validate the effectiveness of these treatments. In this study, antibiotic and antifungal therapies were administered to 39.0% and 4.1% of patients, respectively. The most commonly used antibiotic agent was moxifloxacin. The antibiotic and antifungal treatments were primarily administered as a form of empiric therapy for the prevention of secondary infection. However, it should be noted that inappropriate use of antibiotics, particularly those that stimulate anti-inflammatory activity, may be associated with an inflammatory storm and septic shock in patients with COVID-19 [22]. Glucocorticoids – predominantly used in patients with moderate-to-severe acute respiratory distress syndrome who require high-flow nasal cannula oxygen therapy, non-invasive, or invasive mechanical ventilation – were not frequently administered in this study (13.8%).
At present, the available evidence on the effectiveness of glucocorticoids in the treatment of patients with COVID-19 is relatively weak. Thus, further studies are required to develop a standardized protocol. Some physicians have recommended that doubling the usual dose of hydrocortisone for patients with mild COVID-19 symptoms may be an effective strategy for preserving the early activation of the immune response. In case of symptom deterioration, it is advisable to further increase the dose of hydrocortisone by up to 100 mg. For cases reaching a critical stage (e.g., rapid decrease in oxygenation) or experiencing an adrenal crisis, the management could involve treatment against refractory shock and the continuous intravenous administration of high doses (200 mg) of hydrocortisone [23].
To conclude, patients aged ≥60 years and those with primary diseases are at a higher risk of worse clinical manifestations. The risk factors that contributed to the length of hospital stay are age, the number of days of illness before hospitalization, WBC count and albumin levels at admission, N% at discharge, and antibiotic treatment; these factors could be used for predicting prolonged hospital stay. The most frequently prescribed antiviral drugs were traditional Chinese medicine and arbidol. Further studies are warranted to investigate the effects of these antiviral therapies on patients with COVID-19.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (Grant Number: 2020JJ5918 awarded to Qian-Yi Peng), the National Natural Science Foundation of China (Grant Number: 81974285 awarded to Qian-Yi Peng), and the National Natural Science Foundation of China (Grant Number: 81873956 awarded to Li-Na Zhang).
Ethics Approval and Consent to Participate
This retrospective study was approved by the Institutional Ethics Board of Xiangya Hospital, Central South University (No. 202003049). Informed consent was waived due to the retrospective nature of this study.
Managing Editor: Jingling Bao
Contributor Information
Zhaoxin Qian, Email: xyqzx@csu.edu.cn.
Lina Zhang, Email: zln7095@163.com.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z, Wu D, Han X, Jiang W, Qiu L, Tang R, et al. Different characteristics of critical COVID-19 and thinking of treatment strategies in non-elderly and elderly severe adult patients. Int Immunopharmacol. 2021;92 doi: 10.1016/j.intimp.2020.107343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance, 2020. Available from: https://www.who.int/publications-detail/clinical-managementof-severe-acute-respiratory-infection-when-novelcoronavirus-(ncov)-infection-is-suspected. [Last accessed on 2020 January 31].
- 8.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130(5):390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han H, Xie L, Liu R, Yang J, Liu F, Wu K, et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan. China. J Med Virol. 2020;92(7):819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees EM, Nightingale ES, Jafari Y, Waterlow NR, Clifford S, Pearson CAB, et al. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020;18(1):270. doi: 10.1186/s12916-020-01726-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B, et al. Can we predict the severity of coronavirus disease 2019 with a routine blood test. Pol Arch Intern Med. 2020;130(5):400–406. doi: 10.20452/pamw.15331. [DOI] [PubMed] [Google Scholar]
- 15.National Health Commission of the People's Republic of China. Guideline on diagnosis and treatment of COVID-19 (Trial 6th edition), 2020. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202002/8334a8326dd94d329df351d7da8aefc2.shtml. [Last accessed on 2020 February 23].
- 16.Gao YJ, Wang RB. Progress in the study of traditional Chinese medicine for the treatment of influenza. Clin Med J. 2018;1(1):17–20. doi: 10.3969/j.issn.1672-3384.2018.01.004. [DOI] [Google Scholar]
- 17.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Publicity Department of the People's Republic of China . Press conference of the joint prevention and control mechanism of state council on Feb 17, 2020. 2020. http://www.nhc.gov.cn/xcs/fkdt/202002/f12a62d10c2a48c6895cedf2faea6e1f.shtml Available from: [Last accessed on 2020 February 23] [Google Scholar]
- 20.Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King R, Tuthill C. Immune modulation with thymosin alpha 1 treatment. Vitam Horm. 2016;102:151–178. doi: 10.1016/bs.vh.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Hantoushzadeh S, Norooznezhad AH. Possible cause of inflammatory storm and septic shock in patients diagnosed with (COVID-19) Arch Med Res. 2020;51(4):347–348. doi: 10.1016/j.arcmed.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isidori AM, Arnaldi G, Boscaro M, Falorni A, Giordano C, Giordano R, et al. COVID-19 infection and glucocorticoids: Update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest. 2020;43(8):1141–1147. doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]