Abstract
Background
C‐reactive protein (CRP) is a well‐known acute‐phase protein in dogs that may discriminate bacterial bronchopneumonia from other pulmonary conditions. Bronchopneumonia caused by Bordetella bronchiseptica (Bb) is common but the associated increase in CRP concentration in naturally infected dogs has not been fully explored.
Objective
To compare CRP concentrations of dogs with Bb infection, with or without radiographic pulmonary lesions, to dogs with aspiration bronchopneumonia (ABP).
Animals
Sixteen dogs with Bb infection and 36 dogs with ABP.
Methods
Retrospective study. C‐reactive protein concentrations and thoracic radiographs were available for each dog.
Results
Eleven dogs with Bb infection had alveolar lesions. In all dogs, CRP concentration was mildly increased (14‐38 mg/L). In the 5 dogs without alveolar lesions, CRP concentration was within the reference range in all but 1 dog, in which it was slightly increased. Median CRP concentration was significantly higher in dogs with alveolar lesions (20 mg/L) compared with dogs without alveolar lesions (5 mg/L; p < .002). In dogs with Bb infection, median duration of clinical signs was not different between dogs with normal CRP concentration and dogs with increased concentration. In dogs with Bb infection either with or without alveolar lessions, median CRP concentration was significantly lower (20 mg/L) than in dogs with ABP (118 mg/L; p < .001).
Conclusions and Clinical Importance
In contrast to dogs with APB, CRP was not a good marker for the diagnosis of dogs suspected to have bordetellosis. Confirmation of Bb infection still requires lower airway sampling.
Keywords: aspiration pneumonia, CRP, parenchymal disease, pneumonia, respiratory tract
Abbreviations
- ABP
aspiration bronchopneumonia
- APP
acute‐phase protein
- BALF
bronchoalveolar lavage fluid
- Bb
Bordetella bronchiseptica
- CIRD‐C
canine infectious respiratory disease‐complex
- CRP
C reactive protein
- qPCR
quantitative polymerase chain reaction
- TCC
total cell count
1. INTRODUCTION
Despite vaccination, Bordetella bronchiseptica (Bb) infection is still common. 1 , 2 , 3 , 4 , 5 , 6 , 7 Definitive diagnosis requires specific procedures including bronchoscopy with bronchoalveolar lavage fluid (BALF) sampling for both cytologic examination and bacterial culture. 1 , 2 , 3 , 4 , 5 Quantitative PCR (qPCR) usually is reserved for cases in which Bb infection is suspected despite negative bacterial culture. 4 Treatment of Bb infection can be challenging and in refractory cases, nebulized gentamicin can be beneficial. 8 Further investigation of noninvasive markers is required because infected dogs are at risk for complications with general anesthesia. In addition, such markers also could be used to monitor response to treatment.
In dogs, C‐reactive protein (CRP) is a well‐known marker of inflammation. It is a highly sensitive positive acute‐phase protein (APP) produced by the liver. 9 , 10 , 11 The APP are produced shortly after initiation of infectious, immunologic, neoplastic, or traumatic processes. 9 , 10 , 11 In dogs, CRP is a major APP that increases rapidly and quickly normalizes with recovery. 9 , 10 , 11 , 12 , 13 Today, several automated assays are validated for reliable measurements of CRP in dogs. 14 , 15 Increases in CRP concentrations have been studied primarily in immune‐mediated diseases and in inflammatory or infectious processes in dogs. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 24 , 25
In respiratory diseases of dogs, serum CRP concentration has been shown to be particularly useful in discriminating bacterial bronchopneumonia from other pulmonary diseases. 26 Moreover, in dogs with bacterial pneumonia, serum CRP concentrations decrease rapidly after initiation of antibiotic treatment and the duration of antibiotic treatment can be minimized. 27 However, in the former study, dogs with tracheobronchitis caused by Bb and without alveolar lesions were compared to dogs with bacterial pneumonia. In a previous experimental study, high concentrations of CRP were identified in dogs inoculated with Bb. 28 Thus, the magnitude of increase of serum CRP concentration in naturally infected dogs with Bb and alveolar lesions has not been fully explored.
Our aim was to report serum CRP concentrations in dogs with confirmed subacute or chronic Bb infection, with or without pulmonary lesions on thoracic radiographs, and to compare the magnitude of increased CRP concentration with dogs diagnosed with aspiration bronchopneumonia (ABP).
2. MATERIALS AND METHODS
2.1. Study populations
Medical records from client‐owned dogs referred to the Veterinary Small Animal Teaching Hospitals of the Faculté de Liège (Belgium) and the Ecole Nationale Vétérinaire d'Alfort (France) between September 2016 and April 2019 and diagnosed with Bb lower airway infection were reviewed. Diagnosis of Bb infection was confirmed by positive culture or qPCR on BALF. Dogs in which other bacteria were identified on culture of BALF were excluded from the study. Collected data included signalment, body weight, previous PO antimicrobial treatment, any previous corticosteroid treatment, duration and severity of clinical signs, physical examination findings, results of CBC, radiographic lesions, and BALF analysis.
Bronchoscopy, bronchoalveolar lavage procedure, and BALF processing were performed as described previously. 4 Briefly, dogs were anesthetized using various anesthetic protocols after 5 minutes of preoxygenation. Aliquots of BALF were used for conventional quantitative culture, total cell count (TCC) determination using a hemocytometer and cytospin preparation (centrifugation at 1400 rpm [197g], for 4 minutes at 20°C, Thermo Shandon Cytospin©4) stained with May‐Grünwald‐Giemsa. Samples with cytological evidence of oropharyngeal contamination were not further evaluated. For bacteriological analysis, BALF was plated onto several agar plates at 35°C for isolation of aerobic organisms. The threshold used to define clinically relevant bacterial growth was 1.7 × 103 colony‐forming units per mL of BALF. Bacterial susceptibility testing was performed using the disk diffusion method. Finally, qPCR for Bb testing was requested in dogs with negative bacterial culture results and was performed by commercial veterinary diagnostic laboratories (TDDS Laboratories, University of Exeter, England or Laboratoire Scanelis, Colomiers, France).
Dogs with ABP were selected over the same study period. Dogs were considered to have ABP based on compatible history of recent vomiting or regurgitation, physical examination findings (at least 3 of the following: fever, lethargy, tachypnea, dyspnea, cough), radiographic lesions (ie, cranio‐ventral alveolar lung pattern), and clinical and radiographical resolution with empirical antimicrobial treatment. For these dogs, serum CRP concentrations and 3‐view thoracic radiographs had to be available at the time of diagnosis for inclusion in the study.
For both groups of dogs, 2‐ or 3‐view thoracic radiographs were reviewed by board‐certified radiologists. Moderate and severe alveolar patterns were defined if lesions were located within 1 or >1 lobes respectively.
2.2. CRP assay
Serum samples obtained for CRP analysis were immediately assayed. C‐reactive protein was analyzed using Eurolyser Solo (SCIL Laboratories) immunoassay as previously validated. 29 The normal reference range was <5 mg/L. For results >200 mg/L, dilutions were made to obtain precise concentrations.
2.3. Statistics
Statistical analysis was performed using commercially available software (XLstat software). The Mann‐Whitney test was used to compare median ages, median CRP concentrations between dogs with Bb infection and ABP, median CRP concentrations between Bb dogs with or without alveolar lesion, median durations of clinical signs between Bb dogs with normal or mildly increased CRP concentrations and median durations of clinical signs between dogs with or without alveolar lesions. Proportions of Bb dogs with increased CRP concentrations were compared between dogs with alveolar lesions and dogs without alveolar lesions on thoracic radiographs using a Chi‐squared test. Statistical significance was set at P < .05.
3. RESULTS
Sixteen dogs with Bb infection and 36 dogs with ABP met the inclusion criteria. The breeds of Bb dogs included French Bulldog (n = 3), Chihuahua (n = 3), Toy Spitz (n = 3), Pomeranian (n = 2), Basset Hound (n = 2), Cavalier King Charles Spaniel (n = 2), and Labrador Retriever (n = 1). Breeds of dogs with ABP were mixed breeds (n = 7), French Bulldog (n = 5), Labrador Retriever (n = 5), English Bulldog (n = 4), German Shepherd (n = 4), Australian Shepherd (n = 3), Chow Chow (n = 2), Beagle (n = 2), Belgian Shepherd (n = 2), Cavalier King Charles Spaniel (n = 1), and West Highland White Terrier (n = 1). Median ages of dogs with Bb infection and ABP were 0.6 and 5 years respectively (P < .001). All dogs with Bb infection had clinical signs for >2 weeks (mean, 2.5 months; median, 3 months). Respiratory signs included chronic cough (n = 16) and mild to moderate dyspnea (n = 5). Four dogs had received PO antimicrobial drugs before diagnosis, including cefalexin, amoxicillin/clavulanic acid, and marbofloxacin. For all dogs, antibiotics were discontinued from 5 to 10 days before CRP measurement and airway sampling. Mean and median durations of clinical signs in dogs with ABP were 4 and 5 days.
Bordetella bronchiseptica infection was confirmed by bacterial culture in 10 of 16 dogs and by qPCR in the 6 remaining dogs. Eleven dogs with Bb infection had alveolar lesions on radiographs. Alveolar patterns were observed in 1 pulmonary lobe in 7 dogs (right cranial lobe in 3 dogs, left cranial lobe in 3 dogs, median lobe in 1 dog), in 2 lobes in 3 dogs (right and left cranial lobes) and in 3 lobes in the remaining dog (right and left cranial lobes and median lobe). In all 5 dogs without alveolar lesions, a diffuse broncho‐interstitial pattern was present.
In Bb dogs, 5 of 16 dogs required brief hospitalization for oxygen administration. After CRP measurement and airway sampling, systemic antibiotic treatment was prescribed in 8 dogs with PO doxycycline, and nebulized gentamicin was initiated in 10 dogs. In the ABP group, 30 dogs were hospitalized for oxygen administration and initial IV antibiotic treatment, followed by PO treatment. The remaining 6 dogs were discharged on PO antimicrobials. For all dogs, treatments were started after CRP measurements and thoracic radiographs.
In the Bb group, serum CRP concentration was mildly increased in 11/11 dogs and 1/5 dogs with and without alveolar lesions, respectively, and these proportions were significantly different (P = .002). In the 4 remaining dogs without alveolar lesions, CRP concentrations were within the reference range (<10 mg/L). The median CRP concentration was significantly higher in dogs with alveolar lesions compared with dogs without alveolar lesions (median, 20 mg/L [range, 14‐38] vs median, 5 mg/L [range, 5‐11], Figure 1, P = .002). Median CRP concentrations were not different between dogs with single vs multiple pulmonary lobe involvement (17 mg/L vs 20 mg/L). Bordetella qPCR results were available for 6 dogs and corresponded to very high (cycle threshold <20) or high (cycle threshold from 20.1 to 24) bacterial load in 1 and 5 dogs, respectively. In these 6 dogs, the median CRP concentration was 14.5 mg/L (range, 5‐22) including 2 dogs with a CRP concentration within the reference interval (dogs with cycle threshold of 18 and 23). Among these last 2 dogs, 1 had alveolar lesions on thoracic radiographs.
FIGURE 1.
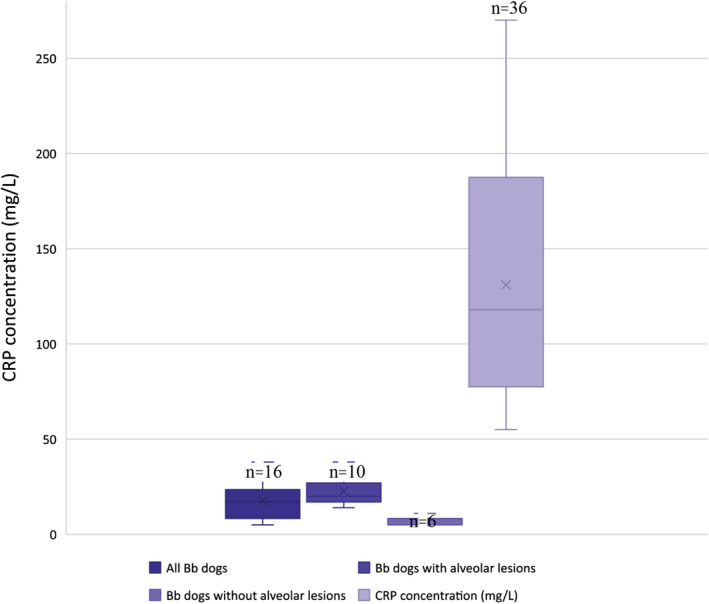
CRP concentrations (mg/L) in all dogs with Bb infection (n = 16), dogs with Bb infection with (n = 10) and without alveolar lesions (n = 6) and in dogs with ABP (n = 36). ABP, aspiration bronchopneumonia; Bb, Bordetella bronchiseptica; CRP, C‐reactive protein
In dogs with Bb infection, CRP concentration was neither associated with duration of clinical signs nor with presence of alveolar lesions.
In dogs with Bb infection and alveolar lesions, median CRP concentration was significantly lower than in dogs with ABP (median, 20 mg/L vs median, 118 mg/L, Figure 1, P < .001), and increases in CRP concentration did not overlap between the 2 groups (range, 14‐38 mg/L and range, 55‐270 mg/L, respectively, Figure 1).
4. DISCUSSION
In our study, dogs with Bb infection with or without alveolar lesions on thoracic radiographs had lower CRP concentrations at the time of diagnosis as compared with dogs diagnosed with ABP. Moreover, CRP concentrations in these 2 groups did not overlap with each other. Also, in 4 dogs with Bb infection, CRP concentrations were within the reference interval defined for healthy dogs. Therefore, a normal CRP concentration does not rule out Bb infection.
The CRP concentrations of dogs diagnosed with ABP in our study were high, which can be explained by the extensive inflammatory process that occurs in ABP. Results obtained were similar to those previously described. 26 In this latter study, a cut‐off for CRP concentration of >100 mg/L was reported to be 100% specific for bacterial pneumonia and a CRP concentration <20 mg/L ruled out bacterial pneumonia. 26 Because our study did not include dogs with other respiratory diseases, sensitivity and specificity of serum CRP concentrations cannot be determined. Nevertheless, based on the concentrations observed in our small group of dogs with ABP, a CRP concentration >55 mg/L in dogs with an alveolar radiographic pattern may be considered to be suggestive of ABP.
Severe increases in serum CRP concentration previously have been identified in dogs inoculated with Bb with concentrations being 23‐95 times preinoculation concentrations within the first day and then returning nearly to normal within 10 days. 28 In the naturally infected young dogs in our study, much lower serum concentrations were measured. Such a discrepancy may be explained by several factors, including differences in bacterial load, bacterial virulence, and host immunity. One of the main reasons for the low serum CRP concentrations in Bb‐infected dogs in our study also could be the chronicity of the condition, because all Bb dogs had a cough for at least 2 weeks. Only dogs with chronic signs were included in both groups, although duration of clinical signs was not different between Bb dogs with normal or increased CRP concentrations. The statistical approach could have been prone to type II error because of low power. Nevertheless, in a recent study, 26 duration of signs did not correlate negatively with serum CRP concentration.
Some dogs included in our study had been treated with corticosteroids before referral. No difference was found in median CRP concentrations between dogs previously treated with corticosteroids and the others (data not shown). In a previous study, pretreatment of experimentally infected dogs with prednisolone also had no effect on production of CRP. 28 On the contrary, in human patients with pneumonia, adjunctive treatment with corticosteroids can lead to a significant decrease in CRP concentrations at the time of diagnosis. 30 Evaluation of the impact of previous corticosteroid treatment on CRP concentrations in bacterial respiratory diseases of dogs requires additional studies.
Moreover, 4 of the dogs with Bb infection in our study had received antimicrobial drugs before diagnosis. However, initial median CRP concentrations were not statistically different between dogs previously treated with antibiotics and untreated dogs (data not shown). Moreover, antibiotics were discontinued at least 5 days before CRP measurement. Thus, the potential impact of previous antimicrobial treatment on serum CRP concentration was considered minimal. In a recent study, 26 the effect of prior antibiotic treatment on serum CRP concentration was not found to be significant in dogs with bacterial tracheobronchitis or pneumonia, in agreement with what has been described in humans with bacterial pneumonia. 31
In our study, some Bb dogs with only mildly increased serum CRP concentrations (<40 mg/L) had severe clinical signs (ie, required a short course of hospitalization and initial oxygen administration), suggesting that CRP concentrations would not be related to disease severity. This observation is in agreement with a previous study 27 that found that serum CRP, serum amyloid A, and haptoglobin concentrations at presentation did not correlate significantly with markers of disease severity, such as arterial PaO2 and duration of hospitalization. 27 Nevertheless, because the acute phase response to inflammatory stimulus in acutely ill animals is rapid, the correlation of serum CRP concentration to indicators of disease severity may be very different in acute bacterial pneumonia and in a chronic Bb infection. Less information is available about the acute phase response to chronic infections in dogs.
The normal or mildly increased serum CRP concentrations (<40 mg/L) observed in dogs with Bb infection are in accordance with laboratory findings in children with Bordetella pertussis infection. In children suffering from nonfebrile cough, no significant increase in CRP concentration was found between children with and without B. pertussis infection and CRP concentrations were within the reference range. 32 However, in this study, children with wheezing or fever were excluded and thoracic radiographs were not performed, limiting further comparison with our results.
In our study, we measured serum CRP concentration rather than haptoglobin or serum amyloid A because of its greater availability and higher performance for indicating infectious processes in children. 33 Because serum amyloid A and haptoglobin were shown to be positively correlated with CRP, 27 we hypothesized that both of these proteins also would have been normal or only mildly increased in our population. However, in dogs with bacterial pneumonia or systemic inflammation, the magnitude of change was more pronounced for serum amyloid A as compared with CRP, emphasizing that measurement of serum amyloid A might be more meaningful. 27 , 34
In humans with community‐acquired pneumonia, serum CRP concentration has been studied for decades. It has been described as an extremely sensitive systemic marker, and its use as a diagnostic and follow‐up biomarker is now largely supported by several meta‐analyses. 35 , 36 , 37 , 38 , 39 Nevertheless, improved performance of this biomarker can be obtained by using it in association with serum procalcitonin concentration. 36 , 37 , 38 , 39 , 40 However, results of procalcitonin concentration in dogs have not been as promising, and the usefulness of this biomarker has yet to be defined in severe infections of dogs. 41
In conclusion, regardless of the presence of alveolar lesions, Bb infection can be suspected in coughing or dyspneic dogs with normal to slightly increased serum CRP concentrations, suggesting that CRP may not be a good marker to facilitate diagnosis in dogs with suspected bordetellosis. Therefore, until other serum biomarkers are investigated for bacterial bronchopneumopathies in dogs, confirmation of Bb infection still requires lower airway sampling.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Canonne AM, Menard M, Maurey C, et al. Comparison of C‐reactive protein concentrations in dogs with Bordetella bronchiseptica infection and aspiration bronchopneumonia. J Vet Intern Med. 2021;35:1519–1524. 10.1111/jvim.16091
REFERENCES
- 1. Radhakrishnan A, Drobatz KJ, Culp WT, King KG. Community‐acquired infectious pneumonia in puppies: 65 cases (1993‐2002). J Am Vet Med Assoc. 2007;230(10):1493‐1497. [DOI] [PubMed] [Google Scholar]
- 2. Priestnall SL, Mitchell JA, Walker CA, Erles K, Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Vet Pathol. 2014;51(2):492‐504. [DOI] [PubMed] [Google Scholar]
- 3. Schulz BS, Kurz S, Weber K. Detection of respiratory viruses and Bordetella bronchiseptica in dogs with acute respiratory tract infections. Vet J. 2014;201(3):365‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canonne AM, Billen F, Tual C, et al. Quantitative PCR and cytology of bronchoalveolar lavage fluid in dogs with Bordetella bronchiseptica infection. J Vet Intern Med. 2016;30(4):1204‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Decaro N, Mari V, Larocca V, et al. Molecular surveillance of traditional and emerging pathogens associated with canine infectious respiratory disease. Vet Microbiol. 2016;30(192):21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joffe DJ, Lelewski R, Weese J, et al. Factors associated with development of canine infectious respiratory disease complex (CIRDC) in dogs in 5 Canadian small animal clinics. Can Vet J. 2016;57(1):46‐51. [PMC free article] [PubMed] [Google Scholar]
- 7. Taha‐Abdelaziz K, Bassel LL, Harness ML, Clark ME, Register KB, Caswell JL. Cilia‐associated bacteria in fatal Bordetella bronchiseptica pneumonia of dogs and cats. J Vet Diagn Invest. 2016;28(4):369‐376. [DOI] [PubMed] [Google Scholar]
- 8. Canonne AM, Roels E, Menard M, Desquilbet L, Billen F, Clercx C. Clinical response to two protocols of aerosolized gentamicin and impact of factors on outcome in 46 dogs with Bordetella bronchiseptica infection (2012–2018). J Vet Intern Med. 2020;34:2078‐2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceron JJ, Eckersall PD, Martýnez‐Subiela S. Acute phase proteins in dogs and cats: current knowledge and future perspectives. Vet Clin Pathol. 2005;34(2):85‐99. [DOI] [PubMed] [Google Scholar]
- 10. Stockham SL. In: Stockham SL, Scott MA, eds. Fundamentals of Veterinary Clinical Pathology. 2nd ed. St Louis, MO: Wiley‐Blackwell; 2008:370‐413. [Google Scholar]
- 11. Eckersall PD, Bell R. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J. 2010;185(1):23‐27. [DOI] [PubMed] [Google Scholar]
- 12. Caspi D, Snel FW, Batt RM, et al. C‐reactive protein in dogs. Am J Vet Res. 1987;48(6):919‐921. [PubMed] [Google Scholar]
- 13. Yamashita K, Fujinaga T, Miyamoto T, Hagio M, Izumisawa Y, Kotani T. Canine acute phase response: relationship between serum cytokine activity and acute phase protein in dogs. J Vet Med Sci. 1994;56(3):487‐492. [DOI] [PubMed] [Google Scholar]
- 14. Hillström A, Hagman R, Tvedten H, Kjelgaard‐Hansen M. Validation of a commercially available automated canine‐specific immunoturbidimetric method for measuring canine C‐reactive protein. Vet Clin Pathol. 2014;43:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klenner S, Bauer N, Moritz A. Evaluation of three automated human immunoturbidimetric assays for the detection of C‐reactive protein in dogs. J Vet Diagn Invest. 2010;22:544‐552. [DOI] [PubMed] [Google Scholar]
- 16. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care. 2009;19(5):450‐458. [DOI] [PubMed] [Google Scholar]
- 17. Lowrie M, Penderis J, Eckersall PD, McLaughlin M, Mello D, Anderson TJ. The role of acute phase proteins in diagnosis and management of steroid‐responsive meningitis arteritis in dogs. Vet J. 2009;182(1):125‐130. [DOI] [PubMed] [Google Scholar]
- 18. Mitchell KD, Kruth SA, Wood RD, Jefferson B. Serum acute phase protein concentrations in dogs with autoimmune hemolytic anemia. J Vet Intern Med. 2009;23(3):585‐591. [DOI] [PubMed] [Google Scholar]
- 19. Baric Rafaj R, Kuleš J, Selanec J, et al. Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis. J Vet Intern Med. 2013;27(5):1172‐1178. [DOI] [PubMed] [Google Scholar]
- 20. McClure V, van Schoor M, Thompson PN, Kjelgaard‐Hansen M, Goddard A. Evaluation of the use of serum C‐reactive protein concentration to predict outcome in puppies infected with canine parvovirus. J Am Vet Med Assoc. 2013;243(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 21. Foster JD, Sample S, Kohler R, Watson K, Muir P, Trepanier LA. Serum biomarkers of clinical and cytologic response in dogs with idiopathic immune‐mediated polyarthropathy. J Vet Intern Med. 2014;28(3):905‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez‐Subiela S, Pardo‐Marín L, Tecles F, Baneth G, Cerón JJ. Serum C‐reactive protein and ferritin concentrations in dogs undergoing leishmaniosis treatment. Res Vet Sci. 2016;109:17‐20. [DOI] [PubMed] [Google Scholar]
- 23. Cantos‐Barreda A, Escribano D, Cerón JJ, et al. Relationship between serum anti‐Leishmania antibody levels and acute phase proteins in dogs with canine leishmaniosis. Vet Parasitol 2018;260:63‐68. [DOI] [PubMed] [Google Scholar]
- 24. Ceron JJ, Pardo‐Marin L, Caldin M, et al. Use of acute phase proteins for the clinical assessment and management of canine leishmaniosis: general recommendations. BMC Vet Res. 2018;14(1):196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Kim HJ, Kang JH, Kang BT, Yang MP. Evaluation of serum C‐reactive protein and high mobility group box 1 concentrations in 22 dogs with acute pancreatitis: a pilot study. Vet Q. 2019. Dec;39(1):122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Viitanen SJ, Laurila HP, Lilja‐Maula LI, Melamies MA, Rantala M, Rajamäki MM. Serum C‐reactive protein as a diagnostic biomarker in dogs with bacterial respiratory diseases. J Vet Intern Med. 2014;28(1):84‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viitanen SJ, Lappalainen AK, Christensen MB, Sankari S, Rajamäki MM. The utility of acute‐phase proteins in the assessment of treatment response in dogs with bacterial pneumonia. J Vet Intern Med. 2017;31(1):124‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamamoto S, Shida T, Honda M, et al. Serum C‐reactive protein and immune responses in dogs inoculated with Bordetella bronchiseptica (phase I cells). Vet Res Commun. 1994;18(5):347‐357. [DOI] [PubMed] [Google Scholar]
- 29. EUROlyser Solo , validated in 2015. [Evaluation of three different point‐of‐care tests for quantitative measurement of canine C‐reactive protein, Jasensky A‐K, Klenner S, Einspanier R, Kohn B. Vet Clin Pathol 2015;44(2):205‐214.] [DOI] [PubMed]
- 30. Kutz A, Grolimund E, Christ‐Crain M, et al. Pre‐analytic factors and initial biomarker levels in community‐acquired pneumonia patients. BMC Anesthesiol. 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bafadhel M, Clark TW, Reid C, et al. Procalcitonin and C‐reactive protein in hospitalized adult patients with community‐acquired pneumonia or exacerbation of asthma or COPD. Chest. 2011;139:1410‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu X, Qiang C, Kai‐Hu Y, et al. Pertussis detection in children with cough of any duration. BMC Pediatr. 2019;19:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dos Anjos BL, Grotto HZ. Evaluation of C‐reactive protein and serum amyloid A in the detection of inflammatory and infectious diseases in children. Clin Chem Lab Med. 2010;48(4):493‐499. [DOI] [PubMed] [Google Scholar]
- 34. Christensen MB, Langhorn R, Goddard A, et al. Comparison of serum amyloid A and C‐reactive protein as diagnostic markers of systemic inflammation in dogs. Can Vet J. 2014;55(2):161‐168. [PMC free article] [PubMed] [Google Scholar]
- 35. Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections‐full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1‐E59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salluh JF, Souza‐Dantas VC, Póvoa P. The current status of biomarkers for the diagnosis of nosocomial pneumonias. Curr Opin Crit Care. 2017;23(5):391‐397. [DOI] [PubMed] [Google Scholar]
- 37. Salluh JF, Souza‐Dantas VC, Póvoa P. Place of biomarkers in the management of pulmonary infections. Rev Mal Respir. 2019;36(3):405‐414. [DOI] [PubMed] [Google Scholar]
- 38. Htun TP, Sun Y, Chua HL, Pang J. Clinical features for diagnosis of pneumonia among adults in primary care setting: a systematic and meta‐review. Sci Rep. 2019;9(1):7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ebell MH, Bentivegna M, Cai X, Hulme C, Kearney M. Accuracy of biomarkers for the diagnosis of adult community‐acquired pneumonia: a meta‐analysis. Acad Emerg Med. 2020. Mar;27(3):195‐206. [DOI] [PubMed] [Google Scholar]
- 40. Karakioulaki M, Stolz D. Biomarkers and clinical scoring systems in community‐acquired pneumonia. Ann Thorac Med. 2019;14(3):165‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goggs R, Milloway M, Troia R, Giunti M. Plasma procalcitonin concentrations are increased in dogs with sepsis. Vet Rec Open. 2018;5:e000255. [DOI] [PMC free article] [PubMed] [Google Scholar]


