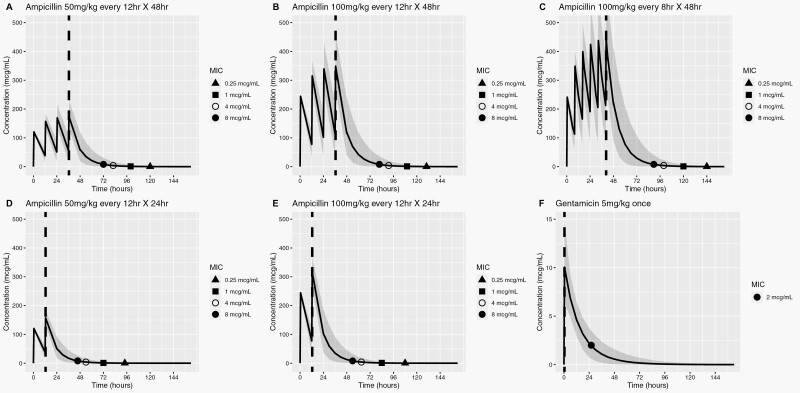
In very low birth weight neonates, ampicillin concentrations remain therapeutic long after the last dose. A short, 2-dose ampicillin course provided therapeutic exposures throughout the typical blood culture incubation period.
Keywords: ampicillin, antimicrobial stewardship, early-onset sepsis, gentamicin, Monte Carlo simulation, neonatal sepsis, neonate, pharmacokinetics
Abstract
Background
Premature, very low birth weight (VLBW) neonates are at risk for early-onset sepsis and receive ampicillin and gentamicin post-birth. Antimicrobial stewardship supports short-course antibiotics, but how long antibiotic concentrations remain therapeutic post-last dose is unknown.
Methods
Using Monte Carlo simulations (NONMEM 7.3), we analyzed antibiotic exposures in a retrospective cohort of 34 689 neonates (<1500 g, 22-27 weeks of gestation). Therapeutic exposure for ampicillin and gentamicin was evaluated relative to the minimum inhibitory concentration (MIC) for common pathogens (MIC 0.25-8 mcg/mL for group B streptococcus [GBS] and Escherichia coli). Post-discontinuation antibiotic exposure (PDAE) was defined as the time from the last dose to time when concentration decreased below MIC.
Results
Neonates had a median (range) gestational age of 26 (22-27) weeks and BW, 790 g (400-1497) . All ampicillin dosing regimens (50-100 mg/kg every 8-12 hours for 2-6 doses) achieved therapeutic exposures > MIC range. After the last dose, the PDAE mean (95% confidence interval [CI]) ranged from 34 to 50 hours (17–79) for E. coli (MIC 8) and 82 to 104 hours (95% CI: 39-122) for GBS (MIC 0.25); longer PDAE occurred with higher dose, shorter interval, and longer course. Short-course ampicillin (2 doses, 50 mg/kg every 12 hours) provided PDAE 34 hours for E. coli and 82 hours for GBS. Single-dose 5 mg/kg gentamicin provided PDAE > MIC 2 for 26 hours.
Conclusions
In VLBW neonates, ampicillin exposure remains therapeutic long after the last dose. Short-course ampicillin provided therapeutic exposures throughout the typical blood culture incubation period.
Early-onset sepsis (EOS) is a potentially life-threatening systemic bacterial infection occurring in 0.5% of newborns within the first 72 hours after birth. In a recent multistate report of 1484 EOS cases, 11% died and 6% of survivors had sequelae at discharge [1]. Group B streptococcus (GBS), Escherichia coli, and Viridans streptococci were the most common pathogens, accounting for 80% of cases. Extremely preterm and very low birth weight (VLBW, birth weight < 1500 g) infants experience a higher incidence of EOS and EOS-attributed mortality that is consistently reported to be at least an order of magnitude higher relative to term infants [1]. Premature VLBW infants who are considered critically ill often exhibit cardiopulmonary insufficiency that can be attributed to premature lung development, sepsis, or both. As a result, most of the VLBW infants receive empiric antibiotics for at least 48 hours after birth, while blood culture results are pending. Even with negative cultures, clinicians are often hesitant to discontinue empiric antibiotics resulting in prolonged courses of antibiotics of ≥5 days [2]. This prolonged antibiotic exposure in the first week after birth has been associated with an increased risk of infectious and noninfectious morbidities and mortality as well as changes in the infant’s microbiome [3, 4].
Antimicrobial stewardship efforts are increasing in neonatal intensive care units (NICUs). Rates of prolonged early antibiotic courses, defined as those initiated within the first 72 hours of life and continued for ≥5 days, have declined to 30%–40% of VLBW neonates [2]. Based on one systematic review, a reduction in antimicrobial utilization through antimicrobial stewardship programs in children’s hospitals did not negatively impact patient outcomes [5]. In light of antimicrobial stewardship programs that support the use of short courses of antibiotics, prompt discontinuation of therapy is prudent once an infection is no longer suspected [6].
When a robust and precise population-based pharmacokinetic model is available in the specific patient population, Monte Carlo simulation is a powerful methodology that provides an opportunity to improve our understanding of the relationship between drug exposure (which is based on drug dosing) and clinical outcomes, encompassing both efficacy and safety. This approach has been applied to the dosing of antimicrobial drugs in neonates, in whom the pharmacodynamic thresholds for therapeutic efficacy are thought to be similar across different neonatal age groups [7, 8].
Traditionally, studies of antibiotic dosing regimens have not considered exposures after discontinuation of therapy. We hypothesized that VLBW infants receiving empiric antibiotics shortly after birth would have prolonged antibiotic exposures after discontinuation due to lower drug clearance. Therefore, this new concept of post-discontinuation antibiotic exposure (PDAE) warranted further exploration in our effort to promote antimicrobial stewardship in this high-risk population. We used Monte Carlo simulation methodology to characterize PDAE of the most common empiric antibiotics, ampicillin and gentamicin, administered shortly after birth among preterm VLBW neonates (<28 weeks of gestation and <1500 g BW) at risk for EOS.
METHODS
Design
We performed a pharmacokinetic-pharmacodynamic simulation study using data from infants cared for in the Pediatrix Medical Group NICUs. The Pediatrix Medical Group Clinical Data Warehouse contains data obtained from admission notes, daily progress notes, and discharge summaries, including demographic data, medications, laboratory results, and diagnoses [9]. This study was approved by the Duke University Institutional Review Board as exempt research.
Subject Selection
Using the Pediatrix Medical Group Clinical Data Warehouse, we created a virtual population of infants who met the following criteria: preterm neonates with gestational age (GA) <28 weeks, postnatal age (PNA) <7 days, VLBW ≤1500 g, and receipt of ampicillin and gentamicin on day 0−1 of age. Demographic data, pertinent covariates (including BW, postmenstrual age, and serum creatinine), antibiotic utilization, and culture results were extracted.
Model Selection
A PubMed search for ampicillin and gentamicin population-based pharmacokinetic models in neonates was performed [10–17]. From the search results, the population-based pharmacokinetic models used in our simulation study were selected based on the demographic characteristics of neonates in the model that most closely fit our target study population: preterm neonates with GA < 28 weeks, PNA < 7 days, and BW ≤ 1500 g (Supplementary Table 1) as well as model robustness and sample sizes included in the original studies. The models with the closest match to our study population were the ampicillin model by Tremoulet et al [16] and the gentamicin model by DiCenzo et al [10].
Monte Carlo Simulations
Simulations were performed using the final population-based pharmacokinetic models to determine the antibiotic exposures during the antibiotic course and the distribution of PDAE after the last dose of antibiotics. Pharmacokinetic parameters, including volume of distribution, clearance, half-life, and elimination rate constant (k), were calculated for each patient. Simulations (ie, one per subject, N = 34 689) were performed using NONMEM 7.3 (Icon, Dublin, Ireland) to generate concentration time profiles for the most commonly used dosing regimens of ampicillin 50−100 mg/kg/dose every 8−12 hours for 24−48 hour courses (ie, 2-6 doses) and 1 dose of 5 mg/kg gentamicin. Concentrations were predicted every 6 hours during therapy and up to 4 days after antibiotic discontinuation (PDAE). Interindividual variability and residual error were integrated into the simulations based on the data from selected models. For gentamicin, we evaluated concentration over time, as well as maximum concentration (Cmax) and area under the curve over 24 hours (AUC24). Total, rather than free, drug concentration was used in this study, due to low protein binding of ampicillin and gentamicin reported in preterm neonates [18].
PDAE definitions were defined as specific to the pharmacodynamics properties of each drug. For ampicillin, the PDAE was defined as the duration of time between the last dose and the time ampicillin concentrations fell below the minimum inhibitory concentration (MIC); this definition ensures that the ampicillin concentration was above the MIC for 100% of the dosing interval. For gentamicin, the PDAE was defined in 2 ways: (1) the duration of time between the single dose administered and the time at which the concentration decreased below the MIC susceptibility breakpoint of 2 mcg/mL for E. coli and (2) the duration of time between the dose of gentamicin and the time at which the AUC24 decreased below 100 mcg-h/L, which has been proposed as a therapeutic target for E. coli [10, 19, 20].
The susceptible interpretative breakpoints were obtained from the United States Committee on Antimicrobial Susceptibility Testing (USCAST; http://www.uscast.org) and European Committee on Antimicrobial Susceptibility Testing (EUCAST; http://www.eucast.org), which had concordant MIC values, except for Listeria monocytogenes with breakpoint obtained from EUCAST. For ampicillin, the MIC susceptible breakpoint values that were evaluated ranged from 0.25 to 8 mcg/mL to cover the most common EOS pathogens, GBS, and E. coli, respectively, as well as to cover the range of MIC susceptibility breakpoints for less common organisms, including L. monocytogenes (MIC 1), V. streptococci (MIC 0.5), and Enterococcus (MIC 8). For gentamicin, the susceptibility breakpoint MIC of 2 mcg/mL was used for E. coli.
Pharmacokinetic Analysis
R version 3.5.0 was used for statistical computation and graphical visualization. Elimination curves were plotted using a decay equation, C = Co * e(−kt), with elimination constant k calculated for each patient from simulation-derived volume of distribution and clearance. The mean and 95% confidence interval (CI) of drug concentration was computed every 6 hours for up to 4 days after the last dose in order to generate visual curve plots of the plasma concentration profiles after a various dosing regimen of ampicillin and 1 dose of gentamicin. The AUC at 24-hour interval for gentamicin was calculated using the trapezoidal rule.
RESULTS
Our study population consisted of 34 689 one-day-old preterm neonates born at a median of 26 weeks’ gestation who met the study criteria and were included in our simulations (Table 1). Applying the published population-based pharmacokinetic models in our study population, the median volume of distribution and clearance for simulated subjects were 0.32 L/kg and 0.032 L/kg/h for ampicillin, and 0.39 L/kg and 0.025 L/kg/h for gentamicin, respectively, which are consistent with reported values in the literature [15, 21]. The concentration profiles of ampicillin (at different doses, dose intervals, and duration) and gentamicin (one 5 mg/kg dose), simulated up to 4 days post-antibiotic discontinuation, are depicted in Figure 1.
Table 1.
Demographic Data of Simulated Population
| Drug | Ampicillin16,a,b | Gentamicin10,a,c |
|---|---|---|
| Sample size | 34,689 | |
| GA (wk) | 26 (22–27) | |
| BW (kg) | 0.790 (0.400-1.497) | |
| Postnatal age (d) | 1 (1) | |
| PMA (wk) | 26 (22-27) | |
| SCR (mg/dL) | 0.90 (0.05-4.7)d | |
| Volume (L/kg) | 0.32 (0.16-0.60) | 0.39 (0.09-1.28) |
| CL (L/h/kg) | 0.032 (0.007-0.159) | 0.025 (0.006-0.080) |
Abbreviations: BW, birth weight; CL, clearance; GA, gestational age; N/A, not applicable or available; PMA, post-menstrual age; SCR, serum creatinine; WTKG, weight in kg; V, volume of distribution
aNumbers, except for sample size, represent median with range in parentheses.
bAmpicillin model: V = 0.399 * WTKG and CL = 0.078 * WTKG * (0.6/SCR)0.428 * (PMA/37)1.34.
cGentamicin model: CL = (0.00504 + [0.00108*GA]) *BW; median V and CL values with respective ranges for GA < 28 weeks were used.
dAt baseline from start of antibiotic therapy, most of the subjects (82%) had SCR < 1 mg/dL, 16% with 1 to 2 mg/dL, and <1% with >2 mg/dL.
Figure 1.
Simulated plasma concentration profiles of ampicillin and gentamicin relative to susceptibility breakpoints of common pathogens. Blue solid line denotes mean drug concentration; shaded area, 95% CI; red dashed line, time at last antibiotic dose; shape symbols indicate time points at which mean antibiotic concentrations decay to susceptibility breakpoint MIC for different bacteria post-antibiotic discontinuation. PDAE represents the time between the red line and symbol. (A–C) represent drug exposure during standard 48-h ampicillin dosing regimens; (D) and (E) represent drug exposure after proposed shortened, 2-dose ampicillin regimens; (F) represents gentamicin exposure after a single, high-dose, extended interval regimen. MIC susceptibility breakpoints for ampicillin range from 0.25 mcg/mL for GBS to 8 mcg/mL for Escherichia coli. Ampicillin MIC susceptible breakpoints for less common organisms include Listeria monocytogenes (MIC 1), Viridans streptococci (MIC 0.5), and Enterococcus (MIC 8). For gentamicin, the susceptibility breakpoint MIC of 2 mcg/mL was used for E. coli. Abbreviations: CI, confidence interval; GBS, group B streptococcus; MIC, minimum inhibitory concentration; PDAE, post-discontinuation empiric antibiotic exposure.
Ampicillin
During therapy, all evaluated ampicillin dosing regimens provided therapeutic exposure above 8 mcg/mL (ie, MIC breakpoint for E. coli). After the last 2–6 doses, the mean (95% CI) duration of PDAE for ampicillin ranged from 34 to 50 hours (17-79) for E. coli MIC 8 mcg/mL and 82 to 104 hours (95% CI: 39-122) for GBS MIC 0.25 mcg/mL (Table 2 and Figure 1A–F), respectively. The PDAE was longest for GBS, due to its low MIC breakpoint. An ampicillin dose increase from 50 to 100 mg/kg or dosing interval decrease from every 12 to 8 hours was associated with an increase in the duration of PDAE. A short course of ampicillin 50 mg/kg every 12 hours × 2 doses achieved a modest mean PDAE of 34 hours for E. coli at a MIC of 8 mcg/mL and 82 hours for GBS at a MIC of 0.25 mcg/mL.
Table 2.
Ampicillin PDAE Among Very Low Birth Weight Infants by Susceptibility Breakpoints of Common Bacterial Pathogensa,b
| 2 Dosesc | 4 Dosesc | 6 Doses | ||||
|---|---|---|---|---|---|---|
| Organism | Susceptible MIC (mcg/mL) | 50 mg/kg Q12 h | 100 mg/kg Q12 h | 50 mg/kg Q12 h | 100 mg/kg Q12 h | 100 mg/kg Q8 h |
| Escherichia coli d | 8 | 34 ± 9.4 (17, 54) | 42 ± 11 (22, 66) | 36 ± 11 (18, 60) | 46 ± 13 (22, 72) | 50 ± 14 (24, 79) |
| 4 | 42 ± 11 (22, 66) | 51 ± 13 (26, 78) | 46 ± 12 (23, 72) | 55 ± 15 (26, 84) | 60 ± 16 (27, 91) | |
| Listeria monocytogenes | 1 | 60 ± 15 (30, 90) | 72 ± 18 (34, 103) | 64 ± 17 (30, 97) | 74 ± 19 (35, 109) | 80 ± 20 (36, 116) |
| Group B Streptococcuse | 0.25 | 82 ± 19 (39, 114) | 96 ± 21 (42, 126) | 84 ± 21 (39, 120) | 94 ± 21 (43, 126) | 104 ± 20 (44, 122) |
Abbreviations: MIC, minimum inhibitory concentration in mcg/mL; PDAE, post-discontinuation empiric antibiotic exposure; Q8, every 8 h; Q12, every 12 h.
aNumbers represent mean ± standard deviation (95% confidence interval) for the duration of therapeutic exposure (hours) from the last dose of ampicillin up to the MIC of bacterial pathogens commonly isolated in early-onset sepsis.
bInfusion time for ampicillin was 15−30 min.
cLast dose was 12 h for 2 doses, 36 h for 4 doses, and 40 h for 6 doses.
dMIC of 8 mcg/mL is also the breakpoint for Enterococcus spp.
eMIC of 0.25 mcg/mL is also the breakpoint for Streptococci groups A, B, C, and G; Viridans streptococci have MIC breakpoint of 0.5 mcg/mL.
Gentamicin
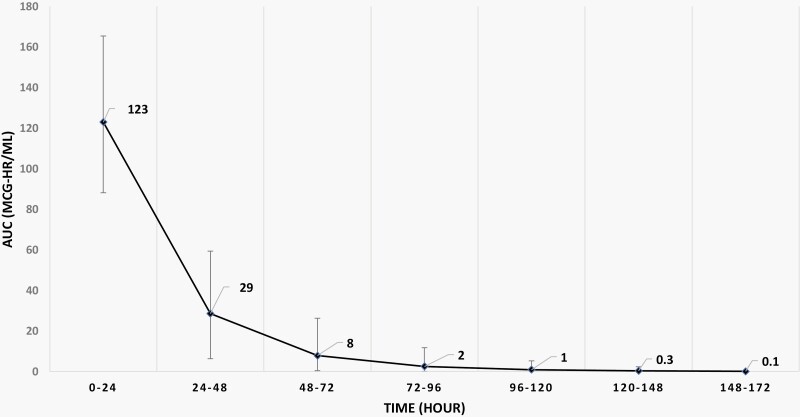
A single 5 mg/kg dose of gentamicin provided concentrations above 2 mcg/mL (MIC breakpoint for E. coli) for a mean PDAE of 26 hours (95% CI: 16-41; Figure 1G). Other therapeutic exposure targets unique to gentamicin include Cmax and AUC24 [7]. The mean steady-state peak concentration was 10 mcg/mL (95% CI: 6.4-15), resulting in a peak-to-MIC ratio of 5 based on an E. coli MIC breakpoint of 2 mcg/mL. The mean AUC24 exposure was 123 mcg-h/mL (95% CI: 88-165; Figure 2), and 89% of all infants achieved AUC24 > 100 mcg-h/L throughout the first 24 hours. If we used the therapeutic target of AUC24 at or above 100 mcg-h/mL, then the PDAE using endpoint AUC24 < 100 was 24 to 30 hours.
Figure 2.
AUC profile after 1-dose gentamicin. AUC profile after 1 dose of gentamicin 5 mg/kg at 24-h interval. Black line represents mean and bar, 95% confidence interval. Abbreviation: AUC, area under the curve.
DISCUSSION
In this study of simulated drug exposures, we demonstrated that VLBW neonates receiving common clinical dosing regimens of ampicillin and gentamicin after birth continue to have therapeutic exposure of ampicillin against common EOS pathogens from 1.5 to 3.5 days after the last dose of a 2-dose, 50mg/kg every 12-hour regimen. Even at a high MIC of 8 mcg/mL, the PDAE at the 2.5% is 17 hours. Prolonged exposure after discontinuing therapy is unnecessary, unlikely to be recognized by clinicians, and potentially harmful. This PDAE concept provides an exposure metric to reevaluate dosing regimens and a new antimicrobial stewardship opportunity for this highly vulnerable population. Additionally, PDAE awareness may help clinicians use shorter empiric antibiotic courses when cultures are negative.
Clinical trials and drug dosing evaluation in neonates are difficult. When robust and precise population pharmacokinetic models exist, then Monte Carlo simulations of large neonatal cohorts provide an ethical and cost-effective opportunity to evaluate novel dosing considerations. We innovatively created this PDAE metric as a method to identify unnecessary antibiotic exposures, reevaluate dosing regimens, and improve antimicrobial stewardship. The prolonged ampicillin PDAE after the traditional 48-hour dosing period was much longer than clinicians would predict and can be explained by both the magnitude of per kilogram dosing and delayed drug clearance. These PDAE simulations demonstrate that a shorter 2-dose ampicillin course, with the last dose 12 hours after a blood culture, would still provide therapeutic ampicillin exposure during the traditional 48-hour blood culture incubation window to rule out bacteremia. Efforts to minimize ongoing PDAE could potentially mitigate the risk of microbiome alteration, development of antimicrobial resistance, and drug toxicity. The clinical impact of the shorter ampicillin courses and limiting PDAE requires further exploration.
VLBW preterm infants with negative cultures at birth are vulnerable to prolonged antibiotic exposures. Prolonged early courses of antibiotics (≥5 days) in preterm neonates with negative blood cultures at birth, while common in clinical practice, are associated with increased risk of late-onset infections, necrotizing enterocolitis, changes in microbiome, and mortality [2]. Additionally, antibiotic use can negatively add selective pressure on the intestinal microbiota for up to 1 year [22, 23]. Minimizing antibiotic exposure in these VLBW infants during their first week of life may decrease their risk of developing infectious and noninfectious morbidities and mortality and preserve the antibiotic armamentarium as they are likely to remain hospitalized for treatment of their underlying medical conditions [3, 4]. Minimization of antibiotic administration and consequent exposure can be achieved by accounting for PDAE in our dosage regimens and encouraging the use of the shortest duration of empiric antibiotics.
From an antimicrobial stewardship perspective, clinician’s awareness of PDAE could help minimize antibiotic exposures in critically ill premature infants with negative cultures. Their cardiopulmonary instability and small blood-volume cultures can make it difficult for clinicians to stop antibiotics even when cultures are negative. A recent study reported that 30%–40% of VLBW infants with negative cultures continue to receive antibiotics for 5 days or longer [2]. Awareness of the ongoing therapeutic PDAE of ampicillin after a 48-hour course could encourage clinicians to comfortably and prudently discontinue antibiotics at least by 48 hours for this challenging population. Molecular diagnostics to provide a more timely and reliable method for detecting bacteremia, although not yet available in most hospitals, could further limit empiric coverage to the shortest possible duration [24–28].
Interpretation and utility of PDAE depend on the pharmacodynamic properties of the antimicrobial drug. For time-dependent antimicrobials like ampicillin, the PDAE relates directly to the time at which the drug concentration decreases below the MIC [8]. For VLBW preterm neonates, a conservative approach to the pharmacodynamics of time-dependent antibiotics typically relies on maintaining exposures above the MIC for 100% of the dosing interval. The PDAE may be even longer if shorter exposures, 50%–75% of the dosing interval, were effective. In contrast, the pharmacodynamics of gentamicin is different and leads to distinct PDAE interpretation.
Gentamicin PDAE, although more difficult to interpret, was not prolonged. The high gentamicin dose with extended interval regimen reflects its pharmacodynamic properties, specifically, the concentration-dependent killing based on the peak-to-MIC ratio >5 and the post-antibiotic effect, which is characterized by ongoing growth suppression after the drug concentration falls below the MIC of an offending organism [8, 29, 30]. Our study confirmed therapeutic gentamicin exposures after a single 5 mg/kg dose based on the peak-to-MIC ratio of 5 for an E. coli MIC breakpoint of 2 mcg/mL [31]. The duration of therapeutic exposure must then account for the time the concentration is above the MIC (PDAE of 26 hours for E. coli with MIC 2 mcg/mL) followed by the post-antibiotic effect, which has been estimated in other studies to continue for at least another 7.5 hours [32, 33]. As such, the total antibacterial protective time after a 5 mg/kg gentamicin dose is predicted to be at least 33 hours. PDAE could also target the alternative AUC24 > 100 mcg-h/mL efficacy metric, which in this study is predicted to be at least 24–30 hours and includes (or does not include) the post-antibiotic effect.
This novel PDAE metric may be a particularly important dosing consideration for neonates. In the NICU, drug dosing is often off-label and may not be supported by rigorous pharmacokinetic and safety studies. Preterm infants are at risk for higher or prolonged drug exposures since they often exhibit delayed drug clearance, due to immature renal function and inefficient drug metabolism. Renal drug clearance is particularly prolonged shortly after birth, a time when most of the VLBW infants receive many medications for their cardiopulmonary morbidities and risk for EOS. For drugs with high renal elimination such as ampicillin and gentamicin, different degrees of renal function will also impact their PDAE (ie, increased PDAE with decreased renal function). Notably, the use of the Pediatrix Medical Group Clinical Data Warehouse that contains a large dataset reflective of authentic patients provides a robust and clinically meaningful distribution of PDAE results. PDAE likely provides an important exposure metric for other antibiotics and for patients of all ages with delayed drug clearance.
There were 2 main limitations of this study. First, while we selected the most robust models that employed population-based pharmacokinetic modeling, the selected models also enrolled older infants by GA and PNA. Nonetheless, the selected models were the closest match to our target study population. Second, we did not assess actual antibiotic concentrations in our subjects, since this is the first-ever attempt to evaluate the presence and extent of PDAE. Notably, the use of a powerful pharmacologic tool to evaluate PDAE with simulated exposures allowed us to mitigate any risks to this vulnerable population. Also, we employed a large national database that identified more than 30 000 subjects for our study, consequently allowing us to minimize the number of simulations per subject, which increased the power of our study and maximized the accuracy of our prediction. Future studies are necessary to confirm PDAE (via pharmacokinetic sampling after antibiotic discontinuation) and to ascertain the safety and effectiveness of dosing practices that account for PDAE.
CONCLUSIONS
PDAE is an innovative metric designed to identify opportunities to reevaluate dose-exposure relationships, enhance antimicrobial stewardship, and minimize unnecessary antibiotic exposure. With the typical ampicillin dosing, simulated exposures demonstrate that premature VLBW neonates had therapeutic exposure for EOS pathogens long after discontinuation of therapy. A short 2-dose course of ampicillin, 50 mg/kg every 12 hours, appears to provide adequate antibacterial coverage pending blood culture results when the PDAE metric of 34 hours was accounted for. Exposure-driven dosing may enhance antimicrobial stewardship and potentially limit morbidities that have been associated with prolonged antibiotic exposure. Prospective studies are necessary to confirm the relationship between PDAE and clinical outcomes.
Supplementary Material
Notes
Acknowledgments
PTN Steering Committee Members: Daniel K. Benjamin Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Scottsdale, AZ; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; and Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD): Perdita Taylor-Zapata and June Lee.
The Emmes Company, LLC (Data Coordinating Center): Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor.
PTN Publications Committee: Chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL.
Financial support. This work was supported by the National Institute of Child Health and Human Development (NICHD) contract (HHSN275201000003I) for the Pediatric Trials Network (PI Danny Benjamin). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Administrative Core Committee of the Best Pharmaceuticals for Children Act–Pediatric Trials Network:
Daniel K Benjamin, Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, Rose Beci, Chi Dang Hornik, Gregory L Kearns, Matthew Laughon, Ian M Paul, Janice Sullivan, Kelly Wade, Paula Delmore, Perdita Taylor-Zapata, June Lee, Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, Lawrence Taylor, and Thomas Green
References
- 1. Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics 2016; 138:e20162013. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg RG, Chowdhury D, Hansen NI, et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res 2019; 85:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 2009; 123:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fjalstad JW, Esaiassen E, Juvet LK, et al. Antibiotic therapy in neonates and impact on gut microbiota and antibiotic resistance development: a systematic review. J Antimicrob Chemother 2018; 73:569–80. [DOI] [PubMed] [Google Scholar]
- 5. Smith MJ, Gerber JS, Hersh AL. Inpatient antimicrobial stewardship in pediatrics: a systematic review. J Pediatric Infect Dis Soc 2015; 4:e127–35. [DOI] [PubMed] [Google Scholar]
- 6. Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am 2014; 28:247–61. [DOI] [PubMed] [Google Scholar]
- 7. Le J, Bradley JS. Pharmacodynamic considerations and special populations: pediatrics. In: Rotschafer J, Andes D, Rodvold K, eds. Antibiotic Pharmacodynamics. Methods in Pharmacology and Toxicology. New York, NY: Humana Press; 2016:561-597. [Google Scholar]
- 8. Le J, Bradley JS. Optimizing antibiotic drug therapy in pediatrics: current state and future needs. J Clin Pharmacol 2018; 58 (Suppl 10:108–22. [DOI] [PubMed] [Google Scholar]
- 9. Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix Babysteps Data Warehouse and the Pediatrix Qualitysteps improvement project system–tools for “meaningful use” in continuous quality improvement. Clin Perinatol 2010; 37:49–70. [DOI] [PubMed] [Google Scholar]
- 10. DiCenzo R, Forrest A, Slish JC, et al. A gentamicin pharmacokinetic population model and once-daily dosing algorithm for neonates. Pharmacotherapy 2003; 23:585–91. [DOI] [PubMed] [Google Scholar]
- 11. Fuchs A, Guidi M, Giannoni E, et al. Population pharmacokinetic study of gentamicin in a large cohort of premature and term neonates. Br J Clin Pharmacol 2014; 78:1090–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García B, Barcia E, Pérez F, Molina IT. Population pharmacokinetics of gentamicin in premature newborns. J Antimicrob Chemother 2006; 58:372–9. [DOI] [PubMed] [Google Scholar]
- 13. Lanao JM, Calvo MV, Mesa JA, et al. Pharmacokinetic basis for the use of extended interval dosage regimens of gentamicin in neonates. J Antimicrob Chemother 2004; 54:193–8. [DOI] [PubMed] [Google Scholar]
- 14. Nielsen EI, Sandström M, Honoré PH, et al. Developmental pharmacokinetics of gentamicin in preterm and term neonates: population modelling of a prospective study. Clin Pharmacokinet 2009; 48:253–63. [DOI] [PubMed] [Google Scholar]
- 15. Stolk LM, Degraeuwe PL, Nieman FH, et al. Population pharmacokinetics and relationship between demographic and clinical variables and pharmacokinetics of gentamicin in neonates. Ther Drug Monit 2002; 24:527–31. [DOI] [PubMed] [Google Scholar]
- 16. Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother 2014; 58:3013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vervelde ML, Rademaker CM, Krediet TG, et al. Population pharmacokinetics of gentamicin in preterm neonates: evaluation of a once-daily dosage regimen. Ther Drug Monit 1999; 21:514–9. [DOI] [PubMed] [Google Scholar]
- 18. Taketomo CK, Hodding JH, Kraus DM.. Pediatric and Neonatal Dosage Handbook. 27th ed. Hudson, Ohio: UpToDate, Inc; 2020. [Google Scholar]
- 19. Stickland MD, Kirkpatrick CM, Begg EJ, et al. An extended interval dosing method for gentamicin in neonates. J Antimicrob Chemother 2001; 48:887–93. [DOI] [PubMed] [Google Scholar]
- 20. Plajer SM, Chin PK, Vella-Brincat JW, et al. Gentamicin and renal function: lessons from 15 years’ experience of a pharmacokinetic service for extended interval dosing of gentamicin. Ther Drug Monit 2015; 37:98–103. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 1976; 58:259–63. [PubMed] [Google Scholar]
- 22. Rashid MU, Zaura E, Buijs MJ, et al. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin Infect Dis 2015; 60(Suppl 2:S77–84. [DOI] [PubMed] [Google Scholar]
- 23. Modi SR, Collins JJ, Relman DA. Antibiotics and the gut microbiota. J Clin Invest 2014; 124:4212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jordan JA, Durso MB. Comparison of 16S rRNA gene PCR and BACTEC 9240 for detection of neonatal bacteremia. J Clin Microbiol 2000; 38:2574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jordan JA, Durso MB. Real-time polymerase chain reaction for detecting bacterial DNA directly from blood of neonates being evaluated for sepsis. J Mol Diagn 2005; 7:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shang S, Chen G, Wu Y, et al. Rapid diagnosis of bacterial sepsis with PCR amplification and microarray hybridization in 16S rRNA gene. Pediatr Res 2005; 58:143–8. [DOI] [PubMed] [Google Scholar]
- 27. El-Amir MI, El-Feky MA, Abo Elwafa DA, Abd-Elmawgood EA. Rapid diagnosis of neonatal sepsis by PCR for detection of 16S rRNA gene, while blood culture and PCR results were similar in. Infect Drug Resist 2019;12:2703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mignard S, Flandrois JP. 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J Microbiol Methods 2006; 67:574–81. [DOI] [PubMed] [Google Scholar]
- 29. Bland CM, Pai MP, Lodise TP. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacotherapy 2018; 38:1229–38. [DOI] [PubMed] [Google Scholar]
- 30. Isemann BT, Kotagal UR, Mashni SM, et al. Optimal gentamicin therapy in preterm neonates includes loading doses and early monitoring. Ther Drug Monit 1996; 18:549–55. [DOI] [PubMed] [Google Scholar]
- 31. Rao SC, Srinivasjois R, Hagan R, Ahmed M. One dose per day compared to multiple doses per day of gentamicin for treatment of suspected or proven sepsis in neonates. Cochrane Database Syst Rev 2011:CD005091. [DOI] [PubMed] [Google Scholar]
- 32. Novelli A, Mazzei T, Fallani S, et al. In vitro postantibiotic effect and postantibiotic leukocyte enhancement of tobramycin. J Chemother 1995; 7:355–62. [DOI] [PubMed] [Google Scholar]
- 33. Gottfredsson M, Erlendsdóttir H, Kolka R, et al. Ultrastructural alterations of bacteria during the postantibiotic effect. Chemotherapy 1993; 39:153–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.