Abstract
Background
Distinctions between HHV-6 primary infection in seronegative patients and HHV-6 reactivation in seropositive patients remains largely undescribed in pediatric liver transplant (LT) recipients.
Methods
We implemented pretransplant serology testing of HHV-6 in a large pediatric hospital and retrospectively assessed the incidence, manifestations and outcomes of HHV-6 infections over a 3-year period.
Results
Among 101 pediatric LT recipients, 96 had pretransplant HHV-6 serologies; 34 (35.4%) were seronegative and 62 (64.6%) seropositive. Posttransplantation, 8/25 (32%) seronegative patients had HHV-6 DNAemia (primary infection) compared to 2/48 (4%) seropositive patients (p=0.002). Compared to seropositive patients, seronegative patients with HHV-6 DNAemia were younger, and had symptoms of fever and/or elevated aminotransferases in association with higher viral loads, in the first month post-transplant. More than 90% of seronegative patients and 77.8% of seropositive patients had HHV-6 detected by PCR in liver biopsy obtained for concerns of allograft rejection, but most had no detectable concomitant DNAemia. Active replication of virus in the liver was confirmed by in situ hybridization in select cases. While HHV-6 infection occurred among patients on prophylaxis doses of antivirals for CMV, HHV-6 DNAemia and presenting symptoms resolved on treatment doses.
Conclusions
HHV-6 DNA-emia occurred more frequently in seronegative pediatric LT recipients, usually in the early posttransplant period, and was subsequently detected in allograft biopsies. HHV-6 cannot be ruled out as a cause of hepatitis in the absence of allograft tissue testing and specialized virological assays, as HHV-6 may disrupt local allograft immune homeostasis while evading traditional screening methods using blood or plasma. The assessment of pre-transplant HHV-6 serological status may be important for risk stratification and post-transplant management of pediatric LT recipients
Keywords: antiviral, HHV-6, ISH, PCR, serology
Human herpesvirus 6 (HHV-6) may be cause of clinical illness in pediatric liver transplant (LT) recipients. Cytomegalovirus prophylaxis does not prevent HHV-6 DNAemia. The assessment of pretransplant HHV-6 serologies may be important for risk stratification and management of LT recipients.
Human herpesvirus 6 (HHV-6) is the collective name for 2 viral species (HHV-6A and HHV-6B) [1] within the subfamily Betaherpesvirinae, of which cytomegalovirus (CMV) and HHV-7 are also members. Primary HHV-6 infection in immunocompetent hosts results in roseola (exanthema subitum) or self-limited febrile illness with seroconversion usually by 2 years of age [2, 3].
HHV-6 has a tropism for T lymphocytes, although it can establish latency in many cell types throughout the body [4–8]. Immunosuppression associated with liver transplantation (LT), which target T lymphocytes to prevent allograft rejection, can cause herpesvirus reactivation, adding to the posttransplant burden. Posttransplant clinical complications such as hepatitis [9], interstitial pneumonitis, encephalitis, bone marrow suppression, and allograft rejection have been associated with HHV-6 infections [6]. HHV-6 also predisposes immunosuppressed hosts to CMV and HHV-7 reactivations after LT [10]. Additionally, HHV-6 is unique in its ability to integrate its genome into the human chromosome, which can be inherited in a Mendelian fashion (vertical transmission) and also lead to viral transmission from donor allograft (horizontal transmission) [6, 11].
The reported incidence of HHV-6 infection after LT ranges widely, from 4.3% to 54.5%, likely depending on pretransplant serostatus, the level of immunosuppression, and sensitivity of laboratory methods used to detect the circulating virus. Additionally, routine CMV prophylaxis can affect the frequency of HHV-6 DNAemia in LT recipients, although its impact on clinically significant HHV-6 disease may be limited [6]. Though clinical features of HHV-6 infection after adult LT have been described and there are evolving pediatric data on the burden of HHV-6 infection in LT [12], further studies are required to document true clinical relevance and outcome of HHV-6 infection in pediatric transplant patients. In addition, data on comprehensive pretransplant HHV-6 serologies and posttransplant testing for HHV-6 in liver tissue by polymerase chain reaction (PCR) and concurrent plasma PCR are limited. Our objectives were to determine pretransplant HHV-6 seroprevalence and retrospectively assess the incidence, clinical manifestations, histopathology, and outcomes of HHV-6 infection in pediatric patients after LT in a large pediatric transplant center.
METHODS
Data Collection and Study Design
This study was approved by the Institutional Review Board at Baylor College of Medicine. From January 2013 to December 2015, 101 pediatric patients less than 18 years of age underwent deceased donor pediatric LT at Texas Children’s Hospital, Baylor College of Medicine. We have incorporated routine pretransplant serological testing for HHV-6 since 2013 to classify patients as seronegative or seropositive as part of our clinical practice. This practice was based on our assessment that HHV-6 is relevant in this population given our previous experience with adverse outcomes of a pediatric LT patient with HHV-6 DNAemia [11, 13]. We retrospectively obtained demographic characteristics, relevant laboratory data, hospital length of stay at LT, posttransplant complications (infection and rejection), and graft and patient outcomes from electronic medical records of the patients during the study period. Mean follow-up was 2.3 years posttransplant. Additionally, we obtained data on plasma HHV-6 PCR, liver biopsy tissue HHV-6 PCR, as well as data on HHV-7, CMV, and Epstein-Barr virus (EBV) PCR when available. Routine surveillance testing for CMV and EBV was performed every 1–2 weeks for the first 3 months after transplantation and subsequently once every 1–2 months for the first-year posttransplant during outpatient clinic visits. Plasma HHV-6 DNA was tested posttransplantation by quantitative PCR (range 188 copies/mL plasma to 1 × 108 copies/mL plasma, Viracor Laboratories Inc., USA) when there was a clinical concern for infection and at the time of evaluation of allograft rejection by liver biopsy. In our institution, liver tissue is only obtained in posttransplant patients with rising liver indices or when there is suspicion for allograft rejection, that is, “for-cause,” with tissue HHV-6 qualitative PCR testing, as well as CMV, EBV, and adenovirus.
Per institution protocol, patients received anti-CMV prophylaxis based on risk factors. Low- and moderate-risk patients (donor−/recipient−, donor−/recipient+, and donor+/recipient+) received 7 days of 5 mg/kg once per day IV ganciclovir, then switched to complete 12 weeks of oral valganciclovir, while high-risk patients (donor+/recipient−) received 14 days of intravenous (IV), ganciclovir and 6 months of oral valganciclovir. All patients received corticosteroids and tacrolimus as part of initial posttransplant immunosuppression.
We compared posttransplant incidence of HHV-6 infection and subsequent graft and clinical outcomes of children who were seropositive for HHV-6 before transplantation (HHV-6 IgG Immunoglobulin G [IgG], positive) with those who were seronegative (HHV-6 IgG negative) during the same period.
Definitions
HHV-6 “DNAemia” was defined as the quantification of DNA in plasma of patients (>188 copies/ml), by PCR-based techniques to measure the virus load. Primary infection was defined as the detection of HHV-6 DNAemia in seronegative patients (pretransplant IgG negative). Reactivation was defined as HHV-6 DNAemia post-LT in previously seropositive patients (IgG positive). Symptomatic infection was defined as the presence of signs and symptoms concerning for infection (eg, fever, hepatitis, and rash) in the absence of other causes, in patients with positive microbiological cultures or viral PCRs. Allograft rejection episodes were defined as liver biopsy-proven rejection based on the evaluation by a pathologist, with grading using the Rejection Activity Index (RAI) score and managed by increased immunosuppression.
Pathology
A single pathologist (blinded to HHV-6 testing data and allograft rejection data) retrospectively reviewed each patient’s initial posttransplantation allograft biopsies tested for HHV-6. Biopsies were scored for RAI (grading from 0 to 9 based on Banff criteria), lobular inflammation (grade 0-3, based on nonalcoholic fatty liver disease data), and central venulitis (present or absent, based on Hepatitis C virus studies) to find if there were histological characteristics of HHV-6 infection in patients who had acute cellular rejection (ACR) along with positive HHV-6 tissue PCR, ACR with negative HHV-6 tissue PCR, and no ACR but with positive HHV-6 tissue PCR [14]. Patients who underwent a biopsy but were not tested for HHV-6 by tissue PCR were excluded from this arm of the study. HHV-6 small noncoding RNA U14 is only expressed when there is active replication of HHV-6 [15–17]. We performed in situ hybridization (ISH) on formalin-fixed paraffin-embedded tissue slides against small noncoding RNA U14 in a subset of patients (with available additional biopsy slides) to further delineate if the HHV-6 found positive on tissue PCR were actively replicating to truly document the burden of tissue infection and viral reactivation [15].
Statistical Analysis
We summarized demographic, clinical characteristics, and complications in post-LT using frequency (%) and median (25th and 75th percentiles). HHV-6-seropositive and HHV-6-seronegative groups were compared using Fisher’s exact test or Mann-Whitney U test. Posttransplant complications were also compared by primary diagnosis using Fisher’s exact test, time to event analysis, or Kruskal-Wallis test as appropriate.
Data analysis was performed using STATA13 (StataCorp LP, College Station, TX) and GraphPad Prism 7.0 (La Jolla, California). A P-value of <.05 was considered statistically significant for our analyses.
RESULTS
In our cohort, 101 patients underwent LT over a 3-year period (January 2013-December 2015; Table 1). The median age of the patients at transplant was 3.1 years and 50 of the 101 (49.5%) patients were females. Biliary atresia (BA) was the most common indication for transplantation (36%), and the median time for follow-up was 2.3 years (interquartile range [IQR]: 1.59, 2.97). Pretransplant HHV-6 IgG testing was performed in 97 of the 101 (96%) patients; 1 patient had an equivocal result; the remaining 96 patients were assigned into 1 of 2 groups: HHV-6 seropositive (IgG positive) or HHV-6 seronegative (IgG negative). None of the patients tested positive for HHV-6 IgM nor did any receive antiviral treatment for HHV-6 or CMV prior to transplant. Every patient received CMV prophylaxis posttransplantation as outlined in the Methods section, and none of the patients required treatment for CMV in the immediate peri-transplant time period. The median donor age was 7.5 in the seropositive and 4 years in the seronegative group.
Table 1.
Demographics of Patients Who Underwent Liver Transplantation (LT) Over a 3-Year Period Classified by Human Herpesvirus 6 (HHV-6) Serostatus Prior to LT (January 2013–December 2015)
| Characteristics | Overalla (n = 101) | HHV-6 Seropositive (n = 62) | HHV-6 Seronegative (n = 34) | P-valuec |
|---|---|---|---|---|
| Female | 50 (49.5%) | 31 (50%) | 17 (50%) | .99 |
| Median age in years (IQR) | 3.12 (1.2, 9.2) | 5.7 (1.6, 13.1) | 1.6 (0.79, 2.7) | <.0001 |
| Median donor age, years (IQR) | 7 (3, 16) | 7.5 (3, 16) | 4 (2,12.8) | .2 |
| Race | .48 | |||
| African American | 15 (14.9%) | 10 (16%) | 4 (11.8%) | |
| Caucasian | 38 (37.6%) | 23 (37%) | 14 (41.2%) | |
| Hispanic | 38 (37.6%) | 21 (34%) | 14 (41.2%) | |
| Asian | 5 (4.9%) | 5 (8%) | 0 | |
| Unknown | 5 (4.9%) | 3 (4.8%) | 2 (5.8%) | |
| Primary liver disease, N (%) | .04 | |||
| Biliary atresia | 36 (35.6%) | 20 (32%) | 16 (47%) | |
| Other cholestatic diseaseb | 15 (14.8%) | 11 (17.7%) | 4 (11.7%) | |
| Fulminant liver failure | 7 (6.9%) | 5 (8%) | 0 | |
| Autoimmune disease | 5 (4.9%) | 3 (4.8%) | 0 | |
| Metabolic | 13 (12.9%) | 5 (8%) | 8 (23.5%) | |
| Tumors/cancer | 15 (14.8%) | 9 (14.5%) | 5 (14.7%) | |
| Others | 10 (9.9%) | 9 (14.5%) | 1 (2.9%) | |
| Insurance private, N (%) | 39 (38.6%) | 23 (37.1%) | 13 (38.2%) | .97 |
aSerology was unavailable for 4 patients and equivocal for 1 patient.
bOther cholestatic liver diseases include progressive familial intrahepatic cholestasis (PFICs), Alagille syndrome, Alpha-1 antitrypsin deficiency, Wilson disease, and cystic fibrosis.
cComparison of seropositive with seronegative groups.
Human Herpesvirus 6 DNAemia
Among the total of 96 patients with pretransplant serologies, 62 of the 96 (64.6%) patients were classified as HHV-6 seropositive and 34 of the 96 (35.4%) patients were HHV-6 seronegative (Table 1). Forty-eight of 62 (77.4%) seropositive patients and 25 of the 34 (73.5%) seronegative patients had plasma HHV-6 PCR testing posttransplant because of concerns for infection or rejection; 2 of the 48 (4.2%) tested seropositive patients developed HHV-6 DNAemia compared with 8 of the 25 (32%) tested seronegative patients (primary infection, P = .002). The median plasma viral load in previously seronegative patients was 23 200 copies/mL, which was higher than the median 1355 copies/mL in seropositive patients; however, the statistical significance was not achieved likely due to the small sample size (P = .08). Seronegative patients tended to be younger and developed HHV-6 DNAemia significantly earlier posttransplant compared with seropositive patients (median 12 vs 52 days, P = .026) (Table 2). The most common clinical manifestations in previously seronegative patients were temporal associations of fever and/or elevated transaminases, with resolution of symptoms after HHV-6 antiviral therapy (twice-a-day dosing of I.V. ganciclovir or oral valganciclovir for 2-4 weeks) (Supplementary Table S1). Chromosomal integrated HHV-6 was not suspected in this cohort based on laboratory results and response to therapy [11, 18]. One patient (patient 84) had concomitant HHV-6 (47 900 copies) and CMV (124 copies) DNAemia despite anti-CMV prophylaxis and was successfully treated with IV ganciclovir (Supplementary Table S1).
Table 2.
HHV-6 in Patients’ Post-LT (Classified by HHV-6 Serostatus Prior to LT)
| Posttransplant Plasma PCR Results | HHV-6 Seropositive Recipients (n = 62) | HHV-6 Seronegative Recipients (n = 34) | P-valuec |
|---|---|---|---|
| Number of patients tested (%) | 48 (77.4%) | 25 (73.5%) | .67 |
| Incidence of DNAemia, % | 2/48, 4% (reactivation)a | 8/25, 32% (primary infection)b | .002 |
| Median viral load copies/mL plasma (IQR) | 1355 (209; 2500) | 23 200 (13 275; 44 275) | .09 |
| Median age of patients with positive PCR result (IQR) | 6.5 years (0.9; 12.2) | 1.2 years (0.4; 2.1) | .38 |
| Time to DNAemia post-LT (range) | 52 days (range 26-78 days) | 13 days (range 9-16 days) | .03 |
| Number of patients with HHV-6 detected in any posttransplant liver biopsy (%) | 21/27 (77.8%) | 13/14 (92.8%) | .39 |
The highlighted terminologies of reactivation and primary infection are to highlight the differences between the groups to the reader.
Abbreviations: HHV-6, human herpesvirus 6; LT, liver transplantation; PCR, polymerase chain reaction.
aReactivation or reinfection: HHV-6 DNAemia post-LT in previously seropositive patients (IgG positive).
bPrimary infection: HHV-6 DNAemia post-LT in previously seronegative patients (IgG negative).
cComparison of seropositive with seronegative groups.
HHV-6 Detection in Liver Biopsy Tissue
A total of 51 out of 96 patients with pretransplant serologies (33 seropositive and 18 seronegative) had for-cause liver biopsies, due to rising liver indices, at a median time of 175 days posttransplantation. HHV-6 was detected by tissue PCR in 21 of the 27 (77.8%) patients tested in the seropositive group. None of the patients had concomitant HHV-6 DNAemia at the time of biopsy, that is, localized reactivation in the liver with no detectable DNAemia (Table 2). In the seronegative group, HHV-6 was detected in liver tissue in 13 of the 14 (92.8%) tested patients (Table 2). Six patients with HHV-6 detected on tissue PCR in the seropositive group were treated with antivirals due to features of viral lobulitis or clinical deterioration, at the discretion of treating hepatologist, in consultation with transplant infectious disease specialist. In seronegative recipients who had posttransplant HHV-6 DNAemia, 5 of the 8 patients had HHV-6 detected in subsequent liver biopsies, while the other 3 did not undergo any for-cause biopsies (Table 2). Of the 2 seropositive patients with DNAemia, one had HHV-6 detected in a subsequent liver biopsy, whereas the other patient did not undergo for-cause liver biopsy. A few of the children with positive HHV-6 tissue PCR concomitantly had features of ACR at the time of liver biopsy prompting the hepatologist to treat the rejection episode with steroids (Supplementary Table S1).
Thirty-three patients had their first posttransplant biopsy examined for HHV-6 by PCR. These liver biopsies were retrospectively reviewed and scored by a pathologist, blinded to patient data, to identify any unique histological findings associated with HHV-6 and to correlate with the presence of cellular rejection. All 9 (100%) seronegative patients had virus detected in their first liver biopsy samples, whereas 17 of the 24 (70.8%) seropositive patients had HHV-6 detected in their first liver biopsy samples (Table 3). There were no statistically significant differences in ACR, RAI score, central venulitis, or lymphocytic lobulitis between either HHV-6 seronegative vs seropositive groups or in patients with HHV-6 detected in liver biopsy vs patients without HHV-6 detected in liver biopsy (Table 3). Ten out of 17 patients in the seropositive group and 7 of the 9 patients in the seronegative group had another virus in addition to HHV-6 detected by tissue PCR in the first posttransplant liver biopsy. EBV was the most commonly identified in 53% of cases, followed by HHV-7 (23%), parvovirus (12%), and adenovirus (12%). None of the patients had concomitant CMV detected by PCR.
Table 3.
Histopathology Results of First Posttransplant Biopsy Stratified by HHV-6 Serology
| First Post-LT Tissue Analysis | HHV-6 Seropositive Group (n = 24 Biopsies) | HHV-6 Seronegative Group (n = 9 Biopsies) | P-valuea |
|---|---|---|---|
| Detection of HHV-6 by PCR in first posttransplant liver biopsy (%) | 17/24 (70.8%) | 9/9 (100%) | .15 |
| ACR on liver biopsy, number of patients (%), composite RAI score | 23/24 (95.8%) median RAI 4 (range 0-9) | 7/9 (77.7%) median RAI 4 (range 0-7) | .87a |
| Central venulitis presence (% of patients) | 15/24 (62.5%) | 3/9 (33.3%) | .24 |
| Lymphocytic lobulitis number of patients (%), median lobulitis grade | 21/24 (87.5%), grade 2 | 7/9 (77.7%), grade 1 | .24b |
Abbreviations: ACR, acute cellular rejection; HHV-6, human herpesvirus 6; LT, liver transplantation; PCR, polymerase chain reaction; RAI, rejection activity index.
aComparison of RAI scores between seropositive with seronegative groups
bComparison of lobulitis (per 200× field): Grade 0: none; grade 1: <2 foci; grade 2: 2-4 foci, and grade 3: >4 foci.
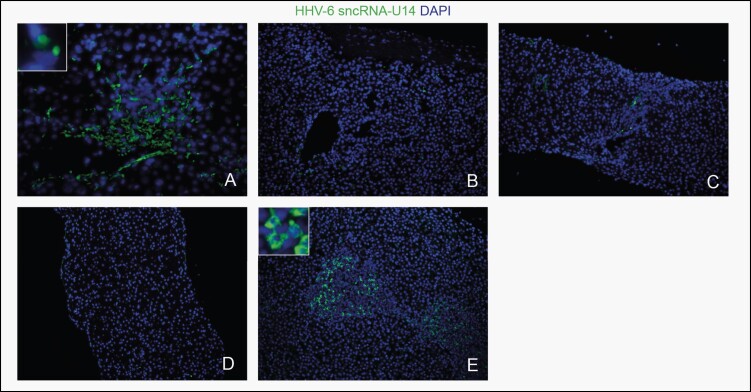
Eleven liver biopsy samples from 7 patients with adequate stored tissue availability (patients 8, 15, 44, 48, 70, 83, and 98) were subsequently analyzed for signs of active HHV-6 replication by ISH (Table 4; Figure 1A-E). Patient 8 had 2 biopsy samples from different time points analyzed: the first was positive for HHV-6 by both PCR and ISH (Figure 1A), and the second was negative for HHV-6 by both PCR and ISH (Figure 1B). She had not been treated with antivirals. Patient 15 had one sample tested, which was positive by PCR and equivocal by ISH (Figure 1C). He had been treated with ganciclovir for HHV-6 DNAemia a year prior to the biopsy with the resolution of DNAemia. Patient 44 had one sample tested that was negative for HHV-6 by PCR and positive by ISH, although the patient had 5 biopsies prior with HHV-6 detected by PCR and had moderate to severe ACR leading subsequently to chronic rejection. Patient 48 had one sample that was positive by PCR and negative by ISH (Figure 1D). Patient 70 had 2 samples from different time points examined: the first was positive by PCR and ISH within 3 weeks after transplant, although this patient was seronegative pretransplantation (Figure 1E), and the second was negative by PCR and equivocal by ISH. She was on valganciclovir prophylaxis at that time and subsequently improved. Patient 83 had 2 samples analyzed: the first was negative by PCR but positive by ISH, whereas the second one was positive for both PCR and ISH. The patient subsequently developed severe ACR and ductopenia. Finally, patient 98 had 2 samples from different time points analyzed: the first was positive by PCR and ISH, and the second was negative by PCR and ISH. She was not treated with antivirals.
Table 4.
HHV-6 PCR in Conjunction With In Situ Hybridization
| Age/Sex (Patient #) | Pre-LT HHV-6 IgG | HHV-6 DNAemia (copies/mL Plasma) | Day of Biopsy Post-LT (HHV-6 PCR Results) | HHV-6 ISH Results | Pathology Results |
|---|---|---|---|---|---|
| 14.5/F (#8) | Pos | Neg | +277 (HHV-6+) | Pos | ACR with central perivenulitis |
| +845 (HHV-6-) | Neg | ||||
| +894 (HHV-6−) | Nd | No rejection | |||
| +975 (HHV-6−) | |||||
| 3.3/M (#15) | Neg | 20 700 (day +17) | +431 (HHV-6+) | Eq | Lobular inflammation, mild focal portal inflammation, likely viral |
| 7.3/M (#44) | Pos | Neg | +504 (HHV-6+) | Nd | ACR |
| +522 (HHV-6+) | |||||
| +538 (HHV-6+) | |||||
| +568 (HHV-6+) | |||||
| +648 (HHV-6+) | Severe ACR, chronic rejection features | ||||
| +767 (HHV-6−) | Pos | No ACR | |||
| 4.0/F (#48) | Pos | Neg | +273 (HHV-6−) | Nd | Suboptimal specimen for grading rejection |
| +351 (HHV-6+) | Neg | ACR with patchy lobular lymphocytic infiltrate | |||
| 2.4/F (#70) | Neg | Neg | +18 (HHV-6+) | Nd | ACR |
| +34 (nd) | |||||
| +67 (nd) | |||||
| +211 (HHV-6+) | Pos | ||||
| +291 (HHV-6−) | Eq | ||||
| 2.6/F (#83) | Pos | Neg | +34 (HHV-6−) | Pos | ACR with centrilobular necrosis and lobulitis |
| +54 (HHV-6+) | Mild ACR, lobular inflammation | ||||
| +99 (HHV-6−) | Nd | ||||
| +183 (HHV-6−) | Severe ACR, ductopenia, fibrosis | ||||
| 0.6/F (#98) | Pos | Neg | +117 (HHV-6+) | Pos | No ACR |
| +132 (HHV-6−) | Neg | ||||
| +146 (HHV-6+) | Nd | ||||
| +183 (eq) | |||||
| +223 (HHV-6+) | Acute cholangitis |
Abbreviations: ACR, acute cellular rejection; HHV-6, human herpesvirus 6; LT, liver transplantation; PCR, polymerase chain reaction; Pos, positive, neg, negative; eq, equivocal (ie, inconclusive); nd, not done (ie, testing not performed).
Figure 1.
In situ hybridization (ISH) results: (A) Positive human herpesvirus 6 (HHV-6) ISH (patient 8: day +277). (B) Negative HHV-6 ISH (patient 8: day +845). (C) Equivocal (Eq; ie, inconclusive) HHV-6 ISH (patient 15: day +431). (D) Negative HHV-6 ISH (patient 48: day +351). (E) Positive HHV-6 ISH (patient 70: day +211). Scale bar represents 100 µm.
DISCUSSION
Our objective was to assess the incidence, manifestations, and outcomes of HHV-6 infections in a cohort of 101 pediatric LT recipients, of whom 96 had pretransplant HHV-6 IgG testing. Based on pretransplant serologies, we identified 34 seronegative patients and 62 seropositive patients. DNAemia occurred in a significantly higher percentage of patients in the seronegative group (primary infection, P = .0021) and these patients tended to be younger, with significantly higher viral loads, and developed DNAemia earlier in the posttransplant period than seropositive patients. These findings suggest that a higher degree of suspicion of HHV-6 infection, particularly in seronegative pediatric LT recipients, is prudent when undergoing evaluation for infections posttransplant. Our results add to previously published literature and confirm findings in a previously published solid organ pediatric transplant recipients [19, 20]. Although the updated 2019 guidelines from the American Society of Transplantation do not recommend routine serological studies for HHV-6 in the evaluation of solid organ transplant candidates [18], results from our cohort highlight the potential benefits of having serological testing. Knowledge about pretransplant HHV-6 serological status can help understand the risk of infection, likely originating from the donor, particularly in young seronegative LT recipients. Seronegative patients had symptoms potentially attributable to HHV-6 infection with high viral loads in the immediate posttransplant period, and both symptoms and DNAemia resolved with twice-a-day IV antiviral therapy. None of the patients in this cohort died due to HHV-6 infection in the posttransplant period, although there are previous reports of such cases in pediatric transplantation [6]. We believe that treating physicians should consider HHV-6 infection in the differential diagnosis for fever and/or hepatitis, particularly in young seronegative pediatric LT recipients.
HHV-6 as a possible cause of hepatitis cannot be ruled out without tissue biopsy testing even when there is no detectable DNAemia: 6 of our patients (HHV-6-seropositive group) without DNAemia but with positive HHV-6 tissue PCR results improved with antiviral treatment. One of the novel findings of the current manuscript is the demonstration of frequent HHV-6 detection from for-cause liver biopsies by both PCR and active viral replication by ISH even in the absence of DNAemia. Additionally, 92.8% of our seronegative patients who had no exposure to HHV-6 prior to transplant had HHV-6 detected in any for-cause liver biopsy albeit without detectable DNAemia (Table 2). The median age of the donor was greater than 2 years suggesting possible donor-derived infection in seronegative recipients with early posttransplant DNAemia. This could also represent an acquired infection in the period of intense immunosuppression or reactivation of latent HHV-6 from donor leucocytes, highlighting that donor serology testing for HHV-6 might provide additional data to risk stratify (low-, intermediate-, and high-risk recipients). Fluorescent in situ hybridization targeting ribonucleic acid molecules (RNA FISH) for small non-coding RNA (sncRNA)-U14 has been shown to be more sensitive than DNA-based PCR as it detects the reactivated state of the virus, which DNA PCR cannot differentiate [17]. On our retrospective analyses of select liver biopsy samples via sensitive methods such as ISH against small noncoding RNA U14 demonstrated that the HHV-6 genome was functionally active in 8 of the 11 cases, strengthening the likelihood that HHV-6 may be contributing to hepatitis (Table 4, Figure 1). In recent studies, HHV-6 reactivation has been associated with major transcriptional changes in host cell as well as changes in mitochondrial architecture suggesting increased mitochondrial senescence and mitophagy in HHV-6-reactivated cells [15, 17]. HHV-6 sncRNA-U14 is detected during both active viral infection and viral reactivation, while latent HHV-6-containing cells did not transcribe these small RNA molecules [15, 16]. Hence, the detection of sncRNA-U14 in liver biopsies suggests the active state of the viral genome. However, the exact role of these RNAs in virus-mediated pathophysiology is incompletely understood. In our study, viruses such as EBV and HHV-7 were detected by tissue PCR, in addition to HHV-6 in the first posttransplant allograft biopsy. While this likely reflects donor-derived infections and individual immune suppression status, it highlights the need for additional testing such as ISH to identify the active replicating virus.
Given that HHV-6 is a lymphotropic virus, one must wonder whether HHV-6 replication causes rejection in a transplant patient with liver dysfunction or is simply an epiphenomenon associated with lymphocytic infiltration of the allograft. HHV-6 could be an innocent bystander in some patients. Our analyses did not show any significant differences in histopathology regarding rejection when stratified by HHV-6 status (Table 4). HHV-6 is likely taking advantage of the immunological microenvironment in these transplant patients by actively replicating, as shown by our ISH analysis, potentially causing abnormal labs, and/or exacerbating preexisting conditions. Regardless, it is crucial to consider HHV-6 in the differential diagnosis when working up for causes of hepatitis in transplant patients. Future routine serological testing of donors and recipients prior to transplant may offer additional risk stratification of pediatric LT recipients.
Past studies have suggested an increased risk of HHV-6 infection in pediatric LT recipients when BA is the underlying disease [12]; this was not reproduced in our study, as there was no significant difference in the incidence of HHV-6 detection (in plasma, liver biopsy tissue, or both) between patients undergoing transplant for BA vs other underlying diseases (P = 1.0; data not shown). It should be noted that all our patients received systematic CMV prophylaxis and underwent deceased donor LT, whereas patients in the study of Yasui et al [12] did not receive anti-CMV prophylaxis and underwent living donor LT. We detected HHV-6 DNAemia at a significantly lower frequency (10/73; 13.6%) compared with the study of Yasui et al [12] (14/33; 42.4%), which falls in line with past studies suggesting that CMV prophylaxis decreases the frequency of HHV-6 DNAemia [6]. Interestingly, only one patient had low-level CMV viremia in addition to HHV-6 DNAemia. Since there are no standard guidelines that recommend prophylaxis against HHV-6 in LT patients, it is presumed that the prophylaxis for CMV also prevents HHV-6 [6]. However, despite systematic CMV prophylaxis in our cohort, HHV-6 remained a significant clinical burden early posttransplant. If detected in a timely manner, HHV-6 infections can still be successfully treated using CMV antivirals at treatment dosages [18, 21, 22].
There are several limitations to our study. Our patients underwent only for-cause liver biopsies limiting the availability of tissue PCR data. Although we had robust pretransplant serology data on patients, systematic posttransplant HHV-6 testing was not done in all our patients. Although we did not have subclassification of HHV-6 into A or B species by our PCR testing, and based on epidemiology, most of the symptomatic infections were likely HHV-6B [23]. Furthermore, we were not able to perform ISH on all our biopsy samples due to limited tissue availability, and thus we were unable to confirm active replication in every case. The tissue PCR and ISH were discordant in certain cases, highlighting the need for clinical validation and the need for determining active viral replication in tissue and further studies of research-based assays such as ISH for U14. Future prospective studies can implement a more systematic approach to determine the true significance of tissue/allograft HHV-6 replication in this patient population, albeit this can be difficult given the invasive nature of performing a liver biopsy. However, we were able to demonstrate active replication of HHV-6 in tissue in several of our patients. This finding, along with resolution of DNAemia with appropriate prompt therapy in select few patients, makes an important case for considering HHV-6 in the differential when working up LT recipients.
In conclusion, our study demonstrates that HHV-6 was temporally associated with fever and/or hepatitis in pediatric LT recipients, and its detection was often associated with markers suggestive of active replication in the peripheral blood or in the tissue. Pretransplant HHV-6 serologies may enable risk stratification of posttransplant infections and prompt quantitative DNA testing when working up symptomatic seronegative children with fever or hepatitis after LT. Furthermore, HHV-6 can be detected in the liver allograft by tissue PCR, in conjunction with ACR, which is not apparent when using typical screening assays in plasma or blood alone. CMV prophylaxis is effective at preventing CMV infections; however, it was not as efficacious in preventing HHV-6 DNAemia in our pediatric patient population. HHV-6 can be successfully treated with antivirals, thus timely detection is a key to prevent complications [13]. Further studies to evaluate the role of tissue HHV-6 detection, its effect on allograft health, and effective antiviral prophylaxis in the prevention of DNAemia are important questions that remain in understanding the pathophysiology of HHV-6 infections in pediatric LT.
Supplementary Material
Notes
Financial support. This work was funded by Cade R Alpard Foundation for Pediatric Liver Disease. K. R. M. was supported by a grant from the National Institutes of Health (grant number T 32DK007664).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ablashi D, Agut H, Alvarez-Lafuente R, et al. . Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol 2014; 159:863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zerr DM, Meier AS, Selke SS, et al. . A population-based study of primary human herpesvirus 6 infection. N Engl J Med 2005; 352:768–76. [DOI] [PubMed] [Google Scholar]
- 3. Briggs M, Fox J, Tedder RS. Age prevalence of antibody to human herpesvirus 6. Lancet 1988; 1:1058–9. [DOI] [PubMed] [Google Scholar]
- 4. Lusso P, De Maria A, Malnati M, et al. . Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T lymphocytes by human herpesvirus 6. Nature 1991; 349:533–5. [DOI] [PubMed] [Google Scholar]
- 5. Galan A, McNiff JM, Choi JN, Lazova R. Fatal HHV6 infection in an immunocompromised patient presenting with skin involvement. J Cutan Pathol 2010; 37:277–81. [DOI] [PubMed] [Google Scholar]
- 6. Phan TL, Lautenschlager I, Razonable RR, Munoz FM. HHV-6 in liver transplantation: a literature review. Liver Int 2018;38:210–23. [DOI] [PubMed] [Google Scholar]
- 7. Potenza L, Luppi M, Barozzi P, et al. . HHV-6A in syncytial giant-cell hepatitis. N Engl J Med 2008; 359:593–602. [DOI] [PubMed] [Google Scholar]
- 8. Fotheringham J, Akhyani N, Vortmeyer A, et al. . Detection of active human herpesvirus-6 infection in the brain: correlation with polymerase chain reaction detection in cerebrospinal fluid. J Infect Dis 2007; 195:450–4. [DOI] [PubMed] [Google Scholar]
- 9. Hill JA, Myerson D, Sedlak RH, et al. . Hepatitis due to human herpesvirus 6B after hematopoietic cell transplantation and a review of the literature. Transpl Infect Dis 2014; 16:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kidd IM, Clark DA, Sabin CA, et al. . Prospective study of human betaherpesviruses after renal transplantation: association of human herpesvirus 7 and cytomegalovirus co-infection with cytomegalovirus disease and increased rejection. Transplantation 2000; 69:2400–4. [DOI] [PubMed] [Google Scholar]
- 11. Bonnafous P, Marlet J, Bouvet D, et al. . Fatal outcome after reactivation of inherited chromosomally integrated HHV-6A (iciHHV-6A) transmitted through liver transplantation. Am J Transplant. 2018;18:1548–51. [DOI] [PubMed] [Google Scholar]
- 12. Yasui T, Suzuki T, Yoshikawa Tet al. . Clinical course of human herpesvirus 6 infection in pediatric living donor liver transplantation. Pediatr Transplant. 2018:e13239. [DOI] [PubMed] [Google Scholar]
- 13. Bonnafous P, Phan TL, Himes R, et al. . Evaluation of liver failure in a pediatric transplant recipient of a liver allograft with inherited chromosomally integrated HHV-6B. J Med Virol 2020; 92:241–50. [DOI] [PubMed] [Google Scholar]
- 14. Demetris AJ, Bellamy C, Hübscher SG, et al. . 2016 Comprehensive update of the Banff Working Group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant 2016; 16:2816–35. [DOI] [PubMed] [Google Scholar]
- 15. Prusty BK, Gulve N, Chowdhury SR, et al. . HHV-6 encoded small non-coding RNAs define an intermediate and early stage in viral reactivation. NPJ Genom Med 2018;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nukui M, Mori Y, Murphy EA. A human herpesvirus 6A-encoded microRNA: role in viral lytic replication. J Virol 2015; 89:2615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schreiner P, Harrer T, Scheibenbogen C, et al. . Human herpesvirus-6 reactivation, mitochondrial fragmentation, and the coordination of antiviral and metabolic phenotypes in myalgic encephalomyelitis/chronic fatigue syndrome. Immunohorizons 2020; 4:201–15. [DOI] [PubMed] [Google Scholar]
- 18. Pellett Madan R, Hand J; AST Infectious Diseases Community of Practice . Human herpesvirus 6, 7, and 8 in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019; 33:e13518. [DOI] [PubMed] [Google Scholar]
- 19. Ylinen E, Lehtinen S, Jahnukainen T, et al. . Human herpes virus 6 infection in pediatric organ transplant patients. Pediatr Transplant 2017;21. doi: 10.1111/petr.12905 [DOI] [PubMed] [Google Scholar]
- 20. Pappo-Toledano A, Dovrat S, Soufiev Z, et al. . Primary infection with human herpes virus type 6, post-pediatric liver transplantation-A pathogen to remember. Transpl Infect Dis 2019; 21:e13014. [DOI] [PubMed] [Google Scholar]
- 21. Toomey D, Phan TL, Nguyen V, Phan TT, Ogata M. Retrospective case analysis of antiviral therapies for HHV-6 encephalitis after hematopoietic stem cell transplantation. Transpl Infect Dis 2020:e13443. [DOI] [PubMed] [Google Scholar]
- 22. Ogata M, Takano K, Moriuchi Y, et al. . Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transplant 2018; 24:1264–73. [DOI] [PubMed] [Google Scholar]
- 23. Tang H, Mori Y. Glycoproteins of HHV-6A and HHV-6B. In: Kawaguchi Y, Mori Y, Kimura H, eds. Human Herpesviruses. Singapore: Springer Singapore; 2018:145–65. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.