Abstract
Background
Nicoletella semolina was identified in the airways of horses and its low prevalence could be because of its difficult differentiation from other Pasteurellaceae.
Objectives
To develop a molecular method for the identification of N. semolina and to evaluate its prevalence in the mouth and the airways of healthy and severe asthmatic horses.
Animals
Six healthy and 6 severely asthmatic horses in phase I, 10 severely asthmatic horses in phase II, and 10 healthy horses in phase III.
Methods
Cohort (phases I and II) and cross‐sectional (phase III) studies. Quantitative polymerase chain reaction primers targeting the sodA gene were optimized. N. semolina was quantified in oral and nasal washes and in bronchoalveolar lavage fluid (BALF; phase I, sampled twice), in nasal washes and BALF (phase II, sampled twice), and in nasal washes (phase III).
Results
N. semolina was found in the nose of 5, 10, and 9 horses in phases I, II, and III, respectively (first sampling for phases I and II). Six BALF from 5 different horses were positive for N. semolina in phase II. In phase I, there was no significant difference in the nasal loads of healthy horses (median (range): 2.04 × 104 copies/mL (0‐2.44 × 105)) and asthmatic horses in exacerbation (3.75 × 102 (0‐4.84 × 106); Wilcoxon's rank sum test, P = .57).
Conclusions and Clinical Importance
N. semolina is commonly found in the airways of horses. The potential pathogenicity of N. semolina remains to be elucidated, but the molecular technique we developed will facilitate future studies.
Keywords: equine, heaves, microbiota, Pasteurellaceae, recurrent airway obstruction
Abbreviations
- BALF
bronchoalveolar lavage fluid
- CFU
colony‐forming units
- DNA
deoxyribonucleic acid
- MALDI‐TOF MS
matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry
- qPCR
quantitative polymerase chain reaction
- rRNA
ribosomal ribonucleic acid
- R5
lung resistance measured at 5 Hz
- R10
lung resistance measured at 10 Hz
- ∆PL
transpulmonary pressure
1. INTRODUCTION
Asthma is a common disease affecting approximately 15% of horses in its severe form, 1 while milder forms of the disease are likely more prevalent. 2 , 3 , 4 Although asthma is primarily considered a non‐infectious disease, bacteria could act as contributing factors by inducing an inappropriate innate response, resulting in excessive inflammation of the airways. 5 Lower airway dysbiosis in horses with severe and moderate asthma suggests that bacteria are related to inflammation. 6 , 7 However, whether dysbiosis perpetuates pulmonary inflammation or occurs secondarily to chronic allergic inflammation is unknown. 8 There is a correlation between bacteria isolated from the trachea of horses and airway inflammation 9 , 10 or the risk of respiratory disease. 11 It remains uncertain if nonspecific bacterial molecular patterns, such as lipopolysaccharides, 12 or specific virulence factors of pathogenic or opportunistic bacteria are a cause, but the presence of certain bacteria in the lower airways has been associated with asthma in horses. These bacteria include Streptococcus equi subsp. zooepidemicus, 6 , 9 , 10 , 13 , 14 S. pneumoniae, 9 , 10 , 14 and bacteria from the Pasteurellaceae family, including Pasteurella spp. and Actinobacillus spp. 9 , 10 , 14 , 15
Recently, a new Pasteurellaceae, Nicoletella semolina, was isolated from horses with and without respiratory diseases. 16 , 17 It is associated with tracheal inflammation 18 and is found in large amounts in horses with respiratory diseases. 17 This suggests that N. semolina could be, as are other Pasteurellaceae, an opportunistic pathogen, 19 and could occasionally act as a contributing factor to exacerbations of asthma in horses. Elucidating N. semolina's role using standard culture is complicated by its fastidious growth and difficult differentiation from other Pasteurellaceae. 16 The development of a molecular method will improve our ability to detect its presence and will help determine its role in the airways of horses. The objectives of the study were (a) to develop a molecular method for the identification of N. semolina and (b) to evaluate its prevalence in the mouth and the airways of healthy and severe asthmatic horses kept in different environments. The hypothesis was that N. semolina is more commonly detected in horses with asthma, especially during exacerbation.
2. METHODS
2.1. Study design
The study was conducted in 3 phases, the first two with research horses and the third one in a private barn. Samples from the Equine Respiratory Tissue Biobank (http://www.ertb.ca/media/html/en_biobank.html) were used in phase I. Briefly, oral and nasal washes and bronchoalveolar lavage fluid (BALF) from 6 controls and 6 severe asthmatic horses, stored at −80°C from a previous project, were analyzed. 7 Samples had been collected when horses were on grass pasture (low antigen exposure) and after being housed indoors and fed poor quality hay (high antigen exposure) for 3 weeks. In phase II, BALF and nasal washes were collected from 10 severe asthmatic horses (different from phase I) when experiencing exacerbation upon being fed poor quality hay (high antigen exposure), and after 6 weeks of being fed either hay soaked for 45 minutes (n = 5) or alfalfa pellets (n = 5) in order to achieve clinical remission (low antigen exposure). Other than having only asthma‐affected horses, phase II differs from phase I by having horses in remission kept indoors, instead of on pasture. In phase III, nasal washes were collected from 10 client‐owned, clinically healthy horses living in the barn of the initial case from which N. semolina was isolated (unrelated to the research facility). All experimental procedures were performed in accordance with the Canadian Council for Animal Care guidelines and were approved by the animal care committee of the Université de Montréal (15Rech1760, 19Rech1995, and 20Rech2082).
2.2. Sample collection and DNA extraction
In phase I, oral, nasal, and BALF samples were obtained as previously described. 7 Briefly, oral and nasal cavities were rinsed using 50 mL of sterile 0.9% saline collected by gravity in a sterile tube, and BALF was collected by passing a 2.5 m videoendoscope through a protective sheath, and by instilling and aspirating 2 boluses of 250 mL of sterile 0.9% saline. In phase II, BALF samples and nasal washes were collected as in phase I, with the exception that a 1.6 m videoendoscope without a protective sheath was used for BALF collection (Olympus, GIF‐H180, Olympus Canada Inc., Richmond Hill, ON, Canada). In phase III, nasal washes were collected without sedation, using a plastic bag around the nose to avoid spills. All samples were kept on ice until freezing at ‐ 80°C within 3 hours of collection.
In phase I, 2 to 5 mL (based on availability) of oral and nasal washes and of BALF samples were used for DNA extraction. Oral washes were filtered (sterile 4‐ply gauze) to eliminate large feed particles. In phase II, 5 mL of nasal wash and 20 mL of BALF were used to increase chances of detection in BALF. In phase III, 5 mL of nasal wash were used for extraction. DNA was initially pelleted by centrifuging samples at 18 400g over 30 minutes, at 4°C. DNA extraction was performed using DNeasy Blood and Tissue kit (Qiagen, Toronto, ON, Canada) following the manufacturer's protocol, with minor modifications to optimize extraction of low biomass samples.
2.3. Initial N. semolina strain
The initial N. semolina strain was obtained from a 2‐month‐old Standardbred colt presented to the Equine Hospital of the Université de Montréal with pneumonia. Culture of the tracheal aspirate and tentative matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) identification of the bacteria present showed a few colonies of Rhodococcus equi and heavy growth (>10 000 CFU/mL) of an unidentified Pasteurellaceae strain. DNA was extracted as described earlier. Complete 16S ribosomal RNA gene (V1‐V9, 1 465 base pairs) was sequenced by Génome Québec (GénomeQuébec Inc., Montreal, QC, Canada). Primers 27F: 5′‐AGAGTTTGATCCTGGCTCAG‐3′ and 1492R: 5′‐GGTTACCTTGTTACGACTT‐3′ were used for amplification and sequencing. Sequencing results were run in Nucleotide BLAST (standard databases) and confirmed the identification of the unknown Pasteurellaceae strain as N. semolina, with 99.85% homology with the 16S ribosomal RNA‐type strain (CCUG43639 16 ), with a 2‐base‐pair difference.
N. semolina‐confirmed strain's spectra were added to the MALDI‐TOF database of the bacteriology diagnostic laboratory of the Complexe de diagnostic et d'épidémiosurveillance vétérinaires du Québec (CDEVQ) of the Université de Montréal. Briefly, MALDI‐TOF technology was used on a Microflex LT/SH mass spectrometer (Bruker Daltonics, Milton, ON, Canada). Generated spectra were compared with those of the RUO Biotyper database (Bruker Daltonics, Milton, ON, Canada) and with a homemade database from the bacteriology diagnostic laboratory, then including the N. semolina spectra of the initially sequenced strain.
2.4. Quantitative PCR
Primers were designed using Geneious Prime software v.2019.2.1 (Biomatters Ltd., San Diego, California), based on available NCBI nucleotide sequences. The primer set amplifies N. semolina, targeting a 130 base‐pair amplicon in the sodA gene (based on DSM16380). Forward primer sodAF 5′‐CAGCGATTGGTCGATTTGGCTCT‐3′ and reverse primer sodAR 5′‐GGATAACCAGACACACCTGCAA‐3′ were further optimized. PCR product was sequenced to confirm gene identification. Primers were tested with 8 other bacteria from the Pasteurellaceae family, 3 other Gram‐negative bacteria, and 1 Gram‐positive bacteria often found in equine airways (S. equi subsp. zooepidemicus). There was no DNA amplification, confirming the high specificity of these primers for N. semolina. Five other isolates of N. semolina identified in the following months using the annotated MALDI‐TOF were tested with the primers and led to DNA amplification in all cases, confirming that our primers could detect more than 1 isolate of N. semolina. A standard curve was made using serial dilutions. An efficiency of 89.63% and R 2 of 0.99 or more were considered adequate. 20
Quantitative PCR (qPCR) was carried out using Rotor‐Gene 3000 (Corbett Research, Mortlake, Australia) with QuantiTect SYBR Green PCR Kit (Qiagen, Toronto, ON, Canada), following the manufacturer's recommendations. Briefly, 2.0 μL of extracted DNA, 0.5 μM of forward and reverse primers (Invitrogen, Thermo Fisher Scientific, Illinois), 10 μL of QuantiTect SYBR Green, and 4 μL of sterile molecular grade water (Wisent, Saint‐Bruno, QC, Canada) were used for a total of 20 μL per reaction. Every run had an initial hold period of 15 minutes at 95°C, followed by 40 cycles of 15 seconds at 94°C, 30 seconds at 59°C, and 30 seconds at 72°C, ending with a melt from 72°C to 95°C. In each qPCR run, all samples were run in duplicates, with inclusion of a water mock and 3 samples of known concentration from the standard curve. When only 1 duplicate was positive, it was run again, and a sample was considered positive if at least 2 runs were positive, and mean value was used for analysis. The standard curve was imported within each run and quantification was reported in gene copies per mL of sample. Melt curves were analyzed to remove nonspecific amplifications.
2.5. Statistical analysis
Statistical analysis and figures were carried out using SAS v9.4 (Cary, North Carolina) and GraphPad Prism software v8.3.0 (San Diego, California). N. semolina loads among horses were not normally distributed. In phase I and BALF samples of phase II, Wilcoxon's signed‐rank tests were used to compare loads as a function of antigen exposure in each group, and Wilcoxon's rank‐sum tests were used to compare loads as a function of groups for each antigen exposure. When groups did not differ statistically, horses were pooled and a Wilcoxon's signed‐rank test was used to compare loads between environments (phase I) or between asthma status (phase II, lung only). For nasal samples of phase II and BALF recovery volumes, a linear mixed model on log‐transformed data was used, with horse identification as the random factor and group and diet as fixed factors. The number of positive horses between level of antigen exposure (pasture and hay in phase I, hay and pellets/soaked hay in phase II) was compared with McNemar's test. Spearman's correlations were used to test the association between BALF neutrophil percentages, lung resistance at 5 Hz over resistance at 10 Hz (R5 to R10 ratio), and N. semolina loads in phase I, between loads and BALF recovery volume and lung function (resistance, elastance, and transpulmonary pressure [∆PL]) in phase II, and between nasal loads and age for all 3 phases. For the correlations with age, only loads of horses housed indoors and eating hay were used for phases I and II. P values of less than .05 were considered statistically significant.
3. RESULTS
3.1. Horses, lung function, and BALF cytology
Lung functions and bronchoalveolar lavage cytology from phase I were previously reported. 7 Horses with asthma developed airway obstruction and severe bronchoalveolar lavage neutrophilia with hay feeding and indoor environment, while controls did not. In phase II, horses were all in exacerbation at baseline, and were in remission or close to remission after 6 weeks of low‐antigen exposure (Westerfeld et al., Effects of soaked hay on lung function and inflammation in horses with severe asthma, Veterinary Comparative Respiratory Society, December 2020). Horses from phase III were deemed healthy based on physical examination and history.
3.2. Phase I: control and asthmatic horses
PCR quantification revealed the presence of N. semolina in 10 oral and 13 nasal samples from 8 different horses (Figure 1A,B). All BALF samples were negative for N. semolina (Figure 1C). Oral and nasal loads were not significantly different between control and asthmatic horses in either environment (oral: P ≥ .46; nasal: P ≥ .57) or between environments in either group (oral: P ≥ .25; nasal: P = .13). Pooling horses from the 2 groups, there was significantly more N. semolina in the nose of horses kept indoors on hay rather than on pasture (P = .008), but not in their mouth (P = .30). When horses were on pasture, 3 oral and 5 nasal washes were positive for N. semolina, while 7 oral and 8 nasal washes were positive when horses were indoors (25 to 66% positive samples overall), with no significant difference between environments in either group (P ≥ .13). All horses positive in the mouth were also positive in the nose and all but 1 horse had higher loads (per mL recovered) in their nose than in their mouth. There was no significant correlation between BALF neutrophils percentage or lung function (R5 to R10 ratio) and N. semolina load in the mouth (P ≥ .26) nor in the nose (P ≥ .16), on pasture and with hay exposure. There was no correlation between age and nasal loads of horses on hay (P = .51).
FIGURE 1.
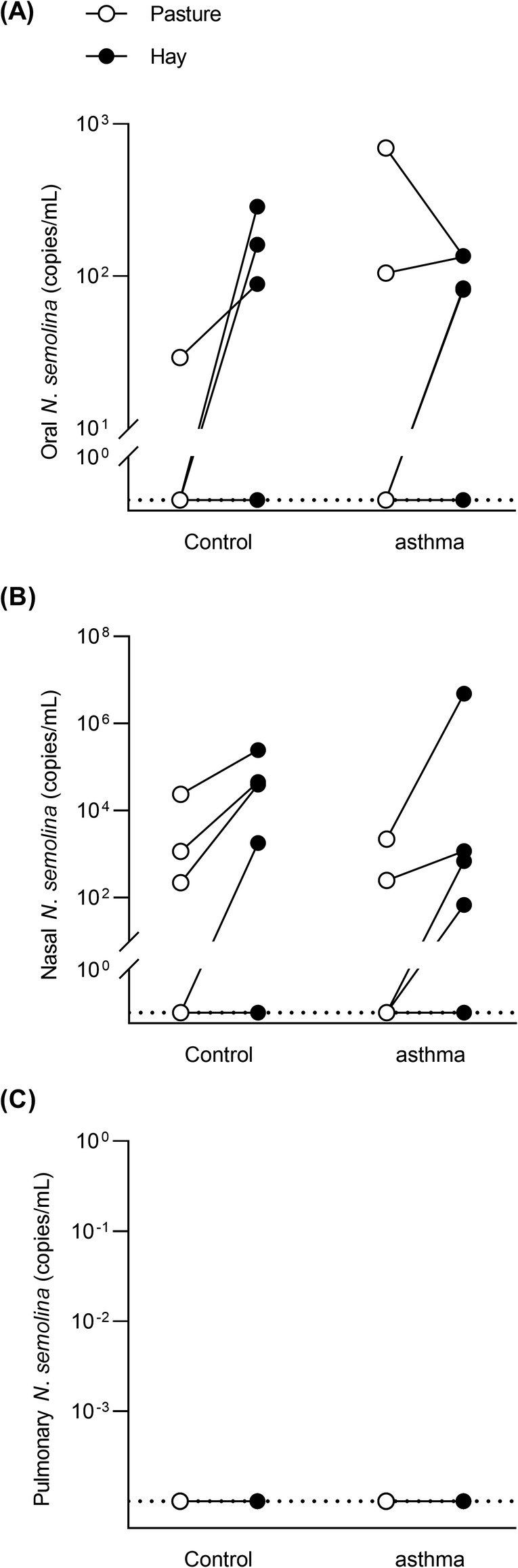
Nicoletella semolina loads in phase I. N. semolina oral (A), nasal (B), and pulmonary (C) loads in gene copies per mL extracted from healthy (n = 6) and asthmatic horses (n = 6) housed on pasture (open circles) and indoors and fed hay (solid circles). There was significantly more N. semolina in the nose of horses on hay (Wilcoxon's signed‐rank test on pooled data; P = .008). No significant difference between groups or environments in the mouth and lungs (P ≥ .25)
3.3. Phase II: asthmatic horses
Nicoletella semolina was detected in all but 1 nasal samples from phase II (9/10 in remission and 10/10 in exacerbation, 95% to 100% positive samples) and in 6 BALF samples (5/10 in remission and 1/10 in exacerbation; Figure 2). The linear mixed model showed no significant effect of asthma status (remission/exacerbation; P = .42), type of diet (P = .33), or interaction between these factors (P = .92) on nasal loads. Pulmonary loads were not different between antigen exposures in either diet group (P ≥ .42), nor between diets in either antigen exposure (P ≥ .25). When horses from both diet groups were pooled, pulmonary loads were not significantly higher in horses in remission (P = .06). Similarly, the percentage of N. semolina‐positive horses in the lungs was not significantly different between remission (5/10, 50%) and exacerbation (1/10, 10%; P = .13). There was no significant correlation between nasal and pulmonary loads (P ≥ .21), and between pulmonary loads and lung function when horses were in exacerbation (P = .86) and in remission (P ≥ .31). In addition, BALF recovery volume did not correlate with lung function (P > .06) and was not different between diet groups or antigen exposure (P ≥ .90). There was no correlation between age and nasal loads of horses in exacerbation (P = .47).
FIGURE 2.
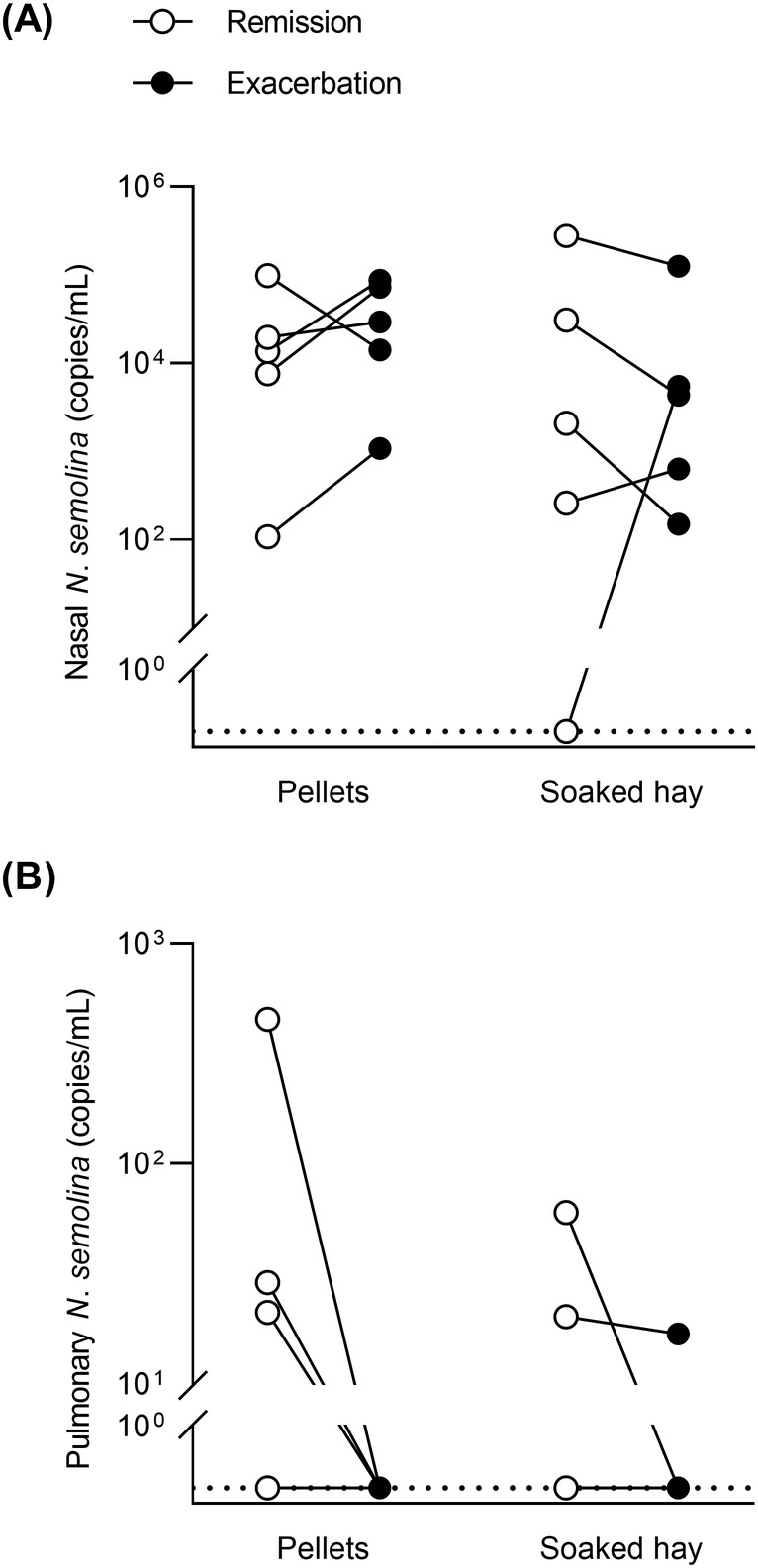
Nicoletella semolina loads in phase II. N semolina nasal (A) and pulmonary (B) loads in gene copies per mL extracted from asthmatic horses fed either pellets (n = 5) or soaked hay (n = 5) during remission (open circles) and exacerbation of asthma (solid circles). No significant effect of feed or asthma status on log‐transformed nasal loads (linear mixed model; P ≥ .33) and no significant difference between diet or asthma status in the lungs (Wilcoxon's signed‐rank tests on pooled data; P > .06)
3.4. Phase III: healthy horses from a different barn
Ten horses, ranging from 6 months to 12 years of age, were sampled in the Fall of 2020, 1 year after the foal with pneumonia had returned home. A total of approximately 20 horses and foals lived on the facility. Not all horses had direct contact with one another, but they shared paddocks, the arena, and the wash stall. N. semolina was identified in the nose of all but 1 horse (9/10 horses). The 4 yearlings who had lived in the same paddock for many months showed the highest loads of N. semolina in their nasal cavities, along with a 6‐month‐old foal. A significant negative correlation was observed between nasal loads and age (r = − .68, P = .04; Figure 3). The foal with pneumonia, who had also been tested at 4 months of age and was now a healthy yearling, remained positive. Its dam, who had also been positive previously, was the only horse in which the bacteria was not detected.
FIGURE 3.
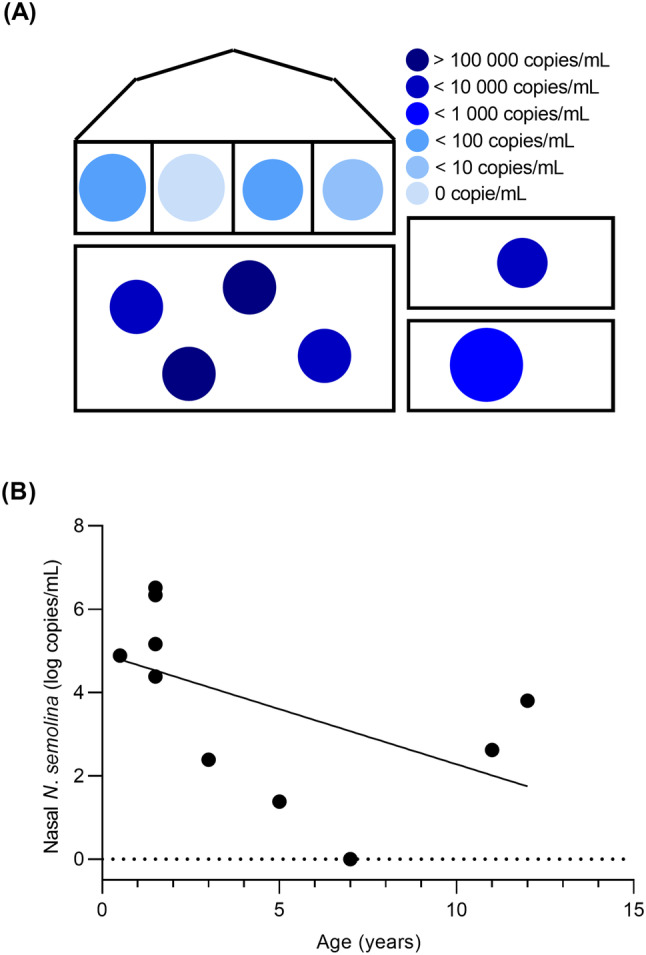
Nicoletella semolina in phase III. Layout showing the horses in the private barn where 10 healthy horses were sampled in the nose (A). Each circle represents a horse; younger horses are represented by smaller circles and darker shades represent horses with higher loads of N. semolina. There is a significant negative correlation between nasal loads and age (Spearman's correlation; r = − .68, P = .04; B)
4. DISCUSSION
This report describes a culture‐independent method for the detection of N. semolina in the airways of horses. There are few reports of cases in North America 21 and a 2012 study included two strains originating from Canada. 22 N. semolina could be an emerging bacterium, but further research would be needed to support this and for now, we hypothesize that N. semolina's fastidious growth and identification prevented accurate identification in the past. Overall, in our study sample of horses in close contact with one another, N. semolina was found in 25% to 58% of oral samples, 41% to 100% of nasal samples, and in up to 30% of BALF samples. N. semolina was also added to the MALDI‐TOF database of the CDEVQ of the Université de Montréal.
4.1. Higher prevalence than previously reported
Using culture, N. semolina is found in the nose of up to 6% (12/207) of healthy horses, 17 and in the trachea of between 1.8% (19/1054) and 5% (49/921) of horses with respiratory diseases. 17 , 18 For comparison, 41% (5/12) and 100% (10/10) of the 22 different horses sampled in phases I and II, respectively, were positive for N. semolina in their nose on their first sampling, with 18 horses having at least 1 positive sample (mouth, nose, or lungs) on at least 1 occasion. N. semolina was also identified in 9 of the 10 horses sampled at an unrelated barn. The use of a molecular method increases the chances of detecting the presence of bacteria compared to culture 23 and could explain, in part, the high prevalence obtained in these 3 samples of horses. Approximately 68% of tracheal aspirates of horses yield growth in standard aerobic culture, whereas all samples are positive for 16S rRNA gene quantification. 24 Similar results are obtained when analyzing nasopharyngeal and sputum samples from humans with bronchial asthma 25 or with pneumonia. 26 The increased sensitivity of molecular methods compared to culture is further enhanced by the fact that Pasteurellaceae show limited survival outside their hosts. 27 In addition to its fastidious growth, 16 N. semolina identification using traditional phenotypical and biochemical characterization is not always possible given the close similarities between Pasteurellaceae. 16 , 27 , 28 It is therefore possible that studies relying on culture for the detection of N. semolina underestimate its prevalence in horses. However, in the current study, samples were not analyzed using standard bacterial culture for a direct comparison between techniques and the high prevalence could be at least in part the result of sampling horses living in the same environment rather than sampling a large number of unrelated horses from different barns, as in previous studies. 17 , 18 In phase III, horses also shared the same environment and all but 1 harbored the bacteria in their nose. In humans, the skin, and to a lesser extent, the oral and gut, microbiota are more similar in individuals living within the same household than between households. 29 This remains true for the skin of genetically unrelated roommates 30 and the fecal microbiota of spouses 31 and of monozygotic twins. 32 It is therefore possible that horses housed together harbor a similar microbiota. The apparent higher prevalence in phase II, where almost all horses harbored N. semolina in their nose, could result from decreased ventilation in the barn as the experiment was carried out during wintertime, which could increase concentration of airborne particles. 33 , 34 , 35 Thus, our results raise the possibility that cohabitation plays a role in N. semolina's carrier status.
4.2. Effect of housing
In phase I, there were significantly more N. semolina in the nose of horses housed inside, and in phase II, when all horses had been indoors for more than a month, 19 out of 20 nasal samples were positive. We hypothesized that indoors housing could induce mucosal inflammation that could favor the growth of N. semolina. Whether nasal mucosa inflammation is present in horses housed indoors remains speculative, but horses with and without asthma develop airway inflammation when housed indoors, at least transiently. 12 , 36 , 37 , 38 Alternatively, hay or pellets could act as a source of bacteria and create a microenvironment inside the barn increasing exposition to airborne particles. If that was the case, we could have seen higher loads in asthmatic horses fed soaked hay, as overall bacterial content tend to increase with soaking, 39 , 40 , 41 but levels were similar whether horses were fed dry hay, soaked hay, or pellets. This points back toward an effect of being housed indoors per se, where horses are more exposed to suspended aerosols and particles, including bacteria that tend to remain suspended in the air for long periods of time. 39 , 42
4.3. Nicoletella semolina in BALF
Initial conclusion from phase I was that there was no N. semolina in the horses' lungs. DNA extraction from a larger BALF volume (20 mL compared to 5 mL) in phase II resulted in the detection of N. semolina in 6 BAL from 5 different horses. Because we did not use a protective sheath in phase II, it is however difficult to determine if N. semolina is a true inhabitant of the lungs or if it was carried over from the upper airways. Lower airway sampling can bring contamination from the upper airways 43 , 44 and a protective sheath can be used to minimize such contamination. 43 However, there was no correlation between nasal and BALF loads and studies in humans show that carry over from the upper airways with unprotected bronchoscopes plays a minimal role in the communities observed in BALF samples. 8 , 45 , 46
Five out of 10 horses in remission in phase II were positive for N. semolina in their lungs as opposed to only 1 when in exacerbation (not statistically significant). Higher BALF recovery volume from decreased fluid trapping with reduced airway obstruction 47 , 48 was explored as a potential explanation, but BALF recovery volume was not different between remission and exacerbation and it was not correlated to lung function. It would have been interesting to quantify N. semolina pulmonary loads in the healthy horses from phase III using the 20 mL volume from phase II and the protective sheath from phase I in order to help understand its significance when detected in horses' lungs. Unfortunately, horses from phase III were client‐owned horses and only minimally invasive procedures could be performed.
4.4. Potential role of N. semolina in the airways
Whether N. semolina is an emerging pathogen, an opportunistic organism or simply is part of the normal microbiota of horses remains unclear, but it does not seem to act as a primary pathogen. It is suggested that infection by N. semolina should be considered in the presence of respiratory signs 16 or tracheal inflammation. 18 However, its isolation alongside S. equi subsp. zooepidemicus and other bacteria 17 , 18 suggests that it might not have been the only driver of inflammation in those cases. Results from phases I and III, as well as a previous report, 17 show that N. semolina can be found in the nose and the mouth of healthy horses and support the hypothesis that this bacteria is a normal inhabitant of respiratory mucosal surfaces of horses, with the capacity of overgrowing when conditions are propitious. 17 , 21 Nevertheless, the negative correlation between nasal loads and age in phase III raises the question whether the development of immunity with age contributes to lower loads as horses get older, as observed with other opportunistic bacteria. 10 , 11 This finding is further supported by the fact that clinical disease in N. semolina‐positive horses is mainly reported in younger horses. 18 , 21 Correlation with age was not observed in phases I and II, where only mature horses were sampled.
The contribution of bacteria in the development and perpetuation of inflammation in asthma is currently unknown. 4 , 8 Albeit no clear diagnosis was established in most cases, horses positive for N. semolina by culture mainly present with cough and nasal discharge. 16 , 18 Pasteurellaceae are associated with airway inflammation, 9 , 10 , 14 , 15 and it is possible that some of these Pasteurella/Actinobacillus‐identified bacteria are in fact N. semolina. Here, we were unable to show an association between N. semolina and severe asthma in horses, but we developed a tool to look at a possible association with mild to moderate asthma on a larger scale.
4.5. Limitations
The main limitation resides in the small number of convenience samples used for both initial phases of the project. This, along with the absence of preliminary data on the detection of N. semolina by qPCR, limited the use of sample size calculation. A larger number of horses would probably have been necessary to detect differences in the percentage of N. semolina‐positive horses between environments or asthma status, should such differences exist. Sampling of feed, pasture, water buckets, ambient air inside the barn, and around a horse's breathing zone could have brought more information in the presence of N. semolina in the environment, even if expected to be minimal. 27 Finally, direct comparison between culture and qPCR on the same samples would have helped comparing our results with previous literature.
In conclusion, N. semolina is highly prevalent in our colony of research horses with and without asthma, and in an unrelated study sample of healthy horses living in a different barn. In both cases however, high prevalence could be due in part to the proximity of these horses living together. We could not show an association between N. semolina and severe asthma, but it is found in higher loads in the nose of horses kept indoors. The potential pathogenicity of N. semolina remains to be elucidated, but the molecular techniques we developed (qPCR and in‐house MALDI‐TOF database annotation) will greatly facilitate future studies in a randomly selected sample of horses.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
All animal manipulations were performed in accordance with the guidelines of the Canadian Council for Animal Care, and the protocol was approved by the Animal Care and Use Committee of the University of Montreal (15Rech1760, 19Rech1995, and 20Rech2082).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study
ACKNOWLEDGMENT
Funding was provided by the Natural Sciences and Engineering Research Council of Canada (06090). The funding source did not have any involvement in the study design, data analysis and interpretation, or writing and publication of the manuscript. The authors acknowledge Marie‐Lou Gauthier and Valérie Dubuc their contribution, as well as Ms Dominique Michel for giving us access to her horses.
Payette F, Charlebois A, Fairbrother J‐H, Beauchamp G, Leclere M. Nicoletella semolina in the airways of healthy horses and horses with severe asthma. J Vet Intern Med. 2021;35:1612–1619. 10.1111/jvim.16140
Funding information Natural Sciences and Engineering Research Council of Canada, Grant/Award Number: 06090
REFERENCES
- 1. Hotchkiss JW, Reid SW, Christley RM. A survey of horse owners in Great Britain regarding horses in their care. Part 2: risk factors for recurrent airway obstruction. Equine Vet J. 2007;39:301‐308. [DOI] [PubMed] [Google Scholar]
- 2. Ivester KM, Couetil LL, Moore GE. An observational study of environmental exposures, airway cytology, and performance in racing thoroughbreds. J Vet Intern Med. 2018;32:1754‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen KJ, Tremaine WH, Franklin SH. Prevalence of inflammatory airway disease in national hunt horses referred for investigation of poor athletic performance. Equine Vet J Suppl. 2006;38:529‐534. [DOI] [PubMed] [Google Scholar]
- 4. Couetil LL, Cardwell JM, Gerber V, et al. Inflammatory airway disease of horses—revised consensus statement. J Vet Intern Med. 2016;30:503‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bullone M, Lavoie JP. Asthma "of horses and men"—how can equine heaves help us better understand human asthma immunopathology and its functional consequences? Mol Immunol. 2015;66:97‐105. [DOI] [PubMed] [Google Scholar]
- 6. Bond SL, Timsit E, Workentine M, Alexander T, Léguillette R. Upper and lower respiratory tract microbiota in horses: bacterial communities associated with health and mild asthma (inflammatory airway disease) and effects of dexamethasone. BMC Microbiol. 2017;17:184‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fillion‐Bertrand G, Dickson RP, Boivin R, Lavoie JP, Huffnagle GB, Leclere M. Lung microbiome is influenced by the environment and asthmatic status in an equine model of asthma. Am J Respir Cell Mol Biol. 2019;60:189‐197. [DOI] [PubMed] [Google Scholar]
- 8. Dickson RP, Erb‐Downward JR, Martinez FJ, Huffnagle GB. The microbiome and the respiratory tract. Annu Rev Physiol. 2016;78:481‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood JL, Burrell MH, Roberts CA, et al. Streptococci and Pasteurella spp. associated with disease of the equine lower respiratory tract. Equine Vet J. 1993;25:314‐318. [DOI] [PubMed] [Google Scholar]
- 10. Chapman PS, Green C, Main JP, et al. Retrospective study of the relationships between age, inflammation and the isolation of bacteria from the lower respiratory tract of thoroughbred horses. Vet Rec. 2000;146:91‐95. [DOI] [PubMed] [Google Scholar]
- 11. Newton JR, Wood JL, Chanter N. A case control study of factors and infections associated with clinically apparent respiratory disease in UK thoroughbred racehorses. Prev Vet Med. 2003;60:107‐132. [DOI] [PubMed] [Google Scholar]
- 12. Pirie RS, Collie DD, Dixon PM, et al. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust‐induced asthma). Clin Exp Allergy. 2003;33:676‐683. [DOI] [PubMed] [Google Scholar]
- 13. Burrell MH, Wood JL, Whitwell KE, et al. Respiratory disease in thoroughbred horses in training: the relationships between disease and viruses, bacteria and environment. Vet Rec. 1996;139:308‐313. [DOI] [PubMed] [Google Scholar]
- 14. Wood JL, Newton JR, Chanter N, et al. Association between respiratory disease and bacterial and viral infections in British racehorses. J Clin Microbiol. 2005;43:120‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ward CL, Wood JL, Houghton SB, et al. Actinobacillus and Pasteurella species isolated from horses with lower airway disease. Vet Rec. 1998;143:277‐279. [DOI] [PubMed] [Google Scholar]
- 16. Kuhnert P, Korczak B, Falsen E, et al. Nicoletella semolina gen. nov., sp. nov., a new member of Pasteurellaceae isolated from horses with airway disease. J Clin Microbiol. 2004;42:5542‐5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansson I, Johansson KE, Persson M, Riihimäki M. The clinical significance of Nicoletella semolina in horses with respiratory disorders and a screening of the bacterial flora in the airways of horses. Vet Microbiol. 2013;162:695‐699. [DOI] [PubMed] [Google Scholar]
- 18. Maillard K, Richard EA, Kuhnert P, Fortier G, Léon A, Pitel PH. Isolation of Nicoletella semolina from equine tracheal washes. J Equine Vet Sci. 2013;33:561‐564. [Google Scholar]
- 19. Bisgaard M. Ecology and significance of Pasteurellaceae in animals. Zentralbl Bakteriol. 1993;279:7‐26. [DOI] [PubMed] [Google Scholar]
- 20. Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. A practical approach to RT‐qPCR‐publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1‐S5. [DOI] [PubMed] [Google Scholar]
- 21. McConachie EL, Hart KA, Whelchel DD, et al. Pulmonary disease potentially associated with Nicoletella semolina in 3 young horses. J Vet Intern Med. 2014;28:939‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhnert P, Bisgaard M, Korczak BM, Schwendener S, Christensen H, Frey J. Identification of animal Pasteurellaceae by MALDI‐TOF mass spectrometry. J Microbiol Methods. 2012;89:1‐7. [DOI] [PubMed] [Google Scholar]
- 23. Boyle AG, Timoney JF, Newton JR, Hines MT, Waller AS, Buchanan BR. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles‐revised consensus statement. J Vet Intern Med. 2018;32:633‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manguin E, Pépin E, Boivin R, Leclere M. Tracheal microbial populations in horses with moderate asthma. J Vet Intern Med. 2020;34:986‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoshii Y, Shimizu K, Morozumi M, et al. Detection of pathogens by real‐time PCR in adult patients with acute exacerbation of bronchial asthma. BMC Pulm Med. 2017;17:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoshii Y, Shimizu K, Morozumi M, et al. Identification of pathogens by comprehensive real‐time PCR versus conventional methods in community‐acquired pneumonia in Japanese adults. Infect Dis (Lond). 2016;48:782‐788. [DOI] [PubMed] [Google Scholar]
- 27. Christensen H, Kuhnert P, Nørskov‐Lauritsen N, et al. The family Pasteurellaceae. In: Rosenberg E, EF DL, Lory S, Stackebrandt E, Thompson F, eds. The Prokaryotes: Gammaproteobacteria. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014:535‐564. [Google Scholar]
- 28. Christensen H, Kuhnert P, Busse HJ, Frederiksen WC, Bisgaard M. Proposed minimal standards for the description of genera, species and subspecies of the Pasteurellaceae. Int J Syst Evol Microbiol. 2007;57:166‐178. [DOI] [PubMed] [Google Scholar]
- 29. Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma A, Richardson M, Cralle L, et al. Longitudinal homogenization of the microbiome between both occupants and the built environment in a cohort of United States Air Force Cadets. Microbiome. 2019;7:70‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finnicum CT, Beck JJ, Dolan CV, et al. Cohabitation is associated with a greater resemblance in gut microbiota which can impact cardiometabolic and inflammatory risk. BMC Microbiol. 2019;19:230‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rosenthal FS, Gruntman A, Couetil LL. A comparison of total, respirable, and real‐time airborne particulate sampling in horse barns. J Occup Environ Hyg. 2006;3:599‐605. [DOI] [PubMed] [Google Scholar]
- 34. Ivester KM, Smith K, Moore GE, et al. Variability in particulate concentrations in a horse training barn over time. Equine Vet J Suppl. 2012;43:51‐56. [DOI] [PubMed] [Google Scholar]
- 35. Webster AJ, Clarke AF, Madelin TM, et al. Air hygiene in stables. 1: effects of stable design, ventilation and management on the concentration of respirable dust. Equine Vet J. 1987;19:448‐453. [DOI] [PubMed] [Google Scholar]
- 36. Leclere M, Lavoie‐Lamoureux A, Gelinas‐Lymburner E, et al. Effect of antigenic exposure on airway smooth muscle remodeling in an equine model of chronic asthma. Am J Respir Cell Mol Biol. 2011;45:181‐187. [DOI] [PubMed] [Google Scholar]
- 37. Pirie RS, Dixon PM, Collie DD, McGorum B. Pulmonary and systemic effects of inhaled endotoxin in control and heaves horses. Equine Vet J. 2001;33:311‐318. [DOI] [PubMed] [Google Scholar]
- 38. Holcombe SJ, Jackson C, Gerber V, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet J. 2001;33:244‐249. [DOI] [PubMed] [Google Scholar]
- 39. Blackman M, Moore‐Colyer MJS. Hay for horses: the effects of three different wetting treatments on dust and nutrient content. Anim Sci. 1998;66:745‐750. [Google Scholar]
- 40. Moore‐Colyer MJS, Fillery BG. The effect of three different treatments on the respirable particle content, total viable count and mould concentrations in hay for horses. In: Saastamoinen M, Fradinho MJ, Santos AS, Miraglia N, eds. Forages and Grazing in Horse Nutrition. Wageningen: Wageningen Academic Publishers; 2012:101‐106. [Google Scholar]
- 41. Moore‐Colyer MJS, Taylor JLE, James R. The effect of steaming and soaking on the respirable particle, bacteria, mould, and nutrient content in hay for horses. J Equine Vet Sci. 2016;39:62‐68. [Google Scholar]
- 42. Clarke AF. A review of environmental and host factors in relation to equine respiratory disease. Equine Vet J. 1987;19:435‐441. [DOI] [PubMed] [Google Scholar]
- 43. Hoffman AM, Viel L, Muckle CA, Tesarowski DB. Evaluation of a guarded bronchoscopic method for microbial sampling of the lower airways in foals. Can J Vet Res. 1991;55:325‐331. [PMC free article] [PubMed] [Google Scholar]
- 44. Bartlett JG, Alexander J, Mayhew J, Sullivan‐Sigler N, Gorbach SL. Should fiberoptic bronchoscopy aspirates be cultured? Am Rev Respir Dis. 1976;114:73‐78. [DOI] [PubMed] [Google Scholar]
- 45. Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet. 2014;384:691‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jean D, Vrins A, Beauchamp G, Lavoie JP. Evaluation of variations in bronchoalveolar lavage fluid in horses with recurrent airway obstruction. Am J Vet Res. 2011;72:838‐842. [DOI] [PubMed] [Google Scholar]
- 48. Derksen FJ, Scott JS, Miller DC, Slocombe RF, Robinson NE. Bronchoalveolar lavage in ponies with recurrent airway obstruction (heaves). Am Rev Respir Dis. 1985;132:1066‐1070. [DOI] [PubMed] [Google Scholar]


