Abstract
Ovarian cancer (OC) is known to be the most lethal cancer in women worldwide, and its etiology is poorly understood. Recent studies show that mitochondrial DNA (mtDNA) content as well as mtDNA and nuclear genes encoding mitochondrial proteins influence OC risk. This review presents an overview of role of mitochondrial genetics in influencing OC development and discusses the contribution of mitochondrial proteome in OC development, progression and therapy. A role of mitochondrial genetics in racial disparity is also highlighted. In-depth understanding of role of mitochondria in OC will help develop strategies toward prevention and treatment and improving overall survival in women with OC.
Introduction
Mitochondria are multifunctional organelles controlling energy production, oxidative stress, metabolic signaling and apoptotic pathways and hence play an important role in cancer. Mitochondrial DNA (mtDNA) is a circular, double-stranded smaller genome of 16.5 kb and encodes total of 37 proteins, which includes 13 protein-coding genes of oxidative phosphorylation (OXPHOS) complexes that forms the respiratory chain complexes, 22 tRNAs and 2 rRNAs (1). It also contains non-coding region, the displacement loop (D-loop), also considered as ‘hot spot’ for mutations in mtDNA, that contains the origin of replication and also the major promoters of transcription (1). Apart from these proteins encoded by mtDNA genes, majority of proteins of respiratory chain complexes are encoded by nuclear genes and many of nuclear-encoded proteins are transported into mitochondria (1). Unlike nuclear DNA (nDNA), mtDNA is unique as it exists in multiple copies per cell and the number depends upon the cell energy demand. Numerous proteins encoded by nDNA are involved in generating new mitochondria (mitochondrial biogenesis), which encodes proteins involved in numerous mitochondrial function such as OXPHOS, apoptosis, and mtDNA replication and gene expression (1). Furthermore, nDNA-encoded mtDNA genes act as regulators of mitochondrial biogenesis and participate in nuclear-mitochondrial DNA crosstalks. Interplay between the two genomes can influence disease processes including cancer (2). Altered energy metabolism is a common feature of cancer and therefore mitochondria may play an important role in cancer including ovarian cancer (OC). In addition, mtDNA when compared with nDNA is more vulnerable to mutations because of lack of histones, inefficient DNA repair mechanism and reactive oxygen species generation in vicinity (2). Furthermore, mitochondria also participate in cellular differentiation, cell signaling, apoptosis, cell cycle and cell growth (2), which are linked to tumorigenesis, and thus emphasizes the crucial role of mitochondria in cancer. Also, any change in mitochondrial genome may activate the mitochondrial retrograde signaling that controls the expression of nuclear genes and hence alters the nuclear gene expression profile. Hence, dysfunctional mitochondria are considered as one of the mechanisms of tumorigenesis including ovarian tumorigenesis. Mitochondrial dysfunction is a hallmark in OC, and mitochondrial determinants have been identified as cause, prognostic biomarkers and therapeutic targets in OC (2). Studies indicate that mitochondrial morphology is significantly changed in OC compared with controls and mitochondria are abundant in OC cells and tissues (3).
OC has complex histopathological phenotypes, and majority are epithelial-derived tumors, which may be serous (SER), mucinous, endometrioid (END), clear cell (CC), transitional, squamous, mixed and undifferentiated types (4). Presently, with no available diagnostic test for OC detection in early stages, majority (~90%) of the women with epithelial OC (EOC) are found with advanced stage with metastasis associated with <30% 5 year survival rates (5). Whereas if detected early in stage I OC without metastasis, 5 year survival rate is 90% (5).
The highest incidence and mortality associated with OC occur in postmenopausal women with a median age of diagnosis at 63. Indeed, 90% of OC is reported to be postmenopausal. Studies suggest that ovarian aging is in menopausal factors involved in the etiology of OC. There is increasing evidence that mitochondrial dysfunction drives ovarian aging and menopause (6). For example, mtDNA content and copy numbers decline in the ovary as women age. Low mtDNA level is also found in menopausal women, the stage associated with the presence of serous tubal intraepithelial carcinoma lesions and detection of most OC subtypes. Decreased mtDNA copy number is also associated with the high-grade subtype of ovarian carcinoma and decreases during cancer progression in whole-blood DNA, cell-free DNA and exosomal DNA. mtDNA deficiency has also been shown to lead to upregulation of genes related to cell proliferation, anti-apoptosis and cancer metastasis, angiogenesis, drug resistance and induction of cancer cell stem-like properties in OC models. Thus, the main goal of this review is to define the role of the mitochondrial dysfunction relevant to the development of OC.
Mitochondrial dysfunction is relevant in all epithelial cancers, as it leads to cancer progression and drug resistance. However, in this review, we focus on OC (particularly EOC) due to unique nature of OC and its mitochondrial dependence. OC is unique as it is formed of solid tumor that develops in the abdominal cavity and metastasizes and recurrence occurs in the same place (7). It has a peculiar microenvironment characterized by ascites, low glucose levels and hypoxia, and due to which OC cells switch to mitochondrial respiration for their survival. Furthermore, compared with other tumors, peritoneal ascitic fluid accumulation is more common in OC and thought to be the major cause of its metastatic spread. As ovarian tumors grow in size, cells into the form of dense spherical clusters (known as spheroids) are shed into the ascitic fluid, which eventually invade surface tissues to form new tumors and are difficult to treat with chemotherapy (7). Spheroids are made up of the outer cellular layer, which is proliferative and can survive with the limited levels of glucose and oxygen available in the ascites. These cells can be selectively eliminated by the standard chemotherapy regimen, a combination of paclitaxel and cisplatin/carboplatin for OC patients (7). However, cells inside of spheroids are dense, hypoxic, poorly vascularized areas become quiescent and not responsive to general chemotherapy regimen treatment. Both quiescent and proliferative layers of spheroids depend on OXPHOS for ATP production for survival. Increased OXPHOS in turn promotes cancer cell survival and proliferation and cause chemoresistance. Highly proliferating cancer cells of spheroids reside in microenvironment with adequate levels of oxygen and glucose but have increased ATP demand and therefore OXPHOS inhibition causes cancer cells to die as the cells are not to be able to meet their high ATP demand despite high glucose. In contrast, quiescent cancer cells reside in microenvironments of low glucose and hypoxia but also have low ATP demand. Hence, OXPHOS inhibition in these cells is absolute lethal as enough glucose is not present to compensate for the loss of ATP needed for cell survival. Independent of microenvironmental factors, mitochondria also play a role in OC metastasis and chemoresistance as genetic mutations in certain gene sets cause dependence on mitochondrial pathways. Ovarian tumors with mutations in genes belonging to SWI/SNF complexes such as ARID1A are more sensitive to inhibition to OXPHOS when compared with wild type (7).
In this review article, we discussed in-detail, various mitochondrial genetic factors that influence OC risk in certain population, cause chemoresistance and also highlight the molecular basis of racial disparity in OC with respect to mitochondrial genome. Furthermore, we also emphasized emerging role of mitochondrial proteins and phosphoproteins in OC, which can be effective diagnostic and prognostic markers and can be druggable therapeutic targets in OC.
mtDNA content in OC
Changes in mtDNA copy number or mtDNA content, calculated as amount of mtDNA relative to nDNA in the cell, have been found to be an important genetic event related to the progression of OC. Wang et al. have found that mtDNA content in OC cells was significantly elevated compared with normal ovary. But average mtDNA copy number in high-grade ovarian tumors is found to be significantly decreased compared with low-grade ovarian tumors (8). Therefore, cellular mtDNA content is emerging as a potential tool to predict prognosis in OC.
To explore the mtDNA-based noninvasive diagnostic biomarker, circulating cell free mtDNA (ccf mtDNA) was evaluated in plasma and was found to be elevated in women with EOC compared with women with benign ovarian diseases and normal healthy control women indicating its diagnostic potential (9). Moreover, ccf mtDNA levels were significantly decreased in plasma of women with EOC after chemotherapy, suggesting its predictive relevance (10). In a study group of women with EOC and age-matched healthy control women, analysis of serum levels of mtDNA79 and mtDNA230 identified to be significantly increased, whereas the levels of the ccf mtDNA integrity (mtDNA230/mtDNA79) were drastically decreased in EOC women compared with healthy control women, suggesting that mtDNA79, mtDNA230 and the mtDNA integrity may help to diagnose women with EOC (11). Moreover, the sensitivity and a specificity to diagnose EOC using ccf mtDNA79 levels were as high as 90.3% and 81.7%, respectively. Furthermore, high levels of ccf mtDNA79 and ccf mtDNA230 as well as a low mtDNA integrity also show significant correlation with grading (lower (G1–2) and higher (G3)) of EOC. Levels of mtDNA79 and mtDNA230 were significantly associated with tumor progression. Highest levels of mtDNA79 and mtDNA230 detected in FIGO III and IV stages suggest a correlation with an increased metastatic potential in advanced EOC. In support of this, ccf mtDNA79 levels in lymph node-positive EOC was found to be significantly higher than that in EOC women who were detected lymph node negative. Furthermore, mtDNA integrity levels show significantly decreasing trend from healthy controls to lymph node-negative EOC and lymph node-positive EOC. In addition, EOC patients with a tumor residue have significantly higher levels of ccf mtDNA79 and mtDNA 230 than in EOC patients without a tumor residue (11). Serum levels of mtDNA79 and mtDNA230 are found to be positively correlated with the values of CA125, current marker of diagnosis, follow response of treatment and predict diagnosis in OC. High levels of mtDNA79 and mtDNA230 were statistically positively correlated with poor overall survival (OS) of the patient, and ccf mtDNA79 has been identified as an independent predictor of OS in EOC patients, showing more prominent role of ccf mtDNA79 diagnostics and prognostics of EOC.
Quantification of mtDNA copy number in whole blood and in plasma (ccf and exosome encapsulated mtDNA) in patients with SER EOC cases revealed that whole-blood mtDNA copy number significantly varies between healthy controls and EOC patients (12). Whole mtDNA levels are found to be significantly decreased among all stages, early stage, advanced stage, as well as with respect to FIGO stages when compared with healthy controls (12). ccf mtDNA copy number was not increased significantly in patients, but mtDNA copy number in exosomal mtDNA was significantly higher in cancer patients in advanced-stage OC patients, in FIGO III and FIGO IV stage patients alone, but not in patients of FIGO I stage (12). There was significant increase in exosomal mtDNA in early- and late-stage OC cancers. In late-stage cancer women, mtDNA content was found to be highest in exosomes, followed by plasma and peripheral blood. The elevated exosomal mtDNA levels in late-stage cancers are obvious as tumor-derived mtDNA molecules promote metastasis (13). This suggests that mtDNA copy number levels in OC can detect OC progression, and whole-blood mtDNA copy numbers can be informative and can diagnose even early-stage SER OC cases.
In a single-center retrospective analysis, ascites mtDNA from patients with newly diagnosed EOC was found to be a substantial variable and correlated with worse progression-free survival in advanced EOC (14).
Various explanations were given for altered mtDNA content in OC. One explanation is that a change in mtDNA content is due to OXPHOS activity defects in OC. Reactive oxygen species generation in mitochondria may lead to mtDNA damage, which may have altered mtDNA content in the cell. It is plausible that significantly different levels of mtDNA content in different grades of tumor may be a result of downregulation of mtDNA replication in the high-grade tumors or upregulation of mtDNA replication in low-grade tumors (8). mtDNA copy number in early and low-grade tumor may be increased via compensatory mechanism for the respiratory system defect due to mutations in mtDNA (15). It may be plausible that mtDNA content decreases due to hypoxia as mitochondrion is highly sensitive to stress including oxygen deficiency (8). So, severe hypoxia microenvironment in high-grade tumors, which have high proliferation rate, leads to downregulation of the mtDNA replication and causes decreased mtDNA content. Explanation for increased ascites mtDNA in advanced OC cancers is due to the fact that mtDNA in ascites acts as damage-associated molecular patterns in and are potential drivers of proinflammatory pathways that may activate neutrophil and platelet responses that facilitate metastasis and obstruct anti-tumor immunity. These pathways are potential prognostic markers and therapeutic targets (14).
mtDNA gene polymorphisms, gene expression and OC risk
D-loop control region
Single-nucleotide polymorphisms (SNPs) in the mtDNA D-loop were identified to be one of the risk factors for OC. Liu et al. in 2016 studied 93 EOC cancer patients and identified that women with D-loop SNPs at various nucleotide positions, 73A/G, 207G/A and 523C/del, were found to be associated with increased susceptibility to EOC (Table 1). Significant association of few other SNPs such as 530C/T 524C/del 476C/A, 441C/A 418C/G 414T/G 411C/G, 366G/A, 275G/A, 259A/G, 254T/G were found with the tendency toward the increased risk for EOC, while women with D-loop alleles 263A/G and 249A/del pose reduced risk of EOC (16). In another study, three SNPs in D-loop region, 309 C/T, 324 C/G and 446 C/A, were identified for prediction of post-operational survival and the two alleles at nucleotide positions 309 and 324 were found to be independent predictors of EOC outcome (17). Kong et al. studied a population-based series consisting of 89 patients with primary EOC identified that age-at-onset in EOC women can be predicted by certain SNPs in the D-loop regions (18). The SNP sites of nucleotide 248, 524 and 16 304 alleles in the D-loop region were found to be associated with age-at-onset. Patients with the minor allele, 248 G and 16 304 T genotype, were found to have lower age of onset than that of patients with 248 A and 16 304 C, respectively, however for minor allele, 524C, age-at-onset of patients was higher than that of patients with higher frequency of 524 deletions (18) (Table 1). Therefore, the study highlights the importance of analysis of mitochondrial genetic polymorphisms in D-loop region in segregating EOC patient subgroups according to high risk of early onset.
Table 1.
Important variants/gene expression alterations in mtDNA genes, nuclear genes regulating mtDNA and genes sharing mitochondrial localization involved in influencing ovarian cancer risk and racial disparity
| Gene characteristics | Genetic region | Gene variants/expression |
|---|---|---|
| mtDNA-encoded genes | D-loop | SNP sites at np 73A/G, 207G/A and 23C/del, 254T/G, 259A/G, 275G/A, 366G/A, 11C/G, 414T/G, 418C/G, 441C/A, 476C/A, 524C/del, 530C/T, 249A/del and 263A/G, 309 C/T, 324 C/G and 446 C/A, D310, 1648delT, 1653A/delT, 1659delT, snp sites at 248, 524 and 16 304 |
| ND2 | G5460A, G5471A | |
| ND4 | SNP rs2857285, 10875TC (p.39LeuPro) | |
| NC5 | CCins at 5899 | |
| TA | C5603T, A5612C, T5655C | |
| TD | C ins at 7505, G7520A, A7523G, C7520T | |
| TG | T del 10045–10046 | |
| 12S rRNA | A772T, 773delT, and 780delC | |
| CO1 | C7256T, G7520A, C7028T, T6777C, G5949A, G5950A, C5953A, A6022G, T ins at 6044 C6054T, C7029G. C7174T. C7275T |
|
| CO III |
C9500T, T9540C, C9391A, A9436T, G9438A C9441A. C9485G, C9488G, T9496C, T9601C, C9520G, A9855G, C9857T |
|
| ATP8 | A8399G, A8411G, T8548G, T8588C | |
| ATP6 | A8860G, T8548G | |
| Nuclear DNA-encoded genes involved in mitochondrial biogenesis/OXPHOS complexes/mitochondrial transport and proteins with mitochondrial localization | PGC1α, TFAM, NRF1, TFB1M | Numerous SNPs |
| ESR2 | rs1256062, rs1256061, and rs12435857 | |
| TUFM | SNP rs9972768 | |
| Nuclear genes encoding OXPHOS complexes | Numerous genes upregulated | |
| SLC25A45 | SNP rs681309 | |
| MGST1 | SNP rs6488840 | |
| AR | Short CAG and GGC repeat length |
Alterations in bold are involved in racial disparity.
Sequencing of D-loop region in 52 different tumor samples of 35 OC cases (17 with bilateral OC), as well as matched normal tissues revealed 86 polymorphisms and 9 different homoplasmic somatic mtDNA mutations in 26% of OC cases. The presence of different mtDNA mutations between paired tumors in 24% of cases with bilateral ovarian tumors suggests that these bilateral tumors have originated from different clonal populations of cancer cells. On the other hand, metastatic tumors revealed identical mtDNA variants found in at least one of the ovarian tumors, suggesting that metastatic tumors arise from same clonal population arising from that ovarian tumor (19).
Gene encoding regions of mtDNA
Analysis of somatic mutations in mtDNA D-loop region in 15 primary ovarian carcinomas and their matched normal controls identified 20% of tumor samples, while complete sequence analysis of the mtDNA genomes of another 10 pairs of primary OC tissues and control tissues revealed somatic mtDNA mutations in 60% (6 of 10) of tumor samples (20). Apart from D-loop region, 12S, 16S rRNA genes and the cytochrome b gene were found to be preferred zone for mtDNA mutation in OC. Most mutations were homoplasmic and transition variants (20).
In a population-based case–control study of participants of European descent (405 invasive SER OC cases) and almost equal age-matched controls from a case–control study of OC in western Canada, a panel of 64 mitochondrial SNPs representing all common variations in mtDNA were genotyped. Mitochondrial SNPs in ND4 gene, rs2857285 (OR = 4.84, 95% CI: 1.03–22.68, P = 0.045), was shown to have significant association with OC (21) (Table 1). However, after adjustment of the Type 1 error, this mitochondrial SNP was not found to be significant. The SNP, rs2857285 was found to be detected in only 2.3% of cases and 0.5% of controls. Other SNPs do not show any significant associations and therefore European haplogroup status (H, U, J, V, K, W, X, I) was not associated with OC in this study (21). It may be possible that European mitochondrial SNPs were underpowered to adequately assess the association and therefore large-scale studies may give more clarification on the role of SNPs in OC risk. Larman et al. identified numerous mtDNA variants with high levels of hetroplasmy in tumor and nontumor tissue pairs obtained from 226 individuals with five different types of cancer including OC (n = 28) (22). Among 28 OC tissues, 236 inherited variants (74 non-synonymous missense and 162 synonymous variants) and 14 somatic variants (12 non-synonymous and 1 synonymous) were identified in protein-coding regions, which include NADH dehydrogenase subunits (complex I), cytochrome b subunits (complex III), cytochrome c oxidase subunits (complex IV) and ATP synthase subunits (complex IV) (22).
mtDNA mutations in OXPHOS complex I genes cause impairment of mitochondrial respiration and are markers of oncocytic transformation (23). Sequencing analysis of whole mitochondrial genome in a 69-year-old SER OC patient revealed missense mutation, m.10875TC in the conserved residue (p.39LeuPro) in the ND4 gene encoding complex 1 subunit (24). The mutation was nearly homoplasmic and found to be probably damaging one by PolyPhen tool and negative immunohistochemistry (IHC) staining obtained of nuclear-encoded CI subunit NDUFB8. The homoplasmic ND4 mutation was present in the post-chemotherapy tissues, but not in the pre-chemotherapy tissues, suggesting that the mutation might have occurred post-treatment (24).
In an another study consisting of EOC samples and matched normal tissues that paired with some of the tumors, sequencing of mtDNA variants revealed 352 mtDNA variants (25). Among these 352 variants, 38.3% were insertions and deletions, suggesting mtDNA instability in EOC. Mutations, A263G, A1438G, A8860G, were found with high frequency (25). Also, variants such as 1648delT, T1653A/delT and 1659delT were found to occur with high frequency among the three EOC subtypes and stages. Mutational hotspot D310, causing mtDNA instability and found in a wide variety of human neoplasms, was present with 97% frequency in the study samples, including the 10 paired tumor samples, suggesting its germline origin. TC insertion at np 310 in D-loop was found in the early stages of SER subtype at a high frequency of 81%, suggesting as a major factor in this particular predominant subtype of EOC. Three mtDNA mutations at np A772T, 773delT and 780delC were identified in the 12S rRNA gene in endometrioid stage III tumors. Furthermore, two mutations, 1657delC in stage IV and 8221delA, were identified in SER subtype in benign cystadenomas and borderline tumors, respectively (25).
Alterations of mtDNA in OC patients with and without chemotherapy revealed a total of 69 novel polymorphisms and 17 mutations. The mean polymorphisms were significantly higher in patients with chemotherapy from those without chemotherapy. The proportion of amino acid changes induced by polymorphisms and the mutation rate differ between two groups (26).
In an another study, 39 mtDNA variants were detected including one somatic variant of C9500T (27). A variant in the CO1 gene, C7028T, was found with high frequency of 75% stages III/IV (75%) but at a frequency of 8% in borderline tumors. Variants, C7256T and G7520A, were found to occur at a frequency of 54% in END OC subtype stage III but were absent in SER OC subtype (27).
Nuclear DNA encoded mitochondrial biogenesis genes
Mitochondrial biogenesis genes include peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PPARGC1A (PGC1α)), transcription factor A, mitochondrial (TFAM) and nuclear respiratory factor1/2 (NRF1/2) play a role to make new mitochondria. PGC1α regulates mitochondrial biogenesis through regulation of nuclear transcription factors, NRF1-2 (28), which in turn regulates TFAM (25). TFAM plays an important role in maintaining structure of mtDNA, mtDNA copy number, as similar to histones in nDNA and efficient transcription of mtDNA-encoded genes. Mitochondrial biogenesis has also been regulated by estrogen (E2) and estrogen receptor alpha (ERα) that impacts mitochondrial function (29). ERα has been found to be expressed in all stages and subtypes of EOC at different levels.
Variants in mitochondrial biogenesis genes found to play role in influencing susceptibility to EOC. In a multi-center, large-scale study of CA EOC cases and controls, 128 SNPs from 22 mitochondrial genetic regions along with 2839 SNPs localized to 138 nuclear-encoded genes involved in mitochondrial biogenesis, steroid hormone metabolism and OXPHOS pathways were investigated to study its associations with EOC risk (30). Analysis of mitochondrial genes revealed significant association of CO1 with EOC risk. A synonymous SNP in CO1, T6777C appeared to decrease risk of EOC. Of the three nDNA pathways studied, 25 genes and 1051 SNPs involved in mitochondrial biogenesis pathway show association with EOC risk. NRF1 showed strongest association followed by MTERF (mitochondrial transcription termination factor), PPARGC1A, ESRRA (estrogen-related receptor alpha) and CAMK2D (calcium/calmodulin-dependent protein kinase D) (30). NRF1 is the nuclear transcription factor and participates in transcription of antioxidant and detoxification genes in response to increased oxidative stress and is called a master regulator of stress responses (31). PPARGC1A, a coactivator for ESRRA, modulates the function of most mitochondrial proteins and induces mitochondrial biogenesis (31). PPARGC1A-ESRRA pathway is known to regulate expression of vascular endothelial growth factor (VEGF) and angiogenesis independent of hypoxia-inducible factor (32). Furthermore, overexpression of PPARGC1A as well as ESRRA has been associated with poor chemoresponse and shorter survival in EOC, indicating therapeutic potential of these genes (33,34). Overexpression of CAMK2D is associated with cisplatin resistance in OC (35). Among SNPs of steroid hormone metabolism gene pathway, four intronic Estrogen Receptor 2 (ESR2) SNPs show individual association and a haplotype block including rs1256062, rs1256061 and rs12435857 in ESR2 was associated with a slightly increased risk of EOC. Further analysis by imputation method revealed undetected signals for TFB1M (transcription factor B1, mitochondrial) with strongest association identified for SNP, rs721101, MAPK1 (mitogen-activated protein kinase 1) and 17 new signals for NR3C1/GR (Nuclear Receptor Subfamily 3 Group C Member 1/glucocorticoid receptor) (30). Other studies also indicate that ESR2 plays an important role in mitochondrial dysfunction and oxidative stress and identifies as susceptibility marker for ovarian carcinogenesis (29,36). Existing data suggest crosstalk between ESR2 and ESRRA (37), suggesting mitochondria alter mitochondrial biogenesis that appears to be mediated by steroids. Among genes of the OXPHOS pathway, seven genes demonstrated were found to have statistically significant association and TUFM (Tu translation elongation factor) shows the strongest association. At the SNP level, each copy of TUFM SNP rs9972768 minor allele showed 11% increased risk for OC (30).
Role of mitochondrial regulation in EOC was examined in a study set of 53 EOC cases in Sweden, and the study revealed that expression of PGC1α and TFAM is different in EOC subtypes and CC subtype shows profile of absent PGC1α/TFAM and low ERα/Ki-67 expression. In contrast, high-grade SER carcinomas subtypes revealed converse state of PGC1α/TFAM expression, ERα positivity and increased Ki-67 expression (37). Furthermore, platinum-resistant non-clear cell EOC cell line was found to have loss of PGC1α/TFAM and ERα expression and showed glycogen accumulation (38).
TFAM is expression has been also observed in nuclei. Study of nuclear TFAM expression in 60 tissue samples of SER OC found that 56.7% of SER OC patients were positive for TFAM and the rest negative (39). Furthermore, TFAM-positive cancer shows significantly poor 5-year survival rate compared with TFAM negative cancer (39). Significant correlation was also observed between nuclear TFAM expression and BCL2L1, both in vitro and in specimens of OC patients. Silencing of TFAM leads to downregulate cellular BCL2L1 and decreased BCL2 promoter activity, which was increased after inducing TFAM expression. TFAM was found to bind with BC2L1 promoter region by Chromatin immunoprecipitation assays (39). Therefore, TFAM may act as an anti-apoptotic factor regulating BCL21 and is a prognostic factor and may be promising molecular therapeutic target for OC treatment.
Genome-wide profiling across the 60-cell lines panel of different cancers indicates mRNA expression of TOP1MT, which encodes the mDNA topoisomerase IB varies widely across these cell lines with the highest levels in various cancers including OC (40). Furthermore, TOP1MT expression is significantly correlated with the other nuclear-encoded mitochondrial genes and a high correlation was observed between TOP1MT and the proto-oncogene MYC (c-myc), an oncogene expression and MYC was found to be a novel regulator of TOP1MT (40).
Expression of energy metabolism genes
Changes in energy metabolism frequently occur within cancer cells and may be potential biomarkers and potent molecules for therapeutic targets against cancer. Several studies demonstrated that the OC progression is associated with diverse pathways involved in energy metabolism (41,42) and that human omental adipocytes provide energy and promote homing, migration and invasion of OC cells mediated by adipokines (43). Study by Wang et al. analyze and characterize the clinical relevance of energy metabolism in OC and identified molecular subtypes and gene signatures of energy metabolism as prognostic markers (44). A total of 39 energy metabolism-related genes significantly associated with OC prognosis were identified, along with distinct three molecular subtypes (C1, C2 and C3). One of the molecular subtype (C1) was associated with poor clinical outcomes of OC with enrichment of genes belonging to the ‘PI3K-Akt signaling pathway’, ‘cAMP signaling pathway’, ‘ECM-receptor interaction’ and other pathways associated with the tumor development and progression (44). Based on final analysis, eight gene signature (tolloid-like 1 gene, type XVI collagen, prostaglandin F2α, cartilage intermediate layer protein 2, kinesin family member 26b, interferon inducible protein 27, growth arrest-specific gene 1 and chemokine receptor 7) independent of other clinical factors were obtained which were associated with OC progression as well as determined to be an independent prognostic factor for patients with OC (44). Association between altered cellular metabolism with cell proliferation and therapeutic relevance had been also reported by other groups (45,46). Interestingly, the three subtypes exhibit mostly different energy metabolism pathways and metabolic patterns. Hence, eight gene signature of energy metabolism-related gene expression levels could stratify high- and low-risk OC patients and may be useful to devise targeted therapies for OC patients based on energy metabolism genes.
Bioenergetic profiling is also shown to distinguish CC subtype from other EOC histological subtypes. CC cells are highly metabolically active, showed ability to form anchorage-independent spheroids suggesting a high bioenergetics, and are characterized by increased expression of oxidative stress and glycolysis-related genes indicative of enhanced mitochondrial OXPHOS and glycolytic rate, respectively (47). In contrast, bioenergetic profiling of non-CC cells (OVCA420), are characterized by mitochondrial dysfunction, accompanied by altered mitochondrial morphology and altered expression of mitochondrial dynamics proteins, Drp1, loss of mitochondrial membrane potential and glycolytic pathway dependence (47,48). Other studies also shown that CC is characterized by high expression of antioxidant enzymes and genes related to glucose metabolism (49,50). Furthermore, CC subtype is also associated with poor survival and resistance to therapy (51).
mtDNA alterations and mechanism associated with OC chemoresistance
Chemotherapeutic drugs generally eliminate tumor cells by inducing apoptosis. Mitochondria, being major regulators of apoptosis, mtDNA mutations may likely to alter responses to cancer therapy. mtDNA depletion activates pro-survival and anti-apoptotic pathways that may lead cancer cells progression to a chemoresistant phenotype (52). Furthermore, mtDNA variants cause reduced mtDNA content that promotes retrograde signaling resulting in increased expression of anti-apoptotic genes such as Bcl2 as well as activation of pro-survival enzymes, such as Akt conferring drug resistance. mtDNA variants leads to mitochondrial dysfunction resulting in ‘metabolic reprogramming’. Evidence of metabolic reprogramming was demonstrated in C13 cells, cisplatin-resistant derivative of 2008 OC cells as demonstrated decreased apoptosis and mitochondrial membrane potential and lower basal oxygen consumption following cisplatin treatment in comparison to 2008 cells which were cisplatin sensitive (52). Depletion of mtDNA in 2008 cells resulted in mitochondrial dysfunction leading to chemoresistance. Metabolic shift was found to be evident in cisplatin resistance when cisplatin-sensitive (SKOV-3 and COV-362) and cisplatin-resistant (SKOV-3-R and COV-362-R) human OC cells following exposure to increasing doses of the chemotherapy were compared. OC resistant cells showed a tremendous shift toward a more oxidative metabolism accompanying with mitochondrial network reorganization and increased mitochondrial compartment and increased functional mitochondria, associated with defect in the electron transport chain/mitochondrial coupling compared to cisplatin-sensitive cells. Mutated mitochondrial genes affecting OXPHOS complex I assembly act in dual manner. Below a threshold level, the cells show pro-tumorigenic role but when reach a critical mutant load, resulting in failure of Complex I assembly show an anti-tumorigenic role. This concept is known as ‘oncojanus’ (52). As mentioned above, in a case of a residual SER OC after carboplatin/ paclitaxel chemotherapy, a mitochondrial oncojanus phenotype was observed as a novel missense mtDNA point mutation, m.10875T>C in ND4 gene was found above the threshold for complex I assembly disruption was only present in the post-chemotherapy tissues (24). Further mtDNA variants may also undergo hetero to homoplasmic shift and confer chemoresistance. Horizontal mitochondrial transfer from endothelial cells to breast and OC cells has also been also suggested to be associated with chemoresistance to treatment with doxorubicin (52).
Targeting glycolysis, glutamine metabolism, mitochondrial retrograde signaling, mitochondrial OXPHOS complexes and mutant mtDNA may provide a novel therapeutic approach toward cancer including OC. Glycolysis inhibitor, ‘lonidamine’ in combination with ‘doxorubicin’ showed better therapeutic efficacy for OC treatment as well as for tumors of breast, prostate, melanoma and brain. Mitochondrial enzyme, ‘Glutaminase’, of glutaminolysis pathway along with mTOR inhibitors sensitizes OC cells to paclitaxel (52). Drug screening study identified electron transport chain inhibitors such as ‘rotenone’, ‘oligomycin A’ and ‘metformin’ as well as mitochondrial uncouplers such as ‘valinomycin’ and ‘dinitrophenol’ to be antineoplastic and open a novel therapeutic window. OXPHOS inhibitor, ‘VLX600’ has been showed to reduce the quiescent cells viability in vivo and cause spheroid reduction, resulting in tumor proliferation inhibition (52). Many antibiotic/anti-parasitic agents as mitochondrial inhibitors such as ‘doxycycline’ and ‘salinomycin’ inhibits the proliferation of OC cells and selectively eliminate cisplatin-resistant OC cells. Pyruvate dehydrogenase activator, ‘CPI-613’ inhibit mitochondrial ATP production and lead to OC cell death (52). ‘Metformin’, complex I inhibitor showed reduction in incidence and improvement of survival in OC. ‘Metformin’ alone inhibits tumor cell proliferation, metastasis and angiogenesis in vivo and its effect was enhanced in combination with cisplatin/paclitaxel (52).
Mitochondrial proteomes
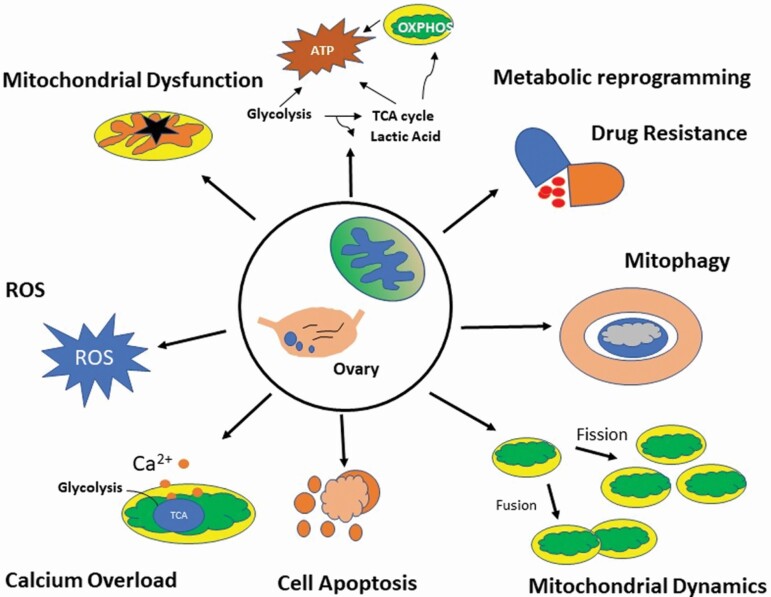
High-throughput mitochondrial proteomic studies identified many potential biomarkers for the development of OC such as ERBB2, PTBP1 and H2AFX and proteins involved in signaling pathways, most importantly mitophagy, energy metabolism and mitochondrial dysfunction pathways and numerous mitochondrial proteins (mitochondrial EFTU and mitochondrial GRP75, APOA1), ATP-α, PRDX3, PHB, ETF, and ALDH) as potential biomarkers of drug-resistant OC (53–57) (Figure 1).
Figure 1.
Schematic diagram depicting role of key mitochondrial proteins involved in development, progression and drug resistant in OC.
Mitochondrial chaperones, heat shock protein 60 (HSP60) and mitochondrial membrane proteins such as SLC25A5, SLC25A, TOMM TIMM, TIMM10 and FAM210B, VDAC families, mitochondrial ribosomal proteins such as MRPL41 and MRPL49, mitochondrial-related antioxidant proteins (NQO1 and SOD2), as well as mitochondrial creatine kinase were identified to be prognostic and therapeutic implications in OC (58–61). Three new mitochondrial gene signatures (MRPL49/UQCRFS1, NDUFA3/UQCRFS1 and NDUFA3/UQCRFS1/PCNA) were identified as prognostic factors (59). Inhibiting mitochondrial pyruvate carrier 1 (MPC1) gene revealed metabolism reprogramming to aerobic glycolysis with reduced ATP production, with increased cell migration and resistance to both chemotherapy and radiotherapy (62). Mitochondrial calcium uptake 1 (MICU1/CBARA1), gatekeeper of mitochondrial Ca2+ uptake that drives aerobic glycolysis, could serve as an important therapeutic target to normalize metabolic aberrations responsible for poor prognosis in OC (63). Furthermore, mitochondrial dynamics proteins such as Mfn1, MFN2, Oma1 are now evolving as a key metabolic player that fuels tumor growth and therapy resistance in OC (64–66) (Figure 1). Mitochondrial sirtuins, SIRT3 and SIRT5, were significantly decreased and increased in SER OC/tubal cancers (PSOCs/PSTCs) compared with that in normal counterparts, respectively, and SIRT3 was found to be an independent favorable prognostic factor for OC (67) Recently, Signorele et al. highlighted a ‘mitochondrial signature’ in OC characterized by cAMP pathway activation, SIRT3 stabilization and increased OPA1, and PHB2 proteins (4). Other mitochondrial proteins such as mitochondrial peroxiredoxins (Prdx3 and Prdx5), which are reactive oxygen species scavengers, mitochondrial ERβ2, survivin, ubiquinol-cytochrome C reductase hinge (UQCRH), mitochondrial tumor suppressor gene (MTUS1), UQCRFS1 and Coenzyme Q0 (CoQ0), are associated with the initiation and progression of various cancer types and can be useful in the development of anticancer drugs with better proficiency and decreased resistance (68–76). Differentially expressed mitochondrial phosphorylated proteins between OC tissue mitochondria relative to controls include EIF2S2, RPLP2, CFL1, VDAC3, MYH10, RPLP0, HSP90, HSPD1, TMX1, VDAC2, TOMM22, PSMA3 and TOMM20 among which phosphorylation sites such as phosphorylated cofilin 1 (p-CFL1) and expressed more in chemoresistant cases (77).
Role of mtDNA in OC racial disparity
Mounting evidences suggest that racial disparity between African American (AA) and Caucasian Americans (CA) women affected with OC. AA women show increased OC mortality rate, increased burdened with late diagnosis of OC, higher recurrence and reduced disease-free survival. In contrast, Hispanic and Asian women are less burdened with advanced OC and diagnosed at a younger age and found to have END and CC subtypes than CA/AA women (78). Racial disparity is not only attributed to socio-economic factors but also biological factors including mitochondrial genetic factors. Alterations in mtDNA-encoded genes (MT-COI and MT-COIII, tRNA and ATPase 6), nuclear genes encoding mtDNA (nuclear genes encoding mitochondrial OXPHOS) as well proteins localized to nuclear and mitochondrial compartments (such as androgen receptor) and mitochondrial transporters (SLC25A45 and MGST1) play a significant role in OC racial disparity (78). Variants in some of these genes such as mitochondrial transporters seem to be regulated by nutrient factors and are linked to different dietary/cooking pattern of the races. It is speculated that variants in the mtDNA genes including OXPHOS complexes in AA might have been a result of natural selection that foster to adapt to different environments but may also contribute to susceptibility toward cancer (78).
Future perspectives
Mitochondrial genetic alterations are critical hallmark of OC. These alterations particularly affect the electron transport chain as OXPHOS pathway was the main pathway found to be altered in OC and other cancers. Involvement of mtDNA alterations in the various stages of tumorigenesis has opened new avenues to study these alterations as diagnostic and prognostic markers in cancers. Mitochondria is abundant and is highly polymorphic in nature. Mutational analysis of mtDNA combined with high-throughput next-generation sequencing techniques may unravel mtDNA alterations including its hetroplasmic levels to be readily detectable in bodily fluids such as blood, urine and saliva and can be used as early diagnostic as well as noninvasive biomarker in cancer. Furthermore, due to smaller genome size, mtDNA sequencing is cost-effective and highly scalable in clinical settings. mtDNA homoplasmic mutations have been identified in early preneoplastic and cancerous lesions. Association between mtDNA copy number alterations and clinicopathological features strengths its role as prognostic biomarkers in cancers. mtDNA mutations have also been shown to be clonal markers to detect metastasis in various tumors as all metastatic lesions showed identical mtDNA changes. Overall, mtDNA variations have important clinical implications and should be studied to detect high-risk individuals, screening of premalignant lesions, tracking cancer progression and predicting prognosis in OC. Additional studies are warranted to characterize the molecular pathways altered due to mtDNA mutations and to explore the clinical relevance to address mitochondria therapies targeted to mutation induced metabolic dysregulation. Also, mitochondrial metabolites may be tested as noninvasive biomarkers for OC prognosis and therapeutic targets. Therapeutic potential for glycolysis inhibitors and the co-treatment with mitochondrial inhibitors in the therapeutic targeting of highly bioenergetics CC and other EOCs must be tailored. A large-scale high-throughput mitochondrial proteomic studies helps to identify potential protein biomarkers and a novel vision in bio-mechanism pertaining to human OC. Mito-signatures may also be useful for selecting new ‘druggable’ targets to prevent treatment failure and improve OS. A comprehensive analysis of mitochondrial phosphoproteome is needed to determine the role of mitochondrial phosphorylation in OC. Furthermore, mitochondria also interact with other organelles such as peroxisomes, endoplasmic reticulum and nucleus through vesicle transport, signal transduction and membrane contact sites. Understanding the inter-organelle communication may provide flexibility and plasticity for cancer treatments, such as radiation sensitivity, multidrug resistance and tumor immune escape mechanism. Studies on identifying mitochondrial determinants in different races may help to identify biomarkers and therapy-related benefit and risk associated with racial disparity in OC patients.
Acknowledgements
KKS also supported by an National Institutes of Health (NIH) grant R01CA204430. PS acknowledge the grant support from ‘Long Term ICMR-DHR International Fellowship for Young Indian Biomedical Scientists 2019–20’.
Glossary
Abbreviations
- CAMK2D
calcium/calmodulin-dependent protein kinase D
- CC
clear cell
- Coenzyme Q0
CoQ0
- END
endometrioid
- EOC
epithelial ovarian cancer
- EOC
Epithelial ovarian cancer
- ERα
estrogen receptor alpha
- ESRRA
estrogen-related receptor alpha
- GR
glucocorticoid receptor
- MAPK1
mitogen-activated protein kinase 1
- MGST1
Microsomal Glutathione S-Transferase 1
- mtDNA
mitochondrial DNA
- MTERF
mitochondrial transcription termination factor
- NR3C1
Nuclear Receptor Subfamily 3 Group C Member 1
- NRF1/2
nuclear respiratory factor1/2
- OC
ovarian cancer
- OS
overall survival
- OXPHOS
oxidative phosphorylation
- p-CFL1
phosphorylated cofilin 1
- PPARGC1A
peroxisome proliferator-activated receptor gamma, coactivator 1 alpha
- SER
serous
- SLC25A45
Solute Carrier Family 25 Member 45
- TFAM
transcription factor A, mitochondrial
- TFB1M
transcription factor B1, mitochondrial
- TUFM
Tu translation elongation factor
- VEGF
vascular endothelial growth factor
Author contribution
KKS conceptualized the idea and edited the manuscript. PS designed and wrote the manuscript.
Conflict of Interest Statement: None declared.
References
- 1. Sharma, P., et al. (2019). Mitochondrial DNA integrity: role in health and disease. Cells, 8, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choudhury, A.R., et al. (2017) Mitochondrial determinants of cancer health disparities. Semin. Cancer Biol., 47, 125–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Signorile, A., et al. (2019). Human ovarian cancer tissue exhibits increase of mitochondrial biogenesis and cristae remodeling. Cancers, 11, 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bell, D.A. (2005) Origins and molecular pathology of ovarian cancer. Mod. Pathol., 18 (Suppl 2), S19–S32. [DOI] [PubMed] [Google Scholar]
- 5. Rauh-Hain, J.A., et al. (2011) Ovarian cancer screening and early detection in the general population. Rev. Obstet. Gynecol., 4, 15–21. [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang, J.L., et al. (2020) Mitochondria in ovarian aging and reproductive longevity. Ageing Res. Rev., 63, 101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emmings, E., et al. (2019). Targeting mitochondria for treatment of chemoresistant ovarian cancer. Int. J. Mol. Sci., 20, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang, Y., et al. (2006) Association of decreased mitochondrial DNA content with ovarian cancer progression. Br. J. Cancer, 95, 1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zachariah, R.R., et al. (2008) Levels of circulating cell-free nuclear and mitochondrial DNA in benign and malignant ovarian tumors. Obstet. Gynecol., 112, 843–850. [DOI] [PubMed] [Google Scholar]
- 10. Kalavska, K., et al. (2018) Prognostic value of various subtypes of extracellular DNA in ovarian cancer patients. J. Ovarian Res., 11, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meng, X., et al. (2019) Circulating mitochondrial DNA is linked to progression and prognosis of epithelial ovarian cancer. Transl. Oncol., 12, 1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keserű, J.S., et al. (2019) Detection of cell-free, exosomal and whole blood mitochondrial DNA copy number in plasma or whole blood of patients with serous epithelial ovarian cancer. J. Biotechnol., 298, 76–81. [DOI] [PubMed] [Google Scholar]
- 13. Ruivo, C.F., et al. (2017) The biology of cancer exosomes: insights and new perspectives. Cancer Res., 77, 6480–6488. [DOI] [PubMed] [Google Scholar]
- 14. Singel, K.L., et al. (2019) Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br. J. Cancer, 120, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee, H.C., et al. (2000) Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J., 348 Pt 2, 425–432. [PMC free article] [PubMed] [Google Scholar]
- 16. Liu, S., et al. (2016) Identification of sequence nucleotide polymorphisms in the D-loop region of mitochondrial DNA as a risk factor for epithelial ovarian cancer. Mitochondrial DNA A DNA Mapp. Seq. Anal., 27, 9–11. [DOI] [PubMed] [Google Scholar]
- 17. Kong, D., et al. (2015) Single nucleotide polymorphisms in the D-loop region of mitochondrial DNA are associated with epithelial ovarian cancer prognosis. Mitochondrial DNA, 26, 848–850. [DOI] [PubMed] [Google Scholar]
- 18. Kong, D., et al. (2016) Single nucleotide polymorphisms in the mitochondrial displacement loop and age-at-onset of epithelial ovarian cancer. Mitochondrial DNA A DNA Mapp. Seq. Anal., 27, 1141–1143. [DOI] [PubMed] [Google Scholar]
- 19. Van Trappen, P.O., et al. (2007) Somatic mitochondrial DNA mutations in primary and metastatic ovarian cancer. Gynecol. Oncol., 104, 129–133. [DOI] [PubMed] [Google Scholar]
- 20. Liu, V.W., et al. (2001) High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Res., 61, 5998–6001. [PubMed] [Google Scholar]
- 21. Earp, M.A., et al. (2013) Inherited common variants in mitochondrial DNA and invasive serous epithelial ovarian cancer risk. BMC Res. Notes, 6, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larman, T.C., et al. ; Cancer Genome Atlas Research Network (2012) Spectrum of somatic mitochondrial mutations in five cancers. Proc. Natl Acad. Sci. USA, 109, 14087–14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasparre, G., et al. (2007) Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl Acad. Sci. USA, 104, 9001–9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerra, F., et al. (2012) Mitochondrial DNA mutation in serous ovarian cancer: implications for mitochondria-coded genes in chemoresistance. J. Clin. Oncol., 30, e373–e378. [DOI] [PubMed] [Google Scholar]
- 25. Aikhionbare, F.O., et al. (2007) Mitochondrial DNA sequence variants in epithelial ovarian tumor subtypes and stages. J. Carcinog., 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aikhionbare, F.O., et al. (2008) mtDNA sequence variants in subtypes of epithelial ovarian cancer stages in relation to ethnic and age difference. Diagn. Pathol., 3, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi HH., et al. (2005) Alterations of mitochondrial DNA in ovarian cancer patients with and without chemotherapy. Zhonghua Fu Chan Ke Za Zhi, 40, 469–471. [PubMed] [Google Scholar]
- 28. Jornayvaz, F.R., et al. (2010) Regulation of mitochondrial biogenesis. Essays Biochem., 47, 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klinge, C.M. (2008) Estrogenic control of mitochondrial function and biogenesis. J. Cell. Biochem., 105, 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Permuth-Wey, J., et al. (2011) Inherited variants in mitochondrial biogenesis genes may influence epithelial ovarian cancer risk. Cancer Epidemiol. Biomarkers Prev., 20, 1131–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raghunath, A., et al. (2018) Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol., 17, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arany, Z., et al. (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature, 451, 1008–1012. [DOI] [PubMed] [Google Scholar]
- 33. Sun, P., et al. (2005) Expression of estrogen receptor-related receptors, a subfamily of orphan nuclear receptors, as new tumor biomarkers in ovarian cancer cells. J. Mol. Med. (Berl), 83, 457–467. [DOI] [PubMed] [Google Scholar]
- 34. Davidson, B., et al. (2009) Expression of the peroxisome proliferator-activated receptors-alpha, -beta, and -gamma in ovarian carcinoma effusions is associated with poor chemoresponse and shorter survival. Hum. Pathol., 40, 705–713. [DOI] [PubMed] [Google Scholar]
- 35. Xu, X., et al. (2018) Overexpression of SMARCA2 or CAMK2D is associated with cisplatin resistance in human epithelial ovarian cancer. Oncol. Lett., 16, 3796–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lurie, G., et al. (2009) Genetic polymorphisms in the estrogen receptor beta (ESR2) gene and the risk of epithelial ovarian carcinoma. Cancer Causes Control, 20, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaillard, S., et al. (2007) Definition of the molecular basis for estrogen receptor-related receptor-alpha-cofactor interactions. Mol. Endocrinol., 21, 62–76. [DOI] [PubMed] [Google Scholar]
- 38. Gabrielson, M., et al. (2014) Expression of mitochondrial regulators PGC1α and TFAM as putative markers of subtype and chemoresistance in epithelial ovarian carcinoma. PLoS One, 9, e107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurita, T., et al. (2012) Mitochondrial transcription factor A regulates BCL2L1 gene expression and is a prognostic factor in serous ovarian cancer. Cancer Sci., 103, 239–444. [DOI] [PubMed] [Google Scholar]
- 40. Zoppoli, G., et al. (2011) Coordinated regulation of mitochondrial topoisomerase IB with mitochondrial nuclear encoded genes and MYC. Nucleic Acids Res., 39, 6620–6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cramer, D.W., et al. (1989) Galactose consumption and metabolism in relation to the risk of ovarian cancer. Lancet, 2, 66–71. [DOI] [PubMed] [Google Scholar]
- 42. Liu, G., et al. (2000) Galactose metabolism and ovarian toxicity. Reprod. Toxicol., 14, 377–384. [DOI] [PubMed] [Google Scholar]
- 43. Nieman, K.M., et al. (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med., 17, 1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang, L., et al. (2020) Identification of an energy metabolism-related gene signature in ovarian cancer prognosis. Oncol. Rep., 43, 1755–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lunt, S.Y., et al. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol., 27, 441–464. [DOI] [PubMed] [Google Scholar]
- 46. Fumarola, C., et al. (2018) Impairing energy metabolism in solid tumors through agents targeting oncogenic signaling pathways. Biochem. Pharmacol., 151, 114–125. [DOI] [PubMed] [Google Scholar]
- 47. Dier, U., et al. (2014) Bioenergetic analysis of ovarian cancer cell lines: profiling of histological subtypes and identification of a mitochondria-defective cell line. PLoS One, 9, e98479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamaguchi, K., et al. (2010) Identification of an ovarian clear cell carcinoma gene signature that reflects inherent disease biology and the carcinogenic processes. Oncogene, 29, 1741–1752. [DOI] [PubMed] [Google Scholar]
- 49. Mandai, M., et al. (2011) Ovarian clear cell carcinoma as a stress-responsive cancer: influence of the microenvironment on the carcinogenesis and cancer phenotype. Cancer Lett., 310, 129–133. [DOI] [PubMed] [Google Scholar]
- 50. Konstantinopoulos, P.A., et al. (2011) Keap1 mutations and Nrf2 pathway activation in epithelial ovarian cancer. Cancer Res., 71, 5081–5089. [DOI] [PubMed] [Google Scholar]
- 51. Tan, D.S., et al. (2013) New perspectives on molecular targeted therapy in ovarian clear cell carcinoma. Br. J. Cancer, 108, 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guerra, F., et al. (2017) Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta. Bioenerg., 1858, 686–699. [DOI] [PubMed] [Google Scholar]
- 53. Li, N., et al. (2018) Quantitative analysis of the mitochondrial proteome in human ovarian carcinomas. Endocr. Relat. Cancer, 25, 909–931. [DOI] [PubMed] [Google Scholar]
- 54. Cruz, I.N., et al. (2017) Proteomics analysis of ovarian cancer cell lines and tissues reveals drug resistance-associated proteins. Cancer Genomics Proteomics, 14, 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li, N., et al. (2019) Signaling pathway network alterations in human ovarian cancers identified with quantitative mitochondrial proteomics. EPMA J., 10, 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen, M., et al. (2015) Quantitative proteomic analysis of mitochondria from human ovarian cancer cells and their paclitaxel-resistant sublines. Cancer Sci., 106, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dai, Z., et al. (2010) Mitochondrial comparative proteomics of human ovarian cancer cells and their platinum-resistant sublines. Proteomics, 10, 3789–3799. [DOI] [PubMed] [Google Scholar]
- 58. Guo, J., et al. (2019) HSP60-regulated mitochondrial proteostasis and protein translation promote tumor growth of ovarian cancer. Sci. Rep., 9, 12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sotgia, F., et al. (2017) Mitochondrial mRNA transcripts predict overall survival, tumor recurrence and progression in serous ovarian cancer: companion diagnostics for cancer therapy. Oncotarget, 8, 66925–66939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muller, P., et al. (2019) Tomm34 is commonly expressed in epithelial ovarian cancer and associates with tumour type and high FIGO stage. J. Ovarian Res., 12, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sun, S., et al. (2017) Loss of the novel mitochondrial protein FAM210B promotes metastasis via PDK4-dependent metabolic reprogramming. Cell Death Dis., 8, e2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li, X., et al. (2017) Mitochondrial pyruvate carrier function determines cell stemness and metabolic reprogramming in cancer cells. Oncotarget, 8, 46363–46380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chakraborty, P.K., et al. (2017) MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun., 8, 14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chakraborty, P.K., et al. (2018) Cystathionine β-synthase regulates mitochondrial morphogenesis in ovarian cancer. FASEB J., 32, 4145–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tanwar, D.K., et al. (2016) Crosstalk between the mitochondrial fission protein, Drp1, and the cell cycle is identified across various cancer types and can impact survival of epithelial ovarian cancer patients. Oncotarget, 7, 60021–60037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kong, B., et al. (2014) p53 is required for cisplatin-induced processing of the mitochondrial fusion protein L-Opa1 that is mediated by the mitochondrial metallopeptidase Oma1 in gynecologic cancers. J. Biol. Chem., 289, 27134–27145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li, J., et al. (2019) Development and validation of SIRT3-related nomogram predictive of overall survival in patients with serous ovarian cancer. J. Ovarian Res., 12, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ismail, T., et al. (2019). Interplay between mitochondrial peroxiredoxins and ROS in cancer development and progression. Int. J. Mol. Sci., 20, 4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang, J., et al. (2016) Evaluation the expression of three genes to epithelial ovarian cancer risk in Chinese population. Afr. J. Tradit. Complement. Altern. Med., 13, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Modena, P., et al. (2003) UQCRH gene encoding mitochondrial Hinge protein is interrupted by a translocation in a soft-tissue sarcoma and epigenetically inactivated in some cancer cell lines. Oncogene, 22, 4586–4593. [DOI] [PubMed] [Google Scholar]
- 71. Bozgeyik, I., et al. (2017) MTUS1, a gene encoding angiotensin-II type 2 (AT2) receptor-interacting proteins, in health and disease, with special emphasis on its role in carcinogenesis. Gene, 626, 54–63. [DOI] [PubMed] [Google Scholar]
- 72. Huang, Y., et al. (2014) Ovarian cancer stem cell-specific gene expression profiling and targeted drug prescreening. Oncol. Rep., 31, 1235–1248. [DOI] [PubMed] [Google Scholar]
- 73. Hseu, Y.C., et al. (2017) Antitumor properties of Coenzyme Q0 against human ovarian carcinoma cells via induction of ROS-mediated apoptosis and cytoprotective autophagy. Sci. Rep., 7, 8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaneko, S.J., et al. (2003) CA125 and UQCRFS1 FISH studies of ovarian carcinoma. Gynecol. Oncol., 90, 29–36. [DOI] [PubMed] [Google Scholar]
- 75. Ciucci, A., et al. (2015) Mitochondrial estrogen receptor β2 drives antiapoptotic pathways in advanced serous ovarian cancer. Hum. Pathol., 46, 1138–1146. [DOI] [PubMed] [Google Scholar]
- 76. Pils, D., et al. (2005) Five genes from chromosomal band 8p22 are significantly down-regulated in ovarian carcinoma: N33 and EFA6R have a potential impact on overall survival. Cancer, 104, 2417–2429. [DOI] [PubMed] [Google Scholar]
- 77. Li, N., et al. (2019) Quantitative analysis of the human ovarian carcinoma mitochondrial phosphoproteome. Aging (Albany. NY), 11, 6449–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shukla, P., et al. (2021) Uncovering mitochondrial determinants of racial disparities in ovarian cancer. Trends Cancer, 7, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]