Abstract
Background
COVID-19 can induce a hyperinflammatory state, which might lead to poor clinical outcomes. We aimed to assess whether patients with a systemic rheumatic disease might be at increased risk for hyperinflammation and respiratory failure from COVID-19.
Methods
We did a retrospective, comparative cohort study of patients aged 18 years or older admitted to hospital with PCR-confirmed COVID-19 at Mass General Brigham (Boston, USA). We identified patients by a search of electronic health records and matched patients with a systemic rheumatic disease 1:5 to comparators. We compared individual laboratory results by case status and extracted laboratory results and COVID-19 outcomes for each participant. We calculated the COVID-19-associated hyperinflammation score (cHIS), a composite of six domains (a score of ≥2 indicating hyperinflammation) and used logistic regression to estimate odds ratios (ORs) for COVID-19 outcomes by hyperinflammation and case status.
Findings
We identified 57 patients with a systemic rheumatic disease and 232 matched comparators who were admitted to hospital with COVID-19 between Jan 30 and July 7, 2020; 38 (67%) patients with a rheumatic disease were female compared with 158 (68%) matched comparators. Patients with a systemic rheumatic disease had higher peak median neutrophil-to-lymphocyte ratio (9·6 [IQR 6·4–22·2] vs 7·8 [4·5–16·5]; p=0·021), lactate dehydrogenase concentration (421 U/L [297–528] vs 345 U/L [254–479]; p=0·044), creatinine concentration (1·2 mg/dL [0·9–2·0] vs 1·0 mg/dL [0·8–1·4], p=0·014), and blood urea nitrogen concentration (31 mg/dL [15–61] vs 23 mg/dL [13–37]; p=0·033) than comparators, but median C-reactive protein concentration (149·4 mg/L [76·4–275·3] vs 116·3 mg/L [58·8–225·9]; p=0·11) was not significantly different. Patients with a systemic rheumatic disease had higher peak median cHIS than comparators (3 [1–5] vs 2 [1–4]; p=0·013). All patients with a peak cHIS of 2 or more had higher odds of admission to intensive care (OR 3·45 [95% CI 1·98–5·99]), mechanical ventilation (66·20 [8·98–487·80]), and in-hospital mortality (16·37 [4·75–56·38]) than patients with a peak cHIS of less than 2. In adjusted analyses, patients with a rheumatic disease had higher odds of admission to intensive care (2·08 [1·09–3·96]) and mechanical ventilation (2·60 [1·32–5·12]) than comparators, but not in-hospital mortality (1.78 [0·79–4·02]). Among patients who were discharged from hospital, risk of rehospitalisation (1·08 [0·37–3·16]) and mortality within 60 days (1·20 [0·58–2·47]) was similar in patients and comparators.
Interpretation
Patients with a systemic rheumatic disease who were admitted to hospital for COVID-19 had increased risk for hyperinflammation, kidney injury, admission to intensive care, and mechanical ventilation compared with matched comparators. However, among patients who survived, post-discharge outcomes were not significantly different. The cHIS identified patients with hyperinflammation, which was strongly associated with poor COVID-19 outcomes in both patients with a rheumatic disease and comparators. Clinicians should be aware that patients with systemic rheumatic diseases and COVID-19 could be susceptible to hyperinflammation and poor hospital outcomes.
Funding
None.
Introduction
Excessive inflammation might be a crucial link between SARS-CoV-2 infection and host immunity that determines COVID-19 severity.1, 2, 3 The effect of COVID-19 in patients with a systemic rheumatic disease is of particular interest due to systemic inflammation. However, the risk of severe COVID-19 outcomes in these patients compared with the general population is unclear. Some previous studies suggested that patients with COVID-19 with a rheumatic disease were at increased risk for mechanical ventilation,4, 5 although this risk decreased in later stages of the pandemic.6, 7 Other studies showed no increased risk in patients with a rheumatic disease relative to the general population.8, 9
Research in context.
Evidence before this study
We evaluated published manuscripts relating to COVID-19 outcomes comparing patients with systemic rheumatic diseases to comparators without rheumatic disease. We searched MEDLINE and Embase for English-language clinical research articles published between Jan 1, 2019, and Feb 21, 2021, using combinations of the following search terms: “rheumatic”, “rheumatology”, “autoimmune”, “COVID”, and “SARS-CoV-2”. We identified only two previous studies that assessed patients with rheumatic diseases who were admitted to hospital for COVID-19 and matched comparators. Both studies included in their comparison patients with other autoimmune conditions or immunosuppressive conditions along with systemic rheumatic diseases. We identified no studies that investigated laboratory trends for patients with systemic rheumatic diseases and matched comparators without rheumatic diseases during their initial hospital stay for COVID-19. We identified no studies that investigated possible differences in post-discharge outcomes for patients with systemic rheumatic diseases and matched comparators. We used the same databases and timeframe and searched for “hyperinflammation”, “inflammation”, “cytokine storm”, “macrophage activation”, “C-reactive protein”, “COVID”, and “SARS-CoV-2” to find previously developed indices that identify patients with hyperinflammation. We identified six possible composite measures to identify hyperinflammation. We eliminated indices that relied on research laboratory measures, only incorporated repeated values of a single laboratory test, or mostly relied on clinical domains that included intensive care unit admission or mechanical ventilation because these were prespecified outcomes of interest. Therefore, we chose to apply the COVID-19-associated hyperinflammation score (cHIS) because this score integrated many laboratory results measuring different domains that are routinely and repeatedly collected during COVID-19 hospital admission.
Added value of this study
In this comparative study, we found that patients with a systemic rheumatic disease who were admitted to hospital with COVID-19 had increased risk for hyperinflammation, admission to intensive care, and mechanical ventilation than did matched comparators. However, risk of poor post-discharge outcomes was similar between patients and comparators.
Implications of all the available evidence
Patients with a systemic rheumatic disease might be susceptible to poor outcomes during the initial COVID-19 hospital stay, but similar outcomes after discharge in patients with or without a rheumatic disease might offer reassurance. Differences in laboratory trends for patients with a rheumatic disease and comparators, particularly for haematological dysfunction and kidney injury, indicate specific domains as potential mechanisms underlying poor COVID-19 outcomes. In both patients with rheumatic diseases and comparators, the cHIS was strongly associated with poor in-hospital COVID-19 outcomes, particularly mechanical ventilation; as such, the cHIS could be used as a tool to identify patients at risk for poor COVID-19 outcomes in clinical care and trials of COVID-19 therapies.
Some patients with a rheumatic disease might be clinically vulnerable to poor outcomes due to underlying propensity for hyperinflammation, which has been previously associated with increased risk of mortality and mechanical ventilation in patients with COVID-19.10 Similarly, in patients admitted to hospital with COVID-19, a rapid rise in C-reactive protein concentrations predicted progression to mechanical ventilation.11 Another study of patients with a rheumatic disease who were admitted to hospital with COVID-19 showed an association between elevated inflammatory markers and moderate-to-severe rheumatic disease activity with mortality.12 It is also possible that baseline use of immunomodulators might dampen the immune response to SARS-CoV-2, resulting in a less severe disease course. By contrast, patients who are immunocompromised might be susceptible to prolonged infection that could result in lengthier hospital stays and more complications.11 Investigating patients admitted to hospital with COVID-19 provides insight into the disease course in those at high risk for severe outcomes. Rates of readmission to hospital are high for patients who survive COVID-1913 but, to our knowledge, no studies have examined readmission among patients with a systemic rheumatic disease.
Therefore, we did a comparative cohort study of patients with a rheumatic disease admitted to hospital with COVID-19 and matched comparators to evaluate laboratory trends, hyperinflammation, and in-hospital and post-discharge outcomes. We hypothesised that patients with a systemic rheumatic disease would be more likely to have hyperinflammation and poor clinical outcomes than patients in a matched comparator group.
Methods
Study design and participants
We did a retrospective, comparative cohort study at Mass General Brigham, a large, multicentre system in the greater Boston area (MA, USA) that includes tertiary care hospitals (Massachusetts General Hospital and Brigham and Women's Hospital), community hospitals, and affiliated outpatient clinics. We did an electronic query of health records to identify patients seen at Mass General Brigham who were aged 18 years or older and had a positive PCR test for SARS-CoV-2 between Jan 30 and July 7, 2020.
We required that all participants be admitted to hospital for COVID-19, either with a known outpatient COVID-19 diagnosis or diagnosed with COVID-19 soon after admission. We excluded patients with emergency department visits that did not result in an inpatient stay, admissions in which SARS-CoV-2 positivity was found incidentally (eg, screening positive upon admission for another indication but asymptomatic), admissions solely for the purpose of quarantining, and patients with iatrogenic SARS-CoV-2 infection after admission for another reason.
Of patients with PCR-positive SARS-CoV-2 infection, we searched the electronic health record for the presence of any International Classification of Diseases (ICD, 9th or 10th edition) diagnosis codes for systemic rheumatic diseases, as previously detailed.4 The index date for matching was the initial date of positive SARS-CoV-2 PCR. We manually reviewed the patients' health records to confirm presence of a systemic rheumatic disease at the time of initial SARS-CoV-2 positivity. Patients with crystalline arthritis, fibromyalgia, or osteoarthritis were not included because these patients are not typically managed with immunosuppression.7 We excluded patients with polymyalgia rheumatica, sarcoidosis, and antiphospholipid syndrome not requiring systemic immunomodulatory medications within the past 5 years.
For each patient with a systemic rheumatic disease (cases), we identified up to five patients with positive SARS-CoV-2 PCR and admission to hospital (comparators) matched at the index date on age (plus or minus 5 years), sex, and date of initial positive PCR test (plus or minus 5 days). We matched on these variables because we considered these to be confounding variables between the status of patients with a rheumatic disease and the clinical outcomes. We chose the ratio of five comparators per case because we expected some comparator participants to be excluded for not meeting the study eligibility requirements after initial matching, and we required each case to have at least three comparators to optimise power.14 We reviewed the comparator participants' health records to confirm hospital admission for PCR-positive COVID-19 and absence of an ICD code for any systemic rheumatic disease.
All aspects of this study were approved by the Mass General Brigham Institutional Review Board. Informed consent was deemed unnecessary due to the retrospective nature of the study.
Procedures
Characteristics gathered by health record review for patients with a systemic rheumatic disease included type of rheumatic disease, baseline type and dose of immunomodulating drugs, disease duration, and disease activity (remission or low, or moderate-to-high).
We extracted all patients' laboratory results, including date and time of collection, from their health records, and we included tests done between the date of hospital admission (including initial values from emergency department or outpatient visits that directly led to hospital admission) to the date of discharge. Laboratory tests included white blood cell count, absolute neutrophil count, absolute lymphocyte count, neutrophil-to-lymphocyte ratio, haemoglobin, platelets, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, creatinine, C-reactive protein, erythrocyte sedimentation rate, ferritin, D-dimer, interleukin (IL)-6, procalcitonin, triglycerides, prothrombin time, international normalised ratio, fibrinogen, and lactate dehydrogenase. Each hospital or site used similar kits that were developed for commercial use and reported laboratory values in the same units.
For analyses of individual laboratory results, we considered the first individual result (baseline) and either the peak or trough value depending on the clinical interpretation (eg, peak C-reactive protein considered as clinically meaningful rather than trough). For laboratory results that were reported as either greater or less than assay limits, we used the extreme value (eg, for C-reactive protein concentrations >300 mg/L, 300 mg/L was used in the analyses).
We calculated the baseline and peak COVID-19 associated hyperinflammation score (cHIS)10 for each patient with a rheumatic disease and the matched comparators. The cHIS comprises six domains: fever, macrophage activation, haematological dysfunction, coagulopathy, hepatic injury, and cytokinaemia. Specific laboratory values and cutpoints on the cHIS are shown in the appendix (p 3). Abnormalities in each domain provide 1 point, such that the cHIS is a semi-continuous scale of discrete integers that ranges from 0 (least inflamed) to 6 (most inflamed). A previous study10 identified that a cHIS of 2 or more was an optimal cutpoint to recognise hyperinflammation that was associated with both mechanical ventilation and mortality. We calculated baseline and peak cHIS using time-updated laboratory results. For patients missing individual laboratory tests, these were assumed to be below the cHIS thresholds when calculating the cHIS because many of the laboratory tests were obtained during suspected clinical deterioration. For analyses considering intensive care unit admission or mechanical ventilation, we stopped updating the cHIS once these clinical outcomes were reached.
We used an electronic query of health records to obtain length of hospital stay and occurrence and dates of intensive care unit admission, mechanical ventilation, and in-hospital death. All participants were either discharged or had died at the time of analysis. For participants who survived the initial hospital stay, health record review determined discharge to rehabilitation or nursing facility and presence and date of rehospitalisation. We reviewed obituaries to identify additional deaths that occurred outside of our hospital system.
We used an electronic query of health records to identify the covariates of age, sex, race, Hispanic or Latinx ethnicity, smoking status (never, past, or current), and body-mass index. Comorbidities (hypertension, diabetes, coronary artery disease, heart failure, asthma, chronic obstructive pulmonary disease, obstructive sleep apnoea, interstitial lung disease, and chronic kidney disease) were obtained from diagnosis codes before the index date. The Charlson Comorbidity Index score was calculated for each participant and dichotomised (≤2 or >2).15 Health record review determined baseline residence in assisted living facility or nursing facility.
Statistical analysis
The baseline of the analysis was the date of initial hospital admission for COVID-19 (or presentation to emergency department that resulted in hospital admission). We report baseline characteristics and used univariate tests to obtain p values comparing cases and comparators.
For each laboratory test, we report median with IQR for baseline and peak or trough values in separate analyses. We compared cases and comparators using Wilcoxon rank sum tests. Patients missing individual laboratory tests were not analysed for that comparison. In a case-only analysis, we stratified by baseline category of immunosuppressive drug. We plotted laboratory value trends over the hospital stay by considering individual laboratory results on each day in hospital and calculating median laboratory values at each day (with 25th and 75th percentiles) for cases and comparators. Because few patients had lengthy hospital stays, we plotted the median laboratory results for the first 14 days of the hospital stay. Generalised linear mixed models compared the repeated continuous laboratory results over the entire hospital stay for cases and comparators.
We assessed the relationships between diagnosis of a systemic rheumatic disease, cHIS score, and in-hospital COVID-19 clinical outcomes. We report the median, IQR, and proportion of patients with a cHIS score of 2 or more for cases and comparators, which were compared using the Wilcoxon rank sum test or χ2 test. We then investigated associations of the continuous and dichotomised cHIS scores with the outcomes of intensive care unit admission, mechanical ventilation, and in-hospital mortality using logistic regression to obtain odds ratios (ORs) and 95% CIs. Multivariable models adjusted for age, sex, and whether the patient was a case or a comparator. Additional analyses stratified cases and comparators and adjusted for age and sex.
We compared cases with matched comparators using conditional logistic regression to obtain ORs and 95% CIs for the in-hospital outcomes of intensive care unit admission, mechanical ventilation, and mortality in separate models. The base model was unadjusted. The main multivariable model adjusted for race, smoking status, and days from initial PCR positivity for SARS-CoV-2 to hospital admission for COVID-19 because this was unbalanced at baseline. We used other multivariable models that adjusted for Charlson Comorbidity Index score, body-mass index, chronic kidney disease, and interstitial lung disease, which did not materially affect results. We considered these as potentially mediating factors between rheumatic diseases and clinical outcomes, so they were not included in the main model.
Among patients who survived the initial hospital stay, we did similar analyses for discharge to rehabilitation or nursing facility, and rehospitalisation within 60 days. We did additional analyses that included death occurring in-hospital or within 60 days after discharge, and death occurring in-hospital or at any point after discharge.
Subgroup analyses were done to further examine associations between cHIS and the clinical outcomes of intensive care unit admission, mechanical ventilation, and in-hospital mortality among patients who were using glucocorticoids at baseline, with users of rituximab excluded, and with stratification by immunosuppressive drug use, disease activity, and sex, all relative to matched comparators.
We considered a two-sided p value of less than 0·05 as statistically significant in all analyses. There was no adjustment for multiple comparisons. All analyses were done using SAS version 9.4.
Role of the funding source
There was no funding source for this study.
Results
We identified 57 patients with a systemic rheumatic disease and 232 matched comparators who were admitted to hospital with PCR-confirmed COVID-19 between Jan 30 and July 7, 2020 (table 1 ). Mean age was 66·8 years (SD 15·5) for patients with a systemic rheumatic disease and 67·5 years (14·5) for matched comparators; 38 (67%) patients with a systemic rheumatic disease were female compared with 158 (68%) matched comparators. More patients with a systemic rheumatic disease than comparators had interstitial lung disease (difference of proportion 10·6% [95% CI 2·9–22·1]) and chronic kidney disease (difference of proportion 9·3% [1·1–19·8]), but other individual comorbidities and the Charlson Comorbidity Index scores were similar between the groups. A higher proportion of patients with a systemic rheumatic disease were admitted to intensive care on day 1 after hospital admission than comparators (16 [28%] vs 36 [16%]; p=0·027). Changes to baseline immunosuppressive drugs and medications prescribed to treat COVID-19 among patients with a systemic rheumatic disease are shown in the appendix (pp 6–7).
Table 1.
Baseline characteristics at the time of initial admission to hospital for COVID-19 for patients with a systemic rheumatic disease and matched comparators
| Patients with a systemic rheumatic disease (n=57) | Comparators*(n=232) | p value | ||
|---|---|---|---|---|
| Age, years | 66·8 (15·5) | 67·5 (14·5) | 0·77 | |
| Sex | ||||
| Female | 38 (67%) | 158 (68%) | 0·84† | |
| Male | 19 (33%) | 74 (32%) | .. | |
| Race | ||||
| White | 30 (53%) | 121 (52%) | 0·95‡ | |
| Non-White | 27 (47%) | 111 (48%) | .. | |
| Hispanic or Latinx | 3 (5%) | 24 (10%) | 0·24 | |
| Timing of PCR positivity and hospital admission | ||||
| PCR positive before hospital stay day 1 | 16 (28%) | 31 (13%) | 0·0015§ | |
| PCR positive at hospital stay day 1 | 39 (68%) | 157 (68%) | .. | |
| PCR positive after hospital stay day 1 | 2 (4%) | 44 (19%) | .. | |
| Smoking status | ||||
| Never | 27 (47%) | 112 (48%) | 0·26¶ | |
| Past | 24 (42%) | 73 (31%) | .. | |
| Current | 2 (4%) | 12 (5%) | .. | |
| Data missing | 4 (7%) | 35 (15%) | .. | |
| Body-mass index, kg/m2 | 30·1 (7·9) | 28·6 (6·5) | 0·17 | |
| Comorbidities | ||||
| Hypertension | 30 (53%) | 101 (44%) | 0·22 | |
| Diabetes | 10 (18%) | 39 (17%) | 0·89 | |
| Coronary artery disease | 12 (21%) | 29 (13%) | 0·10 | |
| Heart failure | 10 (18%) | 31 (13%) | 0·42 | |
| Asthma | 7 (12%) | 13 (6%) | 0·084 | |
| Chronic obstructive pulmonary disease | 7 (12%) | 19 (8%) | 0·33 | |
| Obstructive sleep apnoea | 6 (11%) | 15 (6%) | 0·27 | |
| Interstitial lung disease | 7 (12%) | 4 (2%) | 0·0014 | |
| Chronic kidney disease | 10 (18%) | 19 (8%) | 0·035 | |
| Charlson Comorbidity Index category | ||||
| ≤2 | 22 (39%) | 112 (48%) | 0·37‖ | |
| >2 | 20 (35%) | 63 (27%) | .. | |
| Data missing | 15 (26%) | 57 (25%) | .. | |
| Assisted living or nursing home residence | 10 (18%) | 48 (21%) | 0·60 | |
| Intensive care unit admission on hospital stay day 1 | 16 (28%) | 36 (16%) | 0·027 | |
Data are mean (SD) or n (%).
Comparators were matched to cases by age, sex, and calendar date of initial PCR positivity for SARS-CoV-2.
p value for the comparison between cases and comparators across both sexes.
p value for the comparison between cases and comparators across both race groups.
p value for the comparison between cases and comparators across all PCR positivity timings.
p value for the comparison between cases and comparators across all smoking status groups.
p value for the comparison between cases and comparators across all Charlson Comorbidity Index categories.
Of the patients with a systemic rheumatic disease, 26 (46%) had rheumatoid arthritis, 14 (25%) had systemic lupus erythematosus, and five (9%) had psoriatic arthritis (appendix pp 4–5). 34 (60%) patients with a systemic rheumatic disease had active disease and 15 (26%) were being treated with biological or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) at baseline. 20 (35%) patients with a systemic rheumatic disease were on conventional synthetic DMARDs and 25 (44%) were on glucocorticoids (median dose of prednisone 5 mg/day [IQR 5–10]) at baseline (appendix pp 4–5).
We analysed 39 900 individual laboratory results (median 85 per patient [IQR 51–152]). The baseline laboratory results are shown in the appendix (p 8). Patients with a systemic rheumatic disease had a lower median haemoglobin concentration and a higher triglyceride concentration than comparators (appendix p 8). There were no other significant differences in baseline median laboratory results between the two groups.
The peak or trough laboratory values are shown in table 2 . Patients with a systemic rheumatic disease had a lower median trough haemoglobin concentration and absolute lymphocyte count, as well as higher median peak neutrophil-to-lymphocyte ratio, triglycerides concentration, lactate dehydrogenase concentration, creatinine concentration, and blood urea nitrogen concentration than comparators. Other median peak or trough laboratory values were similar in both groups, including peak C-reactive protein and IL-6 concentrations.
Table 2.
Peak or trough laboratory values for patients with a systemic rheumatic disease and matched comparators during initial hospital admission for COVID-19
| Patients with a systemic rheumatic disease (n=57) | Comparators (n=232) | p value | ||
|---|---|---|---|---|
| White blood cell count peak, 1000 per μL | 57; 9·0 (6·6–20·3) | 232; 8·6 (6·2–13·0) | 0·34 | |
| Absolute neutrophil count peak, 1000 per μL | 57; 7·7 (4·5–12·6) | 231; 6·3 (4·4–10·1) | 0·32 | |
| Absolute lymphocyte count trough, 1000 per μL | 57; 0·5 (0·3–0·7) | 231; 0·7 (0·4–1·0) | 0·0049 | |
| Neutrophil-to-lymphocyte ratio peak | 57; 9·6 (6·4–22·2) | 231; 7·8 (4·5–16·5) | 0·021 | |
| Haemoglobin concentration trough, g/dL | 57; 10·1 (7·5–11·4) | 232; 10·7 (9·3–12·4) | 0·0040 | |
| Platelet count, 1000 per μL | ||||
| Peak | 57; 308 (233–420) | 232; 314 (216–420) | 0·80 | |
| Trough | 57; 175 (128–204) | 232; 182 (135–228) | 0·11 | |
| AST peak, U/L | 56; 55 (34–107) | 229; 49 (31–99) | 0·45 | |
| ALT peak, U/L | 56; 32 (20–90) | 229; 41 (21–75) | 0·69 | |
| Blood urea nitrogen peak, mg/dL | 57; 31 (15–61) | 232; 23 (13–37) | 0·033 | |
| Creatinine peak, mg/dL | 57; 1·2 (0·9–2·0) | 232; 1·0 (0·8–1·4) | 0·014 | |
| C-reactive protein peak, mg/L | 56; 149·4 (76·4–275·3) | 222; 116·3 (58·8–225·9) | 0·11 | |
| Erythrocyte sedimentation rate peak, mm/h | 37; 75 (35–116) | 180; 56 (37–91) | 0·15 | |
| Ferritin peak, μg/L | 57; 765 (353–1219) | 219; 628 (335–1211) | 0·46 | |
| D-dimer peak, ng/mL | 56; 2056 (710–3785) | 22; 1352 (650–2820) | 0·072 | |
| Interleukin-6 peak, pg/mL | 23; 53·6 (23·6–201·6) | 59; 28·5 (10·6–73·3) | 0·17 | |
| Procalcitonin peak, ng/mL | 2; 3·1 (3·0–3·3) | 23; 0·3 (0·1–2·4) | 0·21 | |
| Triglycerides peak, mg/dL | 23; 273 (210–462) | 61; 208 (113–402) | 0·026 | |
| Prothrombin time peak, s | 49; 14·9 (13·8–16·6) | 187; 14·6 (13·5–16·4) | 0·28 | |
| International normalised ratio peak | 49; 1·2 (1·1–1·4) | 187; 1·2 (1·1–1·4) | 0·36 | |
| Fibrinogen peak, mg/dL | 37; 611 (510–795) | 114; 631 (493–805) | 0·84 | |
| Lactate dehydrogenase peak, U/L | 54; 421 (297–528) | 222; 345 (254–479) | 0·044 | |
Data are n; median (IQR). ALT=alanine aminotransferase. AST=aspartate aminotransferase.
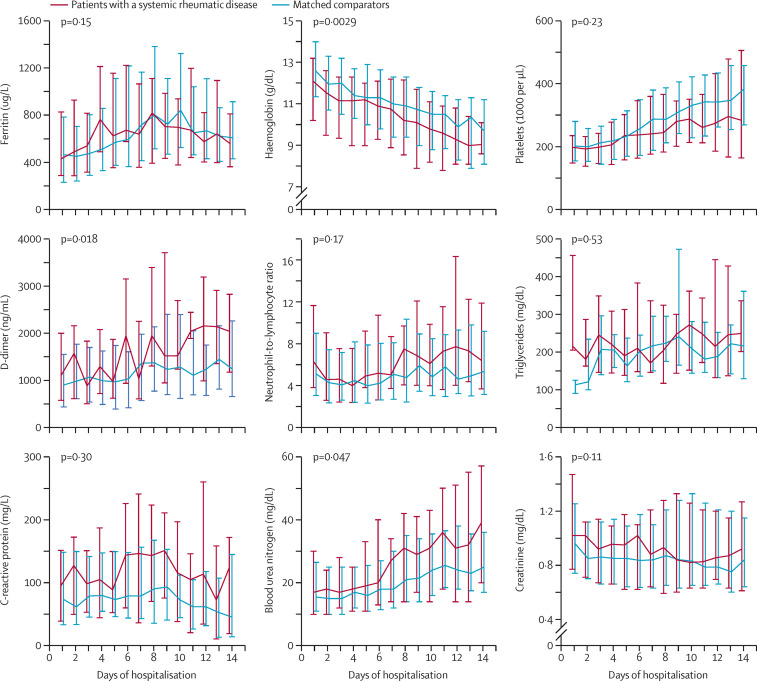
Patients with a systemic rheumatic disease had lower haemoglobin concentrations (p=0·0029), higher blood urea nitrogen concentrations (p=0·047), and lower D-dimer concentrations (p=0·018) during the first 14 days of the hospital stay than comparators (figure ). Although the patients with a systemic rheumatic disease had numerically higher median C-reactive protein concentrations on each hospital day than the comparators, there was no significant difference between the groups (p=0·30; figure). There was no difference between the groups for the other laboratory values during the first 14 days of the hospital stay (figure).
Figure.
Laboratory trends for selected components of the cHIS, blood urea nitrogen, and creatinine for patients with a systemic rheumatic disease and matched comparators for each day during the first 14 days of hospitalisation for COVID-19
Components of the cHIS included are ferritin concentration, haemoglobin concentration, platelets, D-dimer concentration, neutrophil-to-lymphocyte ratio, triglycerides concentration, and C-reactive protein concentration. Median values are connected. Whiskers represent the 25th and 75th percentiles. cHIS=COVID-19-associated hyperinflammation score.
At baseline, patients with a systemic rheumatic disease had higher cHIS than comparators (appendix p 11). Median peak cHIS was 3 (IQR 1–5) for patients with a systemic rheumatic disease and 2 (1–4) for comparators (p=0·013). At baseline, a significantly higher proportion of patients with a systemic rheumatic disease than comparators had a cHIS of 2 or higher (appendix p 11). 41 (72%) patients with a systemic rheumatic disease had hyperinflammation (peak cHIS ≥2), compared with 136 (59%) comparators (p=0·065; table 3 ).
Table 3.
Associations of peak cHIS with COVID-19 hospital admission outcomes
| Number of patients |
Intensive care unit admission |
Mechanical ventilation |
In-hospital mortality |
||||
|---|---|---|---|---|---|---|---|
| n (%) with outcome | Adjusted OR (95% CI) | n (%) with outcome | Adjusted OR (95% CI) | n (%) with outcome | Adjusted OR (95% CI) | ||
| All patients | |||||||
| Continuous cHIS per unit | 289 | 108 (37%) | 1·74 (1·48–2·04) | 67 (23%) | 4·55 (3·11–6·64) | 50 (17%) | 2·09 (1·63–2·68) |
| cHIS <2 | 112 | 23 (21%) | 1 (ref) | 1 (1%) | 1 (ref) | 3 (3%) | 1 (ref) |
| cHIS ≥2 | 177 | 85 (48%) | 3·45 (1·98–5·99) | 66 (37%) | 66·20 (8·98–487·80) | 47 (27%) | 16·37 (4·75–56·38) |
| Patients with a systemic rheumatic disease | |||||||
| Continuous cHIS per unit | 57 | 29 (51%) | 1·98 (1·35–2·92) | 22 (39%) | 4·66 (2·20–9·85) | 12 (21%) | 1·57 (1·01–2·44) |
| cHIS <2 | 16 | 3 (19%) | 1 (ref) | 0 | 1 (ref) | 1 (6%) | 1 (ref) |
| cHIS ≥2 | 41 | 26 (63%) | 9·01 (2·05–39·57) | 22 (54%) | ..* | 11 (27%) | 7·72 (0·81–73·35) |
| Comparators | |||||||
| Continuous cHIS per unit | 232 | 79 (34%) | 1·68 (1·41–2·02) | 45 (19%) | 4·55 (2·92–7·10) | 38 (16%) | 2·32 (1·71–3·13) |
| cHIS <2 | 96 | 20 (21%) | 1 (ref) | 1 (1%) | 1 (ref) | 2 (2%) | 1 (ref) |
| cHIS ≥2 | 136 | 59 (43%) | 2·90 (1·59–5·30) | 44 (32%) | 47·58 (6·40–353·70) | 36 (26%) | 20·83 (4·67–92·91) |
Data are n (%) or OR (95% CI). Adjusted for age and sex (and diagnosis of systemic rheumatic disease for the all patients analysis). Reference values are for the comparison between the dichotomised cHIS groups. cHIS=COVID-19-associated hyperinflammation score. OR=odds ratio.
Model could not be run because no mechanical ventilation outcomes occurred in the reference group.
Continuous cHIS and hyperinflammation defined by the dichotomised cHIS variable were each strongly associated with all outcomes across both groups (table 3). After adjusting for age, sex, and whether the patient had a systemic rheumatic disease, patients with peak cHIS of 2 or more had higher odds of intensive care unit admission (OR 3·45 [95% CI 1·98–5·99), mechanical ventilation (66·20 [8·98–487·80]), and in-hospital mortality (16·37 [4·75–56·38]) compared with those with a cHIS of less than 2 (table 3). Results were similar in the analyses restricted to cases and comparators. All 22 patients with a systemic rheumatic disease who required mechanical ventilation and 44 (98%) of 45 comparators who required mechanical ventilation had peak cHIS of 2 or more.
The median length of hospital stay was 7 days (IQR 5–18) for patients with a systemic rheumatic disease and 8 days (5–13) for comparators (p=0·46). 29 (51%) patients with a systemic rheumatic disease were admitted to intensive care compared with 79 (34%) comparators (table 4 ). After adjusting for race, smoking status, and the days between PCR positivity and hospital admission, patients with a systemic rheumatic disease had greater odds of being admitted to intensive care than comparators (adjusted OR 2·08 [95% CI 1·09–3·96]); table 4). Patients with a systemic rheumatic disease were also more likely to require mechanical ventilation than comparators (2·60 [1·32–5·12]). Results were similar when additionally adjusting for comorbidities (including chronic kidney disease and interstitial lung disease; appendix p 27). There was no difference in in-hospital deaths between the two groups (table 4).
Table 4.
Associations between systemic rheumatic disease or matched comparator status and COVID-19 hospital admission outcomes
| Patients with a systemic rheumatic disease (n=57) | Comparators (n=232) | ||
|---|---|---|---|
| Outcomes at initial admission to hospital | |||
| Intensive care unit admission | 29 (51%) | 79 (34%) | |
| Unadjusted OR (95% CI) | 2·00 (1·09–3·67) | 1 (ref) | |
| Adjusted model OR (95% CI) | 2·08 (1·09–3·96) | 1 (ref) | |
| Mechanical ventilation | 22 (39%) | 45 (19%) | |
| Unadjusted OR (95% CI) | 2·59 (1·35–4·94) | 1 (ref) | |
| Adjusted model OR (95% CI) | 2·60 (1·32–5·12) | 1 (ref) | |
| In-hospital mortality | 12 (21%) | 38 (16%) | |
| Unadjusted OR (95% CI) | 1·48 (0·69–3·17) | 1 (ref) | |
| Adjusted model OR (95% CI) | 1·78 (0·79–4·02) | 1 (ref) | |
| Outcomes after discharge* | |||
| Discharged to rehabilitation or nursing facility | 16 (36%) | 80 (41%) | |
| Unadjusted OR (95% CI) | 0·79 (0·40–1·54) | 1 (ref) | |
| Adjusted model OR (95% CI) | 0·90 (0·43–1·88) | 1 (ref) | |
| Readmission to hospital within 60 days | 5 (11%) | 22 (11%) | |
| Unadjusted OR (95% CI) | 0·98 (0·35–2·74) | 1 (ref) | |
| Adjusted model OR (95% CI) | 1·08 (0·37–3·16) | 1 (ref) | |
| Mortality during and after hospital stay | |||
| In-hospital mortality or within 60 days after discharge | 13 (23%) | 50 (22%) | |
| Unadjusted OR (95% CI) | 1·08 (0·54–2·15) | 1 (ref) | |
| Adjusted model OR (95% CI) | 1·20 (0·58–2·47) | 1 (ref) | |
| In-hospital mortality or at any point after discharge | 17 (30%) | 57 (25%) | |
| Unadjusted OR (95% CI) | 1·30 (0·69–2·48) | 1 (ref) | |
| Adjusted model OR (95% CI) | 1·48 (0·75–2·91) | 1 (ref) | |
Data are n (%) or OR (95% CI). Matching factors were age, sex, and date of initial positive PCR for SARS-CoV-2. Model adjusted for race, smoking status, and days between initial PCR positivity for SARS-CoV-2 and COVID-19 hospital admission. OR=odds ratio.
n=45 for patients with a systemic rheumatic disease; n=194 for comparators.
Among the participants who survived the initial hospital stay, the proportion who were discharged to rehabilitation or a nursing facility did not differ between the patients with a systemic rheumatic disease and the comparators (adjusted OR 0·90 [95% CI 0·43–1·88]; table 4). There was also no difference between the groups in the number of patients who were re-admitted to hospital within 60 days or in the number deaths in-hospital or within 60 days of discharge (table 4). Results were similar when considering death at any time after initial hospital admission.
Associations with poor COVID-19 outcomes were stronger among patients with a systemic rheumatic disease on glucocorticoids at baseline (appendix p 13), on immunosuppressive medications (appendix p 23), and with moderate or high disease activity (appendix p 19). Results stratified by sex are shown in the appendix (pp 25, 28).
Discussion
In this comparative cohort study, we showed that patients with a systemic rheumatic disease who were admitted to hospital for COVID-19 were at increased risk of developing hyperinflammation compared with matched comparators. Patients with a systemic rheumatic disease were also more likely to have elevated markers of kidney injury. cHIS was strongly associated with poor in-hospital outcomes, including intensive care admission, mechanical ventilation, and mortality, providing, to our knowledge, the first external application of the cHIS. Our results suggest that propensity for hyperinflammation might be a potential mechanism for poor outcomes in patients with a rheumatic disease.4, 5 The cHIS might be a useful tool for clinicians or in clinical trials to risk stratify patients admitted to hospital with COVID-19. To our knowledge, ours is the first study to investigate post-hospital discharge outcomes for patients with a rheumatic disease. We found similar rates of discharge to a nursing facility, rehospitalisation, and out-of-hospital mortality between patients with a systemic rheumatic disease and comparators, although the sample size was small.
Our study adds to the growing literature showing an association between rheumatic disease and serious COVID-19 outcomes compared with the general population.16, 17 Among more than 17 million patients in OpenSAFELY, those with rheumatoid arthritis, systemic lupus erythematosus, or psoriasis had a 19% increase in COVID-19 mortality relative to the general public.18 A Swedish study found excess COVID-19 mortality for patients with rheumatoid arthritis and other inflammatory joint diseases,19 and a study from Wuhan, China, suggested the risk for mechanical ventilation due to COVID-19 was three times greater for patients with rheumatic diseases than for other patients.5 We previously showed that patients with a systemic rheumatic disease had similar odds of hospital admission but that their risk of mechanical ventilation or admission to intensive care was three times higher than for matched comparators.4 A follow-up study 6 months into the COVID-19 pandemic showed improvements over calendar time, but patients with a rheumatic disease still had a higher risk for mechanical ventilation before adjusting for comorbidities.7 Studies using TriNETx, a multicentre electronic health records network, showed that patients with a rheumatic disease had increased risk for several poor COVID-19 outcomes, including hospital admission, admission to intensive care, acute kidney injury, and venous thromboembolism,20 and these risks might also be mediated by comorbidities and have improved over calendar time.6 Our results were similar after adjusting for comorbidities, suggesting that these did not explain the relationships that we observed.
Only a few studies have compared patients with a rheumatic disease with the general population for outcomes among patients admitted to hospital for COVID-19. In a study of 2121 consecutive patients admitted to hospital for COVID-19, 108 patients were classified as immunosuppressed due to medication use.21 That study reported no association between immunosuppression and in-hospital outcomes including mechanical ventilation, in-hospital mortality, or length of stay.21 A Spanish registry study analysed patients with immune-mediated diseases admitted to hospital for COVID-19 and found significantly lower rates of admission to intensive care and mechanical ventilation compared with controls,9 the opposite of our findings. These differences might be due to the inclusion of patients with diseases other than rheumatic diseases that might have introduced heterogeneity. Most other studies of patients with a rheumatic disease and COVID-19 did not include comparator groups without rheumatic diseases. The Global Rheumatology Alliance reported that older age, glucocorticoid use, and comorbidities were associated with higher odds of hospital admission, whereas the use of biological or targeted synthetic DMARDs was associated with lower odds of hospital admission.22 Another study from the Global Rheumatology Alliance investigated factors associated with mortality and found that rituximab use was strongly associated with increased COVID-19 mortality.23 Few patients in our study were on biologic DMARDs at baseline, and the majority of those who were on biologic DMARDs were on rituximab. However, our results were similar in the sensitivity analysis that removed patients who were on rituximab, suggesting that our findings were not solely due to the use of this medication.
To our knowledge, our study is the first to examine laboratory trends and hyperinflammation during the initial COVID-19 hospital admission in patients with a rheumatic disease, and the first external application of the cHIS. Many of the individual cHIS laboratory markers were numerically higher in patients with a rheumatic disease than comparators, suggesting a propensity for hyperinflammation or so-called cytokine storm. Consistent with the initial derivation study,10 high cHIS values were strongly associated with poor in-hospital outcomes in our study,3, 11, 24, 25, 26 particularly mechanical ventilation. To our knowledge, we are the first to report post-hospital discharge outcomes for patients with a rheumatic disease and COVID-19. Reassuringly, post-hospital discharge outcomes were not different between these patients and comparators. However, rates of poor post-discharge outcomes were high, emphasising that the COVID-19 disease course might span well beyond initial clinical recovery.13, 27 Further studies are needed to investigate whether there are other post-acute sequelae of COVID-19 that could be amplified in patients with rheumatic diseases.
The sample size in our study might have been too small to detect some true associations, particularly those that occurred infrequently. This also limited our ability to assess individual rheumatic diseases or specific medications. Patients on baseline glucocorticoids or immunosuppressive medications were at increased risk for poor COVID-19 outcomes relative to comparators. We also observed poor outcomes for patients with moderate-to-high rheumatic disease activity, so it is difficult to disentangle which was responsible for severe outcomes. Although we matched on calendar time, the study was mostly done early in the COVID-19 pandemic and outcomes have since improved.6, 7 For multivariable analyses, we adjusted for baseline factors rather than for events occurring after hospital admission because post-baseline factors might have been mediators. We were unable to adjust for characteristics of the underlying rheumatic disease, including medications, because these factors are not applicable to comparators. We also did not adjust for treatment during the hospital stay, such as glucocorticoids,28 but treatments were similar among patients with a rheumatic disease and comparators.4 There could be a potential for selection bias because we required all patients to be admitted to hospital. However, our previous study found no association of rheumatic disease with hospital admission for COVID-19.4 More patients with a rheumatic disease were admitted to intensive care on day 1 of their hospital admission, suggesting worse severity of COVID-19 at presentation. Future studies should investigate whether our findings could be due to prolonged viral replication in immunocompromised patients.11 We did not adjust for multiple comparisons, so some findings might be due to chance. Finally, we did our study at a single centre, so the findings might not be generalisable.
In conclusion, patients with a rheumatic disease admitted to hospital for COVID-19 were at increased risk for hyperinflammation, kidney injury, admission to intensive care, and mechanical ventilation compared with matched comparators. We found similar rates of post-discharge outcomes such as discharge to a nursing facility, rehospitalisation, and mortality. These results suggest that patients with a rheumatic disease might be vulnerable to poor outcomes during the initial hospital stay for COVID-19.
Data sharing
Data are not publicly available due to protected health information. Data request proposals, including de-identified requests, can be submitted to the corresponding author with appropriate regulatory approval and signed data use agreement.
Declaration of interests
HKC has received research support from AstraZeneca and consultancy fees from Takeda, Selecta, GlaxoSmithKline, and Horizon. EMG has an editor position at New England Journal of Medicine and has received royalties from the textbook Rheumatology. ZSW has received research support from Bristol-Myers Squibb and Principia and consulting fees from Viela Bio and MedPace. JAS has received research support from Amgen and Bristol-Myers Squibb and consultancy fees from Bristol-Myers Squibb, Gilead, Inova, Janssen, Optum, and Pfizer. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
KMD and NJP are supported by the National Institutes of Health (NIH) Ruth L Kirschstein Institutional National Research Service Award (grant number T32-AR-007258). KMD is supported by the Rheumatology Research Foundation Scientist Development Award. AAM is supported by the NIH Ruth L Kirschstein Institutional National Research Service Award (grant number T32-AR-007530-35). HKC is funded by the NIH (grant number P50-AR-060772). ZSW is funded by the NIH and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; grant numbers K23AR073334 and L30 AR070520). JAS is funded by the NIH and NIAMS (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253, and P30 AR072577), the Rheumatology Research Foundation R Bridge Award, the Brigham Research Institute, and the R Bruce and Joan M Mickey Research Scholar Fund.
Contributors
TY-TH, KMD, NJP, ZSW, and JAS designed the study, were responsible for the acquisition, analysis, and interpretation of data, and drafted and revised the article. JW, AAM, and XF were involved in data analysis and interpretation and revision of the manuscript. LP, LM, KMMV, AZ, and CC were involved in data acquisition. HKC, YZ, and EMG were involved in data analysis and interpretation and revision of the manuscript. All authors approved the final version of the article. All authors had full access to the data in the study and had final responsibility to submit for publication. JAS and TH have accessed and verified the data.
Supplementary Material
References
- 1.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gianfrancesco MA, Leykina LA, Izadi Z, et al. Association of race and ethnicity with COVID-19 outcomes in rheumatic disease: data from the COVID-19 global rheumatology alliance physician registry. Arthritis Rheumatol. 2021;73:374–380. doi: 10.1002/art.41567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye C, Cai S, Shen G, et al. Clinical features of rheumatic patients infected with COVID-19 in Wuhan, China. Ann Rheum Dis. 2020;79:1007–1013. doi: 10.1136/annrheumdis-2020-217627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorge A, D'Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol. 2021;3:e131–e137. doi: 10.1016/S2665-9913(20)30422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-219279. published online Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarzi-Puttini P, Marotto D, Caporali R, et al. Prevalence of COVID infections in a population of rheumatic patients from Lombardy and Marche treated with biological drugs or small molecules: a multicentre retrospective study. J Autoimmun. 2021;116 doi: 10.1016/j.jaut.2020.102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarmiento-Monroy JC, Espinosa G, Londoño MC, et al. A multidisciplinary registry of patients with autoimmune and immune-mediated diseases with symptomatic COVID-19 from a single center. J Autoimmun. 2021;117 doi: 10.1016/j.jaut.2020.102580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi B, Choudhary MC, Regan J, et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos CS, Morales CM, Álvarez ED, Castro CA, Robles AL, Sandoval TP. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39:2789–2796. doi: 10.1007/s10067-020-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2020 doi: 10.7326/M20-5661. M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor JM. Choosing the number of controls in a matched case-control study, some sample size, power and efficiency considerations. Stat Med. 1986;5:29–36. doi: 10.1002/sim.4780050106. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Grainger R, Machado PM, Robinson PC. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: epidemiology and outcomes. Best Pract Res Clin Rheumatol. 2021;35 doi: 10.1016/j.berh.2020.101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol. 2020;32:434–440. doi: 10.1097/BOR.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 18.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bower H, Frisell T, Di Giuseppe D, et al. Impact of the COVID-19 pandemic on morbidity and mortality in patients with inflammatory joint diseases and in the general population: a nationwide Swedish cohort study. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-219845. published online Feb 23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Silva KM, Jorge A, Cohen A, et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases (SARDs) compared to the general population: a US multi-center comparative cohort study. Arthritis Rheumatol. 2020 doi: 10.1002/art.41619. published online Dec 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen KM, Mehta HB, Palamuttam N, et al. Association between chronic use of immunosuppresive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1488. published online Jan 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2020-219498. published online Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro VM, McCoy TH, Perlis RH. Laboratory findings associated with severe illness and mortality among hospitalized individuals with coronavirus disease 2019 in eastern Massachusetts. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro-Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a Case series. Arthritis Rheumatol. 2020;72:1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caricchio R, Gallucci M, Dass C, et al. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-218323. [DOI] [PubMed] [Google Scholar]
- 27.Logue JK, Franko NM, McCulloch DJ, et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not publicly available due to protected health information. Data request proposals, including de-identified requests, can be submitted to the corresponding author with appropriate regulatory approval and signed data use agreement.