Abstract
The viewpoint proposes a carbon-neutral biochemical cycle in the cytosol of plant leaves that is up-regulated by reactive oxygen species. Cycling provides NADPH and dissipates energy to counteract oxidative stress.
Keywords: Cytosolic oxidation–reduction cycle, energy metabolism, energy status, futile carbon cycling, glyceraldehyde-3-phosphate dehydrogenase, NADPH, oxidative stress, primary carbon metabolism, reactive oxygen species, redox status
Leaf cytosol contains non-phosphorylating and phosphorylating glyceraldehyde-3-phosphate dehydrogenase (np-GAPDH and p-GAPDH, respectively). From the viewpoint of carbon metabolism, np-GAPDH is redundant. However, mutants lacking np-GAPDH show significant metabolic adjustments and decreased growth, suggesting that np-GAPDH has central functions in plant metabolism. Here, I propose a cytosolic oxidation–reduction cycle. In its forward direction, np-GAPDH supplies NADPH. In the reverse direction, phosphoglycerate kinase and p-GAPDH consume ATP and NADH. Thus, the cytosolic oxidation–reduction cycle may constitute a central hub in energy metabolism.
In the light, NADPH is primarily synthesized in chloroplasts. However, NADPH is highly compartmentalized (Heber and Santarius, 1965). Chloroplastic NAD+ carrier proteins in reconstituted systems show low affinities for this reductant (Palmieri et al., 2009). Thus, NADPH export to the cytosol is not straightforward.
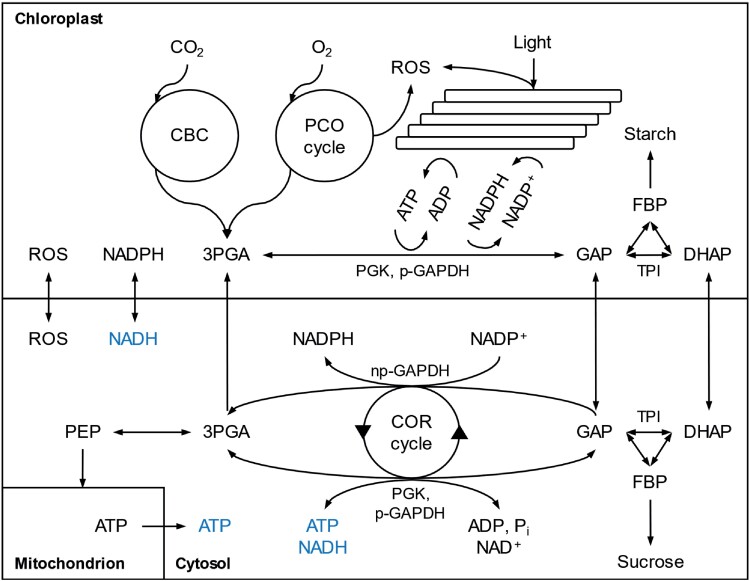
Currently, it is thought that glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and NADP+-dependent isocitrate dehydrogenase supply cytosolic NADPH (Geigenberger and Fernie, 2014). Additionally, carbon cycling around non-phosphorylating (np)-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was suggested to provide cytosolic NADPH (Fig. 1; Kelly and Gibbs, 1973a, b; Scagliarini et al., 1990). In this cycle, (i) dihydroxyacetone phosphate is exported from chloroplasts to the cytosol by the triose phosphate translocator and converted to glyceraldehyde 3-phosphate (GAP) by triose phosphate isomerase, (ii) GAP is oxidized to 3-phosphoglyceric acid (3PGA) and NADP+ is reduced to NADPH by np-GAPDH, and (iii) 3PGA is reimported into chloroplasts by the triose phosphate translocator and reduced to dihydroxyacetone phosphate by chloroplastic phosphoglycerate kinase (PGK) and phosphorylating (p)-GAPDH. A similar cycle involving p-GAPDH and PGK in the forward direction was suggested to provide cytosolic NADH and ATP (Fig. 1; Stocking and Larson, 1969). However, Heber and Santarius (1970) questioned its occurrence in vivo.
Fig. 1.
Carbon and energy metabolism in plant leaves. Parts of the PCO cycle reside outside chloroplasts, in peroxisomes and mitochondria; notably, ROS synthesizing glycolate oxidase is peroxisomal. Abbreviations: 3PGA, 3-phosphoglyceric acid; ADP, adenosine diphosphate; ATP, adenosine triphosphate; CBC, Calvin–Benson cycle; COR cycle, cytosolic oxidation–reduction cycle; DHAP, dihydroxyacetone phosphate; FBP, fructose 1,6-bisphosphate; GAP, glyceraldehyde 3-phosphate; np-GAPDH, non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase; PCO, photosynthetic carbon oxidation; PEP, phosphoenolpyruvate; p-GAPDH, phosphorylating glyceraldehyde-3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; Pi, inorganic phosphate; ROS, reactive oxygen species; TPI, triose phosphate isomerase.
Here, another route supplying cytosolic NADPH is proposed: the cytosolic oxidation–reduction (COR) cycle (Fig. 1). In its forward direction, cytosolic np-GAPDH oxidizes GAP to 3PGA and reduces NADP+ to NADPH. In the reverse direction, cytosolic PGK and p-GAPDH reduce 3PGA to GAP, dephosphorylate ATP to ADP and Pi, and oxidize NADH to NAD+. COR cycling was shown to be operational in reconstituted enzyme systems (Serrano et al., 1993; Arutyunov and Muronetz, 2003). Here, the COR cycle is discussed as an actual metabolic route with benefits for plant functioning. Specifically, I address (i) how differences in biochemical properties of np-GAPDH and p-GAPDH may promote COR cycling, (ii) how COR cycle requirements may be met, and (iii) physiological benefits of the COR cycle. My aim is to raise awareness that COR cycling may occur in vivo and encourage assessments of its metabolic feasibility, e.g. by flux network modelling (cf. Shameer et al., 2019) and the development of methodology enabling flux quantification such as stable isotope techniques.
Biochemical properties of GAPDH enzymes promote COR cycling
The cytosol of plant leaves contains two distinct GAPDHs. While np-GAPDH catalyses the irreversible conversion of GAP to 3PGA, p-GAPDH together with PGK catalyses the same reaction in a reversible manner (Fig. 1). Thus, from the viewpoint of carbon metabolism, np-GAPDH is redundant. However, np-GAPDH null mutants of Arabidopsis showed delayed growth compared with wild type (Rius et al., 2006). Additionally, these mutants exhibited 3.5-fold higher transcript levels, 5.8-fold higher mRNA levels, and a 2.5-fold increased activity of p-GAPDH, indicating a compensation effect for the lack of np-GAPDH. Thus, np-GAPDH seems to be required for optimal plant functioning and growth (Rius et al., 2006).
At the sequence level, np-GAPDH and p-GAPDH are entirely unrelated (Habenicht et al., 1994; Michels et al., 1994). I argue that a comparison of differences in enzyme properties may point to the physiological necessity for np-GAPDH.
GAPDH energetics, substrate affinity, and activity
Conversion of GAP to 3PGA by np-GAPDH is energetically favourable over conversion by p-GAPDH and PGK (ΔG0′=−22.1 versus −13.3 kcal mol−1, respectively). Additionally, np-GAPDH from various sources has an ~10-fold higher affinity for GAP than p-GAPDH (Km of np-GAPDH between 17 and 40 µM, Km of p-GAPDH between 239 and 400 µM; Rosenberg and Arnon, 1955; Kelly and Gibbs, 1973b; Duggleby and Dennis, 1974; Speranza and Gozzer, 1978; Iglesias and Losada, 1988; Scagliarini et al., 1990). Furthermore, np-GAPDH reportedly exceeded the activity of p-GAPDH in a cell-free extract from Pisum sativum shoots at in vivo levels of GAP and reductants (Kelly and Gibbs, 1973b). Since np-GAPDH exhibits a higher affinity for GAP, Flügge and Heldt (1984) hypothesized cytosolic NADPH synthesis by np-GAPDH may be prioritized over NADH and ATP synthesis by p-GAPDH and PGK. I argue that growth delays and substantial biochemical compensation effects in np-GAPDH null mutants (Rius et al., 2006) suggest a significant contribution of np-GAPDH to catalysing GAP to 3PGA conversions.
Reported concentrations of GAP and 3PGA in suspension-cultured cells of Catharanthus roseus correspond to equilibrium conditions around p-GAPDH and PGK (Kubota and Ashihara, 1990) even though np-GAPDH works against this equilibrium. Thus, p-GAPDH and PGK may readjust their disturbed equilibrium by more frequently catalysing the reverse reaction (3PGA to GAP) than the forward reaction (GAP to 3PGA), which results in COR cycling.
Activation of np-GAPDH by reactive oxygen species
In natural settings, the cellular redox balance is regularly disturbed, e.g. by low chloroplastic CO2 concentrations (Cc) due to drought. Low Cc promotes an energy excess in chloroplasts due to decreased consumption of ATP and NADPH by the Calvin–Benson cycle but continued electron input by light-harvesting complexes (Wilhelm and Selmar, 2011). The resulting lack of electron acceptors promotes the generation of reactive oxygen species (ROS) especially superoxide and H2O2. Additionally, low Cc promotes photorespiration and the generation of the photorespiratory side product H2O2. Under low Cc, photorespiration was estimated to generate >70% of all H2O2 (Noctor et al., 2002). Thus, low Cc promotes oxidative stress leading to increased oxidation of cytosolic NADPH by antioxidant systems. In vivo, the NADPH concentration proposedly exerts primary control over np-GAPDH activity with decreasing concentrations activating np-GAPDH (Kelly and Gibbs, 1973b; Iglesias and Losada, 1988; Scagliarini et al., 1990). Thus, decreasing NADPH due to oxidative stress at low Cc causes np-GAPDH activation and promotes the GAP to 3PGA forward reaction of COR cycling.
Inhibition of p-GAPDH by reactive oxygen species
np-GAPDH is 63 times less susceptible to inhibition by ROS than p-GAPDH, with H2O2 being a particularly potent inhibitor of p-GAPDH (Piattoni et al., 2013). Rising H2O2 levels are believed to progressively inhibit the reversible p-GAPDH (Hancock et al., 2005; Bedhomme et al., 2012; Piattoni et al., 2013). This is corroborated by reported increases of glycolytic downstream metabolites including 3PGA under oxidative conditions (Baxter et al., 2007; Lehmann et al., 2009, 2012; Rabara et al., 2017). At PGK and p-GAPDH, increased 3PGA levels would promote the reverse reaction (3PGA to GAP). Thus, the COR cycling flux mode may be promoted under oxidative conditions.
Interestingly, inactive p-GAPDH functions as transcription factor triggering the induction of genes encoding antioxidant enzymes. For more information on GAPDH regulation including moonlighting functions see Scheibe et al. (2019) and references therein.
Requirements of the COR cycle
Reverse reactions require ATP and NADH
COR cycling reverse reactions (3PGA to GAP) catalysed by PGK and p-GAPDH require cytosolic ATP and NADH (Fig. 1). This requirement may increase with oxidative stress, e.g. due to low Cc (see above). Much of the cytosolic ATP is believed to derive from the mitochondrial oxidation of photorespiratory glycine (Shameer et al., 2019). Low Cc promotes photorespiratory glycine oxidation. Moreover, low Cc is often associated with excess amounts of NADPH in chloroplasts (see above). Excess NADPH in chloroplasts is shuttled out to the cytosol by the malate valve as NADH. Flux through the valve is regulated strictly by the activity of chloroplastic malate dehydrogenase, which increases with [NADPH]/[NADP+] ratios (Fridlyand et al., 1998). Under normal conditions, flux is low, yet kinetic modelling predicts much enhanced rates at high [NADPH]/[NADP+] ratios, e.g. due to low Cc under drought (Fridlyand et al., 1998). Drought was shown to cause significantly increased activity of chloroplastic malate dehydrogenase in Triticum aestivum particularly under high light and moderate ambient CO2 levels of 350 ppm (Biehler et al., 1996). Thus, export of ATP and NADH to the cytosol is generally feasible. Mechanisms increasing ATP and NADH supply coincide with increased NADPH demands from COR cycling for ROS scavenging.
Retaining activity of p-GAPDH under oxidative conditions
Reactive oxygen species inhibit p-GAPDH (see above). If all p-GAPDH were in its oxidized inactive state, COR cycling would halt. Hence, cycling requires retained p-GAPDH activity. Bedhomme et al. (2012) proposed cytosolic glutaredoxin and thioredoxin-based mechanisms reversing oxidative deactivation of p-GAPDH. Thus, part of the p-GAPDH population is likely always active.
Benefits of COR cycling for plant functioning
COR cycling involves only three cytosolic enzymes and is carbon neutral. It requires no net carbon input, causes no net carbon loss, and does not produce carbon products that need to be consumed by other processes. Hence, COR cycling is independent of other parts of carbon metabolism as well as transmembrane transport of triose or pentose phosphates. This provides flexibility to its functions.
In vivo, NADPH concentration is believed to exert primary control over np-GAPDH activity (see above). Thus, COR cycling may help to maintain NADPH concentrations at high levels to steadily support all NADPH-consuming processes in the cytosol.
Optimal plant functioning requires a well-balanced energy supply versus consumption. Under most conditions, NADPH and ATP supply exceed metabolic demands, and several processes dissipating excess energy have been proposed (Wilhelm and Selmar, 2011). Export of reductant and ATP to the cytosol can remove excess energy from chloroplasts and mitochondria, respectively. COR cycling can dissipate this energy because each turn produces one molecule NADPH but consumes one molecule of ATP and NADH. Thus, COR cycling may counteract the generation of ROS. Additionally, NADPH from COR cycling may fuel cytosolic antioxidant systems and thus support ROS scavenging.
Glossary
Abbreviations
- 3PGA
3-phosphoglyceric acid
- COR
cytosolic oxidation–reduction
- GAP
glyceraldehyde 3-phosphate
- np-GAPDH
non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase
- p-GAPDH
phosphorylating glyceraldehyde-3-phosphate dehydrogenase
- PGK
phosphoglycerate kinase
- ROS
reactive oxygen species
References
- Arutyunov DY, Muronetz VI. 2003. The activation of glycolysis performed by the non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase in the model system. Biochemical and Biophysical Research Communications 300, 149–154. [DOI] [PubMed] [Google Scholar]
- Baxter CJ, Redestig H, Schauer N, Repsilber D, Patil KR, Nielsen J, Selbig J, Liu J, Fernie AR, Sweetlove LJ. 2007. The metabolic response of heterotrophic Arabidopsis cells to oxidative stress. Plant Physiology 143, 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedhomme M, Adamo M, Marchand CH, Couturier J, Rouhier N, Lemaire SD, Zaffagnini M, Trost P. 2012. Glutathionylation of cytosolic glyceraldehyde-3-phosphate dehydrogenase from the model plant Arabidopsis thaliana is reversed by both glutaredoxins and thioredoxins in vitro. The Biochemical Journal 445, 337–347. [DOI] [PubMed] [Google Scholar]
- Biehler K, Migge A, Fock HP. 1996. The role of malate dehydrogenase in dissipating excess energy under water stress in two wheat species. Photosynthetica 32, 431–438. [Google Scholar]
- Duggleby RG, Dennis DT. 1974. Nicotinamide adenine dinucleotide-specific glyceraldehyde 3-phosphate dehydrogenase from Pisum sativum: assay and steady state kinetics. Journal of Biological Chemistry 249, 167–174. [PubMed] [Google Scholar]
- Flügge UI, Heldt HW. 1984. The phosphate-triose phosphate-phosphoglycerate translocator of the chloroplast. Trends in Biochemical Sciences 9, 530–533. [Google Scholar]
- Fridlyand LE, Backhausen JE, Scheibe R. 1998. Flux control of the malate valve in leaf cells. Archives of Biochemistry and Biophysics 349, 290–298. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR. 2014. Metabolic control of redox and redox control of metabolism in plants. Antioxidants & Redox Signaling 21, 1389–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habenicht A, Hellman U, Cerff R. 1994. Non-phosphorylating GAPDH of higher plants is a member of the aldehyde dehydrogenase superfamily with no sequence homology to phosphorylating GAPDH. Journal of Molecular Biology 237, 165–171. [DOI] [PubMed] [Google Scholar]
- Hancock JT, Henson D, Nyirenda M, Desikan R, Harrison J, Lewis M, Hughes J, Neill SJ. 2005. Proteomic identification of glyceraldehyde 3-phosphate dehydrogenase as an inhibitory target of hydrogen peroxide in Arabidopsis. Plant Physiology and Biochemistry 43, 828–835. [DOI] [PubMed] [Google Scholar]
- Heber UW, Santarius KA. 1965. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochimica et Biophysica Acta 109, 390–408. [DOI] [PubMed] [Google Scholar]
- Heber U, Santarius KA. 1970. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Zeitschrift fur Naturforschung 25, 718–728. [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Losada M. 1988. Purification and kinetic and structural properties of spinach leaf NADP-dependent nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase. Archives of Biochemistry and Biophysics 260, 830–840. [DOI] [PubMed] [Google Scholar]
- Kelly GJ, Gibbs M. 1973a. A mechanism for the indirect transfer of photosynthetically reduced nicotinamide adenine dinucleotide phosphate from chloroplasts to the cytoplasm. Plant Physiology 52, 674–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly GJ, Gibbs M. 1973b. Nonreversible D-glyceraldehyde 3-phosphate dehydrogenase of plant tissues. Plant Physiology 52, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota K, Ashihara H. 1990. Identification of non-equilibrium glycolytic reactions in suspension-cultured plant cells. Biochimica et Biophysica Acta 1036, 138–142. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Laxa M, Sweetlove LJ, Fernie AR, Obata T. 2012. Metabolic recovery of Arabidopsis thaliana roots following cessation of oxidative stress. Metabolomics 8, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Schwarzländer M, Obata T, et al. 2009. The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Molecular Plant 2, 390–406. [DOI] [PubMed] [Google Scholar]
- Michels S, Scagliarini S, Della Seta F, Carles C, Riva M, Trost P, Branlant G. 1994. Arguments against a close relationship between non-phosphorylating and phosphorylating glyceraldehyde-3-phosphate dehydrogenases. FEBS Letters 339, 97–100. [DOI] [PubMed] [Google Scholar]
- Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH. 2002. Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Annals of Botany 89, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F, Rieder B, Ventrella A, et al. 2009. Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins. The Journal of Biological Chemistry 284, 31249–31259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattoni CV, Guerrero SA, Iglesias AA. 2013. A differential redox regulation of the pathways metabolizing glyceraldehyde-3-phosphate tunes the production of reducing power in the cytosol of plant cells. International Journal of Molecular Sciences 14, 8073–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabara RC, Tripathi P, Rushton PJ. 2017. Comparative metabolome profile between tobacco and soybean grown under water-stressed conditions. BioMed Research International 2017, 3065251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius SP, Casati P, Iglesias AA, Gomez-Casati DF. 2006. Characterization of an Arabidopsis thaliana mutant lacking a cytosolic non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase. Plant Molecular Biology 61, 945–957. [DOI] [PubMed] [Google Scholar]
- Rosenberg LL, Arnon DI. 1955. The preparation and properties of a new glyceraldehyde-3-phosphate dehydrogenase from photosynthetic tissues. The Journal of Biological Chemistry 217, 361–371. [PubMed] [Google Scholar]
- Scagliarini S, Trost P, Valenti V, Pupillo P. 1990. Glyceraldehyde 3-phosphate:NADP reductase of spinach leaves: steady state kinetics and effect of inhibitors. Plant Physiology 94, 1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R. 2019. Maintaining homeostasis by controlled alternatives for energy distribution in plant cells under changing conditions of supply and demand. Photosynthesis Research 139, 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Mateos MI, Losada M. 1993. ATP-driven transhydrogenation and ionization of water in a reconstituted glyceraldehyde-3-phosphate dehydrogenases (phosphorylating and non-phosphorylating) model system. Biochemical and Biophysical Research Communications 197, 1348–1356. [DOI] [PubMed] [Google Scholar]
- Shameer S, Ratcliffe RG, Sweetlove LJ. 2019. Leaf energy balance requires mitochondrial respiration and export of chloroplast NADPH in the light. Plant Physiology 180, 1947–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza ML, Gozzer C. 1978. Purification and properties of NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase from spinach leaves. Biochimica et Biophysica Acta 522, 32–42. [DOI] [PubMed] [Google Scholar]
- Stocking CR, Larson S. 1969. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochemical and Biophysical Research Communications 37, 278–282. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Selmar D. 2011. Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. Journal of Plant Physiology 168, 79–87. [DOI] [PubMed] [Google Scholar]