Abstract
The development of innovator biologics and now their biosimilars has created some unique challenges in oncology practice. The oncology advanced practitioner (OAP) must understand the key differences between the innovator biologic and biosimilars in regard to efficacy, safety, and immunogenicity. In addition, the OAP must be able to evaluate and successfully navigate factors that may affect the adoption of biosimilars, such as the perceived cost-benefit and clinician and patient acceptance.
A biosimilar product is highly similar to a U.S. Food & Drug Administration (FDA)-approved biologic product and does not have clinically meaningful differences in regards to safety, purity, and potency (FDA, 2018). Multiple surveys reveal health-care providers have inadequate knowledge about biosimilars, including basic information about drug development, the FDA regulatory process, and the safety and efficacy of biosimilars, particularly among office-based physicians (Leonard et al., 2019). It is also known that adequate health-care provider knowledge and patient education will lead to the most successful prescribing changes (Chan et al., 2019). The oncology advanced practitioner (OAP) must be knowledgeable about biosimilars and play an active role in assessing the agents for integration into clinical practice. Throughout this article, we will describe current challenges to integration and discuss potential solutions.
Background
According to the FDA, a biosimilar product is "highly similar to an FDA-approved reference biological product although there may be minor differences in clinically inactive components" (FDA, 2018). There should be no clinically meaningful differences in regards to safety, purity, and potency of the product between the biosimilar and the reference biological product (FDA, 2018).
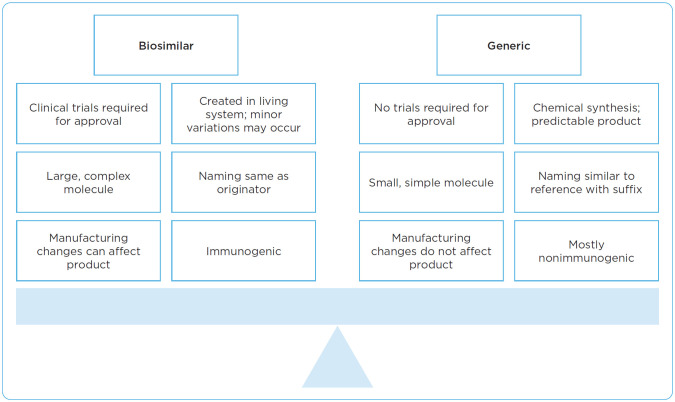
Biosimilars are not considered generics. To understand this difference, it is helpful to first consider how biologic products and small molecules differ with respect to their fundamental properties and manufacturing processes (Figure 1). In terms of structure and size, small-molecule drugs are simple structures with low molecular weight in comparison with biologic products that have high molecular weight, complex structures and, on average, are 100- to 1,000-fold larger than small-molecule drugs (FDA, 2018).
Figure 1.
Biosimilar product and generic product comparison. Information from FDA (2017).
In addition, the process of developing a biosimilar is far more complicated than a standard generic equivalent of a small-molecule medication. Manufacturing biologic products is a complex, multistage process involving cloning of relevant protein of interest, transfection into host cells, cell screening and selection, and lastly, large-scale protein expression and purification (Tinsley et al., 2018). During the manufacturing process, biologic agents are unable to precisely duplicate the proteins, which leads to slight differences in the resulting products; this is expected and occurs with reference or biosimilar products. Those components of biologic products could be affected by changes in temperature or sterility.
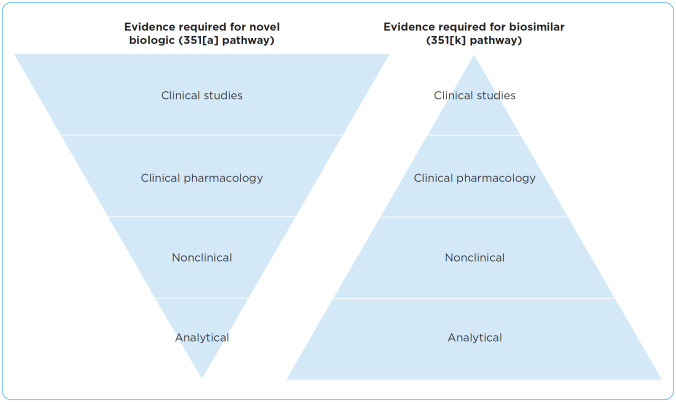
There is an abbreviated pathway for obtaining FDA approval for biosimilars compared with that for novel biologics. The 351(k) pathway for the approval of biosimilars in the United States was established by the Biologics Price Competition and Innovation Act of 2009. The four major elements required in approval are analytical analysis, nonclinical studies, clinical pharmacology, and clinical studies, which can be seen in Figure 2 (Lucio, 2018). Each of these elements are accomplished through a step-wise approach to determine the totality of evidence for the biosimilar. During the analytical analysis, the structure and function of the molecule is confirmed. Nonclinical studies assess the biosimilar mechanism of action and associated toxicities. The assessment of pharmacokinetic and pharmacodynamics markers occurs during the clinical pharmacology component. Lastly, if needed, the efficacy, safety, and immunogenicity is analyzed with clinical studies.
Figure 2.
Biosimilar approval process. Adapted from FDA (2017).
Needs in Clinical Practice
One of the primary challenges to the successful adoption of biosimilars is inadequate knowledge on the part of the health-care provider. Table 1 describes major gaps in provider knowledge (Cohen et al., 2016). Literature reviews show that self-study, peer-reviewed journals, and professional guidelines are the most trusted resources for health-care professionals in regards to educating themselves on biosimilars (Leonard et al., 2019). Lack of awareness and understanding of biosimilars can result in decreased access or increased costs to health systems and patients.
Table 1. Knowledge Gaps of Health-Care Providers Regarding Biosimilars.
|
Note. Information from Cohen et al. (2016); Leonard et al. (2019).
Key Concepts
Interchangeability or substitution is one of the key concepts for the OAP to understand. Interchangeability between pharmaceutical agents means that the interchanged drug is expected to produce the same clinical results as the reference product in any given patient (FDA, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 2019).
Generic products are generally considered interchangeable to their reference product. To date, no biosimilar has been deemed interchangeable with their reference product. This may change as more products come to market and with increasing clinical evidence for currently available agents. Although the FDA designates interchangeable status, state laws are the authority regarding substitution of products (Ventola, 2013). The National Conference of State Legislators maintains a database of state laws for easy reference (http://www.ncsl.org/research/health/state-laws-and-legislation-related-to-biologic-medications-and-substitution-of-biosimilars.aspx#2013-14). Every OAP should be familiar with their state laws regarding interchangeability.
Formulary Approaches
There are various approaches to the review, approval, and inclusion of biosimilars into clinical practice. In most instances, the Pharmacy & Therapeutics (P&T) committee reviews products for inclusion onto hospital/organizational formulary and approves policies and procedures regarding these. The P&T committee may include subcommittees for specific areas such as an oncology subcommittee or a biosimilar subcommittee. P&T committees may rely on analytical or scientific equivalence data to review rather than clinical data in the case of biosimilars (Ventola, 2013). There are several questions that are considered by P&T committees, which are noted in Table 2. (Note that this list is not all inclusive.)
Table 2. Questions Considered by the P&T Committee Regarding Biosimilars.
|
Note. REMS = Risk Evaluation and Mitigation Strategies. Information from Ventola (2013).
Electronic Medical Record Integration
Electronic medical record integration of biosimilars may present some unique challenges. First, initial decisions about the “default” drug must be made. For example, a facility may choose to default to the reference product. Insurance will remain a driver of product selection, which may require the organization to modify plans on a per patient basis from the defaulted product. If the reference product remains the default, the organization may avoid the personnel costs related to modifying all treatment plans, but may miss out on cost savings from those payors who consider the products at parity.
Some facilities may choose to default to a biosimilar product. This has the potential for cost savings to the practice but will require work up front. If insurance will not cover the chosen biosimilar or if the patient cannot tolerate it, then changes are made on a per patient basis. This may result in large cost savings to the organization, but does result in higher personnel costs to make modifications to the plans up front.
A third choice of facilities might be to include all choices, both reference product and all biosimilars. There is potential for cost savings if providers choose biosimilars. However, this relies on the health-care provider to be knowledgeable about all the options for informed decision-making and may put additional pressure on the provider to seek out information regarding the patient’s insurance preferences prior to making a selection. Lastly, this decision will require the pharmacy to stock all therapeutic options. This can result in higher drug budgets and requires additional space in the pharmacy, which is already a commodity in most hospital pharmacies.
Financial Considerations: It’s Not Just About the Cost of the Drug
There are also financial factors for health-care providers to consider. As discussed previously, there are costs related to plan maintenance or updates, either up front or on a per patient basis. If the organization chooses to carry all products, this may result in higher drug budgets and requires additional space in the pharmacy.
In terms of dollar-for-dollar costs, drug pricing depends on purchasing agreements and contracting opportunities. However, the purchasing price is only half of the equation. Reimbursement is also an important factor to consider. Biosimilars are eligible for pass-through payment status through the outpatient prospective payment system (OPPS) rule for drugs acquired under the 340B Drug Pricing Program (Fein, 2018). For organizations that purchase drugs under the 340B program, this may provide for a larger profit margin as compared with the reference product.
Provider Concerns: Efficacy Considerations
Oncology health-care providers may have some concerns regarding the integration of biosimilars into their practice. As noted earlier, many providers have inadequate knowledge of biosimilars, and this lack of knowledge may lead to conflict within the health-care system during the integration process. Some concerns expressed from health-care providers regard efficacy, immunogenicity, and staff knowledge.
The FDA approval pathway allows for a biosimilar product to be approved in an indication without direct studies of the biosimilar in that indication (see Table 3 for FDA-approved biosimilars in oncology). If the total body of evidence submitted to the FDA supports the biosimilarity for at least one reference product indication, the FDA allows for approval to other indications through "extrapolation." Extrapolation is the approval of a biosimilar product for use in an indication held by the originator product, but that was not directly studied in a comparative clinical trial with the biosimilar (Tesser et al., 2017). The FDA works with each biosimilar manufacturer to determine what data is needed to support extrapolation (FDA, 2017). Not all indications qualify for extrapolation, such as orphan drug status, leading to “skinny labels” or labels that are missing some indications when compared to the reference product.
Table 3. FDA-Approved Biosimilars in Oncology.
| Reference biological product | Approved biosimilar product(s) | Date approved |
|---|---|---|
| Neupogen (filgrastim) | Nivestym (filgrastim-aafi) Zarxio (filgrastim-sndz) | July 20, 2018 March 6, 2015 |
| Epogen/Procrit (epoetin alfa) | Retacrit (epoetin alfa-epbx) | May 15, 2018 |
| Neulasta (pegfilgrastim) | Fulphila (pegfilgrastim-jmdb) Udenyca (pegfilgrastim-cbqv) Ziextenzo (pegfilgrastim-bmez) Nyvepria (pegfilgrastim-apgf) | June 4, 2018 November 2, 2018 November 4, 2019 June 11, 2020 |
| Herceptin (trastuzumab) | Ogivri (trastuzumab-dkst) Herzuma (trastuzumab-pkrb) Kanjinti (trastuzumab-anns) Ontruzant (trastuzumab-dttb) Trazimera (trastuzumab-qyyp) | December 1, 2017 December 14, 2018 June 13, 2019 January 18, 2019 March 11, 2019 |
| Avastin (bevacizumab) | Mvasi (bevacizumab-awwb) Zirabev (bevaziumab-bvzr) | September 14, 2017 June 27, 2019 |
| Rituxan (rituximab) | Truxima (rituximab-abbs) Ruxience (rituximab-pvvr) Riabni (rituximab-arrx) | November 28, 2018 July 23, 2019 December 17, 2020 |
Note. Information from FDA (2021)
As a result, in considering efficacy, there may be apprehension about the data for indications or the lack of superiority testing of biosimilars (for example, if the reference product had an objective response of 75.5% at 26 weeks compared to the biosimilar with 71.7% objective response). Another example might be if studies were done in the metastatic setting, but the product would also be used in the curative setting.
There may also be a concern surrounding immunogenicity as these are biologic products. Immunogenicity is the propensity of the therapeutic protein product to generate immune responses to itself (FDA, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 2019). This may lead to neutralizing antibodies or a cytokine release. While there are tools for detecting immunogenicity, they lack precision (FDA, 2014). Other immunogenicity concerns include the potential loss of efficacy or safety and possible serious adverse reactions. Variations in manufacturing must be minimized so as to decrease the conceivable changes to the structure of the protein.
Lastly, differences in administration must be considered. For example, if the reference product may be given at rapid infusion rates, can that data be extrapolated to the biosimilar? Biosimilars have been approved and in use in Europe for a longer period of time, so we may need to rely on international data to help to guide our decisions. Health-care providers must also understand if there are differences in concentrations, fluids, or tubing to ensure that safety measures and smart-pump technologies are accurate as health-care providers begin use of a new product.
Developing a Practice Process
While considering all these factors as well as education needs and concerns, the facility must develop a thorough integration practice process. One of the best examples is the Berkshire West experience (Chan et al., 2019). This study reported the outcomes of a facility switching from a reference product to a biosimilar. Initially, the practice set up a biosimilar working group that defined the integration plan that was more patient focused. Letters and educational materials were sent to patients, providers had personal discussions with patients, educational events were held for patients, and patients’ financial factors were considered. Results of this study indicated a high percentage of successful switches compared with practices that did not utilize this process. There were no increased adverse events, and the facility was able to decrease costs.
The integration process developed by the facility or practice must address both educational gaps as well as potential challenges. This mutually agreed-upon process must be written, clear, and transparent prior to its initiation. This process must begin with clinician and staff education as a priority. Communication about biosimilar insertion into practice should be part of each step, transparent to all stakeholders, and address concerns. Education regarding biosimilars must begin with clinicians and then staff must soon follow (Cuellar, 2020). Thorough and consistent patient education is a vital key. Education to financial staff is also crucial. Close monitoring of patients’ cost is critical. Each biosimilar should be monitored closely for postapproval efficacy and safety, with providers promptly reporting and addressing any potential concerns. The integration process should include a feedback system with regular reports given to all stakeholders at their educational level.
Patient Concerns
In many settings, OAPs are an integral provider of patient education. Like some providers, patients, too, have inadequate knowledge about biosimilars. Fears must be addressed with patients and their questions must be answered to ensure their satisfaction. Media reports of patent litigations may fuel patient fears. Education must be at the patient’s educational level. Most biosimilar educational materials, including those of the FDA, are written at reading levels too high for most patients. For instance, the FDA resource, "What is a Biosimilar?" is written at a 14 grade level by Flesch-Kincaid scoring (FDA, 2019). Pharmaceutical patient educational materials may be written at an even higher level. As a result, many health systems have developed their own patient education materials (Figure 3).
Figure 3.
Example of a patient handout on biosimilars. Used with permission from Wendy Vogel, MSN, FNP, AOCNP®, and Matthew Brignola, PharmD.
Another factor to be considered by health-care providers is the nocebo effect. The nocebo effect is the negative effect of treatment as a result of a patient’s perceived expectations (Kabir et al., 2019). This effect can negatively impact adherence rates and can be minimized by adequate patient education.
Summary
Biosimilars will continue to play an important role in the future care of oncology patients. Oncology advanced practitioners must understand and be able to articulate what biosimilars are and what they mean to colleagues, patients, and staff. It is imperative that OAPs perform an objective analysis of comparative data between each biosimilar product and the reference product in terms of efficacy, safety, administration, and cost considerations. Oncology advanced practitioners should be able to appropriately educate patients on the benefits and risks of utilizing biosimilar products in their cancer treatment. Educational materials developed by the OAP should be at an appropriate grade level for the learner. Oncology advanced practitioners are integral to the success of biosimilar integration into oncology practice.
Footnotes
The authors have no conflicts of interest to disclose.
Reference
- Chan A., Kitchen J., Scott A., Pollock D., Marshall R., & Herdman L. (2019).Implementing and delivering a successful biosimilar switch programme – The Berkshire West experience. Future Healthcare Journal, 6(2), 143–145. 10.7861/futurehosp.6-2-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H.Beydoun, D., Chein D., Lessor T., McCabe D., Muenzberg M., … Uy J. (2016).Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Advances in Therapy, 33,2160–2172. 10.1007/s12325-016-0431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuellar S. (2020).Integrating trastuzumab-biosimilars and HER2-directed therapies into HER2-positive breast cancer management. American Journal of Managed Care, 26(2 Suppl), S32–S40. 10.37765/ajmc.2020.42900 [DOI] [PubMed] [Google Scholar]
- Fein A. J. (2018).The mysteries of pass-through status: Why Medicare and seniors are now paying more for lower-cost biosimilars at hospitals. https://www.drugchannels.net/2018/04/the-mysteries-of-pass-through-status.html [Google Scholar]
- Kabir E. R., Moreino S. S., & Siam M. K. (2019).The breakthrough of biosimilars: A twist in the narrative of biological therapy. Biomolecules, 9(9), 410–444. 10.3390/biom9090410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E., Wascovich M., Oskouei S., Gurz P., & Carpenter D. (2019).Factors affecting health care provider knowledge and acceptance of biosimilar medicines: A systematic review. Journal of Managed Care and Specialty Pharmacy, 25(1), 102–112. 10.18553/jmcp.2019.25.1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio S. (2018).The complexities of biosimilars and the regulatory approval process. American Journal of Managed Care, 24(11_Suppl),S231–S236.https://pubmed.ncbi.nlm.nih.gov/29957908/ [PubMed] [Google Scholar]
- Tesser J. R., Furst D. E., & Jacobs I. (2017).Biosimilars and the extrapolation of indications for inflammatory conditions. Biologics, 11,5–11. 10.2147/BTT.S124476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley S. M., Grande C., Olson K., Plato L., & Jacobs I. (2018).Potential of biosimilars to increase access to biologics: Considerations for advanced practice providers in oncology. Journal of the Advanced Practitioner in Oncology, 9(7), 699–716. 10.6004/jadpro.2018.9.7.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food & Drug Administration. (2014).Guidance for industry on immunogenicity assessment for therapeutic protein products. https://www.govinfo.gov/content/pkg/FR-2014-08-14/pdf/2014-19267.pdf
- U.S. Food & Drug Administration. (2017).Biosimilar development, review, and approval. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval
- U.S. Food & Drug Administration. (2018).Biosimilar and interchangeable products: The U.S. FDA perspective.https://www.fda.gov/media/112818/download
- U.S. Food & Drug Administration. (2019).What is a biosimilar?. https://www.fda.gov/media/108905/download
- U.S. Food & Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. (2019).Considerations in demonstrating interchangeability with a reference product: Guidance for industry. https://www.fda.gov/media/124907/download [Google Scholar]
- U.S. Food & Drug Administration. (2021).Purple Book: Database of Licensed Biological Products. https://purplebooksearch.fda.gov/
- Ventola C. L. (2013).Biosimilars: Part 2: Potential concerns and challenges for P & T committees. P & T, 38(6), 329–335.https://pubmed.ncbi.nlm.nih.gov/23946628/ [PMC free article] [PubMed] [Google Scholar]