Abstract
The development of the control of breathing begins in utero and continues postnatally. Fetal breathing movements are needed for establishing connectivity between the lungs and central mechanisms controlling breathing. Maturation of the control of breathing, including the increase of hypoxia chemosensitivity, continues postnatally. Insufficient oxygenation, or hypoxia, is a major stressor that can manifest for different reasons in the fetus and neonate. Though the fetus and neonate have different hypoxia sensing mechanisms and respond differently to acute hypoxia, both responses prevent deviations to respiratory and other developmental processes. Intermittent and chronic hypoxia pose much greater threats to the normal developmental respiratory processes. Gestational intermittent hypoxia, due to maternal sleep-disordered breathing and sleep apnea, increases eupneic breathing and decreases the hypoxic ventilatory response associated with impaired gasping and autoresuscitation postnatally. Chronic fetal hypoxia, due to biologic or environmental (i.e. high-altitude) factors, is implicated in fetal growth restriction and preterm birth causing a decrease in the postnatal hypoxic ventilatory responses with increases in irregular eupneic breathing. Mechanisms driving these changes include delayed chemoreceptor development, catecholaminergic activity, abnormal myelination, increased astrocyte proliferation in the dorsal respiratory group, among others. Long-term high-altitude residents demonstrate favorable adaptations to chronic hypoxia as do their offspring. Neonatal intermittent hypoxia is common among preterm infants due to immature respiratory systems and thus, display a reduced drive to breathe and apneas due to insufficient hypoxic sensitivity. However, ongoing intermittent hypoxia can enhance hypoxic sensitivity causing ventilatory overshoots followed by apnea; the number of apneas is positively correlated with degree of hypoxic sensitivity in preterm infants. Chronic neonatal hypoxia may arise from fetal complications like maternal smoking or from postnatal cardiovascular problems, causing blunting of the hypoxic ventilatory responses throughout at least adolescence due to attenuation of carotid body fibers responses to hypoxia with potential roles of brainstem serotonin, microglia, and inflammation, though these effects depend on the age in which chronic hypoxia initiates. Fetal and neonatal intermittent and chronic hypoxia are implicated in preterm birth and complicate the respiratory system through their direct effects on hypoxia sensing mechanisms and interruptions to the normal developmental processes. Thus, precise regulation of oxygen homeostasis is crucial for normal development of the respiratory control network.
Introduction
Survival and proper development of the fetus and neonate depend on the maintenance of oxygen homeostasis, including the development of the respiratory system (51). Exposure to hypoxia poses a major threat to its development. The fetus relies on maternal blood through the placenta compared to independent breathing that occurs in the neonate. As such there are differing homeostatic regulatory mechanisms for responding to hypoxia between the fetus and neonate. This implies that the causes of, and ventilatory response to acute, intermittent, and/or chronic hypoxia are also different between the fetus and neonate. Given the nature of ongoing respiratory system development beginning in utero and extending throughout postnatal life, exposure to different degrees of hypoxia, whether during fetal or neonatal periods, can cause lasting changes to respiratory control.
The definitions of terms relating to oxygen homeostasis in this article are hypoxemia, which refers to abnormally, and relatively, low levels of dissolved oxygen in blood. Physiologically and clinically, hypoxemia is determined by measuring the pressure (mmHg) of oxygen (PaO2). Hypoxia refers to insufficient oxygen supply to the entire body or a body region (tissue hypoxia) to meet metabolic needs. Hypoxia can also refer to low levels of oxygen in the air, which is the case for alveolar hypoxia prevalent at high-altitude or in regions of atelectatic lungs in lung diseases. Alveolar hypoxia can cause hypoxemia and generally, hypoxemia suggests hypoxia. However, increasing oxygen delivery or reducing oxygen consumption can compensate for hypoxemia. Oxygenation is the process of passive oxygen diffusion across the alveolarcapillary barrier where it enters the blood, and either dissolves or binds to hemoglobin (oxyhemoglobin). Dissolved oxygen plus the amount of oxyhemoglobin (defined as the percentage of oxygen saturating hemoglobin, or SaO2) defines oxygen content (CaO2; often measured as mL O2/dL of blood). Poor oxygenation and/or hypoxemia and anemia can cause reductions in oxygen content (hypoxemia and/or decrease in SaO2).
Herein, we will provide an overview of the fetal and neonatal control of breathing with regards to the ventilatory responses to different frequencies of hypoxia exposures and the long-term implications of such exposures. In doing so, we will discuss the underlying hypoxia sensing mechanisms present in the fetus and neonate. Additionally, we will highlight areas of potential future research throughout this article.
Basics of Fetal Respiratory Physiology
The neural respiratory control network is composed of specialized populations of cells throughout the pons and medulla that contribute to the regulation of respiratory pattern and rhythm generation, peripheral chemo- and mechano-sensory integration, central pH/CO2 chemosensitivity, and neuromodulation. Although fetal oxygenation is independent of alveolar ventilation, fetal breathing movements (FBMs) generated by the developing respiratory control network are critical to the developmental process of the respiratory system indicated from preclinical (3, 92, 117, 127, 128, 173, 308) and clinical studies (37, 83, 104).
Fetal oxygenation
Fetal oxygenation is dependent on gas exchange within the placenta between maternal and fetal blood until birth. Studies in near-term gestation human fetuses (257) using phase-contrast magnetic resonance imaging (MRI) and fetal lambs (268) have demonstrated low PaO2 (20 ± 1 mmHg compared to postnatal standards (168)). However, fetuses are not hypoxic as they deliver adequate oxygen to their tissues because of: (i) high hemoglobin concentrations (19.3±2g/dL) (198); (ii) the presence of fetal hemoglobin (~72% HbF) (197); and (iii), the double Bohr effect within the placenta where high levels of fetal CO2 diffuse into the maternal blood increasing the release of maternal O2 to the fetal blood (i.e. rightward shift of maternal oxygen dissociation curve) and the concomitant increase in fetal O2 binding affinity (i.e. leftward shift of fetal oxygen dissociation curve); and (iV) high cardiac output, relative to the postnatal period (Figure 1). The steep portion of the fetal oxygen dissociation curve enables significant unloading of oxygen at relatively hypoxemic levels. The umbilical venous (oxygenated) PO2 levels in human neonates at birth is 28 mmHg (median PO2 is 3.7 kPa in umbilical venous blood where 2.3–5.5kPa is the 2.5th–97.5th percentile) with SO2 of 61% (24.2%–86.5% SO2 is the 2.5th–97.5th percentile, respectively) (Figure 1). Umbilical (deoxygenated) arterial samples at birth have a PO2 of 21 mmHg (2.3 kPa -median; 1.2—4–2.5th–97.5th percentile) with SO2 of 28.3% (8%–64.9%—2.5th–97.5th percentile). With a small difference in PO2 (7 mmHg) between oxygenated and deoxygenated blood, the term fetus achieves a SO2 difference of 33% with a median oxygen content difference of 3.1 mM/liter (5.9 in venous vs. 2.8 mM/liter on arterial samples) or 6.1mL/dL (203). This difference compares to the fluctuation in PO2 between arterial and venous circulation in adult humans which is approximately 57 mmHg to achieve a similar difference in oxygen content. With advancing gestation, hemoglobin concentration increases and PaO2 levels decrease in human fetuses to maintain a relatively constant CaO2 (283). The precise mechanism by which a fetus regulates CaO2 is not known.
Figure 1.
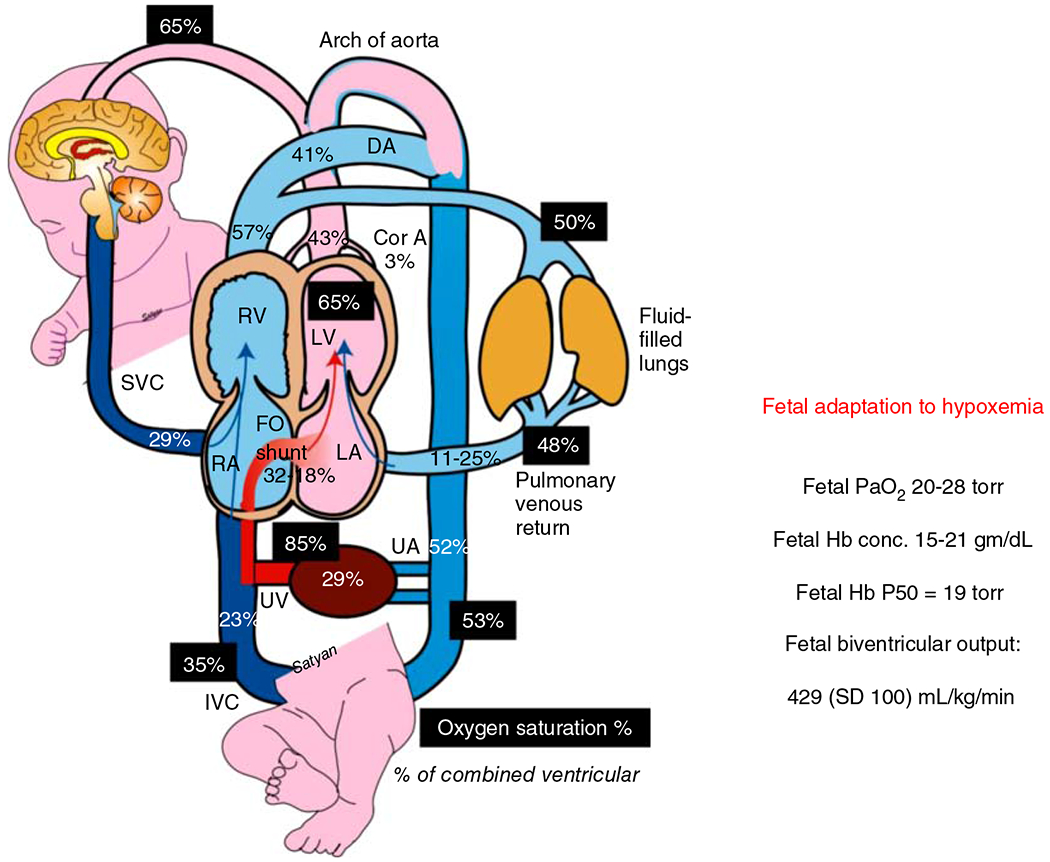
Adaptation of the fetus to low oxygen environment present in utero. Fetal oxygen supply is provided by maternal blood bathing the chorionic villi in the placenta. The umbilical vein carries the oxygenated blood to inferior vena cava (IVC) and eventually across the foramen ovale to left atrium and left ventricle to be pumped into coronary and cerebral circulations. The oxygen saturation of fetal blood in different sites are indicated by dark shaded boxes. The percent of cardiac output distributed to each organ is indicated in plain text. The mean values for fetal biventricular output, range of normal Hb concentrations and fetal PaO2 are indicated in the text box to the right, along with the HbP50 for fetal Hb (197, 257, 268). Reused, with permission, from Richard A. Polin and William W Fox, 2016, Fetal and Neonatal Physiology, Ed: Polin, Abman, Rowitch, Benitz and Fox, 5th edition, Lakshminrusimha and Steinhorn “Pathophysiology of PPHN,” pp 1576-1587. Copyright Satyan Lakshminrusimha.
Fetal breathing movements
The fetus generates breathing-like behaviors called FBMs which are regulated by developing areas of the respiratory control network within the pons and medulla of the brainstem (66, 236, 281, 293, 295). Even though fetal oxygenation does not depend on pulmonary gas exchange, FBMs are critical because they promote lung development, retention of fluid within the lung to assure adequate intraluminal pressures, and establish connectivity among neural control mechanisms and respiratory pump muscles, such as the diaphragm and intercostal muscles (2, 9, 157).
Human FBMs are detectable as early as 10 weeks of gestation and change their frequency throughout gestation (227, 242). FBMs occur only during low voltage electrocortical activity, which is associated with rapid eye movement (REM) sleep, and accounts for approximately 40% of a fetal sheep’s life (similar in the human fetus) in the last trimester (66) and reviewed in Ref. 293. How and why FBMs occur only during low voltage electrocortical activity in the fetus is not known. FBMs occur infrequently early in gestation (112) and become more regular and episodic closer to term where periods of FBMs (55, 300) and the intervening apneic periods are longer (13, 266). In the developing fetal rat, single FBMs are first observed at E16 at low frequencies (~8FBMs/h) and increase in frequency at E18 (~40 FBMs/h), reaching a maximum frequency of approximately 80 FBMs/h by E20 (150). Episodic (not single) FBMs are first observed in the fetal rat at E18 (40 episodes/h) (150). The age of onset and maturation of FBMs in mice (E16) is similar to the rat (228). These in vivo measurements coincide with in vitro rat pup electrophysiology data that indicate commencement of inspiratory drive at E17 with continual increases in motor output throughout the remaining gestational development (72, 113). During the last days of gestation (E19-21), the respiratory neurons functionally mature to the level of the neonate’s (235), with the spatio-temporal patterning of respiratory neuronal activity from E20 to 21 reflecting that of the postnatal rat (237). The transition to more mature-like respiratory activity is associated with age-dependent changes in chloride conductance through respiratory neurons that cause respiratory neuron excitation preceding E19 but thereafter inhibiting respiratory neurons (261).
The maturation of FBM rhythmicity is reflected in the development of the preBötzinger complex (preBötC) (66, 281, 293) and parafacial respiratory group/retrotrapezoid nucleus (pFRG/RTN) (236), two important regions controlling breathing rhythmicity in the fetus and neonate. Because transcriptional regulation contributes to development of these neuron populations, measuring expression levels and experimental manipulations of specific genes are used to understand their functional roles. For example, the subpopulations of glutamatergic and NK1R (Substance P receptor) expressing neurons that contribute to breathing rhythmicity of the preBötC (238) are transcriptionally regulated by developing brain homeobox protein 1 (Dbx1) and roundabout homolog 3 (Robo3). Dbx1facilitates glutamatergic preBötC neuron development whereas Robo3 connects the bilateral preBötC nuclei necessary for synchronization of breathing rhythmicity (40, 110). PreBötC neurons become terminally differentiated in the second half of gestation (E12-13 of the rat) and then migrate to their final location in the ventrolateral medulla (E16.5-E18; E15.5 in the mouse (295)) at which point FBMs demonstrate episodic rhythmic activity (238).
pFRG/RTN neurons also demonstrate breathing rhythmicity (237, 295), where the most rostral portion of these neurons contribute specifically to pre-inspiratory activity (234). Rhythmicity of pFRG neurons begins at E14.5, and within 24 h, pFRG respiratory rhythmogenesis is coupled with the preBötC neurons in mice (295). The development of pFRG neurons is dependent on the Egr2 (also known as Krox20) and Phox2b transcription factors (295). Disruption to Egr2 expression during embryogenesis causes significant loss of rhombomere 3 and 5 differentiation, portions of the hindbrain that give rise to these rhythmogenic nuclei (275). Disrupting Egr2 expression causes slowing of rhythmogenic activity mediated by a subpopulation of Phox2b expressing pFRG/RTN neurons and impairs the subsequent coupling of respiratory rhythmogenesis with the preBötC (295). Thus, the pFRG and preBötC neurons have critical roles in establishing neural respiratory network connectivity during fetal development in addition to establishing the onset of FBM rhythmicity.
FBM rhythmicity occurs through activation of AMPA (2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid) receptors expressed on these rhythmic neurons. These receptors can be modulated by a variety of other neurochemicals including serotonin (5-HT), substance P, thryotropin releasing hormone (TRH), adenosine triphosphate (ATP), noradrenaline, adenosine, γ-aminobutyric acid, and glycine, and a variety of different transmembrane proteins (transporters and receptors) as reviewed previously (112). FBMs are also modulated by chemosensory input, such as acute hypoxia as discussed below (136, 155, 161, 162).
Thus, while FBMs are not needed for fetal gas exchange, they play a vital role in mediating the development of the respiratory system. Their onset occurs early in gestation where they present as single “breaths” and while maturation progresses, they take on more mature-like/neonatal-like breathing patterns. Such maturation corresponds with the development of two key regions in the brainstem important for breathing rhythmogenesis, the preBötC and pFRG/RTN. These processes, and other related developmental aspects of the respiratory system, can be acutely or chronically altered by fetal hypoxia, as will be discussed below.
Types and Impact of Fetal Hypoxia on the Control of Breathing
Fetal hypoxia, also known as intrauterine hypoxia, occurs from a variety of complications of maternal, placental, or fetal origin. Kingdom and Kaufmann categorized fetal hypoxia into three subtypes based on the physiologic origins contributing to hypoxia (146). They are (i) preplacental hypoxia (mother and fetus are hypoxic), (ii) uteroplacental hypoxia, and (iii) postplacental hypoxia (fetus is hypoxic) (146). Fetal hypoxia can be acute, intermittent, or chronic in nature due to a variety of causes (101, 152). Common causes for preplacental hypoxia include chronic hypoxic environments, such as high-altitude residence and preexisting maternal cardiovascular diseases including pulmonary hypertension, cyanotic heart disease, and heart failure (146). Pregnant women suffering from obstructive sleep apnea and sleep-disordered breathing often exhibit intermittent hypoxia, which contributes to gestational intermittent hypoxia (GIH) (135). Abnormal placental development and placental vascular disease contribute to uteroplacental hypoxia (146). Causes for postplacental hypoxia include impaired uterine blood flow, fetal anemia, fetal cardiac failure, and genetic anomalies (146). The normal, healthy fetus maintains sufficient oxygen consumption in response to acute reductions in blood flow to the fetus and/or oxygen-carrying capacity by increasing oxygen extraction from the placenta (67, 78, 129, 263) (and as reviewed previously (51)). However, longer reductions in blood flow or oxygen-carrying capacity cause reduction in fetal growth (219). Further, while acute hypoxia does not cause major changes to the fetus, gestational intermittent, and chronic hypoxia can be pathologic (263).
Acute hypoxia
The carotid bodies are the main peripheral oxygen sensing organ in the fetus and neonate located bilaterally at the bifurcation of the common carotid artery composed of type 1 and type 2 glomus cells of the carotid body that are stimulated by low arterial PO2 levels (159, 165, 167, 254). Hypoxic activation of fetal carotid bodies occurs at a drastically lower set-point relative to the neonate and adult due to the naturally lower PaO2 in the fetus (~25mmHg PaO2) and is mediated by A2a receptor activation by adenosine (153, 155, 161). The innervating carotid sinus nerve sends afferent input to the respiratory control network through the nucleus of the solitary tract (NTS) and increases firing rate when PaO2 drops below approximately 15 mmHg in the fetus (64). In fetal lambs, carotid chemoreceptor firing rate increases from approximately 13 Hz at 25 mmHg PaO2 to 42 Hz at 10 mmHg PaO2 at 108 days gestation whereas it increases even more at 140 days (term birth for sheep is ~145days (103)) gestation (from 21 Hz at 25 mmHg PaO2 to 50 Hz at 10 mmHg PaO2 (35)). Throughout this period, the carotid bodies are tonically active and are responsive to hypercapnia induced by 1 to 2 mL bolus injection of CO2-saturated saline into the lingual artery in the fetus (35).
In the fetus, acute hypoxia depresses FBMs (38, 159). However, the carotid bodies do not contribute to the FBM depression in response to acute hypoxia as carotid sinus nerve transection has no effect on FBM depression (208). Rather, acute hypoxemic stimulation of carotid bodies in the fetus (induced by ewe FIO2 of 95% N2 with 5% O2 or occlusion of ewe’s hypogastric artery (15)) causes a cardiovascular response represented by a decrease in heart rate and increase in peripheral vascular resistance which are abolished with carotid body denervation (15). These cardiovascular effects occur through increased vagal activity and vasoconstriction of peripheral blood vessels through increased sympathetic tone without an increase in FBMs (15, 35, 102, 130). The combination of FBM depression and vasoconstriction is likely a protective response referred to as fetal brain sparing which decreases oxygen consumption in, and redirects blood flow away from nonvital organs (e.g. lungs, gut, kidneys, and liver) and to the brain (101).
The depression of FBMs is mediated by central mechanisms as decerebration eliminates the hypoxic ventilatory depression (195). Similarly, decerebration in neonatal rabbits abolished the depressive component of the biphasic neonatal hypoxic ventilatory response (HVR) indicating the neonatal hypoxic ventilatory decline may be mediated by similar mechanisms as hypoxic FBM depression (195). Thus, investigating the mechanism of neonatal hypoxic ventilatory decline may help elucidate mechanism of hypoxic FBM depression. Such studies have led to the identification of multiple brain regions implicated in hypoxia sensing. For example, the midbrain red nucleus appears to be involved in central hypoxia sensing since electrolytic lesions of this region attenuate hypoxia-induced ventilatory depression (measured with FIO2: 0.1–0.12) in 26 day old rabbits (305). Alternatively, lesioning the parafascicular nuclear complex within the thalamus of lambs (164) (Figure 2; lambs < 8 days old) and fetal sheep (161) also eliminates hypoxia-induced ventilatory depression, as does lesioning the lateral pons (136, 137). Moreover, the subcoeruleus nucleus of the pons is selectively activated in response to hypoxia (induced by ewe FIO2: 0.08–0.09 for 2 h) during fetal development but not postnatally (43).
Figure 2.
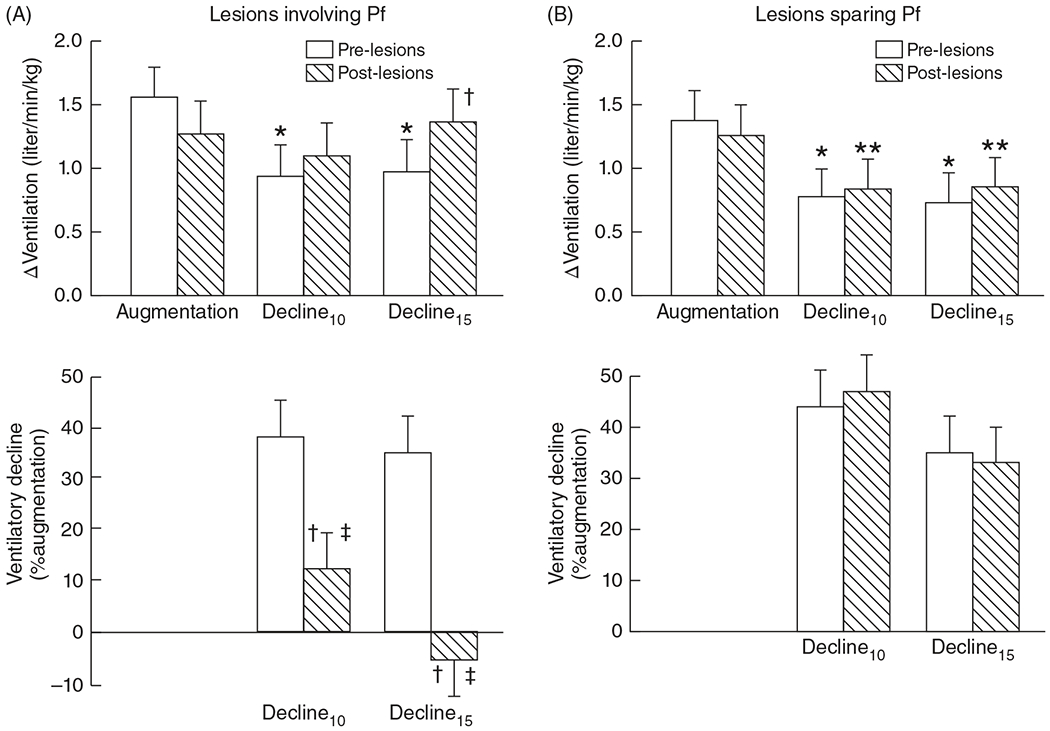
Effects of ibotenic acid lesioning within or sparing the thalamic parafascicular (Pf) nuclear complex in neonatal lambs on the depression phase of the hypoxic ventilatory response. Lesioning the Pf removes the ventilatory depression at 10 and 15 min (decline 10 and 15, respectively) expressed as a change in ventilation from prelesion values (upper left) or as a percent of the augmentation phase (lower left) (A). Lesions sparing the Pf have no effect on the hypoxic ventilatory decline (B). *P < 0.005, **P < 0.03 compared with augmentation phase. †P < 0.005 versus prelesion at same time, ‡ P < 0.05 versus thalamic lesions sparing Pf at same time. Adapted, with permission, from Koos BJ, et al., 2016 (164).
Like the carotid bodies, adenosine and A2a receptors appear to be key factors involved in central hypoxia sensing. The hypoxic depression of FBMs is likely mediated by adenosine release in this area, since lesioning this area in fetal sheep removes the hypoxic or adenosine-mediated depression of FBMs (Figures 3A and 3B; (155, 161, 162)). This acute response lasts for 12 to 16 h of hypoxia (Figure 4). Thereafter, FBM activity resumes as FBMs are necessary for respiratory development. In summary, because FBMs require oxygen consumption and are not vital for fetal oxygenation, depression of FBMs is a defense mechanism against acute hypoxia.
Figure 3.
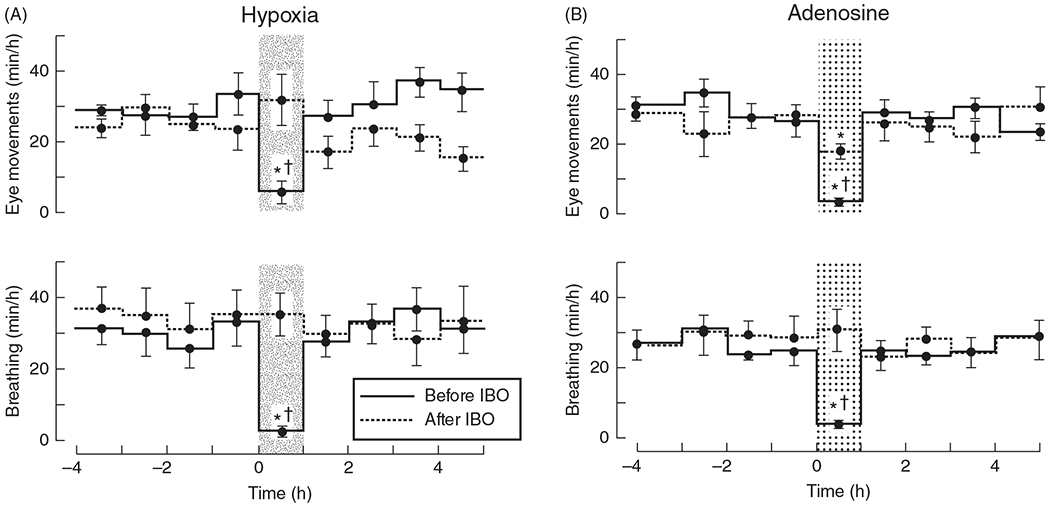
The thalamic parafascicular nuclear region mediates hypoxic depression of fetal breathing movements in fetal sheep. Hypoxia-induced suppression of breathing and associated eye movements is completely removed following lesioning of the thalamic parafascicular nuclear region by ibotenic (IBO) acid (A). Adapted, with permission, from Koos BJ, 2002 (163). In a similar experiment using exogenous adenosine, the known neurochemical mediating hypoxia-induced suppression of fetal breathing suppresses breathing and associated eye movements before IBO injection but is significantly impaired following IBO lesioning of the thalamic parafascicular nuclear region (B). Adapted, with permission, from Koos BJ, et al., 2000 (162).
Figure 4.
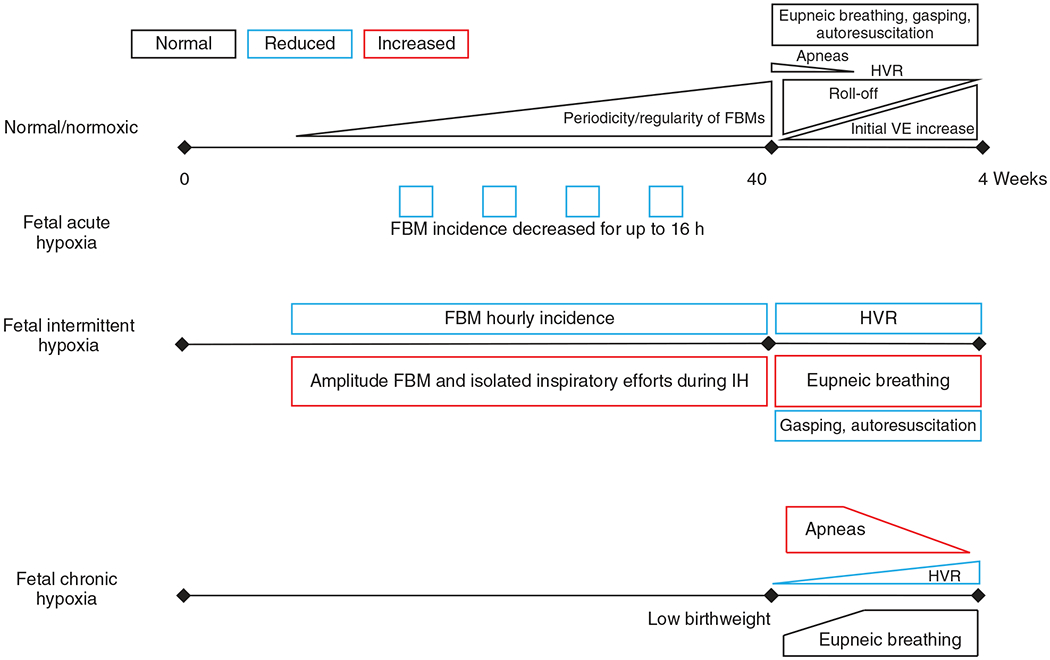
Schematic summary of in-text descriptions of the effects fetal normoxia and fetal acute, intermittent, and chronic hypoxia have on breathing. During normal development, the periodicity and regularity of FBM activity steadily rises and approaches postnatal breathing regularity at birth. Upon birth, few apneas are present and quickly diminishes with age. The hypoxic ventilatory response (HVR) begins with a significant secondary role-off pertaining to a decrease in metabolic rate following an initial increase in ventilation (VE). This biphasic HVR quickly matures (within weeks of birth) to a sustained increase in ventilation as the metabolic roll-off diminishes in effect. Fetal acute hypoxia causes a decrease in FBM incidence. Fetal intermittent hypoxia, also called gestational intermittent hypoxia, decreases FBM hourly incidence but increases FBM amplitude and inspiratory efforts during the hypoxic episodes. Postnatally, fetal intermittent hypoxia reduces the HVR and capacity for gasping and autoresuscitation and an increase in eupneic breathing. Fetal chronic hypoxia is typically observed in high-altitude births where infants are born with lower weight. Infants also have increased apneas at birth associated with diminished and delayed development of the HVR. Eupneic breathing is fairly normal aside from the apneas. See text for references.
Adenosine and adenosine receptors
Adenosine and adenosine A2A receptors are involved in carotid body and central hypoxia sensing. Fetal carotid bodies express A2A receptor messenger ribonucleic acid or messenger RNA (mRNA) (307). The carotid bodies are excited by oligomycin, an ATPase inhibitor, adenosine, or by activating the A2A receptor, resulting in marked increases in tidal volume and breathing frequency (153, 161). Additionally, the cardiovascular responses caused by fetal (117–120 days gestation) hypoxia (9.3–15 mmHg PaO2 for 5–60 min) are blocked by both a nonselective adenosine receptor antagonist (103, 154) and a selective A2A receptor antagonist (155).
Adenosine and adenosine receptors are also important in central hypoxia sensing (258). A2A receptor mRNA is expressed throughout fetal rat brains in regions implicated in hypoxia-induced FBM depression (152, 314). Like the carotid body, reduction in ATP produced by mitochondria within the brain is involved in central hypoxic inhibition of FBMs (158). Furthermore, hypoxia increases adenosine levels in the brain (160), and administration of exogenous adenosine inhibits FBMs like hypoxia (156). Blocking central A2A receptors prevents the inhibition of FBMs (163). Prematurely born infants may retain the inhibitory effects caused by A2A receptors unlike term infants since methylxanthines (i.e. caffeine), which are A2A receptor antagonists that effectively stimulate breathing and thereby ameliorate apnea of prematurity.
Pulmonary neuroendocrine cells and neuroepithelial bodies
Alternative peripheral mechanisms that may control fetal breathing are pulmonary neuroepithelial cells (PNECs) which can exist as cell clusters called neuroepithelial bodies (NEBs). NEBs are sensitive to hypoxia (fetal rabbit cultures exposed to 25–30 mmHg PO2), hypercapnia (neonatal hamster slice, 20% CO2 and pH 6.8), and inflammation (42, 61, 179, 287, 317) and are selectively innervated by P2RY1 expressing vagal afferents that project directly to the central respiratory network through the nucleus of the solitary tract (54, 239). Selective and continuous activation of P2YR1-expressing vagal neurons in anesthetized mice induces complete cessation of breathing, or apnea (54). Whether NEBs play a role in regulating FBM in response to hypoxia remains unknown. However, neuroendocrine cell hyperplasia is associated with chronic fetal hypoxia in humans leading to death in Hemoglobin Bart-induced Hydrops Fetalis (290). Additionally, NEB hyperplasia and hypertrophy are found in conditions commonly associated with impaired hypoxic sensitivity such as bronchopulmonary dysplasia (BPD), which occurs when infants are born prematurely during the saccular stage of lung development, and sudden infant death syndrome (SIDS) (62, 63). Additional research is needed to determine if NEBs have any role in mediating hypoxia-induced FBM depression.
Gestational intermittent hypoxia
Maternal sleep-disordered breathing and sleep apnea cause repetitive and intermittent bouts of hypoxemia causing GIH. During fetal development, GIH reduces the hourly incidence of FBMs and increases the FBM amplitude and inspiratory efforts (306) (Figure 4). Though understanding the effects of GIH on the control of breathing is an emerging area of research, early studies are indicating impact of GIH to the control of breathing postnatally (135). Rat pups exposed to GIH (dams exposed to 90 s iterations of 21% and 10% O2) demonstrate increased eupneic ventilation and decreased HVRs from postnatal day 5 to 30 (109). Also, rat pups exposed to GIH have impaired gasping and autoresuscitation (108), though, motor output measured from C4 is less irregular (135) (Figure 4). Preliminary transcriptomic profiles from GIH (dams exposed to 2 min iteration of 21% and 10.5% O2 for 8h/day from 10 to 21 days gestation) exposed adult rats (8–12 weeks old) indicate major transcriptomic differences in microglia compared to adult rats not exposed to GIH, suggesting potential long-term effects of GIH to the resident inflammatory cells within the respiratory control system (86). Furthermore, the preliminary data indicate more differentially expressed genes in GIH females than GIH males within the spinal cord microglia. The potential sex-differences of GIH is further supported by full-data sets published by Johnson et al. in 2018 (135) showing that GIH (dams exposed to 2 min iteration of 21% and 10.5% O2 for 8 h/day from 10 to 21 days gestation) in male, but not female, rat spinal cord tissue is associated with upregulated expression of cyclooxygenase 2 (COX-2). Also, subsequent postnatal immune challenges (P2.5–3.5) are differentially affected where IL-1β and Tnfα mRNA in the brainstem, and COX-2 in the spinal cord, are significantly reduced in female GIH relative to female non-GIH rats (135). Though IL-1β, Tnfα, and COX-2 gene expression was not apparently changed in males, male rats exposed to GIH (and unlike female GIH rats) had decreased C4 neuron burst frequency in response to postnatal LPS treatment (135). Together, these data highlight the postnatal impact GIH has on cells within respiratory control centers and indicate these effects occur in a sex-specific manner. They also implicate neuroinflammatory processes in mediating these changes. Whether these changes have a functional impact remain unknown; future studies are needed to further understand the effects GIH has on fetal, neonatal, and adult control of breathing and the roles inflammation and microglia have in mediating/modulating these potential effects.
Chronic hypoxia and high altitude
Barometric pressure decreases with increasing altitudes, thereby decreasing oxygen pressure and atmospheric oxygen content causing hypobaric hypoxia in residents living at high-altitudes. Noticeable physiologic changes occur at altitudes greater than 2500 m where arterial PO2 is between 60 and 70 mmHg and the SpO2 is close to the steep portion of the oxygen dissociation curve (138). People living at altitudes above 2500 m, including pregnant women, are thus exposed to chronic hypoxia. Although pregnancy-associated ventilatory changes partially benefit the mother and fetus at high-altitude (138), the fetus is still at greater risk for chronic hypoxia, increasing the risk for growth restriction and premature birth associated with impairments to fetal control of breathing (204).
IUGR and prematurity
Pregnancy itself is associated with changes to the control of breathing due to changes in hormonal patterns that act on the carotid bodies and central respiratory network, including hyperventilation and increased hypoxic and hypercapnic sensitivities (102, 114, 182, 186). Because oxygen saturation is on the flat part of the oxyhemoglobin curve at sea-level, hyperventilation elevates PaO2 without much change in oxygen saturation. At high-altitude, where PaO2 and SpO2 are lower, a comparable hyperventilation is able to increase PaO2 and SpO2 (206, 303). This increase in ventilation is beneficial in some high-altitude pregnancies (Coloradan) causing an increase in maternal arterial O2 content and thus maintaining oxygen delivery to the uterus (207). Other high-altitude populations, like Tibetans, rely on redistribution of blood flow in the uterine and common iliac arteries without increases in maternal arterial O2 content to maintain oxygen delivery to the uterus (204). Failure of minute ventilation to increase over the course of gestation is associated with smaller sized babies (206). In response, the fetus decreases metabolic activity to reduce oxygen consumption, consequently contributing to reduced fetal growth (see Ref. 263). For these reasons, high-altitude pregnancies are, in general, associated with higher rates of intrauterine growth restriction [IUGR, also known as fetal growth restriction (FGR)]. IUGR refers to a fetus that has failed to reach its growth potential and is defined as a newborn weighing less than the 10th percentile (17). Statistically, for every 1000 m of elevation over 2500 m, infant birthweight is lowered by an average of 120 g (134) and is associated with three times the occurrence of IUGR compared to sea level births, contributing to low birth weight (140, 302).
IUGR and low birth weight affect the development of the control of breathing as indicated from animals exposed to prenatal hypoxia (by uterine artery ligation or exposure of pregnant animals to atmospheric hypoxia). In lambs, prenatal hypoxia-induced growth restriction (50% uterine blood flow restricted) decreases the postnatal HVRs but not the hypercapnic ventilatory response (218), which are similar findings in rats (118, 176, 218). Also, the hypoxic but not the hypercapnic ventilatory response is reduced in premature birth (not caused by prenatal hypoxia) (65), likely due to a delay in the maturation of the carotid bodies (65). In a similar study, prenatal hypoxia (10% O2 exposure of dams starting embryonic day 5–20) eliminated the ventilatory decline of the HVR which is normally present within the first week of life in rats (250). Furthermore, prenatal hypoxia increases postnatal eupneic breathing (251, 299) and its irregularity (250) (Figure 4). These ventilatory control changes are driven by the effects of prenatal hypoxia on the carotid bodies and central respiratory network; it alters the developmental pattern of catecholaminergic activity with the carotid bodies, the petrosal ganglion, and brainstem catecholaminergic cell groups (251). The increases in respiratory rhythm measured by whole-body plethysmography, C4 activity from en bloc preparations, and in acute brainstem slices following FGR (10% O2 exposure of dams starting embryonic day 5–20) is likely mediated by changes in catecholaminergic (indicated by increased levels of levodopa or l-3,4-dihydroxyphenylalanine (L-DOPA)) activity within the brainstem (299). This is likely at the level of the pons as blockade of A2 adrenergic receptors within the pons and not medulla reversed the increases in C4 firing frequency relative to controls (299).
Prenatal hypoxia and growth restriction are also associated with abnormal myelination (297), decreased Substance P in the spinal trigeminal nucleus but increased Substance P+ neuronal density in the NTS, increased met-enkephalin in the hypoglossal and ventral medulla, increased astrocyte proliferations in the dorsal motor nucleus of the vagus (DMV), NTS, and around blood vessels throughout the brainstem (296). Increased density of mu-opioid receptor binding sites is also found throughout the brainstem in IUGR rats (177). These functional and histological changes to the control of breathing may in part be secondary to impairments in lung development associated with IUGR (118) as infants with IUGR are at greater risk for premature birth and BPD (7, 39, 98, 105, 169, 252). High-altitude (>400 m) is also associated with significantly higher BPD rats compared to infants born <33 weeks gestation at altitudes lower than 400 m (171). Both premature birth and BPD are associated with respiratory control abnormalities (274, 286) and for every 100 m of altitude increase over 400 m, infants born <33 weeks gestation in high-altitude theirs odds of BPD increase by 8% and their odds for BPD/death increase by 9% (171).
In the case of premature infants with BPD, the lungs and the respiratory control system are underdeveloped, indicated by periodic breathing, apneas, and reduced HVRs (16, 46, 56). The impact BPD has on the control of breathing is likely multifactorial, owing to immature respiratory and antioxidant systems coupled with environmental exposure to high supplemental oxygen levels and/or other therapeutic interventions (16, 69, 111, 223, 226). These infants are also subject to intermittent hypoxia due to lung and respiratory center immaturity (56). BPD in humans is associated with reduced oxygen sensitivity (46, 143) which is attributed to time spent on mechanical ventilators (143). However, infants with BPD are also exposed to therapeutic hyperoxia which may also contribute to reduced oxygen sensitivity given that HVRs are blunted following perinatal hyperoxia exposure in animal studies (19, 23, 115, 175). Evidence suggests that there are long-term respiratory control abnormalities in infants with BPD following hospitalization: the breathing pattern in BPD infants between 36 and 42 weeks postmenstrual age show significantly decreased inspiratory and expiratory times with no changes in tidal volumes, and elevated peak inspiratory and expiratory flow rates, respiratory rate, minute ventilation, and ventilatory drive (tidal volume/inspiratory time) compared to age-matched, non-BPD infants (274). Children (8–12 years old) with moderate/severe BPD have expiratory airflow limitations and decreased oxygen uptake with increased ventilatory responses (189). BPD adults have significantly greater expiratory flow limitation compared to non-BPD preterm and full-term adults (possibly due to lung or lung and breathing control abnormalities) (185). Long-term changes to the control of breathing in BPD remains incompletely understood. However, eupneic ventilation does not appear altered in BPD adults, which may be due to compensatory changes within the neural control of breathing in response to BPD. For example, in a set of recent animal studies, BPD induced by neonatal hyperoxia exposure (0–10 days of life) in rats caused sustained lung disease through day 60 of life (223). By day 12 and through day 60 BPD rats were hyperventilating to sustain normal levels of oxygenation. This was sustained through day 60 and was associated with increased glial marker expression and altered protein expression levels (224, 225). These observations indicate potential compensatory responses within the central respiratory network to lung disease. Although breathing may not be apparently different in adult BPD patients, the mechanisms generating their breathing might be different.
IUGR in native and nonnative high-altitude residents
IUGR disproportionally affects more nonnative than native high-altitude residents. Nonnative high-altitude residents (i.e. Europeans) have fivefold greater incidence of IUGR compared to indigenous high-altitude residents (i.e. Andeans) (138) (Figure 5). Andean birthweights at 3600 m are reduced by 236 g whereas the reduction is 418 g for European newborns (318). These effects on birthweight are independent of any potential socioeconomic factors (134). This dichotomy may be driven by different pregnancy adaptations in high-altitude native populations (138). Specifically, uteroplacental blood flow is greater in high-altitude natives (e.g. Andeans) compared to nonnatives (e.g. European) (138). This limits the potential for fetal hypoxia and associated changes to the development of the control of breathing (Figure 5B) (138). Furthermore, while Andean newborns have lower cerebral and regional oxygen saturation, their transcutaneous vessel density is 14% higher compared to nonnative newborns; microvascular vessel density is higher in newborns born to high-altitude dwelling mothers compared to sea level counterparts suggesting such increases in microvascularization occur during fetal development as an additional adaptive mechanism to the lower oxygen environment at high-altitude relative to sea-level (99).
Figure 5.
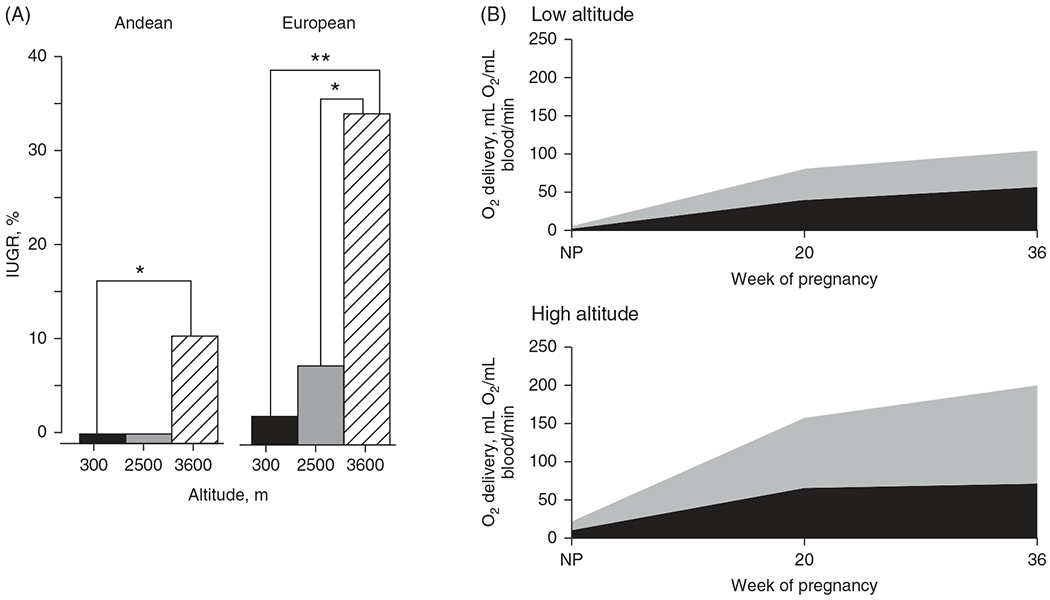
Rate of IUGR at altitude and differences in uterine blood flow during pregnancy between nonnative (European) and native (Andean) high-altitude populations. The rate of IUGR increases with altitude and at high-altitude Europeans have a fivefold greater occurrence of IUGR compared to Andeans after adjusting for other fetal growth factors (shown in the graph are unadjusted values; A). A potential explanation for the IUGR rate disparity at altitude between Andeans and Europeans is the compensatory twofold greater increase in uteroplacental oxygen delivery in Andean compared to European women at 36 weeks’ gestation indicating potential genetic adaptions across generations (B). NP, nonpregnant. *P < 0.05, **P < 0.01. Reused, with permission, from Julian CG, 2011 (138).
While clear differences in IUGR are present between native and nonnative high-altitude residents, there is also evidence of different adaptations during pregnancy among high-altitude populations, such as those from Tibet, Peru, and Colorado (Leadville). For example, pregnancy adaptations in Tibetan women do not reflect the increased levels of arterial O2 content measured in pregnant women from Peru or Colorado yet birthweight is highest in Tibetan neonates, perhaps due to redistribution of blood flow to the uterine circulation (204). Furthermore, Tibetans have lower hemoglobin concentrations than Andeans (27) while Andeans have lower HVRs but higher resting ventilation compared to Tibetans (28). Despite the differences among Andeans and Tibetans, both demonstrate increases in uteroplacental blood flow and protection from IUGR compared to nonnative high-altitude residents (see above).
The physiologic differences among high-altitude natives likely arise from genetic adaptations. For example, gene expression adaptations have been found in Tibetan and Andean high-altitude natives, such as the downregulation of endothelial PAS domain-containing protein 1 (EPAS1) [gene encoding hypoxia-inducible factor 2α (Hif-2α)] and EGLN1 [gene encoding prolyl hydroxylase 2, prolyl hydroxylase domain 2 (PHD2), which degrades hypoxia inducible factors (Hifs)] (31, 184, 244). While both populations show evidence for these two genes to be involved in adaptations to chronic hypoxia, only Tibetans had EGLN1 and EPAS1 gene variants that associate with low hemoglobin concentrations (29, 31, 244). Indeed, EPAS1 knockdown mice have blunted physiological responses to chronic hypoxia, similar to high-altitude Tibetan natives, including lower hemoglobin levels that protect against polycythemia and lower pulmonary vasoconstriction (244). Also, unlike wild-type erythroid progenitors, hypoxia does not stimulate proliferation in erythroid progenitors expressing EGLN1 with a Tibetan-based mutation (184). Together, these data highlight divergent evolutionary physiologic adaptations to chronic hypoxia apparent in high-altitude natives that give rise to protective adaptations during pregnancy. These data also begin to demonstrate the gene expression adaptations that underlie such physiologic adaptations. Future research will unravel the link between genetic (or epigenetic (139)) variations that give rise to other phenotypic differences among the populations discussed above.
Basics of Neonatal Respiratory Physiology
Upon birth of term infants, the respiratory system immediately transitions to independent alveolar ventilation. Ventilatory responses to acute hypoxia are different between the fetus and neonate, where breathing movements decrease in the fetus but breathing increases in the neonate. Also, exposure to prolonged durations of hypoxia has different effects on the development of the neonate’s neural respiratory control network, causing both short and long-term effects on the regulation of breathing.
Neonatal oxygenation
The transition to alveolar ventilation causes a gradual increase in SpO2 over the first 10 to 15 min after birth (192). Due to elevated pulmonary vascular resistance and right-to-left shunting at the ductus arteriosus, there may be a difference between preductal and postductal SpO2 (192) in the first few minutes after birth. However, by 15 min after birth, most term infants have SpO2 values in the high 80 s to 90 s and no differences between pre- and postductal saturations (192). This increase in oxygenation occurs due to rapid decrease in pulmonary vascular resistance in the neonate.
With increased use of delayed umbilical cord clamping, the effect of placental transfusion on neonatal oxygenation can be studied. For example, Ashish et al. have reported that with delayed cord clamping, preductal SpO2 is 18% higher at 1 min, 13% higher at 5 min, and 10% higher at 10 min when compared to infants whose cords were clamped within 60 s of birth (144). Following umbilical cord clamping, lungs are the sole site of gas exchange. With the onset of rapid pulmonary vasodilation, there is an eightfold increase in pulmonary blood flow and a switch in ductal shunt to left-right. Neonates continue to possess high concentrations of fetal hemoglobin resulting in increased oxygen content and delivery (273). With advancing postnatal age, pulmonary vascular resistance continues to decrease, and fetal hemoglobin is gradually replaced by adult hemoglobin, leading to gas exchange physiology and oxygen-hemoglobin dissociation curve similar to that of adults (271, 291).
Neonatal control of breathing
The neural respiratory network, comprised of cell populations throughout the pons and medulla, are sufficiently “ready” in the neonate to work in concert as a network to maintain blood gas homeostasis through the regulation of alveolar ventilation (49, 286). Unlike the breathing activity during fetal development, postnatal breathing is constant and required for survival. Across states of vigilance and activity, respiratory rhythm is characterized by inspiration, postinspiration, and expiration (282). Chemosensory information and mechanical stretch receptors of the lungs provide continual sensory input to the central respiratory network necessary to generate proper motor output to the respiratory muscles to meet metabolic demands. Although “ready,” the neural respiratory network in neonates is still physiologically immature, indicated by increased apneas, insufficient chemoreflexes, ventilatory instability/irregularity, and other control of breathing abnormalities (1, 48). Within a few weeks to months, these irregularities in term infants are diminished highlighting that the control of breathing undergoes further development postnatally. For example, as early as 1 week after birth the second phase of the HVR transitions from respiratory depression to a sustained increase in ventilation (41).
Any infant born before 37 weeks of gestation is considered preterm. Infants born prematurely have not fully completed fetal respiratory development. Therefore, they are less prepared for independent breathing than term infants. Extremely preterm infants (<28 weeks gestation at birth) in particular have highly irregular breathing, characterized by increased apneas which are mainly central in origin (Figure 6; (170)), increased breath-to-breath variability, hypoxemia, and altered chemoreflexes, especially during sleep as recently reviewed (5). Additionally, many very preterm infants (28–32 weeks) and extremely preterm infants have underdeveloped lungs which complicate oxygenation (217). Thus, the entire respiratory system is insufficiently prepared for independent breathing often necessitating respiratory interventions in premature infants.
Figure 6.
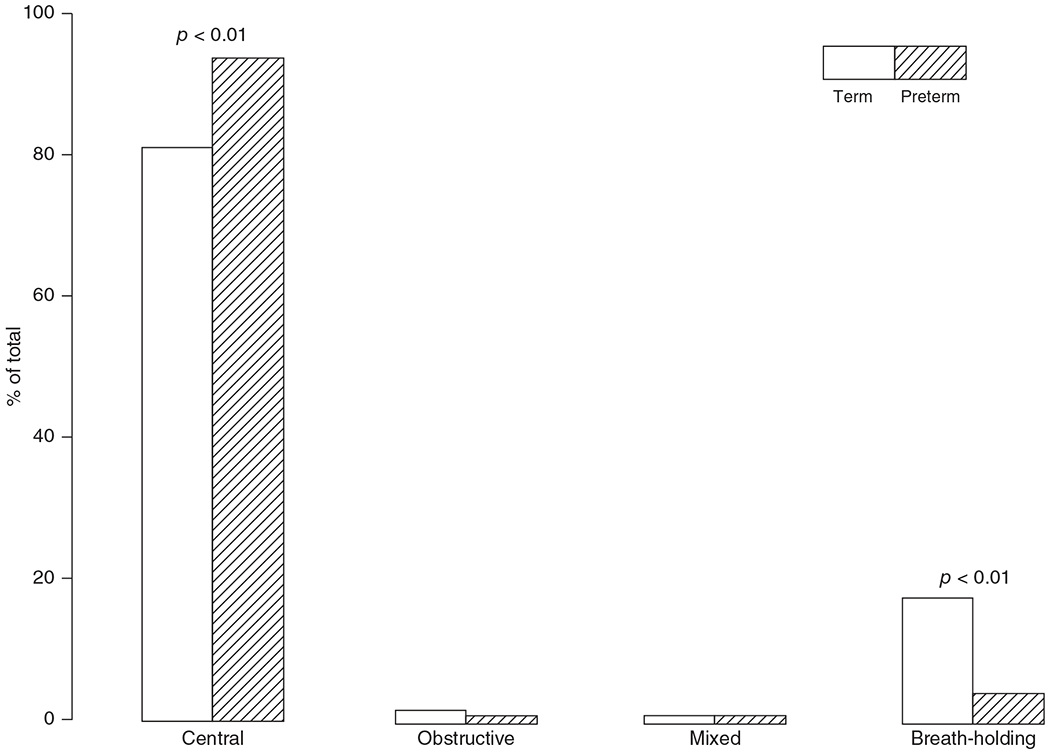
Distribution of apnea types lasting 3 to 15 s in eight term (gestational age: 39.5±0.3 weeks) and eight preterm (gestational age: 34.3±0.4 weeks) infants measured between birth and 56 weeks old. A total of 783 and 4086 apneas were recorded in term and preterm infant groups, respectively. Reused, with permission, from Lee D, et al., 1987 (170). © 1987, Springer Nature.
Types and Impact of Neonatal Hypoxia on the Control of Breathing
Various conditions, environments, and multiple factors impact the development of hypoxia sensing in the neonate. Such factors include exposure to intermittent or chronic hypoxia, hyperoxia, inflammation or infection, and premature birth. These factors may arise during fetal development, contribute to premature birth, and last throughout neonatal or adult life. Exposure to certain factors, like hypoxia, within a specific developmental window (and not at any other time), can cause lasting changes to respiratory control, a concept referred to as developmental respiratory plasticity (see review (18)).
Acute hypoxia
The carotid body chemoreceptors initiate the HVR in the neonate (see review (165)). The HVR in the neonate is different than in utero and undergoes postnatal development lasting between 3 and 4 weeks (variable across species) (50). The HVR is biphasic for up to 1 month of age characterized by a short initial increase in ventilation followed by a decrease in breathing to levels at or below control values (264). Postnatal development of the HVR is generally the same across mammals including lambs, cats, piglets, and rats, where the developmental age dictates the magnitude of the two phases of the HVR (reviewed in Ref. 293). Generally, the initial increase in breathing is low immediately after birth and progressively increases, while the ventilatory roll-off (the second phase) progressively diminishes until full maturation of the HVR, characterized by an initial and sustained increase in breathing (264, 293).
A decrease in metabolic rate is a major contributing factor to the neonatal HVR (210, 213), where neonates decrease O2 consumption to a greater extent than during a mature HVR (293). The decrease in metabolism occurs because brown fat mobilization for thermogenesis is reduced in the neonate (209–211, 213). Because the decrease in metabolic rate is greater than the decrease in breathing, the neonatal HVR effectively causes hyperventilation regardless of the magnitude of the HVR (210, 211,213). As the acute hypoxic response approaches maturation (see review for details (293)), the degree to which metabolism decreases, and the increase in ventilation align more closely, proportionate to the metabolic needs. In other words, hyperpnea becomes the predominant feature of the mature HVR.
Intermittent hypoxia
Intermittent hypoxia is the repeated episodic drop in blood oxygen saturation typically under 80% with greater prevalence in premature infants due to multiple factors including immaturity of the respiratory system, lung disease, and anemia (133). In preterm infants born between 24 and 27 weeks gestation, episodes of intermittent hypoxia are relatively low in the first 1 to 2 weeks of life but progressively increase by weeks 3 to 4 where it plateaus through 10 weeks of life (Figure 7; (71, 133)). Approximately 50% of preterm (<37 weeks gestation) infants have intermittent hypoxic episodes (253).
Figure 7.
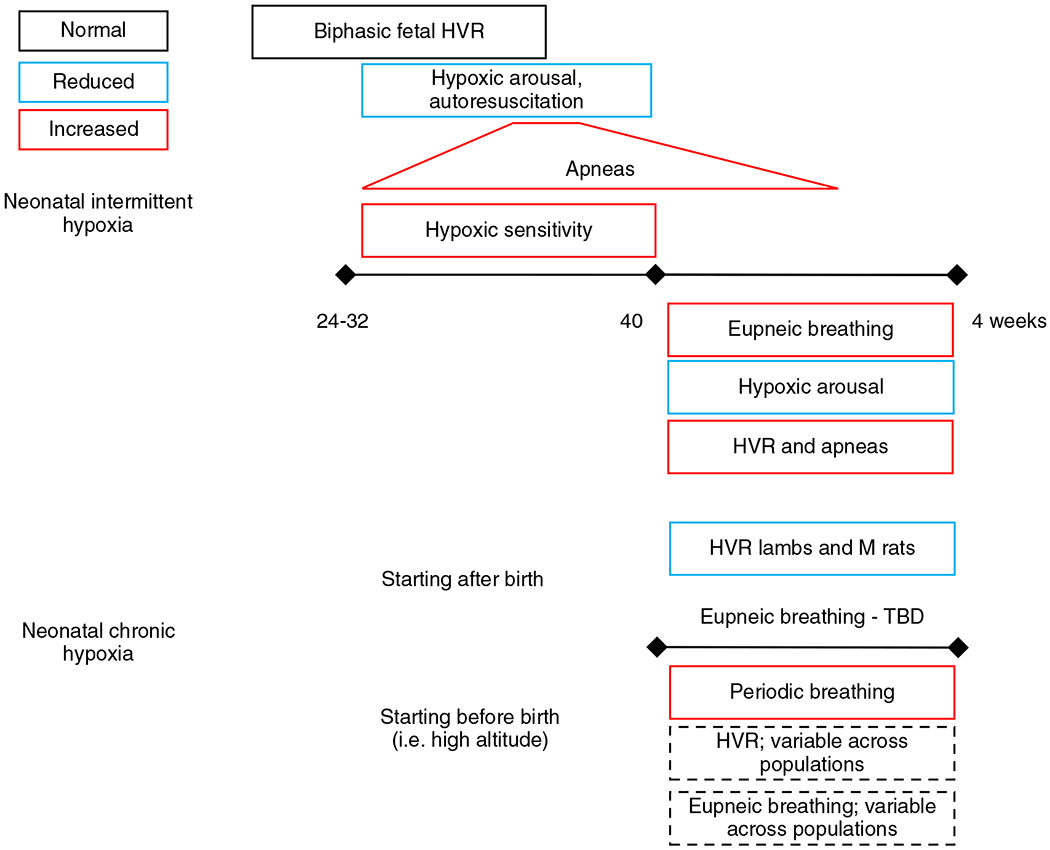
Schematic summary of in-text descriptions of the effects neonatal intermittent and chronic hypoxia have on breathing. Infants born premature (24-32 weeks) retain a biphasic fetal hypoxic ventilatory response (HVR) which is associated with decreased hypoxic arousal and autorescuscitation but increased number of apneas (which plateaus around 10 weeks after birth). The increased apneas are a reflection of apnea of prematurity but also enhanced hypoxic sensitivity which can cause ventilatory overshoots and trigger the CO2 apneic threshold. Term infants exposed to neonatal intermittent hypoxia have increased eupneic breathing, HVR, and more apneas though arousal from hypoxia is reduced. Exposure to chronic neonatal hypoxia commencing after birth is associated with reduced HVR [in lambs and male (M) rats]. The effects on eupneic breathing are equivocal and thus remain to be fully elucidated. Infants that continue to be exposed to chronic hypoxia after birth (i.e. high-altitude births) demonstrate increased periodic breathing but the effects on the HVR and eupneic breathing are variable across high altitude populations. See text for references.
Immature hypoxia sensing in the preterm infant, indicated by the persistence of the fetal biphasic respiratory response to hypoxia, effectively causes episodes of hypoxia (193). However, elevated hypoxic sensitivity may also contribute to the generation of apneas in preterm infants (4,47), consistent with the role of carotid bodies in causing apneas, particularly during sleep (68, 280). Though seemingly paradoxical, an enhanced hypoxic sensitivity causes ventilatory overshoot whereby sufficient oxygen is inhaled, but excessive carbon dioxide is exhaled to, or below, the apneic threshold increasing the apneic index (number of apneas per hour) (141). Aside from hypoxic sensing, preterm infants suffer from apnea of prematurity, a consequence of immature respiratory drive to breathe characterized by the repeated cessation of breathing for more than 20 s or a shorter pause in breathing but with bradycardia and/or oxygen desaturations (80). Lastly, though apneas are mainly central rather than obstructive in origin (95), reduced upper airway muscle tone may contribute to apneas (310). The effects of intermittent hypoxia in preterm infants are summarized schematically in Figure 7.
Intermittent hypoxic episodes alter the HVR, but also eupneic breathing and capacity for respiratory plasticity. While apneas typically precede decreases in oxygen saturation (133), exposure to intermittent hypoxia during the neonatal period can enhance hypoxic sensitivity and thus contribute to more apneas (141). For example, in 2 day old rat pups, intermittent hypoxia (15 s of 5% O2 with 5 min recovery at 21% O2, 9 times/h for 8 h/day) increased the hypoxic ventilatory chemoreflex, mediated by faster and stronger firing discharge measured from ex vivo carotid bodies (245). A similar finding of augmented carotid body hypoxic sensitivity occurs after 10 days of a similar intermittent hypoxic regimen (9 episodes/h; 8 h/day) in P10 rat pups (243). Furthermore, in a similar study, intermittent hypoxia (21%–5% O2 within 100 s then back to 21% in 140 s, repeated 6times/day) for the first 10 postnatal days in rats augments the HVR and it increases the apneic index and duration (141). This finding is consistent with the positive correlation between the number of apneas (i.e. periods of intermittent hypoxia) and the degree of hypoxic sensitivity in preterm infants born less than 30 weeks gestation (231). The carotid bodies participate in mediating the increase in hypoxia sensitivity reflected by increased firing rate in response to hypoxia and hyperplasia of the chemosensitive glomus cells (243). These functional and morphological changes to the carotid bodies may also explain the increased eupneic ventilation following intermittent hypoxia (21% then 10% O2 every 90 s for first 30 days of life in rat) (259) given that carotid bodies are vital for sustaining breathing in neonates, indicated by enhanced mortality of neonates (276) but not adults after carotid sinus nerve transection (96, 124, 220) However, the increased eupneic breathing may also be mediated by central mechanisms in the neonate as indicated by a sustained increase in fictive respiratory frequency after repetitive anoxia (95% O2/5% CO2–95% N2/5% CO2 for 3 min) exposures measured in acute neonatal (P0-7) mouse brain sections (36). Thus, the augmentation of eupneic breathing is likely mediated by multiple mechanisms. The interplay between these mechanisms remains to be tested, especially in awake, unrestrained states.
The sensitivity to intermittent hypoxia of the neonatal carotid bodies is greater than adult carotid bodies. The hypoxic response in neonates is augmented after 72 intermittent hypoxic episodes (1day of 15 s of 5% O2 with 5 min recovery at 21% O2, 9 times/h for 8h/day), while 720 episodes (10 days of intermittent hypoxic episodes) are needed to enhance the hypoxic response in the adult rat (Figures 8A and 8B; (243)). The enhanced adult rat HVR following intermittent hypoxia is reversed after re-exposure to just 10 days of normoxia, whereas, the neonate sustains an increased hypoxic response for up to 2 months after the last intermittent hypoxic episode (Figures 8C and 8D; (243)), together indicating that the neonatal carotid bodies are more sensitive than adult carotid bodies to chronic intermittent hypoxia. This is further supported by selective hyperplasia of chemosensitive glomus cells in the neonate but not the adult after chronic intermittent hypoxia, a finding that may underlie the greater sensitization of the carotid body hypoxic response in neonatal compared to adult rats (243).
Figure 8.
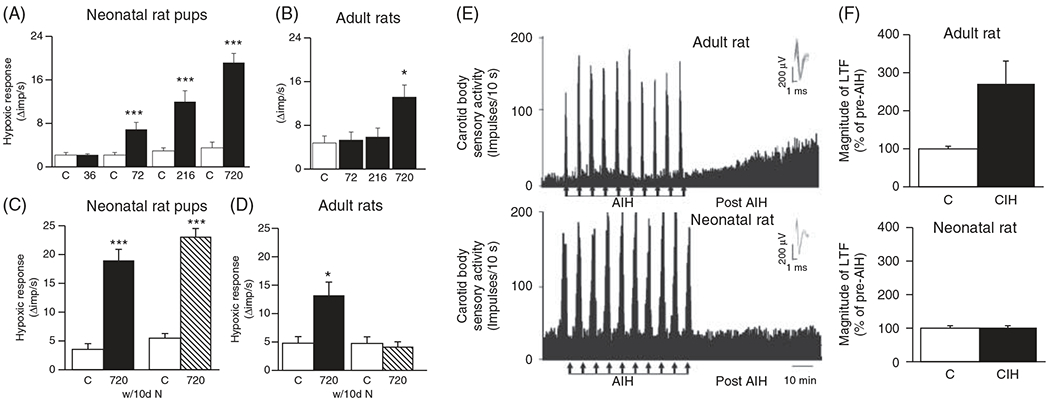
Effects of intermittent hypoxic episodes on carotid body hypoxic sensitivity and long-term facilitation in neonatal versus adult rats. Progressive increases in intermittent hypoxic episodes (36, 72, 216, and 720) cause progressive hypoxic sensitization of the carotid bodies in neonates (A) whereas hypoxic sensitization requires 720 episodes in adults and is not as robust as in neonates (B). The hypoxic sensitization in the neonatal (C) but not the adult (D) rats is sustained after their return to normoxia for 10 days. Intermittent hypoxia causes long-term facilitation in adult rats (E) but not in neonatal rats (F) despite having enhanced carotid body hypoxic sensitivity. Reused, with permission, from Pawar A, et al., 2008 (243).
While the HVR and eupneic breathing are augmented following multiple days (i.e. “chronic”) of intermittent hypoxia in neonates, presumably via morphological changes to the carotid bodies and/or other central mechanisms, the capacity for respiratory plasticity is impaired (145, 243, 260). For example, sensory long-term facilitation (LTF), a form of respiratory plasticity (294) that describes a sustained increase in baseline neuronal activity following episodes of “acute” (within a single day) intermittent hypoxia, can be induced in adult (246) but not neonatal rats previously exposed to chronic intermittent hypoxia (243). Indeed, unlike the LTF observed in the adult ex vivo carotid bodies previously exposed to chronic intermittent hypoxia, acute intermittent hypoxia [10 episodes of 30 s hypoxia (35mmHg PO2) then 5 min of baseline (390mmHg PO2)] has no effect (243), or may even cause a reduction in LTF in the neonate previously exposed to chronic intermittent hypoxia (Figures 8E and 8F; (260)). Though the carotid body may be mediating the LTF it may not be the only site and mechanism as carotid sinus denervated adult rats also demonstrate LTF, albeit to a lesser degree than intact rats, in response to acute intermittent hypoxia (3, 5 min episodes of isocapnic hypoxia (PaO2 = 40 mmHg) (21). These data suggest central mechanisms may also be mediating LTF in adults. The propensity for LTF to be present in the adult but not the neonatal carotid bodies after chronic intermittent hypoxia may be reflected in the lack of serotonin (5-HT) and/or 5-HT receptors, known mechanisms implicated in adult carotid body sensory LTF (247) and in LTF observed elsewhere in the respiratory system (8, 36, 289) Thus, 5-HT and 5-HT receptors may be reduced or not expressed in neonatal carotid bodies which may not permit LTF to occur, although this needs to be tested.
In summary, neonatal chronic intermittent hypoxia causes significant changes to the development of the control of breathing, causing increased hypoxic sensitivity and baseline ventilation, though impairs LTF. These effects of chronic intermittent hypoxia are longer-lasting than and distinct from the adult compared to the neonate. These distinct changes between the neonate and adult suggest, intermittent hypoxia is a stimulus for developmental plasticity, a concept that refers to a stimulus presented during a period of development, but not later in life, that causes sustained phenotypic changes as reviewed elsewhere (20).
Chronic hypoxia
Chronic neonatal hypoxia (<15% FIO2) in rats, cats, and lambs blunts the HVR (79,116, 214, 279, 313). Rats exposed to chronic neonatal hypoxia (FIO2: 0.13–0.15 from birth) for the first 14 days of life have blunted hypoxic responses compared to age-matched normoxic rats (79) as do lambs exposed to chronic hypoxia (FIO2: 0.10 from birth) for the first 12 days of life, through the subsequent 47 days of life (279). A later study reported that male but not female adult rats exposed to neonatal hypoxia (FIO2: 0.10 from birth) have blunted HVRs, indicating potential sexual dimorphic responses (22) (Figure 7). In 2013, Mayer and colleagues (200) exposed neonatal rats first to chronic hypoxia (FIO2: 0.11 from P1 to 5) followed by intermittent hypoxia (FIO2: 0.05 for 5 min, 8 h/day from P6 to 15) to model the oxygenation of the premature infant (288). This led to a blunted HVR, augmented excitatory postsynaptic potentials of neurons within the nucleus of the solitary tract, and attenuated single carotid body fiber responses to hypoxia (199, 200). Thus, chronic neonatal hypoxia impairs peripheral carotid bodies and areas of central sensory integration.
The effects of chronic neonatal hypoxia on eupneic ventilation are less resolved. Rats exposed to chronic hypoxia (FIO2: 0.10) for the first week of life hyperventilate 43 days after being returned to normoxic conditions (212, 232, 233). However, in a later study, Bavis et al. found rats did not hyperventilate following chronic neonatal hypoxia (22), a finding more consistent with sheep (279). Although there are disparities in the effects of chronic neonatal hypoxia on eupneic ventilation, both studies in rats report blunting of the HVR. The effects of chronic neonatal hypoxia on eupneic breathing remain to be determined (Figure 7).
The age in which neonates are exposed to chronic hypoxia influences whether changes in ventilatory control occur acutely, chronically, or are not altered. Exposure to chronic neonatal hypoxia (FIO2: 0.11) from 11 to 15 days of postnatal age (P11-P15) attenuated both the hypoxic and hypercapnic ventilatory responses which were associated with significant increases in mortality, affects not observed in neonatal rats exposed to sustained hypoxia from 1 to 5 or 21 to 25 days of postnatal age (201). These findings are consistent with day P12 to 13 being a “critical window” of respiratory development in the rat (178, 311, 312), which is a specific developmental age in which the respiratory control network has greater vulnerability to environmental stressors (178, 201). Similarly, neonatal but not 7 weeks old rats exposed to the same level and duration of chronic hypoxia hyperventilate (233), an example of developmental respiratory plasticity (20). The specific sensitivity of the P12 to 13 age range appears to be system wide as the increase in mortality and reduced rat HVRs following chronic hypoxia exposure between 11 and 15 days of life are associated with reduced serotonin immunoreactivity and increased microglia in two key respiratory nuclei, which is prevented with minocycline, an inhibitor of microglia (188). Serotonin is a key respiratory neuromodulator with functional importance in providing an excitatory drive to breathing, chemoreception, sleep arousal, auto resuscitation, and trophic effects (45, 53, 74, 122, 316). Irregularities in this system are often identified in the brainstem tissue of infants that succumbed to SIDS, which is also associated with peak incidence at 2 to 4 months of age, perhaps the human correlate of a “critical window” (44, 76, 147).
Together, these data demonstrate that chronic neonatal hypoxia exposure blunts the development of the HVR unlike intermittent neonatal hypoxia. Furthermore, chronic neonatal hypoxia has age-specific effects on the control of breathing peripherally and centrally, where P12 to 13 appears to be a uniquely specific age range for hypoxic stressors to alter the course of respiratory development.
High-altitude
High-altitude is a natural chronic hypoxic environment and high-altitude residents provide an opportunity to study the effects it has on the development of the control of breathing. Infants born at high-altitude have increased periodic breathing after birth, coinciding with lowering of arterial oxygen saturation levels (230). Term infants born at 3100 m in Leadville, Colorado demonstrate four distinct phases to the acute HVR unlike the characteristic bi-phasic neonatal response (57). Peruvian neonates born at 3850 m do not have the hypoxic ventilatory depression as observed in sea-level newborns, although eupneic and hypercapnic ventilation are equivalent (166) (Figure 7). In adults, eupneic ventilation and metabolic rate are similar between natives of the Bolivian cities, Santa Cruz (400 m), or La Paz (3800 m). However, high-altitude natives have deeper and slower breathing patterns (215). It remains to be determined if similar changes in tidal volume and breathing frequency occur during eupneic ventilation in newborns at high-altitude.
High-altitude neonates appear to have greater vagal input to respiratory centers related to the Herring-Breuer inspiratory reflex and lower vagal output during expiration compared to low-land neonates (216). However, these changes may not be ubiquitous across high-altitude populations as different high-altitude populations at similar altitudes (and presumably similar neonatal conditions), have significantly different levels of alveolar ventilation, as indicated between Andean (4216 m) and Tibetan (4203 m) adult natives (205) (Figure 7). Differences in genetics have been identified to contribute to some differences between these populations as discussed in earlier sections (29, 31, 184, 244). The ventilatory adaptations across specific high-altitude populations are reviewed elsewhere in more detail (229).
Inflammation
Inflammation is a common occurrence, especially in premature infants which can be induced by hypoxemia (82) and exacerbate underlying respiratory abnormalities (119). Infants born prematurely have more apneas, periods of hypoxemia, and are at greater risk for infection and sudden death (87, 119, 126, 256). Infection is highly correlated with central apneas in premature infants (120) suggesting infection may be a causative factor for apneas. Indeed, neonatal inflammation can impact the developing respiratory system as recently reviewed (309). It also impairs respiratory control later in life indicated by the reduced capacity for respiratory motor plasticity in adult rats that were exposed to a single bout of intraperitoneal (IP) and lipopolysaccharide (LPS) on day 4 of life (121). Furthermore, to P10 but not P5- or P20-day old rats have reduced HVRs when treated with intr LPS (262). This reduction may be mediated by Il-1β as pretreatment with an IL-1β receptor antagonist delivered intracerebroventricularly but not by intraperitoneal injection prevents intratracheal LPS mediated reduction of the HVR (262,267). These observations implicate a central mechanism of the HVR as the susceptible mechanism to inflammation and suggest the presence of a potential lung-neural axis in mediating the effects of intratracheal LPS. Similarly, I.P. treatment with IL-1β in P9 mice impairs the hypoxic and hypercapnic ventilatory responses and autoresuscitation after anoxia exposure in neonates (123, 278).
Although IL-1β induced by systemic or intratracheal LPS administration alters the control of breathing, IL-1β does not readily cross the blood-brain barrier and requires binding to the IL-1β receptor on the intraluminal membrane of cerebral blood vessels, activating a series of arachidonic acid and prostaglandin E2 generating enzymes (81, 123). prostaglandin E2 (PGE2) is released into the nucleus of the solitary tract, rostroventrolateral medulla, and preBötC microenvironment where it binds to the prostaglandin EP3 (EP3) receptor, causing reduced breathing, impaired autoresuscitation (123) or modulation of eupneic breathing, sighs, and gasping (151). Mice lacking the EP3 receptor do not have reduced breathing or impaired autoresuscitation (278), indicating a potential therapeutic target for neonatal respiratory disorders and a key mediator of respiratory effects originating from LPS or other pro-inflammatory stimuli. Furthermore, levels of cerebral spinal fluid PGE2 are significantly correlated with the neonatal apneic index and with the systemic infection marker, C-reactive protein (CRP), in human neonates (123), indicating a potential biomarker to screen for neonates at risk for respiratory control disorders.
Alternatively, IL-1β may not require binding to receptors to transmit its effect into the brain. Rather, strong evidence suggests potential induction of brainstem IL-1β expression via pulmonary vagal fibers. For example, vagotomy reduces IL-1β mRNA expression in the brain induced by intratracheal LPS in the neonate (12) and blunts the HVR to similar degrees in carotid sinus nerve intact or denervated 10 to 12 day old rats (11). Furthermore, intratracheal administration of bleomycin is another model of airway inflammation and acute lung injury that also causes increases in brainstem cytokine expression without any evidence of systemic inflammation in the blood and associated changes in breathing patterns, though in adult rats (132). Inflammation may also impair the control of breathing during development through its effects on hindering carotid body development, a topic reviewed elsewhere (100). Thus, airway and/or more wide-spread peripheral inflammation can alter the neural control of breathing at multiple levels or sites of the neonatal respiratory system and alter the neural control of breathing—at the blood-brain barrier, pulmonary vagal nerves, or carotid body afferents (100).
Microglia
Chronic neonatal hypoxia exposure around the critical developmental periods (P11–15) in rats also increases microglia cell numbers which reduces serotonin levels within the nucleus of the solitary tract and dorsal motor nucleus, thereby impairing acute HVRs and increasing mortality, effects that are ameliorated with minocycline (188). These data indicate that microglia play a role in modulating the activity of other cell types within respiratory nuclei. The role of microglia in respiratory control is a major area of ongoing investigation.
Neonatal mechanisms of hypoxia sensing and ventilatory responses
The neonatal HVR is biphasic. The change from biphasic to sustained increases in ventilation indicates maturation of the hypoxic sensing mechanisms. While peripheral carotid bodies are the major drivers of the HVR (221, 222, 276), recent data have unveiled hypoxia sensing cells within central respiratory control nuclei (6, 106, 107, 258, 277).
Carotid bodies
Development of carotid body chemoreceptors occurs following birth where oxygen sensitivity is low and increases within 1 to 2 weeks of life. This “resetting” may represent the adaptation of oxygen sensing of the carotid bodies from in utero to ex utero life where there is four times higher oxygen tension (50). The maturation of oxygen sensitivity may occur due to increases in anatomical maturation of chemosensitive type 1 glomus cells of the carotid bodies, maturation of the secretory responses of the glomus cells (increase in intracellular calcium and catecholamine secretion), or along the transduction pathway of oxygen sensing within glomus cells, as reviewed previously (10, 50, 73). Despite the low oxygen sensitivity during neonatal life, the carotid bodies appear more critical during neonatal development than adulthood as denervation of the carotid sinus nerve in neonatal rats causes significant mortality unlike carotid sinus nerve denervation in adult rats (221, 222, 276). This mortality is likely a function of loss of tonic excitatory input from the carotid bodies to the central respiratory centers in the brainstem rather than a relationship with hypoxia sensing, given that carotid body denervation occurred during low carotid body oxygen sensitivity.
Upon maturation, carotid bodies sense hypoxia primarily by the Type 1 versus the Type 2 glomus cells as supported by a large body of literature (reviewed in Ref. 165). The specific sensing mechanisms are extensively reviewed by Prabakar and Semenza (255). In brief, cellular sensing of hypoxia occurs through an interaction with carbon monoxide (CO) and hydrogen sulfide (H2S). O2 sensitive heme oxygenase 2, with O2 as a substrate, generates CO which under normoxic conditions, inhibits carotid bodies but under hypoxic conditions activates the carotid bodies. Type 1 cell expression of cystathionine-γ-lyase (CSE) generates H2S. CO leads to the inhibition of CSE, reducing H2S generation and thereby inhibiting mitochondria and potassium channels, leading to increases in intracellular calcium and depolarization. This causes release of excitatory neurotransmitters onto the innervating carotid sinus nerve, increasing its firing rate and ultimately leading to an increase in ventilation.
Central hypoxia sensing
Recent studies indicate that there are central mechanisms contributing to HVRs which contrast a longstanding belief that the central nervous system in animals lacks hypoxic sensory mechanisms capable of driving HVRs (106). Although evidence for central hypoxia sensing in the intact neonate is lacking, evidence from in vitro cell culture or brain slices (acute or organotypic) from the neonate, with or without corresponding in vivo adult animal studies, indicate that astrocytes play a key role in central hypoxia sensing and contribute to respiratory rhythm generation and the HVR (6, 106, 107, 258, 277). For example, from these studies, it was shown that hypoxia induces ATP release, a signaling mechanism of astrocytes, on the ventral medullary surface produced from the ventral respiratory column of the brainstem (107). The ATP production and release occurs independent of peripheral sensory input from the vagal, aortic, and carotid sinus nerves (107). Functionally, this ATP release maintains respiratory activity during hypoxia (10% O2 for 5 min) as blockade of ATP receptors in the ventral lateral medulla reduced respiratory activity (107). It was later shown that ATP released from astrocytes, specifically within the preBötC, contribute to attenuating the hypoxic ventilatory depression (258). A contribution of elevated intracellular calcium levels, reactive oxygen species (ROS), and PLC-IP3 signaling pathways are proposed leading mechanisms in central hypoxia sensing (Figure 9; (6, 106, 258)). Hypoxia inhibits mitochondrial respiration, causing depolarization of the mitochondrial plasma membrane leading to changes in redox state. This in turn activates signaling mechanisms triggering intracellular calcium release of ATP onto P2Y1 receptor on preBötC neurons (6, 258). Blocking vesicular release from astrocytes within the preBötC increases breathing even without carotid body input during hypoxia (6). Furthermore, HVRs are blunted when ATP release is inhibited and hypoxic ventilatory depression is enhanced after blocking P2Y1 receptors (258). Together, these data indicate a central hypoxic sensing mechanism capable of eliciting a centrally mediated HVR via astrocytic release of ATP onto P2Y1 receptors on preBötC neurons. Further, this response appears to be independent of the carotid bodies (6). However, there is contention that the HVR is dependent on a central hypoxia sensing component as discussed in a recent view-point exchange, which also points out the extent of carotid body denervation from Angelova et al. (6) was not verified and that neuroplastic changes may underly the hypoxic responses measured (97, 292). While there is strong evidence of a role for central hypoxia sensing mechanisms in respiratory control, whether such mechanisms are present and to what extent in the intact neonate remains to be determined.
Figure 9.
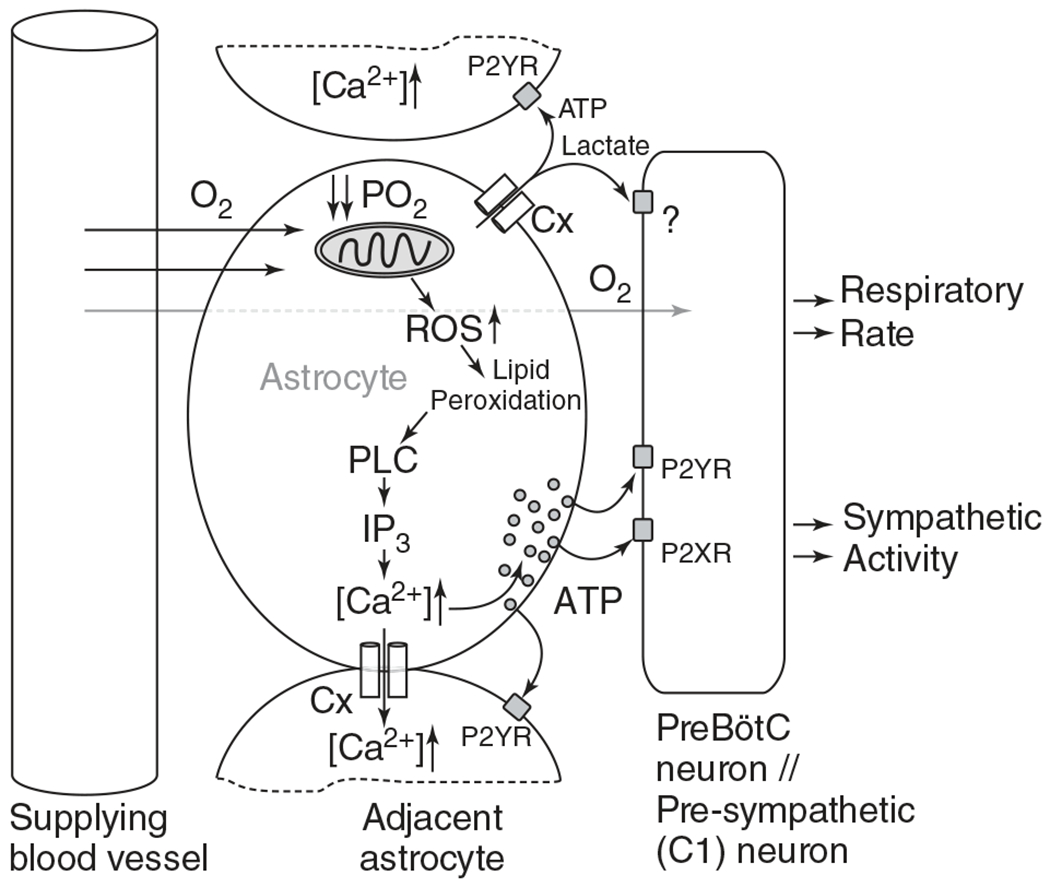
Schematic of the hypothesized cellular and molecular mechanisms of central oxygen sensing by the astrocyte. Hypoxia sensed from perfusing blood by the astrocyte inhibits the mitochondria and thus stimulating mitochondrial reactive oxygen species (ROS) production, leading to an increase in lipid peroxidation. PLC-IP3 signaling releases intracellular calcium stores and releases ATP onto nearby pre-BötC neurons that express two ATP receptors, P2YR and P2XR. In parallel, hypoxia may cause opening of the connexin (Cx) hemichannel leading to release of ATP and lactate, the latter with unknown stimulatory effects on the preBötC neurons. Release of ATP causes further release of ATP in autocrine and paracrine manners, increasing respiratory rate and sympathetic activity through preBötC neurons. Reused, with permission, from Gourine AV and Funk GD, 2017 (106). © 1985, The American Physiological Society.
Hypoxia-inducible factor (HIF)
Hypoxia-inducible factor (HIF) is a transcription factor implicated in various cellular and systemic responses to hypoxia (77, 181). Various isoforms exist but HIF-1α and HIF-2α are the most commonly studied in mammals, each with distinct actions (77, 125, 149, 181). PHD containing enzymes of which there are three isoforms, PHD 1 to 3, regulate HIF isoform protein levels (77, 142, 190). PHD2 primarily regulates HIF-1α (30) whereas PHD1 and 3 regulate HIF-2α (270). Homozygous deletion of either HIF isoform causes embryonic lethality or extremely impaired developmental phenotypes (77, 131). Thus, heterozygote animal models are used to study the role of these isoforms in the control of breathing (248, 249). Heterozygote HIF-1α (149, 248) or HIF-2α mice (249) have different effects on the control of breathing. HIF-1α +/− mice have normal baseline breathing but impaired acute carotid body hypoxic responses and reduced acclimatization to chronic hypoxia (149). HIF-2α heterozygotes have abnormal baseline breathing patterns and greater acute hypoxic sensitivity (249). Heterozygote PHD2, but not homozygote PHD1 or 3, deficient mice causes carotid body hyperplasia and greater HVRs (34). Homozygote PHD1 and PHD3 mice causes NEB hyperplasia (240). The NEBs of PHD1 null mice appear more sensitive to hypoxia based on the increase in neurotransmitters released in response to hypoxia (180). Whether PHD3 null mice have augmented NEB hypoxia sensitivity remains unknown. These studies indicate an important role of HIF and PHD oxygen-sensitive proteins in mediating hypoxic responses within the main peripheral carotid body chemoreceptors and in secondary hypoxia sensitive cells of the NEBs. Furthermore, HIF and PHD proteins are expressed at the early embryonic period and play important roles during fetal development (33, 58, 265, 269). Their expression may be epigenetically regulated (75), but their contributions to the control of breathing are not fully understood.
Arousal and autoresuscitation
Arousal is a first-line defense mechanism preventing severe hypoxemia or hypercapnia during sleep which may occur in neonates with diseases associated with sleep-disordered breathing like BPD or airway obstruction (196, 202, 304). Arousal is a stepwise process of activating subcortical to cortical brain regions beginning with a sigh, then behavioral thrashing movements, to awakening (174). Progression to awakening is not always necessary to correct blood gas levels back to homeostasis, permitting the preservation of the sleep state (174). Experimental data from 3-day old lambs indicate that rapidly developing hypoxemia significantly alters the arousal response (88, 90). Sleep state sensitivities to hypoxia and hypercapnia are significantly altered following repetitive epochs of hypoxemia during sleep (90). Arousal times from quiet and active sleep are prolonged and oxygen saturation levels at the time of arousal are lower in response to hypoxia after recurrent hypoxemia, indicating a shift to higher apneic thresholds (90). However, the influence of hypercapnia on arousal from sleep is much greater following repetitive epochs of hypoxemia (90). Nonetheless, the arousal response from quiet and active sleep is significantly influenced by carotid bodies, as indicated by carotid body denervation studies (91, 93, 94). Impairment to carotid body development due to hyperoxia exposure in very premature infants is thus a contributing factor to impaired arousal responses in these preterm infants. However, central mechanisms also contribute, as indicated by hyperoxia-hypercapnia exposure (transient hyperoxia exposure functionally inhibits peripheral carotid bodies) (89). Furthermore, premature infants, born to mothers who smoked during pregnancy, have increased apneic thresholds and higher arousal thresholds contributing to a greater risk for SIDS (272) and implicating prenatal nicotine exposure to impacting apneic thresholds and the control of breathing. Indeed, the exposure to pre and postnatal nicotine was confirmed to exacerbate autoresuscitation failure in neonatal rats with inherent serotonin deficiencies (172). Thus, neonatal hypoxemia impairs arousal from sleep and this arousal is exacerbated with exposure to prenatal stressors like nicotine.
Autoresuscitation is the last resort mechanism when arousal fails to correct for positional asphyxia or apnea (84). It ensures survival during extreme hypoxemia by driving the spontaneous recovery from hypoxia-induced apnea and bradycardia. This process occurs through gasping initiated by neurons in the preBötC and modulated by microglia (183). Sustained gasping leads to increases in sympathetic activity and recovery from apnea and bradycardia mediated by non-preBötC neurons within the respiratory control network and influenced by microglia. Other neurons critical for autoresuscitation are the brainstem serotonin neurons (14, 59, 60, 74, 85, 316). Intermittent hypoxia is commonly observed in premature infants due to immature respiratory control systems (70, 71, 194) and intermittent hypoxia likely impairs gasping and autoresuscitation (108). Indeed, preterm infants are at a greater risk for SIDS (191) and infants succumbing to SIDS have more occurrences of complex gasps and reduced occurrences of autoresuscitation compared to non-SIDS related infant deaths (174).
Serotonin and nicotine
Near-complete loss of brainstem serotonin (5-HT) does not remove the initiation of gasping, but autoresuscitation is impaired (284, 285, 298, 301). Moreover, perinatal nicotine exposure exacerbates this impairment, perhaps by blunting the activity of any remaining serotonin neurons (52, 172). Indeed, the risk for SIDS is greater if exposed to perinatal nicotine and SIDS is associated with impaired brainstem serotonin systems indicated by postmortem histological analyzes as reviewed by Kinney et al. (76, 148, 241). Similar, and more recent findings in an Australian cohort of SIDS and non-SIDS infant brainstem histologic analyzes corroborate these findings (44).
Advances in genetic technologies have allowed more precise modeling of SIDS brainstem serotonin abnormalities allowing for only partial loss, postnatal loss, or temporary loss of brainstem serotonin in mice or allowing for in vivo inhibition of serotonin neurons (14, 59, 74, 315). Results from these studies indicate that even partial dysfunction (~33%–75%) of serotonin neurons impairs autoresuscitation (14). Moreover, in vivo inhibition of serotonin neurons using a chemo-genetic technique uncouples the recovery of breathing and heart rate recovery after repeated bouts of anoxia (30 min, 97%N2/3%CO2) within a few days after birth in mice (74). Notably, denervation of carotid body chemoreceptors does not impact gasping duration, the number of gasps, or autoresuscitation in neonatal rat pups (5–6 day old), indicating that carotid bodies, even though important for the acute HVR, are not integral to generation of gasping, leading to autoresuscitation in response to hypoxia-induced apnea (187).
Concluding Remarks and Future Directions
Breathing is a vital physiologic process coordinated by the neural respiratory network throughout the medulla and pons. Development of this system begins early in fetal life and continues postnatally. During fetal development, the neural respiratory network establishes connectivity and lung growth facilitated by critical FBMs. After birth, the neural respiratory network independently regulates alveolar ventilation to sustain blood gas homeostasis. Physiologic transition at birth from low to high oxygen environment modulates the transition of the regulatory mechanisms in early postnatal life. Maturation of the system continues during early postnatal life, indicated by a progressive reduction in the number of apneas and periodic breathing and the augmentation of ventilatory chemoreflexes within weeks after birth.
Exposure to hypoxia during fetal and neonatal life poses a major threat for proper development of the neural respiratory control system. Acute fetal hypoxia inhibits rather than excites breathing activity. Chronic fetal hypoxia causes a reduction in metabolism, thereby contributing to IUGR and associated morbidities, such as impaired respiratory control, though some high-altitude natives have evolved genetic adaptations rendering them less susceptible to IUGR. Premature infants are most susceptible to neonatal hypoxia, which can impair the normal development of the carotid body chemoreceptors and central mechanisms controlling breathing. Exposure to repeated bouts or chronic hypoxia at any point during fetal and neonatal development causes lasting changes to the control of breathing. Although not reviewed here, hyperoxia can also impair the development of the control of breathing (19, 20, 23–26, 32). Thus, precise regulation of oxygen homeostasis throughout fetal and neonatal development is critical for proper development of the control of breathing.
Future studies are needed to elucidate the short and long-term impact of fetal and neonatal hypoxia on central nuclei controlling breathing. Specifically, future research is needed to understand the impact hypoxia has on neurons, astrocytes, and microglia within respiratory control nuclei. Understanding possible contributions of airway lung cells may reveal contributing sensory input to the control of breathing, especially under conditions of sustained intermittent or chronic hypoxia. Delineation of this lung-brain axis will enhance our understanding of altered regulatory mechanisms that may contribute to immature/unstable breathing in neonates, especially those born prematurely. Lastly, identification of genetic and epigenetic factors altered in response to various fetal and hypoxic conditions will aid in understanding mechanisms underlying the various physiologic responses to such hypoxic conditions.
Didactic Synopsis.
Major Teaching Points
The development of the control of breathing commences early in fetal life and continues postnatally.
Maintenance of oxygenation of the fetus and neonate is different.
Hypoxia is a major stressor during development, especially of the respiratory control system.
Fetal and neonatal hypoxia is commonly associated with in utero stress, premature birth, respiratory instabilities, and underdeveloped respiratory control systems.
Hypoxia can be acute, intermittent, and chronic, each with differing effects on fetal and neonatal control of breathing.
The acute hypoxic ventilatory response of the fetus is a reduction in fetal breathing movements whereas in the neonates, the ventilatory response is an increase in alveolar ventilation.
Intermittent and chronic hypoxia cause longer-lasting changes to the control of breathing than acute hypoxia exposures in the fetus and neonate.
There remains a large gap in understanding the breadth of impact fetal and neonatal hypoxia have at the cellular and molecular levels within central respiratory nuclei.
References
- 1.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: What’s new? Pediatr Pulmonol 43 (10): 937–944, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W. Experimental pulmonary hypoplasia and oligohydramnios: Relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg 19(6): 658–665, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Alcorn D, Adamson TM, Maloney JE, Robinson PM. Morphological effects of chronic bilateral phrenectomy or vagotomy in the fetal lamb lung. J Anat 130 (Pt 4): 683–695, 1980. [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Matary A, Kutbi I, Qurashi M, Khalil M, Alvaro R, Kwiatkowski K, Cates D, Rigatto H. Increased peripheral chemoreceptor activity may be critical in destabilizing breathing in neonates. Semin Perinatol 28 (4): 264–272, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro R, Rigatto H. Control of breathing in newborns. In: Bracci R, Buonocore G, Weindling M, editors. Neonatology. Milano: Springer, 2012. [Google Scholar]
- 6.Angelova PR, Kasymov V, Christie I, Sheikhbahaei S, Turovsky E, Marina N, Korsak A, Zwicker J, Teschemacher AG, Ackland GL, Funk GD, Kasparov S, Abramov AY, Gourine AV. Functional oxygen sensitivity of astrocytes. J Neurosci 35 (29): 10460–10473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aucott SW, Donohue PK, Northington FJ. Increased morbidity in severe early intrauterine growth restriction. J Perinatol 24 (7): 435–440, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104 (2–3): 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Baguma-Nibasheka M, Gugic D, Saraga-Babic M, Kablar B. Role of skeletal muscle in lung development. Histol Histopathol 27 (7): 817–826, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Bairam A, Basson H, Marchal F, Cottet-Emard JM, Pequignot JM, Hascoet JM, Lahiri S. Effects of hypoxia on carotid body dopamine content and release in developing rabbits. J Appl Physiol (1985) 80(1): 20–24, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Balan KV, Kc P, Hoxha Z, Mayer CA, Wilson CG, Martin RJ. Vagal afferents modulate cytokine-mediated respiratory control at the neonatal medulla oblongata. Respir Physiol Neurobiol 178 (3): 458–464,2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balan KV, Kc P, Mayer CA, Wilson CG, Belkadi A, Martin RJ. Intra-pulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatr 101 (5): 466–471, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcroft J, Barron DH. Movements in midfoetal life in the sheep embryo. J Physiol 91 (3): 329–351, 1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett KT, Dosumu-Johnson RT, Daubenspeck JA, Brust RD, Kreouzis V, Kim JC, Li A, Dymecki SM, Nattie EE. Partial raphe dysfunction in neurotransmission is sufficient to increase mortality after anoxic exposures in mice at a critical period in postnatal development. J Neurosci 36 (14): 3943–3953, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartelds B, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res 34 (1): 51–55, 1993. [DOI] [PubMed] [Google Scholar]
- 16.Bates ML, Pillers DA, Palta M, Farrell ET, Eldridge MW. Ventilatory control in infants, children, and adults with bronchopulmonary dysplasia. Respir Physiol Neurobiol 189 (2): 329–337, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr 71 (2): 159–163, 1967. [DOI] [PubMed] [Google Scholar]
- 18.Bavis RW. Developmental plasticity of the hypoxic ventilatory response after perinatal hyperoxia and hypoxia. Respir Physiol Neurobiol 149 (1–3): 287–299, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Bavis RW, Fallon SC, Dmitrieff EF. Chronic hyperoxia and the development of the carotid body. Respir Physiol Neurobiol 185 (1): 94–104, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bavis RW, MacFarlane PM. Developmental plasticity in the neural control of breathing. Exp Neurol 287 (Pt 2): 176–191, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol (1985) 94 (1): 399–409,2003. [DOI] [PubMed] [Google Scholar]
- 22.Bavis RW, Olson EB Jr, Vidruk EH, Fuller DD, Mitchell GS. Developmental plasticity of the hypoxic ventilatory response in rats induced by neonatal hypoxia. J Physiol 557 (Pt 2): 645–660, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bavis RW, Young KM, Barry KJ, Boller MR, Kim E, Klein PM, Ovrutsky AR, Rampersad DA. Chronic hyperoxia alters the early and late phases of the hypoxic ventilatory response in neonatal rats. J Appl Physiol (1985) 109 (3): 796–803, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavis RW, Kim I, Pradhan N, Nawreen N, Dmitrieff EF, Carroll JL, Donnelly DF. Recovery of carotid body O2 sensitivity following chronic postnatal hyperoxia in rats. Respir Physiol Neurobiol 177 (1): 47–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bavis RW, van Heerden ES, Brackett DG, Harmeling LH, Johnson SM, Blegen HJ, Logan S, Nguyen GN, Fallon SC. Postnatal development of eupneic ventilation and metabolism in rats chronically exposed to moderate hyperoxia. Respir Physiol Neurobiol 198: 1–12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bavis RW, Li KY, DeAngelis KJ, March RJ, Wallace JA, Logan S, Putnam RW. Ventilatory and chemoreceptor responses to hypercapnia in neonatal rats chronically exposed to moderate hyperoxia. Respir Physiol Neurobiol 237: 22–34, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beall CM, Brittenham GM, Macuaga F, Barragan M. Variation in hemoglobin concentration among samples of high-altitude natives in the Andes and the Himalayas. Am J Hum Biol 2 (6): 639–651, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Almasy LA, Decker MJ, Worthman CM, Goldstein MC, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol 104 (4): 427–447, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Wang W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, Zheng YT. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107 (25): 11459–11464, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J 22 (16): 4082–4090, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bigham AW, Wilson MJ, Julian CG, Kiyamu M, Vargas E, Leon- Velarde F, Rivera-Chira M, Rodriquez C, Browne VA, Parra E, Brutsaert TD, Moore LG, Shriver MD. Andean and Tibetan patterns of adaptation to high altitude. Am J Hum Biol 25 (2): 190–197, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Bisgard GE, Olson EB Jr, Bavis RW, Wenninger J, Nordheim EV, Mitchell GS. Carotid chemoafferent plasticity in adult rats following developmental hyperoxia. Respir Physiol Neurobiol 145 (1): 3–11, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Bishop T, Ratcliffe PJ. HIF hydroxylase pathways in cardiovascular physiology and medicine. Circ Res 117 (1): 65–79, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop T, Talbot NP, Turner PJ, Nicholls LG, Pascual A, Hodson EJ, Douglas G, Fielding JW, Smith TG, Demetriades M, Schofield CJ, Robbins PA, Pugh CW, Buckler KJ, Ratcliffe PJ. Carotid body hyperplasia and enhanced ventilatory responses to hypoxia in mice with heterozygous deficiency of PHD2. J Physiol 591 (14): 3565–3577, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanco CE, Dawes GS, Hanson MA, McCooke HB. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol 351: 25–37, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blitz DM, Ramirez JM. Long-term modulation of respiratory network activity following anoxia in vitro. J Neurophysiol 87 (6): 2964–2971, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Blott M, Greenough A, Nicolaides KH, Campbell S. The ultrasonographic assessment of the fetal thorax and fetal breathing movements in the prediction of pulmonary hypoplasia. Early Hum Dev 21 (3): 143–151, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Boddy K, Dawes GS, Fisher R, Pinter S, Robinson JS. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol 243 (3): 599–618, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A, Extremely Low Gestational Age Newborn Study I. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124 (3): e450–e458, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chedotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci 13 (9): 1066–1074, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Brady JP, Ceruti E. Chemoreceptor reflexes in the new-born infant: Effects of varying degrees of hypoxia on heart rate and ventilation in a warm environment. J Physiol 184 (3): 631–645, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science 351 (6274): 707–710, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breen S, Rees S, Walker D. Identification of brainstem neurons responding to hypoxia in fetal and newborn sheep. Brain Res 748 (1–2): 107–121, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Bright FM, Byard RW, Vink R, Paterson DS. Medullary serotonin neuron abnormalities in an Australian cohort of sudden infant death syndrome. J Neuropathol Exp Neurol 76 (10): 864–873, 2017. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci USA 107 (37): 16354–16359, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calder NA, Williams BA, Smyth J, Boon AW, Kumar P, Hanson MA. Absence of ventilatory responses to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: Implications for the risk of sudden infant death. Pediatr Res 35 (6): 677–681, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Cardot V, Chardon K, Tourneux P, Micallef S, Stephan E, Leke A, Bach V, Libert JP, Telliez F. Ventilatory response to a hyperoxic test is related to the frequency of short apneic episodes in late preterm neonates. Pediatr Res 62 (5): 591–596, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Carroll JL. Developmental plasticity in respiratory control. J Appl Physiol (1985) 94 (1): 375–389, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Carroll JL, Agarwal A. Development of ventilatory control in infants. Paediatr Respir Rev 11 (4): 199–207, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol 149 (1–3): 201–215, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Carter AM. Placental gas exchange and the oxygen supply to the fetus. Compr Physiol 5 (3): 1381–1403, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Cerpa VJ, Aylwin Mde L, Beltran-Castillo S, Bravo EU, Llona IR, Richerson GB, Eugenin JL. The alteration of neonatal raphe neurons by prenatal-perinatal nicotine. Meaning for sudden infant death syndrome. Am J Respir Cell Mol Biol 53 (4): 489–499, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerpa VJ, Wu Y, Bravo E, Teran FA, Flynn RS, Richerson GB. Medullary 5-HT neurons: Switch from tonic respiratory drive to chemoreception during postnatal development. Neuroscience 344: 1–14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang RB, Strochlic DE, Williams EK, Umans BD, Liberles SD. Vagal sensory neuron subtypes that differentially control breathing. Cell 161 (3): 622–633, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clewlow F, Dawes GS, Johnston BM, Walker DW. Changes in breathing, electrocortical and muscle activity in unanaesthetized fetal lambs with age. J Physiol 341: 463–476, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: Bronchopulmonary dysplasia and more. Ann Am Thorac Soc 15 (5): 530–538, 2018. [DOI] [PubMed] [Google Scholar]
- 57.Cotton EK, Grunstein MM. Effects of hypoxia on respiratory control in neonates at high altitude. J Appl Physiol Respir Environ Exerc Physiol 48 (4): 587–595, 1980. [DOI] [PubMed] [Google Scholar]
- 58.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu C-J, Labosky PA, Simon MC, Keith B. HIF-2α regulates Oct-4: Effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20 (5): 557–570, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol 589 (Pt 21): 5247–5256, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: Implications for SIDS. J Appl Physiol 111 (3): 825–833, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cutz E Hyperplasia of pulmonary neuroendocrine cells in infancy and childhood. Semin Diagn Pathol 32 (6): 420–437, 2015. [DOI] [PubMed] [Google Scholar]
- 62.Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol 10 (6): 419–435, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: Potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol 10 (2): 106–116, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Darnall RA. The carotid body and arousal in the fetus and neonate. Respir Physiol Neurobiol 185 (1): 132–143, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davey MG, Moss TJ, McCrabb GJ, Harding R. Prematurity alters hypoxic and hypercapnic ventilatory responses in developing lambs. Respir Physiol 105 (1–2): 57–67, 1996. [DOI] [PubMed] [Google Scholar]
- 66.Dawes GS. The central control of fetal breathing and skeletal muscle movements. J Physiol 346: 1–18, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delpapa EH, Edelstone DI, Milley JR, Balsan M. Effects of chronic maternal anemia on systemic and uteroplacental oxygenation in near-term pregnant sheep. Am J Obstet Gynecol 166 (3): 1007–1012, 1992. [DOI] [PubMed] [Google Scholar]
- 68.Dempsey JA. Crossing the apnoeic threshold: Causes and consequences. Exp Physiol 90 (1): 13–24, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Deoras KS, Greenspan KS, Wolfson MR, Keklikian EN, Shaffer TH, Allen JL. Effects of inspiratory resistive loading on chest wall motion and ventilation: Differences between preterm and full-term infants. Pediatr Res 32 (5): 589–594, 1992. [DOI] [PubMed] [Google Scholar]
- 70.Di Fiore JM, Vento M. Intermittent hypoxemia and oxidative stress in preterm infants. Respir Physiol Neurobiol 266: 121–129, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, Walsh M, Finer N, Martin RJ. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr 157 (1): 69–73, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di Pasquale E, Tell F, Monteau R, Hilaire G. Perinatal developmental changes in respiratory activity of medullary and spinal neurons: An in vitro study on fetal and newborn rats. Brain Res Dev Brain Res 91 (1): 121–130, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol 475 (2): 267–275, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dosumu-Johnson RT, Cocoran AE, Chang Y, Nattie E, Dymecki SM. Acute perturbation of Pet1-neuron activity in neonatal mice impairs cardiorespiratory homeostatic recovery. elife 7: e37857, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ducsay CA, Goyal R, Pearce WJ, Wilson S, Hu XQ, Zhang L. Gestational hypoxia and developmental plasticity. Physiol Rev 98 (3): 1241–1334, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS,Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA 303 (5): 430–437, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell 17 (6): 755–773, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Edelstone DI, Caine ME, Fumia FD. Relationship of fetal oxygen consumption and acid-base balance to fetal hematocrit. Am J Obstet Gynecol 151 (7): 844–851, 1985. [DOI] [PubMed] [Google Scholar]
- 79.Eden GJ, Hanson MA. Effects of chronic hypoxia from birth on the ventilatory response to acute hypoxia in the newborn rat. J Physiol 392: 11–19, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eichenwald EC, Committee on Fetus and Newborn, American Academy of Pediatrics. Apnea of prematurity. Pediatrics 137 (1): e20153757, 2016. [Google Scholar]
- 81.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Inflammatory response: Pathway across the blood-brain barrier. Nature 410 (6827): 430–431, 2001. [DOI] [PubMed] [Google Scholar]
- 82.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med 364 (7): 656–665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Endo A, Minato M, Takada M, Takahashi S, Harada K, Yamada T, Takashima S. A case of pulmonary hypoplasia associated with intrauterine brainstem necrosis. Eur J Pediatr 160 (11): 675–676, 2001. [DOI] [PubMed] [Google Scholar]
- 84.Erickson JT. Central serotonin and autoresuscitation capability in mammalian neonates. Exp Neurol 326: 113162, 2020. [DOI] [PubMed] [Google Scholar]
- 85.Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol (1985) 106 (6): 1785–1792, 2009. [DOI] [PubMed] [Google Scholar]
- 86.Ewald AC, Kiernan EA, Baker TL, Watters JJ. The transcriptomic profiles of microglia from respiratory control centers in adult offspring exposed to gestational intermittent hypoxia differ by sex and CNS region. FASEB J 33 (1_supplement): 730.7–730.7, 2019. [Google Scholar]
- 87.Fanaroff AA, Korones SB, Wright LI, Verter J, Poland RL, Bauer CR, Tyson JE, Philips JB 3rd, Edwards W, Lucey JF, Catz CS, Shankaran S, Oh W. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 17 (7): 593–598, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Fewell JE, Baker SB. Arousal from sleep during rapidly developing hypoxemia in lambs. Pediatr Res 22 (4): 471–477, 1987. [DOI] [PubMed] [Google Scholar]
- 89.Fewell JE, Baker SB. Arousal and cardiopulmonary responses to hyperoxic hypercapnia in lambs. J Dev Physiol 12 (1): 21–26, 1989. [PubMed] [Google Scholar]
- 90.Fewell JE, Konduri GG. Repeated exposure to rapidly developing hypoxemia influences the interaction between oxygen and carbon dioxide in initiating arousal from sleep in lambs. Pediatr Res 24 (1): 28–33, 1988. [DOI] [PubMed] [Google Scholar]
- 91.Fewell JE, Konduri GG. Influence of repeated exposure to rapidly developing hypoxaemia on the arousal and cardiopulmonary response to rapidly developing hypoxaemia in lambs. J Dev Physiol 11 (2): 77–82, 1989. [PubMed] [Google Scholar]
- 92.Fewell JE, Lee CC, Kitterman JA. Effects of phrenic nerve section on the respiratory system of fetal lambs. J Appl Physiol 51 (2): 293–297, 1981. [DOI] [PubMed] [Google Scholar]
- 93.Fewell JE, Kondo CS, Dascalu V, Filyk SC. Influence of carotid-denervation on the arousal and cardiopulmonary responses to alveolar hypercapnia in lambs. J Dev Physiol 12 (4): 193–199, 1989. [PubMed] [Google Scholar]
- 94.Fewell JE, Kondo CS, Dascalu V, Filyk SC. Influence of carotid denervation on the arousal and cardiopulmonary response to rapidly developing hypoxemia in lambs. Pediatr Res 25 (5): 473–477, 1989. [DOI] [PubMed] [Google Scholar]
- 95.Finer NN, Barrington KJ, Hayes BJ, Hugh A. Obstructive, mixed, and central apnea in the neonate: Physiologic correlates. J Pediatr 121 (6): 943–950, 1992. [DOI] [PubMed] [Google Scholar]
- 96.Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol 119 (2–3): 199–208, 2000. [DOI] [PubMed] [Google Scholar]
- 97.Funk GD, Gourine AV. CrossTalk proposal: A central hypoxia sensor contributes to the excitatory hypoxic ventilatory response. J Physiol 596 (15): 2935–2938, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 191 (2): 481–487, 2004. [DOI] [PubMed] [Google Scholar]
- 99.Gassmann NN, van Elteren HA, Goos TG, Morales CR, Rivera-Ch M, Martin DS, Cabala Peralta P, Passano Del Carpio A, Aranibar Machaca S, Huicho L, Reiss IK, Gassmann M, de Jonge RC. Pregnancy at high altitude in the Andes leads to increased total vessel density in healthy newborns. J Appl Physiol (1985) 121 (3): 709–715, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gauda EB, Shirahata M, Mason A, Pichard LE, Kostuk EW, Chavez-Valdez R. Inflammation in the carotid body during development and its contribution to apnea of prematurity. Respir Physiol Neurobiol 185(1): 120–131, 2013. [DOI] [PubMed] [Google Scholar]
- 101.Giussani DA. The fetal brain sparing response to hypoxia: Physiological mechanisms. J Physiol 594 (5): 1215–1230, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461: 431–449, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giussani DA, Gardner DS, Cox DT, Fletcher AJ. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol 280 (3): R678–R685, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Goldstein JD, Reid LM. Pulmonary hypoplasia resulting from phrenic nerve agenesis and diaphragmatic amyoplasia. J Pediatr 97 (2): 282–287, 1980. [DOI] [PubMed] [Google Scholar]
- 105.Gortner L, Misselwitz B, Milligan D, Zeitlin J, Kollee L, Boerch K, Agostino R, Van Reempts P, Chabernaud JL, Breart G, Papiernik E, Jarreau PH, Carrapato M, Gadzinowski J, Draper E, Members of the MRG. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: Results from the MOSAIC cohort. Neonatology 99 (2): 112–117, 2011. [DOI] [PubMed] [Google Scholar]
- 106.Gourine AV, Funk GD. On the existence of a central respiratory oxygen sensor. J Appl Physiol (1985) 123 (5): 1344–1349, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: Role in hypoxic ventilatory response. J Neurosci 25 (5): 1211–1218, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gozal D, Gozal E, Reeves SR, Lipton AJ. Gasping and autoresuscitation in the developing rat: Effect of antecedent intermittent hypoxia. J Appl Physiol (1985) 92 (3): 1141–1144, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med 167 (11): 1540–1547, 2003. [DOI] [PubMed] [Google Scholar]
- 110.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci 30 (44): 14883–14895, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Greenspan JS, Wolfson MR, Locke RG, Allen JL, Shaffer TH. Increased respiratory drive and limited adaptation to loaded breathing in bronchopulmonary dysplasia. Pediatr Res 32 (3): 356–359, 1992. [DOI] [PubMed] [Google Scholar]
- 112.Greer JJ. Control of breathing activity in the fetus and newborn. Compr Physiol 2 (3): 1873–1888, 2012. [DOI] [PubMed] [Google Scholar]
- 113.Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J Neurophysiol 67 (4): 996–999, 1992. [DOI] [PubMed] [Google Scholar]
- 114.Hannhart B, Pickett CK, Moore LG. Effects of estrogen and progesterone on carotid body neural output responsiveness to hypoxia. J Appl Physiol (1985) 68 (5): 1909–1916, 1990. [DOI] [PubMed] [Google Scholar]
- 115.Hanson MA, Eden GJ, Nijhuis JG, Moore PJ. Peripheral chemoreceptors and other oxygen sensors in the fetus and newborn. In: Lahiri S, Forster RE, Davies RO, Pack AI, editors. Chemoreceptors and Reflexes in Breathing: Cellular and Molecular Aspects. New York: Oxford University Press, 1989, p. 113–120. [Google Scholar]
- 116.Hanson MA, Kumar P, Williams BA. The effect of chronic hypoxia upon the development of respiratory chemoreflexes in the newborn kitten. J Physiol 411: 563–574, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harding R, Hooper SB, Han VK. Abolition of fetal breathing movements by spinal cord transection leads to reductions in fetal lung liquid volume, lung growth, and IGF-II gene expression. Pediatr Res 34 (2): 148–153, 1993. [DOI] [PubMed] [Google Scholar]
- 118.Harding R, Tester ML, Moss TJ, Davey MG, Louey S, Joyce B, Hooper SB, Maritz G. Effects of intra-uterine growth restriction on the control of breathing and lung development after birth. Clin Exp Pharmacol Physiol 27 (1–2): 114–119, 2000. [DOI] [PubMed] [Google Scholar]
- 119.Herlenius E An inflammatory pathway to apnea and autonomic dysregulation. Respir Physiol Neurobiol 178 (3): 449–457, 2011. [DOI] [PubMed] [Google Scholar]
- 120.Hoch B, Bernhard M. Central apnoea and endogenous prostaglandins in neonates. Acta Paediatr 89 (11): 1364–1368, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Hocker AD, Beyeler SA, Gardner AN, Johnson SM, Watters JJ, Huxtable AG. One bout of neonatal inflammation impairs adult respiratory motor plasticity in male and female rats. elife 8: e45399, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: Neuromodulatory and trophic effects. Respir Physiol Neurobiol 164: 222–232, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hofstetter AO, Saha S, Siljehav V, Jakobsson PJ, Herlenius E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc Natl Acad Sci USA 104 (23): 9894–9899, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Holton P, Wood JB. The effects of bilateral removal of the carotid bodies and denervation of the carotid sinuses in two human subjects. J Physiol 181 (2): 365–378, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu CJ, Poth JM, Zhang H, Flockton A, Laux A, Kumar S, McKeon B, Frid MG, Mouradian G, Li M, Riddle S, Pugliese SC, Brown RD, Wallace EM, Graham BB, Stenmark KR. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur Respir J, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178 (3): 482–489, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Inanlou MR, Kablar B. Contractile activity of skeletal musculature involved in breathing is essential for normal lung cell differentiation, as revealed in Myf5−/−:MyoD−/− embryos. Dev Dyn 233 (3):772–782, 2005. [DOI] [PubMed] [Google Scholar]
- 128.Inanlou MR, Kablar B. Abnormal development of the intercostal muscles and the rib cage in Myf5−/− embryos leads to pulmonary hypoplasia. Dev Dyn 232 (1): 43–54, 2005. [DOI] [PubMed] [Google Scholar]
- 129.Itskovitz J, LaGamma EF, Rudolph AM. The effect of reducing umbilical blood flow on fetal oxygenation. Am J Obstet Gynecol 145 (7): 813–818, 1983. [DOI] [PubMed] [Google Scholar]
- 130.Itskovitz J, LaGamma EF, Bristow J, Rudolph AM. Cardiovascular responses to hypoxemia in sinoaortic-denervated fetal sheep. Pediatr Res 30 (4): 381–385, 1991. [DOI] [PubMed] [Google Scholar]
- 131.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12 (2): 149–162, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE. Lung and brain-stem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol 178 (3): 429–438, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jawdeh EGA. Intermittent hypoxemia in preterm infants: Etiology and clinical relevance. NeoReviews 18 (11): e637–e646, 2017. [Google Scholar]
- 134.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: Independent or interactive effects? Am J Public Health 87 (6): 1003–1007, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Johnson SM, Randhawa KS, Epstein JJ, Gustafson E, Hocker AD, Huxtable AG, Baker TL, Watters JJ. Gestational intermittent hypoxia increases susceptibility to neuroinflammation and alters respiratory motor control in neonatal rats. Respir Physiol Neurobiol 256: 128–142, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Johnston BM, Gluckman PD. Lateral pontine lesions affect central chemosensitivity in unanesthetized fetal lambs. J Appl Physiol (1985) 67 (3): 1113–1118, 1989. [DOI] [PubMed] [Google Scholar]
- 137.Johnston BM, Gluckman PD. Peripheral chemoreceptors respond to hypoxia in pontine-lesioned fetal lambs in utero. J Appl Physiol (1985) 75 (3): 1027–1034, 1993. [DOI] [PubMed] [Google Scholar]
- 138.Julian CG. High altitude during pregnancy. Clin Chest Med 32 (1): 21–31, 2011. [DOI] [PubMed] [Google Scholar]
- 139.Julian CG, Moore LG. Human genetic adaptation to high altitude: Evidence from the Andes. Genes (Basel) 10 (2): 150, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed 92 (5): F372–F377, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Julien C, Bairam A, Joseph V Chronic intermittent hypoxia reduces ventilatory long-term facilitation and enhances apnea frequency in newborn rats. Am J Physiol Regul Integr Comp Physiol 294 (4): R1356–R1366, 2008. [DOI] [PubMed] [Google Scholar]
- 142.Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell 30 (4): 393–402, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Katz-Salamon M, Jonsson B, Lagercrantz H. Blunted peripheral chemoreceptor response to hyperoxia in a group of infants with bronchopulmonary dysplasia. Pediatr Pulmonol 20 (2): 101–106, 1995. [DOI] [PubMed] [Google Scholar]
- 144.Kc A, Singhal N, Gautam J, Rana N, Andersson O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth - randomized clinical trial. Matern Health Neonatol Perinatol 5: 7, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiernan EA, Wang T, Vanderplow AM, Cherukuri S, Cahill ME, Watters JJ. Neonatal intermittent hypoxia induces lasting sex-specific augmentation of rat microglial cytokine expression. Front Immunol 10: 1479, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kingdom JC, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta 18 (8): 613–621, 1997. discussion 623–626. [DOI] [PubMed] [Google Scholar]
- 147.Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Fingers ST, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: A case report. J Neuropathol Exp Neurol 64 (8): 689–694, 2005. [DOI] [PubMed] [Google Scholar]
- 148.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol 4: 517–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 99 (2): 821–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kobayashi K, Lemke RP, Greer JJ. Ultrasound measurements of fetal breathing movements in the rat. J Appl Physiol (1985) 91 (1):316–320, 2001. [DOI] [PubMed] [Google Scholar]
- 151.Koch H, Caughie C, Elsen FP, Doi A, Garcia AJ 3rd, Zanella S, Ramirez JM. Prostaglandin E2 differentially modulates the central control of eupnoea, sighs and gasping in mice. J Physiol 593 (1): 305–319, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Koos BJ. Adenosine A(2)a receptors and O(2) sensing in development. Am J Physiol Regul Integr Comp Physiol 301 (3): R601–R622, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koos BJ, Chau A. Fetal cardiovascular and breathing responses to an adenosine A2a receptor agonist in sheep. Am J Physiol 274 (1): R152–R159, 1998. [DOI] [PubMed] [Google Scholar]
- 154.Koos BJ, Chau A, Ogunyemi D. Adenosine mediates metabolic and cardiovascular responses to hypoxia in fetal sheep. J Physiol 488 (Pt 3): 761–766, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koos BJ, Maeda T. Adenosine A(2A) receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am J Physiol Heart Circ Physiol 280(1): H83–H89, 2001. [DOI] [PubMed] [Google Scholar]
- 156.Koos BJ, Matsuda K. Fetal breathing, sleep state, and cardiovascular responses to adenosine in sheep. J Appl Physiol (1985) 68 (2): 489–495, 1990. [DOI] [PubMed] [Google Scholar]
- 157.Koos BJ, Rajaee A. Fetal breathing movements and changes at birth. Adv Exp Med Biol 814: 89–101, 2014. [DOI] [PubMed] [Google Scholar]
- 158.Koos BJ, Sameshima H, Power GG. Fetal breathing movement, sleep state and cardiovascular responses to an inhibitor of mitochondrial ATPase in sheep. J Dev Physiol 8 (1): 67–75, 1986. [PubMed] [Google Scholar]
- 159.Koos BJ, Sameshima H, Power GG. Fetal breathing, sleep state, and cardiovascular responses to graded anemia in sheep. J Appl Physiol (1985) 63 (4): 1463–1468, 1987. [DOI] [PubMed] [Google Scholar]
- 160.Koos BJ, Mason BA, Punla O, Adinolfi AM. Hypoxic inhibition of breathing in fetal sheep: Relationship to brain adenosine concentrations. J Appl Physiol (1985) 77 (6): 2734–2739, 1994. [DOI] [PubMed] [Google Scholar]
- 161.Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic locus mediates hypoxic inhibition of breathing in fetal sheep. J Neurophysiol 79 (5): 2383–2393, 1998. [DOI] [PubMed] [Google Scholar]
- 162.Koos BJ, Chau A, Matsuura M, Punla O, Kruger L. Thalamic lesions dissociate breathing inhibition by hypoxia and adenosine in fetal sheep. Am J Physiol Regul Integr Comp Physiol 278 (4): R831–R837, 2000. [DOI] [PubMed] [Google Scholar]
- 163.Koos BJ, Maeda T, Jan C, Lopez G. Adenosine A(2A) receptors mediate hypoxic inhibition of fetal breathing in sheep. Am J Obstet Gynecol 186 (4): 663–668,2002. [DOI] [PubMed] [Google Scholar]
- 164.Koos BJ, Rajaee A, Ibe B, Guerra C, Kruger L. Thalamic mediation of hypoxic respiratory depression in lambs. Am J Physiol Regul Integr Comp Physiol 310 (7): R586–R595, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr Physiol 2 (1): 141–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lahiri S, Brody JS, Motoyama EK, Velasquez TM. Regulation of breathing in newborns at high altitude. J Appl Physiol Respir Environ Exerc Physiol 44 (5): 673–678, 1978. [DOI] [PubMed] [Google Scholar]
- 167.Lahiri S, Roy A, Baby SM, Hoshi T, Semenza GL, Prabhakar NR. Oxygen sensing in the body. Prog Biophys Mol Biol 91 (3): 249–286, 2006. [DOI] [PubMed] [Google Scholar]
- 168.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, Morin FC 3rd. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res 62 (3): 313–318, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation: A population-based study. Pediatrics 111 (3): 483–487, 2003. [DOI] [PubMed] [Google Scholar]
- 170.Lee D, Caces R, Kwiatkowski K, Cates D, Rigatto H. A developmental study on types and frequency distribution of short apneas (3 to 15 seconds) in term and preterm infants. Pediatr Res 22 (3): 344–349, 1987. [DOI] [PubMed] [Google Scholar]
- 171.Lee SK, Ye XY, Singhal N, De La Rue S, Lodha A, Shah PS, Canadian Neonatal N. Higher altitude and risk of bronchopulmonary dysplasia among preterm infants. Am J Perinatol 30 (7): 601–606, 2013. [DOI] [PubMed] [Google Scholar]
- 172.Lee SY, Sirieix CM, Nattie E, Li A. Pre- and early postnatal nicotine exposure exacerbates autoresuscitation failure in serotonin-deficient rat neonates. J Physiol 596 (23): 5977–5991, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liggins GC, Vilos GA, Campos GA, Kitterman JA, Lee CH. The effect of spinal cord transection on lung development in fetal sheep. J Dev Physiol 3 (4): 267–274, 1981. [PubMed] [Google Scholar]
- 174.Lijowska AS, Reed NW, Chiodini BA, Thach BT. Sequential arousal and airway-defensive behavior of infants in asphyxial sleep environments. J Appl Physiol (1985) 83 (1): 219–228, 1997. [DOI] [PubMed] [Google Scholar]
- 175.Ling L, Olson EB Jr, Vidruk EH, Mitchell GS. Attenuation of the hypoxic ventilatory response in adult rats following one month of perinatal hyperoxia. J Physiol 495 (Pt 2): 561–571, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu J, Boujedaini N, Cazin L, Mallet E, Clabaut M. Developmental changes in cardio-respiratory responses to hypoxia and hypercapnia in anesthetized low-birth-weight rats. Respir Physiol 123 (3): 189–199, 2000. [DOI] [PubMed] [Google Scholar]
- 177.Liu J, Dong C, Cazin L, Clabaut M, Dubuc I, Costentin J, Coquerel A. Developmental changes of (3)H-labelled mu-opioid receptors in brain-stems of intra-uterine growth-restricted rats. Brain Res Dev Brain Res 126 (2): 211–215, 2001. [DOI] [PubMed] [Google Scholar]
- 178.Liu Q, Lowry TF, Wong-Riley MT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: Implication for a sensitive period. J Physiol 577 (Pt 3): 957–970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Livermore S, Zhou Y, Pan J, Yeger H, Nurse CA, Cutz E. Pulmonary neuroepithelial bodies are polymodal airway sensors: Evidence for CO2/H+ sensing. Am J Physiol Lung Cell Mol Physiol 308 (8): L807–L815, 2015. [DOI] [PubMed] [Google Scholar]
- 180.Livermore S, Pan J, Yeger H, Ratcliffe P, Bishop T, Cutz E. Augmented 5-HT secretion in pulmonary neuroepithelial bodies from PHD1 null mice. Adv Exp Med Biol 860: 309–313, 2015. [DOI] [PubMed] [Google Scholar]
- 181.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells 29 (5): 435–442, 2010. [DOI] [PubMed] [Google Scholar]
- 182.LoMauro A, Aliverti A. Respiratory physiology of pregnancy: Physiology masterclass. Breathe (Sheff) 11 (4): 297–301, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lorea-Hernandez JJ, Morales T, Rivera-Angulo AJ, Alcantara-Gonzalez D, Pena-Ortega F. Microglia modulate respiratory rhythm generation and autoresuscitation. Glia 64 (4): 603–619, 2016. [DOI] [PubMed] [Google Scholar]
- 184.Lorenzo FR, Huff C, Myllymaki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG Jr, Koivunen P, Prchal JT. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46 (9): 951–956, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lovering AT, Elliott JE, Laurie SS, Beasley KM, Gust CE, Mangum TS, Gladstone IM, Duke JW. Ventilatory and sensory responses in adult survivors of preterm birth and bronchopulmonary dysplasia with reduced exercise capacity. Ann Am Thorac Soc 11 (10): 1528–1537, 2014. [DOI] [PubMed] [Google Scholar]
- 186.Lucius H, Gahlenbeck H, Kleine HO, Fabel H, Bartels H. Respiratory functions, buffer system, and electrolyte concentrations of blood during human pregnancy. Respir Physiol 9 (3): 311–317, 1970. [DOI] [PubMed] [Google Scholar]
- 187.Lun R, Zhang C, Fewell JE. Carotid chemoreceptors do not mediate hypoxic-induced gasping and autoresuscitation in newborn rats. Respir Physiol Neurobiol 212-214: 33–38, 2015. [DOI] [PubMed] [Google Scholar]
- 188.MacFarlane PM, Mayer CA, Litvin DG. Microglia modulate brain-stem serotonergic expression following neonatal sustained hypoxia exposure: Implications for sudden infant death syndrome. J Physiol 594 (11): 3079–3094, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.MacLean JE, DeHaan K, Fuhr D, Hariharan S, Kamstra B, Hendson L, Adatia I, Majaesic C, Lovering AT, Thompson RB, Nicholas D, Thebaud B, Stickland MK. Altered breathing mechanics and ventilator response during exercise in children born extremely preterm. Thorax 71 (11): 1012–1019, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40 (2): 294–309, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malloy MH, Hoffman HJ. Prematurity, sudden infant death syndrome, and age of death. Pediatrics 96 (3 Pt 1): 464–471, 1995. [PubMed] [Google Scholar]
- 192.Mariani G, Dik PB, Ezquer A, Aguirre A, Esteban ML, Perez C, Fernandez Jonusas S, Fustinana C. Pre-ductal and post-ductal O2 saturation in healthy term neonates after birth. J Pediatr 150 (4): 418–421, 2007. [DOI] [PubMed] [Google Scholar]
- 193.Martin RJ, DiFiore JM, Jana L, Davis RL, Miller MJ, Coles SK, Dick TE. Persistence of the biphasic ventilatory response to hypoxia in preterm infants. J Pediatr 132 (6): 960–964, 1998. [DOI] [PubMed] [Google Scholar]
- 194.Martin RJ, Di Fiore JM, Macfarlane PM, Wilson CG. Physiologic basis for intermittent hypoxic episodes in preterm infants. Adv Exp Med Biol 758: 351–358, 2012. [DOI] [PubMed] [Google Scholar]
- 195.Martin-Body RL, Johnston BM. Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol 71 (1): 25–32, 1988. [DOI] [PubMed] [Google Scholar]
- 196.Martinez-Garcia MA, Campos-Rodriguez F, Nagore E, Martorell A, Rodriguez-Peralto JL, Riveiro-Falkenbach E, Hernandez L, Banuls J, Arias E, Ortiz P, Cabriada V, Gardeazabal J, Montserrat JM, Carrera C, Corral J, Masa JF, de Terreros JG, Abad J, Boada A, Mediano O, de Eusebio E, Chiner E, Landete P, Mayos M, Fortuno A, Barbe F, Sanchez de la Torre M, Sanchez de la Torre A, Cano I, Gonzalez C, Perez-Gil A, Gomez-Garcia T, Cullen D, Somoza M, Formigon M, Aizpuru F, Navarro C, Selma-Ferrer MJ, Garcia-Ortega A, de Unamuno B, Almendros I, Farre R, Gozal D, Spanish Sleep N. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma: A multicenter observational study in 443 patients. Chest 154 (6): 1348–1358, 2018. [DOI] [PubMed] [Google Scholar]
- 197.Masoumi Z, Familari M, Kallen K, Ranstam J, Olofsson P, Hansson SR. Fetal hemoglobin in umbilical cord blood in preeclamptic and normotensive pregnancies: A cross-sectional comparative study. PLoS One 12 (4): e0176697, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matoth Y, Zaizov R, Varsano I. Postnatal changes in some red cell parameters. Acta Paediatr Scand 60 (3): 317–323, 1971. [DOI] [PubMed] [Google Scholar]
- 199.Mayer CA, Wilson CG, MacFarlane PM. Changes in carotid body and nTS neuronal excitability following neonatal sustained and chronic intermittent hypoxia exposure. Respir Physiol Neurobiol 205: 28–36, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mayer CA, Ao J, Di Fiore JM, Martin RJ, MacFarlane PM. Impaired hypoxic ventilatory response following neonatal sustained and subsequent chronic intermittent hypoxia in rats. Respir Physiol Neurobiol 187 (2): 167–175, 2013. [DOI] [PubMed] [Google Scholar]
- 201.Mayer CA, Di Fiore JM, Martin RJ, MacFarlane PM. Vulnerability of neonatal respiratory neural control to sustained hypoxia during a uniquely sensitive window of development. J Appl Physiol (1985) 116 (5): 514–521, 2014. [DOI] [PubMed] [Google Scholar]
- 202.Milross MA, Piper AJ, Dobbin CJ, Bye PT, Grunstein RR. Sleep disordered breathing in cystic fibrosis. Sleep Med Rev 8 (4): 295–308, 2004. [DOI] [PubMed] [Google Scholar]
- 203.Monneret D, Desmurs L, Zaepfel S, Chardon L, Doret-Dion M, Cartier R. Reference percentiles for paired arterial and venous umbilical cord blood gases: An indirect nonparametric approach. Clin Biochem 67: 40–47, 2019. [DOI] [PubMed] [Google Scholar]
- 204.Moore LG. Maternal O2 transport and fetal growth in Colorado, Peru, and Tibet high-altitude residents. Am J Hum Biol 2 (6): 627–637, 1990. [DOI] [PubMed] [Google Scholar]
- 205.Moore LG. Comparative human ventilatory adaptation to high altitude. Respir Physiol 121 (2–3): 257–276, 2000. [DOI] [PubMed] [Google Scholar]
- 206.Moore LG, Rounds SS, Jahnigen D, Grover RF, Reeves JT. Infant birth weight is related to maternal arterial oxygenation at high altitude. J Appl Physiol Respir Environ Exerc Physiol 52(3): 695–699, 1982. [DOI] [PubMed] [Google Scholar]
- 207.Moore LG, Jahnigen D, Rounds SS, Reeves JT, Grover RF. Maternal hyperventilation helps preserve arterial oxygenation during high-altitude pregnancy. J Appl Physiol Respir Environ Exerc Physiol 52 (3): 690–694, 1982. [DOI] [PubMed] [Google Scholar]
- 208.Moore PJ, Parkes MJ, Nijhuis JG, Hanson MA. The incidence of breathing movements of fetal sheep in normoxia and hypoxia after peripheral chemodenervation and brain-stem transection. J Dev Physiol 11 (3): 147–151, 1989. [PubMed] [Google Scholar]
- 209.Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol 116 (2–3): 95–103, 1999. [DOI] [PubMed] [Google Scholar]
- 210.Mortola JP. Implications of hypoxic hypometabolism during mammalian ontogenesis. Respir Physiol Neurobiol 141 (3): 345–356, 2004. [DOI] [PubMed] [Google Scholar]
- 211.Mortola JP. Influence of temperature on metabolism and breathing during mammalian ontogenesis. Respir Physiol Neurobiol 149 (1–3): 155–164, 2005. [DOI] [PubMed] [Google Scholar]
- 212.Mortola JP, Morgan CA, Virgona V. Respiratory adaptation to chronic hypoxia in newborn rats. J Appl Physiol (1985) 61 (4): 1329–1336, 1986. [DOI] [PubMed] [Google Scholar]
- 213.Mortola JP, Rezzonico R, Lanthier C. Ventilation and oxygen consumption during acute hypoxia in newborn mammals: A comparative analysis. Respir Physiol 78 (1): 31–43, 1989. [DOI] [PubMed] [Google Scholar]
- 214.Mortola JP, Saiki C. Ventilatory response to hypoxia in rats: Gender differences. Respir Physiol 106 (1): 21–34, 1996. [DOI] [PubMed] [Google Scholar]
- 215.Mortola JP, Frappell PB, Frappell DE, Villena-Cabrera N, Villena-Cabrera M, Pena F. Ventilation and gaseous metabolism in infants born at high altitude, and their responses to hyperoxia. Am Rev Respir Dis 146 (5 Pt 1): 1206–1209, 1992. [DOI] [PubMed] [Google Scholar]
- 216.Mortola JP, Trippenbach T, Rezzonico R, Fisher JT, Diaz M, Villena-Cabrera N, Pena F. Hering-Breuer reflexes in high-altitude infants. Clin Sci (Lond) 88 (3): 345–350, 1995. [DOI] [PubMed] [Google Scholar]
- 217.Moss TJ. Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol 33 (3): 280–284, 2006. [DOI] [PubMed] [Google Scholar]
- 218.Moss TJ, Davey MG, McCrabb GJ, Harding R. Development of ventilatory responsiveness to progressive hypoxia and hypercapnia in low-birth-weight lambs. J Appl Physiol (1985) 81 (4): 1555–1561, 1996. [DOI] [PubMed] [Google Scholar]
- 219.Mostello D, Chalk C, Khoury J, Mack CE, Siddiqi TA, Clark KE. Chronic anemia in pregnant ewes: Maternal and fetal effects. Am J Physiol 261 (5 Pt 2): R1075–R1083, 1991. [DOI] [PubMed] [Google Scholar]
- 220.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation (CBD) on ventilation and chemoreflexes in three rat strains. J Physiol 590 (14): 3335–3347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol 590 (14): 3335–3347, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mouradian GC Jr, Liu P, Hodges MR. Raphe gene expression changes implicate immune-related functions in ventilatory plasticity following carotid body denervation in rats. Exp Neurol 287 (Pt 2): 102–112, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mouradian GC Jr, Alvarez-Argote S, Gorzek R, Thuku G, Michkalkiewicz T, Wong-Riley MTT, Konduri GG, Hodges MR. Acute and chronic changes in the control of breathing in a rat model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 316 (3): L506–L518, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mouradian GC Jr, Alvarez-Argote S, Gorzek R, Thuku G, Michalkiewicz T, Wong-Riley MTT, Konduri GG, Hodges MR. Acute and chronic changes in the control of breathing in a hyperoxia-induced model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 316 (3): L506–L518, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mu L, Xia DD, Michalkiewicz T, Hodges M, Mouradian G, Konduri GG, Wong-Riley MTT. Effects of neonatal hyperoxia on the critical period of postnatal development of neurochemical expressions in brain stem respiratory-related nuclei in the rat. Physiol Rep 6 (5): e13627, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mu L, Xia DD, Michalkiewicz T, Konduri GG, Hodges M, Mouradian G, Wong-Riley MTT. Effects of neonatal hyperoxia on the critical period of postnatal development of neurochemical expressions in brain stem respiratory-related nuclei in the rat. Physiol Rep 6 (5): e13627, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Natale R, Nasello-Paterson C, Connors G. Patterns of fetal breathing activity in the human fetus at 24 to 28 weeks of gestation. Am J Obstet Gynecol 158 (2): 317–321, 1988. [DOI] [PubMed] [Google Scholar]
- 228.Niblock MM, Perez A, Broitman S, Jacoby B, Aviv E, Gilkey S. In utero development of fetal breathing movements in C57BL6 mice. Respir Physiol Neurobiol 271: 103288, 2020. [DOI] [PubMed] [Google Scholar]
- 229.Niermeyer S Cardiopulmonary transition in the high altitude infant. High Alt Med Biol 4 (2): 225–239, 2003. [DOI] [PubMed] [Google Scholar]
- 230.Niermeyer S, Shaffer E, Moore LG. Impaired cardiopulmonary transition at high altitude † 1710. Pediatr Res 43 (4): 292, 1998. [Google Scholar]
- 231.Nock ML, Difiore JM, Arko MK, Martin RJ. Relationship of the ventilatory response to hypoxia with neonatal apnea in preterm infants. J Pediatr 144 (3): 291–295, 2004. [DOI] [PubMed] [Google Scholar]
- 232.Okubo S, Mortola JP. Long-term respiratory effects of neonatal hypoxia in the rat. J Appl Physiol (1985) 64 (3): 952–958, 1988. [DOI] [PubMed] [Google Scholar]
- 233.Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Am J Physiol 259 (4 Pt 2): R836–R841, 1990. [DOI] [PubMed] [Google Scholar]
- 234.Onimaru H, Dutschmann M. Calcium imaging of neuronal activity in the most rostral parafacial respiratory group of the newborn rat. J Physiol Sci 62 (1): 71–77, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Onimaru H, Homma I. Development of the rat respiratory neuron network during the late fetal period. Neurosci Res 42 (3): 209–218, 2002. [DOI] [PubMed] [Google Scholar]
- 236.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci 23 (4): 1478–1486, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Onimaru H, Homma I. Developmental changes in the spatio-temporal pattern of respiratory neuron activity in the medulla of late fetal rat. Neuroscience 131 (4): 969–977, 2005. [DOI] [PubMed] [Google Scholar]
- 238.Pagliardini S, Ren J, Greer JJ. Ontogeny of the pre-Botzinger complex in perinatal rats. J Neurosci 23 (29): 9575–9584, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pan J, Yeger H, Cutz E. Innervation of pulmonary neuroendocrine cells and neuroepithelial bodies in developing rabbit lung. J Histochem Cytochem 52 (3): 379–389, 2004. [DOI] [PubMed] [Google Scholar]
- 240.Pan J, Bishop T, Ratcliffe PJ, Yeger H, Cutz E. Hyperplasia and hypertrophy of pulmonary neuroepithelial bodies, presumed airway hypoxia sensors, in hypoxia-inducible factor prolyl hydroxylase-deficient mice. Hypoxia (Auckl) 4: 69–80, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296 (17): 2124–2132, 2006. [DOI] [PubMed] [Google Scholar]
- 242.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of human fetal breathing during the last 10 weeks of pregnancy. Obstet Gynecol 56 (1): 24–30, 1980. [PubMed] [Google Scholar]
- 243.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR. Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol (1985) 104 (5): 1287–1294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Peng Y, Cui C, He Y, Ouzhuluobu, Zhang H, Yang D, Zhang Q, Bianbazhuoma, Yang L, He Y, Xiang K, Zhang X, Bhandari S, Shi P, Yangla, Dejiquzong, Baimakangzhuo, Duojizhuoma, Pan Y, Cirenyangji, Baimayangji, Gonggalanzi, Bai C, Bianba, Basang, Ciwangsangbu, Xu S, Chen H, Liu S, Wu T, Qi X, Su B. Downregulation of EPAS1 transcription and genetic adaptation of Tibetans to high-altitude hypoxia. Mol Biol Evol 34 (4): 818–830, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Peng YJ, Rennison J, Prabhakar NR. Intermittent hypoxia augments carotid body and ventilatory response to hypoxia in neonatal rat pups. J Appl Physiol (1985) 97 (5): 2020–2025, 2004. [DOI] [PubMed] [Google Scholar]
- 246.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: Implications for recurrent apneas. Proc Natl Acad Sci USA 100 (17): 10073–10078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576 (Pt 1): 289–295, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577 (Pt 2): 705–716, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA 108 (7): 3065–3070, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz H, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol 524 (Pt 2): 525–537, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Peyronnet J, Roux JC, Mamet J, Perrin D, Lachuer J, Pequignot JM, Dalmaz Y. Developmental plasticity of the carotid chemoafferent pathway in rats that are hypoxic during the prenatal period. Eur J Neurosci 26(10): 2865–2872, 2007. [DOI] [PubMed] [Google Scholar]
- 252.Pike K, Jane Pillow J, Lucas JS. Long term respiratory consequences of intrauterine growth restriction. Semin Fetal Neonatal Med 17 (2): 92–98, 2012. [DOI] [PubMed] [Google Scholar]
- 253.Poets CF, Samuels MP, Southall DP. Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate 65 (3–4): 211–219, 1994. [DOI] [PubMed] [Google Scholar]
- 254.Prabhakar NR. O2 sensing at the mammalian carotid body: Why multiple O2 sensors and multiple transmitters? Exp Physiol 91 (1): 17–23, 2006 [DOI] [PubMed] [Google Scholar]
- 255.Prabhakar NR, Semenza GL. Oxygen sensing and homeostasis. Physiology 30 (5): 340–348, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Prandota J Possible pathomechanisms of sudden infant death syndrome: Key role of chronic hypoxia, infection/inflammation states, cytokine irregularities, and metabolic trauma in genetically predisposed infants. Am J Ther 11 (6): 517–546, 2004. [DOI] [PubMed] [Google Scholar]
- 257.Prsa M, Sun L, van Amerom J, Yoo SJ, Grosse-Wortmann L, Jaeggi E, Macgowan C, Seed M. Reference ranges of blood flow in the major vessels of the normal human fetal circulation at term by phase-contrast magnetic resonance imaging. Circ Cardiovasc Imaging 7 (4): 663–670, 2014. [DOI] [PubMed] [Google Scholar]
- 258.Rajani V, Zhang Y, Jalubula V, Rancic V, SheikhBahaei S, Zwicker JD, Pagliardini S, Dickson CT, Ballanyi K, Kasparov S, Gourine AV, Funk GD. Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. J Physiol 596 (15): 3245–3269, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Reeves SR, Gozal D. Changes in ventilatory adaptations associated with long-term intermittent hypoxia across the age spectrum in the rat. Respir Physiol Neurobiol 150 (2–3): 135–143, 2006. [DOI] [PubMed] [Google Scholar]
- 260.Reeves SR, Mitchell GS, Gozal D. Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term phrenic facilitation in adult rats. Am J Physiol Regul Integr Comp Physiol 290 (6): R1664–R1671, 2006. [DOI] [PubMed] [Google Scholar]
- 261.Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J Neurosci 26 (14): 3721–3730, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ribeiro AP, Mayer CA, Wilson CG, Martin RJ, MacFarlane PM. Intratracheal LPS administration attenuates the acute hypoxic ventilatory response: Role of brainstem IL-1beta receptors. Respir Physiol Neurobiol 242: 45–51, 2017. [DOI] [PubMed] [Google Scholar]
- 263.Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol 119 (3): 717–723, 1998. [DOI] [PubMed] [Google Scholar]
- 264.Richardson HL, Parslow PM, Walker AM, Harding R, Horne RS. Maturation of the initial ventilatory response to hypoxia in sleeping infants. J Sleep Res 16 (1): 117–127, 2007. [DOI] [PubMed] [Google Scholar]
- 265.Rojas DA, Perez-Munizaga DA, Centanin L, Antonelli M, Wappner P, Allende ML, Reyes AE. Cloning of hif-1alpha and hif-2alpha and mRNA expression pattern during development in zebrafish. Gene Expr Patterns 7 (3): 339–345, 2007. [DOI] [PubMed] [Google Scholar]
- 266.Rosenfeld M, Snyder FF. Direct observation of intrauterine respiratory movements of the fetus and the role of carbon dioxide and oxygen in their regulation. Am J Physiol 119 (1): 153–166, 1937. [Google Scholar]
- 267.Rourke KS, Mayer CA, MacFarlane PM. A critical postnatal period of heightened vulnerability to lipopolysaccharide. Respir Physiol Neurobiol 232: 26–34, 2016. [DOI] [PubMed] [Google Scholar]
- 268.Rudolph AM. Congenital Diseases of the Heart: Clinical-Physiological Considerations (3rd ed). Chichester, UK; Hoboken, NJ: Wiley-Blackwell, 2009. 538 p. [Google Scholar]
- 269.Ryan HE, Lo J, Johnson RS. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J 17 (11): 3005–3015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rytkonen KT, Williams TA, Renshaw GM, Primmer CR, Nikinmaa M. Molecular evolution of the metazoan PHD-HIF oxygen-sensing system. Mol Biol Evol 28 (6): 1913–1926, 2011. [DOI] [PubMed] [Google Scholar]
- 271.Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 3 (1): a011643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sawnani H, Jackson T, Murphy T, Beckerman R, Simakajornboon N. The effect of maternal smoking on respiratory and arousal patterns in preterm infants during sleep. Am J Respir Crit Care Med 169 (6): 733–738, 2004. [DOI] [PubMed] [Google Scholar]
- 273.Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood 112 (10): 3927–3938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Schmalisch G, Wilitzki S, Wauer RR. Differences in tidal breathing between infants with chronic lung diseases and healthy controls. BMC Pediatr 5: 36, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schneider-Maunoury S, Topilko P, Seitandou T, Levi G, Cohen-Tannoudji M, Pournin S, Babinet C, Charnay P. Disruption of Krox-20 results in alteration of rhombomeres 3 and 5 in the developing hindbrain. Cell 75 (6): 1199–1214, 1993. [DOI] [PubMed] [Google Scholar]
- 276.Serra A, Brozoski D, Hedin N, Franciosi R, Forster HV. Mortality after carotid body denervation in rats. J Appl Physiol 91 (3): 1298–1306, 2001. [DOI] [PubMed] [Google Scholar]
- 277.Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC, Gourine AV. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9 (1): 370, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Siljehav V, Shvarev Y, Herlenius E. Il-1beta and prostaglandin E2 attenuate the hypercapnic as well as the hypoxic respiratory response via prostaglandin E receptor type 3 in neonatal mice. J Appl Physiol (1985) 117 (9): 1027–1036, 2014. [DOI] [PubMed] [Google Scholar]
- 279.Sladek M, Parker RA, Grogaard JB, Sundell HW. Long-lasting effect of prolonged hypoxemia after birth on the immediate ventilatory response to changes in arterial partial pressure of oxygen in young lambs. Pediatr Res 34 (6): 821–828, 1993. [DOI] [PubMed] [Google Scholar]
- 280.Smith CA, Chenuel BJ, Henderson KS, Dempsey JA. The apneic threshold during non-REM sleep in dogs: Sensitivity of carotid body vs. central chemoreceptors. J Appl Physiol (1985) 103 (2): 578–586, 2007. [DOI] [PubMed] [Google Scholar]
- 281.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: A brainstem region that may generate respiratory rhythm in mammals. Science 254 (5032): 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: Building blocks and microcircuits. Trends Neurosci 36(3): 152–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther 1 (4): 168–175, 1986. [DOI] [PubMed] [Google Scholar]
- 284.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of alpha1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol (1985) 104 (3): 665–673, 2008. [DOI] [PubMed] [Google Scholar]
- 285.St-John WM, Li A, Leiter JC. Genesis of gasping is independent of levels of serotonin in the Pet-1 knockout mouse. J Appl Physiol (1985) 107 (3): 679–685, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Stojanovska V, Miller SL, Hooper SB, Polglase GR. The consequences of preterm birth and chorioamnionitis on brainstem respiratory centers: Implications for neurochemical development and altered functions by inflammation and prostaglandins. Front Cell Neurosci 12: 26, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360 (6393): eaan8546, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 362 (21): 1959–1969, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Tadjalli A, Mitchell GS. Cervical spinal 5-HT2A and 5-HT2B receptors are both necessary for moderate acute intermittent hypoxia-induced phrenic long-term facilitation. J Appl Physiol (1985) 127 (2): 432–443, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Taweevisit M, Theerasantipong B, Taothong K, Thorner PS. Pulmonary neuroendocrine cell hyperplasia in hemoglobin Bart-induced hydrops fetalis: A model for chronic intrauterine hypoxia. Pediatr Dev Pathol 20 (4): 298–307,2017. [DOI] [PubMed] [Google Scholar]
- 291.Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res 27 (4 Pt 1): 372–378, 1990. [DOI] [PubMed] [Google Scholar]
- 292.Teppema LJ. CrossTalk opposing view: The hypoxic ventilatory response does not include a central, excitatory hypoxia sensing component. J Physiol 596 (15): 2939–2941, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Teppema LJ, Dahan A. The ventilatory response to hypoxia in mammals: Mechanisms, measurement, and analysis. Physiol Rev 90 (2): 675–754, 2010. [DOI] [PubMed] [Google Scholar]
- 294.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189 (1): 57–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat Neurosci 12 (8): 1028–1035, 2009. [DOI] [PubMed] [Google Scholar]
- 296.Tolcos M, Rees S. Chronic placental insufficiency in the fetal guinea pig affects neurochemical and neuroglial development but not neuronal numbers in the brainstem: A new method for combined stereology and immunohistochemistry. J Comp Neurol 379 (1): 99–112, 1997. [DOI] [PubMed] [Google Scholar]
- 297.Tolcos M, Harding R, Loeliger M, Breen S, Cock M, Duncan J, Rees S. The fetal brainstem is relatively spared from injury following intrauterine hypoxemia. Brain Res Dev Brain Res 143 (1): 73–81, 2003. [DOI] [PubMed] [Google Scholar]
- 298.Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol (1985) 103 (1): 220–227, 2007. [DOI] [PubMed] [Google Scholar]
- 299.Tree K, Viemari JC, Cayetanot F, Peyronnet J. Growth restriction induced by chronic prenatal hypoxia affects breathing rhythm and its pontine catecholaminergic modulation. J Neurophysiol 116 (4): 1654–1662, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Trudinger BJ, Knight PC. Fetal age and patterns of human fetal breathing movements. Am J Obstet Gynecol 137 (6): 724–728, 1980. [DOI] [PubMed] [Google Scholar]
- 301.Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: A rhythm dependent on 5-HT2A receptors. J Neurosci 26 (10): 2623–2634, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. JAMA 259 (23): 3427–3432, 1988. [PubMed] [Google Scholar]
- 303.Vargas M, Vargas E, Julian CG, Armaza JF, Rodriguez A, Tellez W, Niermeyer S, Wilson M, Parra E, Shriver M, Moore LG. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude. Am J Physiol Regul Integr Comp Physiol 293 (3): R1303–R1312, 2007. [DOI] [PubMed] [Google Scholar]
- 304.Villa MP, Pagani J, Lucidi V, Palamides S, Ronchetti R. Nocturnal oximetry in infants with cystic fibrosis. Arch Dis Child 84 (1): 50–54, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Waites BA, Ackland GL, Noble R, Hanson MA. Red nucleus lesions abolish the biphasic respiratory response to isocapnic hypoxia in decerebrate young rabbits. J Physiol 495 (Pt 1): 217–225, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Watson CS, Schaefer R, White SE, Homan JH, Fraher L, Harding R, Bocking AD. Effect of intermittent umbilical cord occlusion on fetal respiratory activity and brain adenosine in late-gestation sheep. Reprod Fertil Dev 14 (1–2): 35–42, 2002. [DOI] [PubMed] [Google Scholar]
- 307.Weaver DR. A2a adenosine receptor gene expression in developing rat brain. Brain Res Mol Brain Res 20 (4): 313–327, 1993. [DOI] [PubMed] [Google Scholar]
- 308.Wigglesworth JS, Desai R. Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum Dev 3 (1): 51–65, 1979. [DOI] [PubMed] [Google Scholar]
- 309.Williams PA, Wilson CG. Effects of inflammation on the developing respiratory system: Focus on hypoglossal (XII) neuron morphology, brainstem neurochemistry, and control of breathing. Respir Physiol Neurobiol 275: 103389, 2020. [DOI] [PubMed] [Google Scholar]
- 310.Wilson SL, Thach BT, Brouillette RT, Abu-Osba YK. Upper airway patency in the human infant: Influence of airway pressure and posture. J Appl Physiol Respir Environ Exerc Physiol 48 (3): 500–504, 1980. [DOI] [PubMed] [Google Scholar]
- 311.Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol 164 (1–2): 28–37, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wong-Riley MTT, Liu Q, Gao X. Mechanisms underlying a critical period of respiratory development in the rat. Respir Physiol Neurobiol 264: 40–50, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wyatt CN, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci USA 92 (1): 295–299, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Yan X, Koos BJ, Kruger L, Linden J, Murray TF. Characterization of [125I]ZM 241385 binding to adenosine A2A receptors in the pineal of sheep brain. Brain Res 1096 (1): 30–39, 2006. [DOI] [PubMed] [Google Scholar]
- 315.Yang HT, Cummings KJ. Brain stem serotonin protects blood pressure in neonatal rats exposed to episodic anoxia. J Appl Physiol (1985) 115 (12): 1733–1741, 2013. [DOI] [PubMed] [Google Scholar]
- 316.Young JO, Geurts A, Hodges MR, Cummings KJ. Active sleep unmasks apnea and delayed arousal in infant rat pups lacking central serotonin. J Appl Physiol (1985) 123 (4): 825–834, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature 365 (6442): 153–155, 1993. [DOI] [PubMed] [Google Scholar]
- 318.Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol 582 (Pt 2): 883–895, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


