Abstract
Interpretation of untargeted metabolomics data from both in vivo and physiologically relevant in vitro model systems continues to be a significant challenge for toxicology research. Potency-based modeling of toxicological responses has served as a pillar of interpretive context and translation of testing data. In this study, we leverage the resolving power of concentration-response modeling through benchmark concentration (BMC) analysis to interpret untargeted metabolomics data from differentiated cultures of HepaRG cells exposed to a panel of reference compounds and integrate data in a potency-aligned framework with matched transcriptomic data. For this work, we characterized biological responses to classical human liver injury compounds and comparator compounds, known to not cause liver injury in humans, at 10 exposure concentrations in spent culture media by untargeted liquid chromatography-mass spectrometry analysis. The analyte features observed (with limited metabolites identified) were analyzed using BMC modeling to derive compound-induced points of departure. The results revealed liver injury compounds produced concentration-related increases in metabolomic response compared to those rarely associated with liver injury (ie, sucrose, potassium chloride). Moreover, the distributions of altered metabolomic features were largely comparable with those observed using high throughput transcriptomics, which were further extended to investigate the potential for in vitro observed biological responses to be observed in humans with exposures at therapeutic doses. These results demonstrate the utility of BMC modeling of untargeted metabolomics data as a sensitive and quantitative indicator of human liver injury potential.
Keywords: toxicometabolomics, toxicogenomics, benchmark concentration analysis, HepaRG cells, liver injury
A broad range of models, from cell free systems to in vivo animal testing, are used in pharmacology and toxicology research. Systems biology approaches have been developed to integrate the distinct levels of biological complexity in these model systems to explore molecular mechanisms (Butcher et al., 2004; Pujol et al., 2010). While this approach shows promise, computational methods to derive meaningful understanding of chemical-biological interactions lag behind our ability to collect these robust data streams. An understanding of dose-response relationships for pharmacological efficacy, safety, and toxicity is a fundamental component of preclinical drug development. However, concentration-response modeling has been under-utilized in the various “omic” data streams including transcriptomics, proteomics, and metabolomics. In particular, metabolomics provides direct measurements of the biochemical and physiological state of the system under evaluation (Beger et al., 2016). Targeted metabolomics has been well-established as an important data stream to explore the underlying pathobiology of a disease and in the development of drugs used to treat diseases (Beger et al., 2016).
Safety assessments for nonpharmaceutical chemicals use nonlinear regression models of dose-response data to derive a benchmark concentration (BMC), which is the concentration or dose that is associated with a benchmark response (Davis et al., 2011). A benchmark response is a predetermined response level, such as a 10% increase in a specific pathological lesion for dichotomous responses or a 10% increase from controls for continuous responses. The BMC approach replaces the no observable adverse effect level (NOAEL) (Davis et al., 2011). The advantage of the BMC approach, compared to NOAELs, is that BMCs do not depend solely on the exposure levels selected during study design (Davis et al., 2011). The BMC approach also better accounts for variability in the estimate of dose response. One of the challenges in the BMC approach is setting the benchmark response (an adverse outcome used to define a BMC). For quantal or dichotomous data, such as number of tumors, setting a benchmark response that is biologically relevant or adverse is clearer than for continuous responses, such as metabolomic or transcriptomic changes. Once derived, the BMC is used as a point of departure for deriving reference values used in regulatory decision making.
Safety assessment for pharmaceutical and nonpharmaceutical chemicals is costly and time consuming. To streamline the approach, particularly for understanding chronic toxicity, the use of “omics” approaches to estimate safe exposures has been proposed (Thomas et al., 2013a; Kuo et al., 2016; Corton et al., 2020; Kang et al., 2020). Several studies have shown that BMCs for transcriptional changes can be concordant with BMCs for apical endpoints, including cancer and noncancer toxicities (Bhat et al., 2013; Thomas et al., 2013b; Moffat et al., 2015). Recent work at our institute has shown that BMCs for transcriptional changes of kidney and liver tissues from rodent 5-day in vivo studies compare favorably to BMCs determined using apical endpoints in chronic in vivo rodent studies (Gwinn et al., 2020).
While there are emerging examples of the use of transcriptomics in dose-response modeling, far fewer examples have been reported with metabolomic changes. Metabolomics may be a useful technique in toxicology since toxic substances are almost certain to cause downstream effects in endogenous metabolites (Robertson et al., 2011; Bouhifd et al., 2013). However, the application of metabolomics in toxicology research has been limited. The goals of the present study were 3-fold. First, determining how to apply concentration-response analysis to mass spectrometry-based untargeted metabolomics. Next, testing the utility of the concentration-response analysis with untargeted metabolomics to screen liver injury compounds to derive BMCs reflective of chemical-induced perturbation at therapeutic doses. Finally, comparing metabolomics and transcriptomics methods to differentiate between liver injury and nonliver injury compounds, and characterize their respective relationships to human exposure scenarios that build towards estimation of the likelihood for human response.
MATERIALS AND METHODS
Materials
Liquid chromatography-mass spectrometry (LCMS) grade water and acetonitrile were purchased from Fisher Scientific (Atlanta, Georgia). Formic acid was obtained from ThermoFisher Scientific (Suwanee, Georgia). Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, Missouri). Tamoxifen, ritonavir, rifampicin, chlorpromazine, sucrose, and potassium chloride were procured from MRIGlobal (Kansas City, Missouri). Detailed information about these compounds can be found in Supplementary Table 1.
Cell culture
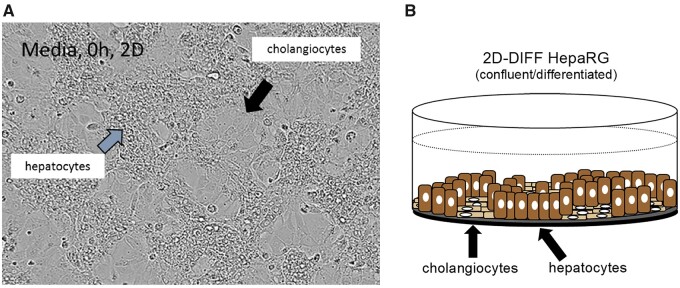
Cryopreserved human HepaRG cells (Lonza, Walkersville, Maryland) were thawed and approximately 20,000 cells per well were seeded onto collagen coated 384-well plates (Corning, New York, New York). Cells were cultured at 37°C and 5% CO2 for 10 days to recover differentiation prior to compound exposures. Cells were plated using Williams E medium (ThermoFisher, Suwanee, Georgia) supplemented with plating additive (Lonza). Five vials (11.8 ml per vial) of plating additive were added to 500 ml of Williams E medium. Culture media was exchanged 24 h after plating, then exchanged on Monday, Wednesday, and Friday for the duration of the cultures. HepaRG cells differentiated into hepatocyte-like and cytokeratin 19 (CK-19) positive “cholangiocyte-like” cells as shown in Figure 1.
Figure 1.
(A) Representative photomicrograph of two-dimensional (2D) HepaRG cultures from vehicle control-treatment over 96 h exposure. Photomicrographs for each culture well at various time points were captured using an Incucyte Zoom (Essen BioSciences). HepaRG cells differentiate into hepatocyte-like and cholangiocyte-like cells as depicted in (B).
Compound exposure
Compounds were procured from MRIGlobal, and the compounds and their CAS numbers are provided in Supplementary Table 1. Stock solutions at 500X the concentration of the highest exposure concentration for each compound were prepared in 100% DMSO. These stocks were serially diluted in DMSO in 96-well plates for a total of 10 concentrations with half-log spacing (Table 1). The concentration range for each compound was determined based on cell viability experiments. From these stocks, 2 µl of each diluted stock solution were transferred into separate 96-well plates containing 1 ml (1/500 dilution) of Williams E medium (ThermoFisher) supplemented with maintenance additive (Lonza) using a Vialflo 96/384 semi-automated pipettor (Integra Biosciences, Hudson, New Hampshire). Compound dilutions were mixed thoroughly prior to 50 µl transfers to cell culture plates. Culture media were exchanged after a 48 h exposure period with refreshed compound exposures as described above. Three independent technical replicates for each treatment group were performed in this study.
Table 1.
Compounds Used in This Study Were Evaluated at Exposure Concentrations With Half-Log Spacing Ranging From the Tabulated High to Low Exposure Concentrations
| Chemical | Low Exposure Concentration (µM) | High Exposure Concentration (µM) | Liver Injury Classification Category |
|---|---|---|---|
| Tamoxifen | 0.002 | 93 | Liver injury |
| Ritonavir | 0.009 | 300 | Liver injury |
| Rifampicin | 0.009 | 300 | Liver injury |
| Chlorpromazine | 0.009 | 300 | Liver injury |
| Sucrose | 0.009 | 300 | Rarely associated with liver injury |
| KCl | 0.009 | 300 | Rarely associated with liver injury |
Each compound was classified as either liver injury or rarely associated with liver injury (negative controls).
After each 48 h exposure period, a 25 µl sample from spent culture media was removed from each well and frozen at −80°C for metabolomics experiments. Spent media culture samples at the 96 h time point were analyzed via metabolomics. Samples were removed from −80°C freezer and thawed on ice. After thawing samples were centrifuged for 5 min at 2000 × g. A 15 µl sample of spent media culture was transferred to a 96-well plate (Thermo Fisher Scientific Inc., Waltham, Massachusetts). Cold acetonitrile (45 µl) was added to spent media culture and plates were centrifuged for 10 min at 3000 × g. A 50 µl sample was then transferred to a 96-well plate and stored in autosampler at 4°C for LCMS analysis. At the final 96 h time point, cells were lysed and assayed via high throughput transcriptomics (see Transcriptomics Methods section) (Ramaiahgari et al., 2019).
LCMS methods
Ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) analyses were performed using a Thermo Vanquish UPLC system with a Thermo Hypersil GOLD aq C18 column (100 × 2.1 mm, 1.9 µm particle size, Thermo Fisher Scientific Inc.) coupled to a Thermo Q Exactive Plus mass spectrometer (Thermo Fisher Scientific Inc.) with an electrospray ionization (ESI) source. Gradient elution was used in the chromatographic separations using water with 0.1% formic acid (mobile phase A) and acetonitrile with 0.1% formic acid (mobile phase B), with the following gradient program: 0–10 min, 10%–100% B; 10–17 min, 100% B; 17–19 min, 100–10% B; and 19–20 min, 10% B. The flow rate was held constant at 0.3 ml/min. Before each run, the column was re-equilibrated for 2 min at 10% B. The injection volume was 2 µl. The column temperature was held at ambient temperature while the autosampler tray temperature was set at 4°C. The first 1.5 min of each LCMS run was directed to waste to minimize potential instrument contamination from cell culture media.
The mass spectrometer was operated in positive ion mode with a spray voltage of 3.00 kV. The S-lens radio frequency (RF) level was set at 60.0. Capillary and auxiliary gas heater temperatures were set at 300 and 413°C, respectively. Sheath gas, auxiliary gas, and sweep gas flow rates were set at 48, 11, and 2, respectively. The mass spectrometer was calibrated with Thermo Fisher Scientific positive mode calibration solution. Full MS scans were collected for the scan range of 150–1500 m/z at a resolution of 70,000, automatic gain control (AGC) target of 1E6, and maximum ionization time of 50 ms. Data-dependent MS/MS scans were collected at a resolution of 17,500, AGC target of 1E5, maximum ionization time of 50 ms. A maximum of 10 peaks were selected for data-dependent fragmentation if a signal intensity threshold of 1.6E5 was reached. MS/MS fragmentation used a 4.0 m/z isolation window and a collision energy of 30.
LCMS data analysis
LCMS data files (Thermo RAW files) were converted to mzXML file formats using MSConvert part of ProteoWizard Tools (Adusumilli and Mallick, 2017). The XCMS online platform (www.xcmsonline.scripps.edu) was used for data analysis of the uploaded mzXML files including peak detection, retention time correction, profile alignment, and isotope annotation (Tautenhahn et al., 2012). Data were analyzed using pair-wise (a direct comparison of two datasets) comparisons to investigate differences in metabolites for the high (without overt cytotoxicity) and low exposure concentrations for each compound. Cytotoxicity data for these experiments can be found in previously published data from our institute (Ramaiahgari et al., 2019). This analysis was completed to determine whether expected changes in endogenous metabolites were occurring based on putative mode of action of the various test articles. After confirming that expected changes were occurring, the data were then processed using a single data analysis method (all data were analyzed together without statistical comparisons). This method allows for multiple experimental conditions to be examined at a single time, in this case, multiple exposure concentrations, instead of being limited to two experimental conditions as is the case with the pair-wise data analysis method. Data were processed for both analysis methods using the following parameters: feature detection using centWave (Δm/z = 5 ppm, minimum peak width = 5 s, and maximum peak width = 20 s); retention time correction using obiwarp (profStep = 1); profile alignment (mzwid = 0.025, minfrac = 0.5, and bw = 5). Unpaired parametric Welch t-test (p-value ≤ 0.05 and fold-change ≥ 1.5) was used to investigate changes in metabolite features between high and low exposure concentrations. Output results were exported from XCMS online and included extracted ion chromatograms and pairwise cloud plots (Patti et al., 2013). Area of extracted ion chromatograms were used for relative quantitative information.
Dose-response analysis of metabolomics data
Peak lists of m/z values and EIC areas were exported from XCMS online and extracted ion chromatograms areas were transformed using a Log2 transformation. Chemical concentrations that caused cytotoxicity or decrease in cell numbers were excluded from the metabolomic analysis. Log2 transformed data were floored to a value of 8 (based on data from blank samples). Data were then loaded into BMDExpress 2 (version 2.20.0180) for dose-response modeling. First, a pre-filtered step was applied to identify dose-response features; specifically, a William’s Trend Test (p < .05) in combination with a 2-fold (up- or down-regulation) cutoff was used. The use of a combination of fold change cut off with a nominal p-value is used in lieu of adjusted p-values based on a recommendation by the MicroArray Quality Control project to obtain the most reproducible set of features (Shi et al., 2008). This is the typical method used for our transriptomics analyses and was used here for metabolomics to keep consistency across omics methods (National Toxicology Program, 2018). Further, it should be noted that application of an unadjusted p-value and fold change threshold is just the first step in feature filtering in our analysis pipeline. In addition to the noted criteria, for a feature to be reported as responsive to chemical treatment the data must also (1) fit a concentration response curve with a high degree of accuracy (global goodness of fit p-value > 0.1), (2) have a BMC potency estimate less than the highest dosed concentration, (3) have convergent estimates of BMC, BMCU (BMC upper bound) and BMCL (BMC lower bound), and (4) have a low uncertainty in the potency estimate (ie, BMCU/BMCL<40). Hence in our analysis pipeline there are multiple layers of feature filtering that act in an orthogonal/complementary manner to most effectively identify features that are plausibly responding to chemical treatment.
Next, these dose-responsive features were fit to 8 different mathematical models (eg, Hill, Power, Linear, Polynomial 2, Exponential 2, Exponential 3, Exponential 4, and Exponential 5) to determine the best fitting BMC value for each metabolite feature using a one standard deviation threshold from vehicle control levels within a given dataset. A benchmark response factor of 1 standard deviation was used. The best-fit model for each fitted probe was then selected based on the lowest AIC (Akaike information criterion). Models that did not demonstrate convergent BMC, BMCL, and BMCU values were considered unacceptable fits. Furthermore, if the Hill model demonstrated a k parameter less than 1/3 of the lowest dose it was not considered as a suitable model fit and was excluded from further consideration. The specific parameter settings, selected from the BMDExpress software when performing “probe set-level” BMC analysis, were as follows: maximum iterations—250, confidence level—0.95, BMR factor—1 (the multiplier of the SD that defined the BMC), restrict power—>1, and constant variance—selected. The specific model selection setting in the BMDExpress software when performing “probe set-level” BMC analysis were as follows: best poly model test—AIC; flag Hill model with “k” parameters—<1/3 the lowest positive dose; and best model selection with flagged Hill model—exclude flagged Hill model. Aggregated BMC data were visualized using BMC accumulation plots, which depict the sequential accumulation of metabolites at each respective BMC across the exposure concentration range.
Transcriptomics methods
After the 96 h compound exposure period, exposure media were removed, and cells were washed once with 50 µl of phosphate-buffered saline (ThermoFisher), followed by addition of 20 µl of 1X TempoSeq lysis buffer (Biospyder, Carlsbad, California) to the culture plates. Culture plates were subsequently incubated for 15 min at room temperature, and frozen at −80°C. TempO-Seq analysis was performed as described previously using the S1500+ targeted gene set (Yeakley et al., 2017; Mav et al., 2018; Ramaiahgari et al., 2019).
Mapped read counts provided by the service provider, Biospyder, were CPM (counts per million) normalized (read count normalized for each culture well, then multiplied by 106). These data were floored with a read count of 5, log2 transformed, and viewed in principal component analysis to remove outlier samples.
Dose-response analysis of transcriptomics data
Dose-response analysis of normalized transcriptomic data was performed using BMDExpress 2 (version 2.20. 0180). All steps in the analysis process were the same as what was described above for the metabolomics data.
Determination of lowest consistent response dose
When evaluating potency of response over many features such as performed here there are often challenges with model outlier/anomalous potency estimates that are significantly offset from the totality of the potency estimates. We have derived a metric called the lowest consistent response dose (LCRD) that is used to identify the most sensitive nonoutlier feature that is the plausibly representative lowest dose level where a consistent response in biological features is observed and is therefore of plausible toxicological relevance. In order to determine this value, the BMC values are ranked from lowest to highest and a ratio (rank n + 1/rank n) of the BMC values is determined. This ratio is representative of the relative degree of change in the BMC between each ranked feature. A search of the ratio values is then performed starting with the lowest BMC to identify the lowest BMC where all subsequent changes (ie, ratio value) in down-rank BMCs are <1.66. BMCs in the down-rank group are declared the “consistent response group of BMCs” (CRGB) because all sequential BMCs in this group have at least one BMC that is <1/4 log difference in value. The lowest BMC in the CRGB is the LCRD. It is anticipated that the exposure levels in the range of CRGB are more likely to be of toxicological relevance due to the more consistent rate of change as a function of dose in the biological system, which is more likely to lead to adversity. A visual illustration of what the LCRD and CRGB represent can be found in Supplementary Figure 1.
Descriptive statistics and analysis
Descriptive statistics (eg, median, first and third quartiles of BMCs) were determined using TIBCO Spotfire version 7.8.0 (TIBCO Software Inc., Palo Alto, California). Comparison of BMC values from metabolomics and transcriptomics was performed in Microsoft Excel 365 (Microsoft Corp, Redmond, Washington) using the T.TEST function using a 2 tailed distribution (“2”) and a two-sample unequal variance (“3”).
All raw and analyzed metabolomics/transcriptomics data are available at: https://doi.org/10.22427/NTP-DATA-002-00058-0003-0000-8.
RESULTS
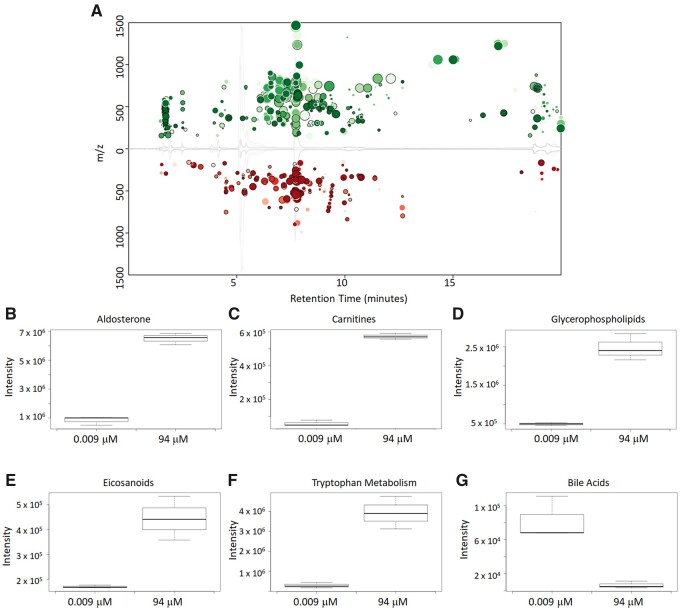
The first fundamental step in this study was to determine if the LCMS analysis of spent culture media samples from HepaRG cultures detected changes in analyte levels consistent with known pharmacological activities. Media samples from ritonavir-exposed HepaRG cultures (96 h exposures) were analyzed by untargeted LCMS, and Figure 2A depicts a summary of these data represented in XCMS cloud plot format. The color of the circles identifies if the feature increased (green) or decreased (red) in intensity. Fold change is shown by the size of the circle with a larger circle relating to a larger fold change, while the depth of color represents the p-value with a darker color correlating to a lower p-value. These data represent pairwise comparisons of the low exposure (0.009 µM) and highest exposure without overt cytotoxity (94 µM) concentrations, and clearly indicate a concentration-related effect with ritonavir. Overall, a total of 635 metabolite features were changed (fold change >1.5 and p-value < 0.05) when comparing the lowest and highest exposures of ritonavir. HepaRG cells exposed to ritonavir were found to have significant changes in various metabolites and metabolite pathways.
Figure 2.
(A) Cloud plot representation of LCMS untargeted metabolomics run of 2D HepaRG cells exposed with ritonavir in positive mode using reverse-phase C18 separation method. Samples were spent culture media samples at 96 h. Data show statistically significant differences between the low and high exposure (without overt cytotoxicity) samples. Box-plot comparison of the intensity of metabolites between the low and high exposures (without overt cytotoxicity) of ritonavir treated samples: (B) aldosterone, (C) carnitines, (D) glycerophospholipids, (E) eicosanoids, (F) tryptophan metabolism, and (G) bile acids.
Based on the Metabolomics Standards Initiative (MSI) classification system, all metabolites identified in this work would be classified as MSI Level 2 or 3 (Sumner et al., 2007). An MSI level 2 identification was possible where tandem mass spectrometry data were available for spectral matching. Tandem mass spectrometry data acquired in this work were searched against the METLIN metabolite database within XCMS online. MSI level 3 identification corresponds to metabolite features that are identified putatively to known compounds of a chemical class. Metabolite features that had hits in the METLIN database with a mass error less than 10 ppm (parts per million) and without tandem mass spectrometry data often resulted in ten or more potential matches. For many features, these potential matches were all compounds of a distinct chemical class and identification of a specific chemical compound was not possible due to absence of tandem mass spectrometry data. Therefore, the data are presented in terms of chemical classes in lieu of referring to specific metabolites by name.
Figures 2B–G show box plot intensity comparisons between the lowest and highest exposure groups for representative metabolites from various metabolite classes. Metabolites in the steroid synthesis pathway were found to increase at higher exposures of ritonavir. Shown in Figure 2B is the increase in amount of aldosterone at the higher exposure concentration. Definitive identification was not possible to distinguish other steroid metabolites but increases in 18-hydroxycorticosterone/cortisol and corticosterone/11-desoxycortisol were also observed. Changes in lipid metabolism have been previously shown to occur in the treatment of HIV patients with ritonavir and other protease inhibitors (Zha et al., 2013; da Cunha et al., 2015). Increases in lipids were observed in the samples with higher ritonavir exposure with example lipid metabolites from various classes including carnitines, glycerophospholipids, and eicosanoids shown in Figures 2C–E. Decreases in the amount of bile acids was found at the higher exposure concentration with a representative bile acid metabolite shown in Figure 2G, which has been previously shown in in vitro hepatocyte studies with HIV protease inhibitor exposure (Griffin et al., 2013). Figure 2F is an example of a change in a nonlipid metabolite for which definitive identification was not possible; however, possible matches in the METLIN metabolite data suggest that it is a metabolite in the tryptophan metabolism pathway. All of these observed changes in metabolites compare favorably to metabolite changes observed in a previous plasma metabolomics study of HIV patients being treated with protease inhibitors such as ritonavir (Cassol et al., 2013).
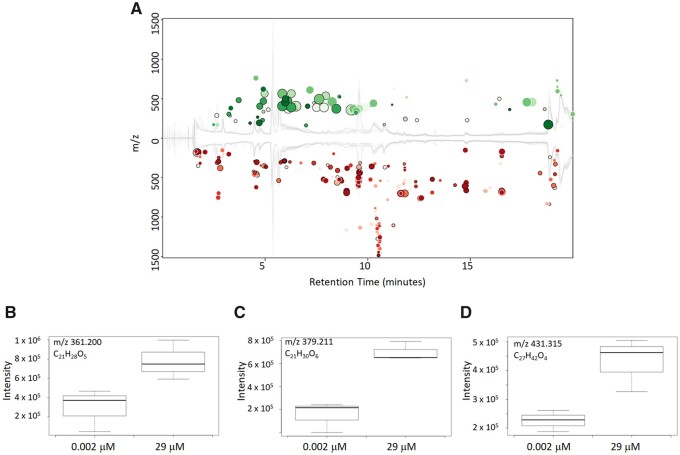
To further explore the utility of the method, spent culture media samples exposed to the lowest (0.002 µM) and highest exposure without overt cytotoxicity (29 µM) concentrations of tamoxifen were compared using pairwise data analysis as shown in Figure 3A. A total of 303 metabolite features were significantly changed (fold change > 1.5 and p-value < 0.05) between the lowest and highest exposures. Numerous studies have shown that tamoxifen causes lipid accumulation in the human liver and the dataset was mined to determine if there were changes in endogenous metabolites that agreed with this previous finding. Primary cultures of human hepatocytes are known to model this tamoxifen-induced lipid accumulation that includes morphological changes (Amacher and Chalasani, 2014). In this study, spent culture media samples from HepaRG cell cultures exposed to 29 µM tamoxifen were observed to have increased amounts of lipids most notably increases in oxysterols and oxysteroids compounds which has been previously shown (Kumar et al., 2017; Raselli et al., 2019). Shown in Figures 3B–3D are examples of these findings. While definitive identification was not possible metabolites represented in Figures 3B and 3C were able to be classified as either oxysterols or oxysteroids compounds. The metabolite represented in Figure 3D could be identified as a lipid species, but specific class of lipid could not be determined. This metabolite is either an oxysterol or a monoglyceride. Monoglycerides are precursors to triglycerides which have been shown to increase in tamoxifen-treated liver cells (Zhao et al., 2014).
Figure 3.
(A) Cloud plot representation of LCMS untargeted metabolomics run of 2D HepaRG cells exposed with tamoxifen in positive mode using reverse-phase C18 separation method. Samples are spent media samples at 96 h. Data show statistically significant differences between the low and high exposure (without overt cytotoxicity) samples. (B–D) Box-plot comparison of the intensity of lipid metabolites between the low and high exposures (without overt cytotoxicity) of tamoxifen-treated samples.
Table 2 gives an overview of the number of statistically significant features shown to be altered after low and high dose (without overt cytotoxicity) chemical exposure. Of major importance is the fact that 68%–88% of these features have no potential hits with a mass error less than 10 ppm in the metabolite database. In addition, those features that do have potential database matches in most cases have greater than 10 potential compound matches thus hampering our ability to positively identify most of these metabolite features. One goal of this study is to show how unidentified metabolite data can still be used to generate toxicologically relevant information.
Table 2.
Summary of Significant Metabolite Features Changed When Comparing Low and High Dose Chemical Exposure and Metabolite Identification Challenges
| Chemical | Metabolite Features Changeda | Database Hits Within Mass Errorb |
|---|---|---|
| Chlorpromazine | 392 | 152 |
| Rifampicin | 1551 | 352 |
| Tamoxifen | 303 | 97 |
| Ritonavir | 635 | 215 |
| Sucrose | 120 | 37 |
| Potassium Chloride | 166 | 35 |
A change is considered significant if p-value ≤.05 and fold-change ≥1.5.
An acceptable mass error was considered to be ≤10 ppm.
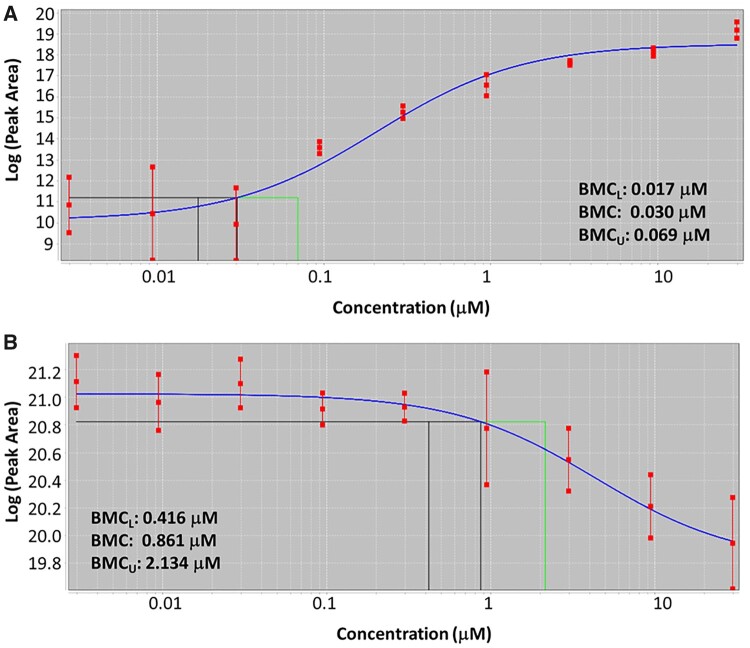
Concentration-response analysis is used to identify chemical-induced perturbations and estimate points of departure for use in risk assessments (Farmahin et al., 2017). Figure 4 shows two example concentration-response curves for tamoxifen-exposed HepaRG cultures at the 96 h time point via LCMS analysis. Both curves were fit using an exponential model. The three vertical lines on the curves represent (from left to right) the lower bound of the 95% confidence interval of the BMCL, the BMC, and the upper bound of the 95% confidence interval of the benchmark concentration BMCU.
Figure 4.
Representative dose-response curves for two features found in the spent media samples for tamoxifen exposed 2D HepaRG cells (A) m/z 406.237 and (B) m/z 613.181. An exponential model is fit to the data using BMDExpress. These representative features were chosen to show that intensities of features can either increase or decrease with chemical concentration. The three vertical lines represent (from left to right) the lower bound of the 95% confidence interval of the BMCL, the BMC, and the upper bound of the 95% confidence interval of the BMCU.
The concentration-response curve in Figure 4A shows an increase in response (peak area) for this specific metabolite as the concentration of tamoxifen was increased. This metabolite had a mass-to-charge value of 406.237, which corresponds to a chemical formula of C26H31NO3, and is a hydroxylation product of tamoxifen and is consistent with Phase 1 metabolism. Tamoxifen has a chemical formula of C26H29NO, thus the metabolite differs by H2O2, and was likely formed via the processes of oxidation and hydration. Analysis of the MS/MS spectra of tamoxifen and this tamoxifen metabolite showed very similar fragmentation patterns, further lending support to this identification. The BMCL, BMC, and BMCU for this metabolite were determined to be 0.017 µM, 0.030 µM, and 0.069 µM, respectively.
Figure 4B shows an example of a concentration-response curve where there was a decrease in peak area for this metabolite as the tamoxifen concentration was increased. This metabolite had a mass-to-charge value of 613.181 and was noted as an M + 3 isotope of the metabolite at a mass-to-charge value of 610.183. Analysis of MS and MS/MS spectra for this metabolite led to inconclusive results as to the identity of this metabolite. Assignment of a chemical formula to this metabolite was also not possible with a high-level of confidence, thus this metabolite is an unknown, currently. The BMCL, BMC, and BMCU for this unknown metabolite was determined to be 0.416, 0.861, and 2.134 µM, respectively. The concentration response analysis included both identified metabolites and nonidentified features. The informatics of successful identification of metabolites, while improving, can still be an issue and a goal of this work was to show that nonidentified features can still be useful pieces of information in a metabolomics-based toxicity assessment.
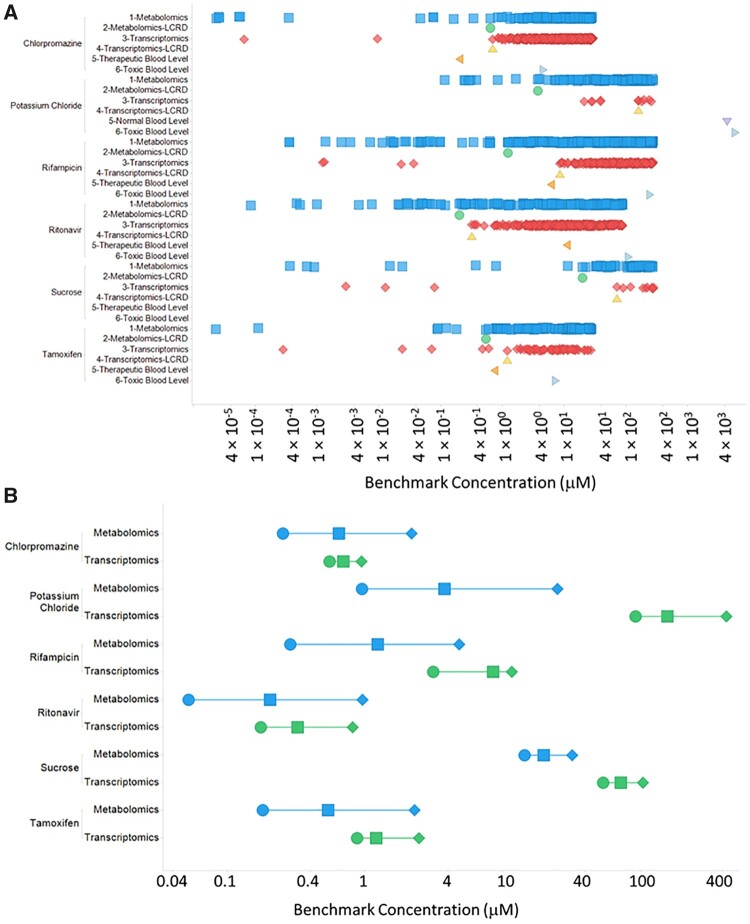
Figure 5 shows the BMC distribution scatter plots for results from metabolomics and transcriptomics analyses of HepaRG cultures treated with 6 different agents, 4 of which are known hepatoxic drugs in humans, tamoxifen, ritonavir, chlorpromazine, and rifampicin. A corresponding summary table of the results presented in Figure 5 can be found in Table 3.
Figure 5.
A, BMC distribution scatter plots showing BMC values for metabolomic features (blue squares), metabolomic LCRD values (green circles), transcriptomic features (red diamonds), transcriptomic LCRD values (light yellow triangles), therapeutic/normal blood levels (if available; orange sideways triangles) and toxic blood levels (if available; light blue sideways triangles). B, Metabolomic and transcriptomic LCRD range plot showing the 95% lower (BMCL) (circles) and upper bound (BMCU) (diamonds) around the BMC that corresponds to the LCRD (squares).
Table 3.
Comparison of Metabolomics and Transcriptomics Data, Including Median, First Quartile (Q1), and Lowest Consistent Response Dose (LCRD) Benchmark Concentrations (in µM) as well as the Number of Features Changed
| Metabolomics |
Transcriptomics |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical | No of Features Changed | Median | Q1 | LCRD | No of Features Changed | Median | Q1 | LCRD | Therapeutic/Normal Blood Levelsa | Toxic Blood Levels1 | T-test of all BMC values (p-value) | T-test of BMC values in CRGB (p-value) |
| Chlorpromazine | 150 | 12.63 | 5.57 | 0.65 | 970 | 11.07 | 5.71 | 0.70 | 0.20 | 4.69 | 0.6 | 0.4 |
| KCl | 185 | 127.30 | 45.74 | 3.78 | 11 | 159.38 | 33.84 | 159.38 | 4400.00 | 6000.00 | 0.7 | 0.004 |
| Rifampicin | 464 | 80.13 | 18.39 | 1.23 | 293 | 79.68 | 43.06 | 8.56 | 6.14 | 247.89 | 0.9 | 0.5 |
| Ritonavir | 184 | 12.73 | 1.80 | 0.20 | 544 | 13.80 | 7.28 | 0.32 | 11.10 | 110.97 | 0.07 | 0.002 |
| Sucrose | 91 | 149.37 | 61.99 | 20.02 | 13 | 187.88 | 72.74 | 72.74 | — | — | 0.6 | 0.09 |
| Tamoxifen | 137 | 10.89 | 2.72 | 0.54 | 67 | 7.33 | 3.29 | 1.20 | 0.74 | 7.40 | 0.06 | 0.07 |
For Chlorpromazine, Rifampicin, Ritonavir, and Tamoxifen therapeutic dose levels were derived from Schulz et al. (2012) Normal and toxic levels of potassium were derived from standard clinical guidance. Toxic levels of Chlorpromazine and Rifampicin were taken from Schulz et al. (2012). Toxic dose levels of Ritonavir and Tamoxifen were not identified by search the literature and were therefore estimated by multiplying the therapeutic dose level by 10. Therapeutic/Normal and toxic dose levels of sucrose were not found when searching the literature.
For chlorpromazine, the metabolomics experiment had a median BMC of 12.6 µM, a BMC of 5.6 µM for the first quartile and a LCRD of 0.647 µM. The transcriptomics data for chlorpromazine exposed cultures had a median BMC of 11.1 µM, a BMC of 5.7 µM for the first quartile, and an LCRD of 0.696 µM. There was no significant difference in all BMC values or just those within the CRBG when comparing metabolomics and transcriptomics from chlorpromazine treatment cells (Table 3). A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for chlorpromazine metabolomic and transcriptomic BMC values showed overlap, further supporting the observation that equivalent estimates of biological potency are obtained from both technologies. The therapeutic blood level of chlorpromazine (0.20 µM) was well below both the metabolomic (0.65 µM) and transcriptomic (0.70 µM) LCRD and the toxic blood level (4.69 µM) is well within range of both the metabolomic and transcriptomic CRGB.
For rifampicin, the metabolomics experiment had a median BMC of 80.1 µM a BMC of 18.4 µM for the first quartile and a LCRD of 1.2 µM. The transcriptomics data for rifampicin exposed cultures had a median BMC of 79.7 µM, a BMC of 43.1 µM for the first quartile, and an LCRD of 8.6 µM. There was no significant difference (p < 0.05) in all BMC values or just those within the CRGB when comparing metabolomics and transcriptomics from rifampicin treatment cells (Table 3). A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for rifampicin metabolomic and transcriptomic BMC values showed overlap, further supporting the observation that equivalent estimates of biological potency are obtained from both technologies. The therapeutic blood level of rifampicin (6.14 µM) was below the transcriptomic LCRD (8.56 µM), however, it was higher than the metabolomic LCRD (1.23 µM). The toxic blood level of rifampicin (247.89 µM) was within range of both the metabolomic and transcriptomic CRGB.
For ritonavir, the metabolomics experiment had a median BMC of 12.7 µM, a BMC of 1.8 µM for the first quartile and a LCRD of 0.204 µM. The transcriptomics data for ritonavir exposed cultures had a median BMC of 13.8 µM, a BMC of 7.3 µM for the first quartile, and an LCRD of 0.324 µM. When comparing all BMC values between metabolomics and transcriptomics for ritonavir there was no significant difference (p < 0.05), however, when the comparison was performed with BMC values in the CRBG the metabolomics BMC values were significantly lower than the transcriptomics BMC values. A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for ritonavir metabolomic and transcriptomic BMC values showed overlap, supporting the observation that equivalent estimates of biological potency are obtained from both technologies. Unexpectedly therapeutic (11.10 µM) and estimated toxic blood level (110.97 µM) of ritonavir were both well above the LCRD for both metabolomics (0.20 µM) and transcriptomics (0.32 µM). This latter observation may be related to unique distribution kinetics of ritonavir that makes comparison between in vitro BMC values and blood levels of the drug in humans difficult (Ferguson et al., 2011).
For tamoxifen, the metabolomics experiment had a median BMC of 10.9 µM, a BMC of 2.7 µM for the first quartile and a LCRD of 0.539 µM. The transcriptomics data for tamoxifen exposed cultures had a median BMC of 7.3 µM, a BMC of 3.3 µM for the first quartile, and an LCRD of 1.2 µM. There was no significant difference (p < 0.05) in all BMC values or just those within the CRGB when comparing metabolomics and transcriptomics from tamoxifen-treated cells (Table 3). Although no significant difference in BMC values were observed, there did appear to be a trend suggesting metabolomics produced lower BMC values. A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for tamoxifen metabolomic and transcriptomic BMC values showed overlap, supporting the observation that equivalent estimates of biological potency are obtained from both technologies. The therapeutic blood level of tamoxifen (0.74 µM) was below transcriptomic LCRD (1.20 µM) and slightly above the metabolomic LCRD (0.54 µM). The toxic blood level for tamoxifen (7.40 µM) was well within range of the CRGB.
For potassium chloride, the metabolomics experiment had a median BMC of 127.3 µM, a BMC of 45.7 µM for the first quartile and a LCRD of 3.8 µM. The transcriptomics data for potassium chloride exposed cultures had a median BMC of 159.4 µM, a BMC of 33.8 µM for the first quartile, and an LCRD of 159.4 µM. There was a significant difference (p < 0.05) in all BMC values or just those within the CRGB when comparing metabolomics and transcriptomics from potassium chloride treatment cells (Table 3) with metabolomics producing relatively lower BMC values. A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for potassium chloride metabolomic and transcriptomic BMC values showed metabolomic responses were more sensitive (ie, lower LCRD). The toxic blood level for potassium chloride (6000 µM) was well outside the upper side of the range of the CRGB. Notably, there was a striking difference in the number of responsive features with potassium chloride with metabolomics having approximately 18X the number of dose-responsive features compared to transcriptomics. This latter observation may be related to potassium ion adducts of certain metabolites that can show a dose-related increase with potassium chloride. This latter concern confounds the metabolomics findings with potassium chloride and therefore the metabolomics findings with this chemical should be interpreted with caution.
For sucrose, the metabolomics experiment had a median BMC of 149.4 µM, a BMC of 61.9 µM for the first quartile and a LCRD of 20.0 µM. The transcriptomics data for sucrose exposed cultures had a median BMC of 187.9 µM, a BMC of 72.7 µM for the first quartile, and an LCRD of 72.7 µM. There was no significant difference (p < 0.05) in all BMC values or just those within the CRGB when comparing metabolomics and transcriptomics from sucrose treatment cells (Table 3). A comparison of the LCRD range (BMCL-BMC[=LCRD]-BMCU) for sucrose metabolomic and transcriptomic BMC values showed metabolomic responses were more sensitive (ie, lower LCRD).
The general trend in the data suggests that metabolomics may be a slightly more sensitive means of detecting dose-related changes in biological systems. Furthermore, the data give a preliminary suggestion that, assuming no unique distribution kinetics as in the case with ritonavir, blood levels near or below the LCRD are likely to have limited acute hepatoxic effects. To further investigate this, a comparison of typical internal human plasma levels (Cmax) to LCRD was made for both metabolomics and transcriptomics data as shown in Table 4.
Table 4.
C max/LCRD Ratios for Metabolomics and Transcriptomics Experiments to Determine Likelihood of Therapeutic Doses Resulting in High Enough Exposure Levels to Cause Observed Responses in Humans
| Chemical | Metabolomics BMC LCRD (µM) | Transcriptomics BMC LCRD (µM) | Reference Human Plasma Concentrations in µMa | Metabolomics Cmax/LCRD | Transcriptomics Cmax/LCRD |
|---|---|---|---|---|---|
| Chlorpromazine | 0.65 | 0.70 | 0.47 | 0.73 | 0.68 |
| KCl | 3.78 | 159.38 | — | — | — |
| Rifampicin | 1.23 | 8.56 | 13.00 | 10.53 | 1.52 |
| Ritonavir | 0.20 | 0.32 | 15.50 | 75.98 | 47.84 |
| Sucrose | 20.02 | 72.74 | — | — | — |
| Tamoxifen | 0.54 | 1.20 | 0.32 | 0.60 | 0.27 |
Reference human plasma concentrations were obtained from Drug Matrix, Goodman & Gilman, and the Physician’s Desk Reference.
Ratios of human Cmax and LCRD were used to determine if therapeutic doses would be sufficiently high to cause the observed in vitro biological responses in humans. A Cmax/LCRD ratio >1 represents a higher probability that internal human plasma levels using therapeutic doses would be sufficiently high to cause the observed biological responses in humans, while a Cmax/LCRD ratio < 0.1 represents very low internal human plasma levels using therapeutic doses and would likely not result in sufficiently high enough exposures to cause observed biological responses. A Cmax/LCRD ratio that falls between 0.1 and 1 would be indicative of a moderate probability that internal human plasma levels would be high enough to cause the observed responses in humans at therapeutic doses.
Results showed that both ritonavir and rifampicin would have a higher probability of causing observed response in humans at therapeutic doses (Cmax/LCRD ratio >1), with metabolomics data resulting in slighted elevated Cmax/LCRD ratio values, likely due to the enhanced sensitivity of metabolomics compared to transcriptomics as previously discussed. Data for chlorpromazine and tamoxifen showed Cmax/LCRD ratio values that fall between 0.1 and 1, suggesting a moderate probability of therapeutic doses causing the observed biological responses in humans. Cmax/LCRD ratio values were not calculated for the control compounds sucrose and potassium chloride as therapeutic Cmax values are unavailable.
DISCUSSION
Our mass spectrometry-based untargeted metabolomics method was shown to be successful in identifying anticipated changes in endogenous metabolites in HepaRG cultures exposed to known liver injury compounds. Increases in lipid species, specifically oxysterols, in tamoxifen exposed cultures were observed as the concentration of tamoxifen was increased. Tamoxifen is known to cause lipid accumulation in the liver. Ritonavir exposed HepaRG cells were shown to have changes in numerous classes of metabolites (lipids, bile acids, and nonlipid metabolites) which have been previously shown to occur in both in vitro cultures as well as in human plasma samples.
This method employs a unique approach with untargeted metabolomics to initially focus on perturbed features rather than comprehensive metabolite identification for biological interpretation. Modifications to the LCMS methods used herein could result in the increase of identified metabolites. Most importantly, modifications to data-dependent tandem mass spectrometry settings would likely result in more fragmentation data which would result in more metabolite identifications. In addition, future efforts are ongoing to create an internal retention time and tandem mass spectrometry library to improve identification. However, the work herein provides an example of how metabolomics data without a focus on metabolite identification can still be a useful method for toxicity assessment. In this study, all features found in the untargeted analysis were included as inputs for concentration-response modeling. This enabled application of the toxicological proven power of concentration-response modeling to identify and discriminate biologically perturbed features using BMD Express 2.30 with liver injury compounds. The results derived BMCs in response to chemical exposure in metabolically competent liver cell cultures displaying physiologically relevant hepatic receptor signaling (eg, CAR, PXR, FXR, and PPARα).
It is important to note that the derived BMCs are based on nominal concentrations (dosed concentrations) which may not directly represent the true free concentration in an in vitro system. A variety of factors have been shown to affect the true available concentration in vitro including binding to plastic, ability of chemical to bind to proteins, etc. (Armitage et al., 2014; Kisitu et al., 2020). Adsorption to plastic can play a major role in decreasing the free concentration, however, this is chemical dependent. For the compounds used in this study the model by Armitage et al. (2014) was used to predict the amount of chemical bound to plastic (Supplementary Table 2). Predictions using the Armitage model were calculated in the High-Throughput Toxicokinetics R package (Pearce et al., 2017). These predictions showed that chemical binding to plastic is not an issue for certain compounds (sucrose), but for other compounds the percentage of chemical bound to plastic was predicted to be between 5% and 25%. This would correspond to derived BMCs in this study being 5%–25% higher than they are in actuality. Future studies on deriving in vitro BMCs could best address this by experimental measurement of chemical concentration in in vitro culture systems thus resulting in more accurate BMCs.
Analysis of liver injury versus nonliver injury compounds revealed all four liver injury compounds studied gave rise to a steep increase in the number of BMCs as the concentration of the chemical exposure increased. Sucrose and KCl, nonliver injury compound controls, did not have this steep increase. Thus, untargeted metabolomics data with physiologically relevant in vitro liver models appear to be a useful tool for distinguishing liver injury from nonliver injury compounds.
Comparisons of Cmax values to LCRD values from metabolomics and transcriptomics were made to determine the probability of therapeutic doses causing high enough exposures to cause in vitro observed biological responses in humans. Rifampicin and ritonavir were determined to have a higher probability of causing these responses in humans at therapeutic doses, while chlorpromazine and tamoxifen showed a moderate probability. These comparisons serve as a method to determine potential risk of hepatoxic compounds using in vitro methods. A key component of this analysis was to confirm that the metabolite feature that corresponds to the LCRD value was not related to the drug compounds since drugs and drug metabolites were not removed prior to BMC analysis. While definitive identification of the metabolites associated with LCRD values was not possible, it was confirmed that none of the metabolomics LCRD values for this study were known drug metabolites or parent drugs. This confirmed that the metabolite features related to the LCRD value were of an unknown endogenous origin and were not related to exogenous (dosed) drugs. This potential issue could be avoided in future studies using metabolomics data for determining BMCs by removing all features related to dosed compounds prior to BMC analysis.
Finally, a direct comparison for this set of compounds between matched metabolomics and transcriptomics data revealed for three of the four liver injury compounds that metabolomics was more potently sensitive than transcriptomics at the same time point (96 h). However, the magnitude of perturbed features was higher with transcriptomics in each case. For one compound, tamoxifen, the transcriptomics, and metabolomics data had very similar potencies in the overall BMC profile revealed in accumulation plots. Further analysis to match biological response pathways in transcriptomics and perturbed metabolites within each respective pathway is warranted.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Alison Harrill and Dr Fred Parham for review of this manuscript. They thank Bradley Collins and Suramya Waidyanatha of National Toxicology Program (NTP) for assistance with chemical procurement from MRIGlobal. They also thank Molly Vallant of NTP and Barney Sparrow of Battelle for coordinating the analysis of samples using TempO-Seq at Biospyder Inc. They also would like to thank Jennifer Fostel (NTP) and the Vistronix team for organizing the raw data associated with this study on NIEHS public data repository CEBS (Chemical Effects in Biological Systems) website.
FUNDING
National Institute of Environmental Health Sciences (NIEHS) of National Institutes of Health (NIH).
Disclaimer: This article may be the work product of an employee or group of employees of the NIEHS, NIH; however, the statements contained herein do not necessarily represent the statements, opinions, or conclusions of the NIEHS, NIH of the U.S. Government. The content of this publication does not necessarily reflect the views or the policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- Adusumilli R., Mallick P. (2017). Data conversion with proteowizard msconvert. In Proteomics: Methods and Protocols (Comai L., Katz J. E., Mallick P., Eds.), pp. 339–368. Springer New York, New York, NY. [DOI] [PubMed] [Google Scholar]
- Amacher D. E., Chalasani N. (2014). Drug-induced hepatic steatosis. Semin. Liver Dis. 34, 205–214. [DOI] [PubMed] [Google Scholar]
- Armitage J. M., Wania F., Arnot J. A. (2014). Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 48, 9770–9779. [DOI] [PubMed] [Google Scholar]
- Beger R. D., Dunn W., Schmidt M. A., Gross S. S., Kirwan J. A., Cascante M., Brennan L., Wishart D. S., Oresic M., Hankemeier T., et al. (2016). Metabolomics enables precision medicine: “A white paper, community perspective”. Metabolomics 12, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat V. S., Hester S. D., Nesnow S., Eastmond D. A. (2013). Concordance of transcriptional and apical benchmark dose levels for conazole-induced liver effects in mice. Toxicol. Sci. 136, 205–215. [DOI] [PubMed] [Google Scholar]
- Bouhifd M., Hartung T., Hogberg H. T., Kleensang A., Zhao L. (2013). Review: Toxicometabolomics. J. Appl. Toxicol. 33, 1365–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C., Berg E. L., Kunkel E. J. (2004). Systems biology in drug discovery. Nat. Biotechnol. 22, 1253–1259. [DOI] [PubMed] [Google Scholar]
- Cassol E., Misra V., Holman A., Kamat A., Morgello S., Gabuzda D. (2013). Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect. Dis. 13, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corton J. C., Hill T. III, Sutherland J. J., Stevens J. L., Rooney J. (2020). A set of six gene expression biomarkers identify rat liver tumorigens in short-term assays. Toxicol. Sci. 177, 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Cunha J., Maselli L. M., Stern A. C., Spada C., Bydlowski S. P. (2015). Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World J. Virol. 4, 56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. A., Gift J. S., Zhao Q. J. (2011). Introduction to benchmark dose methods and U.S. Epa's benchmark dose software (bmds) version 2.1.1. Toxicol. Appl. Pharmacol. 254, 181–191. [DOI] [PubMed] [Google Scholar]
- Farmahin R., Williams A., Kuo B., Chepelev N. L., Thomas R. S., Barton-Maclaren T. S., Curran I. H., Nong A., Wade M. G., Yauk C. L. (2017). Recommended approaches in the application of toxicogenomics to derive points of departure for chemical risk assessment. Arch. Toxicol. 91, 2045–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. S., Black C. B., Baucom C., Peterson A., Wright J., Freeman K., Easterwood L., Lowrance L., Hill J., LeCluyse E. L. (2011). Ritonavir and troleandomycin: Time-course assessment of induction and inhibition incultures of primary human hepatocytes. Society of Toxicology 50th Annual Meeting & ToxExpo. Washington, DC. [Google Scholar]
- Griffin L. M., Watkins P. B., Perry C. H., St Claire R. L. 3rd, Brouwer K. L. (2013). Combination lopinavir and ritonavir alter exogenous and endogenous bile acid disposition in sandwich-cultured rat hepatocytes. Drug Metab. Dispos. 41, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn W. M., Auerbach S. S., Parham F., Stout M. D., Waidyanatha S., Mutlu E., Collins B., Paules R. S., Merrick B. A., Ferguson S., et al. (2020). Evaluation of 5-day in vivo rat liver and kidney with high-throughput transcriptomics for estimating benchmark doses of apical outcomes. Toxicol. Sci. 176, 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W., Podtelezhnikov A. A., Tanis K. Q., Pacchione S., Su M., Bleicher K. B., Wang Z., Laws G. M., Griffiths T. G., Kuhls M. C., et al. (2020). Development and application of a transcriptomic signature of bioactivation in an advanced in vitro liver model to reduce drug-induced liver injury risk early in the pharmaceutical pipeline. Toxicol. Sci. 177, 121–139. [DOI] [PubMed] [Google Scholar]
- Kisitu J., Hollert H., Fisher C., Leist M. (2020). Chemical concentrations in cell culture compartments (c5) - free concentrations. Altex 37, 693–708. [DOI] [PubMed] [Google Scholar]
- Kumar A., Blackshear C., Subauste J. S., Esfandiari N. H., Oral E. A., Subauste A. R. (2017). Fatty liver disease, women, and aldosterone: Finding a link in the Jackson heart study. J. Endocr. Soc. 1, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo B., Francina Webster A., Thomas R. S., Yauk C. L. (2016). Bmdexpress data viewer - a visualization tool to analyze bmdexpress datasets. J. Appl. Toxicol. 36, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mav D., Shah R. R., Howard B. E., Auerbach S. S., Bushel P. R., Collins J. B., Gerhold D. L., Judson R. S., Karmaus A. L., Maull E. A., et al. (2018). A hybrid gene selection approach to create the s1500+ targeted gene sets for use in high-throughput transcriptomics. PLoS One 13, e0191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat I., Chepelev N., Labib S., Bourdon-Lacombe J., Kuo B., Buick J. K., Lemieux F., Williams A., Halappanavar S., Malik A., et al. (2015). Comparison of toxicogenomics and traditional approaches to inform mode of action and points of departure in human health risk assessment of benzo[a]pyrene in drinking water. Crit. Rev. Toxicol. 45, 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. 2018. NTP research reports. NTP research report on national toxicology program approach to genomic dose-response modeling: research report 5. Durham (NC): National Toxicology Program. [PubMed]
- Patti G. J., Tautenhahn R., Rinehart D., Cho K., Shriver L. P., Manchester M., Nikolskiy I., Johnson C. H., Mahieu N. G., Siuzdak G. (2013). A view from above: Cloud plots to visualize global metabolomic data. Anal. Chem. 85, 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce R. G., Setzer R. W., Strope C. L., Wambaugh J. F., Sipes N. S. (2017). Httk: R package for high-throughput toxicokinetics. J. Stat. Softw. 79, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol A., Mosca R., Farres J., Aloy P. (2010). Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol. Sci. 31, 115–123. [DOI] [PubMed] [Google Scholar]
- Ramaiahgari S. C., Auerbach S. S., Saddler T. O., Rice J. R., Dunlap P. E., Sipes N. S., DeVito M. J., Shah R. R., Bushel P. R., Merrick B. A., et al. (2019). The power of resolution: contextualized understanding of biological responses to liver injury chemicals using high-throughput transcriptomics and benchmark concentration modeling. Toxicol. Sci. 169, 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raselli T., Hearn T., Wyss A., Atrott K., Peter A., Frey-Wagner I., Spalinger M. R., Maggio E. M., Sailer A. W., Schmitt J., et al. (2019). Elevated oxysterol levels in human and mouse livers reflect nonalcoholic steatohepatitis. J. Lipid Res. 60, 1270–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. G., Watkins P. B., Reily M. D. (2011). Metabolomics in toxicology: preclinical and clinical applications. Toxicol. Sci. 120(Suppl. 1), S146–S170. [DOI] [PubMed] [Google Scholar]
- Schulz M., Iwersen-Bergmann S., Andresen H., Schmoldt A. (2012). Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Critical Care 16, R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Jones W. D., Jensen R. V., Harris S. C., Perkins R. G., Goodsaid F. M., Guo L., Croner L. J., Boysen C., Fang H., et al. (2008). The balance of reproducibility, sensitivity, and specificity of lists of differentially expressed genes in microarray studies. BMC Bioinformatics 9, S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner L. W., Amberg A., Barrett D., Beale M. H., Beger R., Daykin C. A., Fan T. W. M., Fiehn O., Goodacre R., Griffin J. L., et al. (2007). Proposed minimum reporting standards for chemical analysis chemical analysis working group (cawg) metabolomics standards initiative (msi). Metabolomics 3, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautenhahn R., Patti G. J., Rinehart D., Siuzdak G. (2012). Xcms online: a web-based platform to process untargeted metabolomic data. Anal. Chem. 84, 5035–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. S., Philbert M. A., Auerbach S. S., Wetmore B. A., Devito M. J., Cote I., Rowlands J. C., Whelan M. P., Hays S. M., Andersen M. E., et al. (2013a). Incorporating new technologies into toxicity testing and risk assessment: Moving from 21st century vision to a data-driven framework. Toxicol. Sci. 136, 4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. S., Wesselkamper S. C., Wang N. C., Zhao Q. J., Petersen D. D., Lambert J. C., Cote I., Yang L., Healy E., Black M. B., et al. (2013b). Temporal concordance between apical and transcriptional points of departure for chemical risk assessment. Toxicol. Sci. 134, 180–194. [DOI] [PubMed] [Google Scholar]
- Yeakley J. M., Shepard P. J., Goyena D. E., VanSteenhouse H. C., McComb J. D., Seligmann B. E. (2017). A trichostatin a expression signature identified by tempo-seq targeted whole transcriptome profiling. PLoS One 12, e0178302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha B. S., Wan X., Zhang X., Zha W., Zhou J., Wabitsch M., Wang G., Lyall V., Hylemon P. B., Zhou H. (2013). HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS One 8, e59514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Xie P., Jiang J., Zhang L., An W., Zhan Y. (2014). The effect and mechanism of tamoxifen-induced hepatocyte steatosis in vitro. Int. J. Mol. Sci. 15, 4019–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.