Abstract
Background
In inflammatory bowel disease (IBD) in humans, phosphorylated signal transducer and activator of transcription 3 (pSTAT3) is upregulated in mucosal epithelial cells and correlates with clinical severity.
Hypothesis/Objective
To investigate the expression pattern of pSTAT3 in the mucosa of dogs with chronic inflammatory enteropathy (CIE) and explore correlations between its expression and clinical and histopathological severity scoring.
Animals
Twenty‐eight canine CIE patients grouped into food‐responsive enteropathy (FRE; 9), steroid‐responsive enteropathy (SRE; 10), and protein‐losing enteropathy (PLE; 9). Ten healthy beagle dogs served as controls (CO).
Methods
Retrospective case control study. Immunohistochemistry was used to detect pSTAT3 in canine duodenal mucosa samples.
Results
Compared to CO, SRE (P < .001) and PLE (P < .001) dogs had significantly higher pSTAT3 expression in the villus epithelium. The SRE group had a significantly higher expression in the villus lamina propria (VLP) compared to controls (P = .009). In the crypt epithelium (CE), all CIE dogs had significantly higher pSTAT3 expression (FRE, P = .002; SRE, P = .003; PLE, P < .001) compared to CO. In the lamina propria crypt region (CLP), dogs with FRE (P = .04) and SRE (P = .03) had significantly upregulated pSTAT3 compared to controls. A positive correlation was found between canine chronic enteropathy clinical activity index (CCECAI) scoring and pSTAT3 expression for both epithelial (rho = .541; P < .001) and crypt regions (rho = .32; P = .02).
Conclusions and Clinical Importance
pSTAT3 is upregulated in CIE in dogs, correlates with clinical severity, and may be helpful as a clinical marker in dogs with CIE.
Keywords: canine, chronic inflammatory enteropathy, IBD, STAT3
Abbreviations
- CCECAI
canine chronic enteropathy clinical activity index
- CD
Crohn's disease
- CEP
crypt epithelial region
- CIE
chronic inflammatory enteropathy
- CLP
crypt lamina propria region
- CO
control dogs
- FRE
food responsive enteropathy
- GSD
German Shepherd dogs
- ICC
intraclass correlation
- PLE
protein losing enteropathy
- pSTAT3
phosphorylation STAT3
- STAT3
transcription 3
- SRE
steroid responsive enteropathy
- VE
villus epithelial region
- VLP
villus lamina propria region
- WSAVA
World Small Animal Veterinary Association
1. INTRODUCTION
Chronic inflammatory enteropathies (CIE) have been observed with increasing frequency in dogs. 1 , 2 After exclusion of infectious, endocrine and neoplastic diseases, CIE are diagnosed based on chronic gastrointestinal signs and histologically‐confirmed inflammation in the intestinal mucosa. 1 , 3
Similar to chronic enteropathies in humans, 4 the underlying pathogenesis in dogs with CIE seems to be multifactorial including genetic predisposition, 5 environmental factors such as dietary antigens, 6 microbiome dysbalances, 7 , 8 , 9 and hyperactivity of the mucosal immune response. 10 Loss of oral tolerance seems to be a key feature, with disruption of the gut‐associated lymphoid tissue (GALT) followed by dysregulation of secreted cytokines and transcription factors leading to chronic inflammation. 1 , 10 , 11
The mucosal inflammation in dogs with CIE is most commonly of lymphocytic‐plasmacytic origin, 12 , 13 , 14 and CD4+ T cells, which have been shown to be a mediator of signal transducer and activator of transcription 3 (STAT3) upregulation, 15 seem to be activated in both species. 13 , 16 , 17
Studies in humans determined that STAT3 plays an important role in the pathogenesis of IBD. 18 It is activated by phosphorylation STAT3 (pSTAT3) and regulates intestinal homeostasis and intestinal wound healing by stimulating the release of several anti‐inflammatory cytokines. 18 , 19 In addition to its regulatory role, activation of STAT3 in the acquired or innate immune system by certain cytokines may lead to prolonged resistance to apoptosis in intestinal T cells and colonic epithelial cells. 18 , 20
Studies in CD patients 15 and mouse models 21 have shown that mucosal pSTAT3 expression serves as a biomarker, because a positive correlation exists among histological findings, severity, clinical activity index 22 and pSTAT3 expression. Compared to healthy controls, upregulation of pSTAT3 in the mucosa of human IBD patients was detected in the lamina propria and epithelium 23 using immunofluorescence 19 or immunohistochemistry. 22
The role of pSTAT3 in the pathogenesis of CIE in dogs is unknown. The purpose of our retrospective study therefore was to investigate the expression profile of pSTAT3 in the mucosa of dogs diagnosed with CIE. Our hypothesis was (a) upregulation of pSTAT3 occurs in the mucosal epithelium and lamina propria of dogs with CIE as compared with healthy dogs, (b) that significant differences in pSTAT3 expression exist among different subtypes of CIE dogs, and (c) that a correlation exists among clinical activity scores, histopathological severity as measured by World Small Animal Veterinary Association (WSAVA) scoring 24 and the mucosal expression of pSTAT3.
2. MATERIALS AND METHODS
Animal protocols for this study were approved by the Institutional Ethics Committee, the Advisory Committee for Animal Experiments (§8 of Law for Animal Experiments, Tierversuchsgesetz [TVG]), and the Federal Ministry for Science and Research [reference number: GZ 68.205/0201‐II/3b/2010].
2.1. Study groups
2.1.1. Control dogs
Ten healthy intact female beagles from an unrelated study were included as a control group.
All laboratory dogs were regularly (every 3 months) treated for gastrointestinal parasites. All dogs underwent clinical examination and a CBC, serum biochemistry, fecal flotation and abdominal ultrasound examination were performed and the dogs deemed healthy before the intestinal samples were taken. They were clinically scored using the previously described Canine Chronic Enteropathy Clinical Activity Index (CCECAI) scoring system 25 by the primary clinician. Intestinal biopsy samples were taken for an unrelated study and paraffin‐embedded slides of biopsy samples were used as the controls in this study.
2.1.2. Chronic inflammatory enteropathy dogs
Twenty‐eight dogs diagnosed with CIE were included in this retrospective study. Ten dogs were presented to the Clinic of Internal Medicine at the Veterinary University of Vienna and 18 dogs to the Royal Veterinary College University of London. Chronic 26 gastrointestinal signs had been present over a period of at least 6 weeks in all dogs at the time of presentation. According to response to treatment, the 28 dogs were retrospectively grouped as food‐responsive enteropathy (FRE; 9) if they responded to an elimination or hydrolyzed diet within 10 days after diagnosis. Dogs that had not responded to dietary treatment alone and with an serum‐albumin concentration within the reference range (28‐35 g/L) were treated additionally with prednisolone at immunosuppressive dosages (2.2 mg/kg/day) for 10 days followed by a tapering dosage, and were defined as steroid‐responsive enteropathy dogs (SRE; 10). Nine dogs were classified as having protein‐losing enteropathy (PLE; 9) because of the presence of panhypoproteinemia with clinically relevant hypoalbuminemia (range 10‐18 g/L).
2.2. Clinical and histopathological scoring
All dogs were clinically scored using the previously described CCECAI scoring system 25 by the primary clinician. To rule out parasitic or systemic disease, all dogs underwent clinical examination, CBC, serum biochemistry, fecal flotation, and abdominal ultrasound examination.
After clinical evaluation, gastroduodenoscopy was performed under general anesthesia and 10 to 15 duodenal biopsy samples were retrieved, placed in 4% neutral buffered formaldehyde and embedded in Paraplast. The paraffin‐processed samples were cut, stained with hematoxylin and eosin and examined histologically by a single independent board‐certified pathologist using the WSAVA grading system. 3
2.3. Cell lines, treatment, and immunoblot analyses
To assess the specificity of the pSTAT3 antibody, in vitro experiments were performed using Madin‐Darby canine kidney (MDCK) cells. The MDCK cells were seeded into 6 wells in growth medium containing 10% fetal calf serum (FCS). One day before treatment with a STAT3 inhibitor (DR‐005, gift R. Moriggl), cells were switched to starvation medium containing 0.5% FCS or were further incubated in growth medium. Inhibitor was added at the indicated concentrations for 6 hours. Then, cells were lysed with radioimmunoprecipitation (RIPA) buffer with protease and phosphatase inhibitors. Samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoreseis (SDS‐PAGE) and immunoblotting was performed using a pSTAT3 specific antibody (1 : 1000 dilution; Cell Signaling Technology #9131). This antibody recognizes STAT3 only when it is phosphorylated at tyrosine residue 705. There is no cross reactivity with other STAT proteins. The antibody is predicted to cross‐react with pSTAT3 of different species including dog because of 100% sequence homology. After overnight incubation at 4°C, blots were treated with horseradish peroxidase‐coupled secondary antibodies and developed using the enhanced chemiluminescent (ECL) reagent (GE Healthcare) on a Chemidoc device (Bio Rad). After stripping, the membrane was reprobed using an antibody recognizing total STAT3 (1 : 1000 dilution; Cell Signaling Technology # 12640S) independent of its phosphorylation status (Figure 1).
FIGURE 1.
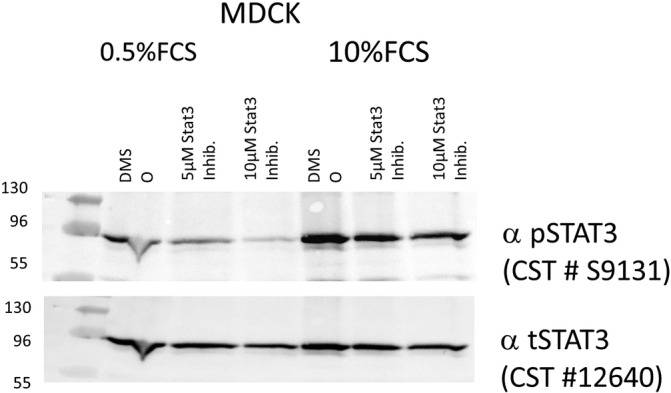
Immunoblot analysis of total signal transducer and activator of STAT3 and pYSTAT3 specific antibody in Madin‐Darby Canine Kidney (MDCK) cells under starvation and growth conditions treated with different concentrations of a STAT3 specific inhibitor to prove the specificity of STAT3 antibodies in dogs. There is no cross reactivity with other STAT proteins
2.4. Immunohistochemical staining and grading system
Immunohistochemistry was used to detect pSTAT3 in CIE patients and CO. Slides from the same formalin‐fixed and paraffin‐embedded mucosal samples as described above were deparaffinized (2× Xylene, 2× 100%, 96%, 70% ethanol) and treated with methanol/0.6% H2O2‐blocking solution to minimize false‐positive signals associated with expression of endogenous peroxidases. Heat‐induced epitope retrieval (HIER) was performed with TRIS‐EDTA buffer at pH 9 to enable binding of the primary antibody. Protein blocking was carried out for 30 minutes using normal goat serum (1.5% normal goat serum in 0.1 M phosphate buffered saline [PBS]) followed by incubation with the primary rabbit antibody anti‐pSTAT3 (Phospho‐Stat3, Tyr705; D3A7, XP Rabbit mAb #9145; Cell Signaling, Frankfurt/Main, Germany; diluted 1 : 300) overnight at 4°C. After washing, the slides were incubated with BrightVision Poly‐HRP anti‐rabbit IgG (ImmunoLogic, Duiven, Netherlands) for 60 minutes at room temperature and the signal was developed with diaminobenzidine (D5905, Sigma‐Aldrich, Vienna, Austria). Finally, cell nuclei were stained with hematoxylin, and slides were mounted with dibutylphthalate polystyrene xylene (DPX, Sigma‐Aldrich) for evaluation. The slides were examined using a Zeiss AxioCam MRc5 camera mounted onto a Zeiss AxioImager Z2 epifluorescence microscope (both Carl Zeiss, Jena, Germany). Mammary carcinoma tissue was used as a positive control and both the mammary carcinoma tissue and a duodenum biopsy sample from a healthy beagle dog were used as negative controls for nonspecific antibody. To determine whether the results were caused by nonspecific IgG binding of the primary antibody, both mammary carcinoma and randomly selected samples of CIE dogs were incubated with rabbit polyclonal IgG isotype control (online GmbH #ABIN400074, Aachen, Germany) at a concentration of 0.001 μg/μL (Figure 2).
FIGURE 2.
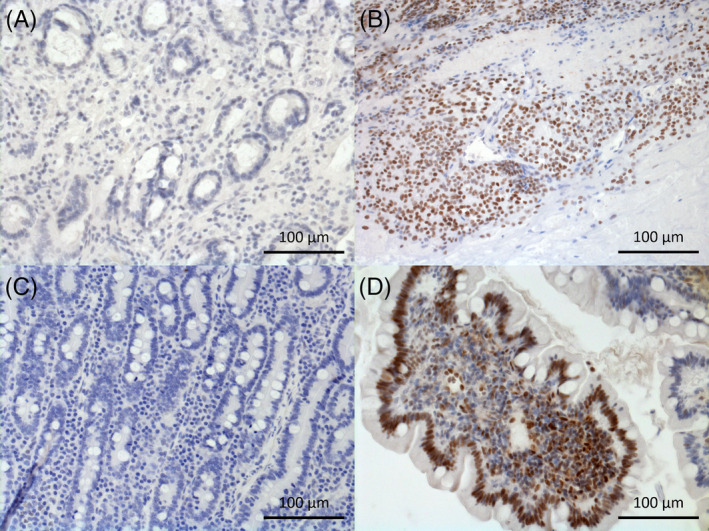
Immunohistochemical staining of phosphorylated signal transducer and activator of pSTAT3 in duodenal mucosa of, A, negative control; B, positive control (mammary carcinoma); C, control (beagle) dog crypt region; D, SRE dog villus region; cell nuclei positive for pSTAT3 are stained in brown, negative ones are stained in blue with hematoxylin
Three to 4 biopsy samples per dog were examined and imaged. Two photographs of each slide per sample were taken at 200× (20 × 10) magnification of the lamina propria and the lamina epithelialis in the crypt and villus area. The number of positive pSTAT3 cells was manually counted using the cell counter plug‐in of the software FIJI, 27 and scoring of pSTAT3 expression was applied as previously published (Table 1). 23 One of the authors was blinded to the different groups and analyzed all slides. One‐third of the slides then were double‐counted in a randomized fashion by a second person blinded to the different groups to verify the results.
TABLE 1.
Grading scale used to determine immunohistochemical positive pSTAT3 cells in the villus and crypt area
| Grade | Epithelial region | Lamina propria region |
|---|---|---|
| 0 | No staining | No staining |
| 1 | 1‐5 positive cells | 1‐50 positive cells |
| 2 | 5‐10 positive cells | 50‐100 positive cells |
| 3 | >10 positive cells | >100 positive cells |
Notes: The scoring of pSTAT3 expression was applied for the lamina propria and the epithelial regions as previously published. 23
2.5. Statistical analysis
Data was analyzed using IBM SPSSv24 (IBM Corporation, Armonk, New York) and GraphPad Prism 7.0 scientific statistic software (GraphPad Prism, GraphPad Software, Inc, San Diego, California). The assumption of normal distribution was tested using the Shapiro Wilk‐Test. Because a normal distribution of the data could not be assumed for WSAVA and CCECAI, a Kruskal‐Wallis H‐Test was applied followed by Mann‐Whitney U‐tests to analyze the differences among groups, using Bonferroni's alpha correction procedure. For all other variables (epithelia and lamina propria for villus and crypt) an analysis of covariance (ANCOVA) was applied to analyze the differences among groups controlling for age and body weight because of significant differences in age and body weight among groups. Post hoc comparisons were performed using Bonferroni's alpha correction procedure. The intraclass correlation (ICC) was used to evaluate the reliability of the pSTAT3 count between the 2 investigators. Correlations between CCECAI and WSAVA grading systems and immunohistochemistry staining were calculated using the nonparametric Spearman correlation coefficient. Statistical significance was set at P < .05.
3. RESULTS
3.1. Description of the different cohorts
Ten intact female Beagle dogs acted as CO. The age of the dogs was 1 year (mean, 1.0 ± 0 years), with weight ranging from 9 to 13 kg (mean, 10.8 ± 1.3 kg).
Twenty‐eight CIE dogs were included and grouped as follows: 9 dogs with FRE, 10 dogs with SRE and 9 dogs with PLE.
The age of the FRE dogs ranged from 0.5 to 7 years (mean, 3.3 ± 1.9 years) and body weight from 6 to 28.8 kg (mean, 18 ± 9.2 kg). For the SRE group, ages ranged from 3.5 to 10.5 years (mean, 6.2 ± 2.3 years) with body weights from 13.5 to 38 kg (mean, 24.7 ± 8.4 kg). Ages in the PLE group ranged from 2 to 11 years (mean, 6.5 ± 3.2 years) with body weights from 3.5 to 27 kg (mean, 11.6 ± 7.3 kg). Breed and sex distribution of each CIE group is outlined in Table 2.
TABLE 2.
Breed and sex distribution in the control and CIE groups
| CO | FRE | SRE | PLE | |
|---|---|---|---|---|
| Breeds: | 10 Beagles |
1 Boxer 1 CKCS 1 Cockapoo 2 Cross‐breeds 1 French Bulldog 1 Groenendael 1 Jack Russel Terrier 1 Labrador |
1 American Bulldog 1 Am. Staff. Terrier 1 Boxer 1 Cross‐breed 1 English Bullterrier 1 Galgo Español 1 German Shepard 1 Lurcher 1 Rottweiler 1 Shar Pei |
1 Beagle 1 Border Terrier 1 Cross‐breed 1 Kerry Blue Terrier 1 Papillon 1 Pug 1 Rottweiler 1 Staffordshire Bullterrier 1 Tibetan Terrier |
| Gender | 10 intact females |
2 intact females 5 neutered females 2 neutered males |
4 neutered females 1 neutered male 5 intact males |
1 intact female 5 neutered females 1 neutered male 2 males |
Abbreviations: Am. Staff. Terrier, American Staffordshire Terrier; CIE, chronic inflammatory enteropathy; CKCS, Cavalier King Charles Spaniel; CO, control group (N = 10); FRE, food responsive enteropathy (N = 9); PLE, protein losing enteropathy (N = 9); SRE, steroid responsive enteropathy (N = 10).
All groups differed significantly in age (P < .0001) and body weight (P < .001).
3.2. Clinical scoring
All dogs were clinically assessed using the CCECAI scoring system. 25 All CO dogs had a CCECAI score of 0. In the CIE groups, the CCECAI scores of the FRE group ranged from 2 to 11 (median, 4), in the SRE group from 5 to 9 (median, 7) and in the PLE group from 8 to 15 (median, 10). The CCECAI scores of each CIE group were significantly higher compared to the CO group (P < .0001; Figure 3). Significant differences in CCECAI also were detected between FRE vs SRE (P < .01), FRD vs PLE (P < .002), and SRE vs PLE (P < .001).
FIGURE 3.
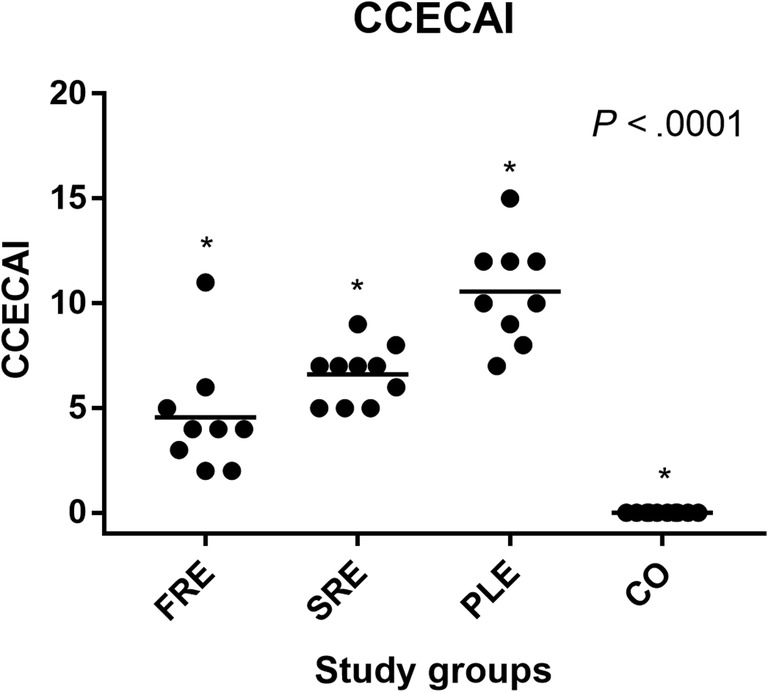
Canine chronic enteropathy clinical activity index for dogs in each study group, FRE group (n = 9); SRE group (n = 10); PLE (n = 9); and CO (n = 10). Each dot represents an individual dog score. The horizontal lines show the mean score in each study‐group. Significant differences were found between all groups of dogs. *P < .0001
3.3. Histopathologic scoring
The mucosal biopsy samples were examined using the WSAVA scoring system. 3 All control dogs (CO) were assigned a WSAVA score of 1. The WSAVA score for the FRE group ranged from 1 to 3 (median, 1.0), for the SRE group from 1 to 5 (median, 2.0), and for the PLE group from 1 to 2 (median, 1.0). All CIE dogs showed significant differences among the groups of the histological WSAVA scores compared with the CO group (P < .01, Figure 4). Significant differences for WSAVA scores within the CIE group were detected between the FRE vs SRE group (P = .01) and SRE vs PLE group (P < .03).
FIGURE 4.
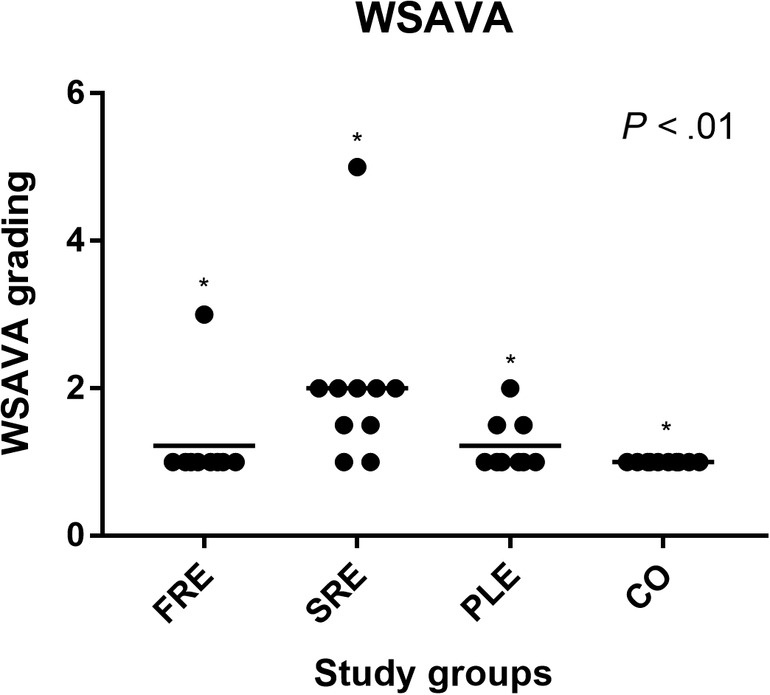
World Small Animal Veterinary Association histology scores for each study group, FRE group (n = 9); SRE group (n = 10); PLE (n = 9); and CO (n = 10). The horizontal lines represent the mean score in each study‐group. Significant differences were found between all groups of dogs. *P < .01
3.4. pSTAT3 expression in the villus area of mucosal biopsies
Phosphorylation STAT3 expression was evaluated in 3‐4 biopsy samples per dog from the CO and CIE groups and analyzed using a previously published grading scale (see Table 1).
In the villus epithelialis (VE) region of the CO group, the mean score ± SD of pSTAT3 was 0.81 ± 0.55, in the FRE group 1.55 ± 0.81, in the SRE group 2.15 ± 0.77, and in the PLE group 2.08 ± 0.61. The SRE and PLE groups had significantly higher expression of pSTAT3 (SRE, P = .001; PLE, P = .001) in the epithelium compared to the control group (Table 3). No significant differences were detected among the subtypes of CIE dogs in the VE region.
TABLE 3.
Immunohistochemical score of pSTAT3 in the villus and crypt area of CIE groups compared to CO
| Group | VE mean ± SD | P‐value | VLP mean ± SD | P‐value | CEP mean ± SD | P‐value | CLP mean ± SD | P‐value |
|---|---|---|---|---|---|---|---|---|
| CO N = 10 | 0.81 ± 0.55 | 1.08 ± 0.44 | 1.20 ± 0.64 | 1.21 ± 0.33 | ||||
| FRE N = 9 | 1.55 ± 0.81 | .01* | 1.67 ± 0.61 | .05* | 2.55 ± 0.73 | <.001*** | 1.85 ± 0.69 | .04* |
| SRE N = 10 | 2.15 ± 0.77 | <.001** | 2.08 ± 0.81 | .009** | 2.36 ± 0.65 | <.001*** | 2.15 ± 0.58 | .03* |
| PLE N = 9 | 2.08 ± 0.61 | .001** | 1.42 ± 0.31 | .37 | 2.56 ± 0.45 | <.001*** | 1.53 ± 0.38 | .79 |
Notes: Significant difference between groups *P < .05; ** P < .01; ***P < .001.
Abbreviations: CEP, crypt epithelium; CIE, chronic inflammatory enteropathy; CLP, crypt lamina propria; CO, control group; FRE, food responsive enteropathy; PLE, protein losing enteropathy; SRE, steroid responsive enteropathy; VE, villus epithelium; VLP, villus lamina propria.
In the villus lamina propria (VLP) region, the CO dogs had a mean pSTAT3 score of 1.08 ± 0.44. The CIE dogs had a mean pSTAT3 score of 1.67 ± 0.61 for the FRE group, a mean of 2.08 ± 0.81 for the SRE group and a mean of 1.42 ± 0.31 for the PLE group.
Only the SRE group showed significantly higher expression of pSTAT3 (P = .009) compared to the CO group in the VLP region. No significant differences were detected among the subtypes of CIE dogs in the VLP region.
3.5. pSTAT3 expression in the crypt area of mucosal biopsies
In the crypt epithelium (CEP), the CO dogs had a mean pSTAT3 score of 1.20 ± 0.64. For the CIE dogs, the mean pSTAT3 score was 2.55 ± 0.73 for the FRE, 2.36 ± 0.65 for the SRE and 2.56 ± 0.45 for the PLE group. Significantly higher expression of pSTAT3 was observed in the CEP in the combined CIE group compared to the CO group (FRE, P = .001; SRE, P = .001; PLE, P < .001). No significant differences were detected for pSTAT3 score in the CEP among the subtypes of the CIE groups.
The mean pSTAT3 score for the crypt lamina propria (CLP) region in the control group was 1.21 ± 0.33. The CIE dogs had a mean ± SD score of 1.85 ± 0.69 for the FRE group, 2.15 ± 0.58 for the SRE group and 1.53 ± 0.38 for the PLE group. pSTAT3 was significantly upregulated in FRE (P = .04) and SRE (P = .03) compared to the CO group.
No significant differences in pSTAT3 scores were detected in the CLP region among the subtypes of CIE dogs, except for the SRE vs PLE (P = .01) group (Table 3).
3.6. Correlation between pSTAT3 expression and clinical and histological scoring; ICC determination
A statistically significant positive correlation was found between the CCECAI and the expression of pSTAT3 in the crypt and villus regions of the lamina propria (rho = .32; P = .022; Figure 5A) and epithelium (rho = .54; P < .001; Figure 5B) of all CIE dogs.
FIGURE 5.
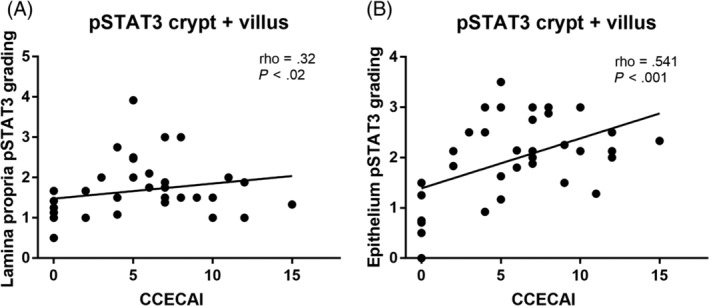
A, Spearman correlation between phosphorylated signal transducer of activation and pSTAT3 and the Canine chronic enteropathy clinical activity index for the crypt and villus lamina propria in 28 dogs with CIE. B, Spearman correlation between phosphorylated signal transducer of activation and transcription 3 (pSTAT3) and the CCECAI for the crypt and villus epithelium in 28 dogs with CIE
A significant positive correlation was detected between the WSAVA scoring and pSTAT3 expression in the villus and crypt lamina propria (rho = .31; P < .03; Figure 6).
FIGURE 6.
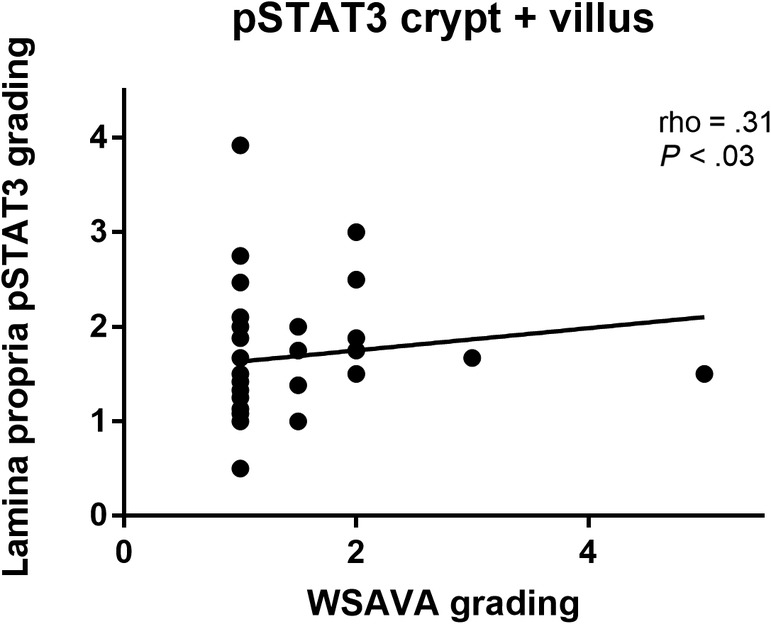
Spearman correlation between phosphorylated signal transducer of activation and pSTAT3 and WSAVA histology scores for the lamina propria in the crypt and villus area in 28 dogs with CIE
The ICC determined for the epithelial region was 0.2 and for the lamina propria region 0.7, and showed more interobserver individuality for the epithelial than for the lamina propria region.
4. DISCUSSION
Transcription 3, an important transcription factor in the JAK/STAT pathway, is activated by several cytokines and growth factors 15 , 19 with variable effect, depending on the cell types. In human patients with chronic gastrointestinal inflammatory diseases such as ulcerative colitis and Crohn's disease (CD), 15 , 28 activated STAT3 (pSTAT3) is used as a mucosal inflammatory biomarker for both acquired immune cells such as T cells 15 and innate cells such as colonic epithelial cells. 23 The role and utility of STAT3 activation (pSTAT3) in dogs with CIEs currently is unknown. Ours is the first study describing pSTAT3 expression in dogs with different CIEs. Similar to studies in humans, 15 , 28 our findings suggest that pSTAT3 could act as a potential tissue marker in dogs with CIEs and that pSTAT3 expression correlates with clinical scoring.
Response to treatment determines the division of CIE into further subgroups such as FRE, 6 antibiotic‐responsive enteropathy (ARE), 29 or SRE. 1 , 25 To date no markers are known that can be used to improve diagnosis and treatment for CIE. In addition, the histopathological findings of the different CIE subtypes have been shown to be very similar in inflammatory cellular infiltration and distribution in the different layers. 24
Using immunohistochemical analyses, we found significant activation of STAT3 in the duodenal mucosa in all CIE groups compared to CO, although the pattern of upregulation indicated differences in the significance among subgroups. There was more pronounced expression in the epithelium and lamina propria of the crypt area in the FRE group whereas in the PLE group, pSTAT3 upregulation was more dominant in the epithelium of the crypt and villus area. Only the SRE group featured pSTAT3 upregulation in both areas compared to the CO group, as well as significant upregulation in the lamina propria of the crypt region compared to the PLE group. One explanation for the significant upregulation exclusively in the villus and crypt epithelial region of the PLE group compared to CO may be a consequence of possible lymphangiectasia, inflammatory lymphatic disease, possible crypt dilatation or abscessation, which are common in PLE patients. 30 These circumstances could result in or lead to decreased cell counts.
The predominant cytokine milieu in the gut is crucial for STAT3 activation and results from T helper cell (Th) polarization, epithelial cell activation or both. 10 The consistent and significant upregulation of pSTAT3 in our study poses the question of whether STAT3 is involved in the pathogenesis of CIE. The variable pSTAT3 expression pattern in the dogs of our study could reflect different local cytokine milieus among the CIE groups compared to the CO group. Previous studies in CIE dogs failed to detect signature cytokines during chronic enteric inflammation, 31 , 32 or a Th17 polarization 33 , 34 as in CD in humans. 4 However, more recent studies in dogs have described differences in the cytokine expression profiles of intestinal epithelial cells in subgroups of dogs with CIEs 35 and show involvement of Th2 cytokines in German Shepherd dog (GSD) enteropathy. 36 Other factors such as polymorphisms in the STAT3 locus as described in humans with CD and UC 37 or single nucleotide polymorphisms in genes encoding cytokines, such as those recently detected in GSD, also could play a role but were not investigated in our study.
We speculate that the strong overall upregulation of pSTAT3 in the SRE group compared to CO dogs may be pathognomonic for the SRE group. This information may help guide treatment decisions in the future in dogs with high mucosal pSTAT3 expression after food trials. Further investigations of pSTAT3 including larger cohorts are necessary to clarify whether a predictive threshold of pSTAT3 expression exists and to allow for differentiation among the 3 subgroups of CIE.
Similar to CD in humans and UC, 22 we also found a positive correlation between pSTAT3 expression and clinical severity scoring. This finding also supports the notion that pSTAT3 is an important clinical marker for active mucosal inflammation in CIE.
Although in studies of humans pSTAT3 activation directly correlated with the histological severity of inflammation, 15 we were unable to confirm this correlation in our study. There are many sources of possible variability in histopathology scores for CIE that have been described previously, such as biopsy sample thickness, the area of the gastrointestinal tract sampled, number of samples obtained, quality of the biopsy samples or of the staining, as well as interobserver variability 38 and the age of the dogs. 39 Recently, the WSAVA working group provided a simplified histopathologic scoring index, 40 which found significant correlations between CCECAI/CIBDAI and summative histopathologic scores for the duodenum and colon. 41 Therefore, larger cohorts of dogs in each subgroup may have resulted in a significant correlation between pSTAT3 and the WSAVA scoring in our study as well. Based on studies in humans 22 indicating that active inflammatory bowel disease shows higher pSTAT3 expression than does disease in remission, it would be interesting to investigate pSTAT3 as a marker during follow‐up of CIE, because previous studies failed to correlate other tissue markers 42 and histological scoring 43 despite treatment response. 44
Our study had some limitations. One is the retrospective design of our study. Several different primary clinicians obtained the history of the dogs and assessed the clinical score. As a referral center, we advised the referral veterinarians to treat the patients for intestinal parasites before endoscopy and examine a 3‐day fecal sample for parasites 1 day before scheduling the endoscopy procedure. However, the exact deworming history was not recorded in all patients in our study. Furthermore there was a lack of history in some cases regarding other routine screening test such basal cortisol, cobalamin or cTLI (canine trypsin‐like‐immunoreactivity) concentrations. A further limitation is the fact that we did not include ileal and colonic biopsy samples of the dogs in our study. Because it previously has been shown that histopathological scoring can be significantly different between duodenum and ileum in the same dog, 45 , 46 it is possible that omitting ileal samples in our study could have affected the results. Furthermore, the immunohistochemical analysis was performed retrospectively, in a semiquantitative manner, and interobserver variability could have confounded the results. However, the primary aim of our study was to evaluate pSTAT3 involvement in the inflammatory process of CIE, which was confirmed. The CO group in our study consisted of 10 young female beagle dogs from a research colony, which therefore were not age‐, sex‐, and breed‐matched to the CIE group. Because age‐related changes in CIE previously have been reported in the literature, 39 a matched CO group would have been more representative and comparable to the patient group, but unfortunately was not available for our study.
In conclusion, we identified significant pSTAT3 upregulation in all CIE groups compared to healthy CO dogs as well as a correlation of pSTAT3 expression and the clinical score in CIE dogs. We were unable however to identify significant differences in pSTAT3 scores among the CIE subgroups. Nevertheless, as in human medicine, additional studies are warranted to compare pSTAT3 expression in CIE dogs, and in those with lymphoma 47 and colorectal cancer. 48 , 49 We therefore conclude that pSTAT3 may be useful as an additional activity marker of inflammation in dogs with CIE and may act as a possible therapeutic target in the future. Additional studies are needed to better evaluate the effect of treatment on pSTAT3 expression.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. This study was presented as an abstract at the ECVIM‐CA Congress 2016.
Manz A, Allenspach K, Kummer S, et al. Upregulation of signal transducer and activator of transcription 3 in dogs with chronic inflammatory enteropathies. J Vet Intern Med. 2021;35:1288–1296. 10.1111/jvim.16141
REFERENCES
- 1. German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17(1):8‐20. 10.1111/j.1939-1676.2003.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 2. Volkmann M, Steiner JM, Fosgate GT, Zentek J, Hartmann S, Kohn B. Chronic diarrhea in dogs—retrospective study in 136 cases. J Vet Intern Med. 2017;31(4):1043–1055. 10.1111/jvim.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Washabau RJ, Day MJ, Willard MD, et al. ACVIM consensus statement IAD. J Vet Intern Med. 2010;24:10‐26. 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 4. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91‐99. 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kathrani A, Werling D, Allenspach K. Canine breeds at high risk of developing inflammatory bowel disease in the south‐eastern UK. Vet Rec. 2011;169(24):635. 10.1136/vr.d5380. [DOI] [PubMed] [Google Scholar]
- 6. Gaschen FP, Merchant SR. Adverse food reactions in dogs and cats. Vet Clin North Am—Small Anim Pract. 2011;41(2):361‐379. 10.1016/j.cvsm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7. Suchodolski JS, Xenoulis PG, Paddock CG, Steiner JM, Jergens AE. Molecular analysis of the bacterial microbiota in duodenal biopsies from dogs with idiopathic inflammatory bowel disease. Vet Microbiol. 2010;142(3–4):394‐400. 10.1016/j.vetmic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7(12):e51907. 10.1371/journal.pone.0051907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honneffer JB, Minamoto Y, Suchodolski JS. Microbiota alterations in acute and chronic gastrointestinal inflammation of cats and dogs. World J Gastroenterol. 2014;20(44):16489‐16497. 10.3748/wjg.v20.i44.16489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allenspach K. Clinical immunology and immunopathology of the canine and feline intestine. Vet Clin North Am—Small Anim Pract. 2011;41(2):345‐360. 10.1016/j.cvsm.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 11. Jergens AE. Understanding gastrointestinal inflammation—implications for therapy. J Feline Med Surg. 2002;4(3):179‐182. 10.1053/jfms.2002.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. German AJ, Hall EJ, Day MJ, Cases C. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 2001;15:14‐25. [DOI] [PubMed] [Google Scholar]
- 13. German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 2009;15(1):14‐25. 10.1111/j.1939-1676.2001.tb02292.x. [DOI] [PubMed] [Google Scholar]
- 14. Cerquetella M, Spaterna A, Laus F, et al. Inflammatory bowel disease in the dog: differences and similarities with humans. World J Gastroenterol. 2010;16(9):1050‐1056. 10.3748/wjg.v16.i9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lovato P, Brender C, Agnholt J, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278(19):16777‐16781. 10.1074/jbc.M207999200. [DOI] [PubMed] [Google Scholar]
- 16. Pallone F, Fais S, Squarcia O, Biancone L, Pozzilli P, Boirivant M. Activation of peripheral blood and intestinal lamina propria lymphocytes in Crohn's disease. In vivo state of activation and in vitro response to stimulation as defined by the expression of early activation antigens. Gut. 1987;28(6):745‐753. 10.1136/gut.28.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101(4):1020‐1030. 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 18. Sugimoto K. Role of STAT3 in inflammatory bowel disease. World J Gastroenterol. 2008;14(33):5110‐5114. 10.3748/wjg.14.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neufert C, Pickert G, Zheng Y, et al. Activation of epithelial STAT3 regulates intestinal homeostasis. Cell Cycle. 2010;9(4):652‐655. 10.4161/cc.9.4.10615. [DOI] [PubMed] [Google Scholar]
- 20. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798‐809. 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL‐22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465‐1472. 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carey R, Jurickova I, Ballard E, et al. Activation of an IL‐6:STAT3‐dependent transcriptome in pediatric‐onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(4):446‐457. 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wick EC, LeBlanc RE, Ortega G, et al. Shift from pStat6 to pStat3 predominance is associated with inflammatory bowel disease‐associated dysplasia. Inflamm Bowel Dis. 2012;18(7):1267‐1274. 10.1002/ibd.21908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med. 2010;24(3):10–26. 10.1111/j.1939-1676.2009.0443.x. [DOI] [PubMed] [Google Scholar]
- 25. Allenspach K, Wieland B. Gröne a, Gaschen F. chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21(4):700‐708. 10.1111/j.1939-1676.2007.tb03011.x. [DOI] [PubMed] [Google Scholar]
- 26. Dandrieux JRS. Inflammatory bowel disease versus chronic enteropathy in dogs: are they one and the same? J Small Anim Pract. 2016;57(11):589–599. 10.1111/jsap.12588. [DOI] [PubMed] [Google Scholar]
- 27. Schindelin J, Arganda‐carreras I, Vasco P, et al. Fiji: an open‐source platform for biological‐ image analysis. Nat Methods. 2019;9:676‐682. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Musso A, Dentelli P, Carlino A, et al. Signal transducers and activators of transcription 3 signaling pathway. Inflamm Bowel Dis. 2005;11(2):91‐98. 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 29. Hall EJ. Antibiotic‐responsive diarrhea in small animals. Vet Clin North Am—Small Anim Pract. 2011;41(2):273‐286. 10.1016/j.cvsm.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 30. Dossin O, Lavoué R. Protein‐losing enteropathies in dogs. Vet Clin North Am—Small Anim Pract. 2011;41(2):399‐418. 10.1016/j.cvsm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 31. Yu T, Ohta H, Yokoyama N, et al. Evaluation of selected cytokine gene expression in colonic mucosa from dogs with idiopathic lymphocytic‐plasmacytic colitis. J Vet Med Sci. 2014;76(10):1407‐1410. 10.1292/jvms.13-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters IR, Helps CR, Calvert EL, Hall EJ, Day MJ. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real‐time reverse transcriptase polymerase chain reaction. J Vet Intern Med. 2005;19(5):644‐653. 10.1892/0891-6640(2005)19[644:CMQIDM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33. German AJ, Hall EJ, Kelly DF, Watson ADJ, Day MJ. An immunohistochemical study of histiocytic ulcerative colitis in boxer dogs. J Comp Pathol. 2000;122(2–3):163‐175. 10.1053/jcpa.1999.0353. [DOI] [PubMed] [Google Scholar]
- 34. Jergens Sonea IM, O'Connor AM, Kauffman LK, Grozdanic SD, Ackermann MR, Evans RB. AE. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta‐analysis with critical appraisal. Comp Med. 2009;59(2):153‐162. [PMC free article] [PubMed] [Google Scholar]
- 35. Osada H, Ogawa M, Hasegawa A, et al. Expression of epithelial cell‐derived cytokine genes in the duodenal and colonic mucosae of dogs with chronic enteropathy. J Vet Med Sci. 2017;79(2):393‐397. 10.1292/jvms.16-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kathrani A, Lezcano V, Hall EJ, et al. Interleukin‐13 and interleukin‐33 mRNA are underexpressed in the duodenal mucosa of German Shepherd dogs with chronic enteropathy. J Vet Intern Med. 2019;33(4):1660‐1668. 10.1111/jvim.15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cénit MC, Alcina A, Márquez A, et al. STAT3 locus in inflammatory bowel disease and multiple sclerosis susceptibility. Genes Immun. 2010;11(3):264‐268. 10.1038/gene.2010.10. [DOI] [PubMed] [Google Scholar]
- 38. Willard M, Mansell J. Correlating clinical activity and histopathologic assessment of gastrointestinal lesion severity: current challenges. Vet Clin North Am—Small Anim Pract. 2011;41(2):457‐463. 10.1016/j.cvsm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 39. Baum B, Meneses F, Kleinschmidt S, Nolte I, Hewicker‐Trautwein M. Age‐related histomorphologic changes in the canine gastrointestinal tract: a histologic and immunohistologic study. World J Gastroenterol. 2007;13(1):152‐157. 10.3748/wjg.v13.i1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jergens AE, Evans RB, Ackermann M, et al. Design of a simplified histopathologic model for gastrointestinal inflammation in dogs. Vet Pathol. 2014;51(5):946‐950. 10.1177/0300985813511123. [DOI] [PubMed] [Google Scholar]
- 41. Jergens AE, Schreiner CA, Frank DE, et al. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17(3):291‐297. 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 42. Luckschander N, Hall JA, Gaschen F, et al. Activation of nuclear factor‐κB in dogs with chronic enteropathies. Vet Immunol Immunopathol. 2010;133(2–4):228‐236. 10.1016/j.vetimm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 43. Okanishi H, Kabeya H, Maruyama S, Kagawa Y, Watari T. Activation of nuclear factor‐kappa B and cell adhesion molecule mRNA expression in duodenal mucosa of dogs with lymphocytic‐plasmacytic enteritis. Vet Immunol Immunopathol. 2013;154(3–4):145‐152. 10.1016/j.vetimm.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 44. Orden C, Blanco JL, Álvarez‐Pérez S, et al. Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole‐resistant strains. Anaerobe. 2017;43:78‐81. 10.1016/j.anaerobe.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 45. Willard MD, Murray JK, Hall EJ, Taylor SS, Day MJ. Comparison of histopathologic findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med. 2010;24(1):80‐83. [DOI] [PubMed] [Google Scholar]
- 46. Procoli F, Mo PF, Keyte SV, Priestnall S, Allenspach K. Comparison of histopathologic findings in duodenal and ileal endoscopic biopsies in dogs with chronic small intestinal enteropathies. J Vet Intern Med. 2013;27:268‐274. [DOI] [PubMed] [Google Scholar]
- 47. Assumpção ALFV, Jark PC, Hong CC, et al. STAT3 expression and activity are up‐regulated in diffuse large B cell lymphoma of dogs. J Vet Intern Med. 2018;32(1):361‐369. 10.1111/jvim.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dobi E, Monnien F, Kim S, et al. Impact of STAT3 phosphorylation on the clinical effectiveness of anti‐egfr‐based therapy in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2013;12(1):28‐36. 10.1016/j.clcc.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 49. Li Y, de Haar C, Chen M, et al. Disease‐related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis‐related carcinogenesis. Gut. 2010;59(2):227‐235. 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]


