Abstract
Scope:
Bacterial infection induces mucus overproduction, contributing to acute exacerbations and lung function decline in chronic respiratory diseases. A diet enriched in apples may provide protection from pulmonary disease development and progression. This study examined whether phloretin, an apple polyphenol, inhibits mucus synthesis and secretion induced by the predominant bacteria associated with chronic respiratory diseases.
Methods and results:
The expression of the mucus constituent mucin 5AC (MUC5AC) in FVB/NJ mice and NCI-H292 epithelial cells was analyzed. Nontypeable Haemophilus influenzae (NTHi)-infected mice developed increased MUC5AC mRNA, which a diet containing phloretin inhibited. In NCI-H292 cells, NTHi, Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas aeruginosa increased MUC5AC mRNA, which phloretin inhibited. Phloretin also diminished NTHi-induced MUC5AC protein secretion. NTHi-induced increased MUC5AC required toll-like receptor 4 (TLR4) and NADH oxidase 4 (NOX4) signaling and subsequent activation of the epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase (MAPK) pathway. Phloretin inhibited NTHi-induced TLR4/NOX4 and EGFR/MAPK signaling, thereby preventing increased MUC5AC mRNA. EGFR activation can also result from increased EGFR ligand synthesis and subsequent ligand activation by matrix metalloproteinases (MMPs). In NCI-H292 cells, NTHi increased EGFR ligand and MMP1 and MMP13 mRNA, which phloretin inhibited.
Conclusions:
In summary, phloretin is a promising therapeutic candidate for preventing bacterial-induced mucus overproduction.
Keywords: Mucin, Reactive oxygen species, Toll-like receptor 4, Matrix metalloproteinase, Epidermal growth factor
INTRODUCTION
Mucus secretion is a critical component of the innate immune system that traps and clears pathogens from the airway [1, 2]. However, bacterial-induced mucus overproduction can contribute to airflow limitation and lung function decline, particularly in chronic respiratory diseases [3, 4]. Apples and other hard fruits may provide protection from pulmonary disease development and progression. Butland and colleagues found that persons eating five or more apples per week had better lung function, as indicated by increased forced expiratory volume in 1 second (FEV1), compared to non-consumers [5]. Because the protection associated with apple consumption was independent of vitamin C or E intake, these investigators suggested this might be due to other antioxidant constituents of apples such as polyphenols. Apple polyphenols include phloridzin and its metabolite phloretin [3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl) propan-1-one] [6]. Phloretin is a promising candidate for protecting against bacterial infection due to its antimicrobial and anti-inflammatory activity [7].
We sought to examine whether phloretin could protect against mucus overproduction induced by nontypeable Haemophilus influenzae (NTHi), Moraxella catarrhalis (M. catarrhalis), Streptococcus pneumoniae (S. pneumoniae), and Pseudomonas aeruginosa (P. aeruginosa), which have been implicated in mucus production in chronic obstructive pulmonary disease (COPD) [3, 8]. We specifically analyzed the mucus constituent mucin 5AC, oligomeric mucus/gel-forming (MUC5AC). MUC5AC is a major component of airway mucus, produced and secreted by surface goblet cells and submucosal glands to trap and clear microbes and particles from the lung [9]. MUC5AC production is regulated by the epidermal growth factor receptor (EGFR) and mitogen-activated protein kinase (MAPK) signaling pathway [10–12].
Previously, bacteria and viruses have been found to increase MUC5AC production through EGFR-MAPK signaling [13, 14]. Phloretin has been reported to inhibit cigarette smoke-induced mucus overproduction in mice through EGFR inhibition, suppressing phosphorylation of MAPK family members MAPK3/1 (aka ERK1/2) and MAPK14 (aka p38) [15]. In this study, we examined whether phloretin altered pathogen-induced cell signaling pathways that mediate MUC5AC overproduction.
EXPERIMENTAL SECTION
A detailed description of methods and reagents is listed in the Supporting Information. Briefly, male and female FVB/NJ mice were provided a diet supplemented with or without phloretin (667 ppm) 1 week prior to NTHi exposure. Studies with mice were conducted with approval of the Institutional Animal Use and Care Committee of the University of Pittsburgh (Protocol# 16088599). Mouse pulmonary MUC5AC mRNA (RT-qPCR) was measured. MUC5AC mRNA was measured in human airway epithelial cells (NCI-H292) incubated with or without phloretin and exposed to the pathogens NTHi, M. catarrhalis, S. pneumoniae, or P. aeruginosa (strain PAO1). The time course of NTHi-induced MUC5AC mRNA and secreted MUC5AC protein (ELISA) was determined. To evaluate if NTHi exposure induced reactive oxygen species (ROS) production in NCI-H292 cells, dihydroethidium (DHE) was measured (flow cytometry). TLR4 and NADPH oxidase 4 (NOX4) protein (Western blot) and MUC5AC mRNA were measured in NTHi-exposed NCI-H292 cells treated with TAK-242 (resatorvid), a TLR4 inhibitor, diphenyleneiodonium (DPI), a NOX inhibitor, or propyl gallate (PG), a ROS scavenger. EGFR, MAPK3/1, and MAPK8 protein (Western blot) and MUC5AC mRNA was measured in NCI-H292 cells treated with phloretin or inhibitors of EGFR kinase (AG-1478), MAPK3/1 (FR180204), and MAPK8 (SP600125) prior to NTHi exposure. The continued activation of the pathway was assessed by measuring matrix metalloproteinases (MMP 1, 2, 3, 7, 8, 9, 10, 12, and 13) by using biotinylated antibodies and RT-qPCR. EGFR ligands (epidermal growth factor (EGF), epiregulin (EREG), amphiregulin (AREG), heparin-binding EGF (HBEGF), transforming growth factor-α (TGFA), epigen (EPGN), and betacellulin (BTC)) were measured by RT-qPCR. Results are expressed as means ± SEM with significance (p<0.05) determined by analysis of variance (ANOVA) followed by Dunnett’s, Tukey’s, or Holm-Sidak’s multiple comparisons tests.
RESULTS
Phloretin inhibits pathogen-induced MUC5AC in mice and NCI-H292 cells:
In mice, intratracheal and intranasal instillation of NTHi induces pulmonary histopathological changes including bronchial, bronchiolar, and alveolar epithelial injury and inflammatory cell infiltration [16–19]. In this study, intratracheal NTHi also induced inflammatory cell influx into the airways, particularly neutrophils, which a phloretin-enriched diet inhibited (Figure S1, Supporting Information). Previously, phloretin was found to protect against ovalbumin [20] and cigarette smoke-induced [15] MUC5AC production in mice. To examine whether phloretin protects against pathogen-induced MUC5AC, mice were supplied a diet supplemented with phloretin and intratracheally exposed to NTHi. NTHi induced MUC5AC, which the phloretin diet inhibited (Figure 1A).
Figure 1. Phloretin inhibits pathogen-induced mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) increase in mouse lung and respiratory cells.
A. Phloretin inhibits nontypeable Haemophilus influenzae (NTHi)-induced MUC5AC mRNA increase in FVB/NJ mouse lung. Mice were supplied food without or with 667 ppm phloretin supplementation ad libitum for 1 wk and intratracheally exposed to control treatment (PBS) or 105 CFU NTHi. After 24h, lung specimens were collected and MUC5AC and RPL32 transcripts were measured by RT-qPCR (n=8–9/group, male (closed symbols) and female (open symbols)). Values are log2 fold change of MUC5AC relative to standard diet control treated mice and normalized to RPL32. Lines indicate mean ± SEM. *Significantly different (p<0.05) in NTHi-treated mice with standard diet exposure compared to phloretin diet exposure, as determined by ANOVA followed by Tukey’s multiple comparison method. B. Phloretin inhibits increased MUC5AC mRNA induced by several common respiratory pathogens in NCI-H292 cells. NCI-H292 cells were treated with 100 μM phloretin (1 h, 37 °C) and exposed to multiplicity of infection (MOI)=7.5 NTHi, MOI=40 Moraxella catarrhalis, MOI=5 Streptococcus pneumoniae, or MOI=0.07 Pseudomonas aeruginosa strain PAO1 (24h, 37 °C). MUC5AC and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=6–9) are log2 fold change of MUC5AC relative to vehicle control treated cells and normalized to RPL32. *Significantly different (p<0.05) in pathogen + phloretin compared to pathogen alone treated cells, as determined by ANOVA followed by Tukey’s multiple comparison method. C. Phloretin inhibits NTHi-induced increased MUC5AC mRNA in a time-dependent manner. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h, 37°C) and exposed to MOI 7.5 NTHi. At indicated times, mRNA was isolated and MUC5AC and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n = 6–12) are log2 fold change of MUC5AC normalized to RPL32. *Significantly different (p<0.05) in NTHi compared to vehicle control treated cells, as determined by ANOVA followed by Tukey’s multiple comparison method. D. Phloretin inhibits NTHi-induced increased MUC5AC protein in a time-dependent manner. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h, 37°C) and exposed to medium control or MOI 7.5 NTHi. At indicated times, supernatant was collected and MUC5AC was measured by ELISA. Values (mean ± SEM, n=6–12) are fold change of MUC5AC of phloretin- and pathogen-treated cells relative to vehicle control treated cells. *Significantly different (p<0.05) in NTHi compared to vehicle control treated cells, as determined by ANOVA followed by Dunnett’s multiple comparisons test. E. Phloretin inhibits NTHi-induced MUC5AC mRNA in NCI-H292 cells similarly to or greater than other polyphenols. NCI-H292 cells were treated with 100 μM phloretin, 100 μM resveratrol, 10 μM epigallocatechin gallate (EGCG), or 100 μM curcumin (1 h) and exposed to NTHi (24 h). MUC5AC and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=6–9) are log2 fold change of MUC5AC normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) from NTHi alone treated cells, as determined by ANOVA followed by Tukey’s multiple comparison method. Tests were performed on ≥2 occasions.
To further examine the inhibitory mechanism of phloretin on increased mucin formation, NCI-H292 cells, a human pulmonary epithelial cell line, were exposed to the pathogens (NTHi, M. catarrhalis, S. pneumoniae, and P. aeruginosa strain PAO1) with and without phloretin pretreatment. Each pathogen increased MUC5AC mRNA in NCI-H292 cells (Figure 1B). Phloretin suppressed MUC5AC induced by these pathogens relative to vehicle-control treated cells (Figure 1B). Phloretin inhibited NTHi-induced MUC5AC mRNA (Figure 1C) and protein (Figure 1D) in NCI-H292 cells in a time dependent manner. Phloretin is anti-tumorigenic, reducing cancer cell proliferation partially through its inhibition of glucose transport [21]. To demonstrate that the suppression of MUC5AC did not result from cytotoxicity of the NCI-H292 cancer cell line, lactate dehydrogenase (LDH) release and ATP was measured (Figure S2, Supporting Information). Phloretin treatment did not increase LDH release or reduce ATP levels compared to vehicle treated cells.
Previous studies have found that other polyphenols, including resveratrol, epigallocatechin gallate (EGCG), and curcumin, can inhibit MUC5AC induction in mouse lung and other cells following various insults [22–24]. We pretreated cells with polyphenol concentrations that exhibited maximum bioactivity without toxicity. Relative to the other polyphenols, phloretin had comparable or greater inhibition of NTHi-induced MUC5AC mRNA (Figure 1E).
Phloretin protects against EGFR activation by TLR4/NOX4-mediated ROS:
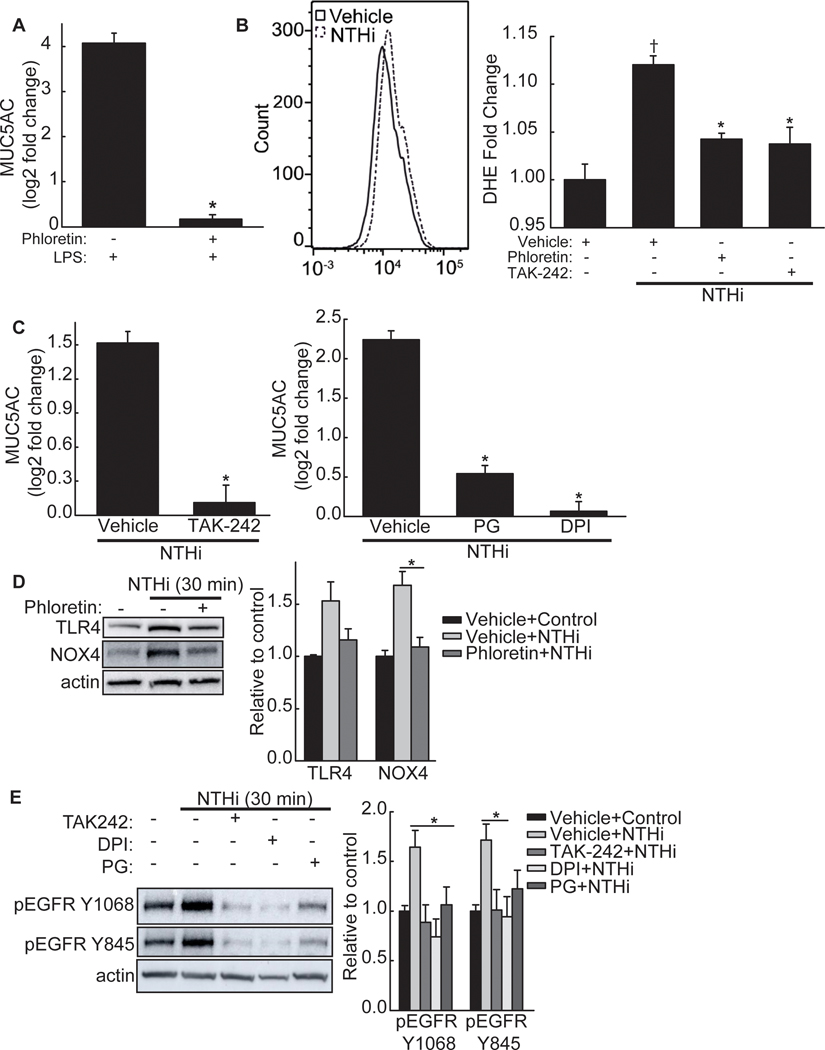
TLR4 rapidly recognizes and responds to microbial insults by inducing an inflammatory cascade [25] and is essential for mediating the response to the pathogens we examined in this study [18, 26–29]. Lipopolysaccharide (LPS) is a pathogen-associated molecular pattern on gram-negative bacteria that is recognized by TLR4, eliciting an innate immune response [25]. To determine the involvement of the TLR4 in MUC5AC transcript expression, we treated NCI-H292 cells with P. aeruginosa derived LPS. LPS increased MUC5AC mRNA, suggesting a role of TLR4 in mucin transcript increase (Figure 2A). Phloretin inhibited this increase (Figure 2A).
Figure 2. Phloretin inhibits NTHi-induced phosphorylation of the epidermal growth factor receptor (EGFR)-MAPK pathway through toll-like receptor 4 (TLR4)-NADPH oxidase 4 (NOX4) signaling.
A. Lipopolysaccharide (LPS) induces mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) mRNA in NCI-H292 cells, which is inhibited by phloretin. NCI-H292 cells were treated without or with 100 μM phloretin and exposed to 1 μg/mL LPS (24 h). MUC5AC and RPL32 mRNA was measured by RT-qPCR. Values (mean ± SEM, n=9) are log2 fold change of MUC5AC mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) in LPS + phloretin compared to LPS alone treated cells, as determined by ANOVA followed by Tukey’s multiple comparison method. B. NTHi increases reactive oxygen species (ROS) production in NCI-H292 cells, which is inhibited by phloretin and a TLR4 inhibitor, TAK-242. NCI-H292 cells were exposed to vehicle, 100 μM phloretin, or 30 μM TAK-242 (1 h) and stimulated with NTHi (30 min). Intracellular ROS production was detected by phycoerythrin (PE)-conjugated dihydroethidium (DHE) using flow cytometry (excitation 488 nm, emission 585 nm). (Left Panel) Representative result (10,000 cells) of increased fluorescence following NTHi treatment (dash line) compared to control (solid line). (Right Panel) Fluorescence intensity (mean ± SEM, n=4) of vehicle alone, NTHi, NTHi + phloretin, and NTHi + TAK-242 treated cells. †Significantly different (p<0.05) from vehicle control as determined by ANOVA followed by Dunnett’s multiple comparison method. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Dunnett’s multiple comparison method. C. Inhibition of TLR4 and ROS reduces NTHi-induced MUC5AC mRNA. NCI-H292 cells were incubated (1h) with (Left Panel) 30 μM TAK-242 or (Right Panel) 10 μM diphenyleneiodonium (DPI), a NADPH oxidase inhibitor, or 100 μM propyl gallate (PG), a ROS scavenger, and stimulated with NTHi (8h). MUC5AC and RPL32 mRNA were measured by RT-qPCR. Values (mean ± SEM, n=9–18) are log2 fold change of MUC5AC normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Tukey’s multiple comparison method. D. NTHi increases TLR4 and NOX4 protein, which phloretin inhibits. NCI-H292 cells were pretreated with vehicle or 100 μM phloretin (1 h) and exposed to NTHi (30 min). (Left Panel) Cell protein extract was collected and immunoblotted with anti-TLR4, anti-NOX4, or anti-β-actin antibodies. (Right Panel) Values (mean ± SEM, n=4) are fold change in protein expression relative to vehicle control normalized to the β-actin control. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Tukey’s multiple comparisons method. E. Inhibition of TLR4 and ROS inhibits NTHi-induced EGFR phosphorylation. NCI-H292 cells were pretreated with TAK-242, DPI, or PG (1 h) and exposed to NTHi (30 min). (Left Panel) Cell protein extract was collected and immunoblotted for phosphorylated EGFR (anti-Y1068 or anti-Y845) or anti-β-actin. (Right Panel) Values (mean ± SEM, n=4) are fold change in protein expression relative to vehicle control normalized to the β-actin control. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Tukey’s multiple comparisons method. Tests were performed on ≥2 occasions.
Activated TLR4 can interact with NOX4, an enzyme that generates superoxide [30]. To examine the role of NOX4 in NTHi-induced MUC5AC transcript level increase, we first determined whether NTHi could increase ROS in NCI-H292 cells. NTHi increased intracellular ROS levels, as measured by dihydroethidium (Figure 2B). TLR4 involvement was measured by pretreating cells with TAK-242, a TLR4 inhibitor. TAK-242 inhibited NTHi-induced increased ROS, as did phloretin (Figure 2B). Phloretin did not reduce baseline ROS in cells (Figure S3A, Supporting Information). Furthermore, inhibition of TLR4 and ROS by diphenyleneiodonium (DPI), a NOX inhibitor, and propyl gallate (PG), a ROS scavenger, inhibited an increase in MUC5AC mRNA induced by NTHi (Figure 2C). To further determine whether NOX4 is involved in generating ROS in response to NTHi, we measured protein expression by Western blot. Activation of TLR4 does not require an increase in receptor protein levels, however, we found that NTHi induced an increase in TLR4 as well as NOX4 protein in NCI-H292 cells, which phloretin reduced (Figure 2D). This is in agreement with other exposures that have induced increases in TLR4 protein in vitro, including LPS [31] and cigarette smoke [32]. Phloretin did not affect baseline expression of TLR4 or NOX4 (Figure S3B, Supporting Information).
EGFR is a redox-sensitive receptor [33] that responds to numerous exposures (including bacteria, viruses, and cigarette smoke) to facilitate mucus production in the airway [13]. We examined if NTHi could activate TLR4-NOX4 signaling and whether the resulting ROS could lead to EGFR activation. To examine whether ROS generated by TLR4/NOX4 signaling could induce EGFR activation, we measured EGFR phosphorylation after NTHi exposure in the presence of TLR4, NOX, or ROS inhibitors. Upon stimulation, EGFR creates homo- and heterodimers, leading to its autophosphorylation at several C-terminal tail sites within its kinase domain, including Tyr-1068 [33]. Furthermore, EGFR can be activated by tyrosine kinases (e.g. SRC), which transphosphorylate its kinase domain at Tyr-845 [33]. NTHi increased autophosphorylation (Y1068) and transphosphorylation (Y845) of EGFR within 30 min, which the inhibitors reduced, supporting the involvement of TLR4/NOX4 signaling (Figure 2E). Thus, phloretin suppression of NTHi-induced MUC5AC expression in NCI-H292 cells involves TLR4/NOX4 signaling that leads to ROS formation and EGFR phosphorylation. The inhibitors did not alter baseline expression of EGFR phosphorylation (Figure S3C, Supporting Information).
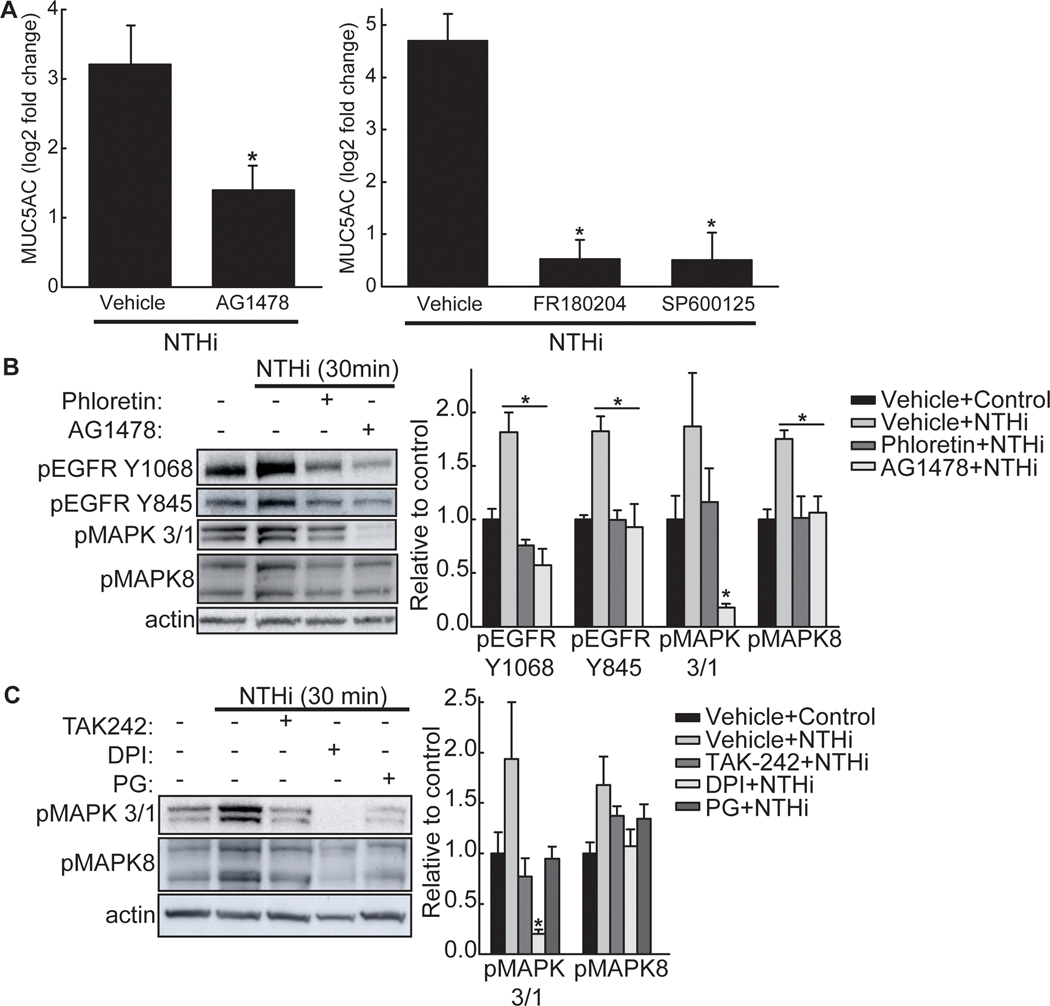
Phloretin inhibits NTHi-induced increased MUC5AC by inhibiting EGFR-MAPK signaling cascade:
To measure EGFR involvement in MUC5AC transcript increase following NTHi exposure, NCI-H292 cells were treated with AG-1478, an EGFR kinase inhibitor, and MUC5AC mRNA was measured (Figure 3A). AG-1478 decreased NTHi-induced MUC5AC mRNA (Figure 3A). In addition, FR180204 and SP600125, inhibitors to MAPK3/1 (aka ERK1/2) and MAPK8 (aka JNK), respectively, inhibited NTHi-induced MUC5AC transcript increase (Figure 3A). We next investigated whether phloretin inhibition of MUC5AC is through the EGFR-MAPK pathway. Phloretin inhibited NTHi-mediated EGFR phosphorylation (Figure 3B). Phloretin also decreased NTHi-induced phosphorylation of MAPK3/1 and MAPK8 protein (Figure 3B), but not MAPK14 (aka p38) (Figure S4, Supporting Information). Furthermore, AG-1478 pretreatment inhibited MAPK3/1 and MAPK8 phosphorylation, but did not alter MAPK14 phosphorylation, thereby linking activation of EGFR by NTHi to MAPK3/1 and MAPK8 signaling. Thus, phloretin inhibits NTHi-induced MUC5AC by suppressing the EGFR-MAPK signaling cascade.
Figure 3. Phloretin inhibits NTHi-induced mucin 5AC, oligomeric mucus/gel-forming (MUC5AC) as mediated by epidermal growth factor receptor (EGFR)-mitogen-activated protein kinase (MAPK) signaling.
A. Inhibition of EGFR phosphorylation, MAPK3/1, and MAPK8 reduces NTHi-induced MUC5AC. NCI-H292 cells were incubated (1h) with vehicle or (Left panel) 1 μM AG-1478, an EGFR kinase inhibitor, or (Right Panel) 30 μM FR180204, a MAPK3/1 (aka ERK1/2) inhibitor, or 100 μM SP600125, a MAPK8 (aka JNK) inhibitor and stimulated with NTHi (8h). MUC5AC and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=6–12) are log2 fold change of MUC5AC mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) compared to NTHi alone treated cells as determined by ANOVA followed by Tukey’s multiple comparison method. B. NTHi increases phosphorylation of EGFR, MAPK3/1, and MAPK8, which phloretin and AG-1478 inhibits. NCI-H292 cells were pretreated with vehicle, 100 μM phloretin, or 1 μM AG-1478 (1 h) and exposed to NTHi (30 min). (Left Panel) Cell protein extract was collected and immunoblotted with antibodies for β-actin (protein loading control) or phosphorylated EGFR, MAPK3/1, or MAPK8 protein. (Right Panel) Values (mean ± SEM, n=4) are fold change in protein expression relative to vehicle control normalized to the β-actin control. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Tukey’s multiple comparisons method. C. Inhibition of TLR4 or ROS inhibits downstream NTHi-induced MAPK signaling. NCI-H292 cells were incubated (1h) with 30 μM TAK-242, a TLR4 inhibitor, 10 μM diphenyleneiodonium (DPI), a NADPH oxidase inhibitor, or 100 μM propyl gallate (PG), a ROS scavenger, and stimulated with NTHi (30 min). (Right Panel) Values (mean ± SEM, n=4) are fold change in protein expression relative to vehicle control normalized to the β-actin control. *Significantly different (p<0.05) from NTHi treated cells as determined by ANOVA followed by Tukey’s multiple comparisons method. Tests were performed on ≥2 occasions.
To connect the TLR4 and NOX4-induced ROS activation of EGFR to the MAPK pathway, we inhibited TLR4 (by TAK-242), NOX activity (by DPI), and ROS (by PG). All inhibitors reduced NTHi-induced phosphorylation of MAPK3/1 and MAPK8 (Figure 3C). Therefore, phloretin inhibits NTHi activation of TLR4/NOX4 signaling and the subsequent EGFR/MAPK pathway.
Phloretin protects against NTHi-induced increased MMP and EGFR ligand expression:
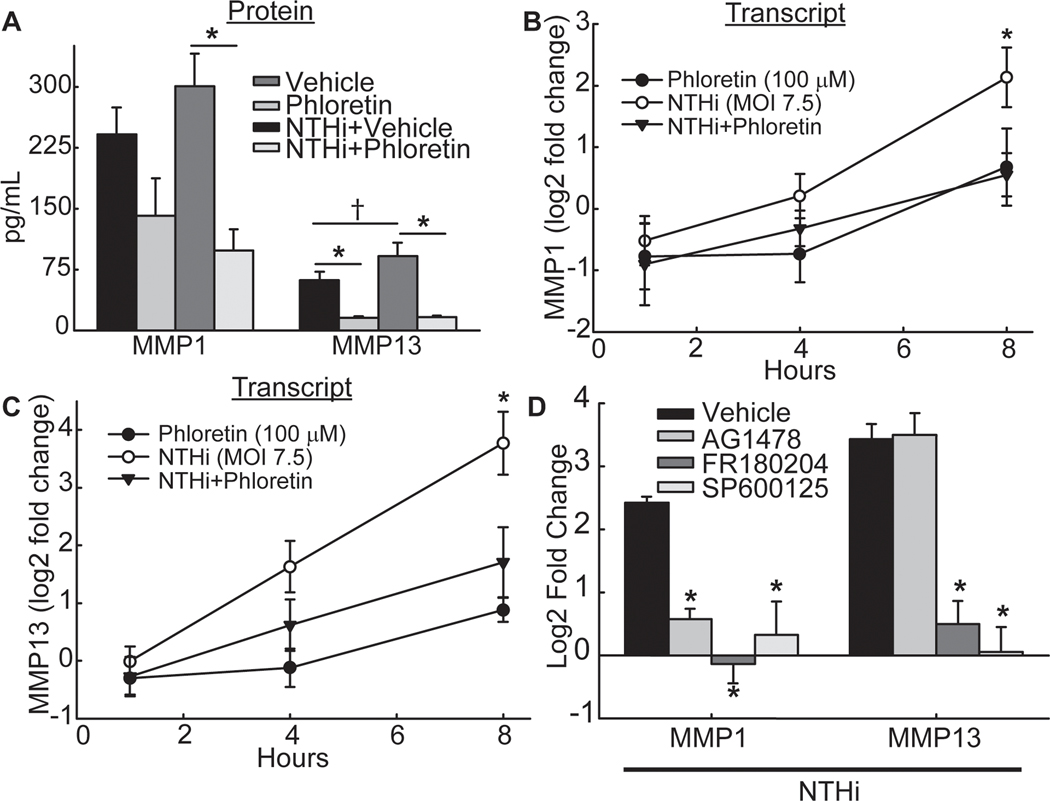
MMPs cleave EGFR ligands, which are synthesized as transmembrane precursors [34]. Subsequently, EGFR ligands induce EGFR dimerization and autophosphorylation of endogenous protein tyrosine residues [33]. An array of MMP proteins (MMP 1, 2, 3, 7, 8, 9, 10, 12, and 13) were measured in NCI-H292 cell culture supernatant following treatment with vehicle control, phloretin, NTHi, or NTHi with phloretin exposure. Phloretin decreased baseline levels of MMP13 protein and NTHi-induced MMP1 and MMP13 protein (Figure 4A). Phloretin also decreased baseline protein levels of MMP7 and MMP10 (Table S2, Supporting Information). However, NTHi exposure did not induce release of these MMP proteins into the cell culture supernatant (Table S2, Supporting Information). In addition, NTHi increased MMP1 and MMP13 transcripts and phloretin inhibited these increases (Figure 4B and 4C).
Figure 4. Phloretin inhibits matrix metalloproteinase (MMP) production stimulated by NTHi induced epidermal growth factor receptor (EGFR)-mitogen-activated protein kinase (MAPK) signaling.
A. Phloretin decreases baseline levels of MMP13 protein and NTHi-induced MMP1 and MMP13 protein. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h) and exposed to NTHi (8 h). Secreted MMP1 and MMP13 protein were measured in cell supernatant by Luminex multiplex assay. Values (mean ± SEM, n = 9–10) are protein concentration (pg/mL). †Significant difference (p<0.05) between vehicle and NTHi-treated cells as determined ANOVA followed by Holm-Sidak multiple comparisons method. *Significantly different (p<0.05) from NTHi treated cells, as determined ANOVA followed by Holm-Sidak multiple comparison method. B. Phloretin inhibits NTHi-induced increased MMP1 transcript. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h) and exposed to NTHi (1, 4, and 8 h). MMP1 and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n =8–9) are log2 fold change of MMP1 mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) in NTHi compared to vehicle control treated cells, as determined by ANOVA followed by Tukey’s multiple comparisons method. C. Phloretin inhibits NTHi-induced increased MMP13 transcript. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h) and exposed to NTHi (1, 4, and 8 h). MMP13 and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=8–9) are log2 fold change of MMP13 mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) in NTHi compared to vehicle control treated cells, as determined by ANOVA followed by Tukey’s multiple comparisons method. D. Inhibition of MMP1 is through the EGFR-MAPK pathway whereas inhibition of MMP13 is through MAPK signaling. NCI-H292 cells were pretreated (1 h) with 1 μM AG-1478, an EGFR inhibitor, 30 μM FR180204, a MAPK3/1 inhibitor, or 100 μM SP600125, a MAPK8 inhibitor, and exposed to NTHi (8 h). MMP1, MMP13 and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=6–12) are log2 fold change of MMP1 and MMP13 mRNA normalized to RPL32 relative vehicle control treated cells. *Significantly different (p<0.05) from NTHi treated cells, as determined by ANOVA followed by Tukey’s multiple comparisons method. Tests were performed on ≥2 occasions.
To determine the involvement of the EGFR-MAPK pathway in MMP production, NCI-H292 cells were pretreated with inhibitors of EGFR phosphorylation (AG-1478), MAPK3/1 (FR180204), or MAPK8 (SP600125) (Figure 4D). Inhibition of EGFR phosphorylation and downstream MAPK3/1 and MAPK8 inhibited NTHi-induced increased MMP1 mRNA (Figure 4D). NTHi-induced MMP13 transcripts were not affected by inhibition of EGFR phosphorylation, but were reduced by MAPK3/1 and MAPK8 inhibition (Figure 4D).
EGFR ligand mRNAs were measured by RT-qPCR 8h after NTHi exposure of NCI-H292 cells. NTHi exposure increased EREG, AREG, and HBEGF mRNA, which phloretin inhibited (Figure 5A). NTHi also increased TGFA, which phloretin treatment did not alter. NTHi induced a decrease in EGF and did not affect EPGN and BTC mRNA (Table S3, Supporting Information).
Figure 5. Phloretin inhibits NTHi-induced ligand-dependent epidermal growth factor receptor (EGFR)-mitogen-activated protein kinase (MAPK) signaling.
A. NTHi increases EGFR ligand transcripts, which phloretin inhibits. NCI-H292 cells were pretreated without or with 100 μM phloretin (1 h) and exposed to NTHi (8 h). Epiregulin (EREG), amphiregulin (AREG), heparin-binding EGF-like growth factor (HBEGF), transforming growth factor (TGFA), and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n=9) are log2 fold change of EGFR ligand mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) from NTHi treated cells, as determined by ANOVA followed by Tukey’s multiple comparisons method. B. NTHi-induced EREG, AREG, and HBEGF transcripts are inhibited by EGFR and MAPK signaling inhibitors. NCI-H292 cells were pretreated (1 h) with vehicle (control), 1 μM AG-1478, an EGFR inhibitor, 30 μM FR180204, a MAPK3/1 inhibitor, or 100 μM SP600125, a MAPK8 inhibitor, and exposed to NTHi (8 h). EREG, AREG, HBEGF, and RPL32 transcripts were measured by RT-qPCR. Values (mean ± SEM, n= 6–12) are log2 fold change of EGFR ligand mRNA normalized to RPL32 relative to vehicle control treated cells. *Significantly different (p<0.05) from NTHi treated cells, as determined by ANOVA followed by Tukey’s multiple comparisons method. Tests were performed on ≥2 occasions.
Inhibition of EGFR phosphorylation (AG-1478) inhibited NTHi-induced increased EREG, AREG, and HBEGF mRNA (Figure 5B). Transcript levels of NTHi-induced EREG and AREG were reduced by MAPK3/1 (FR180204) and MAPK8 (SP600125) inhibition, whereas those of HBEGF were not (Figure 5B). Therefore, phloretin inhibits NTHi-induced EGFR-MAPK activation of MMPs and EGFR ligands, preventing the continued activation of this cascade.
DISCUSSION
Epidemiological studies have found that apples are protective against cough and phlegm development [35], lung function decline [5, 36], and the development of chronic lung disorders [37]. In this study, we offer a mechanism by which the apple polyphenol phloretin can alter pathogen-induced MUC5AC induction in airway epithelial cells. We found that NTHi initially induced MUC5AC by TLR4/NOX4 mediated ROS and EGFR phosphorylation. Subsequently, increased MUC5AC mRNA was maintained via EGFR signaling through increased metalloproteinase and EGFR ligand production. Consistent with previous studies in mice [16–19], intratracheal NTHi exposure in our study induced pulmonary inflammation, particularly neutrophil influx, which phloretin inhibited. Moreover, phloretin inhibited the increase in MUC5AC induced by NTHi in mouse lung and human airway epithelial cells. In human airway epithelial cells, phloretin inhibited TLR4 and NOX4 signaling, reducing ROS and downstream EGFR phosphorylation.
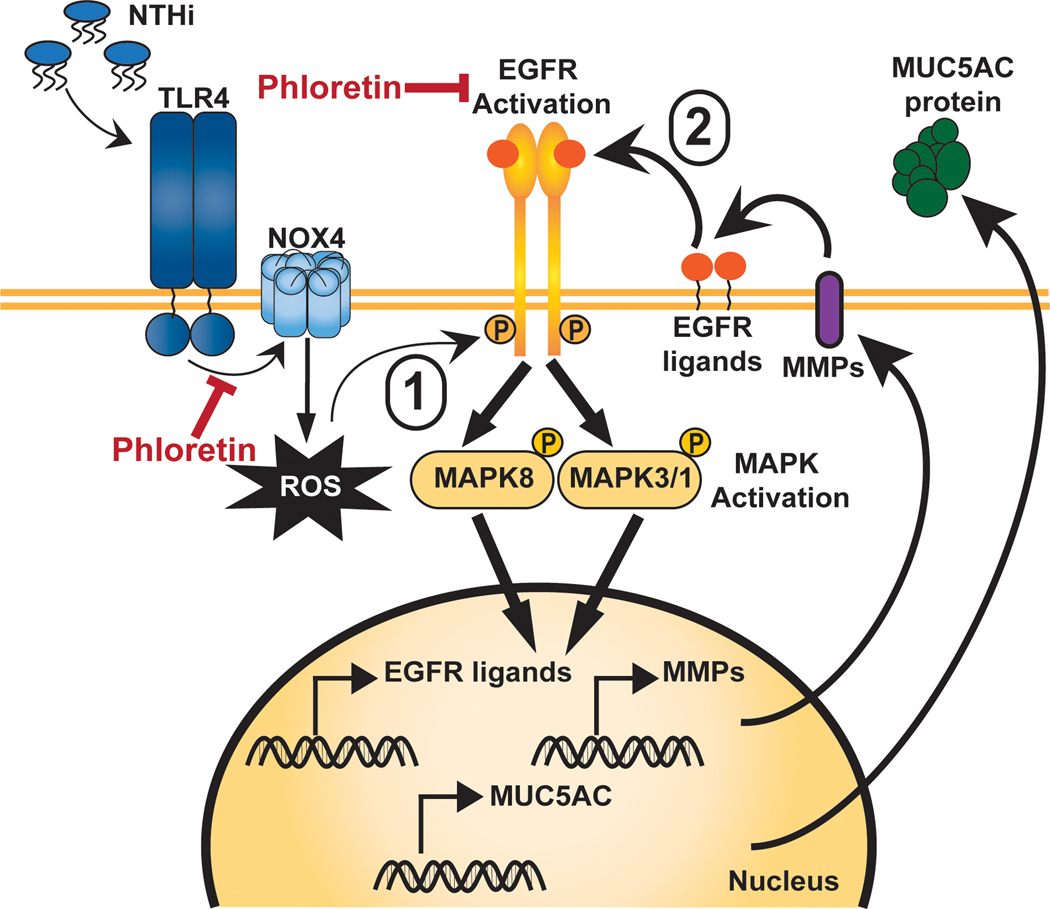
Previously, Kim et al. found that phloretin can directly interact with the TLR2 receptor and inhibit heterodimerization of the receptor with TLR1, preventing its activation [38]. We propose that phloretin could be directly interacting with the TLR4 receptor or inhibiting downstream TLR4 activated signaling cascades (Figure 6).
Figure 6. Proposed mechanism of phloretin protection against pathogen-induced mucin 5AC, oligomeric mucus/gel-forming (MUC5AC).
NTHi infection induces reactive oxygen species (ROS) by toll-like receptor 4 (TLR4)-NADPH oxidase 4 (NOX4) signaling (inhibited by phloretin, TAK-242, DPI, and PG), resulting in the phosphorylation of epidermal growth factor receptor (EGFR) tyrosine residues (inhibited by phloretin and AG-1478). This results in the downstream phosphorylation of the mitogen-activated protein kinase (MAPK) members MAPK3/1 (inhibited by phloretin and FR180204) and MAPK8 (inhibited by phloretin and SP600125), which leads to induction of MUC5AC expression. Stimulation of this pathway induces matrix metalloproteinase (MMP) and EGFR ligand production, resulting in the continued activation of the EGFR-MAPK cascade and MUC5AC production, which phloretin inhibits.
In addition, phloretin inhibited NTHi-induced increases in MMP1 and MMP13 mRNA. Matrix metalloproteinases (MMPs) activate membrane bound EGFR-ligands [34], and phloretin also inhibited NTHI-induced increased EREG, AREG, and HBEGF mRNA. Thus, phloretin was effective in reducing both the initial activation and maintenance of EGFR-MAPK signaling that sustained MUC5AC induction.
Previously, two mechanisms of bacterial-induced increased MUC5AC mRNA have been proposed. The first involves TLR2 stimulation, leading to MAPK14 (aka p38) and NF-κB activation [39–43]. This pathway was identified using bacterial whole cell lysate [41–43] or lysates of NTHi cytoplasmic proteins [40] and outer membrane lipoprotein P6 [39, 41]. The second involves nicotinamide adenine dinucleotide phosphate (NADPH) oxidase generated ROS that may lead to ligand–dependent activation of EGFR [33]. Cigarette smoke- or hydrogen peroxide-induced oxidative stress also can increase MUC5AC mRNA through NADPH oxidase, leading to the activation of ligand-dependent and ligand-independent EGFR activation [44–46]. In some incidences, both MAPK14 and MAPK 3/1 pathways can be activated and act synergistically to induce MUC5AC production [47, 48]. In this case, MUC5AC induction is mediated by MAPK3/1 (ERK1/2) activation.
Consistent with these studies, we found that NTHi interaction with the TLR4 receptor resulted in subsequent NOX4 mediated ROS production that, in turn, induced phosphorylation of the Y1068 and Y845 EGFR tyrosine kinase sites. We determined that MAPK3/1 and MAPK8 are essential for NTHi-induced MUC5AC in human airway epithelial cells. In addition, we found that NTHi exposure leads to a delayed increase in MMP1 and MMP13 transcripts and EGFR ligands, which could sustain the induction of MUC5AC by further EGFR stimulation.
Previously, phloretin was found to directly inhibit EGFR phosphorylation by interacting with the ATP binding pocket of EGFR, preventing tyrosine kinase activity [49]. In this study, we found that phloretin also attenuated EGFR phosphorylation indirectly through upstream NOX4 signaling inhibition. In addition, phloretin diminished NTHi induced collagenases MMP13 and MMP1 and EGFR ligand formation. MMP13 can activate MMP2 and MMP9, and proMMP13 activation can be achieved by autoactivation or by other MMPs such as MMP2, MMP3, and MMP14, and it is inhibited by tissue inhibitors of MMPs (TIMPs) [50–53]. Activation of MMP9 can lead to MUC5AC formation [12, 54, 55].
Importantly, increased MMP1 also has been associated with alveolar destruction in numerous infectious and chronic respiratory diseases, including Mycobacterium tuberculosis [56] and emphysema [57–61]. The role of phloretin in preventing alveolar disruption requires further investigation, however. Nonetheless, phloretin may provide protection from bacterial-induced airway mucus overproduction by inhibiting MUC5AC and MMP13, and may provide protection from alveolar proteinase imbalance by inhibiting MMP1.
Apples contain approximately 5.6 mg of phloretin and its glycosides per 100 g of fresh fruit (approximately the weight of one apple) [6]. In humans, Schulze et al. found that venous blood glucose and plasma insulin decreased after an oral glucose tolerance test with oral supplementation of 2.8 g apple extract containing 448 mg phloretin [62], which is equivalent to roughly 80 apples. Therefore, a clinically relevant dietary intervention in humans would require an apple extract or phloretin isolate supplement, which are both commercially available. Our findings indicate that phloretin supplementation may have clinical significance for use against pathogen-induced mucus overproduction.
Supplementary Material
Acknowledgements:
We thank Dr. Jadranka Milosevic and Dr. Gaowei Mao for their scientific assistance.
Abbreviations:
- EGFR
Epidermal growth factor receptor
- MMP
Matrix metalloproteinase
- MAPK
Mitogen-activated protein kinase
- M. catarrhalis
Moraxella catarrhalis
- MUC5AC
Mucin 5AC oligomeric mucus/gel-forming
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NOX4
Nicotinamide adenine dinucleotide phosphate oxidase 4
- NTHi
Nontypeable Haemophilus influenzae
- P. aeruginosa
Pseudomonas aeruginosa
- ROS
Reactive oxygen species
- S. pneumoniae
Streptococcus pneumoniae
- TLR4
Toll-like receptor 4
Footnotes
Data availability statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclosures: Authors have no disclosures to report.
REFERENCES
- [1].Bustamante-Marin XM, Ostrowski LE, Cilia and Mucociliary Clearance. Cold Spring Harb Perspect Biol 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ali MS, Pearson JP, Upper airway mucin gene expression: a review. Laryngoscope 2007, 117, 932–938. [DOI] [PubMed] [Google Scholar]
- [3].Sethi S, Murphy TF, Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008, 359, 2355–2365. [DOI] [PubMed] [Google Scholar]
- [4].Sze MA, Hogg JC, Sin DD, Bacterial microbiome of lungs in COPD. Int J Chron Obstruct Pulmon Dis 2014, 9, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Butland BK, Fehily AM, Elwood PC, Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax 2000, 55, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY, Major phenolics in apple and their contribution to the total antioxidant capacity. J Agric Food Chem 2003, 51, 6516–6520. [DOI] [PubMed] [Google Scholar]
- [7].Behzad S, Sureda A, Barreca D, Nabavi SF, Rastrelli L, Nabavi SM, Health effects of phloretin: from chemistry to medicine. Phytochemistry Reviews 2017, 16, 527–533. [Google Scholar]
- [8].Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ, Joint Taskforce of the European Respiratory, S., European Society for Clinical, M., Infectious, D., Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 2011, 17 Suppl 6, E1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leiva-Juarez MM, Kolls JK, Evans SE, Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol 2018, 11, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I, Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem 2002, 277, 32258–32267. [DOI] [PubMed] [Google Scholar]
- [11].Basbaum C, Li D, Gensch E, Gallup M, Lemjabbar H, Mechanisms by which gram-positive bacteria and tobacco smoke stimulate mucin induction through the epidermal growth factor receptor (EGFR). Novartis Found Symp 2002, 248, 171–176; discussion 176–180, 277–182. [PubMed] [Google Scholar]
- [12].Deshmukh HS, McLachlan A, Atkinson JJ, Hardie WD, Korfhagen TR, Dietsch M, Liu Y, Di PY, Wesselkamper SC, Borchers MT, Leikauf GD, Matrix metalloproteinase-14 mediates a phenotypic shift in the airways to increase mucin production. Am J Respir Crit Care Med 2009, 180, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Burgel PR, Nadel JA, Epidermal growth factor receptor-mediated innate immune responses and their roles in airway diseases. Eur Respir J 2008, 32, 1068–1081. [DOI] [PubMed] [Google Scholar]
- [14].Hewson CA, Haas JJ, Bartlett NW, Message SD, Laza-Stanca V, Kebadze T, Caramori G, Zhu J, Edbrooke MR, Stanciu LA, Kon OM, Papi A, Jeffery PK, Edwards MR, Johnston SL, Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur Respir J 2010, 36, 1425–1435. [DOI] [PubMed] [Google Scholar]
- [15].Wang H, Yang T, Wang T, Hao N, Shen Y, Wu Y, Yuan Z, Chen L, Wen F, Phloretin attenuates mucus hypersecretion and airway inflammation induced by cigarette smoke. Int Immunopharmacol 2018, 55, 112–119. [DOI] [PubMed] [Google Scholar]
- [16].Venuprasad K, Theivanthiran B, Cantarel B, Intra-tracheal Administration of Haemophilus influenzae in Mouse Models to Study Airway Inflammation. J Vis Exp 2016, e53964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Andrews CS, Matsuyama S, Lee BC, Li JD, Resveratrol suppresses NTHi-induced inflammation via up-regulation of the negative regulator MyD88 short. Sci Rep 2016, 6, 34445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wieland CW, Florquin S, Maris NA, Hoebe K, Beutler B, Takeda K, Akira S, van der Poll T, The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol 2005, 175, 6042–6049. [DOI] [PubMed] [Google Scholar]
- [19].Gaschler GJ, Skrtic M, Zavitz CC, Lindahl M, Onnervik PO, Murphy TF, Sethi S, Stämpfli MR, Bacteria challenge in smoke-exposed mice exacerbates inflammation and skews the inflammatory profile. Am J Respir Crit Care Med 2009, 179, 666–675. [DOI] [PubMed] [Google Scholar]
- [20].Huang WC, Fang LW, Liou CJ, Phloretin Attenuates Allergic Airway Inflammation and Oxidative Stress in Asthmatic Mice. Front Immunol 2017, 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].de Oliveira MR, Phloretin-induced cytoprotective effects on mammalian cells: A mechanistic view and future directions. BioFactors (Oxford, England) 2016, 42, 13–40. [DOI] [PubMed] [Google Scholar]
- [22].Chen J, Yang X, Zhang W, Peng D, Xia Y, Lu Y, Han X, Song G, Zhu J, Liu R, Therapeutic Effects of Resveratrol in a Mouse Model of LPS and Cigarette Smoke-Induced COPD. Inflammation 2016, 39, 1949–1959. [DOI] [PubMed] [Google Scholar]
- [23].Liang Y, Liu KWK, Yeung SC, Li X, Ip MSM, Mak JCW, (−)-Epigallocatechin-3-gallate Reduces Cigarette Smoke-Induced Airway Neutrophilic Inflammation and Mucin Hypersecretion in Rats. Front Pharmacol 2017, 8, 618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Konduru AS, Matsuyama S, Lee BC, Komatsu K, Li JD, Curcumin Inhibits NTHi-Induced MUC5AC Mucin Overproduction in Otitis Media via Upregulation of MAPK Phosphatase MKP-1. Int J Inflam 2017, 2017, 4525309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Park BS, Lee JO, Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med 2013, 45, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang X, Moser C, Louboutin JP, Lysenko ES, Weiner DJ, Weiser JN, Wilson JM, Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J Immunol 2002, 168, 810–815. [DOI] [PubMed] [Google Scholar]
- [27].Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin S, van der Poll T, Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect Immun 2004, 72, 788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hassan F, Ren D, Zhang W, Merkel TJ, Gu XX, Moraxella catarrhalis activates murine macrophages through multiple toll like receptors and has reduced clearance in lungs from TLR4 mutant mice. PLoS One 2012, 7, e37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gugliandolo E, Fusco R, Ginestra G, D’Amico R, Bisignano C, Mandalari G, Cuzzocrea S, Di Paola R, Involvement of TLR4 and PPAR-α Receptors in Host Response and NLRP3 Inflammasome Activation, Against Pulmonary Infection With Pseudomonas Aeruginosa. Shock (Augusta, Ga.) 2019, 51, 221–227. [DOI] [PubMed] [Google Scholar]
- [30].Bernard K, Hecker L, Luckhardt TR, Cheng G, Thannickal VJ, NADPH oxidases in lung health and disease. Antioxid Redox Signal 2014, 20, 2838–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang P, Han X, Mo B, Huang G, Wang C, LPS enhances TLR4 expression and IFNgamma production via the TLR4/IRAK/NFkappaB signaling pathway in rat pulmonary arterial smooth muscle cells. Mol Med Rep 2017, 16, 3111–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pace E, Ferraro M, Siena L, Melis M, Montalbano AM, Johnson M, Bonsignore MR, Bonsignore G, Gjomarkaj M, Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 2008, 124, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heppner DE, van der Vliet A, Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol 2016, 8, 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singh B, Carpenter G, Coffey RJ, EGF receptor ligands: recent advances. F1000Res 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Butler LM, Koh WP, Lee HP, Yu MC, London SJ, Dietary fiber and reduced cough with phlegm: a cohort study in Singapore. Am J Respir Crit Care Med 2004, 170, 279–287. [DOI] [PubMed] [Google Scholar]
- [36].Garcia-Larsen V, Potts JF, Omenaas E, Heinrich J, Svanes C, Garcia-Aymerich J, Burney PG, Jarvis DL, Dietary antioxidants and 10-year lung function decline in adults from the ECRHS survey. Eur Respir J 2017, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kaluza J, Larsson SC, Orsini N, Linden A, Wolk A, Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax 2017, 72, 500–509. [DOI] [PubMed] [Google Scholar]
- [38].Kim J, Durai P, Jeon D, Jung ID, Lee SJ, Park YM, Kim Y, Phloretin as a Potent Natural TLR2/1 Inhibitor Suppresses TLR2-Induced Inflammation. Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen R, Lim JH, Jono H, Gu XX, Kim YS, Basbaum CB, Murphy TF, Li JD, Nontypeable Haemophilus influenzae lipoprotein P6 induces MUC5AC mucin transcription via TLR2-TAK1-dependent p38 MAPK-AP1 and IKKbeta-IkappaBalpha-NF-kappaB signaling pathways. Biochem Biophys Res Commun 2004, 324, 1087–1094. [DOI] [PubMed] [Google Scholar]
- [40].Wang B, Lim DJ, Han J, Kim YS, Basbaum CB, Li JD, Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J Biol Chem 2002, 277, 949–957. [DOI] [PubMed] [Google Scholar]
- [41].Shuto T, Xu H, Wang B, Han J, Kai H, Gu XX, Murphy TF, Lim DJ, Li JD, Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha /beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc Natl Acad Sci U S A 2001, 98, 8774–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jono H, Xu H, Kai H, Lim DJ, Kim YS, Feng XH, Li JD, Transforming growth factor-beta-Smad signaling pathway negatively regulates nontypeable Haemophilus influenzae-induced MUC5AC mucin transcription via mitogen-activated protein kinase (MAPK) phosphatase-1-dependent inhibition of p38 MAPK. J Biol Chem 2003, 278, 27811–27819. [DOI] [PubMed] [Google Scholar]
- [43].Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, Guan YL, Han J, Cato AC, Lim DJ, Akira S, Li JD, Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem 2002, 277, 17263–17270. [DOI] [PubMed] [Google Scholar]
- [44].Shao MX, Nakanaga T, Nadel JA, Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol 2004, 287, L420–427. [DOI] [PubMed] [Google Scholar]
- [45].Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA, Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 2000, 164, 1546–1552. [DOI] [PubMed] [Google Scholar]
- [46].Kim HJ, Park YD, Moon UY, Kim JH, Jeon JH, Lee JG, Bae YS, Yoon JH, The role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cells. Am J Respir Cell Mol Biol 2008, 39, 598–609. [DOI] [PubMed] [Google Scholar]
- [47].Jono H, Lim JH, Xu H, Li JD, PKCtheta synergizes with TLR-dependent TRAF6 signaling pathway to upregulate MUC5AC mucin via CARMA1. PLoS One 2012, 7, e31049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Shen H, Yoshida H, Yan F, Li W, Xu F, Huang H, Jono H, Li JD, Synergistic induction of MUC5AC mucin by nontypeable Haemophilus influenzae and Streptococcus pneumoniae. Biochem Biophys Res Commun 2008, 365, 795–800. [DOI] [PubMed] [Google Scholar]
- [49].Yang EB, Guo YJ, Zhang K, Chen YZ, Mack P, Inhibition of epidermal growth factor receptor tyrosine kinase by chalcone derivatives. Biochim Biophys Acta 2001, 1550, 144–152. [DOI] [PubMed] [Google Scholar]
- [50].Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G, Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 1996, 271, 17124–17131. [DOI] [PubMed] [Google Scholar]
- [51].Leeman MF, Curran S, Murray GI, The structure, regulation, and function of human matrix metalloproteinase-13. Crit Rev Biochem Mol Biol 2002, 37, 149–166. [DOI] [PubMed] [Google Scholar]
- [52].Barthelemi S, Robinet J, Garnotel R, Antonicelli F, Schittly E, Hornebeck W, Lorimier S, Mechanical forces-induced human osteoblasts differentiation involves MMP-2/MMP-13/MT1-MMP proteolytic cascade. J Cell Biochem 2012, 113, 760–772. [DOI] [PubMed] [Google Scholar]
- [53].Tardif G, Reboul P, Pelletier JP, Martel-Pelletier J, Ten years in the life of an enzyme: the story of the human MMP-13 (collagenase-3). Mod Rheumatol 2004, 14, 197–204. [DOI] [PubMed] [Google Scholar]
- [54].Deshmukh HS, Case LM, Wesselkamper SC, Borchers MT, Martin LD, Shertzer HG, Nadel JA, Leikauf GD, Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 2005, 171, 305–314. [DOI] [PubMed] [Google Scholar]
- [55].Deshmukh HS, Shaver C, Case LM, Dietsch M, Wesselkamper SC, Hardie WD, Korfhagen TR, Corradi M, Nadel JA, Borchers MT, Leikauf GD, Acrolein-activated matrix metalloproteinase 9 contributes to persistent mucin production. Am J Respir Cell Mol Biol 2008, 38, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, Edwards DR, Robertson BD, D’Armiento J, Friedland JS, MMP-1 drives immunopathology in human tuberculosis and transgenic mice. The Journal of clinical investigation 2011, 121, 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dalal S, Imai K, Mercer B, Okada Y, Chada K, D’Armiento JM, A role for collagenase (Matrix metalloproteinase-1) in pulmonary emphysema. Chest 2000, 117, 227S–228S. [DOI] [PubMed] [Google Scholar]
- [58].Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, Wright C, Sin D, Pare PD, Pierce JA, Pierce RA, Patterson A, Cooper J, Hogg JC, Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010, 181, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mercer BA, Wallace AM, Brinckerhoff CE, D’Armiento JM, Identification of a cigarette smoke-responsive region in the distal MMP-1 promoter. Am J Respir Cell Mol Biol 2009, 40, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Koo HK, Hong Y, Lim MN, Yim JJ, Kim WJ, Relationship between plasma matrix metalloproteinase levels, pulmonary function, bronchodilator response, and emphysema severity. Int J Chron Obstruct Pulmon Dis 2016, 11, 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wallace AM, Mercer BA, He J, Foronjy RF, Accili D, Sandford AJ, Pare PD, D’Armiento JM, Functional characterization of the matrix metalloproteinase-1 cigarette smoke-responsive region and association with the lung health study. Respir Res 2012, 13, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Schulze C, Bangert A, Kottra G, Geillinger KE, Schwanck B, Vollert H, Blaschek W, Daniel H, Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res 2014, 58, 1795–1808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.