Abstract
This article comments on:
Fernández Farnocchia RB, Benech-Arnold RL, Mantese A, Batlla D. 2021. Optimization of timing of next-generation emergence in Amaranthus hybridus is determined via modulation of seed dormancy by the maternal environment. Journal of Experimental Botany 72, 4283–4297.
Keywords: Agricultural weed management, climate change, dormancy trait plasticity, maternal environment, soil seed bank dynamics, weed emergence timing
Predicting weed emergence in crop production systems is a global challenge that requires understanding mechanisms of weed ecology and trait evolution in response to climate change and altered agricultural practices. Seed dormancy is a highly adaptive trait that controls this by defining the environmental conditions in which the seed is able to germinate (Finch-Savage and Leubner-Metzger, 2006). Weed soil seed bank persistence and the timing of seedling emergence depend on dormancy (Baskin and Baskin, 2006; Walck et al., 2011; Batlla et al., 2020). Integrating mechanisms of seed dormancy dynamics in variable field environments and across generations with population-based models and realistic ecophysiological simulations (Fernández Farnocchia et al., 2021) are essential for more sustainable weed management strategies.
Charles Darwin wrote in his letter to Joseph Hooker (12 April 1857) ‘I have been interested in my ‘weed garden’ of 3×2 feet square: I mark each seedling as it appears, and I am astonished at number that come up.’ The timing of weed seedling emergence, often as seasonal flushes, has critical and agronomical implications as weeds produce the highest potential yield loss (30–40%) in the major crop production systems (Oerke, 2006). Weed soil seed bank dynamics depend on seed dormancy, a trait with high plasticity in weed species and with enormous adaptive value to adjust the population to a cropping system (Baskin and Baskin, 2006; Westwood et al., 2018; Schwartz-Lazaro and Copes, 2019; Batlla et al., 2020). The control of germination timing is achieved by seed dormancy, which can be considered as block(s) to the completion of germination of an intact viable seed under otherwise favourable conditions, namely after the seed becomes non-dormant (Finch-Savage and Leubner-Metzger, 2006). Primary dormancy is established during seed maturation prior to dispersal, whereas secondary dormancy refers to the acquisition of dormancy in a mature seed after dispersal and after the loss of primary dormancy (Graeber et al., 2012; Finch-Savage and Footitt, 2017; Penfield and MacGregor, 2017). The molecular mechanisms underpinning the seasonal seed dormancy cycling to time germination in variable field environments have been investigated with Arabidopsis thaliana ecotypes adapted to different climates (Finch-Savage and Footitt, 2017). Seeds continually adjust their dormancy status by sensing a range of environmental signals. Temperature is related to slow seasonal change and used for temporal sensing to determine the time of year and adjust the depth of dormancy accordingly. This alters seed sensitivity to signals related to the spatial environment, including light and soil moisture. The sensing of these signals is more ultimate as they indicate if conditions are suitable for germination and therefore trigger dormancy release. Molecular mechanisms and large-scale molecular datasets of A. thaliana seed dormancy states (see references in Finch-Savage and Footitt, 2017) and of weed trait plasticity (Maroli et al., 2018) require integration by using threshold population-based models and realistic ecophysiological simulations (Batlla and Benech-Arnold, 2014; Donohue et al., 2015; Finch-Savage and Footitt, 2017). These models and simulations provide an ecophysiological framework and are especially complex if seed dormancy regulation is investigated across weed generations to capture how it maximizes weed population fitness.
Using the summer annual weed Amaranthus hybridus (smooth pigweed), Fernández Farnocchia et al. (2021) provide a sophisticated and well-integrated analysis of how the primary dormancy level at dispersal established during maturation in different maternal environments synchronizes next-generation seedling emergence timing to maximize weed population fitness. Nine Amaranthus (pigweed) species, including A. hybridus, A. retroflexus, and A. palmeri, are listed as invasive or noxious weeds, with Palmer amaranth being the most troublesome herbicide-resistant weed in south-eastern USA (Trucco et al., 2009; Ward et al., 2013; Assad et al., 2017). Fernández Farnocchia et al. (2021) found that primary seed dormancy depth was lower when harvested from late season mother plants where seed maturation occurred in a short photoperiod maternal environment (Box 1). However, these observed variations in dormancy depth in the laboratory experiments did not affect seedling emergence timing in the field experiments. To interpret these results, Fernández Farnocchia et al. (2021) developed threshold population-based models and performed realistic simulations which generated a better ecophysiological framework for predicting seedling emergence patterns under natural conditions. Their major conclusion is that it is crucial to consider the effects of distinct maternal environments leading to variations in the depth of primary dormancy for correctly predicting weed soil seed bank dynamics, and how these contribute to the synchronization of next-generation emergence timing to maximize population fitness. Other well-investigated examples where regulation of seed dormancy by the maternal environment, in particular photoperiod and temperature during maturation (Box 2), was instrumental for maximizing population fitness in the field are A. thaliana (MacGregor et al., 2015; Huang et al., 2018; Footitt et al., 2020) and the weed Polygonum aviculare (Batlla and Benech-Arnold, 2014; Fernández Farnocchia et al., 2019; Batlla et al., 2020).
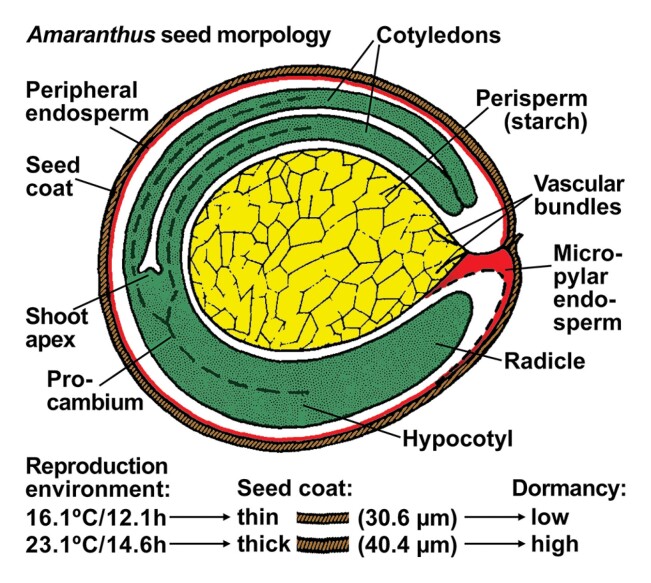
Box 1. Amaranthus seed structure with peripheral embryo and perisperm, and maternal effects on seed cost thickness.
The typical seed of the Amaranthaceae and of many other core Caryophyllales families evolved in the early Cretaceous and is characterized by a peripheral embryo curved around a central starchy perisperm (dead storage tissue) (Baskin and Baskin, 2019). Most Amaranthus species, including the weeds A. hybridus, A. retroflexus, and A. palmeri, and the amaranth food crops A. caudatus and A. cruentus, disperse seeds from one-seeded dehiscent fruits which open at maturity (Irving et al., 1981; Trucco et al., 2009; Ward et al., 2013; Assad et al., 2017; Ninfali et al., 2020; Fernández Farnocchia et al., 2021). The inner seed coat consists of a sclerified parenchyma layer with osteosclereids on either side. The outer seed coat layer of palisade sclereids can vary considerably in thickness. Fernández Farnocchia et al. (2021) found that the maternal environment during seed maturation on the mother plant determined seed coat thickness and depth of primary physiological dormancy. The seed coat morphological and physicochemical properties are most important for mediating the interactions between the embryo and the ambient environment. Other core Caryophyllales species with perispermic seeds disperse one-seeded indehiscent fruits (Sukhorukov et al., 2015) in which the fruit coat (pericarp) properties serve this role; an example for this from the Amaranthaceae family is sugar beet (Hermann et al., 2007). The maternal environment during reproduction also affects the primary dormancy depth of the dispersed fruits of the Caryophyllales (Polygonaceae) weed P. aviculare (Fernández Farnocchia et al., 2019), but the possible effects on pericarp properties have not been investigated. The figure shows a drawing of A. cruentus seed structure modified from Irving et al. (1981), with permission from the publisher John Wiley and Sons; seed coat thickness of A. hybridus from Fernández Farnocchia et al. (2021).
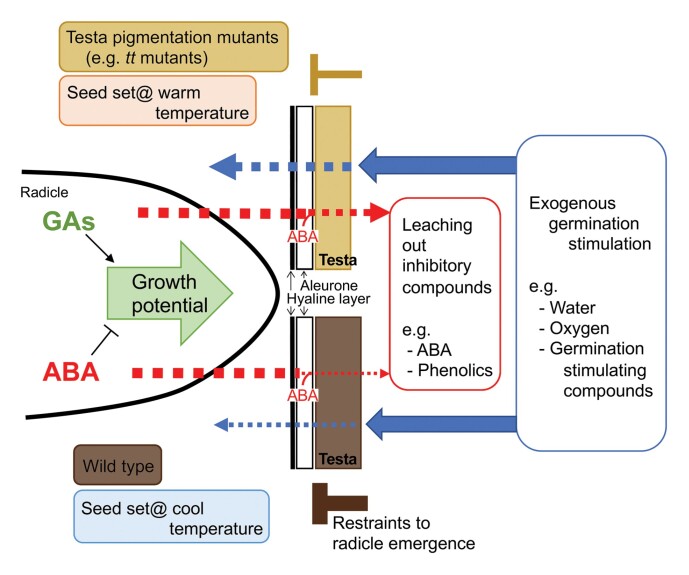
Box 2. Physicochemical seed coat properties determine the flux of compounds required for the control of germination.
Physicochemical properties of seed and fruit coats have been shown to play important roles in the control of seed germination by providing permeability and/or mechanical restraints on germination processes (Steinbrecher and Leubner-Metzger, 2017). The outer seed coverings consist mostly of dead tissues and represent the seed’s interphase with the external environment. In addition to providing mechanical restraint, coat-associated mechanisms control or even prevent water uptake, leaching of inhibitors for embryo elongation such as abscisic acid (ABA), or gaseous exchange which may cause oxygen deficiency within the embryo. An excellent example to illustrate this are the transparent testa (tt) mutants of Arabidopsis thaliana which exhibit lighter testa (seed coat) colour (see figure) due to defects in flavonoid metabolism and in turn reduced proanthocyanidin biosynthesis (Lepiniec et al., 2006). During A. thaliana seed coat development, proanthocyanidins accumulate in the endothelium, the innermost cell layer of the inner integument, while the outermost cell layer of the outer integument differentiates into mucilage-producing cells; and at seed maturity the testa consists entirely of dead tissue with oxidized proanthocyanidins as brownish pigments. In many mutants, reduced pigmentation often led to thinner testa and increased permeability for hormones or other compounds (see figure), and this was associated with reduced dormancy phenotypes of the tt mutants (Debeaujon et al., 2000). Flavonoid biosynthesis during seed coat development was shown to be higher when seeds were matured in cool conditions (see figure), which was associated with a less permeable testa and increased primary dormancy (MacGregor et al., 2015). Furthermore, the seed coat of many tt mutants remained permeable even when matured under low temperature. These results clearly indicate that temperature regulation for increased primary dormancy involves altering testa properties by accumulation of flavonoids. Increased permeability not only permits a greater influx of water and oxygen, but also allows leaching out of endogenous compounds which are inhibitory to germination or embryo growth (see figure). Similarly to Arabidopsis where temperature has been demonstrated to be a major factor, the maternal environmental signalling and dormancy control in Amaranthus seem to be an interaction between embryo and seed coat, with photoperiod during reproduction as the major factor (Fernández Farnocchia et al., 2021).
Of particular interest from a mechanistic point of view is the finding that the maternal environment, photoperiod (Fernández Farnocchia et al., 2021) and temperature (MacGregor et al., 2015), affected primary dormancy depths, at least in part, by altering seed coat morphological (Box 1) and physicochemical (Box 2) properties. In species with coat-imposed dormancy, the seed and fruit coat properties are a decisive component of this trait (Finch-Savage and Leubner-Metzger, 2006; Lepiniec et al., 2006; Steinbrecher and Leubner-Metzger, 2017; Francoz et al., 2018). In cereal grains and in A. thaliana, proanthocyanidins (tannins, brownish pigments) accumulate during seed coat development on the mother plant. The extent of this and thereby primary dormancy depths varies with temperature during seed production (MacGregor et al., 2015), and transparent testa (tt) mutants (Debeaujon et al., 2000) have reduced dormancy and altered permeability properties (Box 2). The typical seed of the Amaranthaceae (Box 1) and of many other Caryophyllales species has a peripheral embryo curved around a central starchy perisperm (dead storage tissue) evolved in the early Cretaceous (Baskin and Baskin, 2019). Amaranthus hybridus seed coat thickness and primary dormancy depths were affected by the reproduction environment on the mother plant (Fernández Farnocchia et al., 2021). Dormancy is, however, not the only trait affected by seed coat thickness: a comparison of several weed species demonstrated that seed mortality in the soil seed bank is related to seed coat thickness (Gardarin et al., 2010). In this work, the estimated annual seed mortality rates in the soil seed bank and the associated seed coat thicknesses of A. hybridus and A. thaliana were very similar, ranking in the middle tier of 18 species. Seed coats are indeed more than a protective shield formed of dead cell layers (Francoz et al., 2018). They play important roles in seed germination, dormancy, longevity, and the persistence of the soil seed bank. As maternal tissues, the interaction between the mother plant’s genotype and the maternal environment during reproduction is decisive in maximizing population fitness across generations. This knowledge is required not only for developing more sustainable weed management strategies (Westwood et al., 2018), but also for better understanding of the underpinning mechanisms of trait plasticity and adaptive evolution upon environmental change.
Acknowledgements
The authors thank Diego Batlla (Ciudad de Buenos Aires, Argentina) for interesting discussions about environmental control of weed seed dormancy. We also acknowledge the financial support of our research projects into weed seed dormancy and seed proanthocyandins by the Biotechnology and Biological Sciences Research Council (BBSRC, grant nos BB/M02203X/1 and BB/M000583/1). For expert information about seed dormancy please visit ‘The Seed Biology Place’ - www.seedbiology.eu.
References
- Assad R, Reshi ZA, Jan S, Rashid I. 2017. Biology of amaranths. Botanical Review 83, 382–436. [Google Scholar]
- Baskin CC, Baskin JM. 2006. The natural history of soil seed banks of arable land. Weed Science 54, 549–557. [Google Scholar]
- Baskin CC, Baskin JM. 2019. Martin’s peripheral embryo—unique but not a phylogenetic ‘orphan’ at the base of his family tree: a tribute to the insight of a pioneer seed biologist. Seed Science Research 29, 155–166. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2014. Weed seed germination and the light environment: implications for weed management. Weed Biology and Management 14, 77–87. [Google Scholar]
- Batlla D, Ghersa CM, Benech-Arnold RL. 2020. Dormancy, a critical trait for weed success in crop production systems. Pest Management Science 76, 1189–1194. [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. 2000. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiology 122, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Burghardt LT, Runcie D, Bradford KJ, Schmitt J. 2015. Applying developmental threshold models to evolutionary ecology. Trends in Ecology & Evolution 30, 66–77. [DOI] [PubMed] [Google Scholar]
- Fernández Farnocchia RBF, Benech-Arnold RL, Batlla D. 2019. Regulation of seed dormancy by the maternal environment is instrumental for maximizing plant fitness in Polygonum aviculare. Journal of Experimental Botany 70, 4793–4805. [DOI] [PubMed] [Google Scholar]
- Fernández Farnocchia RB, Benech-Arnold RL, Mantese A, Batlla D. 2021. Optimization of timing of next-generation emergence in Amaranthus hybridus is determined via modulation of seed dormancy by the maternal environment. Journal of Experimental Botany 72, 4283–4297. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Footitt S. 2017. Seed dormancy cycling and the regulation of dormancy mechanisms to time germination in variable field environments. Journal of Experimental Botany 68, 843–856. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171, 501–523. [DOI] [PubMed] [Google Scholar]
- Footitt S, Walley PG, Lynn JR, Hambidge AJ, Penfield S, Finch-Savage WE. 2020. Trait analysis reveals DOG1 determines initial depth of seed dormancy, but not changes during dormancy cycling that result in seedling emergence timing. New Phytologist 225, 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francoz E, Lepiniec L, North HM. 2018. Seed coats as an alternative molecular factory: thinking outside the box. Plant Reproduction 31, 327–342. [DOI] [PubMed] [Google Scholar]
- Gardarin A, Dürr C, Mannino MR, Busset H, Colbach N. 2010. Seed mortality in the soil is related to seed coat thickness. Seed Science Research 20, 243–256. [Google Scholar]
- Graeber K, Nakabayashi K, Miatton E, Leubner-Metzger G, Soppe WJ. 2012. Molecular mechanisms of seed dormancy. Plant, Cell & Environment 35, 1769–1786. [DOI] [PubMed] [Google Scholar]
- Hermann K, Meinhard J, Dobrev P, Linkies A, Pesek B, Heß B, Machackova I, Fischer U, Leubner-Metzger G. 2007. 1-Aminocyclopropane-1-carboxylic acid and abscisic acid during the germination of sugar beet (Beta vulgaris L.)—a comparative study of fruits and seeds. Journal of Experimental Botany 58, 3047–3060. [DOI] [PubMed] [Google Scholar]
- Huang Z, Footitt S, Tang A, Finch-Savage WE. 2018. Predicted global warming scenarios impact on the mother plant to alter seed dormancy and germination behaviour in Arabidopsis. Plant, Cell & Environment 41, 187–197. [DOI] [PubMed] [Google Scholar]
- Irving DW, Betschart AA, Saunders RM. 1981. Morphological studies on Amaranthus cruentus. Journal of Food Science 46, 1170–1174. [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. 2006. Genetics and biochemistry of seed flavonoids. Annual Review of Plant Biology 57, 405–430. [DOI] [PubMed] [Google Scholar]
- MacGregor DR, Kendall SL, Florance H, Fedi F, Moore K, Paszkiewicz K, Smirnoff N, Penfield S. 2015. Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytologist 205, 642–652. [DOI] [PubMed] [Google Scholar]
- Maroli AS, Gaines TA, Foley ME, Duke SO, Dogramaci M, Anderson JV, Horvath DP, Chao WS, Tharayil N. 2018. Omics in weed science: a perspective from genomics, transcriptomics, and metabolomics approaches. Weed Science 66, 681–695. [Google Scholar]
- Ninfali P, Panato A, Bortolotti F, Valentini L, Gobbi P. 2020. Morphological analysis of the seeds of three pseudocereals by using light microscopy and ESEM-EDS. European Journal of Histochemistry 64, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerke E-C. 2006. Centenary Review: Crop losses to pests. Journal of Agricultural Science 144, 31–43. [Google Scholar]
- Penfield S, MacGregor DR. 2017. Effects of environmental variation during seed production on seed dormancy and germination. Journal of Experimental Botany 68, 819–825. [DOI] [PubMed] [Google Scholar]
- Schwartz-Lazaro LM, Copes JT. 2019. A review of the soil seedbank from a weed scientists perspective. Agronomy 9, 369. [Google Scholar]
- Steinbrecher T, Leubner-Metzger G. 2017. The biomechanics of seed germination. Journal of Experimental Botany 68, 765–783. [DOI] [PubMed] [Google Scholar]
- Sukhorukov AP, Mavrodiev EV, Struwig M, Nilova MV, Dzhalilova KK, Balandin SA, Erst A, Krinitsyna AA. 2015. One-seeded fruits in the core Caryophyllales: their origin and structural diversity. PLoS One 10, e0117974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco F, Tatum T, Rayburn AL, Tranel PJ. 2009. Out of the swamp: unidirectional hybridization with weedy species may explain the prevalence of Amaranthus tuberculatus as a weed. New Phytologist 184, 819–827. [DOI] [PubMed] [Google Scholar]
- Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. 2011. Climate change and plant regeneration from seed. Global Change Biology 17, 2145–2161. [Google Scholar]
- Ward SM, Webster TM, Steckel LE. 2013. Palmer amaranth (Amaranthus palmeri): a review. Weed Technology 27, 12–27. [Google Scholar]
- Westwood JH, Charudattan R, Duke SO, Fennimore SA, Marrone P, Slaughter DC, Swanton C, Zollinger R. 2018. Weed management in 2050: perspectives on the future of weed science. Weed Science 66, 275–285. [Google Scholar]


