Abstract
A 6‐year‐old neutered male German shepherd dog was evaluated for obtundation, blindness, and bilateral exophthalmos. A magnetic resonance imaging scan of the brain was performed and identified an arteriovenous malformation (AVM) with several feeding arterial branches, and venous drainage through the cavernous sinus. Venous vessels rostral to the AVM were severely distended and extended into the retrobulbar spaces. Liquid embolization by injection of ethylene vinyl alcohol copolymer was performed from access points in the maxillary arteries and internal carotid arteries. No intraprocedural complications were encountered, and the dog was discharged the next day. Bilateral enucleation eventually was performed because of exposure keratopathy. At 31 months post‐embolization, owners reported that the dog was doing very well clinically with high activity level and normal appetite, and the dog also appeared to be pain free. Although intracranial AVMs are very rare in companion animals, successful treatment using liquid embolization is possible and should be considered.
Keywords: angiography, interventional neurology, interventional radiology, neurovascular, vascular anomaly
Abbreviations
- AVM
arteriovenous malformation
- DMSO
dimethyl sulfoxide
- EVOH
ethylene vinyl alcohol copolymer
- MRI
magnetic resonance imaging
- UCD‐VMTH
University of California‐Davis, Veterinary Medical Teaching Hospital
1. CASE DESCRIPTION
A 6‐year‐old neutered male German shepherd dog (41.5 kg) was evaluated at the University of California‐Davis Veterinary Medical Teaching Hospital (UCD‐VMTH) for obtundation, blindness, and bilateral exophthalmos. Additionally, the dog was experiencing substantial periocular pain secondary to exposure keratitis. Before evaluation at the UCD‐VMTH, the dog had a history of exocrine pancreatic insufficiency and was eating a specialized diet (Instinct Limited Diet‐Lamb) and receiving a psyllium supplement (1 tsp PO q12h), loperamide hydrochloride (0.1 mg/kg q12h; Imodium, Johnson & Johnson, New Brunswick, NJ), and pancreatic enzyme (1.5 tsp q12h; PancrePlus Powder, VetOne, Boise, ID). Additionally, the dog was receiving the following ocular medications: timolol maleate 0.5% (3 drops OU q12h; Sandoz, Holzkirchen, Germany) and diclofenac sodium 0.1% (3 drops OU q12h; GlaxoSmithKline, Mumbai, India).
A magnetic resonance imaging (MRI) scan of the head was performed, and the following series were obtained: transverse T2, proton density, FLAIR images, sagittal and transverse time‐of‐flight, and pre‐ and post‐contrast T1‐weighted images and dorsal post‐contrast T1 images (Figures 1, 2, 3). Findings included a large, irregular semilobulated oval mass‐like structure (arteriovenous malformation or AVM) encompassing the right side of the dorsum sella and pituitary fossa, adjacent to the cavernous sinus and dorsally displacing the arterial circle. The AVM extended partly across midline into the left cavernous sinus and consisted of multiple distended tubular vessels that were hypointense on most of the imaging sequences and hyperintense on the time‐of‐flight 3D multislab series. The venous vessels rostral to the AVM were severely distended and extended into the right and left retrobulbar space pushing on the eyes, maxilla, and mandible. The basilar artery caudal to the AVM was also distended. The right internal carotid artery was distended and tortuous as it made the hairpin turn at the foramen lacerum. The pituitary gland was displaced dorsally and to the left by the AVM. The thalamus was dorsally displaced and compressed by the mass; the remainder of the brain parenchyma and ventricular system were unremarkable.
FIGURE 1.
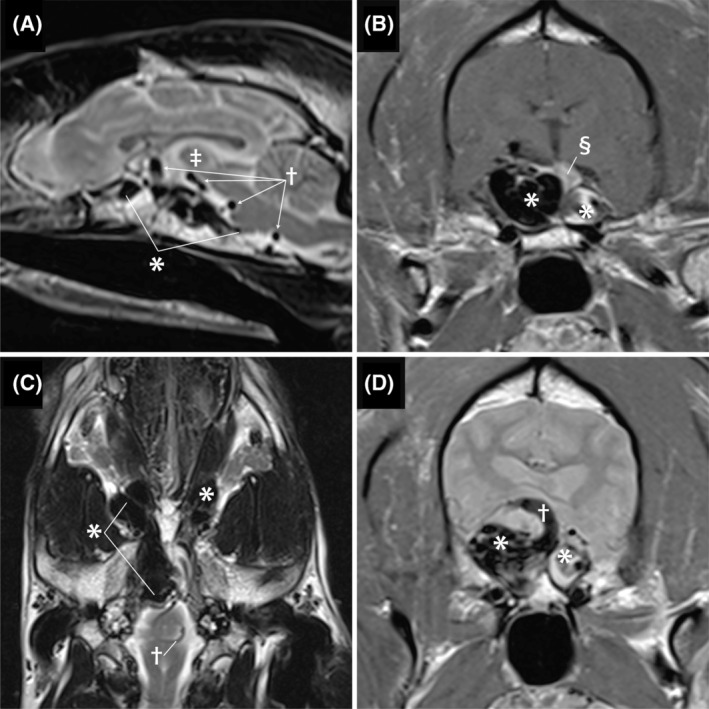
Pretreatment of magnetic resonance images. Image A is a sagittal T2‐weighted image. The complex aggregation of hypoechoic structures along the ventral calvaria (*) represents the arteriovenous malformation (AVM). The AVM is causing a mass effect that is dorsally deviating the intrathalmic adhesion (double dagger). The round structures with a signal void indicated by the cross are the engorged serpentine basilar artery. Image B is a transverse post‐contrast T1‐weighted image. The large bilobed shape of the AVM is indicated by the *. The right side is larger and represents the confluence of multiple abnormal vessels including the cavernous sinus and the left side represents distention of the contralateral cavernous sinus. The contrast enhancing pituitary indicated by the double S symbol is dorsally displaced. Image C is a dorsal plane T2‐weighted image. The hypointense structures indicated by the * represent severe dilatation of the ophthalmic plexus that is worse on the right and the cause of exophthalmos. Image D Is a transverse plane image. The bilobed mass (*) causes dorsal elevation and compression of the thalmus. The dorsally curving vessel (cross) is the basilar artery connecting with the AVM
FIGURE 2.
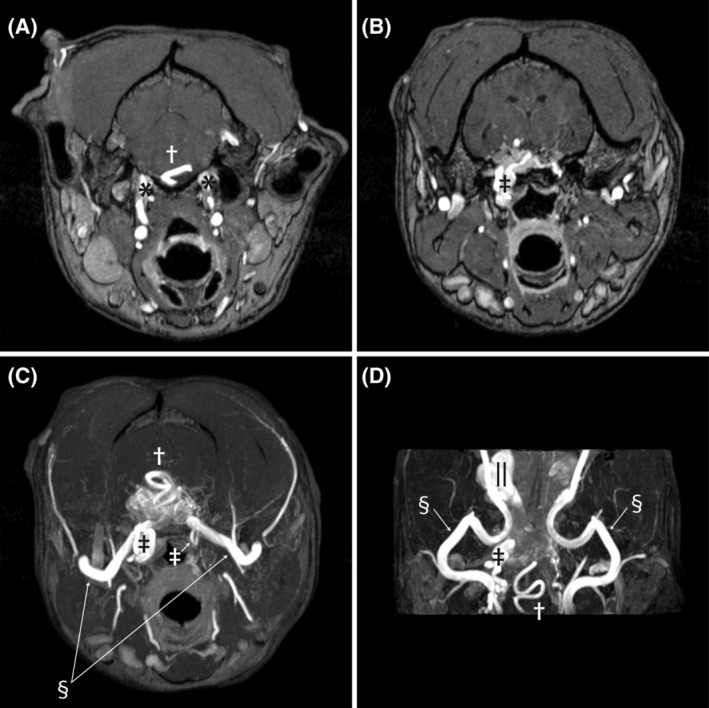
Pretreatment time‐of‐flight sequences demonstrating abnormal vasculature. Image A is at the level of the petroccipital fissure as the internal carotid arteries enter the calvaria (*). The engorged basilar artery is indicated by the cross. Image B is at the level of the foramen lacerum, where the internal carotid arteries normally exit the calvaria and make a hairpin turn and re‐enter the calvaria. The internal carotid artery on the right side is enlarged (double dagger), tortuous, and continuous with the nest of vessels comprising the AVM. Image C is a transverse maximum intensity time‐of‐flight image that shows the confluence of multiple abnormal vessels within the calvaria. The internal carotid arteries can be seen as they make the hairpin turn at the foramen lacerum (double daggers). The left is normal in size and makes a simple loop, while the right side is enlarged and tortuous. The maxillary arteries are bilaterally visible (double S) as they enter the calvaria. The basilar artery can be seen as it dives into the AVM (cross). Image D is a dorsal plane time‐of‐flight image at the base of the skull. The maxillary arteries (double S) can be seen along their length as they course from laterally toward midline where they go through the alar canals and then extend rostrally to the retrobulbar space. The enlarged right internal carotid artery is visible (cross) where it makes the hairpin turn at the foramen lacerum. The serpentine basilar artery is indicated by the cross. Finally, the dilated ophthalmic plexus on the right (||) is visible as the saccular dilatation
FIGURE 3.
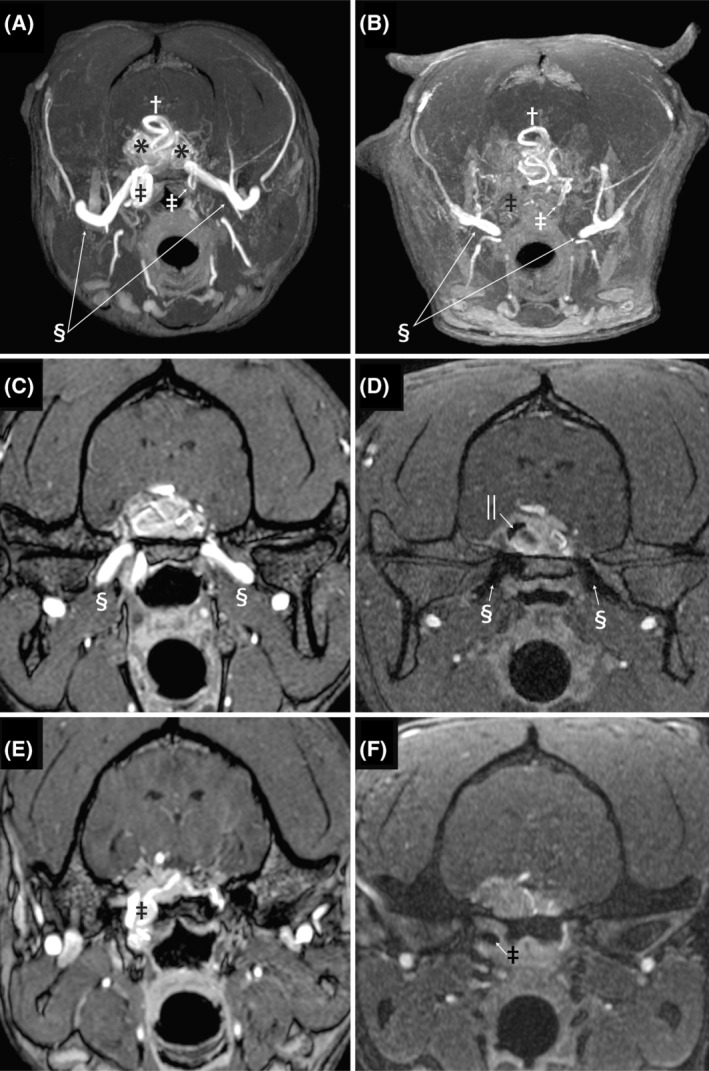
Images A and B are pre‐ and post‐embolization transverse maximum intensity time‐of‐flight images. On image A, the maxillary arteries are clearly visible indicated by the double S symbol, and after embolization (B) both the left and right maxillary arteries abruptly truncate before entering the calvaria. The previously dilated and tortuous right‐sided internal carotid artery (black double dagger) at the level of the foramen lacerum is absent in the post‐embolization image, while the small normally sized left internal carotid artery (white double dagger) can be seen to take a hairpin loop at this level on both the pre‐ and post‐embolization images. Images C and D are time‐of‐flight images at the level of the oval foramen. The left and right maxillary arteries have flow present on the pre‐embolization images as indicated by the double S symbol. After embolization, both the maxillary arteries are occluded with hypointense material. Additional hypointense embolization material is evident within the AVM (||). Images E and F are transverse time‐of‐flight images at the level of the foramen lacerum. Image E is pre‐embolization and the dilated, tortuous internal carotid artery is evident (double dagger). Image F is post‐embolization and there is hypointense embolization material and no flow is identified in the internal carotid artery (double dagger) at that level
Approximately 3 months later, the dog was evaluated at the UCD‐VMTH. The dog was obtunded and lacked a menace response in both eyes, as well as having decreased pupillary light reflexes OU. Additionally, the dog exhibited bilateral exophthalmos and was nonvisual. The owners reported that the dog's pain had been increasing progressively and was being manifested as increased lethargy, poor appetite and attempts to tuck in or hide its head.
Because of worsening clinical signs and the MRI findings, an endovascular approach to decreasing or eliminating the abnormal blood flow through the AVM was recommended. After anesthetic induction and intubation, and preparation with aseptic technique, the right femoral artery was digitally palpated through the skin, and a 1‐cm incision over the femoral artery was made. Two 3‐0 polydioxanone (PDS, Ethicon US, LLC, Bridgewater, NJ) sutures were placed around the femoral artery. An 18‐gauge over‐the‐needle catheter (Becton, Dickinson and Company, Franklin Lakes, NJ) was introduced into the femoral artery, and the needle was removed. A 0.035‐in. × 180‐cm‐long hydrophilic guidewire (Weasel Wire, Infiniti Medical, Redwood City, CA) was introduced into the catheter and subsequently into the femoral artery. A 5 Fr. vascular access sheath and dilator (Introducer Sheath and Dilator, Infiniti Medical, Redwood City, CA) were introduced into the femoral artery over the guidewire. The dilator was then removed over the guidewire, and the sheath was secured to the skin using 2‐0 nylon suture (Ethilon, Ethicon US, LLC, Bridgewater, NJ).
The guidewire was passed from the femoral artery through the external iliac artery to the abdominal aorta. The guidewire was then passed cranially into the thoracic aorta. A 4 Fr.‐angled catheter (Berenstein Catheter, Infiniti Medical, Redwood City, CA) was then placed into the vascular access sheath over the guidewire. The brachiocephalic trunk was accessed using the guidewire and angled catheter combination. The guidewire was then directed into the left common carotid artery, and digital subtraction angiography was performed using a mixture of 50% saline and 50% non‐ionic iodinated contrast medium (Isovue‐370, Bracco Diagnostics Inc., Princeton, NJ). A high‐flow AVM was diagnosed by direct angiography. Abnormal blood flow through the left internal carotid artery and a branch off of the left external carotid artery (maxillary artery) was noted, and contrast medium was immediately drained into the cavernous sinuses from those arteries after passing through an AVM nidus (Figure 4A‐D). After exiting the cavernous sinuses, contrast medium drained into the facial veins bilaterally and then into both the left and right external jugular veins (Figures 4E‐H). An arteriogram was then performed from the right common carotid artery after selection, and the angiogram results identified abnormal blood vessels branching from the right internal carotid artery and right maxillary artery. The left vertebral artery was then selected with the guidewire and angled catheter, and an angiogram was performed. An additional abnormal communication between the basilar artery and the cavernous sinuses was also noted. In summary, abnormal arteriovenous communications from the right and left internal carotid arteries, right and left maxillary arteries and basilar artery were diagnosed.
FIGURE 4.
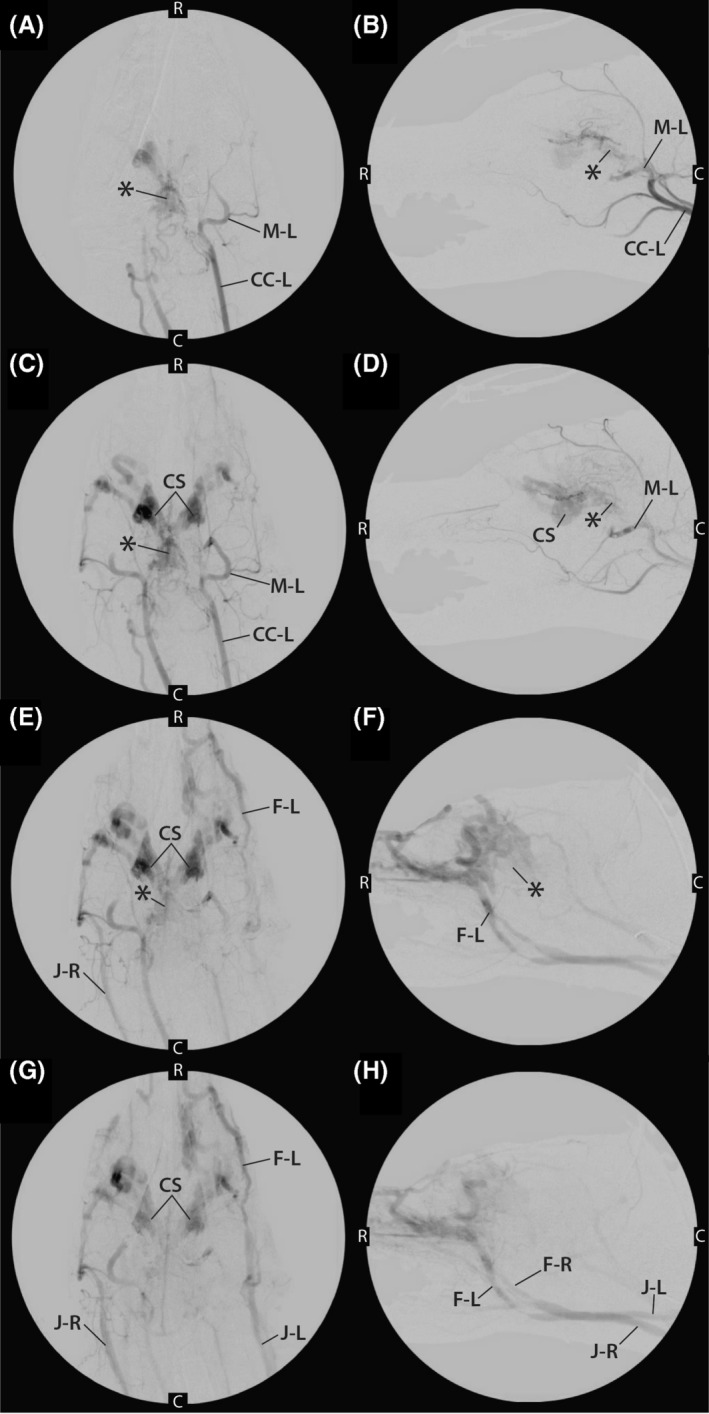
A,B, Early phase angiograms performed from injection of contrast medium into left common carotid artery (CC‐L) in both dorsal (A) and lateral (B) recumbency. During this injection, the contrast enhancement of the left maxillary artery (M‐L) and AVM nidus (*) can be visualized. C, caudal; R, rostral. C,D, mid‐phase angiogram after injection of contrast medium into left common carotid artery (CC‐L) in both dorsal (C) and lateral (D) recumbency. The left maxillary artery (M‐L) and AVM nidus (*) can still be seen as can the cavernous sinus (CS). C, caudal; R, rostral. E,F, Late‐phase angiogram after injection of contrast medium into left common carotid artery in both dorsal (E) and lateral (F) recumbency. At this point, contrast enhancement of the nidus (*) is only faintly identified, and contrast medium is clearing from the CS and draining into the left facial vein (F‐L) and right jugular vein (J‐R). C caudal; R, rostral. G,H, Late‐phase angiogram after injection of contrast medium into the left common carotid artery in both dorsal (G) and lateral (H) recumbency. Bilateral venous drainage of the contrast medium can be visualized after contrast medium has passed through the AVM. The CS, left facial vein (F‐L), right facial vein (F‐R), left external jugular vein (J‐L), and right external jugular vein (J‐R) are visualized. C caudal; R, rostral
The right common carotid artery was again selected with the angled catheter and guidewire. The guidewire and catheter were directed into the right external carotid artery. The 0.035‐in. guidewire was removed, and a dimethyl sulfoxide (DMSO)‐compatible microcatheter (Marathon, ev3, Plymouth, MN) and microwire (RUNTHROUGH, Terumo Medical Corporation, Shibuya City, Tokyo, Japan) were coaxially introduced into the angled catheter and passed into the right maxillary artery, and then further into the AVM nidus (Figure 5). Once the microcatheter was in position in the nidus, the microwire was removed and angiography (100% contrast medium) was performed to confirm appropriate location. The contrast medium was then irrigated from the microcatheter using saline.
FIGURE 5.
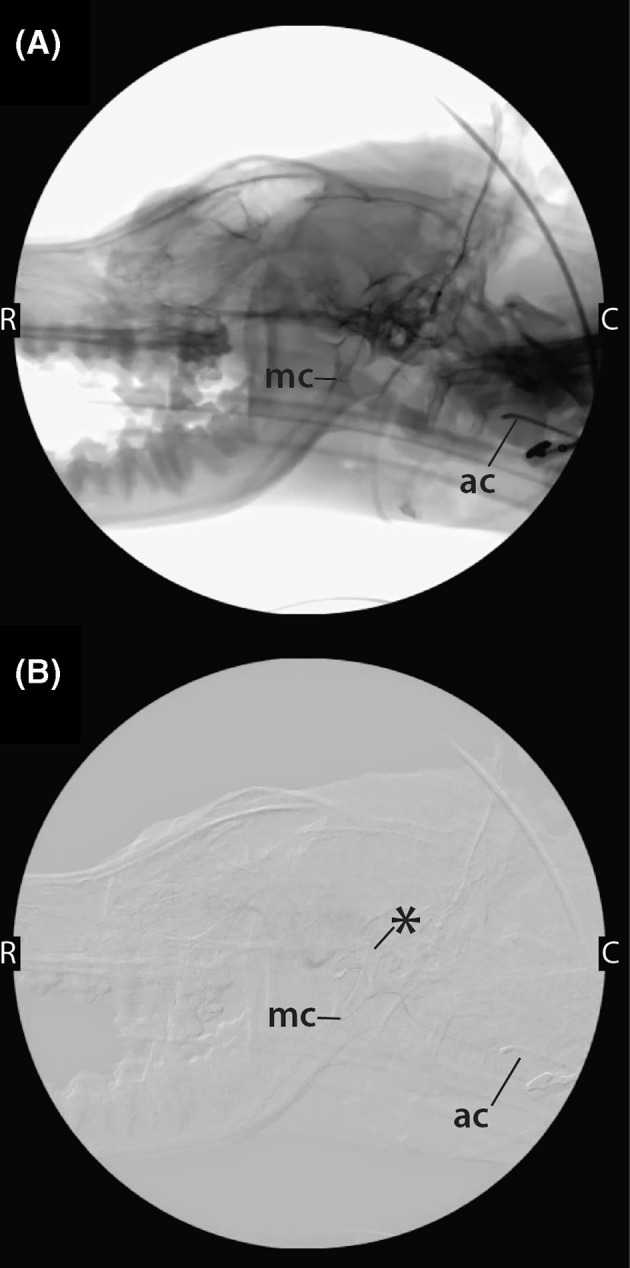
Nonsubtracted (A) and subtracted (B) fluoroscopic images obtained with the dog in lateral recumbency during placement of instrumentation for the performance of liquid embolization. The 4Fr.‐angled catheter (ac) can be visualized in the maxillary artery, and a microcatheter (mc) has been coaxially introduced into the angled catheter and passed into the AVM nidus (*). C caudal; R, rostral
Liquid embolization was performed using a commercially available nonadhesive, radio‐opaque liquid embolic agent: ethylene vinyl alcohol copolymer (EVOH) dissolved in DMSO and suspended micronized tantalum powder (Onyx, ev3, Plymouth, MN). Preparation of the EVOH‐DMSO was performed according to manufacturer recommendations. After preparation, a 1 mL syringe containing EVOH‐DMSO was immediately connected to the hub. The syringe was held vertically and the EVOH‐DMSO was injected at a slow, steady rate using thumb pressure under fluoroscopic observation. The injection was continued until the portion of the AVM that could be visualized at this site was completely occluded with EVOH‐DMSO. Once complete, mild aspiration was applied and the microcatheter was gently pulled to separate it from the embolic cast. This same procedure was performed in the abnormal branch from the left maxillary artery and abnormal branches from the left and right internal carotid arteries. Angiograms performed from the common carotid arteries after the 4 treatments indicated no blood flow through the AVM. The abnormal branch from the basilar artery was not treated because of concern for compromising blood flow to the brain after the previous treatments. The angled catheter and introducer sheath were removed and the femoral artery ligated. The SC tissue and skin were closed routinely. Total anesthesia time was 350 minutes with an embolization procedure time of 220 minutes.
The dog recovered without incident from anesthesia and was discharged the next day. Over the next week, the owners reported that the dog was experiencing a decrease in his pain level and lethargy and was more interactive. Four months after embolization, the dog was reevaluated at the UCD‐VMTH. The owners reported a substantial improvement in the swelling in the ocular region, improved mentation, and noted that the dog was no longer painful. An MRI scan was repeated. The basilar artery remained enlarged and tortuous from the occiput to the rostral extent. The area of the AVM had mixed signal intensities from flow and hypointense embolic material. The maxillary arteries were occluded bilaterally and truncated caudal to the alar canal (Figure 3). Both maxillary arteries were truncated at the level of the oval foramen and no longer extended through the orbital fissure. The previously identified severe dilatation of the ophthalmic venous plexus had improved. The diameter of the internal carotid arteries at the entrance to the petrous occipital fissure was bilaterally decreased in size compared to previously. An ovoid 4‐mm hypointense structure was identified within the right internal carotid artery at the level of the foramen lacerum and represented embolic material, and no flow in the right internal carotid artery was identified at this level. The venous distention in the retrobulbar space had decreased in size.
Over the next 6 months, the dog was reported to have improved behavior with higher activity level and more interaction. The dog was evaluated by an ophthalmologist 7 months after embolization for pain associated with the left eye, which manifested with clinical signs of blepharospasm, hyperemia and ocular discharge. An infected corneal ulcer was diagnosed, and eye lubricant (applied q8‐12 hours daily), moxifloxacin 0.5% (1 drop OS q4‐6 hours daily), and meloxicam (0.2 mg/kg PO q24h) were prescribed. Improvement was noted for approximately 4 months, at which time, the dog was re‐evaluated for conjunctivitis OU and for pawing at its eyes. Because of worsening exposure keratitis and associated pain, bilateral enucleation was recommended using a subconjunctival approach with use of a vessel‐sealing system (LigaSure, Medtronic, Minneapolis, MN) for hemostatic control and transection of the optic nerve. Bilateral enucleation was performed successfully approximately 1 month later, and the dog recovered from the procedure without incident. The dog is now 16 months post‐enucleation (31 months post‐embolization), and the owners report that the dog is doing very well clinically. It appears not to be experiencing any pain, its appetite is normal, and its activity level is high.
2. DISCUSSION
Liquid embolization delivered via an endovascular technique was performed successfully to treat a complex, high‐flow intracranial AVM in a dog. No complications related to the procedure were encountered in either the short term or long term. Dramatic improvement in clinical signs was noted post‐embolization, and MRI findings were suggestive of improved intracranial blood flow.
The arterial blood supply to the brain in dogs is delivered from 2 major sources, the basilar artery (branch of the vertebral artery) and the right and left internal carotid arteries (branches of the right and left common carotid arteries, respectively). 1 , 2 The basilar artery is the largest contributor of blood to the brain via the circulus arteriosus cerebri. The venous drainage from the cerebrum can be divided into cortical and central veins. 1 Blood from these veins passes through the sinuses of the dura mater before draining into the paired maxillary, internal jugular, and vertebral veins as well as the ventral internal vertebral venous plexuses. 1
Abnormal vascular connections between an artery and a vein are uncommonly diagnosed in companion animals. The most common location for AVMs is the liver, and both surgical and endovascular techniques have been described for treatment. 3 , 4 , 5 , 6 A naturally occurring intracranial AVM has not been reported previously in dogs, to our knowledge, but an AVM in the spinal cord and an arteriovenous fistula of the temporal branches of the external carotid artery have been reported in single case reports. 7 , 8
In the dog of our report, most clinical signs occurred likely secondary to the space‐occupying effect of the additional abnormal blood vessels of the AVM. These vessels caused compression of multiple intracranial organs, likely leading to pain, lethargy, and obtundation. Treatment with embolization resulted in improved blood flow to cerebral tissues, and owners reported improved cognitive function, activity levels, and interest in play. The ocular signs can be explained by the presence of large abnormal veins in the retrobulbar space exerting pressure on the globes and leading to exophthalmos. The exophthalmos resulted in exposure keratitis and subsequent discomfort associated with this condition. Eventually, the trauma to the corneas was severe enough that enucleation was required to eliminate the pain. In the 16 months since enucleation, the dog apparently has been pain free.
Brain AVMs in humans usually are divided into dural AVMs and cerebral AVMs. 9 Dural AVMs are thought to be acquired lesions, but the pathogenesis is not understood. 9 The concept that cerebral AVMs are congenital is controversial; the major argument against this origin is that AVMs can recur, even after complete surgical removal in humans. 10 , 11 , 12 The pathogenesis of intracranial AVMs in humans is unknown, but some studies suggest that variation in the hemodynamic forces of the flow of blood could alter cellular metabolism as well as impact epigenetic factors of the endothelial cells. 13 If epigenetic modifications of the endothelial cells occur, they may cause blood vessels that were destined to be either an artery or a vein to become an aberrant phenotype, as previously reported. 13
Treatment options for brain AVMs in humans are microsurgical resection, stereotactic radiosurgery, and embolization. Alternatively, many brain AVMs are not treated, and monitoring with medical management is elected instead. 14 The use of liquid embolization with EVOH in humans with brain AVMs has been well‐documented. 14 It provides an increased level of control during injection as well as more complete obliteration of AVMs as compared to glue embolization. 14 Additionally, the improved ease of use offered by EVOH has allowed for more types of cases to be treated endovascularly. 14
When embolization is pursued, liquid embolic agents including cyanoacrylate and ethylene vinyl alcohol generally are chosen, but particles and coils also can be considered. 5 , 15 Before the last 20 years, cyanoacrylate (particularly n‐butyl cyanoacrylate) was the embolic liquid of choice. Cyanoacrylate polymerizes upon contact with blood, and this embolic agent is challenging to use because polymerization occurs quickly and unpredictably. More recently, liquid embolic agents that are EVOH‐ and DMSO‐based have been shown to polymerize more slowly and in a more controlled manner. 16 This ability to control the distribution of the embolic agent and rate of polymerization is highly advantageous, especially when treating AVMs in highly challenging locations such as deep in the brain. In companion animals, descriptions of the use of EVOH to treat AVMs have included a peripheral AVM of a hind limb and hepatic AVMs in dogs. 5 , 15 In these reports, treatment with EVOH was well tolerated. 5 , 15
Limitations of this technique and report should be recognized. This procedure is very challenging technically, and a strong understanding of anatomy and endovascular instrumentation is essential. Furthermore, the instrumentation and materials used for embolization can be cost‐prohibitive. Complications were not encountered during the follow‐up time period for this dog, but recurrence of abnormal vascular communications or clinical signs is possible. Additionally, in this location, a nontarget embolization event could be life‐threatening or could cause long‐term neurologic or visual deficits because the feeder vessels provide the major blood supply to the brain. Finally, complete obliteration of AVMs in humans using endovascular embolization is uncommon. Similarly, the dog of this report did not have the AVM completely obliterated because of concern over compromising blood supply to the brain.
The dog of this report was successfully treated using endovascular liquid embolization. No complications were encountered, and improvement in the angiographic findings and clinical signs was achieved. Despite the extremely rare nature of this disease in companion animals, further evaluation of potential treatment options and assessment of outcome is indicated.
CONFLICT OF INTEREST DECLARATION
Some of the authors teach in laboratories where some of the equipment described in this report are utilized.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge and thank Chrisoula Toupadakis Skouritakis for assistance with the development of figures.
Culp WTN, Gratzek A, Burtch M, et al. Ethylene vinyl alcohol copolymer to treat an intracranial arteriovenous malformation in a dog. J Vet Intern Med. 2021;35:1558–1565. 10.1111/jvim.16120
REFERENCES
- 1. Bezuidenhout AJ. Heart and arteries. In: Evans HE, de Lahunta A, eds. Miller's Anatomy of the Dog. 4th ed. St. Louis, MO: Elsevier; 2013. [Google Scholar]
- 2. Tanaka T, Akiyoshi H, Mie K. Anatomical variations in the circle of Willis in canines. Anat Histol Embryol. 2018;47:609‐612. [DOI] [PubMed] [Google Scholar]
- 3. Case JB, Boston SE, Porter EP, et al. Endovascular treatment of a high‐flow hepatic arteriovenous malformation with secondary portal hypertension in a dog. J Am Vet Med Assoc. 2017;251:824‐828. [DOI] [PubMed] [Google Scholar]
- 4. Chanoit G, Kyles AE, Weisse C, et al. Surgical and interventional radiographic treatment of dogs with hepatic arteriovenous fistulae. Vet Surg. 2007;36:199‐209. [DOI] [PubMed] [Google Scholar]
- 5. Ryan SD, Nambiar A, Maingard J, et al. Endovascular embolization of canine hepatic arteriovenous malformations using precipitating hydrophobic injectable liquid (PHIL) liquid embolic agent: a proof of concept study. CVIR Endovasc. 2019;2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey MQ, Willard MD, McLoughlin MA, et al. Ultrasonographic findings associated with congenital hepatic arteriovenous fistula in three dogs. J Am Vet Med Assoc. 1988;192:1099‐1101. [PubMed] [Google Scholar]
- 7. Suter PF, Gourley IM, Rhode EA. Arteriovenous fistula of the temporal branches of the external carotid artery in a dog. J Am Vet Med Assoc. 1971;158:349‐357. [PubMed] [Google Scholar]
- 8. Cordy DR. Vascular malformations and hemangiomas of the canine spinal cord. Vet Path. 1979;16:275‐282. [DOI] [PubMed] [Google Scholar]
- 9. Davidson AS, Morgan MK. The embryologic basis for the anatomy of the cerebral vasculature related to arteriovenous malformations. J Clin Neuro. 2011;18:464‐469. [DOI] [PubMed] [Google Scholar]
- 10. Gabriel EM, Sampson JH, Wilkins RH. Recurrence of a cerebral arteriovenous malformation after surgical excision. Case Report J Neurosurgery. 1996;84:879‐882. [DOI] [PubMed] [Google Scholar]
- 11. Hashimoto N, Nozaki K. Do cerebral arteriovenous malformations recur after angiographically confirmed total extirpation? Crit Rev Neurosurg. 1999;9:141‐146. [DOI] [PubMed] [Google Scholar]
- 12. Freudenstein D, Duffner F, Ernemann U, et al. Recurrence of a cerebral arteriovenous malformation after surgical excision. Cerebrovasc Dis. 2001;11:59‐64. [DOI] [PubMed] [Google Scholar]
- 13. Thomas JM, Surendran S, Abraham M, et al. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin Epigenetics. 2016;8:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unnithan A. Overview of the current concepts in the management of arteriovenous malformations of the brain. Postgraduate Med J. 2020;96:212‐220. [DOI] [PubMed] [Google Scholar]
- 15. Culp WT, Glaiberman CB, Pollard RE, et al. Use of ethylene‐vinyl alcohol copolymer as a liquid embolic agent to treat a peripheral arteriovenous malformation in a dog. J Am Vet Med Assoc. 2014;245:216‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR. 2007;28:172‐177. [PMC free article] [PubMed] [Google Scholar]


