Abstract
Bisphenol F (BPF) is increasingly substituting bisphenol A in manufacturing polycarbonates and consumer products. The cardiometabolic effects of BPF in either humans or model organisms are not clear, and no studies to date have investigated the role of genetic background on susceptibility to BPF-induced cardiometabolic traits. The primary goal of this project was to determine if BPF exposure influences growth and adiposity in male N:NIH heterogeneous stock (HS) rats, a genetically heterogeneous population. Littermate pairs of male HS rats were randomly exposed to either vehicle (0.1% ethanol) or 1.125 µg/ml BPF in 0.1% ethanol for 5 weeks in drinking water starting at 3 weeks-of-age. Water consumption and body weight was measured weekly, body composition was determined using nuclear magnetic resonance, urine and feces were collected in metabolic cages, and blood and tissues were collected at the end of the study. BPF-exposed rats showed significantly increased body growth and abdominal adiposity, risk factors for cardiometabolic disease. Urine output was increased in BPF-exposed rats, driving a trend in increased creatinine clearance. We also report the first relationship between a bisphenol metabolizing enzyme and a bisphenol-induced phenotype. Preliminary heritability estimates of significant phenotypes suggest that BPF exposure may alter trait variation. These findings support BPF exposure as a cardiometabolic disease risk factor and indicate that the HS rat will be a useful model for dissecting gene by BPF interactions on metabolic health.
Keywords: bisphenols, ; environmental exposure, gene expression, obesity, heterogeneous stock rats, adiposity
Bisphenols are used in the manufacturing of polycarbonates and epoxy resins and in consumer products, such as plastic baby bottles, water bottles, dental sealants, and thermal receipt paper (Chen et al., 2016; Milner, 2017). Considerable evidence demonstrates the presence of bisphenols in environmental samples, food, and food containers (Chen et al., 2016), and they are ubiquitously found in human populations worldwide (Jacobson et al., 2019; Lehmler et al., 2018; Liu et al., 2019; Michalowicz, 2014; Verbanck et al., 2017). The most well-studied bisphenol is bisphenol A (BPA). As an endocrine disruptor, epidemiological and animal studies implicate BPA in sex-specific adverse outcomes involving obesity and related metabolic processes as well as the cardiovascular system (cardiometabolic disease; Han and Hong, 2016; Ranciere et al., 2015; Richter et al., 2007) even at doses lower than the allowable daily intake (ADI; Le Magueresse-Battistoni et al., 2018; Vom Saal and Vandenberg, 2021). Because of human health concerns and public demand, the use of BPA-based plastics in baby bottles is prohibited in Canada, the European Union, and the United States (Escalante et al., 1989, 1991; Fang et al., 1995), and the U.S. Food and Drug Administration (FDA) has set the ADI for BPA to be 50 µg/kg body weight/day (USEPA, 2010). BPA’s use in plastic drinking bottles and other consumer products has been phased out, only to be replaced by structurally similar chemicals, including bisphenol F (BPF). Bisphenol metabolism is influenced by phases I and II xenobiotic processing (XP) enzymes, but they somewhat differ between BPA and BPF. In in vitro studies with human XP enzymes, BPA has been shown to be metabolized by CYP2C9 and CYP2C19 (Niwa et al., 2001; Schmidt et al., 2013), SULT1A1 and SULT2A1 (Nishiyama et al., 2002), and UGT1A9 and UGT2B15 (Hanioka et al., 2011), while BPF was shown to be metabolized by CYP2C9, CYP2E1 (Schmidt et al., 2013), UGT1A10, and UGT2A1 (Gramec Skledar et al., 2015). Hydroxylated, sulfonated, and glucuronidated metabolites of both BPA and BPF have been reported, but in vivo studies in rats indicate that the major metabolite of BPA and BPF is glucuronidated (Snyder et al., 2000) and sulfonated (Cabaton et al., 2006), respectively. Although the majority of BPA is excreted in the feces (Snyder et al., 2000), BPF is excreted mostly in the urine (Cabaton et al., 2006), likely due to the greater solubility of BPF in water. BPF also seems to exhibit enterohepatic cycling, a feature not seen with BPA (Cabaton et al., 2006).
The presence of BPA structural analogs in human biomonitoring samples are rising (Ye et al., 2015) despite insufficient data supporting their safety. Exposure to BPF in children and adolescent boys is positively associated with obesity in recent epidemiological studies (Jacobson et al., 2019; Lehmler et al., 2018; Liu et al., 2019). Most BPF exposure studies to date use in vitro or nonmammalian models to investigate its endocrine disrupting potential. These studies consistently demonstrate that BPF is a more potent endocrine disruptor than BPA, with effects on adipose, thyroid, and neuroendocrine functions and oxidative stress (Boucher et al., 2016; Chen et al., 2016; Michałowicz et al., 2015; Rosenfeld, 2017; Verbanck et al., 2017; Zhang et al., 2018; Zhu et al., 2018). Although relatively few in vivo mammalian studies have investigated the physiological effects of BPF exposure, they implicate BPF exposure with anxiety/depressive behaviors in mice (Ohtani et al., 2017), neurotoxicity in zebrafish (Gu et al., 2020), and thyroid dysfunction in frogs (Zhang et al., 2018). In vivo exposure studies in classical rat lines have shown that BPF exposure causes reproductive dysfunction in adult Sprague Dawley rats (Ijaz et al., 2019; Ullah et al., 2018a,b, 2019a,b) and effects both the dopamine and serotonin pathways in the perinatal brain of female Wistar rats (Castro et al., 2015). There are no published studies dedicated to the metabolic health effects of BPF exposure. Given the growing exposure of humans to BPF and the paucity of in vivo studies on BPF, there is a critical knowledge gap that warrants further investigation on the cardiometabolic impact of BPF exposure using a state-of-the-animal in vivo model.
There are major caveats to traditional inbred and outbred rodent models for translation to human health and for gene by environment (GxE) studies. Commercially available outbred strains often used for toxicology studies are not genetically defined, can differ dramatically between vendors, and lack high genetic diversity (Aldinger et al., 2009; Fitzpatrick et al., 2013; Gileta et al., 2018); however, inbred models lack any genetic diversity, meaning that such studies may not produce generalizable conclusions. Conflicting results from bisphenol exposure studies (Lejonklou et al., 2017) may be due to genetic differences. A previous study reported strain differences in BPA metabolism, with F344 female rats showing an increased capacity for BPA glucuronidation and urinary excretion compared with SD female rats (Snyder et al., 2000), suggesting that the F344 strain may be relatively resistant to BPA-induced effects (and possibly to other analogs) compared with the SD strain due to genetic differences. Additionally, the metabolism of bisphenols were shown to be impacted by genetic variation in human XP enzymes (Trdan Lusin et al., 2012). These data call for defined population models in bisphenol exposure studies to better mimic human population variability and the effects of genome variation.
N:NIH Heterogeneous stock (HS) rats (Solberg Woods, 2014; Woods and Mott, 2017) are a population-based animal model that simulates the genetic diversity in human populations but are amenable to genetic studies (Solberg Woods and Palmer, 2019). They are a powerful tool to overcome significant weaknesses of conventional animal models in GxE interaction studies relevant to complex human disease. HS rats were created as resource population for experimental and selection studies (Hansen and Spuhler, 1984) by crossing together 8 inbred rat strains and maintaining the colony in a manner that minimizes inbreeding (Supplementary Figure 1). The HS colony founding inbred strains were chosen based on genetic and phenotypic differences and include: ACI/N, BN/N, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N (Hansen and Spuhler, 1984). After more than 90 generations of breeding, each HS rat represents a genetic mosaic of the 8 founders, with genetic diversity more closely representing human populations (Woods and Mott, 2017) compared with inbred strains and with high phenotypic diversity for nearly any phenotype studied. HS rats are an ideal model for identifying the genetic causes of complex traits, including addiction behavior, obesity, cardiovascular, and metabolic diseases (Alam et al., 2011; Chitre et al., 2020; Holl et al., 2018; Keele et al., 2018; Rat Genome Sequencing and Mapping Consotorium et al., 2013; Solberg Woods et al., 2010, 2012). To our knowledge, there is no report of the HS rats being studied in toxicology nor of the HS rats being exposed to any bisphenol, including BPA. In pilot this study, we propose that BPF exposure in HS rats will alter body weight and adiposity, providing proof-of-principle evidence that this population-based model is ideal for exposure and GxE studies.
MATERIALS AND METHODS
Animals
Twenty-five pairs of male and female HS (NMcwiWfsm: HS) were sent from the Wake Forest School of Medicine to the University of Iowa at approximately 2 months of age. At the time of this study, the HS colony at Wake Forest consisted of 45 breeder pairs maintained using a breeding strategy that considers degree of relatedness to mate only distantly related individuals. Males and females were selected from the colony for breeding using a kinship coefficient threshold of 0.165 obtained from the HS pedigree information (Woods and Mott, 2017). Litters (4–13 pups) were culled to only male offspring upon sexing at 7–12 days of age (2–7 males). Breeders and offspring were provided ad libitum drinking water and a diet formulated to be devoid of phytoestrogens (ENVIGO, Indianapolis, Indiana, Teklad 2920X, <20 mg/kg isoflavones). Animals were housed in polysulfone microisolator cages (Thoren Caging Systems, Inc., Hazelton, Pennsylvania) on a 12-h light/dark cycle and were provided iso-BLOX (ENVIGO, T.6060) as environmental enrichment. The study design included 2 rats per exposure group per litter to attempt preliminary heritability estimates. When only 1 rat per exposure group was available from a litter, a subsequent litter was generated from the same breeder pair to generate the second matched pair. A total of 88 male HS rats (Vehicle = 45 and BPF = 43) from 27 litters of 23 breeder pairs were studied. The number of animals that completed the phenotyping protocol were not equal between the exposure groups because 2 males (1 vehicle- and 1 BPF-exposed) died within 1 week after weaning due to unknown causes, 2 animals in the BPF-exposed group were incorrectly sexed as male, 2 males were given the wrong exposure water at 6 weeks-of-age, and 1 BPF-exposed male had numerous congenital deformities at tissue collection. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals in a protocol approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Drinking Water
BPF was administered with the drinking water because oral ingestion is likely a major route of human exposure to BPF (Liao and Kannan, 2013). Moreover, the method of exposing animals to environmental bisphenols, such as BPA, via the drinking water is well established, is noninvasive, less stressful to the animal (vs. gavage eg,), and has been used in numerous animal studies (Al-Hiyasat et al., 2004; Funabashi et al., 2004; Kabuto et al., 2004; Kubo et al., 2003; Rubin et al., 2001; Takao et al., 1999, 2003). Because our hypothesis is that genetic background influences the outcomes of exposure, a single exposure level was selected to test the hypothesis that, while external exposure can be constant, genetic variability in BPF absorption, distribution, metabolism, and excretion (ADME) could impact physiological or clinical outcome variability. BPF was obtained from Angene International Limited, Nanjing, Jiangsu, China (Batch No.: AGN2017-11809-001) and had a purity > 99%, as determined by gas chromatography-gas chromatography (Supplementary Figure 2). Because there is no FDA ADI for BPF, the target exposure was 50 µg BPF/kg body weight/day, the current FDA ADI for BPA. Based on preliminary water consumption data from 14-week-old rats, we calculated that the concentration of BPF in the exposure water should be 1.125 mg BPF/ml to achieve a targeted dosing schedule of approximately 50 µg BPF/kg body weight/day. The BPF dosing solution was prepared by dissolving 281.25 mg of BPF in 250 ml of ethanol (U.S.P. dehydrated ethanol; Spectrum, New Brunswick, New Jersey) to create a solution of 1.125 mg/ml BPF in ethanol. Ten milliliters of this solution was diluted with 9990 ml Milli-Q water (Milli-Q Advantage A10 Water Purification System, Millipore Corporation, Burlington, Massachusetts) in a 10 l polypropylene carboy to give a final concentration of 1.125 ppm (1.125 µg/ml) in 0.1% ethanol. BPF was soluble at this concentration, as assessed using a published light scattering method (Supplementary Figure 3). The vehicle control solution (0.1% ethanol) was prepared by combining 10 ml of ethanol with 9990 ml Milli-Q water in a 10 l polypropylene carboy (ThermoFisher Scientific, Waltham, Massachusetts). All dosing solutions were stored at 4°C until provided to the animals.
Exposure and Phenotyping Protocol
Exposure
Littermate male HS rats were weaned at 3 weeks-of-age and were randomly assigned to vehicle control or exposure group by a random number generator (Google random number generator). Animals were exposed to vehicle (0.1% ethanol) or BPF (1.125 mg/l BPF in 0.1% ethanol) in drinking water contained in glass water bottles to avoid other possible exposures due to leaching from plastic water bottles. HS rats were singly housed and exposed via ad libitum drinking during the 5-week phenotyping protocol. Water consumption was monitored weekly to calculate estimated daily consumption and exposure. The consumption did not account for possible leakage or evaporation from the water bottles. Since BPF exposure in children and adolescent boys is positively associated with obesity (Jacobson et al., 2019; Lehmler et al., 2018; Liu et al., 2019), exposure lasted from wean to 8 weeks-of-age, the end of the juvenile period for male rats.
Phenotyping
Body weight was measured weekly during exposure beginning with a baseline body weight at wean. Blood glucose was measured from the saphenous vein in unfasted, unanesthetized animals at wean using a handheld glucometer (Contour blood glucose meter, Bayer HealthCare LLC, Leverkusen, Germany). Time-domain nuclear magnetic resonance (NMR) was performed at 7 weeks-of-age in unfasted, unanesthetized animals to assess body composition (fat, lean, and fluid body masses as percentages and absolute masses) using a LF90II BCA-Analyzer (Bruker, Billerica, Massachusetts) prior to placing the animals in metabolic cages. Body composition measurements were taken between 10:00 and 13:00. Animals were placed in metabolic cages (Tecniplast S.p.A., Buguggiate, Italy) at 7 weeks-of-age and provided drinking water (containing vehicle or BPF) and food (Teklad 2920X) ad libitum. After a 24-h acclimation period, 24-h urine and fecal samples were weighed, collected, and stored at −80°C. Food and drinking water consumed during the 24-h period was determined. All 24-h measurements were taken between 11:00 and 13:00 on collection day. At 8 weeks-of-age, blood glucose was measured from the saphenous vein in unfasted, unanesthetized animals at using a handheld glucometer. After an overnight fast, the 8-week-old animals were humanely euthanized by CO2 and thoracotomy. Body length measures (nose to rump and nose to tip of tail) were taken. Tissues (liver, left ventricle of the heart, kidney, testes, gonadal white adipose tissue [GWAT], perirenal white adipose tissue [PWAT], inguinal white adipose tissue [IWAT], skeletal muscle, hypothalamus, cerebrum, and brown adipose tissue [BAT]) were harvested and weights collected for liver, left ventricle of the heart, kidney, testes, GWAT, PWAT, IWAT, and BAT. Tissue samples were either snap frozen in liquid nitrogen and/or placed in RNAlater (Invitrogen, Carlsbad, California) at −80°C for RT-R. Blood samples were collected by cardiac puncture, processed into serum (BD Microtainer, SST-Amber, Franklin Lakes, New Jersey) and plasma samples (BD Microtainer, Tubes with K2E, Franklin Lakes, New Jersey), aliquoted, and stored at −80°C.
Creatinine Levels
Urine samples collected in metabolic cages were centrifuged at 3000 × g for 5 min to pellet particulate matter, such as food. Creatinine levels (mg/dl) were determined in the urine samples following the manufacturer protocol (Enzo, Farmingdale, New York). Samples were diluted appropriately in deionized water (Invitrogen). Plasma creatinine levels (mg/dl) were determined in fasted animals from the terminal blood draw following the manufacturer’s protocol (Arbor Assay, Ann Arbor, Michigan). Plasma samples were centrifuged at 14 000 × g for 15 min to pellet cellular particulates prior to conducting the assay.
Calorimetry
Bomb calorimetry was performed on fecal samples as previously described (Grobe, 2017) to quantitatively assess digestive efficiency and caloric absorption. Briefly, quantitative fecal samples collected in metabolic cages were desiccated in an oven and weighed prior to (wet mass) and after (dry mass) desiccation. Desiccated samples were pressed into pellets and weighed. Digestive efficiency and total daily caloric absorption were determined using a semi-microbomb calorimeter (Parr Instrument Co., Moline, Illinois). Fecal samples were burned to completion in the bomb calorimeter according to the manufacturer’s protocol. Desiccated, powdered Teklad 2920X diet from the same lot was also analyzed by bomb calorimetry to determine total caloric density.
Urine Osmolality
Urine osmolality was measured by freezing-point depression osmometry (OsmoPRO multi-sample micro-osmometer, Advanced Instruments, Norwood, Massachusetts). 24-h urine samples collected during metabolic cages were diluted by 1:4 in deionized water to reliably obtain values for rodents within the measurable range of the instrument. Clinitrol 290 reference solution (Advanced Instruments) was measured first and then after every 19 samples until all samples were measured to confirm accurate system conditions (Clinitrol standard [mean ± SD, coefficient of variation]: 290 mOsm ± 0.8 mOsm/kg H2O, 0.3%).
Urinary Microalbumin
Microalbumin content of 24-h urine samples was measured at the Physiology Biochemical Analysis Core at the Medical College of Wisconsin. Albumin Blue 580 is an anionic dye that binds strongly and specifically to albumin giving off a red fluorescence (590ex 616em). A standard curve was prepared, mixed with a working solution of Albumin Blue 580 in buffer and ispropanol in microplate format. The 96-well plate was read on Biotek Synergy HT (Bio-Tek Instruments, Winooski, Vermont). Sample values are read directly from nonlinear standard curve, useful range for rat urine is 0.033–1.0 mg/ml. Samples were diluted 20 µl urine into 180 µl standard diluent.
Total RNA Isolation
Liver or PWAT tissue in RNAlater was thawed on ice. 30–60 or 200–250 mg of sample was homogenized in 1 or 4 ml of TRIzol Reagent (Invitrogen), respectively, using a Fisherbrand Bead Mill 4 Homogenizer (ThermoFisher Scientific). Homogenization was repeated, and the homogenates were cooled on ice. Samples were centrifuged at 21 100 × g for 3 min to pellet unhomogenized tissue. PWAT homogenates were left at room temperature for 10 min prior to centrifugation. The supernatant and 200 or 800 µl of Chloroform (ThermoFisher Scientific) for liver or PWAT samples, respectively, were mixed rapidly by inversion, incubated at room temperature for approximately 3 min, and centrifuged at 4°C at 12 000 × g for 15 min. Total RNA was purified from the aqueous layer following the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. PWAT samples were treated with DNase I from the RNase-Free DNase Set (Qiagen) according to the manufacturer’s protocol to remove any remaining genomic DNA. RNA was quantified by a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific). RNA samples were stored at −80°C.
Real-Time Quantitative PCR
Purified RNA (approximately 1µg) was reverse-transcribed using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California). All real-time quantitative PCR (RT-qPCR) assays were prepared following the manufacturer protocol using Prime Time Gene Expression Master Mix gene expression assay (Integrated DNA Technologies, Coralville, Iowa). Liver experiments were conducted on a StepOnePlus RT-PCR System, and PWAT experiments were conducted on a QuantStudio 6 Flex RT-PCR System (Applied Biosystems, Foster City, California). Primer sequences for all genes (Liver: Ugt1a9 and Gapdh; PWAT: Ccl2, Fabp4, Fas, Hif1a, Lpl, Ppar-γ, and Rplp0) are listed in Supplementary Table 1. Gapdh and Rplp0 were used as endogenous controls to normalize gene expression for each target gene in liver and PWAT, respectively (Supplementary Figure 4 and Table 2). Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method (Pfaffl, 2004) on the average of 3 technical replicates per sample. PCR grade water (Invitrogen) was used as a negative control replacing the reverse transcriptase, and rat universal RNA (Biochain, Newark, California) was used as a positive control.
Calculations
To remove body weight as a confounding variable in tissue masses and metabolic measures, all absolute tissue masses, food and water consumption, and feces and urine output were normalized to body weight (g) to provide a “relative” value in units/g body weight. Creatinine excretion rate (mg/day) was calculated by multiplying the urinary creatinine concentration by the 24-h urine output. Creatinine clearance (ml/min) to estimate glomerular filtration rate (Olea-Herrero et al., 2014) was calculated by multiplying the creatinine excretion rate by a factor to convert to mg/min and diving the product by the plasma creatinine concentration. To assess energy balance, digestive efficiency and energy efficiency was calculated following equations in (Grobe, 2017). Dry:Wet Feces (g/g) was calculated by taking the ratio of dry feces mass from calorimetry results to wet feces mass from metabolic cages. Water in Feces (g/day) was calculated by subtracting the Dry:Wet Feces (g/g) from 1 and multiplying the result by the 24-h wet feces mass. Water lost to feces and water lost to urine were calculated as the percentage of Water in Feces (g/day) or Urine (g/day) to water consumed in 24 h in metabolic cages. Water lost to other (%) was calculated by subtracting water lost to feces (%) and water lost to urine (%) from 100. To calculate average daily BPF exposure in HS rats, the following equation was used:
where T1 is time point 1, T2 is time point 2, BW is body weight (g), W is water (ml). Hydration (%) was calculated by multiplying fat-free mass (%) by 0.732 as most mature animals have hydration levels at approximately 73.2% of their fat-free body mass (Sheng and Huggins, 1979).
Statistical Analysis
Outlier analysis
Outliers were removed from the raw dataset, body weight relative data, and other calculations by ROUT analysis (Motulsky and Brown, 2006; Q = 1%) that is integrated into the GraphPad Prism v8.3.0 for Windows (GraphPad Software, www.graphpad.com, San Diego, California). (See Supplementary Table 3 for details regarding outlier removal and other impacts on experimental noise.)
Statistical modeling
Statistical significance was determined using multivariate linear mixed-effect modeling. The following model was used for the analysis of independent phenotypes:
where yklp is the response variable for the kth animal of the lth litter of the pth breeder pair, Blp is the nested random effect of the lth litter of the pth breeder pair, E is the fixed effect of exposure of the kth animal, Sl is the size of the lth litter at birth as a covariate, and eklp is a residual term.
A separate univariate model was constructed for the analysis of body weight gain as longitudinal data. The maximal random effects structure supported by the data tested the linear random effect, quadratic fixed effect, and quadratic random effect for week, as well as both linear and quadratic interaction terms for week and exposure. ultimately, the following model was used for the analysis of longitudinal data:
where yklpw is the response variable for the kth animal of the lth litter of the pth breeder pair at the wth time point, Blpw is the nested random effect of the lth litter of the pth breeder pair including a linear effect of time, W2 is the fixed quadratic effect of time, W*E is the fixed linear interaction effect of time and exposure of the kth animal, E is the fixed effect of exposure of the kth animal, Sl is the size of the lth litter at birth as a covariate, and eklpw is a residual term.
Each mixed-effects model was fit using the lmer function in the lme4 package (Bates et al., 2015) in R (64-bit v3.5.1; R Core Team, 2018) with p-values and fit indices estimated using the Likelihood Ratio Test, Chi-Square distribution on the fixed effect of exposure. Litter size was chosen a priori as a covariate. Exposure was effect coded as 0 for Vehicle and 1 for BPF-exposed. All results are reported as mean ± SEM unless otherwise specified.
Model residuals were tested for normality using the Shapiro-Wilk test (α = 0.05) and homoscedasticity using the Breusch-Pagan test (α = 0.05). Data with nonnormally distributed residuals were log transformed, and the residuals retested for normality and homoscedasticity after model fitting as previously described. After selective log transformation, all data residuals except Nose:Rump body length and Dry:Wet Feces was normally distributed and homoscedastic.
Permutation testing
Nose:Rump and Dry:Wet Feces data were tested by permutation testing because of the failure of the dataset to be normally distributed and homoscedastic after log transformation. The actual difference between the vehicle and BPF-exposed groups means was calculated. Forty-three (vehicle n) random data points from the observed Nose:Rump and Dry:Wet Feces dataset were selected to represent the vehicle group, whereas the remaining data points represented the BPF-exposed group. The difference between the means of the permuted vehicle and BPF-exposed groups was calculated. This permutation was repeated 10 000 times. A p-value (α = 0.05) was calculated using a 2-tailed test: the proportion of the number of instances that the permutation returned an absolute difference greater than or equal to the actual difference to the number of permutations. Permutation testing was completed in R (64-bit v3.5.1).
Linear regression
Linear regressions were calculated and plotted in GraphPad Prism v8.3.0 for Windows (GraphPad Software, www.graphpad.com) to compare gene expression-phenotype correlations between vehicle- and BPF-exposed male HS rats. Regressions were tested for different slopes and y-intercept values.
RESULTS
Water Consumption and Dosing
Weekly water consumption was tracked in all animals over the study protocol. From those data, an average daily water consumption was determined to be 17 ± 0 ml/day and did not significantly differ by exposure group (Supplementary Figure 5 and Table 4). Average daily BPF exposure was 153 ± 3 µg BPF/kg BW/day based on water consumption for the entire 5-week exposure period (Supplementary Figure 6 and Table 4).
To compare the HS rat BPF exposure dose to a human dose, the dosimetric adjustment factor equation, endorsed by the USEPA as a method to extrapolate toxicologically equivalent doses of orally administered agents from laboratory animals to humans (USEPA, 2011), was implemented. Using the BW3/4 equation and a human body weight of 70 kg, the average HS rat dose of 152.5 µg BPF/kg BW/day was comparable to a human dose of 32 µg BPF/kg BW/day.
Body Growth and Adiposity
Body weight gain from the initiation of exposure was determined (Table 1 and Figure 1A). Three weeks of BPF exposure resulted in an increase in body weight gain compared with littermate controls which was sustained through the end of the study (Table 1, Figs. 1A and 1B, and Supplementary Table 5). Body length was also modestly increased in the BPF-exposed rats (Table 1 andFigure 1C).
Table 1.
Weight Parameters and Other Phenotypes in Male HS Rats (Mean ± SEM [n])
| Phenotype | Vehicle | BPF-Exposed | Likelihood Ratio Test p-Value |
|---|---|---|---|
| Body size measurements | |||
| Body weight (g): week 8 | 206 ± 3 (45) | 212 ± 4 (43) | .12 |
| Body weight gain (g): week 8 | 154 ± 3 (45) | 161 ± 3 (43) | .03* |
| Nose:Rump (cm) | 20.1 ± 0.2 (45) | 20.5 ± 0.2 (43) | .06a |
| Adiposity measurements | |||
| NMR fat mass (g) | 14 ± 1 (45) | 15 ± 1 (42) | .05 |
| NMR fat % mass | 7.8 ± 0.3 (45) | 8.4 ± 0.3 (43) | .06 |
| NMR fat free mass (g) | 164.2 ± 2.8 (45) | 166.7 ± 2.8 (43) | .41 |
| NMR fat free mass % | 92.1 ± 0.3 (45) | 91.7 ± 0.3 (43) | .16 |
| GWAT (g) | 1.59 ± 0.08 (44) | 1.71 ± 0.08 (42) | .08 |
| GWAT (mg/g) | 7.58 ± 0.29 (44) | 8.37 ± 0.34 (43) | .01* |
| PWAT (g) | 2.23 ± 0.12 (45) | 2.51 ± 0.12 (41) | .01* |
| PWAT (mg/g) | 10.58 ± 0.49 (45) | 12.21 ± 0.54 (42) | .003** |
| BAT (mg) | 225 ± 6 (45) | 232 ± 6 (42) | .35 |
| BAT (mg/g) | 1.10 ± 0.03 (45) | 1.12 ± 0.03 (43) | .45 |
| Metabolic cage measurements | |||
| Food consumed (g/day) | 18.89 ± 0.35 (45) | 19.39 ± 0.37 (43) | .23 |
| Wet feces (g/day) | 5.67 ± 0.16 (45) | 6.07 ± 0.15 (43) | .06 |
| Dry:Wet Feces (g/g) | 0.515 ± 0.005 (43) | 0.509 ± 0.004 (40) | 1.00 |
| Digestive efficiency (%) | 85.2 ± 0.3 (41) | 84.6 ± 0.3 (41) | .24 |
| Energy efficiency (mg/kcal) | 69.0 ± 1.2 (44) | 70.6 ± 1.4 (42) | .39b |
| Water consumed (g/day) | 18.11 ± 0.44 (43) | 18.11 ± 0.52 (41) | .87b |
| Urine (g/day) | 5.93 ± 0.41 (44) | 6.83 ± 0.33 (40) | .03* |
| Water in feces (g/day) | 2.76 ± 0.09 (44) | 3.00 ± 0.09 (42) | .04a |
| Water lost to feces (%) | 15.3 ± 0.6 (42) | 17.0 ± 0.6 (40) | .04* |
| Water lost to urine (%) | 31.4 ± 1.9 (41) | 38.2 ± 1.6 (39) | .001** |
| Water lost to other (%) | 53.3 ± 1.9 (41) | 45.0 ± 1.8 (39) | <.0001*** |
| Hydration (%) | 67.4 ± 0.2 (45) | 67.1 ± 0.2 (43) | .15 |
| Creatinine measurements | |||
| Urine creatinine (mg/dl) | 68.6 ± 2.9 (43) | 65.58 ± 2.80 (42) | .35 |
| Creatinine excretion (mg/day) | 3.75 ± 0.20 (42) | 4.41 ± 0.19 (41) | .0009*** |
| Plasma creatinine (mg/dl) | 0.63 ± 0.02 (36) | 0.56 ± 0.02 (40) | .009** |
| Creatinine clearance (mg/min) | 0.45 ± 0.03 (39) | 0.50 ± 0.03 (39) | .11 |
Relative tissue masses in mg/g are mg tissue per g body weight; NMR body composition measurements are shown as absolute g and as percent of total body weight.
Abbreviations: GWAT, gonadal white adipose tissue; PWAT, perirenal white adipose tissue; BAT, brown adipose tissue.
Model residuals not normally distributed in raw or log10 transformed data, data were tested by permutation testing multivariate linear mixed model Likelihood Ratio Test.
Model residuals not normally distributed, data were log10 transformed for analysis.
p < .05;
p < .01; and
p < .001.
Figure 1.
Body growth in male heterogeneous stock rats. A, Body weight gain curves were constructed during the study based on body weight of vehicle- and bisphenol F (BPF)-exposed males throughout the study. BPF-exposed rats had significantly increased body weight gain compared with vehicle-exposed males (univariate longitudinal linear mixed model and Likelihood Ratio Test). B, BPF-exposed males had significantly increased body weight gain at 8 weeks-of-age. C, At 8 weeks-of-age, BPF-exposed rats have significantly increased nose to rump body length. (mean ± SEM, multivariate linear mixed model Likelihood Ratio Test *p < .05 and **p < .01).
To determine an effect of BPF exposure on adiposity, body composition was determined at 7 weeks-of-age using NMR (Table 1 andFigure 2). BPF exposure increased adiposity in HS males: there were strong trends in increased % fat mass (Figure 2A) and fat mass as absolute grams (Figure 2B) in BPF-exposed HS rats. Fat-free mass was unaffected by BPF exposure (Figs. 2C and 2D), suggesting an overall increase in whole-body adiposity. At the end of the study, fat pads were weighed from GWAT, PWAT, and intrascapular BAT (Table 1 andFigure 3) to determine if abdominal adiposity specifically was increased. Indeed, BPF-exposed HS rats had significantly increased relative GWAT and PWAT masses, corresponding to approximately 11% and approximately 15% increases in fat pad masses, respectively (Figure 3A), while there was no difference in relative BAT mass (Figure 3B). To attempt to understand the cause of the increased relative PWAT mass, gene expression of widely used biomarkers for adipogenesis (Fabp4 [Kloting and Bluher, 2014; Yang et al., 2016], Ppar-γ [Dusserre et al., 2018; Somm et al., 2009; Yang et al., 2016]), lipogenesis (Fas [Somm et al., 2009], Lpl [Lejonklou et al., 2017; Somm et al., 2009]), fibrosis (Hif1a [Kloting and Bluher, 2014; Xu et al., 2017]), inflammation (Il6 [Appari et al., 2018; Kloting and Bluher, 2014; Yang et al., 2016]), and macrophage infiltration (Ccl2 [Appari et al., 2018; Kloting and Bluher, 2014; Yang et al., 2016]) were assessed. No significant difference in expression of any of these genes was found (Supplementary Figure 7 and Table 2).
Figure 2.
Body composition of 7-week-old male heterogeneous stock rats. At 7 weeks-of-age, fat (A and B) and fat-free (C and D) body mass was measured by nuclear magnetic resonance. bisphenol F-exposed rats tend to have increased absolute and % fat body mass compared with vehicle-exposed rats. There was no difference in absolute or % fat-free body mass. (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05).
Figure 3.
Adipose tissue relative masses in 8-week-old male heterogeneous stock rats. At 8 weeks-of-age, abdominal fat depots and brown adipose tissue were harvested, weighed, and adjusted for body weight. Bisphenol F (BPF)-exposed rats had significantly increased gonadal white adipose tissue and perirenal white adipose tissue compared with vehicle-exposed males (A). BPF-exposed rats had no difference in BAT mass (B). (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05).
Feces Output and Energy Efficiency
The growth differences between vehicle- and BPF-exposed rats may have been caused by changes in energy homeostasis, the balance between “energy in,” like food consumption and energy efficiency, and “energy out,” like physical activity and metabolic rate. To test the hypothesis that BPF exposure alters male HS rats’ ability to extract calories from food, 24-h fecal samples were collected in metabolic cages at 7 weeks-of-age, weighed, and analyzed by bomb calorimetry, a method for caloric density determination (Table 1 andFigure 4). Although there was no difference in food consumed over the 24-h collection period (Figure 4A), there was a strong trend in increased fecal output (Figure 4B). Digestive efficiency, the proportion of calories absorbed from consumed food, did not differ between the exposure groups (Figure 4C). Energy efficiency, the amount of weight gained per calorie absorbed over time, calculated using the calorimetry results showed that there was no difference between vehicle- and BPF-exposed rats (Figure 4D).
Figure 4.
Metabolic cage food consumption, fecal output, and metabolic efficiency calculations in 7-week-old male heterogeneous stock rats. A–C, At 7 weeks-of-age, feeding behavior was assessed for 24 h using metabolic caging. Feeding behavior was unaffected by bisphenol F (BPF) exposure (A); however, BPF-exposed rats showed a trend in increased feces output (B). Digestive efficiency (C) and energy efficiency (D) calculated from bomb calorimetry results showed no difference between vehicle- and BPF-exposed rats. (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05).
Water Loss, Urine Output, and Creatinine Measures
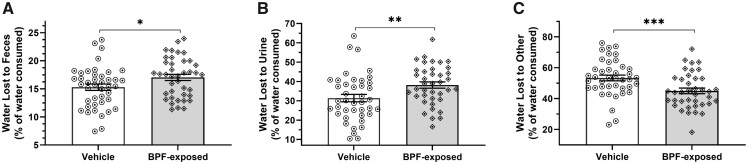
In parallel with food consumption, 24-h water consumption did not differ between exposure groups in metabolic cages at 7 weeks-of-age (Table 1 and Figure 5A), consistent with the water consumption data taken from the entirety of the study. However, there was a significant increase in urine output (Table 1 andFigure 5B) by approximately 15% in the BPF-exposed rats. A closer examination of the calorimetry results showed that despite no difference in the ratio of dry fecal mass to wet fecal mass (Dry:Wet Mass; Table 1 andFigure 6A), there was also a significant increase in water content in the entire fecal sample in BPF-exposed rats compared with vehicle-exposed rats (Table 1 andFigure 6B). The increased urine rate and increased fecal water content combined showed a significant change in water loss over the 24-h period, with the BPF-exposed rats having increased water loss to both the urine and feces and decreased water loss to other sources relative to 24-h water consumption compared with the vehicle-exposed rats (Table 1 andFigure 7).
Figure 5.
Metabolic cage water consumption and urine output in 7-week-old male heterogeneous stock rats and 7 weeks-of-age. At 7 weeks-of-age, drinking behavior was assessed and urine collected for 24 h using metabolic caging. Drinking behavior was unaffected by bisphenol F (BPF) exposure (A). However, BPF-exposed rats had significantly increased urine output (B). (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05).
Figure 6.
Water content in male heterogeneous stock rat fecal samples. Water content of fecal samples collected at 7 weeks-of-age was determined using the calorimetry results. Although there was no difference in the ratio of dry fecal sample mass to wet fecal sample mass (A), there was a significant increase in water content in the entire fecal sample in the bisphenol F-exposed rats (B). (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05).
Figure 7.
Water loss profiles in 7-week-old male heterogeneous stock rats. Water loss to feces, urine, and other evaporative sources were calculated using data collected at 7 weeks of age in metabolic cages. Bisphenol F-exposed rats show increases in both water lost to feces (A) and urine (B) with a proportion decrease in water lost to other sources (C) compared with vehicle-exposed rats. (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test *p < .05, **p < .01, and ***p < .001).
To test whether the increase in urine output was due to more dilute urine production, urinary creatinine levels and urine osmolality were determined (Table 1 andFigure 8). Although absolute urinary creatinine level did not differ (Figure 8A), creatinine excretion was significantly increased in BPF-exposed rats (Figure 8B). Urine osmolality also did not differ between vehicle- and BPF-exposed rats (Supplementary Figure 8 and Table 2). Both measures indicated no change in urine concentration between vehicle- and BPF-exposed male HS rats, rather only increased urinary output, suggesting a state of mild dehydration. To assess whether the BPF-exposed HS rats were dehydrated, we calculated the hydration of the rats using the body composition measurements at 7 weeks-of-age (Table 1). There was a trend in decreased hydration in the BPF-exposed rats compared with the vehicle-exposed rats, supporting the increased water loss to urine and feces.
Figure 8.
Urinary and plasma creatinine levels and creatinine excretion and clearance in male heterogeneous stock rats. Creatinine levels were assessed by colorimetric detection assay in 24-h urine samples collected at 7 weeks-of-age in metabolic cages and in plasma samples from a terminal blood draw at 8 weeks-of-age. Bisphenol F (BPF)-exposed rats show no change in urine creatinine levels (A), a significant increase in creatinine excretion compared with vehicle-exposed rats (B), and a significant decrease in plasma creatinine levels (C). Taken together, the BPF-exposed rats exhibit a trend in increased creatinine clearance rate (D). (mean ± SEM; multivariate linear mixed model Likelihood Ratio Test **p < .01, ***p < .001).
Creatinine clearance is an estimate of glomerular filtration rate and can be used to evaluate kidney function (Olea-Herrero et al., 2014). To calculate creatinine clearance, plasma creatinine levels from the terminal blood draw was determined using the same creatinine assay. BPF-exposed rats had significantly lower plasma creatinine levels than vehicle-exposed rats (Figure 8C). Creatinine clearance calculations showed a weak trend in increased creatinine clearance in the BPF-exposed male HS rats (Figure 8D). Possible kidney damage was assessed by measuring urinary microalbumin. There was no difference in urinary microalbumin levels between vehicle- and BPF-exposed rats (vehicle-exposed mean = 0.086, SEM = 0.011, n = 24 and BPF-exposed mean = 0.084, SEM = 0.008, n = 24); this is not unexpected as proteinuria is typically consistent with decreased rather than increased creatinine clearance.
Liver Ugt1a9 Expression Versus BPF-Induced Phenotypes
Bisphenols are processed primarily in the liver by phases I and II metabolizing enzymes (Cabaton et al., 2006, 2008; Snyder et al., 2000) before being excreted as inactive, conjugated compounds. Human UGT1A10, equivalent to rat Ugt1a9, is one of the most active phase II enzymes responsible for the glucuronidation of BPF in humans (Gramec Skledar et al., 2015; Hanioka et al., 2011). Gene expression of liver Ugt1a9 was assessed in HS rats by RT-qPCR. There was a nonsignificant decrease in gene expression in the BPF-exposed rats compared with the vehicle-exposed rats (Supplementary Figure 9 and Table 2).
To further test the hypothesis that expression of Ugt1a9 in the liver impacts significant phenotypes in BPF-exposed rats, linear regressions were calculated between body growth, adiposity, and other metabolic data and Ugt1a9 gene expression. Ugt1a9 was negatively associated with % fat mass from body composition at 7 weeks-of-age (Figure 9A) and relative GWAT mass (Figure 9B) in the BPF-exposed rats but not in the vehicle-exposed rats. When tested, the slopes of the linear regressions in % fat mass and relative GWAT mass were significantly different between the vehicle- and BPF-exposed rats (% Fat Mass: Vehicle- R2 = 0.0042, BPF-exposed R2 = 0.1597, Pslope = 0.02; GWAT (mg/g): Vehicle R2 = 0.0011, BPF-exposed R2 = 0.2325, Pslope = 0.02). Although Ugt1a9 expression had no impact on the vehicle exposed rats, there was a significant association between decreased Ugt1a9 expression and increased adiposity due to BPF exposure.
Figure 9.
Liver Ugt1a9 expression—adiposity phenotype regression analysis in male heterogeneous stock (HS) rats. Liver gene expression of Ugt1a9 was assessed in HS rats by real time quantitative PCR. Linear regressions were calculated and compared between adiposity measures and Ugt1a9 expression as ΔCt. Liver Ugt1a9 expression significantly correlated with NMR % fat mass (A) and with gonadal white adipose tissue (mg/g) (B) in the bisphenol F-exposed rats (n = 43) but not in the vehicle-exposed rats (n = 45). Comparison of linear regressions of the data showed significantly different slopes in both Ugt1a9-adiposity datasets (*p < .05).
DISCUSSION
Our data demonstrate that BPF exposure increases body weight, body growth, abdominal adiposity, and urine output in adolescent male HS rats. It further suggests that decreased Ugt1a9 expression plays a role in BFP-induced adiposity. BPF is often used in the manufacturing of common consumer products. Traditional in vivo toxicity studies do not consider the genetic backgrounds of inbred animals nor outbred animals; GxE interactions may be the origin of conflicting results reported from exposure studies in different animal models. The use of genetically heterogenous animals to study toxicological endpoints may be more difficult due to greater experimental “noise” (Festing, 1993); however, this noise (ie, population phenotypic variation) is a reflection of the genetic variation that may contribute to additive responses to exposures, and more closely models human population diversity in genetic and phenotypic variability. The impact of genetic variation on susceptibility to bisphenol-induced disease is unknown and requires population-based models to better approximate human population genetic variation. We used the HS rats to identify the metabolic impact of postnatal BPF exposure in juvenile males, a particularly at-risk human demographic. Our proof-of-principle BPF exposure study on the HS rats is the first to incorporate population-level genetic variation in exposure studies. The BPF-exposed male HS rats were exposed to a dose comparable to an adult human dose of 32 µg BPF/kg/day, which is below the current FDA ADI for BPA, making our dose relevant to human health concerns.
Three weeks into exposure, BPF-exposed males had increased body weight gain by 5% on average that was maintained for the study’s duration. Although 5% difference may seem modest from the perspective of an inbred or a gene-edited animal model, a decrease in body weight by 5% in human populations is a significant phase II endpoint for drug therapies (Apovian et al., 2015), making the BPF-induced 5% increase in body weight relevant to human health outcomes. Also, at the human population level, the combination of approximately 100 identified human genetic loci associated with body weight accounts for <3% of body weight variation (Locke et al., 2015). BPF-exposed male HS rats also had modestly increased body length, suggesting that BPF exposure in male HS rats caused accelerated overall body growth. Our studies found the increase in body weight and adiposity was not due to changes in energy intake, digestive efficiency, or energy efficiency, possibly pointing to other mechanisms such as decreased resting or active energy expenditure to be determined in future studies.
Five weeks of BPF exposure in drinking water significantly increased adiposity in male HS rats as seen in the change in body composition and in abdominal fat mass. There was an increase in total body % fat mass, and both relative GWAT and PWAT masses were significantly greater in the BPF-exposed male HS rats compared with the vehicle-exposed by approximately 11% and 15% on average, respectively. Increased adiposity and obesity in children and adolescents are risk factors for developing cardiometabolic traits in adulthood (Di Cesare et al., 2019; Must and Strauss, 1999). Future studies will study the effects of adolescent exposure in adult and aged animals. There was no difference in gene expression of the markers we assessed for cellular processes that could explain the significant increase in relative PWAT mass in the BPF-exposed rats. Future studies will evaluate other biomarkers and incorporate histological examination of adipose tissues or cultured adipocytes to investigate the underlying mechanisms of BPF-induced adiposity.
Very few studies exist regarding BPF, but the increase in body weight gain and adiposity phenotypes are consistent with some BPA exposure studies (Lejonklou et al., 2017; Rubin et al., 2001; Somm et al., 2009; van Esterik et al., 2014; Yang et al., 2016). However, there are also reports in male mice and rats of no change (Chianese et al., 2018; Kovanecz et al., 2014; Lejonklou et al., 2017; Peerapanyasut et al., 2019; Takao et al., 1999; Ullah et al., 2018a) or a decrease in body weight (Drobna et al., 2019; Kobroob et al., 2018; Nakamura et al., 2010) as well as no change in adiposity (Drobna et al., 2019; van Esterik et al., 2014). It is possible that the exposure timing may impact bisphenol effects; however, bisphenol studies on male rodents aged similarly to our study still report diverse body weight and adiposity results supporting a role for an effect of genetic background. A study where 4-week-old Wistar/ST male rats were exposed to 57.1 and 114.2 mg BPA/kg/day for 6 weeks reported a decrease in body weight (Nakamura et al., 2010). A study in male C57BL/6 mice 5 weeks-of-age exposed to BPA for 30 days showed body weight and adiposity increases at all tested doses (Yang et al., 2016). The genetic variants that underly susceptibility to the negative effects of bisphenol exposure are unknown, so interpretation of results found in different animal models must be done with caution.
Blood glucose is often measured to assess the development of glucose-handling disorders, such as diabetes mellitus (Solberg Woods et al., 2012; Tsaih et al., 2014). Optimal measures of blood glucose involve food deprivation to test how the body responds to fasting. We measured unfasted glucose at 8 weeks-of-age and saw a modest but statistically significant increase in blood glucose in the BPF-exposed males (data not shown). It is unclear if this increase represents a biologically significant change in glucose handling given the fed state of the animals. A longer exposure period may lead to more robust physiological outcomes. For example, a study exposing CD-1 male mice from birth until adulthood to approximately 0.5 µg BPA/kg/day reported no difference in fasted blood glucose at 8 weeks-of-age but found a significant increase in fasted blood glucose at 10 weeks-of-age compared with the vehicle-control mice (Ke et al., 2016). Future studies on BPF exposure will incorporate fasted blood glucose, insulin and glucagon measures and glucose tolerance tests in older, chronically exposed animals.
The significant increase in both urine output by approximately 15% and water in feces after 4 weeks of exposure led to an overall increase in water lost to nonevaporative sources and possibly mild dehydration in the BPF-exposed male HS rats. Increased urine output by the BPF-exposed males drove the significant increase in urinary creatinine excretion. The urine output increase was not balanced by an increase in water consumption, suggesting that thirst was not altered during BPF exposure. Increased urine output and urinary creatinine excretion are inconsistent with most previous BPA exposure results, suggesting differences between BPA and BPF. A study in C57BL/6J male mice exposed to 25 µg BPA/kg by a subcutaneous injection at PND10 showed significantly decreased 24-h urine output and no change to creatinine excretion (Esplugas et al., 2018). Interestingly, there was a significant decrease in plasma creatinine in BPF-exposed males, which may be a result of liver damage or decreased physical activity (not measured in this study). Because there was no difference in food consumption or energy efficiency, our results suggest that the energy balance between intake and output is skewed toward decreased energy output, including physical activity and resting metabolic rate (not measured in this study), which may explain the increases in both body weight and adiposity in the BPF-exposed rats. Future studies will address how BPF exposure impacts physical activity and metabolic rate.
Creatinine clearance approximates glomerular filtration rate, a widely used measure of kidney health (Olea-Herrero et al., 2014). The combination of increased creatinine excretion and decreased circulating creatinine resulted in a trending creatinine clearance increase, suggesting altered kidney function like creatinine hyperfiltration. Consistent with our findings, increased creatinine clearance has been shown in CD-1 male mice exposed to 50 mg BPA/kg for 5 weeks (Olea-Herrero et al., 2014) and in adult male Wistar rats exposed for 5 weeks with 50 mg BPA/kg (Peerapanyasut et al., 2019). However, decreased creatinine clearance and increased serum creatinine has been reported in adult male Wistar rats exposed to 5 weeks of 50, 100, and 150 mg BPA/kg/day (Kobroob et al., 2018). Increased serum creatinine was also reported in a study of male C57BL/6 mice exposed to 500 µg BPA/kg/day for 8 weeks (Tong et al., 2019). We also report no change in urinary microalbumin between the exposure groups, indicating no overt kidney damage at the study timepoint, although the hyperfiltration could lead to damage in the adult animal. Additional animal studies on the effect of BPF on the kidney must be conducted to interrogate this creatinine dysfunction.
Human UGT1A10, the protein most structurally similar to rat Ugt1a9, is a phase II metabolism uridine 5-diphospho-glucuronosyltransferase that has been implicated in BPF glucuronidation in humans, as compared with UGT2B15 and UGT2A1 in BPA glucuronidation (Gramec Skledar et al., 2015; Hanioka et al., 2011). Because Ugt1a9 activity increases target compound solubility and promotes its excretion, Ugt1a9 activity can be a protective factor against BPF exposure. Conceptually, greater Ugt1a9 activity causes faster removal of the active compounds and lessens exposure effects. We demonstrated that liver Ugt1a9 expression is negatively related to % fat mass and relative GWAT mass (mg/g) in BPF-exposed but not in vehicle-exposed rats. These results suggest that without BPF-exposure, no relationship existed between liver Ugt1a9 expression and adiposity in male HS rats, but with BPF-exposure, male HS rats with lower liver Ugt1a9 expression showed increased adiposity. This is the first report of a relationship between a bisphenol metabolizing enzyme and a bisphenol-induced phenotype. The relationship between Ugt1a9 and BPF-induced adiposity is based on gene expression, however, gene expression may not extrapolate to protein expression or activity. Future studies will determine if liver Ugt1a9 protein expression and activity differ between vehicle- and BPF-exposed male HS rats and affect adiposity.
The HS rats are an exemplary model for population-based studies that represent human phenotypic and genotypic diversity seen in complex, quantitative traits. Furthermore, HS are ideal for quantitative genetic studies because of the careful breeding of unrelated individuals to create a genetically diverse rat population (Solberg Woods, 2014; Woods and Mott, 2017). Heritability of complex traits can differ between populations and depend on the population’s genetic makeup and the environment (Visscher et al., 2008). Estimations of narrow-sense heritability (h2), which describes the proportion of trait variance accounted for by additive genetic effects, incorporate genetic and environmental variance. Although the exposed cohort was intended to identify BPF-induced influences on body weight and adiposity, it was not intended to perform genetic mapping to identify the loci regulating BPF-induced phenotypes or to accurately estimate h2. However, to set the state for future genetic studies of BPF exposure in HS rats, we were able to calculate preliminary h2 estimates on significant phenotypes to estimate how much of a trait’s variability in the HS rats is due to interindividual genetic differences as opposed to environmental factors. The most striking difference in preliminary h2 estimations between vehicle- and BPF-exposed male HS rats was in body weight gain after 5 weeks of BPF exposure, where the BPF-exposed h2 was less than the vehicle-exposed h2 (vehicle-exposed mean = 0.91, SD = 0.14 and BPF-exposed mean = 0.69, SD = 0.25). These preliminary data suggest genetic variation in the HS population interacts with BPF (ie, GxE interactions) to impact this phenotype. Future work on defining the impact of genetic variation on BPF-exposure will be conducted in larger cohorts of HS rats.
This study had some experimental design limitations. It is well known that males and females can show different responses to endocrine disruptors, including BPA (Diamanti-Kandarakis et al., 2009; Yang et al., 2017), while our studies were limited to males. Epidemiological data suggest greater positive associations between BPF exposure and obesity in human juvenile males compared with females (Jacobson et al., 2019; Liu et al., 2019). Previous BPF research reports that exposure impacted both male and female zebrafish reproductive health but showed male-specific alternations in steroid hormone levels and hypothalamus-pituitary-gonadal axis and liver gene expression (Yang et al., 2017). Our study limited investigation to male rats at a comparable age to an at-risk human demographic. This study will be replicated using female HS rats to define the impact of BPF on female growth and metabolism. To assess BPF exposure of individual animals, this study singly housed the HS rats throughout the entire study. Isolation is known to impact animal behavior and can lead to anxiety and depression in rodents (Hurst et al., 1997). It is unclear how single housing impacted the HS males or if there are any interactions between isolation and BPF exposure affecting the phenotypes measured in this study.
In this proof-of-principle study, we found that 5 weeks of BPF exposure in HS rats through drinking water increased total body growth and increased abdominal adiposity, which are risk factors for cardiometabolic disease. We report that BPF exposure caused creatinine dysfunction in male HS rats. We also demonstrate that liver gene expression of Ugt1a9 is negatively related to adiposity degree in BPF-exposed male HS rats, possibly implicating xenobiotic metabolism in BPF-induced outcomes. Furthermore, we show preliminary evidence that BPF exposure may alter trait heritability in male HS rats, suggesting possible interactions between genetic background and bisphenol exposure. These findings support BPF exposure as a cardiometabolic disease risk factor and indicate that the HS rat will be a useful model for dissecting gene by BPF interactions on metabolic health.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ms Nicole Pearson for performing the calorimetry assay, Camille Taylor for performing the microalbumin assay, as well as Dr John Reho for performing the urine osmolality assay.
FUNDING
University of Iowa Environmental Health Sciences Research Center (NIH P30ES005605), the National Institutes of Health Predoctoral Training Grant (NIH T32GM008629), the Mechanisms of Health and Disease at the Behavioral and Biomedical Interface Training Program (NIH T32GM108540), and the National Institutes of Health Program Project Grant (NIH P01HL084207).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Al-Hiyasat A. S., Darmani H., Elbetieha A. M. (2004). Leached components from dental composites and their effects on fertility of female mice. Eur. J. Oral Sci. 112, 267–272. [DOI] [PubMed] [Google Scholar]
- Alam I., Koller D. L., Sun Q., Roeder R. K., Canete T., Blazquez G., Lopez-Aumatell R., Martinez-Membrives E., Vicens-Costa E., Mont C., et al. (2011). Heterogeneous stock rat: A unique animal model for mapping genes influencing bone fragility. Bone 48, 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger K. A., Sokoloff G., Rosenberg D. M., Palmer A. A., Millen K. J. (2009). Genetic variation and population substructure in outbred cd-1 mice: Implications for genome-wide association studies. PLoS One 4, e4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian C. M., Aronne L. J., Bessesen D. H., McDonnell M. E., Murad M. H., Pagotto U., Ryan D. H., Still C. D., Endocrine S.; Endocrine Society. (2015). Pharmacological management of obesity: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 100, 342–362. [DOI] [PubMed] [Google Scholar]
- Appari M., Channon K. M., McNeill E. (2018). Metabolic regulation of adipose tissue macrophage function in obesity and diabetes. Antioxid. Redox. Signal. 29, 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 48. [Google Scholar]
- Boucher J. G., Ahmed S., Atlas E. (2016). Bisphenol S induces adipogenesis in primary human preadipocytes from female donors. Endocrinology 157, 1397–1407. [DOI] [PubMed] [Google Scholar]
- Cabaton N., Chagnon M. C., Lhuguenot J. C., Cravedi J. P., Zalko D. (2006). Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J. Agric. Food Chem. 54, 10307–10314. [DOI] [PubMed] [Google Scholar]
- Cabaton N., Zalko D., Rathahao E., Canlet C., Delous G., Chagnon M. C., Cravedi J. P., Perdu E. (2008). Biotransformation of bisphenol F by human and rat liver subcellular fractions. Toxicol. In Vitro 22, 1697–1704. [DOI] [PubMed] [Google Scholar]
- Castro B., Sanchez P., Torres J. M., Ortega E. (2015). Bisphenol A, bisphenol F and bisphenol S affect differently 5alpha-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ. Res. 142, 281–287. [DOI] [PubMed] [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y. L., Wu Y., Widelka M. (2016). Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-a review. Environ. Sci. Technol. 50, 5438–5453. [DOI] [PubMed] [Google Scholar]
- Chianese R., Viggiano A., Urbanek K., Cappetta D., Troisi J., Scafuro M., Guida M., Esposito G., Ciuffreda L. P., Rossi F., et al. (2018). Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via sirt1 modulation. Sci. Rep. 8, 2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitre A. S., Polesskaya O., Holl K., Gao J., Cheng R., Bimschleger H., Garcia Martinez A., George T., Gileta A. F., Han W., . et al. (2020). Genome‐Wide Association Study in 3,173 Outbred Rats Identifies Multiple Loci for Body Weight, Adiposity, and Fasting Glucose. Obesity 28, 1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare M., Sorić M., Bovet P., Miranda J. J., Bhutta Z., Stevens G. A., Laxmaiah A., Kengne A.-P., Bentham J. (2019). The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Medicine 17, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J. P., Giudice L. C., Hauser R., Prins G. S., Soto A. M., Zoeller R. T., Gore A. C. (2009). Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 30, 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobna Z., Talarovicova A., Schrader H. E., Fennell T. R., Snyder R. W., Rissman E. F. (2019). Bisphenol F has different effects on preadipocytes differentiation and weight gain in adult mice as compared with bisphenol A and S. Toxicology 420, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusserre C., Mollergues J., Lo Piparo E., Smiesko M., Marin-Kuan M., Schilter B., Fussell K. (2018). Using bisphenol A and its analogs to address the feasibility and usefulness of the CALUX-PPARgamma assay to identify chemicals with obesogenic potential. Toxicol. In Vitro 53, 208–221. [DOI] [PubMed] [Google Scholar]
- Escalante B., , Sacerdoti D., , Davidian M. M., , Laniado-Schwartzman M., and , McGiff J. C. (1991). Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension (Dallas, Tex. : 1979) 17, 776–779. [DOI] [PubMed] [Google Scholar]
- Escalante B., , Sessa W. C., , Falck J. R., , Yadagiri P., and , Schwartzman M. L. (1989). Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. The Journal of Pharmacology and Experimental Therapeutics 248, 229–232. [PubMed] [Google Scholar]
- Esplugas R., MI L. L., Belles M., Serra N., Vallve J. C., Domingo J. L., Linares V. (2018). Renal and hepatic effects following neonatal exposure to low doses of bisphenol-A and (137) Cs. Food Chem. Toxicol. 114, 270–277. [DOI] [PubMed] [Google Scholar]
- Fang X., , VanRollins M., , Kaduce T. L., and , Spector A. A. (1995). Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. Journal of Lipid Research 36, 1236–1246. [PubMed] [Google Scholar]
- Festing M. F. (1993). Genetic variation in outbred rats and mice and its implications for toxicological screening. J. Exp. Anim. Sci. 35, 210–220. [PubMed] [Google Scholar]
- Fitzpatrick C. J., Gopalakrishnan S., Cogan E. S., Yager L. M., Meyer P. J., Lovic V., Saunders B. T., Parker C. C., Gonzales N. M., Aryee E., et al. (2013). Variation in the form of pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: Sign-tracking vs. Goal-tracking. PLoS One 8, e75042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T., Kawaguchi M., Furuta M., Fukushima A., Kimura F. (2004). Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology 29, 475–485. [DOI] [PubMed] [Google Scholar]
- Gileta A., Fitzpatrick C., Chitre A., Pierre C., Joyce E., Maguire R., McLeod A., Gonzales N., Williams A., Morrow J., . et al. (2018). Genetic characterization of outbred Sprague Dawley rats and utility for genome-wide association studies. bioRxiv. Available at: https://doi.org/10.1101/412924. Accessed September 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramec Skledar D., Troberg J., Lavdas J., Peterlin Masic L., Finel M. (2015). Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica 45, 511–519. [DOI] [PubMed] [Google Scholar]
- Grobe J. L. (2017). Comprehensive assessments of energy balance in mice. Methods Mol. Biol. 1614, 123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Wu J., Xu S., Zhang L., Fan D., Shi L., Wang J., Ji G. (2020). Bisphenol F exposure impairs neurodevelopment in zebrafish larvae (Danio rerio). Ecotoxicol. Environ. Saf. 188, 109870. [DOI] [PubMed] [Google Scholar]
- Han C., Hong Y. C. (2016). Bisphenol A, hypertension, and cardiovascular diseases: Epidemiological, laboratory, and clinical trial evidence. Curr. Hypertens. Rep. 18, 11. [DOI] [PubMed] [Google Scholar]
- Hanioka N., Oka H., Nagaoka K., Ikushiro S., Narimatsu S. (2011). Effect of UDP-glucuronosyltransferase 2B15 polymorphism on bisphenol A glucuronidation. Arch. Toxicol. 85, 1373–1381. [DOI] [PubMed] [Google Scholar]
- Hansen C., Spuhler K. (1984). Development of the national institutes of health genetically heterogeneous rat stock. Alcohol Clin. Exp. Res. 8, 477–479. [DOI] [PubMed] [Google Scholar]
- Holl K., He H., Wedemeyer M., Clopton L., Wert S., Meckes J. K., Cheng R., Kastner A., Palmer A. A., Redei E. E., et al. (2018). Heterogeneous stock rats: A model to study the genetics of despair-like behavior in adolescence. Genes Brain Behav. 17, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J. L., Barnard C. J., Nevison C. M., West C. D. (1997). Housing and welfare in laboratory rats: Welfare implications of isolation and social contact among caged males. Anim. Welf. 6, 329–347. [Google Scholar]
- Ijaz S., Ullah A., Shaheen G., Jahan S. (2019). Exposure of BPA and its alternatives BPB, BPF and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicol. Mech. Methods 30, 60–72. [DOI] [PubMed] [Google Scholar]
- Jacobson M. H., Woodward M., Bao W., Liu B., Trasande L. (2019). Urinary bisphenols and obesity prevalence among U.S. Children and adolescents. J. Endocr. Soc. 3, 1715–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabuto H., Amakawa M., Shishibori T. (2004). Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 74, 2931–2940. [DOI] [PubMed] [Google Scholar]
- Ke Z. H., Pan J. X., Jin L. Y., Xu H. Y., Yu T. T., Ullah K., Rahman T. U., Ren J., Cheng Y., Dong X. Y., et al. (2016). Bisphenol A exposure may induce hepatic lipid accumulation via reprogramming the DNA methylation patterns of genes involved in lipid metabolism. Sci. Rep. 6, 31331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele G. R., Prokop J. W., He H., Holl K., Littrell J., Deal A., Francic S., Cui L., Gatti D. M., Broman K. W., et al. (2018). Genetic fine-mapping and identification of candidate genes and variants for adiposity traits in outbred rats. Obesity (Silver Spring) 26, 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloting N., Bluher M. (2014). Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 15, 277–287. [DOI] [PubMed] [Google Scholar]
- Kobroob A., Peerapanyasut W., Chattipakorn N., Wongmekiat O. (2018). Damaging effects of bisphenol A on the kidney and the protection by melatonin: Emerging evidences from in vivo and in vitro studies. Oxid. Med. Cell Longev. 2018, 3082438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanecz I., Gelfand R., Masouminia M., Gharib S., Segura D., Vernet D., Rajfer J., Li D. K., Kannan K., Gonzalez-Cadavid N. F. (2014). Oral bisphenol A (BPA) given to rats at moderate doses is associated with erectile dysfunction, cavernosal lipofibrosis and alterations of global gene transcription. Int. J. Impot. Res. 26, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S. (2003). Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 45, 345–356. [DOI] [PubMed] [Google Scholar]
- Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. (2018). Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell Endocrinol. 475, 74–91. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Liu B., Gadogbe M., Bao W. (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. Adults and children: The national health and nutrition examination survey 2013-2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejonklou M. H., Dunder L., Bladin E., Pettersson V., Ronn M., Lind L., Walden T. B., Lind P. M. (2017). Effects of low-dose developmental bisphenol A exposure on metabolic parameters and gene expression in male and female fischer 344 rat offspring. Environ. Health Perspect. 125, 067018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Kannan K. (2013). Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J. Agric. Food Chem. 61, 4655–4662. [DOI] [PubMed] [Google Scholar]
- Liu B., Lehmler H. J., Sun Y., Xu G., Sun Q., Snetselaar L. G., Wallace R. B., Bao W. (2019). Association of bisphenol A and its substitutes, bisphenol F and bisphenol S, with obesity in United States children and adolescents. Diabetes Metab. J. 43, 59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke A. E., Kahali B., Berndt S. I., Justice A. E., Pers T. H., Day F. R., Powell C., Vedantam S., Buchkovich M. L., Yang J., et al. ; The LifeLines Cohort Study. (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowicz J. (2014). Bisphenol A–sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 37, 738–758. [DOI] [PubMed] [Google Scholar]
- Michałowicz J., Mokra K., Bąk A. (2015). Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol. In Vitro 29, 1464–1472. [DOI] [PubMed] [Google Scholar]
- Milner L. (2017). Chemical profiles: Europe Bisphenol A. In ICIS Chemical Business. Available at: https://www.icis.com/explore/resources/news/2017/02/02/10075664/chemical-profile-europe-bisphenol-a/. Accessed March 29, 2021. [Google Scholar]
- Motulsky H. J., Brown R. E. (2006). Detecting outliers when fitting data with nonlinear regression - A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics 7, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A., Strauss R. S. (1999). Risks and consequences of childhood and adolescent obesity. Int. J. Obes. Relat Metab. Disord. 23(Suppl. 2), S2–S11. [DOI] [PubMed] [Google Scholar]
- Nakamura D., Yanagiba Y., Duan Z., Ito Y., Okamura A., Asaeda N., Tagawa Y., Li C., Taya K., Zhang S.-Y., et al. (2010). Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett. 194, 16–25. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ogura K., Nakano H., Kaku T., Takahashi E., Ohkubo Y., Sekine K., Hiratsuka A., Kadota S., Watabe T. (2002). Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug Metab. Pharmacokinet. 17, 221–228. [DOI] [PubMed] [Google Scholar]
- Niwa T., Fujimoto M., Kishimoto K., Yabusaki Y., Ishibashi F., Katagiri M. (2001). Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol. Pharm. Bull. 24, 1064–1067. [DOI] [PubMed] [Google Scholar]
- Ohtani N., Iwano H., Suda K., Tsuji E., Tanemura K., Inoue H., Yokota H. (2017). Adverse effects of maternal exposure to bisphenol F on the anxiety- and depression-like behavior of offspring. J. Vet. Med. Sci. 79, 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olea-Herrero N., Arenas M. I., Munoz-Moreno C., Moreno-Gomez-Toledano R., Gonzalez-Santander M., Arribas I., Bosch R. J. (2014). Bisphenol-A induces podocytopathy with proteinuria in mice. J. Cell Physiol. 229, 2057–2066. [DOI] [PubMed] [Google Scholar]
- Peerapanyasut W., Kobroob A., Palee S., Chattipakorn N., Wongmekiat O. (2019). Activation of sirtuin 3 and maintenance of mitochondrial integrity by N-acetylcysteine protects against bisphenol A-induced kidney and liver toxicity in rats. Int. J. Mol. Sci. 20, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. 2004. Quantification strategies in real-time pcr. In The Real-Time PCR Encyclopedia A-Z of Quantitative PCR (Bustin S., Ed), pp. 87–112. International university Line; La Jolla, CA. [Google Scholar]
- Ranciere F., Lyons J. G., Loh V. H., Botton J., Galloway T., Wang T., Shaw J. E., Magliano D. J. (2015). Bisphenol A and the risk of cardiometabolic disorders: A systematic review with meta-analysis of the epidemiological evidence. Environ. Health 14, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rat Genome Sequencing and Mapping Consotorium.Baud A., Hermsen R., Guryev V., Stridh P., Graham D., McBride M. W., Foroud T., Calderari S., et al. (2013). Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat. Genet. 45, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. (2007). In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 24, 199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C. S. (2017). Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 47, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. S., Murray M. K., Damassa D. A., King J. C., Soto A. M. (2001). Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ. Health Perspect. 109, 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Kotnik P., Trontelj J., Knez Z., Masic L. P. (2013). Bioactivation of bisphenol A and its analogs (Bpf, Bpaf, BPZ and DMBPA) in human liver microsomes. Toxicol. In Vitro 27, 1267–1276. [DOI] [PubMed] [Google Scholar]
- Sheng H. P., Huggins R. A. (1979). A review of body composition studies with emphasis on total body water and fat. Am. J. Clin. Nutr. 32, 630–647. [DOI] [PubMed] [Google Scholar]
- Snyder R. W., Maness S. C., Gaido K. W., Welsch F., Sumner S. C., Fennell T. R. (2000). Metabolism and disposition of bisphenol A in female rats. Toxicol. Appl. Pharmacol. 168, 225–234. [DOI] [PubMed] [Google Scholar]
- Solberg Woods L. C. (2014). QTL mapping in outbred populations: Successes and challenges. Physiol. Genomics 46, 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Palmer A. A. (2019). Using heterogeneous stocks for fine-mapping genetically complex traits. Methods Mol. Biol. 2018, 233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Holl K. L., Oreper D., Xie Y., Tsaih S. W., Valdar W. (2012). Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol. Genomics 44, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods L. C., Stelloh C., Regner K. R., Schwabe T., Eisenhauer J., Garrett M. R. (2010). Heterogeneous stock rats: A new model to study the genetics of renal phenotypes. Am. J. Physiol. Renal Physiol. 298, F1484–F1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E., Schwitzgebel V. M., Toulotte A., Cederroth C. R., Combescure C., Nef S., Aubert M. L., Huppi P. S. (2009). Perinatal exposure to bisphenol A alters early adipogenesis in the rat. Environ. Health Perspect. 117, 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao T., Nanamiya W., Nagano I., Asaba K., Kawabata K., Hashimoto K. (1999). Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 65, 2351–2357. [DOI] [PubMed] [Google Scholar]
- Takao T., Nanamiya W., Nazarloo H. P., Matsumoto R., Asaba K., Hashimoto K. (2003). Exposure to the environmental estrogen bisphenol A differentially modulated estrogen receptor-alpha and -beta immunoreactivity and mRNA in male mouse testis. Life Sci. 72, 1159–1169. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Tong S., Yang S., Li T., Gao R., Hu J., Luo T., Qing H., Zhen Q., Hu R., Li X., et al. (2019). Role of neutrophil extracellular traps in chronic kidney injury induced by bisphenol-A. J. Endocrinol. doi: 10.1530/JOE-18-0608. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Trdan Lusin T., Roskar R., Mrhar A. (2012). Evaluation of bisphenol A glucuronidation according to UGT1A128 polymorphism by a new LC-MS/MS assay. Toxicology 292, 33–41. [DOI] [PubMed] [Google Scholar]
- Tsaih S. W., Holl K., Jia S., Kaldunski M., Tschannen M., He H., Andrae J. W., Li S. H., Stoddard A., Wiederhold A., et al. Meta-Analyses of Glucose and Insulin-Related Traits Consortium (MAGIC) Investigators. (2014). Identification of a novel gene for diabetic traits in rats, mice, and humans. Genetics 198, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A., Pirzada M., Afsar T., Razak S., Almajwal A., Jahan S. (2019a). Effect of bisphenol F, an analog of bisphenol A, on the reproductive functions of male rats. Environ. Health Prev. Med. 24, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah A., Pirzada M., Jahan S., Ullah H., Khan M. J. (2019b). Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health 35, 294–303. [DOI] [PubMed] [Google Scholar]
- Ullah A., Pirzada M., Jahan S., Ullah H., Shaheen G., Rehman H., Siddiqui M. F., Butt M. A. (2018a). Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere 209, 508–516. [DOI] [PubMed] [Google Scholar]
- Ullah A., Pirzada M., Jahan S., Ullah H., Turi N., Ullah W., Siddiqui M. F., Zakria M., Lodhi K. Z., Khan M. M. (2018b). Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 121, 24–36. [DOI] [PubMed] [Google Scholar]
- USEPA. (2010). Bisphenol A Action Plan (casrn 80-05-7). Available at: https://www.epa.gov/sites/production/files/2015-09/documents/bpa_action_plan.pdf. Accessed June 1, 2019.
- USEPA. (2011). Recommended use of body weight 3/4 as the default method in derivation of the oral reference dose. Office of the Science Advisor, Washington, DC. Risk Assessment Forum No. EPA/100/R11/0001. Available at: https://www.epa.gov/sites/production/files/2013-09/documents/recommended-use-of-bw34.pdf. Accessed September 28, 2020.
- van Esterik J. C., Dolle M. E., Lamoree M. H., van Leeuwen S. P., Hamers T., Legler J., van der Ven L. T. (2014). Programming of metabolic effects in C57BL/6JxFVB mice by exposure to bisphenol A during gestation and lactation. Toxicology 321, 40–52. [DOI] [PubMed] [Google Scholar]
- Verbanck M., Canouil M., Leloire A., Dhennin V., Coumoul X., Yengo L., Froguel P., Poulain-Godefroy O. (2017). Low-dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS One 12, e0179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. M., Hill W. G., Wray N. R. (2008). Heritability in the genomics era–concepts and misconceptions. Nat. Rev. Genet. 9, 255–266. [DOI] [PubMed] [Google Scholar]
- Vom Saal F. S., Vandenberg L. N. (2021). Update on the health effects of bisphenol A: Overwhelming evidence of harm. Endocrinology 162, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods L. C., Mott R. (2017). Heterogeneous stock populations for analysis of complex traits. Methods Mol. Biol. 1488, 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Wang X., Wu N., He S., Yi W., Xiang S., Zhang P., Xie X., Ying C. (2017). Bisphenol A induces proliferative effects on both breast cancer cells and vascular endothelial cells through a shared GPER-dependent pathway in hypoxia. Environ. Pollut. 231, 1609–1620. [DOI] [PubMed] [Google Scholar]
- Yang M., Chen M., Wang J., Xu M., Sun J., Ding L., Lv X., Ma Q., Bi Y., Liu R., et al. (2016). Bisphenol A promotes adiposity and inflammation in a nonmonotonic dose-response way in 5-week-old male and female C57BL/6J mice fed a low-calorie diet. Endocrinology 157, 2333–2345. [DOI] [PubMed] [Google Scholar]
- Yang Q., Yang X., Liu J., Ren W., Chen Y., Shen S. (2017). Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic-pituitary-gonadal axis of zebrafish. Environ. Sci. Pollut. Res. Int. 24, 21311–21322. [DOI] [PubMed] [Google Scholar]
- Ye X., Wong L. Y., Kramer J., Zhou X., Jia T., Calafat A. M. (2015). Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. Adults during 2000-2014. Environ. Sci. Technol. 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Ren X. M., Li Y. Y., Yao X. F., Li C. H., Qin Z. F., Guo L. H. (2018). Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environ. Pollut. 237, 1072–1079. [DOI] [PubMed] [Google Scholar]
- Zhu M., Chen X. Y., Li Y. Y., Yin N. Y., Faiola F., Qin Z. F., Wei W. J. (2018). Bisphenol F disrupts thyroid hormone signaling and postembryonic development in Xenopus laevis. Environ. Sci. Technol. 52, 1602–1611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.