Abstract
BNT162b2 is a vaccine developed to prevent coronavirus disease 2019 (COVID-19). BNT162b2 is a lipid nanoparticle formulated nucleoside-modified messenger RNA (mRNA) encoding the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein locked in its prefusion conformation. A developmental and reproductive toxicity study was conducted in rats according to international regulatory guidelines. The full human BNT162b2 dose of 30 μg mRNA/dose (>300 times the human dose on a mg/kg basis) was administered intramuscularly to 44 female rats 21 and 14 days prior to mating and on gestation days 9 and 20. Half of the rats were subject to cesarean section and full fetal examination at the end of gestation, and the other half were allowed to deliver and were monitored to the end of lactation. A robust neutralizing antibody response was confirmed prior to mating and at the end of gestation and lactation. The presence of neutralizing antibodies was also confirmed in fetuses and offspring. Nonadverse effects, related to the local injection site reaction, were noted in dams as expected from other animal studies and consistent with observations in humans. There were no effects of BNT162b2 on female mating performance, fertility, or any ovarian or uterine parameters nor on embryo-fetal or postnatal survival, growth, physical development or neurofunctional development in the offspring through the end of lactation. Together with the safety profile in nonpregnant people, this ICH-compliant nonclinical safety data supports study of BNT162b2 in women of childbearing potential and pregnant and lactating women.
Keywords: BNT162b2, COVID-19 vaccine, Pregnancy, Rat, Developmental toxicity, Fertility
1. Introduction
Coronavirus disease 2019 (COVID-19) has affected tens of millions of people globally since it was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 [1]. Older adults, persons with certain coexisting conditions, and front-line workers are at highest risk for COVID-19 and its complications, and, as such, the Centers for Disease Control recommended prioritizing these populations for vaccination. Recent data have shown that other populations, including pregnant women, may also be at increased risk [2,3]; however, pregnant women were excluded from initial clinical trials [4,5] until sufficient safety data in nonpregnant adults had been demonstrated.
Because of changes in adaptive immunity and physiology associated with pregnancy, there is a theoretical basis to suggest that pregnant women may be at an increased risk of severe COVID-19 [[6], [7], [8], [9]]. While not yet conclusive, studies have shown that pregnancy is associated with a greater risk of severe disease among women presenting with symptomatic COVID-19 compared to nonpregnant counterparts [10,11], and fatality rates in pregnant women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections have been reported to be nearly 14-fold higher than those of similarly aged individuals [12]. These reports are consistent with observations of other respiratory viral illnesses, such as pandemic H1N1v influenza, for which associated deaths were > 5-fold higher among pregnant women than among nonpregnant individuals [13]. Additionally, available data to date suggest that pregnant women with COVID-19 are potentially also at risk for adverse pregnancy outcomes, such as preterm birth [12,14,15].
With the development of COVID-19 vaccines, there is the potential to alleviate the higher risk related to COVID-19 during pregnancy. The first vaccine authorized for emergency use in the United States and conditional approval in Europe, BNT162b2, a lipid nanoparticle (LNP) formulated nucleoside-modified messenger RNA (mRNA) encoding the SARS-CoV-2 spike protein, developed by Pfizer-BioNTech, has demonstrated 95 % efficacy in a pivotal clinical trial and ≥90 % effectiveness in real world use studies in preventing COVID-19 in adults aged 16 and older [[16], [17], [18]]. Data demonstrate maternal transfer of neutralizing antibodies to neonates (as measured in umbilical cord blood), which could also protect infants [19]. Other COVID-19 vaccines have been developed and authorized for use in the United States, Europe, and other countries. Available data in pregnant women indicate that the vaccines have been well tolerated and that immunity (antibodies) maybe transferred to infants [[19], [20], [21]]. At the time this manuscript was submitted the safety and effectiveness in pregnancy have not been demonstrated in clinical trials with any of these vaccines; however, a clinical trial of BNT162b2 in pregnant women is ongoing (ClinicalTrials.gov Identifier: NCT04754594).
Before conducting clinical trials in humans, the safety of drugs and vaccines must be evaluated in nonclinical safety studies that are designed to support the specific requirements of the clinical trial design, including age, sex, and reproductive status of the participants, duration of treatment, and treatment indication. General toxicology studies in young adult rats showed BNT162b2 was well tolerated, elicited an immune response and resulted in expected inflammatory changes. The International Council for Harmonisation (ICH) and WHO guidelines describe expectations for the nonclinical study that is necessary prior to performing a clinical trial of a vaccine in pregnant women [22,23]. This article reports the design and results of the nonclinical developmental and reproductive toxicity (DART) study to support initiation of a clinical trial in pregnant women with BNT162b2.
2. Materials and methods
All animal care and experimental procedures were conducted in compliance with guidelines for the care and use of laboratory animals [[24], [25], [26]] and were approved by the ethical committee of Charles River Laboratories France Safety Assessment SAS. The facility where this study was conducted is accredited by Association for Assessment and Accreditation of Laboratory Animal Care International.
2.1. Vaccine
BNT162b2 is an LNP-formulated, nucleoside-modified mRNA vaccine that encodes a prefusion stabilized, membrane-anchored SARS-CoV-2 full length spike protein. The lipid components include an ionizable lipid, cholesterol, a polyethylene glycol conjugated lipid, and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC). BNT162b2 was manufactured and supplied by Polymun Scientific, Klosterneuburg, Austria. The saline control consisted of 0.9 % sterile saline for injection, USP (Lavoisier, France).
2.2. Study design
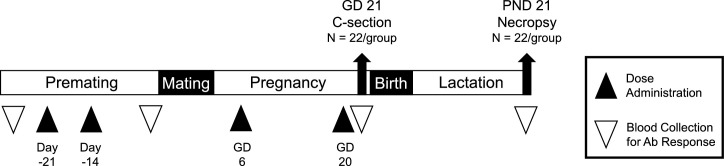
The study was conducted by Charles River Laboratories France Safety Assessment SAS. An overview of the study design is presented in Fig. 1 . Virgin female Wistar Han rats CRL:WI(Han) (Charles River Laboratories France; approximately 11 weeks old and 179–265 g at initiation of dosing) were acclimated, randomly assigned to groups in two main cohorts (n = 22 per dose group in the Caesarean cohort and n = 22 per dose group in the delivery cohort). Female rats were administered saline or BNT162b2 (30 μg mRNA/dose) intramuscularly (IM; 0.06 mL/dose) 21 and 14 days prior to the start of mating and on gestation days (GD) 9 and 20, for a total of 4 doses. The 30 μg mRNA/dose is the full human dose but represents greater than 300 times the human dose on a mg/kg basis (based on 220 g rat and 70 kg human).
Fig. 1.
Overview of the study design. Female rats were administered four intramuscular injections (2 prior to cohabitation with the males and 2 during gestation) of saline (control) or BNT162b2 (44/group). On each dosing day, animals were administered the full human dose (30 μg mRNA/dose) by intramuscular injection into the quadriceps muscle. Approximately half the rats (n = 22/group) underwent cesarean section on gestation day (GD) 21. The remaining rats (n = 22/group) were allowed to deliver naturally, and the maternal animals and offspring were followed through to the end of weaning. Blood was collected for measurement of antibody response in maternal animals prior to the first dose, at mating, end of gestation, and end of lactation. Blood was collected from fetuses at the end of gestation and from the littered offspring at the end of lactation.
Wistar Han rats CRL:WI(Han) were group housed (up to 5 per cage) until paired for mating at which time females were housed 1:1 with a non-treated breeder male. Following evidence of mating, the females were individually housed through gestation and lactation. Rats were provided with Complete Rodent Diet (Safe, France) available ad libitum. Locally sourced water (softened and filtered) was available ad libitum. Environmental conditions across studies were set to maintain relative humidity ≥35 % and temperature of 66 °F to 77 °F with room lighting set to provide a 12-h light/dark cycle.
2.3. Observations and measurements
Clinical signs, body weight, and food consumption were monitored throughout the study. Vaginal smears were collected daily and used to determine the cycle stage from 14 days prior to administration of the initial dose and continued until positive evidence of mating was observed (sperm present in a smear of vaginal contents or presence of copulatory plug). The day on which evidence of mating was observed was designated as GD 0.
Rats in the Caesarean cohort were euthanized on GD 21 via CO2 asphyxiation followed by exsanguination. A gross examination of the abdominal, thoracic, and pelvic viscera was performed. The gravid uterus was removed and weighed, and the number of corpora lutea in each ovary, and the number, type, and position of implantation sites were recorded. Uteri of apparently non-pregnant animals were stained with 10 % aqueous (v/v) ammonium sulfide to confirm absence of implantation sites. Viable fetuses were removed from the uteri and individually weighed. Live fetuses were euthanized by oral administration of sodium pentobarbital (0.05 mL of 182.2 mg/mL; Vetoquinol, France) or by decapitation (for purposes of blood collection). A detailed external examination of each fetus was conducted, including assessment of palatal closure and determination of sex. External, visceral, and skeletal findings were recorded as developmental abnormalities, variations or malformations. Approximately half of the fetuses were examined for visceral abnormalities using a modification of the Staples technique [27]. The heads from approximately half of the rats from each litter were removed and fixed in Harrison’s solution and subsequently examined by serial sectioning [28]. The remaining fetuses were eviscerated, macerated in potassium hydroxide, and stained with alizarin red [29] for skeletal examinations. All fetal morphological observations were recorded in general accordance with standardized terminology [30]. As defined by the Test Facility, fetal observations were classified as either malformations (structural defects that are rare in the control population and are thought to be life threatening or of major physiological consequence), anomalies (minor abnormalities or defects that are relatively rare in the control population and/or are considered not to be of major physiological consequence), or variations (minor abnormalities, defects or alternative forms that are either common in the control population or are of no known physiological consequence).
In the littering cohorts, dams were evaluated for natural delivery parameters including duration of gestation, litter size, maternal behavior (e.g. nursing, nesting) and pup viability at birth. Litters were reduced to 8 pups per litter when possible on postnatal day (PND) 4 by intraperitoneal injection of sodium pentobarbital (0.1 mL of 182.2 mg/mL; Vetoquinol, France). Rat pups that were not selected for continued observation underwent necropsy and gross examination of the abdominal, thoracic, and pelvic viscera. Pups were evaluated for external abnormalities, and body weights and age obtainment of physical developmental landmarks (pinna unfolding and eye opening) were monitored. Pinna unfolding and eye opening were evaluated once daily from PND 1 and PND 12, respectively, until both pinna were unfolded and both eyes were open. Neurofunctional development was evaluated by recording auditory and pupillary reflexes on PND 21 in response to a click sound and light source, respectively. Dams were euthanized on LD 21 via CO2 asphyxiation followed by exsanguination, and the ovaries and uteri were examined, as well as the abdominal and thoracic cavities, for any gross lesions. The number of implantation sites in the uteri were also recorded. Offspring were euthanized by CO2 asphyxiation on PND 21. Following euthanasia, blood was collected from at least 1 pup/sex/litter as described below, and a gross examination of the abdominal, thoracic, and pelvic viscera was performed.
2.4. Antibody analysis
Blood was collected from the dams prior to study initiation, before mating on the day of cohabitation, on GD 21 (Cesarean cohort) and on Lactation Day (LD) 21 (littering cohort). Rat fetal blood samples were collected on GD 21 from arbitrarily selected fetuses by decapitation, and blood samples were pooled by litter (minimum of 1 male and 1 female). Additionally, blood samples were collected on PND 21 from at least 2 pups per litter (1 male and 1 female where possible) via intracardiac puncture following euthanasia and were pooled by litter.
Samples were collected into tubes without anticoagulant and centrifugated at 1800 g and 4 °C, for 10 min. The resultant serum was removed, and serum samples were frozen at −80 °C prior to functional antibody analysis. Each serum sample was tested in duplicate for serological detection of SARS-CoV-2 specific neutralizing antibodies by VisMederi (Siena, Italy). The SARS-CoV-2 microneutralization cytopathic effect (CPE) based assay is a 4–5 day manual 96-well assay. On Day 0, Vero E6 cells (CRL-1586, American Type Culture Collection) were seeded into 96-well tissue culture plates. On Day 1, serial dilutions of test sera were incubated with SARS-CoV-2 2019 nCOV ITALY/INMI1 infectious virus to allow any antigen-specific antibodies to bind to the virus. The serum-virus mixture was then transferred onto the Vero cell monolayer and allowed to incubate for 3–4 days to allow for infection by non-neutralized virus to occur. Plates were visualized under an inverted light microscope and a sample microneutralization titer (MNt) was determined. The MNt was defined as the reciprocal of the highest serum dilution that protects at least 50 % of the cells from CPE.
2.5. Statistics and data analysis
Continuous data (e.g. body weights, body weight changes, food consumption, and litter averages for percent nonviable conceptuses) were analyzed using Levene’s test to assess the homogeneity of group variances. The groups were compared using a Dunnett’s test if Levene’s test was not significant or Dunn’s test if it was significant. Pre-coital interval, estrous cycle and F1 offspring functional test data were analyzed using Levene’s test to test the equality of variance across groups and Shapiro-Wilk's test was used to assess the normality of the data distribution in each group. Data with homogeneous variances and normal distribution in all groups were analyzed using ANOVA followed by Dunnett’s test. Data showing non-homogeneous variances or a non-normal distribution in at least 1 group were analyzed using Kruskal-Wallis test followed by Wilcoxon’s rank sum test. Litter percent of fetuses with abnormalities was evaluated as nonparametric data using Dunn’s test. Incidence data (reflex and physical development, mating performance, fertility indices, and parental indices) were assed using a Fisher’s exact test to conduct pairwise group comparisons of interest.
3. Results
3.1. Virus neutralizing antibody response
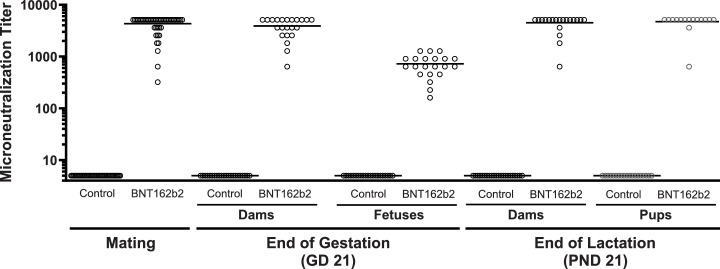
Administration of BNT162b2 elicited SARS-CoV-2 neutralizing antibody responses in all dams and all offspring (Fig. 2 ). Neutralizing antibody titers against SARS-CoV2 measured as MNt were detected in all dams at approximately 14 days following the second dose administration just prior to cohabitation, and titers remained elevated on GD 21 and LD 21. Similar to the dams, SARS-CoV2 neutralizing antibody titers were observed in all offspring (fetuses on GD 21 and pups on PND 21).
Fig. 2.
Functional antibody response against SARS-CoV2, as measured by microneutralization titer (MNt), over time after administration of saline (control) or BNT162b2 to female rats. See methods section for details on the method. MNt in dams were measured just prior to cohabitation for the mating phase, at the end of gestation on GD21, and at the end of lactation on LD 21. There were no detectable titers in females prior to first dose (data not shown). MNt in offspring were measured on GD 21 (fetuses) and PND 21 (pups). Titer data for each individual animal are shown with their respective means.
3.2. Assessment of female fertility and pregnancy
There were no BNT162b2-related effects on female fertility, as evidenced by lack of effects on estrous cyclicity, pre-coital interval, and mating, fertility or pregnancy indices (Table 1 ). In the BNT162b2 group, all of these mating performance and fertility endpoints were comparable to concurrent control.
Table 1.
Summary of fertility data from female rats administered control (saline) or BNT162b2.
| Control (saline) | BNT162b2 | |
|---|---|---|
| Fertility (n)a | 44 | 44 |
| Mean Estrous Cycle Length (days)b, c | 4.02 ± 0.19 | 4.00 ± 0.11 |
| Females with Acyclic Periodd | 8/44 (18.2 %) | 8/44 (18.2 %) |
| Days in Cohabitationb | 3.0 ± 2.2 | 2.8 ± 1.7 |
| Mating (Copulation) Indexe | 44/44 (100 %) | 44/44 (100 %) |
| Fertility Indexf | 43/44 (98 %) | 42/44 (95 %) |
| Pregnancy Rateg | 43/44 (98 %) | 42/44 (95 %) |
*p ≤ 0.05, **p ≤ 0.001, (g) = grams.
Combined data from both cesarean section and delivery cohorts.
Data presented as mean per group ± standard deviation.
Estrous cycle length is determined by counting the days from the first day of estrous and the next cycle, and only complete cycles are counted. Estrous cycle length calculation excluded females with no complete cycles (acyclic) (n = 8 in control and BNT162b2 groups).
Any cycle with length > 6 days is considered an acyclic period, and females with no complete cycles were considered acyclic.
Calculated as number of mated females/number paired × 100.
Calculated as number pregnant/number paired × 100.
Calculated as number pregnant/number mated × 100.
All F0 females in both the cesarean section and littering subgroups survived to scheduled euthanasia on GD 21 and LD 21, respectively. The only clinical sign associated with BNT162b2 administration was transient swelling at the injection site following administration of each dose during the premating and gestation periods. The swelling was associated with limping and/or piloerection in some animals for 1 or 2 days after the second dose only. These clinical signs were not considered adverse because the overall health of the animals was not impacted by these transient clinical signs. At maternal necropsy on both GD 21 and LD 21, macroscopic findings localized to the injection site (firm area, enlarged, edematous area and/or pale) were noted, which are consistent with administration of a vaccine and an inflammatory/ immune response.
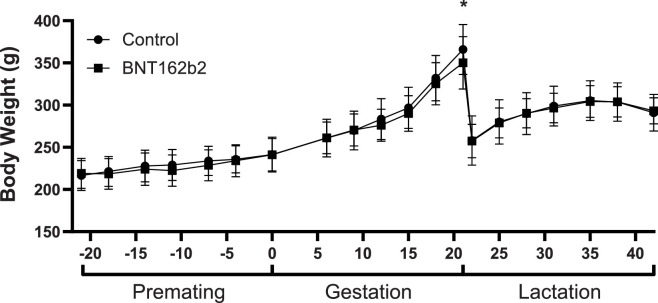
The only other maternal effects that were related to BNT162b2 administration were the slight, transient decreases in body weight (Fig. 3 ) and food consumption (data not shown) that followed each dose administration. The slight body weight loss or reduced body weight gain noted after each dose administration corresponded with the reduced mean food consumption that was noted after the first 3 dose administrations. Complete recovery of the body weight and food consumption effects was noted between each of the dose administrations such that absolute mean body weight and mean food consumption was comparable with the control group within a week or two following each dose administration.
Fig. 3.
Body weight of female rats (n = 44/group) following administration of saline (control) or BNT162b2 during the premating, gestation, and lactation phases. Significant decrease compared to control (p < 0.05) indicated by (*).
3.3. Cesarean section evaluation
There were no BNT162b2-related effects on any ovarian or uterine parameter evaluated at GD 21 cesarean section (Table 2 ). The numbers of corpora lutea and implantation sites in the control and BNT162b2 groups were both slightly higher than the normal range of historical control (HC). Early embryonic survival was not affected by BNT162b2 administration. Although the mean percentage pre-implantation loss (difference between number of corpora lutea [CL] and implantation sites divided by CL) was statistically (p < 0.05) higher in the BNT162b2 group (9.77 % compared with 4.09 % in the control group), the value was within the historical control data range (1.4–16.2 %) and likely due to the numerically higher number of CL in the BNT162b2 group (14.7 and 15.5 CL in the control and BNT162b2 groups, respectively). However, as there were no differences in the number of implantation sites (14.1 and 14.0 implantation sites/female in the control and BNT162b2 groups, respectively), live fetuses (13.2 and 13.1 live fetuses/dam in the control and BNT162b2 groups, respectively) or live pups in the delivery cohort (13.0 in both the control and BNT162b2 groups) the higher pre-implantation loss was considered to represent normal biological variation. BNT162b2 also had no impact on post-implantation embryo-fetal survival. The mean percentage post-implantation loss and the mean live litter size were comparable in the control and BNT162b2 groups and consistent with the historical control data. There were no effects on mean fetal body weights.
Table 2.
Cesarean section observations and fetal weights from the female rats in the cesarean section cohort administered control (saline) or BNT162b2.
| Control (saline) | BNT162b2 | CRL-Lyon HC Mean (min–max)a | |
|---|---|---|---|
| C-Section Cohort (n)b | 21 | 21 | – |
| Gravid uterine weight (g) | 86.32 ± 7.69c | 87.65 ± 13.48 | 75.6 (64.6–86.8) |
| Corpora lutea | 14.7 ± 1.6 | 15.5 ± 2.1 | 13.2 (11.6–14.3) |
| Implantation sites | 14.1 ± 1.6 | 14.0 ± 2.2 | 12.1 (10.4–13.8) |
| Pre-implantation loss (%) | 4.09 ± 6.56 | 9.77 ± 8.09* | 8.4 (1.4–16.2) |
| Post-implantation loss (%) | 6.10 ± 7.64 | 5.85 ± 7.28 | 8.8 (2.4–17.3) |
| Number live fetuses | 13.2 ± 1.6 | 13.1 ± 2.1 | 11.0 (9.3–12.7) |
| Mean fetal body weight (g) | 4.89 ± 0.23 | 4.90 ± 0.30 | 5.09 (4.87–5.24) |
p ≤ 0.05, (g) = grams.
Historical control data for 24 studies from the test facility from years.2017–2018.
In this C-section cohort, 1 dam from each group was not pregnant.
Data presented as mean per group ± standard deviation.
3.4. Fetal examinations
At GD 21 cesarean section, fetuses were evaluated for potential effects of BNT162b2 on fetal morphological development. There were no BNT162b2-related effects on fetal external, visceral, or skeletal morphology (Table 3 ). Two fetuses in the BNT162b2 group were noted with external observations (1 fetus with gastroschisis and 1 fetus with small mouth and agnathia). The fetus with agnathia and a small mouth had associated skeletal malformations of short and fused mandibles. These external malformations are normal background findings in this strain (are within HC incidence) and only occurred in single fetuses and thus were not considered to be related to BNT162b2. The visceral malformation of right sided aortic arch was seen in 1 fetus in the BNT162b2 and not in control fetuses. This finding has not been observed in the testing facility HC data with Wistar rats; however, it has been reported in the HC from CRL Den Bosch in the same strain and source (2 fetuses in 2 litters, with a maximum incidence of 0.8 % fetuses/litter), and therefore this single finding was not considered related to BNT162b2 administration. All other findings noted at fetal visceral and skeletal examination occurred at an incidence within the normal background incidence noted for this rat strain at the performing laboratory, and therefore were considered incidental (Table 3).
Table 3.
Summary of rat fetal examination data from the embryo fetal development study with control (saline) and BNT162b2 (n = 21 rats per group).
| Control (saline) | BNT162b2 | CRL-Lyon HCa | |
|---|---|---|---|
| External (n) | 21/277b | 21/276 | |
| Agnathia with small mouth – [M] | – | 1/1 (0.4)b, c | 1 (1.7) |
| Gastroschisis – [M] | – | 1/1 (0.4) | 1 (NA) |
| Visceral (n) | 21/133 | 21/132 | |
| Aortic arch, right sided – [M] | – | 1/1 (0.8)d | 2 (0.8)e |
| Azygous vein, transposed – [A] | 1/1 (0.8) | – | 1 (NA) |
| Umbilical artery, transposed – [V] | 6/7 (5.3) | 8/13 (9.8)d | 239 (21.9) |
| Liver, abnormal lobation – [A] | 1/1 (0.8) | – | 2 (1.9) |
| Absent lung lobe – [A] | – | 1/1 (0.8)d | 1 (1.1) |
| Skeletal (n) | 21/144 | 21/144 | |
| Hyoid, incomplete ossification – [A] | – | 1/1 (0.7) | 4 (2.0) |
| Interparietal, incomplete ossification – [V] | 3/3 (2.1) | 3/4 (2.8) | 113 (15.1) |
| Parietal, incomplete ossification – [V] | – | 3/3 (2.1) | 107 (16.2) |
| Presphenoid, incomplete ossification – [A] | 1/1 (0.7) | – | 1 (1.1) |
| Squamosal, incomplete ossification – [V] | – | 1/1 (0.7) | 36 (11.2) |
| Supraoccipital, incomplete ossification – [V] | – | 2/2 (1.4)c | 44 (8.9) |
| 27 presacral vertebral arches – [A] | – | 1/1 (0.7) | 4 (1.5) |
| Forepaw phalanx, unossified – [A] | 7/9 (6.3) | 3/6 (4.2) | 51 (15.7) |
| Hindpaw phalanx | |||
| 1st digit, metatarsal, unossified – [V] | 3/3 (2.1) | 3/3 (2.1) | 11 (NA) |
| 2nd-5th digit, unossified – [V] | 11/46 (31.9) | 7/22 (15.3) | 236 (NA) |
| Ribs | |||
| Supernumerary cervical – [A] | 3/3 (2.1) | – | 11 (4.5) |
| Supernumerary lumbar – [A] | 3/3 (2.1) | 6/12 (8.3) | 17 (9.7) |
| Supernumerary lumbar, short – [V] | 17/57 (39.6) | 18/71 (49.3) | 500 (56.1) |
| Thick – [A] | 1/2 (1.4) | 3/4 (2.8) | 57 (11.2) |
| Wavy – [A] | – | 1/1 (0.7) | 13 (3.4) |
| Sternebra | |||
| Asymmetric – [A] | 1/1 (0.7) | – | 12 (2.8) |
| Minor fusion – [A] | 1/1 (0.7) | – | – |
| Incompletely ossified, 1st/3rd – [A] | 1/1 (0.7) | 1/1 (0.7) | 11 (2.1) |
| Incompletely ossified, 2nd/4th – [V] | 1/1 (0.7) | 2/2 (1.4) | 34 (6.9) |
| Caudal vertebra, less than 5 – [A] | – | 2/2 (1.4) | 19 (6.3) |
| Cervical vertebra | |||
| Arch, incomplete ossification – [A] | – | 2/2 (1.4)c | 12 (5.8) |
| Odontoid process, incomplete ossification – [V] | 7/9 (6.3) | 4/6 (4.2) | 65 (13.0) |
| Centrum, unossified – [V] | 3/3 (2.1) | 2/2 (1.4) | 111 (32.8) |
| 7 Lumbar vertebrae – [A] | 1/1 (0.7) | 2/3 (2.1) | 12 (3.2) |
| Thoracic vertebral centrum | |||
| Incomplete ossification, 1st-9th – [A] | 1/1 (0.7) | 3/3 (2.1)c | 8 (2.8) |
| Incomplete ossification, 10th-13th – [A] | 5/6 (4.2) | 9/9 (6.3)c | 12 (5.0) |
[M] = Malformation; [A] = Anomaly; [V] = Variation; - = not observed; NA = not available.
Historical control data in the CRL-WI rat from the test facility from years 2013–2019, data presented as fetal incidence (maximum % fetuses affected in a control group).
Data presented as number of litters affected/number of fetuses affected (mean % fetuses affected).
Multiple findings observed in this specific fetus.
Multiple findings observed in this specific fetus.
Historical control data in the CRL:WI(Han) rat from Charles River Den Bosch from years.2014–2019.
3.5. Assessment of Parturition, lactation and offspring
BNT162b2 had no effects on parturition or gestation length (Table 4 ). The number of pups delivered per litter, mean number of live pups at birth, and the live birth index in the BNT162b2 group were comparable to control. Survival through PND 21 was also not affected by BNT162b2, as demonstrated by the lack of difference in the viability index (percent of pups surviving from PND 0 to PND 4; pre-cull) and weaning index (percent of pups surviving from PND 4 through PND 21; post-cull) as compared to control. BNT162b2 administration had no effects on offspring growth, physical development or neurofunctional development. There were no clinical signs related to BNT162b2 administration and no effects on pup body weights during the preweaning period. There were no effects on preweaning physical or functional development of the F1 pups (Table 5 ). In the BNT162b2 group, the age at which pinna unfolding and eye opening was attained was comparable to the control group. Similarly, all pups in the control and BNT162b2 groups exhibited auditory and pupillary reflexes when evaluated on PND 21. At scheduled necropsy on PND 21, there were no BNT162b2-related macroscopic observations in the pups (data not shown).
Table 4.
Summary of maternal delivery and pup data from the female rats in the delivery cohort administered control (saline) or BNT162b2.
| Control (saline) | BNT162b2 | |
|---|---|---|
| Delivery Cohort (n)a | 22 | 21 |
| Gestation Length (days) | 22.1 ± 0.4b | 22.0 ± 0.7 |
| Number of implantation sites | 14.3 ± 2.2 | 14.2 ± 2.2 |
| Pups delivered per litter (PND 0) | 13.3 ± 2.5 | 13.1 ± 3.1 |
| Number live pups at birth (mean/litter) | 13.0 ± 2.5 | 13.0 ± 3.1 |
| Live Birth Indexc | 98 % | 99.3 % |
| Pup Viability Index (PND 0–4)d | 99 % | 98.9 % |
| Pup Weaning Index (PND 4–21)e, f | 99.4 % | 100 % |
| Pup Mortality (PND 0–21) | 10 | 5 |
| Pup body weight on PND 4 (g)f | 9.60 ± 1.25 | 9.75 ± 1.31 |
| Pup body weight on PND 21(g)f | 54.75 ± 4.07 | 55.23 ± 2.71 |
(g) = grams; LD = lactation day; PND = postnatal day.
In this Delivery cohort, 1 dam in the BNT162b2 group was not pregnant.
Data presented as mean per group ± standard deviation.
Calcluated as (number of pups born alive/ number of pups born) × 100.
Calculated as (number of live pups on PND 4 (preculling)/ number of liveborn pups on PND 1) × 100.
Calculated as (number of live pups on PND 21 (weaning)/ number of liveborn pups on PND 4) × 100.
Data after post-cull.
Table 5.
Pup preweaning physical and neurofunctional development. For pinna unfolding (pre-cull), the total number of litters/pups evaluated were 22/285 and 21/273 for the control and BNT162b2 groups, respectively. For eye opening (post-cull), the total number evaluated were 22/176 and 21/163 for the control and BNT162b2 groups, respectively. For both reflex endpoint (post-cull), the total number evaluated were 22/175 and 21/163 for the control and BNT162b2 groups, respectively.
| Control (saline) | BNT162b2 | |
|---|---|---|
| Pinna unfolding (day at which 100 % pups attained landmark) | 4 | 4 |
| Eye opening (day at which 100 % pups attained landmark) | 16 | 16 |
| Auditory reflex (% pups positive) | 100 | 100 |
| Pupillary reflex (% pups positive) | 100 | 100 |
4. Discussion
Experimental studies in animals are currently the best available tools to predict the potential for developmental toxicity of vaccines in humans [31] and are generally considered prerequisites to support clinical studies in pregnant women [32] or registration in target populations that include women of childbearing potential [33]. The current BNT162b2 DART study was conducted based on the design described in the 2006 FDA Guidance, Considerations for Developmental Toxicity Studies for Preventative and Therapeutic Vaccines for Infectious Disease Indications [33], the 2005 World Health Organization guidelines on nonclinical evaluation of vaccines [23], and the recently updated ICH S5(R3) guideline, which now includes vaccines for infectious disease [22]. The purpose of this DART vaccine study was to detect any adverse effects on development regardless of the mechanism (e.g. vaccine product itself and/or immune response to that vaccine) [33,34]. The endpoints in this rat DART vaccine study design have been used for more than 20 years with other therapeutics in female fertility, embryo-fetal development, and pre- and postnatal developmental toxicity studies, and thus this experience and historical control data provide confidence in the suitability of these animal models and endpoints in detection of effects relevant for human risk [22,[35], [36], [37], [38], [39]].
No adverse effects of BNT162b2 or its associated immune response, were detected on embryo-fetal or postnatal survival, growth, or development in the offspring through the end of lactation. Vaccination of female rats with BNT162b2 twice prior to mating and twice more during gestation resulted in a robust, neutralizing maternal antibody response that was noted prior to mating, throughout gestation and lactation, as well as in fetuses at the end of gestation and pups at the end of lactation. There were no effects on female rat fertility and reproduction. The lack of female fertility effects is consistent with the lack of microscopic effects in female reproductive organs in non-pregnant rats administered BNT162b2 in prior general toxicology studies (data not shown). The only observations in adult females in the current DART study were non-adverse clinical signs and macroscopic findings localized to the injection site as well as transient, non-adverse body weight and food consumption effects after each dose administration. These findings were consistent with BNT162b2 studies in non-pregnant rats (data not shown) and are related to the inflammatory response following vaccine administration.
Therapeutic success in humans using LNP to deliver RNA has been demonstrated using Onpattro (patisiran) [40]. The recommended dosage of Onpattro for patients weighing 100 kg or more is 30 mg by intravenous infusion compared with 30 μg by intramuscular injection for BNT162b2. Onpattro is not a vaccine but a double-stranded RNA that has its therapeutic effect by interfering with the mRNA of a pharmacological target protein in a specific patient population. Regarding nonclinical safety in pregnancy, there were no adverse pre- and postnatal developmental effects associated with Onpattro except for embryofetal lethality in rabbits at maternally toxic doses, which may be associated with a pharmacodynamic-related decrease in Vitamin A (considered essential for embryo-fetal development). With Onpattro there was no fetal transfer of RNA, PEG-C-DMG (lipid component of the LNP), or DLin-MC3-DMA (a different lipid component of the LNP) in rabbit, but in rat approximately 0.4 % fetal transfer of DLin-MC3-DMA was observed. While Onpattro was not designed for in utero delivery, this fetal exposure is consistent with efforts to evaluate different mRNA-LNP formulations for mRNA expression in the fetus, in the absence of maternal toxicity or fetal loss [41]. In addition, while RNA delivered by Onpattro was not detected in milk of lactating rats, both PEG-C-DMG and DLin-MC3-DMA were present. Overall, for Onpattro no fetal or milk transfer of RNA was observed, and the limited transfer of LNP components observed did not correlate with any observed outcome in the pre- and postnatal developmental studies [42].
LNP formulated mRNA vaccines are administered intramuscularly, twice with a 3- or 4-week inter-dose interval and at a much lower dose (30 μg/ intramuscular injection for BNT162b2) compared to 15000–21,000 μg/ intravenous injection for Onpattro [40] based on 50–70 kg individuals. As compared with intravenous and intraperitoneal administration, relatively low amounts of mRNA-LNPs are expected to reach the maternal blood stream via lymphatic draining when administered intramuscularly [43]. Moreover, the maternal-placental blood circulation and the fetal-placental blood circulation are separate circulatory systems providing an additional barrier to the fetus for mRNA-LNP delivery. Therefore, the potential for meaningful fetal exposure to mRNA-LNP vaccines following maternal intramuscular vaccination is expected to be very low. However, due to the robust maternal SARS-CoV-2 neutralizing antibody titers it is expected that there would be both placental and lactation transfer of those maternal antibodies to offspring [44,45] as observed in both rat fetuses and pups (Fig. 3).
These DART data with BNT162b2 represent some of the first published data evaluating functional outcomes following administration of an LNP-formulated, nucleoside-modified mRNA vaccine during gestation. Adverse effects on female fertility and pre/postnatal development with BNT162b2 were not observed. The lack of effect on female fertility and development was also observed in a separate female rat DART study with mRNA-1273, another LNP-mRNA vaccine used to prevent COVID-19 [46]. Both COVID-19 LNP-mRNA vaccines use similar lipid components (an ionizable lipid, cholesterol, a polyethylene glycol conjugated lipid, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]) and an mRNA encoding the prefusion spike (S) glycoprotein of the SARS-CoV-2 virus.
As pregnant women were excluded from the initial vaccine clinical trials, these nonclinical BNT162b2 DART study data presented here, coupled with broad clinical experience including nonpregnant women, enabled the initiation of clinical evaluation of BNT162b2 in pregnant women. Historically, clinical trials have not often been conducted in pregnant women, and thus critical data to inform benefit/risk in human pregnancy have been lacking. However, several vaccines are recommended for administration to pregnant women, including vaccines protecting against influenza and tetanus, diphtheria, and pertussis. The absence of specific clinical trial data of BNT162b2 in pregnant women resulted in limited uptake of BNT162b2 vaccination in pregnant women, and this vaccine hesitancy is putting mothers and fetuses at higher risk of pregnancy complications related to COVID-19 [47]. Disease risk with respiratory viruses is particularly high in pregnant women, where the resultant reduction in lung capacity can be compounded by pregnancy-induced changes in the maternal immune system, leading to increased susceptibility to complications from infection [9]. Experience from the 2009 influenza pandemic demonstrated the increased benefit-risk ratio of vaccines to infectious disease during pregnancy. Pregnant women with influenza were shown to be at increased risk of influenza-associated complications, including morbidity and death, and their newborn infants were shown to be at increased risk of adverse outcomes such as preterm birth and low birthweight [48,49]. There have been many studies demonstrating the safety and immunogenicity of influenza vaccination for pregnant women, including evidence of maternal antibody transfer, confirming the clinical benefit of maternal influenza vaccination both for the mother and the infant, over any perceived vaccination risks [50,51].
Similar to influenza, COVID-19 in pregnant women carries a higher risk of severe illness compared with infection in nonpregnant women, and increased severity of this illness has been associated with adverse outcomes including preterm birth [[2], [3], [4], [5],[10], [11], [12],14,15,52,53]. The limited data available suggest that SARS-CoV-2 rarely crosses from mother to fetus, although some data indicate that the virus may adversely affect the placenta with potential consequences to the fetus [53,54]. Natural COVID-19 infection appears to elicit limited transfer of SARS-CoV-2 induced antibodies to the fetus [55,56], whereas COVID-19 vaccine-induced antibodies have more consistently been detected in cord blood [19,20,57]. This transplacental transfer may offer protection to infants from COVID-19 shortly after birth. There are also data suggesting that, although virus is rarely detectable in breast milk, SARS-CoV-2 induced antibodies are present [55,56]. In addition, although RNA from COVID-19 vaccines has not been found in breastmilk [58], available data show that SARS-CoV-2 specific antibodies are present in breast milk following COVID-19 vaccination, including BNT162b2, during lactation [19,59,60]. This is consistent with presence of SARS-CoV-2 specific antibodies in rat pups from the current DART study following maternal vaccination, and the lack of toxicity in these rat pups may support safety of vaccine-induced antibodies during lactation.
Because pregnant women are at increased risk of severe COVID-19 symptoms and associated complications, it is important to provide more definitive data on the benefit-risk of maternal COVID-19 vaccination in order to aid informed decision-making based on individual vaccine recipient considerations [61]. Currently, there is a positive profile of efficacy and safety in nonpregnant women [17,62], and the DART study presented here demonstrates safety of BNT162b2 based on apical endpoints in a guideline-designed toxicity study in animals. This DART study evaluated the clinical dose of 30 μg mRNA/dose, which represents greater than 300 times the human dose on a mg/kg basis. The lack of findings with BNT162b2 in animals supports the safety of using this vaccine in women of childbearing potential and supports the clinical trial in pregnant women (ClinicalTrials.gov Identifier: NCT04754594) that is ongoing at the time this manuscript was written. This prospective study with BNT162b2 in pregnant women coupled with existing and available data being collected to monitor COVID-19 vaccine safety in pregnant women [21] (including the FDA and CDC’s Vaccine Adverse Event Reporting System [VAERS] and the CDC’s smartphone-based pregnancy registry [V-safe]) will help improve the benefit-risk decision-making for BNT162b2 vaccination in pregnant women.
5. Conclusion
The vaccine BNT162b2 was developed in response to the COVID-19 pandemic. To support use in women of childbearing potential, a developmental and reproductive toxicity study was conducted in rats according to regulatory guidelines. Intramuscular administration of the full human BNT162b2 dose of 30 μg mRNA (>300 times the human dose on a mg/kg basis) to female rats twice prior to mating and twice during gestation caused no effects on embryo-fetal or postnatal survival, growth, or development in the offspring through the end of lactation. A robust neutralizing immune response was confirmed prior to mating and at the end of gestation and lactation. Neutralizing antibodies were also confirmed in fetuses and offspring. No effects of BNT162b2 were observed on female mating performance, fertility, or any ovarian or uterine parameters. The only findings were nonadverse maternal effects related to the local injection site reaction that were expected from other animal studies and consistent with those observed in humans. In concert with the safety profile in nonpregnant people from ongoing vaccination programs, this nonclinical study enabled initiation of a clinical study in pregnant women.
Declaration of Competing Interest
CJB, NRC, GDC, SNC, MWC, CMR, RS are currently employed by and hold stock in Pfizer, Inc. CL and JD are currently employed by and hold stock in BioNTech SE. MB is currently employed by Charles River Laboratories.
Acknowledgements
Funding for this work came from BioNTech and Pfizer, Inc. The authors would like to thank Giulia Lapini (VisMederi) for the serum antibody analysis, Christine Stethem (Pfizer) for data review, Hendrik Gille (BioNTech) for study design discussions, Edward Marsden (Charles River Laboratories) for data and manuscript review, and Ugur Sahin (BioNTech), Kathrin Jansen (Pfizer), Phil Dormitzer (Pfizer), and Mark Boaz (Pfizer) for critical review of the manuscript.
Handling Editor: Dr. Anna Bal-Price
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wastnedge E.A.N., Reynolds R.M., van Boeckel S.R., Stock S.J., Denison F.C., Maybin J.A., Critchley H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021;101(1):303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zambrano L.D., Ellington S., Strid P., Galang R.R., Oduyebo T., Tong V.T., Woodworth K.R., Nahabedian J.F., 3rd, Azziz-Baumgartner E., Gilboa S.M., Meaney-Delman D., C.C.-R. Pregnancy, T. Infant Linked Outcomes Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(44):1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi D.W., Kaeser L., Cernich A.N. Involving pregnant individuals in clinical research on COVID-19 vaccines. JAMA. 2021;325(11):1041–1042. doi: 10.1001/jama.2021.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin R. Pregnant people’s paradox-excluded from vaccine trials despite having a higher risk of COVID-19 complications. JAMA. 2021;325(11):1027–1028. doi: 10.1001/jama.2021.2264. [DOI] [PubMed] [Google Scholar]
- 6.Clarke A.G., Kendall M.D. The thymus in pregnancy: the interplay of neural, endocrine and immune influences. Immunol. Today. 1994;15(11):545–551. doi: 10.1016/0167-5699(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 7.Sel G. In: Practical Guide to Oral Exams in Obstetrics and Gynecology. Sel G., editor. Springer; Cham, Switzerland: 2020. Physiological changes during pregnancy; pp. 29–37. [Google Scholar]
- 8.Vale A.J.M., Fernandes A.C.L., Guzen F.P., Pinheiro F.I., de Azevedo E.P., Cobucci R.N. Susceptibility to COVID-19 in pregnancy, labor, and postpartum period: immune system, vertical transmission, and breastfeeding. Front. Glob. Women’s Health. 2021;2:1–16. doi: 10.3389/fgwh.2021.602572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morelli S.S., Mandal M., Goldsmith L.T., Kashani B.N., Ponzio N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015;6:171–189. [Google Scholar]
- 10.Oakes M.C., Kernberg A.S., Carter E.B., Foeller M.E., Palanisamy A., Raghuraman N., Kelly J.C. Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am. J. Obstet. Gynecol. MFM. 2021;3(3) doi: 10.1016/j.ajogmf.2021.100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villar J., Ariff S., Gunier R.B., Thiruvengadam R., Rauch S., Kholin A., Roggero P., Prefumo F., do Vale M.S., Cardona-Perez J.A., Maiz N., Cetin I., Savasi V., Deruelle P., Easter S.R., Sichitiu J., Soto Conti C.P., Ernawati E., Mhatre M., Teji J.S., Liu B., Capelli C., Oberto M., Salazar L., Gravett M.G., Cavoretto P.I., Nachinab V.B., Galadanci H., Oros D., Ayede A.I., Sentilhes L., Bako B., Savorani M., Cena H., Garcia-May P.K., Etuk S., Casale R., Abd-Elsalam S., Ikenoue S., Aminu M.B., Vecciarelli C., Duro E.A., Usman M.A., John-Akinola Y., Nieto R., Ferrazi E., Bhutta Z.A., Langer A., Kennedy S.H., Papageorghiou A.T. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lokken E.M., Huebner E.M., Taylor G.G., Hendrickson S., Vanderhoeven J., Kachikis A., Coler B., Walker C.L., Sheng J.S., Al-Haddad B.J.S., McCartney S.A., Kretzer N.M., Resnick R., Barnhart N., Schulte V., Bergam B., Ma K.K., Albright C., Larios V., Kelley L., Larios V., Emhoff S., Rah J., Retzlaff K., Thomas C., Paek B.W., Hsu R.J., Erickson A., Chang A., Mitchell T., Hwang J.K., Erickson S., Delaney S., Archabald K., Kline C.R., LaCourse S.M., Adams Waldorf K.M., C.-i.P.C. Washington State Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2020.12.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosby L.G., Rasmussen S.A., Jamieson D.J. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am. J. Obstet. Gynecol. 2011;205(1):10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Woodworth K.R., Olsen E.O., Neelam V., Lewis E.L., Galang R.R., Oduyebo T., Aveni K., Yazdy M.M., Harvey E., Longcore N.D., Barton J., Fussman C., Siebman S., Lush M., Patrick P.H., Halai U.A., Valencia-Prado M., Orkis L., Sowunmi S., Schlosser L., Khuwaja S., Read J.S., Hall A.J., Meaney-Delman D., Ellington S.R., Gilboa S.M., Tong V.T., Pregnancy C.C.-R., T. Infant Linked Outcomes, C.-. Pregnancy, T. Infant Linked Outcomes Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69(44):1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee J., Mullins E., Townson J., Playle R., Shaw C., Kirby N., Munnery K., Bourne T., Teoh T.G., Dhanjal M., Poon L., Wright A., Lees C. Pregnancy and neonatal outcomes in COVID-19: study protocol for a global registry of women with suspected or confirmed SARS-CoV-2 infection in pregnancy and their neonates, understanding natural history to guide treatment and prevention. BMJ Open. 2021;11(1) doi: 10.1136/bmjopen-2020-041247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., Kuntz J.L., Dunnigan K., Odean M.J., Hegmann K.T., Stefanski E., Edwards L.J., Schaefer-Solle N., Grant L., Ellingson K., Groom H.C., Zunie T., Thiese M.S., Ivacic L., Wesley M.G., Lamberte J.M., Sun X., Smith M.E., Phillips A.L., Groover K.D., Yoo Y.M., Gerald J., Brown R.T., Herring M.K., Joseph G., Beitel S., Morrill T.C., Mak J., Rivers P., Harris K.M., Hunt D.R., Arvay M.L., Kutty P., Fry A.M., Gaglani M. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers — Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Tureci O., Nell H., Schaefer A., Unal S., Tresnan D.B., Mather S., Dormitzer P.R., Sahin U., Jansen K.U., Gruber W.C., Group C.C.T. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernan M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., Baez A.M., Shook L.L., Cvrk D., James K., De Guzman R.M., Brigida S., Diouf K., Goldfarb I., Bebell L.M., Yonker L.M., Fasano A., Rabi S.A., Elovitz M.A., Alter G., Edlow A.G. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert P., Rudnick C. Newborn Antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination. medRxiv. 2021 doi: 10.1186/s12887-021-02618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimabukuro T.T., Kim S.Y., Myers T.R., Moro P.L., Oduyebo T., Panagiotakopoulos L., Marquez P.L., Olson C.K., Liu R., Chang K.T., Ellington S.R., Burkel V.K., Smoots A.N., Green C.J., Licata C., Zhang B.C., Alimchandani M., Mba-Jonas A., Martin S.W., Gee J.M., Meaney-Delman D.M., CDC v-safe COVID-19 Pregnancy Registry Team Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ICH . 2020. S5(R3) Guidance on Detection of Reproductive and Developmental Toxicity for Human Pharmaceuticals. [Google Scholar]
- 23.WHO . WHO Technical Report Series, No. 927; 2005. WHO Guidelines on Nonclinical Evaluation of Vaccines. [Google Scholar]
- 24.National Research Council . eighth edition. The National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 25.2013-118, Decree No . 2013. Relating to the Protection of Animals Used in Scientific Experiments Described in the Journal Officiel De La Republique Francaise. [Google Scholar]
- 26.2010/63/EU, Directive . 2010. European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. [Google Scholar]
- 27.Staples R.E. Detection of visceral alterations in mammalian fetuses. Teratology. 1974;9(3):A37–A38. [Google Scholar]
- 28.Wilson J.G. In: Teratology: Principles and Techniques. Wilson J.G., editor. University of Chicago Press; Chicago, IL: 1965. Methods for administering agents and detecting malformations in experimental animals; pp. 262–277. [Google Scholar]
- 29.Staples R.E., Schnell V.L. Refinements in rapid clearing technic in the koh-alizarin red S method for fetal bone. Stain Technol. 1964;39:61–63. [PubMed] [Google Scholar]
- 30.Makris S.L., Solomon H.M., Clark R., Shiota K., Barbellion S., Buschmann J., Ema M., Fujiwara M., Grote K., Hazelden K.P., Hew K.W., Horimoto M., Ooshima Y., Parkinson M., Wise L.D. Terminology of developmental abnormalities in common laboratory mammals (version 2) Congenit. Anom. (Kyoto) 2009;49(3):123–246. doi: 10.1111/j.1741-4520.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 31.Gruber M.F. Maternal immunization: US FDA regulatory considerations. Vaccine. 2003;21(24):3487–3491. doi: 10.1016/s0264-410x(03)00357-8. [DOI] [PubMed] [Google Scholar]
- 32.Roberts J.N., Gruber M.F. Regulatory considerations in the clinical development of vaccines indicated for use during pregnancy. Vaccine. 2015;33(8):966–972. doi: 10.1016/j.vaccine.2014.12.068. [DOI] [PubMed] [Google Scholar]
- 33.FDA . 2006. Guidance for Industry: Considerations for Developmental Toxicity Studies for Preventative and Therapeutic Vaccines for Infectious Disease Indications. [Google Scholar]
- 34.Barrow P. Developmental and reproductive toxicity testing of vaccines. J. Pharmacol. Toxicol. Methods. 2012;65(2):58–63. doi: 10.1016/j.vascn.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 35.FDA . 2011. Guidance for Industry: Reproductive and Developmental Toxicities - Integrating Study Results to Assess Concerns. [Google Scholar]
- 36.Bailey G.P., Wise L.D., Buschmann J., Hurtt M., Fisher J.E. Pre- and postnatal developmental toxicity study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86(6):437–445. doi: 10.1002/bdrb.20217. [DOI] [PubMed] [Google Scholar]
- 37.Wise L.D., Buschmann J., Feuston M.H., Fisher J.E., Hew K.W., Hoberman A.M., Lerman S.A., Ooshima Y., Stump D.G. Embryo-fetal developmental toxicity study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86(6):418–428. doi: 10.1002/bdrb.20214. [DOI] [PubMed] [Google Scholar]
- 38.Lerman S.A., Hew K.W., Stewart J., Stump D.G., Wise L.D. The nonclinical fertility study design for pharmaceuticals. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86(6):429–436. doi: 10.1002/bdrb.20221. [DOI] [PubMed] [Google Scholar]
- 39.Sun Y., Gruber M., Matsumoto M. Overview of global regulatory toxicology requirements for vaccines and adjuvants. J. Pharmacol. Toxicol. Methods. 2012;65(2):49–57. doi: 10.1016/j.vascn.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Alnylam Pharmaceuticals, Inc.; 2020. Onpattro (patisiran) [United States Package Insert] Revised 02. [Google Scholar]
- 41.Riley R.S., Kashyap M.V., Billingsley M.M., White B., Alameh M.G., Bose S.K., Zoltick P.W., Li H., Zhang R., Cheng A.Y., Weissman D., Peranteau W.H., Mitchell M.J. Ionizable lipid nanoparticles for in utero mRNA delivery. Sci. Adv. 2021;7(3) doi: 10.1126/sciadv.aba1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Food and Drug Administration, Center for Drug Evaluation and Research; 2018. Onpattro (patisiran) [Multi-discipline review] NDA 210922. [Google Scholar]
- 43.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bowman C.J., Breslin W.J., Connor A.V., Martin P.L., Moffat G.J., Sivaraman L., Tornesi M.B., Chivers S. Placental transfer of Fc-containing biopharmaceuticals across species, an industry survey analysis. Birth Defects Res. B Dev. Reprod. Toxicol. 2013;98:459–485. doi: 10.1002/bdrb.21089. [DOI] [PubMed] [Google Scholar]
- 45.DeSesso J.M., Williams A.L., Ahuja A., Bowman C.J., Hurtt M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012;42(3):185–210. doi: 10.3109/10408444.2011.653487. [DOI] [PubMed] [Google Scholar]
- 46.2021. COVID-19 Vaccine Moderna (mRNA-1273) [European Medicines Agency EPAR, Annex I, Summary of Product Characteristics] last updated 03. [Google Scholar]
- 47.Brent R.L. Risks and benefits of immunizing pregnant women: the risk of doing nothing. Reprod. Toxicol. 2006;21(4):383–389. doi: 10.1016/j.reprotox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Haberg S.E., Trogstad L., Gunnes N., Wilcox A.J., Gjessing H.K., Samuelsen S.O., Skrondal A., Cappelen I., Engeland A., Aavitsland P., Madsen S., Buajordet I., Furu K., Nafstad P., Vollset S.E., Feiring B., Nokleby H., Magnus P., Stoltenberg C. Risk of fetal death after pandemic influenza virus infection or vaccination. N. Engl. J. Med. 2013;368(4):333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasmussen S.A., Jamieson D.J., Uyeki T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012;207(3 Suppl):S3–8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 50.Jamieson D.J., Kissin D.M., Bridges C.B., Rasmussen S.A. Benefits of influenza vaccination during pregnancy for pregnant women. Am. J. Obstet. Gynecol. 2012;207(3 Suppl):S17–20. doi: 10.1016/j.ajog.2012.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E., Omer S.B., Shahid N.S., Breiman R.F., Steinhoff M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008;359(15):1555–1564. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 52.Khoury R., Bernstein P.S., Debolt C., Stone J., Sutton D.M., Simpson L.L., Limaye M.A., Roman A.S., Fazzari M., Penfield C.A., Ferrara L., Lambert C., Nathan L., Wright R., Bianco A., Wagner B., Goffman D., Gyamfi-Bannerman C., Schweizer W.E., Avila K., Khaksari B., Proehl M., Heitor F., Monro J., Keefe D.L., D’Alton M.E., Brodman M., Makhija S.K., Dolan S.M. Characteristics and outcomes of 241 births to women with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection at five New York City Medical Centers. Obstet. Gynecol. 2020;136(2):273–282. doi: 10.1097/AOG.0000000000004025. [DOI] [PubMed] [Google Scholar]
- 53.Subbaraman N. How Does COVID Affect Mother and Baby? Nature. 2021;591:193–195. doi: 10.1038/d41586-021-00578-y. [DOI] [PubMed] [Google Scholar]
- 54.Edlow A.G., Li J.Z., Collier A.Y., Atyeo C., James K.E., Boatin A.A., Gray K.J., Bordt E.A., Shook L.L., Yonker L.M., Fasano A., Diouf K., Croul N., Devane S., Yockey L.J., Lima R., Shui J., Matute J.D., Lerou P.H., Akinwunmi B.O., Schmidt A., Feldman J., Hauser B.M., Caradonna T.M., De la Flor D., D’Avino P., Regan J., Corry H., Coxen K., Fajnzylber J., Pepin D., Seaman M.S., Barouch D.H., Walker B.D., Yu X.G., Kaimal A.J., Roberts D.J., Alter G. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu F., Zozaya C., Zhou Q., De Castro C., Shah P.S. SARS-CoV-2 genome and antibodies in breastmilk: a systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021 doi: 10.1136/archdischild-2020-321074. [DOI] [PubMed] [Google Scholar]
- 56.Pace R.M., Williams J.E., Jarvinen K.M., Belfort M.B., Pace C.D.W., Lackey K.A., Gogel A.C., Nguyen-Contant P., Kanagaiah P., Fitzgerald T., Ferri R., Young B., Rosen-Carole C., Diaz N., Meehan C.L., Caffe B., Sangster M.Y., Topham D., McGuire M.A., Seppo A., McGuire M.K. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio. 2021;12(1) doi: 10.1128/mBio.03192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. medRxiv. 2021 doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golan Y., Prahl M., Cassidy A., Lin C.Y., Ahituv N., Flaherman V.J., Gaw S.L. COVID-19 mRNA vaccine is not detected in human milk. medRxiv. 2021 doi: 10.1001/jamapediatrics.2021.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baird J.K., Jensen S.M., Urba W.J., Fox B.A., Baird J.R. SARS-CoV-2 antibodies detected in human breast milk post-vaccination. medRxiv. 2021 doi: 10.1177/08903344211030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedman M.R., Kigel A., Bahar Y., Yogev Y., Dror Y., Lubetzky R., Many A., Wine Y. BNT162b2 COVID-19 mRNA vaccine elicits a rapid and synchronized antibody response in blood and milk of breastfeeding women. medRxiv. 2021 doi: 10.1038/s41467-021-26507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minkoff H., Ecker J. Balancing risks: making decisions for maternal treatment without data on fetal safety. Am. J. Obstet. Gynecol. 2021 doi: 10.1016/j.ajog.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.Y., Tureci O., Tompkins K.R., Lyke K.E., Raabe V., Dormitzer P.R., Jansen K.U., Sahin U., Gruber W.C. Safety and immunogenicity of two RNA-Based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]