Abstract
OBSESSIVE–COMPULSIVE DISORDER (OCD) IS A COMMON and debilitating neuropsychiatric disorder. Although it is widely believed to have a genetic basis, no specific genetic factors have been conclusively identified as yet, leading researchers to look for environmental risk factors that may interact with an underlying genetic susceptibility in affected individuals. Recently, there has been increasing interest in a possible link between streptococcal infections and the development of OCD and tic disorders in children. It has been suggested that OCD in some susceptible individuals may be caused by an autoimmune response to streptococcal infections, that is, a similar biological mechanism to that associated with Sydenham's chorea. The term “pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections” (PANDAS) has been used to describe a subset of children with abrupt onset or exacerbations of OCD or tics, or both, following streptococcal infections. Affected children have relatively early symptom onset, characteristic comorbid symptoms and subtle neurological dysfunction. Neuroimaging studies reveal increased basal ganglia volumes, and the proposed cause involves the cross-reaction of streptococcal antibodies with basal ganglia tissue. Vulnerability to developing PANDAS probably involves genetic factors, and elevated levels of D8/17 antibodies may represent a marker of susceptibility to PANDAS. Prophylactic antibiotic treatments have thus far not been shown to be helpful in preventing symptom exacerbations. Intravenous immunoglobulin therapy may be an effective treatment in selected individuals. Further understanding of the role of streptococcal infections in childhood-onset OCD will be important in determining alternative and effective strategies for treatment, early identification and prevention of this common and debilitating psychiatric disorder.
Obsessive–compulsive disorder (OCD) is a common and debilitating disorder, which an estimated 2%–4% of individuals will develop before the age of 18 years.1 The pathogenesis is presumed to involve basal ganglia dysfunction2,3 and underlying genetic factors.4,5 Compared with adults, children with OCD are more likely to be male1 and to have comorbid Tourette's syndrome, which is another highly familial disorder attributed to basal ganglia dysfunction6 that many investigators believe may result from the same underlying diathesis.7,8
Recently, there has been increasing interest in a possible link between streptococcal infections and OCD and tic disorders in children.9 A subtype of childhood OCD known as “pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections” (PANDAS)9,10 has been postulated. The purpose of this review is to provide clinicians with an overview of the PANDAS concept and information regarding the clinical presentation, prevalence, pathophysiology, predisposing factors and treatment of this condition.
Sydenham's chorea: a medical model for OCD
Sydenham's chorea, which is a neuropsychiatric syndrome that usually occurs in prepubertal children, may represent a “medical model” of onset of OCD in childhood. Sydenham's chorea develops following group A β-hemolytic streptococcal infections and is one manifestation of rheumatic fever according to the Jones criteria.11 In a process known as “molecular mimicry,” antistreptococcal antibodies cross-react with basal ganglia proteins, triggering an inflammatory response and producing the symptoms of Sydenham's chorea.9
In addition to the chorea that gives the syndrome its name, there are characteristic psychiatric symptoms, including a syndrome similar to attention-deficit hyperactivity disorder and emotional lability.12,13,14 Obsessive–compulsive symptoms, which were first described in patients with Sydenham's chorea by Osler,15 have been well documented in prospective studies of the syndrome14,16 leading to the suggestion that OCD may represent a “forme fruste,” or an atypical, incomplete form, of Sydenham's chorea.17
PANDAS: a proposed OCD subtype
Longitudinal studies of children with OCD identified a subgroup with a course of illness characterized by the dramatic onset of symptoms or their exacerbation, or both,9,10 one-third of whom exhibited “choreiform” movements resembling the chorea of Sydenham's chorea.18 The exacerbations of symptoms in some of these children were correlated with a history of recent streptococcal infections.9,10 Studies of children presenting with tics19 also raised the possibility that some children with neuropsychiatric disorders have symptoms caused or exacerbated by streptococcal infections.
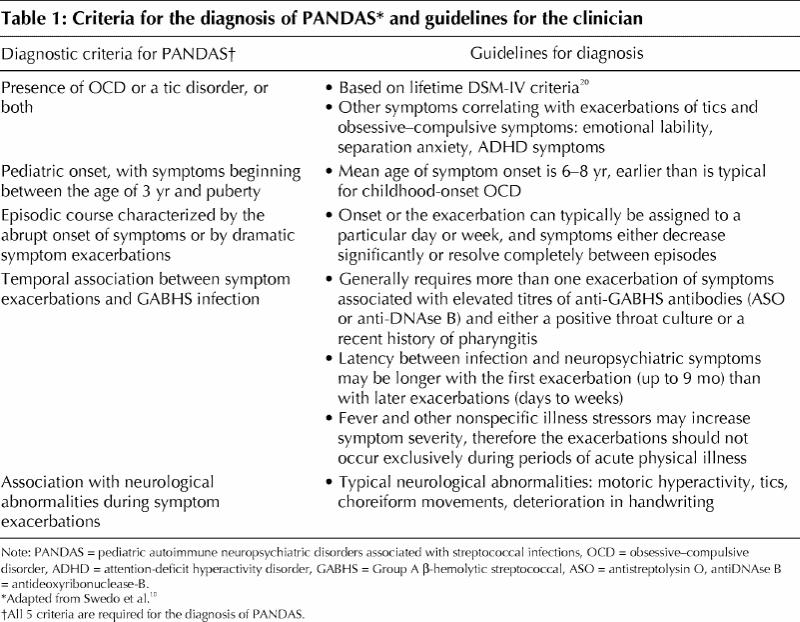
Following up on these early clinical observations, a group based at the US National Institute of Mental Health recruited children with presumed streptococcal-induced OCD based on a set of diagnostic criteria that they had developed. The term “pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections” (PANDAS) was coined to describe a putative subtype of children with OCD and tic disorders who met all 5 working criteria.9 Table 1 summarizes these criteria and other diagnostic guidelines gleaned from the current literature.10,20
Table 1
The prevalence of PANDAS is unknown. There is one report on the prevalence of streptococcal-induced exacerbations in a group of children presenting with tics, 11% of whom had a history of exacerbations within 6 weeks of a streptococcal infection.21 Limitations of this study include its setting in a highly specialized clinic, which limits its generalizability to other populations with tic disorders, and its reliance on retrospective historical data instead of objective laboratory findings such as antistreptococcal antibodies. Two studies have evaluated antistreptococcal antibodies in subjects with Tourette's syndrome compared with controls, and in both of these studies significantly elevated titres were observed in the subjects with Tourette's syndrome.22,23 Antistreptococcal antibody levels were found to correlate with severity of symptoms in one report.22
The PANDAS syndrome is believed to result from antistreptococcal antibodies that have cross-reacted with basal ganglia tissue, as has been demonstrated in Sydenham's chorea. Consistent with this model, the following findings have been reported: significantly elevated antineuronal antibodies in children with PANDAS24 and related neuropsychiatric disorders,25,26,27 increased basal ganglia volumes on volumetric MRI28 in subjects with PANDAS and higher antistreptococcal antibodies that correlated with increased basal ganglia volumes in subjects with either attention-deficit hyperactivity disorder or OCD.29 Taken together, these findings are consistent with autoimmune-mediated inflammation of the basal ganglia, although further research is required to confirm this association, and many unanswered questions remain. For example, it is not clear whether this autoimmune mechanism is specific to OCD and tic disorders or has implications for other childhood neuropsychiatric disorders. This was highlighted by a recent study reporting a significant correlation of antistreptococcal antibody titres with a diagnosis of attention-deficit hyperactivity disorder, but not with OCD or tic disorders.29
Predisposing factors for PANDAS
Streptococcal infections are ubiquitous in childhood, however, neuropsychiatric disorders are not, suggesting that only certain individuals are predisposed to develop the PANDAS phenotype.9 Vulnerable individuals may have a genetic predisposition to developing neurotransmitter dysfunction or to formation of antistreptococcal antibodies that cross-react with neuronal proteins, or may have a form of immune dysregulation, or both.
D8/17, which is a monoclonal antibody that identifies a specific B lymphocyte cell-surface marker, is one possible susceptibility factor for PANDAS. Significantly elevated levels of this marker have been found in individuals with rheumatic fever and to a lesser extent in their family members when compared with controls. It is, thus, considered a possible trait marker, indicating genetic susceptibility to rheumatic fever (and by inference, to Sydenham's chorea).30,31 To date, elevated levels of antibodies that recognize the D8/17 marker have been demonstrated in children with PANDAS,32 and more generally in subjects with early onset OCD and tic disorders.33,34 In a sample of children with autism, D8/17 expression was shown to be significantly correlated with compulsive behaviour.35 Although the D8/17 data are suggestive, the functional significance of this marker is unknown,30,31 and further investigation is needed to clarify its etiological and clinical significance.
Elevated rates of tic disorders and OCD have been reported in first-degree relatives of children with PANDAS, which is comparable to previous observations in relatives of individuals with OCD and tic disorders, indicating that genetic factors may be important in conferring vulnerability to the PANDAS subtype.36 However, although a number of candidate genes have been implicated in OCD,37,38,39,40,41 there are no published reports of molecular genetics studies of individuals with PANDAS.
A few studies have measured cytokines or immune cells in subjects with OCD. In addition to acting as protein messengers between immune cells, cytokines are also known to influence central nervous system signalling and, therefore, may play a role in the pathophysiology of a number of psychiatric and neurological disorders.42 A relative skewing toward type 1 cytokine production in cerobrospinal fluid has been demonstrated in pediatric patients with OCD.43 Various immunological abnormalities reported in adult subjects with OCD44,45,46,47 have not been consistently replicated, and other studies have produced negative findings.48,49,50
In summary, although it is presumed that individuals who develop PANDAS in the presence of streptococcal infections are vulnerable as a result of a genetic predisposition or a form of immune dysregulation, or both, this has yet to be clearly demonstrated. Interesting preliminary findings in PANDAS that require further investigation include the presence of elevated levels of D8/17 antibodies, elevated rates of tic disorders and OCD in first-degree relatives, and immunological abnormalities reported in individuals with OCD.
Approach to the diagnosis of PANDAS
We recommend evaluation of all children who present with the sudden onset or exacerbation of obsessive–compulsive symptoms, using the approach summarized in Fig. 1. This diagnostic algorithm, which is based on the literature summarized earlier as well as our clinical judgement, begins with a history-taking, mental status examination and focused physical examination. Initial investigations in children with a history suggestive of streptococcal infection or a strong family history of rheumatic fever, or both, should include throat cultures and antistreptolysin O titres. These titres should be repeated after an interval of approximately 3–4 weeks, because a correlation of symptom severity with changes in antibody levels is far more informative than an isolated antistreptolysin O titre. We recommend antistreptolysin O titre, because the other antistreptococcal test reported in the PANDAS literature, namely, antideoxyribonuclease-B (antiDNAse B), is expensive and not widely available in Canada. The D8/17 marker is an experimental assay that is not available for routine clinical use.

Fig. 1: Assessment and treatment of children presenting with abrupt-onset obsessive–compulsive disorder (OCD) or tic disorders. ADHD = attention-deficit hyperactivity disorder, ASO = antistreptolysin O, CBT = cognitive behavioural therapy, SRIs = serotonin reuptake inhibitors, NIMH = US National Institute of Mental Health, PANDAS = pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections.
Treatment of PANDAS
Current first-line treatments for OCD include pharmacological treatment with serotonin reuptake inhibitors (SRIs) and cognitive behavioural therapy. Researchers have reported response rates of between 50% and 75% for pharmacotherapy1 and from 67% to 100% for cognitive behavioural therapy.1,51 With the accumulating evidence that PANDAS represents a distinct autoimmune subtype, it is now possible to examine various therapies for the disorder targeting either the infectious trigger for the illness or the immune response itself. Recently, 2 randomized controlled trials have evaluated these approaches to the treatment of children with PANDAS.52,53 In the first study, patients received either 4 months of penicillin V administered orally followed by 4 months of placebo, or placebo followed by penicillin V. There was no difference between the active and placebo phases in the severity of obsessive–compulsive symptoms or tics. However, the penicillin regimen used was ineffective in preventing infections and, consequently, no conclusions could be drawn regarding the efficacy of penicillin prophylaxis in preventing exacerbations of tics or OCD symptoms.52
In the second study, children with severe PANDAS received one of 3 treatments: plasma exchange, intravenous immunoglobulin, or placebo (sham intravenous immunoglobulin). When subjects were assessed one and 12 months post treatment and compared with the placebo group, both plasma exchange and intravenous immunoglobulin were associated with striking improvements on standardized scales that measure obsessive–compulsive symptoms, anxiety and overall functioning.53 An open trial of plasma exchange in a small group of children with treatment-refractory OCD without a history of streptococcal infections failed to show any therapeutic benefit.54 These findings demonstrate that immunomodulatory treatments may represent an efficacious treatment for PANDAS specifically and are probably ineffective in treating other forms of OCD. Plasma exchange and intravenous immunoglobulin are highly invasive, require admission to hospital and have not been directly compared with more traditional therapies such as serotonergic medications and cognitive behavioural therapy. However, as a result of recent publicity surrounding PANDAS, parents and physicians in the United States have been seeking immunomodulatory treatments for children with OCD and tic disorders despite the potential risks. This has led the US National Institute of Mental Health to issue a warning to parents and clinicians that plasma exchange and intravenous immunoglobulin are not to be used outside research protocols.55
Our recommended approach to treatment, based on the literature summarized above as well as our clinical judgement, is summarized in Fig. 1. Standard therapies shown to be efficacious in the treatment of OCD51 and tic disorders56 should still be used as first-line treatment, accompanied by careful monitoring and early treatment of group A β-hemolytic streptococcal infections. However, in treatment-refractory children with a clear PANDAS course, prophylactic antibiotics might be considered in consultation with a pediatrician. To our knowledge, immunomodulatory treatments are not currently available anywhere in Canada for the treatment of childhood neuropsychiatric disorders. Physicians who are seeking treatment for a child with PANDAS are encouraged to contact the US National Institute of Mental Health directly (www.nimh.nih.gov) regarding ongoing clinical trials.
Childhood-onset obsessive–compulsive disorder .
Lifetime prevalence: 2%–4%
Pathogenesis: genetic causes, basal ganglia dysfunction
Children with this disorder are more likely to be male and to have comorbid Tourette's syndrome or tics
Sydenham's chorea .
Poststreptococcal syndrome that usually occurs in prepubertal children
Pathogenesis: mechanism of “molecular mimicry” in which antistreptococcal antibodies cross-react with basal ganglia proteins
Psychiatric symptoms: obsessive–compulsive behaviour, emotional lability, “attention-deficit hyperactivity disorder–like” syndrome
Evidence regarding the origin and pathogenesis of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) .
Elevated antineuronal antibodies
Increased basal ganglia volume measured using volumetric MRI
Elevated levels of cells expressing the D8/17 marker
Positive family history of obsessive–compulsive disorder and tic disorders
Evidence of response to immunomodulatory therapies
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. Margaret A. Richter, Centre for Addiction and Mental Health, 250 College St., Toronto ON M5T 1R8; fax 416 979-6853; peggy_richter@camh.net
References
- 1.Geller DA, Biederman JS, Jones J, Shapiro S, Schwartz S, Park K. Obsessive-compulsive disorder in children and adolescents: a review. Harv Rev Psychiatry 1998; 5:260-73. [DOI] [PubMed]
- 2.Rapoport JL. Obsessive-compulsive disorder and basal ganglia dysfunction. Psychol Med 1990;20:465-9. [DOI] [PubMed]
- 3.Rapaport JL, Fiske A. The new biology of obsessive-compulsive disorder: implications for evolutionary psychology. Perspect Biol Med 1998;41(2):159-75. [DOI] [PubMed]
- 4.Billett EA, Richter MA, Kennedy JL. Genetics of obsessive-compulsive disorder. In: Swinson RP, Antony MM, Rachman S, Richter MA, editors. Obsessive-compulsive disorder theory, research and treatment. New York: Guilford Press; 1998.
- 5.Pauls DL, Alsobrook JP II. The inheritance of obsessive-compulsive disorder. Child Adolesc Psychiatr Clin N Am 1999;8(3):481-96. [PubMed]
- 6.Barr CL, Sandor P. Current status of genetic studies of Gilles de la Tourette syndrome. Can JPsychiatry 1998;43:351-7. [DOI] [PubMed]
- 7.Pauls DL, Alsobrook JP II, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Evidence supporting a genetic relationship. Am J Psychiatry 1995;152:76-84. [DOI] [PubMed]
- 8.Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ. Gilles de la Tourette's syndrome and obsessive-compulsive disorder. Arch Gen Psychiatry 1986; 43(12):1180-2. [DOI] [PubMed]
- 9.Garvey MA, Giedd J, Swedo S. PANDAS: the search for environmental triggers of pediatric neuropsychiatric disorders. Lessons from rheumatic fever. J Child Neurol 1998;13:413-23. [DOI] [PubMed]
- 10.Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998; 155:264-71. [DOI] [PubMed]
- 11.Harrison TR, Isselbacher KJ. Harrison'sprinciples of internal medicine. 13th ed. New York: McGraw-Hill; 1994. p. 1048.
- 12.Moore DP. Neuropsychiatric aspects of Sydenham's chorea: a comprehensive review. J ClinPsychiatry 1996;57:407-14. [PubMed]
- 13.Marques-Dias M, Mercadante MT, Tucker D, Lombroso P. Sydenham's chorea. Psychiatr Clin NorthAm 1997;20(4):809-20. [DOI] [PubMed]
- 14.Garvey MA, Swedo SE. Sydenham's chorea: clinical and therapeutic update. In: Horaud T, Bouvet A, Leclerq R, de Montclos H, Sicard M, editors. Streptococci and the host. New York: Plenum Press; 1997. p. 115-20. [DOI] [PubMed]
- 15.Osler W. On chorea and choreiform affections. Philadelphia: HK Lewis; 1894. p. 33-5.
- 16.Asbahr FR, Negrao AB, Gentil V, Zanetta DMT, da Paz JA, Marques-Dias MJ, et al. Obsessive-compulsive and related symptoms in children and adolescents with rheumatic fever with and without chorea: a prospective 6-month study. Am J Psychiatry 1998;155:1122-4. [DOI] [PubMed]
- 17.Swedo SE. Sydenham's chorea: a model for childhood autoimmune neuropsychiatric disorders. JAMA 1994;272(2):1788-91. [DOI] [PubMed]
- 18.Denckla MB. Neurological examination. In: Rapoport JL, editor. Obsessive-compulsive disorder in children and adolescents. Washington: American Psychiatric Press; 1989. p. 107-15.
- 19.Hallett JJ, Kiessling LS. Neuroimmunology of tics and other childhood hyperkinesias. Neurol Clin 1997;15(2):333-44. [DOI] [PubMed]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). 4th ed. Washington: The Association; 1994.
- 21.Singer HS, Giuliano JD, Zimmerman AM, Walkup JT. Infection: a stimulus for tic disorders. Pediatr Neurol 2000;22:380-3. [DOI] [PubMed]
- 22.Cardona F, Orefici G. Group A streptococcal infections and tic disorders in an Italian pediatric population. J Pediatr 2001;138:71-5. [DOI] [PubMed]
- 23.Muller N, Reiedel M, Straube A, Gunther W, Wilske B. Increased anti-streptococcal antibodies in patients with Tourette's syndrome. Psychiatry Res 2000; 94: 43-9. [DOI] [PubMed]
- 24.Swedo SE, Kilpatrick K, Shapiro MB, Mannheim G, Leonard H. Antineuronal antibodies (AnA) in Sydenham's Chorea (SC) and obsessive-compulsive disorder (OCD) [abstract]. Pediatr Res 1991;29:364A.
- 25.Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics 1993;92:39-43. [PubMed]
- 26.Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies: tics and obsessive-compulsive symptoms. J Dev Behav Pediatr 1994;15:421-5. [PubMed]
- 27.Singer HS, Giuliano JD, Hansen BH, Hallett JJ, laurino JP, Benson M, et al. Antibodies against human putamen in children with Tourette syndrome. Neurology 1998;50(6):1618-24. [DOI] [PubMed]
- 28.Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry 2000;157:281-3. [DOI] [PubMed]
- 29.Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, et al. Preliminary findings of antistreptococcal antibody titres and basal ganglia volumes in tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders. Arch Gen Psychiatry 2000;57:364-72. [DOI] [PubMed]
- 30.Gibofsky A. The genetics of rheumatic fever: relationship to streptococcal infection and autoimmune disease. J Rheumatol Suppl 1991;30:1-5. [PubMed]
- 31.Khanna AK, Buskirk DR, Williams RC, Gibofsky A, Crow MK, Menon A, et al. Presence of a non-HLA B cell antigen in rheumatic fever patients and their families as defined by a monoclonal antibody. J Clin Invest 1989;83: 1710-6. [DOI] [PMC free article] [PubMed]
- 32.Swedo SE, Leonard HL, Mittleman B, Allen AJ, Rapoport JL, Dow SP, et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry 1997;154:110-2. [DOI] [PubMed]
- 33.Murphy TK, Goodman WK, Fudge MW, Williams RC, Ayoub EM, Dalal M, et al. B lymphocyte antigen D8/17: a peripheral marker for childhood-onset obsessive-compulsive disorder and Tourette's syndrome? Am J Psychiatry 1997; 154: 402-7. [DOI] [PubMed]
- 34.Chapman F, Visvanathan K, Carrano-Manjarrez R, Zabriskie J. A flow cytometric assay for D8/17 B cell marker in patients with Tourette's syndrome and obsessive-compulsive disorder. J Immunol Methods 1998;219:181-6. [DOI] [PubMed]
- 35.Hollander E, DelGiudice-Asch G, Simon L, Schmeidler J, Cartwright C, DeCaria CM, et al. B lymphocyte antigen D8/17 and repetitive behaviours in autism. Am J Psychiatry 1999;156:317-20. [DOI] [PubMed]
- 36.Lougee L, Perlmutter SJ, Nicolson R, Garvey MA, Swedo SE. Psychiatric disorders in first-degree relatives of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). J Am Acad Child Adolesc Psychiatry 2000;39:1120-6. [DOI] [PubMed]
- 37.Bengel D, Greenberg BD, Cora-Locatelli G, Altemus M, Heils A, Li Q, et al. Association of the serotonin transporter promoter regulatory region polymorphism and obsessive-compulsive disorder. Mol Psychiatry 1999;4:463-6. [DOI] [PubMed]
- 38.McDougle CL, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder. Mol Psychiatry 1998;3:270-3. [DOI] [PubMed]
- 39.Billett EA, Richter MA, King N, Heils A, Lesch KP, Kennedy JL. Obsessive compulsive disorder, response to serotonin reuptake inhibitors and the serotonin transporter gene. Mol Psychiatry 1997;2:403-6. [DOI] [PubMed]
- 40.Mundo E, Richter MA, Sam F, Macciardi F, Kennedy JL. Is the 5-HT (1D beta) receptor gene implicated in the pathogenesis of obsessive-compulsive disorder? Am J Psychiatry 2000;157:1160-2. [DOI] [PubMed]
- 41.Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, et al. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry 1999;45:1178-89. [DOI] [PubMed]
- 42.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry 2000;157(5):683-94. [DOI] [PubMed]
- 43.Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM, et al. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol 1997;159(6):2994-9. [PubMed]
- 44.Ravindran AV, Griffiths J, Merali Z, Anisman H. Circulating lymphocyte subsets in obsessive compulsive disorder, major depression and normal controls. J Affect Disord 1999;52:1-10. [DOI] [PubMed]
- 45.Marazziti D, Presta S, Pfanner C, Gemignani A, Rossi A, Sbrana S, et al. Immunological alterations in adult obsessive compulsive disorder. Biol Psychiatry 1999; 46 (6): 810-814. [DOI] [PubMed]
- 46.Brambilla F, Perna G, Bellodi L, Arancio C, Bertani A, Perini G, et al. Plasma interleukin-1β and tumor necrosis factor concentrations in obsessive compulsive disorder. Biol Pscyhiatry 1997;42(11):976-81. [DOI] [PubMed]
- 47.Roy BF, Benkelfat C, Hill JL, Pierce PF, Dauphin MM, Kelly TM, et al. Serum antibody for somatostatin-14 and prodynorphin 209–240 in patients with obsessive-compulsive disorder, schizophrenia, Alzheimer's disease, multiple sclerosis, and advanced HIV infection. Biol Psychiatry 1994;35(5):335-44. [DOI] [PubMed]
- 48.Black JL, Lamke GT, Walikonis JE. Serologic survey of adult patients with obsessive-compulsive disorder for neuron-specific and other autoantibodies. Psychiatry Res 1998;81:371-80. [DOI] [PubMed]
- 49.Barber Y, Toren P, Achiron A, Noy S, Wolmer L, Weizman R, et al. T cell subsets in obsessive-compulsive disorder: Neuropsychobiology 1996;34(2):63-6. [DOI] [PubMed]
- 50.Weizman R, Laor N, Barber Y, Hermesh H, Notti I, Djaldetti M, et al. Cytokine production in obsessive-compulsive disorder. Biol Psychiatry 1996;40 (9): 908-12. [DOI] [PubMed]
- 51.Rapoport JL, Inoff-Germain G. Practitioner review: treatment of obsessive-compulsive disorder in children and adolescents. J Child Psychol Psychiatry 2000; 41 (4): 418-31. [PubMed]
- 52.Garvey MA, Perlmutter SJ, Allen AJ, Hamburger S, Lougee L, Leonard HL, et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry 1999;45:1564-71. [DOI] [PubMed]
- 53.Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999; 354: 1153-8. [DOI] [PubMed]
- 54.Nicolson R, Swedo SE, Lenane M, Bedwell J, Wudarsky M, Gochman P, et al. An open trial of plasma exchange in childhood-onset obsessive-compulsive disorder without post-streptococcal exacerbations. J Am Acad Child Adolesc Psychiatry 2000;39:1313-5. [DOI] [PubMed]
- 55.National Institute of Mental Health (NIMH). Plasma exchange and intravenous immunoglobulin lack proven benefit and carry risk for children with PANDAS, Tourette's Syndrome, or OCD. Available: www.nimh.nih.gov/events/pandaalert.cfm (accessed 2001 Oct 9).
- 56.Robertson MM, Stern JS. Gilles de la Tourette syndrome: symptomatic treatment based on evidence. Eur Child Adolesc Psychiatry 2000;9(Suppl l):160-75. [DOI] [PubMed]


