Abstract
Background
Combatting antimicrobial resistance requires a One Health approach to antimicrobial stewardship including antimicrobial drug (AMD) use evaluation. Current veterinary AMD prescribing data are limited.
Objectives
To quantify companion animal AMD prescribing in primary care and specialty practice across 3 academic veterinary hospitals with particular focus on third‐generation cephalosporins, fluoroquinolones, and carbapenems.
Animals
Dogs and cats presented to 3 academic veterinary hospitals from 2012 to 2017.
Methods
In this retrospective study, AMD prescribing data from 2012 to 2017 were extracted from electronic medical records at each hospital and prescriptions classified by service type: primary care, specialty practice or Emergency/Critical Care (ECC). Hospital‐level AMD prescribing data were summarized by species, service type, AMD class, and drug. Multivariable logistic full‐factorial regression models were used to estimate hospital, year, species, and service‐type effects on AMD prescribing. Estimated marginal means and confidence intervals were plotted over time.
Results
The probability of systemic AMD prescribing for any indication ranged between 0.15 and 0.28 and was higher for dogs than cats (P < .05) apart from 2017 at hospital 1. Animals presented to primary care were least likely to receive AMDs (dogs 0.03‐0.15, cats 0.03‐0.18). The most commonly prescribed AMD classes were aminopenicillins/β‐lactamase inhibitors (0.02‐0.15), first‐generation cephalosporins (0.00‐0.09), fluoroquinolones (0.00‐0.04), nitroimidazoles (0.01‐0.06), and tetracyclines (0.00‐0.03). Among the highest priority classes, fluoroquinolones (dogs 0.00‐0.09, cats 0.00‐0.08) and third‐generation cephalosporins (dogs 0.00‐0.04, cats 0.00‐0.05) were most frequently prescribed.
Conclusions and Clinical Importance
Antimicrobial drug prescribing frequencies were comparable to previous studies. Additional stewardship efforts might focus on fluoroquinolones and third‐generation cephalosporins.
Keywords: antibiotic, antimicrobial drug, antimicrobial prescribing practices, antimicrobial prescription
1. INTRODUCTION
Antimicrobial drug resistance (AMR) is a critically important issue in human and veterinary medicine. The Centers for Disease Control and Prevention (CDC) estimates that resistant bacteria kill >35 000 people in the United States annually with 2.8 million people infected. 1 Resistant bacteria characterized as Urgent or Serious Threats by the CDC also infect pets, including extended‐spectrum β‐lactamase (ESBL) Enterobacterales, methicillin‐resistant Staphylococcus aureus, and multidrug‐resistant Pseudomonas aeruginosa. 2 This might result from the close relationships between people and their pets, for instance an estimated 56% of dogs and 75% of cats share their owners' beds. 3 Given that approximately 43 million households in the United States include dogs and 36 million households include cats, 4 it is imperative that human and animal AMR be considered together. Strategies to combat AMR in people require a One Health approach to antimicrobial stewardship, highlighting the critical importance of collaboration between human and veterinary medicine.
One of the core principles of antimicrobial stewardship is the evaluation of antimicrobial drug (AMD) use practices. 5 In companion animal medicine, documentation of AMD use, resistance patterns, and development of usage guidelines have largely focused on academic teaching hospitals, 6 , 7 , 8 , 9 , 10 which have some advantages for such studies including AMR surveillance systems, written biosecurity policies, searchable electronic medical record (EMR) systems, and personnel focused on AMR. A major limitation of studying AMD use in veterinary teaching hospitals is the predominance of animals with complex or severe illnesses for which AMDs have been previously prescribed. 6 , 11 , 12 Some studies have also focused on prescribing of 1 specific class of AMDs, such as the carbapenems. 13 To date, few data on AMD use in veterinary primary care in North America are available. One study measured outpatient AMD use in small animal community practice involving a single geographic area and relied on additional record keeping, wherein veterinarians were asked to complete a journal for up to 5 animals, monthly for a year, which likely reflected a very small proportion of the AMD prescriptions written. 14 A study from a network of private primary care practices across North America reported AMD usage data for urinary tract infections (UTI) and respiratory tract infections (RTI) for 2015 across 926 general practice clinics. 15 This study leveraged the single unified medical records system shared between all of the practices to report on AMD prescribing for >32 000 episodes of UTI and >35 000 episodes of RTI. However, there are various reports on veterinary AMD use from the United Kingdom, Europe, and Australia made possible by collaborative data‐sharing networks. 16 , 17 , 18 , 19 , 20 , 21 , 22 Alternative and innovative methods for studying AMD prescribing have been described including large‐scale data‐sharing networks, 17 , 19 , 22 use of insurance databases, 23 questionnaire augmented records, 24 and automated natural language processing of electronic medical records. 20 , 21
There is a clear need for additional AMD prescribing data in veterinary medicine across practice types and geographic areas. To address these knowledge gaps, the present study aimed to measure AMD prescribing and use in both specialty and primary care veterinary practice across 3 academic veterinary hospitals with particular focus on common infections of the urinary, respiratory, and integumentary systems in dogs and cats. 25 Additionally, the present study sought to quantify AMD prescriptions across practice types and institutions and to compare AMD prescribing patterns and frequencies between service types within and between hospitals. Particular attention was paid to prescribing of certain AMDs classified as critically important (highest priority or high priority) by both the World Health Organization (WHO) and the US Food and Drug Administration 2020 Concept Paper. 26 , 27 Specifically, we focused on prescribing of third‐generation cephalosporins, fluoroquinolones, and carbapenems. It was hypothesized that the probability of AMD prescribing varied by year, hospital, species and indication, and drug class.
2. MATERIALS AND METHODS
2.1. Participants and sites
Study investigators included veterinary clinicians, microbiologists, epidemiologists, and pharmacologists at the Colleges of Veterinary Medicine at Cornell University (Cornell), North Carolina State University (NCSU), and Texas A&M University (TAMU). The University teaching hospitals at these veterinary colleges were comparable in terms of caseload and availability of specialties and were located in 3 distinct geographic areas within the continental United States (North Atlantic, Mid‐Atlantic, and West South Central). At each site, data were collected regarding AMD prescribing to inpatients and outpatients treated within University teaching hospitals providing specialty and emergency care, and from standalone clinics providing general practitioner care on a first‐opinion basis to animals and clients within their respective communities. Each of these primary care clinics was part of their respective academic institution and shared electronic medical record systems with the parent organization. The primary care clinics at all 3 institutions are staffed by a mixture of experienced general practitioners without board certification, veterinarians with certification in general practice, that is, DABVP, and veterinarians with board certification in a specific field such as internal medicine. It was not possible to separate data on AMD prescribing to inpatients vs outpatients, and hence all AMD prescribing data were analyzed by service type. Local ethical approval was sought as necessary. At all 3 institutions, this study was exempt from Institutional Animal Care and Use Committee approval because it analyzed prescribing data from clinician‐driven care provided to animals at the institution hospitals. Data confidentiality was strictly maintained and no animal or client identifying information is reported.
Investigators at each hospital identified all AMD products in their pharmacy formularies and collectively refined a list of AMDs for which data would be collected (Table S1). Data were collected on all products containing these AMDs, but for subsequent analyses topical preparations were excluded. None of the participating centers had established formal AMD use guidelines. At each site, EMR systems were searched to identify AMD prescription data from 2012 to 2017. Data for all animals, including those not prescribed AMDs, were also collected from 2012 to 2017. At each hospital, EMR systems were queried to provide the total number of hospital visits and the total number of AMD prescriptions for all indications annually during this timeframe. Two hospitals used commercial EMR software (UVIS; UGA, Athens, GA), while the other used in‐house EMR software. Each hospital had different procedures for capturing AMD prescription data. At each site, search strategies to identify AMD prescriptions were evaluated and refined by iterative pilot data collection, comprehensive manual record review, and search parameter revision. Detailed descriptions of search strategies at each site are provided in supplementary materials (Data S1). Standard operating procedures were developed at each hospital to identify standard data fields including prescription and dispensing dates, animal identification numbers, bodyweight, species, sex, date of birth, attending clinical service, attending clinician, AMD product name and identification number, quantity dispensed, and administration directions. These data were collected and collated to enable curation and crosschecking, but they are not reported. All 3 EMR systems used unique identification numbers for each available product and coded drugs dispensed as individual tablets, capsules, or milliliters or in the original manufacturer's packaging such as per bottle or vial.
At each veterinary teaching hospital, study investigators classified AMD prescriptions according to prescribing service as primary care, specialty practice, or Emergency and Critical Care (ECC). Primary care encompassed services such as general surgery or community practice where veterinarians assessed animals for the first time. Specialty services including internal medicine and orthopedic surgery were considered specialty practice, while ECC by definition sees a wide range of case types including cases that could be managed by primary care to specialty level care and was therefore analyzed separately. Primary care cases might have been managed by veterinarians with a range of experience, as described earlier, while specialty and ECC cases were managed by veterinarians with a range of experience (such as interns, residents), but always under the supervision of a board‐certified faculty member. Hospital‐level AMD use data were summarized by species (dog, cat, and other), service type (primary care, specialty, and ECC), AMD class, and drug.
2.2. Data Analysis
The database was restricted to animal visits resulting from care episodes from 2012 to 2017. Hospital visit frequency data included topical drugs; however, topical drugs were excluded from analyses of systemic AMDs. The frequency of systemic AMD prescribing was collapsed to 1 prescription per unique animal visit per AMD class/drug, such that repeated administration of the same drug to the same animal within a visit was represented by a single prescription. Data sets were collapsed to either AMD class or drug by hospital, year, species (dog; cat), and service type (primary care; ECC; specialty practice; pharmacy refill; service type missing). AMD prescriptions coded as pharmacy refills and entries missing a service type were not analyzed; these data were used solely to enable data tallying and crosschecking. Non‐accession numbers (no service attributed) were coded as pharmacy refill in 1 hospital. Occasional services like dogs and cats receiving care by food animal or equine field service clinicians were coded as primary care because these animals were prescribed AMDs on an outpatient basis by veterinarians evaluating animals on a first‐opinion basis. These prescriptions were judged to have similar potential to generate antimicrobial resistance or to be released into the environment as those prescribed to outpatients from a clinic or hospital. Spreadsheets (see supplemental material) for AMD class or drug provide frequency for each combination of hospital by year, by species, and by service type. Multivariable logistic (AMD class, any AMD) full‐factorial regression models were used to analyze hospital, year, species, and service type as factors that affected dispensing and prescribing practices.
The indication for the AMD prescription and site of infection could not be universally identified, for instance, where more than 1 potential indication coexisted or where the medical record was incomplete. Prescribing for perioperative prophylaxis was not specifically recorded. Major problem lists of urinary, respiratory, and skin diseases were totaled by AMD class. These were restricted to 2017 for 1 hospital and 2012 to 2017 for a second hospital. Major problem lists were not available for the third hospital. Marginal means were estimated and plotted over time to view trends and explore differences among the factors. Specifically, these logistic regression models were constructed to account for various fixed effects that might have influenced the likelihood of prescribing, including species, service type, disease type, and hospital location. The point estimates from the logistic regression models were then tabulated and graphed as marginal means of the probability of AMD prescribing and presented along with their 95% confidence intervals (CIs). At each point where the 95% CIs do not overlap, differences between the point estimates differ significantly at P < .05.
3. RESULTS
Visits by unique dogs and cats to each hospital within the study period and their distribution by attending service are summarized in Table 1. Prescriptions of AMDs that were refills were not included in these totals. The total number of visits by hospital and service type are provided in Table 2. The probability of a systemic AMD prescription for any indication was determined for dogs and cats by year for each hospital (Figure 1). Overall, the probabilities ranged between .15 and .28 and were generally higher for dogs than cats. The probability of animals receiving an AMD prescription differed significantly by service and hospital (Figure 2) as indicated where the 95% CIs of the modeled marginal means of the probability of AMD prescribing do not overlap. For all 3 hospitals, animals presented to primary care were less likely to receive AMDs than those presented to specialty services or ECC. At hospital 1, the probability of an animal presented to ECC receiving an AMD was greater than for animals presented to specialty services, while it was the reverse at hospital 2. At hospital 3, the probability of animals presented to specialty services or to ECC of receiving an AMD was similar. These patterns were similar for dogs and cats within each hospital.
TABLE 1.
Unique visits (total unique visits, and only those with systemic antibiotic prescriptions): subclassified by service type and species from 2012 to 2017
| Clinical service type and species | Hospital 1 | Hospital 2 | Hospital 3 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Total unique visits | Visits with systemic AMD prescriptions | Total unique visits | Visits with systemic AMD prescriptions | Total unique visits | Visits with systemic AMD prescriptions | Total unique visits | Visits with systemic AMD prescriptions | |
| Primary care | 15 735 | 1766 (11.2%) | 15 468 | 697 (4.5%) | 30 977 | 3970 (12.8%) | 62 180 | 6433 (10.3%) |
| Primary care dogs | 11 178 | 1324 (11.8%) | 11 155 | 535 (4.8%) | 24 659 | 3120 (12.7%) | 46 992 | 4979 (10.6%) |
| Primary care cats | 4557 | 442 (9.7%) | 4313 | 162 (3.8%) | 6318 | 850 (13.5%) | 15 188 | 1454 (9.6%) |
| ECC | 25 575 | 9577 (37.4%) | 19 042 | 2574 (13.5%) | 22 730 | 5123 (22.5%) | 67 347 | 17 274 (25.6%) |
| ECC dogs | 20 399 | 7877 (38.6%) | 15 185 | 2109 (13.9%) | 19 338 | 4404 (22.8%) | 54 922 | 14 390 (26.2%) |
| ECC cats | 5176 | 1700 (32.8%) | 3857 | 465 (12.1%) | 3392 | 719 (21.2%) | 12 425 | 2884 (23.2%) |
| Specialty | 75 560 | 14 745 (19.5%) | 129 792 | 27 040 (20.8%) | 58 274 | 14 450 (24.8%) | 263 626 | 56 235 (21.3%) |
| Specialty dogs | 65 420 | 13 155 (20.1%) | 114 009 | 23 931 (21.0%) | 51 923 | 13 218 (25.5%) | 231 352 | 50 304 (21.7%) |
| Specialty cats | 10 140 | 1590 (15.7%) | 15 783 | 3109 (19.7%) | 6351 | 1232 (19.4%) | 32 274 | 5931 (18.4%) |
Note: Topical drugs, pharmacy refills, or visits missing, and assigned service types are not included.
Abbreviations: AMD, antimicrobial drug; ECC, emergency and critical care.
TABLE 2.
Total records, unique visits, unique animals, and unique visits per animal; by service type from 2012 to 2017
| Clinical service type | Hospital 1 | Hospital 2 | Hospital 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total records | Uniquevisits | Unique animals | Visits per animal | Total records | Uniquevisits | Unique animals | Visits per animal | Total records | Uniquevisits | Unique animals | Visits per animal | |
| Primary care | 16 948 (10.8%) | 15 735 (12.6%) | 6625 (14.6%) | 2.38 | 15 546 (7.7%) | 15 468 (8.8%) | 3719 (7.6%) | 4.16 | 32 427 (23.0%) | 30 977 (27.6%) | 11 934 (25.4%) | 2.60 |
| ECC | 43 698 (27.8%) | 25 575 (20.4%) | 20 182 (44.6%) | 1.27 | 20 245 (10.1%) | 19 042 (10.9%) | 14 310 (29.3%) | 1.33 | 26 871 (19.1%) | 22 730 (20.3%) | 19 478 (41.5%) | 1.17 |
| Specialty | 87 253 (55.4%) | 75 560 (60.4%) | 28 631 (63.3%) | 2.64 | 152 694 (76.0%) | 129 792 (74.1%) | 37 065 (75.8%) | 3.50 | 81 090 (57.5%) | 58 274 (51.9%) | 21 858 (46.6%) | 2.67 |
| Pharmacy refill | 9269 (5.9%) | 8130 (6.5%) | 3275 (7.2%) | 2.48 | 11 437 (5.7%) | 9839 (5.6%) | 4409 (9.0%) | 2.23 | 577 (0.4%) | 244 (0.2%) | 236 (0.5%) | 1.03 |
| Missing service | 222 (0.1%) | 190 (0.2%) | 154 (0.3%) | 1.23 | 970 (0.5%) | 970 (0.6%) | 697 (0.4%) | 1.39 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 |
| Total | 157 390 | 125 190 | 45 250 | 2.77 | 200 892 | 175 096 | 48 894 | 3.58 | 140 965 | 112 199 | 46 942 | 2.39 |
Note: Includes pharmacy refills and visits missing an assigned service type. Proportions of the column totals are given as percentages in parentheses.
Abbreviations: ECC, emergency and critical care.
FIGURE 1.
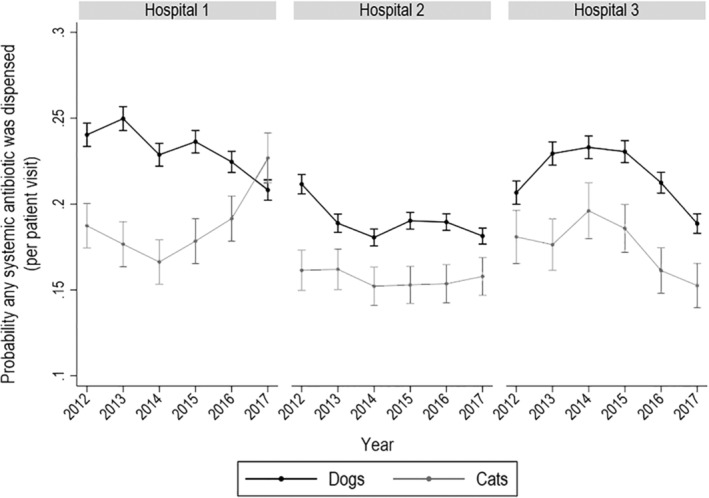
Probability of systemic antimicrobial drug dispensing in dogs and cats by year and hospital. Probability of any systemic antimicrobial drug dispensing in dogs and cats by year and hospital based on logistic regression‐modeled marginal mean predictions with 95% confidence intervals (CIs). At each point where the 95% CIs do not overlap, differences between the point estimates differ significantly at P < .05. These figures do not include topical antimicrobial drugs
FIGURE 2.
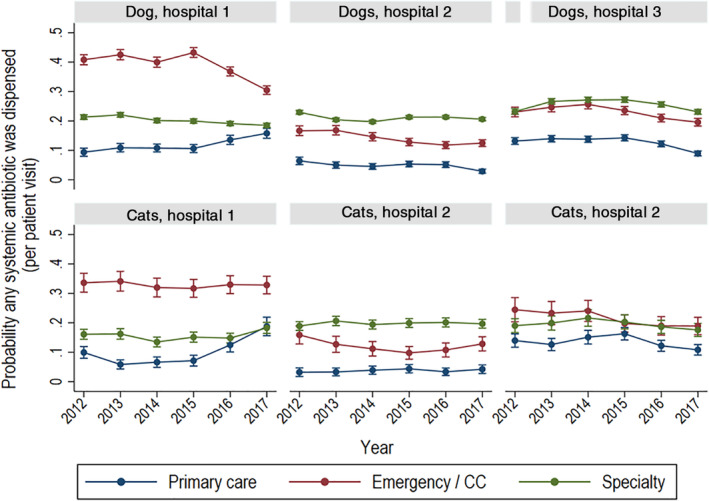
Probability of systemic antimicrobial drug dispensing in dogs and cats by year, hospital, and service type. Probability of systemic antimicrobial drug dispensing in dogs and cats by year, hospital, and type of service based on logistic regression‐modeled marginal mean predictions with 95% confidence intervals (CIs). At each point where the 95% CIs do not overlap, differences between the point estimates differ significantly at P < .05. Service types are primary care, emergency and critical care (CC), and specialty practice. These figures do not include topical antimicrobial drugs
The 5 most commonly prescribed AMD classes aggregated across all 3 hospitals and from 2012 to 2017 inclusive were aminopenicillins/β‐lactamase inhibitors, first‐generation cephalosporins, fluoroquinolones, nitroimidazoles, and tetracyclines (Figure 3). The frequency of AMD prescriptions by class varied by service type (Figure 3). In ECC, aminopenicillins/β‐lactamase inhibitors (dogs n = 6956, 35.9%; cats n = 1842, 49.5%) and nitroimidazoles were most commonly prescribed (dogs n = 3347, 17.3%; cats n = 331, 8.9%), while first‐generation cephalosporins (dogs n = 20 467, 30.9%; cats n = 1139, 14.0%) and tetracyclines (dogs n = 5073, 7.7%; cats n = 522, 6.4%) were more commonly prescribed by specialty services. For all drugs, primary care services had the lowest proportion of AMD prescriptions. Among the critically important AMD classes defined by the WHO (carbapenems, glycopeptides, oxazolidinones, third‐generation cephalosporins, and fluoroquinolones), the probability of prescriptions was greatest for fluoroquinolones and third‐generation cephalosporins (Figure 4), but these drugs accounted for <10% of all AMD prescriptions in both dogs and cats, regardless of hospital. Across all hospitals, carbapenems were prescribed for 0.17% dog visits, and 0.15% of cat visits; oxazolidinones were prescribed for 0.07% of dog and cat visits; and glycopeptides were prescribed for 0.01% of dog and cat visits. For the first‐generation cephalosporins, AMD prescribing practices differed by hospital section. Prescribing for perioperative prophylaxis was not specifically recorded, but 75.5% of the prescriptions for cefazolin in dogs and 59.9% in cats were attributable to surgical services such as orthopedics, soft tissue surgery, general surgery, neurology, and ophthalmology. In contrast, dermatology accounted for 0.4% of the cefazolin prescriptions, but 12.7% of the cephalexin prescriptions (Data S2).
FIGURE 3.
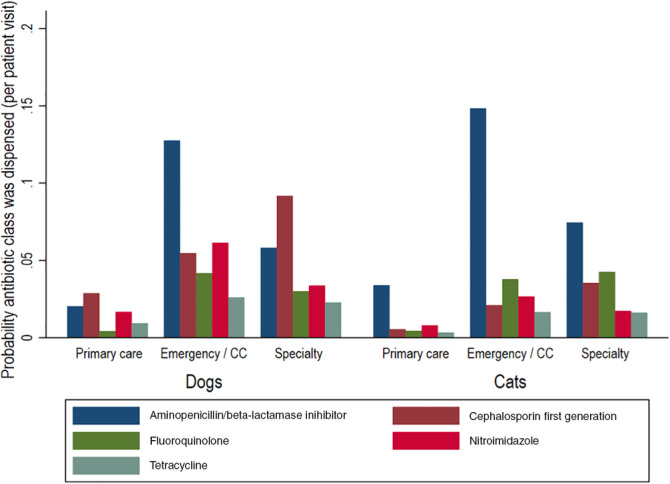
Frequency of dispensing of the top five antimicrobial drug classes by species and service type. The proportion of unique visits in which an antimicrobial drug was dispensed for the 5 most commonly dispensed drugs by service type. Service types are primary care, emergency and critical care (CC), and specialty practice. These figures do not include topical antimicrobial drugs. Results are aggregated across all 3 hospitals and from 2012 to 2017, inclusive
FIGURE 4.
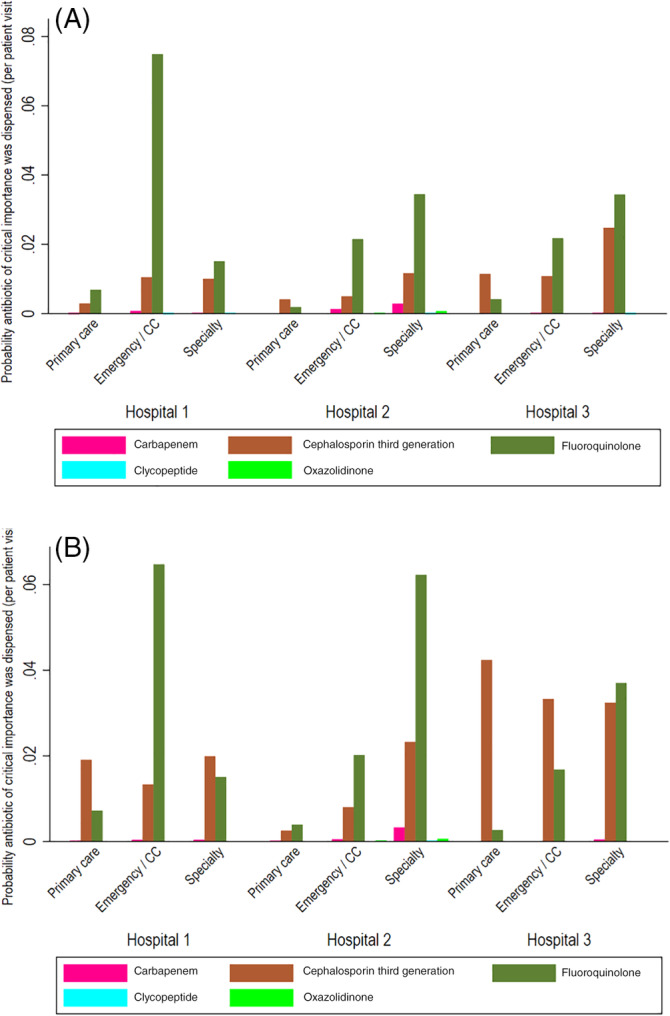
Probability that a critically important antimicrobial drug class was dispensed (by hospital, service type, and species and aggregated across 2012–2017). The proportion of unique visits in which a critically important antimicrobial drug class was dispensed by hospital, species (A, dogs; B, cats), and service type. Service types are primary care, emergency and critical care (CC), and specialty practice. These figures do not include topical antimicrobial drugs. Results are aggregated across 2012 to 2017, inclusive
Within hospitals, the weighted mean annual proportions of prescriptions were greatest for ECC and specialty services relative to primary care (Figure 2). The exception to this was for prescriptions of third‐generation cephalosporin to cats in primary care at hospital 3. This pattern can be observed when the probability of prescribing a third‐generation cephalosporin was modeled by species, year, and hospital (Figure 5). Specifically, the probability of a third‐generation cephalosporin being prescribed to dogs presented to specialty services at hospital 3 decreased over time but was stable in dogs presented to other service types. In the same hospital, the probability of a third‐generation cephalosporin prescription in cats presented to primary care increased from 2014 to 2015 and remained quite consistent from 2015 to 2017. At hospital 2, the probability of a third‐generation cephalosporin prescription to dogs and cats was greater for animals presented to specialty services and increased slightly over time.
FIGURE 5.
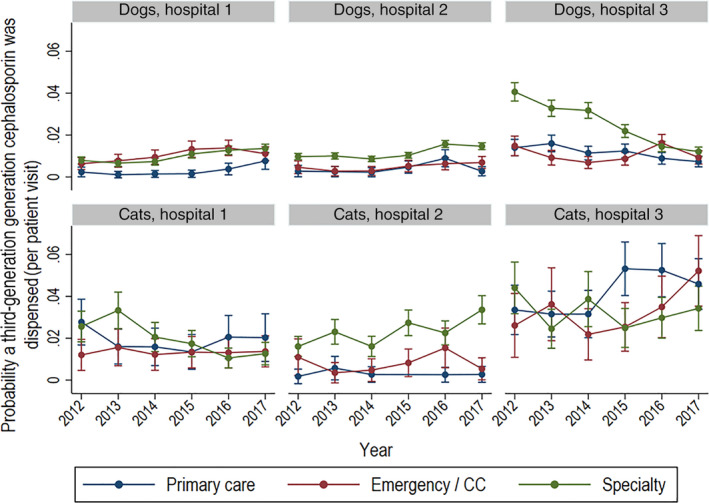
Probability of dispensing of a third‐generation cephalosporin in dogs and cats by year and hospital. probability of third‐generation cephalosporin dispensing per visit in dogs and cats by year, hospital, and type of service based on modeled marginal mean predictions with 95% confidence intervals. Service types are primary care, emergency and critical care, and specialty practice
Prescribing of the third‐generation cephalosporins was dependent primarily on species with some variation in prescribing patterns by hospital (Figure 6). The most frequently prescribed third‐generation cephalosporin in dogs across all 3 hospitals was oral cefpodoxime, which accounted for 76.6% of prescribing of this class of AMDs in dogs. In contrast, the most frequently prescribed third‐generation cephalosporin in dogs across all 3 hospitals was parenteral cefovecin, which accounted for 78.6% of prescribing of this class of AMDs in cats. Prescribing of other parenteral third‐generation cephalosporins was influenced by hospital, with ceftazidime predominating at hospital 1 while cefotaxime predominated at hospital 3. Primary care accounted for only 6.7% of third‐generation cephalosporin prescribing in dogs, while in cats, 23.1% of third‐generation cephalosporins were prescribed in primary care (almost all were cefovecin). Cats were prescribed third‐generation cephalosporins 1.95‐fold more frequently than dogs (9.3% vs 4.8%).
FIGURE 6.
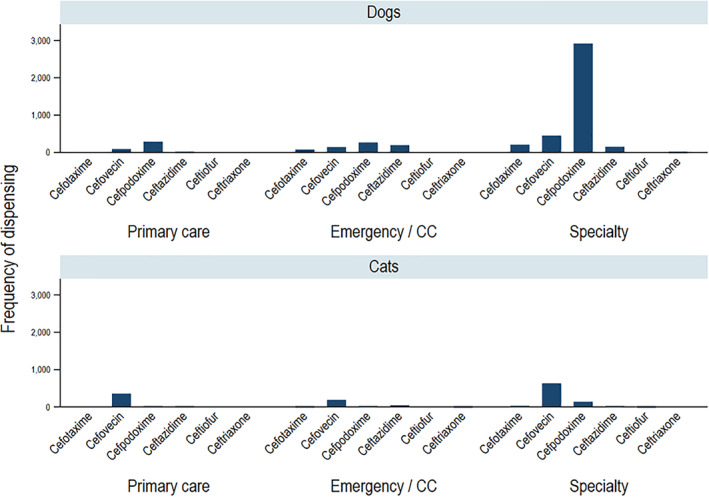
Frequency of dispensing third‐generation cephalosporins by species and service type. The number of unique visits in which a third‐generation cephalosporin was dispensed for the 5 most commonly dispensed drugs by service type. Service types are primary care, emergency and critical care (CC), and specialty practice. These figures do not include topical antimicrobial drugs. Results are aggregated across all 3 hospitals and from 2012 to 2017, inclusive
In assessing the probability of fluoroquinolones prescription over time, the in‐hospital patterns were similar between dogs and cats (Figure 7). Hospitals 2 and 3 had increased probability of a fluoroquinolone prescription for animals presented to specialty services, while the lowest probability of prescription was by primary care services. In hospital 1, there was greater probability of a fluoroquinolone prescription for animals presented to ECC relative to other service types.
FIGURE 7.
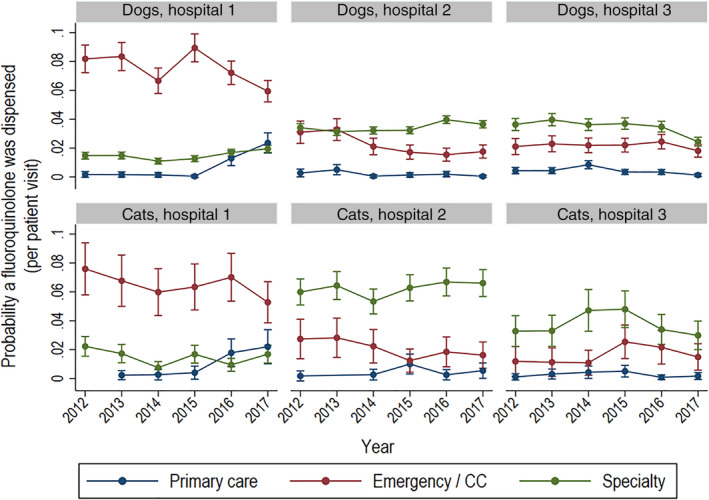
Probability of dispensing of a fluoroquinolone in dogs and cats by year and hospital. probability of fluoroquinolone dispensing per visit in dogs and cats by year, hospital, and type of service based on modeled marginal mean predictions with 95% confidence intervals. Service types are primary care, emergency and critical care, and specialty practice
At hospitals 1 and 3, veterinarian‐coded problem lists were used to determine the indication for AMD prescriptions. The 5 most common classes of AMDs for dogs and cats for each of these indications are presented in Table 3. When data from dogs and cats were combined, there were 3344 AMD prescriptions from 7692 visits for animals with urinary tract infections. The top 5 AMD classes prescribed for urinary tract infections were aminopenicillin/β‐lactamase inhibitor combinations, fluoroquinolones, first‐generation cephalosporins, aminopenicillins, and tetracyclines (Figure 8A). There were 3322 AMD prescriptions from 6453 visits for animals with RTIs; the top 5 AMD classes prescribed (dogs and cats combined) were aminopenicillin/β‐lactamase inhibitor combinations, fluoroquinolones, tetracyclines, first‐generation cephalosporins, and lincosamides (Figure 8B). There were 5016 AMD prescriptions from 14 881 visits for animals with skin infections. The top 5 AMD classes prescribed (dogs and cats combined) were first‐generation cephalosporins, third‐generation cephalosporins, aminopenicillin/β‐lactamase inhibitor combinations, fluoroquinolones, and lincosamides (Figure 8C). Note that when data were separated into dogs and cats, the top 5 AMDs were not identical to the combined, such that the ranks in Table 3 and Figure 8A‐C differ slightly.
TABLE 3.
Top 5 most frequently prescribed antimicrobial drugs in animals with urinary tract infections, respiratory tract infections, and skin infections in dogs and cats
| Dog urinary tract | n | % of prescriptions | % of visits | Cat urinary tract | n | % of prescriptions | % of visits |
| Aminopenicillin/β‐lactamase inhibitor | 842 | 30.9% | 15.8% | Aminopenicillin/β‐lactamase inhibitor | 268 | 43.5% | 11.4% |
| Fluoroquinolone | 448 | 16.4% | 8.4% | Fluoroquinolone | 90 | 14.6% | 3.8% |
| Cephalosporin (first generation) | 377 | 13.8% | 7.1% | Cephalosporin (third generation) | 65 | 10.6% | 2.8% |
| Aminopenicillin | 256 | 9.4% | 4.8% | Aminopenicillin | 54 | 8.8% | 2.3% |
| Nitroimidazole | 192 | 7.0% | 3.6% | Cephalosporin (first generation) | 48 | 7.8% | 2.0% |
| Dog respiratory | n | % of prescriptions | % of visits | Cat respiratory | n | % of prescriptions | % of visits |
| Aminopenicillin/β‐lactamase inhibitor | 832 | 30.5% | 16.2% | Aminopenicillin/β‐lactamase inhibitor | 203 | 43.1% | 15.5% |
| Fluoroquinolone | 589 | 21.6% | 11.5% | Tetracycline | 64 | 13.6% | 4.9% |
| Tetracycline | 421 | 15.4% | 8.2% | Macrolide | 60 | 12.7% | 4.6% |
| Cephalosporin (first generation) | 251 | 9.2% | 4.9% | Fluoroquinolone | 53 | 11.3% | 4.0% |
| Lincosamide | 240 | 8.8% | 4.7% | Cephalosporin (third generation) | 31 | 6.6% | 2.4% |
| Dog skin | n | % of prescriptions | % of visits | Cat skin | n | % of prescriptions | % of visits |
| Cephalosporin (first generation) | 1507 | 34.8% | 11.7% | Cephalosporin (third generation) | 247 | 36.1% | 12.6% |
| Cephalosporin (third generation) | 857 | 19.8% | 6.6% | Aminopenicillin/β‐lactamase inhibitor | 224 | 32.7% | 11.4% |
| Aminopenicillin/β‐lactamase inhibitor | 752 | 17.4% | 5.8% | Fluoroquinolone | 53 | 7.7% | 2.7% |
| Fluoroquinolone | 313 | 7.2% | 2.4% | Cephalosporin (first generation) | 48 | 7.0% | 2.5% |
| Lincosamide | 242 | 5.6% | 1.9% | Lincosamide | 28 | 4.1% | 1.4% |
FIGURE 8.
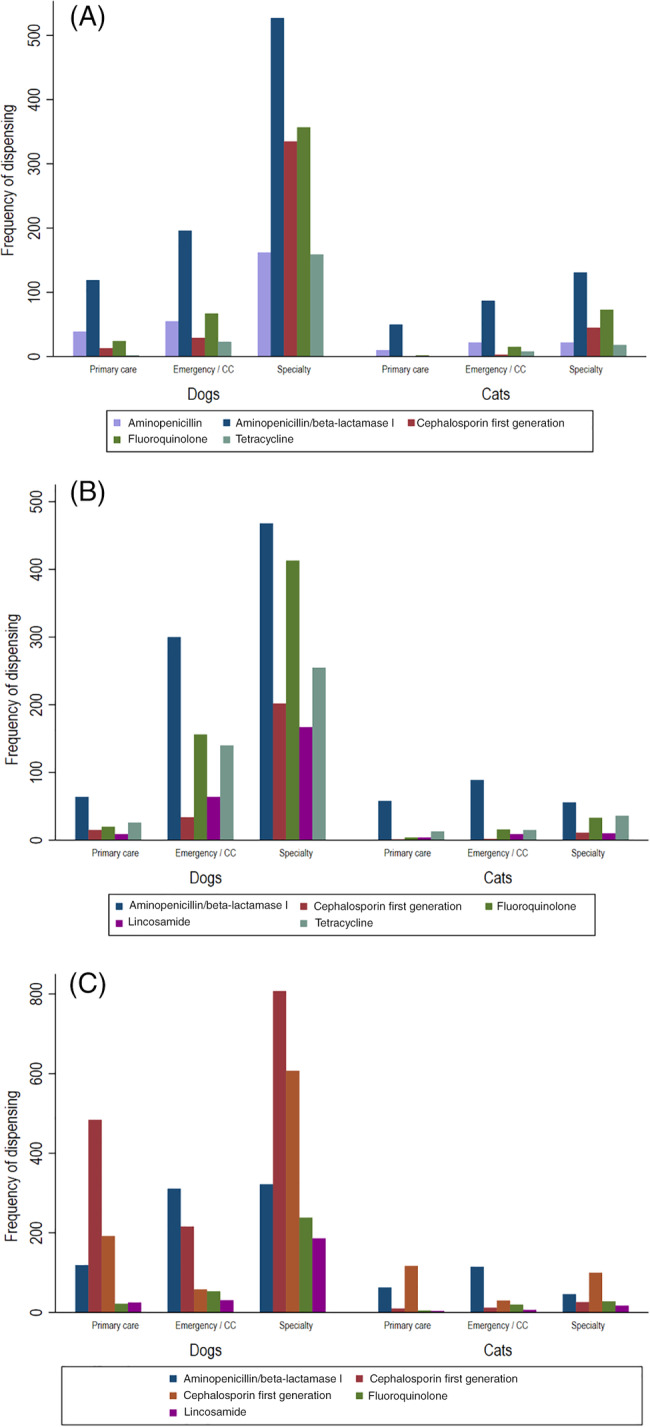
Antimicrobial drug dispensing in animals with urinary tract, respiratory, and skin infections. The frequency of dispensing of the 5 most common antimicrobial drugs by service, in animals with (A) urinary tract infections (n = 3344), (B) respiratory tract infections (n = 3322), and (C) skin infections (n = 5016)
4. DISCUSSION
Our study assessed AMD‐prescribing patterns in primary care, specialty medicine, and ECC services at 3 large veterinary teaching hospitals. Demographics were similar across hospitals with most animals presented to specialty services rather than primary care, consistent with the institutions involved. Across hospitals and species, the probability of AMD prescription was similar to that previously reported. 17 A study from the UK small animal veterinary surveillance network (SAVSNET) gathered data from 22 859 consultations at 16 small animal practices. During the 3‐month study period, the proportion of consults associated with AMD prescribing ranged from 0.26 to 0.55 in dogs and 0.41 to 0.73 in cats. 17 In our study, the probability of AMD prescription by practice type was similar across hospitals except for increased probability of prescription by ECC at hospital 1. The probability of AMD prescription was lowest for primary care across all hospitals, concomitant with frequent prescribing of aminopenicillin/β‐lactamase inhibitors and infrequent prescribing of fluoroquinolones. The cause of the higher prescribing frequency in ECC at hospital 1 is uncertain and likely multifactorial. Possible explanations include dissimilarities in types, patterns and severity of illness, differences in clinician preference, training or willingness to prescribe, and presence of more stringent local restrictions on prescribing in other locations.
In a 4‐year retrospective cohort study using a pet insurance database, the overall rate of AMD prescribing was 5.8 prescriptions per 10 dog‐years and 3.1 prescriptions per 10 cat‐years. 23 In addition, the authors identified seasonality of AMD prescribing, with rates of prescribing increasing in dogs in the spring and summer and decreasing in cats in the summer compared to the winter. This seasonality might reflect local geographic and climatic effects, but it is important when attempting to compare prescribing data collected annually compared to those collected for a limited time period as in the SAVSNET study. 17 Of the prescriptions identified through the insurance database analyses, critically important AMDs accounted for 8% of all the AMDs prescribed. Cats were 4.8‐fold more likely than dogs to be prescribed third‐generation cephalosporins, predominantly because of prescribing of cefovecin. In our study, cats were prescribed third‐generation cephalosporins 1.95‐fold more frequently than dogs, also due to preferential cefovecin prescribing, while cefpodoxime was the most commonly prescribed third‐generation cephalosporin in dogs.
Our study involves nearly 80 000 visits with an AMD prescription, and analyzing data on that scale was challenging. A recent, innovative study used automated natural language processing to identify AMD prescriptions from 4.4 million electronic veterinary medical records. 20 In that study, the rate of AMD prescribing was 0.145 for dogs and 0.108 for cats. Critically important AMDs were prescribed at rates of 0.038 and 0.047 for dogs and cats, respectively. Rates of AMD prescribing in emergency and referral centers were 0.250, significantly greater than those in primary care. The overall rates of prescribing in our study (0.15‐0.28) were comparable, and we also identified clear differences between rates of prescribing in different practice types.
Among the WHO critically important AMD classes, fluoroquinolones and third‐generation cephalosporins were most commonly prescribed. Reassuringly, and consistent with veterinary guidelines, 28 carbapenems, glycopeptides, and oxazolidinones were rarely prescribed in our study. The frequency of carbapenem usage in our study was comparable to that reported from a separate North American veterinary tertiary care hospital where 68 animals were treated with carbapenems in a 12‐month period. 13 On average across all 3 hospitals, 41 visits per year were associated with carbapenem prescribing in the present study. Usage of third‐generation cephalosporins and fluoroquinolones varied between hospitals and services. The probability of third‐generation cephalosporin use was consistent across service types at hospital 1, whereas at hospital 2, use was greater in specialty services. In hospital 3, the pattern was complex, with use in dogs declining from 2013 to 2017, while use in cats increased from 2014 to 2015 in primary care and steadily increased in ECC from 2014 to 2017. These trends might reflect alterations in populations or changes in disease prevalence, but ease of administration, safety, and efficacy of drugs including FDA veterinary approved cefovecin and cefpodoxime might have influenced clinician behavior. 23 , 29 , 30 The probability of fluoroquinolone prescribing was greater for specialty services, except at hospital 1 where the probability was greatest for ECC. These differences might reflect hospital policies, clinician preference, local AMR patterns, or differences in the proportion of first‐ and second‐opinion cases examined at hospital 1 compared to other centers. 31 Geography might affect local disease prevalence and influence the types of cases presented to ECC because of variation in the availability of other veterinary practices in the catchment area of each hospital. Comparably, the probability of fluoroquinolone prescribing to cats seen by specialty services at hospital 2 was greater than for dogs, perhaps reflecting differences in rates of respiratory or urinary tract infections. Categorizing cats as indoor versus outdoor in future studies might provide some insights into these patterns.
Patterns of AMD prescribing in our study were consistent with to previous studies wherein aminopenicillins and aminopenicillin/β‐lactamase inhibitors were most frequently prescribed in companion animals. 7 , 8 , 9 , 17 , 29 , 32 , 33 Although the indication for every AMD prescription could not be definitively determined here, data from 2 hospitals were available to determine the indication for AMD prescriptions in animals with skin infections, UTIs, and RTIs. While noting the limitations imposed by this approach, aminopenicillins and aminopenicillin/β‐lactamase inhibitors were most commonly prescribed in animals with UTI and RTIs across all service types. This is also consistent with data from a network of private practices, 15 and in keeping with recommendations from the International Society for Companion Animal Infectious Diseases (ISCAID). 34 , 35 Previous studies have suggested that veterinarians commonly deviate from published guidelines through excessive prescribing, 29 inadvisable AMD choice, 36 or limited submissions for culture and susceptibility testing. 7 , 32 Tetracyclines, lincosamides, and fluoroquinolones were also commonly prescribed for RTIs in this study. Current guidelines recommend the use of tetracyclines or lincosamides for mild to moderate disease and fluoroquinolones for severe disease. 35 In our study, it was not possible to determine the severity of respiratory disease. Current guidelines were published in March 2017 and would not have been fully implemented within the timeframe of our study. Those guidelines list doxycycline as the first choice for 5 of the most common respiratory conditions in dogs and cats. Given the overall probabilities of fluoroquinolone prescribing in the present study, it seems likely that over‐prescribing occurred. Prescriptions for skin infections were also largely consistent with ISCAID guidelines with aminopenicillin/β‐lactamase inhibitors, first‐generation cephalosporins, and lincosamides commonly prescribed. 37 Skin infections were most commonly treated with first‐generation cephalosporins in primary care and specialty services, consistent with a study from 2010 that reported the most frequently prescribed drugs for bacterial pyoderma were amoxicillin‐clavulanate, cephalexin, clindamycin, and cefovecin. 38 Skin infections were more frequently treated with aminopenicillin/β‐lactamase inhibitors by ECC, which likely resulted from inclusion of wounds within the category of skin infections.
There was a considerable variation in AMD prescribing over time within our study. There were some trends toward reduced prescribing over time, for instance all AMD prescribing in dogs and in ECC in hospital 1 from 2015 to 2017 and overall prescribing in dogs and cats in hospital 3 over the same period. This could relate to improved recognition and use of prescribing guidelines, 34 , 39 an increased focus on stewardship by clinicians at participating hospitals and within the profession in general. This is consistent with trends elsewhere in veterinary medicine. 6 , 30 , 40 Although there remains room for improvement within each hospital and the profession at large, these are encouraging signs. Other potential explanations include alterations in personnel, publication of studies informing prescribing practices, and variations in bacterial antimicrobial resistance patterns. Future studies should focus on evaluating the factors affecting AMD prescribing decisions by veterinarians and the relationship between use of bacterial susceptibility testing and AMD prescribing in veterinary medicine, because it is recognized that AMDs are frequently prescribed in the absence of evidence of bacterial infection. 10 This suggests that future stewardship efforts should also focus on improving education and enhancing the diagnosis and confirmation of bacterial infection in dogs and cats.
Our study has limitations. The AMD prescribing service was classified as primary care, specialty care, or ECC. Classification of cases as primary or specialty care was straightforward, but cases managed by ECC could not be readily separated and hence were described separately. In each participating institution, ECC cases were a mixture of walk‐in emergencies that could reasonably be classified as primary care, and cases referred to as emergencies by other veterinarians. Some referred animals were not critically ill, but rather were referred to as emergencies to secure more urgent specialty care than was achievable by appointment. This might have skewed some of the prescribing behavior reported and the pattern, frequency, and nature of the cases presented to the ECC services at the participating institutions. Varying levels of pharmacy refill data and difficulties in attributing those to original service made these a potential source of differences between hospitals. It was also possible that animals could have been presented to all 3 service types (primary care, ECC, and specialty care) and on more than 1 occasion all for the same problem. It was not possible to discern this using the data collection methods necessary to evaluate 393 000 visits. The likely effect of some animals visiting more than 1 service for the same problem would be to mildly inflate the probability of prescribing estimates, but in the scale of the data this is considered to have a minor effect.
It was not possible to differentiate inpatients from outpatients within our dataset and hence all animals prescribed AMDs are included. The decision to hospitalize animals or discharge them for care at home likely impacted prescribing practices by altering frequency of prescribing, drug choices within a class, for instance ampicillin/sulbactam vs amoxicillin/clavulanate and potentially choice of drug class such as aminoglycosides vs fluoroquinolones. It is likely that most primary care cases were managed on an outpatient basis, while most specialty care cases were hospitalized, with ECC cases likely representing a mixture of both inpatient care and outpatient care. However, the precise distribution cannot be established with certainty and might have varied with hospital and over time. Future studies should consider the impact of inpatient care vs outpatient care on the nature and frequency of AMD prescribing. It was also not possible to differentiate indications for usage of some classes of medications, for instance perioperative prophylaxis vs therapeutic administration. Inferences can be made for some classes such as the first‐generation cephalosporins, but these comparisons could also be biased if different proportions of surgical procedures were occurring in different hospitals or distinct service types.
Future efforts in this field might focus on creating consensus within the profession regarding the nature, extent, and specifics of data on AMD prescribing that should be collected within veterinary EMRs, potentially through engaging professional bodies, large corporate veterinary practices, and veterinary practice management software companies. Financial incentives, altruistic and professional conduct motivations, and regulatory requirements might be necessary to drive establishment of standards for collection of AMD prescription data in veterinary medicine. 41 , 42 , 43 Such efforts will be essential if veterinarians are to fully engage with the challenges of antimicrobial stewardship.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Off‐label use of the following antimicrobials: Amikacin; Amoxicillin; Amoxicillin/Clavulanate; Ampicillin; Ampicillin/Sulbactam; Azithromycin; Bacitracin, Polymyxin B; Cefadroxil; Cefazolin; Cefovecin; Cefoxitin; Cefpodoxime; Ceftazidime; Ceftiofur; Ceftotaxime; Ceftriaxone; Cephalexin; Chloramphenicol; Ciprofloxacin; Clarithromycin; Clindamycin; Doxycycline; Enrofloxacin; Erythromycin; Florfenicol; Gatifloxacin; Gentamicin; Imipenem; Linezolid; Marbofloxacin; Meropenem; Metronidazole; Minocycline; Mupirocin; Neomycin; Neomycin/Polymyxin B; Neomycin/Polymyxin B/Bacitracin; Neomycin/Thiostrepton; Nitrofurantoin; Nitrofurazone; Ofloxacin; Orbifloxacin; Oxytetracycline/Polymyxin B; Penicillin G Potassium; Penicillin G Procaine; Piperacillin/Tazobactam; Polymyxin B; Pradofloxacin; Rifampin; Sulfadiazine; Sulfadimethoxine; Sulfasalazine; Tetracycline; Tobramycin; Trimethoprim/Sulfadiazine; Trimethoprim/Sulfamethoxazole; Tylosin; Vancomycin.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed. The study was exempt from IACUC approval because it analyzed prescribing data from clinician‐driven care provided to animals at the institution hospitals. Confidentiality of animal and client data was strictly maintained and no animal or client identifying information is reported.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENT
This work was supported by the Centers for Disease Control and Prevention (1605554‐DOMAIN 8: RFTOP 2016‐8 Antibiotic use, Resistance and Stewardship in Veterinary Practice). The authors thank the information technology specialists who repeatedly extracted and provided data or created novel search codes for this work, especially Mr. Larry Parlett, Ms. Isabelle Schweitzer, and Mr. Scott Ross at Cornell University, Glenn Waters and Patti Andrews at North Carolina State University, and Ms. Jenny Chen and Mr. Joel Hammond at Texas A&M University. We also thank the pharmacists at Texas A&M University, Drs. Tia Nieuwoudt, Amy Savarino, and Madeline Droog, for helpful conversations.
Goggs R, Menard JM, Altier C, et al. Patterns of antimicrobial drug use in veterinary primary care and specialty practice: A 6‐year multi‐institution study. J Vet Intern Med. 2021;35:1496–1508. 10.1111/jvim.16136
Robert Goggs, Julie M. Menard, Megan E. Jacob, Keri N. Norman, Virginia R. Fajt, H. Morgan Scott, and Sara D. Lawhon contributed equally to this study.
Funding information Centers for Disease Control and Prevention, Grant/Award Number: 1605554‐DOMAIN 8: RFTOP 2016‐8
REFERENCES
- 1. Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States. Atlanta, GA: U.S. Department of Health and Human Services; 2019. [Google Scholar]
- 2. American Veterinary Medical Association . Antimicrobial‐Resistant Pathogens Affecting Animal Health. Schaumburg, IL: AVMA; 2020. [Google Scholar]
- 3. Chomel BB, Sun B. Zoonoses in the bedroom. Emerg Infect Dis. 2011;17:167‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Veterinary Medical Association . U.S. Pet Ownership Demographics Sourcebook. Schaumburg, IL: AVMA; 2012. [Google Scholar]
- 5. American Veterinary Medical Association . Antimicrobial Stewardship Definition and Core Principles. Schaumberg, IL: AVMA; 2020. [Google Scholar]
- 6. Weese JS. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995‐2004. J Am Vet Med Assoc. 2006;228:553‐558. [DOI] [PubMed] [Google Scholar]
- 7. Escher M, Vanni M, Intorre L, Caprioli A, Tognetti R, Scavia G. Use of antimicrobials in companion animal practice: a retrospective study in a veterinary teaching hospital in Italy. J Antimicrob Chemother. 2011;66:920‐927. [DOI] [PubMed] [Google Scholar]
- 8. Rantala M, Huovinen P, Hölsö K, Lilas A, Kaartinen L. Survey of condition‐based prescribing of antimicrobial drugs for dogs at a veterinary teaching hospital. Vet Rec. 2004;155:259‐262. [DOI] [PubMed] [Google Scholar]
- 9. Hölsö K, Rantala M, Lillas A, Eerikäinen S, Huovinen P, Kaartinen L. Prescribing antimicrobial agents for dogs and cats via university pharmacies in Finland—patterns and quality of information. Acta Vet Scand. 2005;46:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wayne A, McCarthy R, Lindenmayer J. Therapeutic antibiotic use patterns in dogs: observations from a veterinary teaching hospital. J Small Anim Pract. 2011;52:310‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weese JS, Faires MC, Frank LA, Reynolds LM, Battisti A. Factors associated with methicillin‐resistant versus methicillin‐susceptible staphylococcus pseudintermedius infection in dogs. J Am vet Med Assoc. 2012;240:1450‐1455. [DOI] [PubMed] [Google Scholar]
- 12. Nienhoff U, Kadlec K, Chaberny IF, et al. Methicillin‐resistant staphylococcus pseudintermedius among dogs admitted to a small animal hospital. Vet Microbiol. 2011;150:191‐197. [DOI] [PubMed] [Google Scholar]
- 13. Smith A, Wayne AS, Fellman CL, Rosenbaum MH. Usage patterns of carbapenem antimicrobials in dogs and cats at a veterinary tertiary care hospital. J Vet Intern Med. 2019;33:1677‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy CP, Reid‐Smith RJ, Boerlin P, et al. Out‐patient antimicrobial drug use in dogs and cats for new disease events from community companion animal practices in Ontario. Can Vet J. 2012;53:291‐298. [PMC free article] [PubMed] [Google Scholar]
- 15. North American Veterinary Conference, Banfield Pet Hospital . Are we doing our part to prevent superbugs? Antimicrobial usage patterns among companion animal veterinarians. Veterinary Emerging Topics (VET) Report. 2017.
- 16. Watson AD, Maddison JE. Systemic antibacterial drug use in dogs in Australia. Aust Vet J. 2001;79:740‐746. [DOI] [PubMed] [Google Scholar]
- 17. Radford AD, Noble PJ, Coyne KP, et al. Antibacterial prescribing patterns in small animal veterinary practice identified via savsnet: the small animal veterinary surveillance network. Vet Rec. 2011;169:310. [DOI] [PubMed] [Google Scholar]
- 18. Hardefeldt L, Hur B, Verspoor K, et al. Use of cefovecin in dogs and cats attending first‐opinion veterinary practices in Australia. Vet Rec. 2020;187:e95. [DOI] [PubMed] [Google Scholar]
- 19. Tompson AC, Chandler CIR, Mateus ALP, O'Neill DG, Chang YM, Brodbelt DC. What drives antimicrobial prescribing for companion animals? A mixed‐methods study of UK veterinary clinics. Prev Vet Med. 2020;183:105117. [DOI] [PubMed] [Google Scholar]
- 20. Hur BA, Hardefeldt LY, Verspoor KM, Baldwin T, Gilkerson JR. Describing the antimicrobial usage patterns of companion animal veterinary practices; free text analysis of more than 4.4 million consultation records. PLoS One. 2020;15:e0230049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hur B, Hardefeldt LY, Verspoor K, Baldwin T, Gilkerson JR. Using natural language processing and vetcompass to understand antimicrobial usage patterns in Australia. Aust Vet J. 2019;97:298‐300. [DOI] [PubMed] [Google Scholar]
- 22. Buckland EL, O'Neill D, Summers J, et al. Characterisation of antimicrobial usage in cats and dogs attending UK primary care companion animal veterinary practices. Vet Rec. 2016;179:489. [DOI] [PubMed] [Google Scholar]
- 23. Hardefeldt LY, Selinger J, Stevenson MA, et al. Population wide assessment of antimicrobial use in dogs and cats using a novel data source—a cohort study using pet insurance data. Vet Microbiol. 2018;225:34‐39. [DOI] [PubMed] [Google Scholar]
- 24. Singleton DA, Noble PJM, Sánchez‐Vizcaíno F, et al. Pharmaceutical prescription in canine acute diarrhoea: a longitudinal electronic health record analysis of first opinion veterinary practices. Front Vet Sci. 2019;6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pedersen K, Pedersen K, Jensen H, Finster K, Jensen VF, Heuer OE. Occurrence of antimicrobial resistance in bacteria from diagnostic samples from dogs. J Antimicrob Chemother. 2007;60:775‐781. [DOI] [PubMed] [Google Scholar]
- 26. World Heath Organization . Critically Important Antimicrobials for Human Medicine. 6th ed. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 27. United States Food & Drug Administration . Concept Paper: Potential Approach for Ranking of Antimicrobial Drugs According to their Importance in Human Medicine: A Risk Management Tool for Antimicrobial New Animal Drugs. Washington, D.C.: US Food & Drug Administration, Health & Human Services; 2020. [Google Scholar]
- 28. American Veterinary Medical Association . AAFP/AAHA Basic Guidelines of Judicious Therapeutic Use of Antimicrobials. In. Schaumburg, IL: AVMA; 2020. [Google Scholar]
- 29. Hardefeldt LY, Holloway S, Trott DJ, et al. Antimicrobial prescribing in dogs and cats in Australia: results of the australasian infectious disease advisory panel survey. J vet Intern Med. 2017;31:1100‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singleton DA, Sánchez‐Vizcaíno F, Dawson S, et al. Patterns of antimicrobial agent prescription in a sentinel population of canine and feline veterinary practices in the United Kingdom. Vet J. 2017;224:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robbins SN, Goggs R, Lhermie G, Lalonde‐Paul DF, Menard J. Antimicrobial prescribing practices in small animal emergency and critical care. Front Vet Sci. 2020;7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Cleven A, Sarrazin S, de Rooster H, Paepe D, van der Meeren S, Dewulf J. Antimicrobial prescribing behaviour in dogs and cats by belgian veterinarians. Vet Rec. 2018;182:324. [DOI] [PubMed] [Google Scholar]
- 33. Kvaale MK, Grave K, Kristoffersen AB, Norström M. The prescription rate of antibacterial agents in dogs in Norway—geographical patterns and trends during the period 2004‐2008. J Vet Pharmacol Ther. 2013;36:285‐291. [DOI] [PubMed] [Google Scholar]
- 34. Weese JS, Blondeau J, Boothe D, et al. International society for companion animal infectious diseases (iscaid) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J. 2019;247:8‐25. [DOI] [PubMed] [Google Scholar]
- 35. Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: antimicrobial guidelines working group of the international society for companion animal infectious diseases. J vet Intern Med. 2017;31:279‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hardefeldt LY, Gilkerson JR, Billman‐Jacobe H, et al. Barriers to and enablers of implementing antimicrobial stewardship programs in veterinary practices. J Vet Intern Med. 2018;32:1092‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hillier A, Lloyd DH, Weese JS, et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (antimicrobial guidelines working group of the international society for companion animal infectious diseases). Vet Dermatol. 2014;25:163‐e143. [DOI] [PubMed] [Google Scholar]
- 38. Summers JF, Hendricks A, Brodbelt DC. Prescribing practices of primary‐care veterinary practitioners in dogs diagnosed with bacterial pyoderma. BMC Vet Res. 2014;10:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weese JS, Giguère S, Guardabassi L, et al. Acvim consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J vet Intern Med. 2015;29:487‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barzelai ID, Whittem T. Survey of systemic antimicrobial prescribing for dogs by victorian veterinarians. Aust Vet J. 2017;95:375‐385. [DOI] [PubMed] [Google Scholar]
- 41. Doyon C. Best practices in record completion. J Med Pract Manage. 2004;20:18‐22. [PubMed] [Google Scholar]
- 42. Stevens JC. Capturing incentives and avoiding penalties: the carrot and the stick. Neurol Clin Pract. 2013;3:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manchikanti L, Benyamin RM, Falco FJ, Hirsch JA. Metamorphosis of medicine in the United States: a carrot and stick policy of electronic medical records. Pain Physician. 2014;17:E671‐E680. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Supporting Information
Supporting Information


