Abstract
Correction for ‘Palladium-catalysed 5-endo-trig allylic (hetero)arylation’ by Bara Singh et al., Chem. Sci., 2020, 11, 4948–4953, DOI: 10.1039/D0SC01932A.
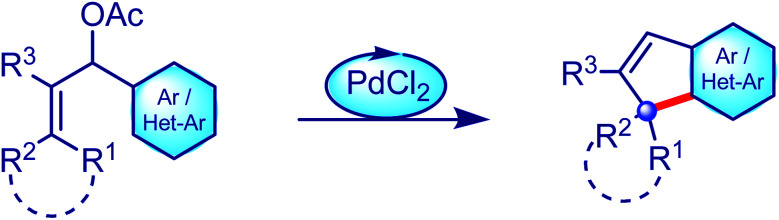
After the publication of our manuscript, a reader suggested that the transformation (shown in Scheme 1) can also be described as a Nazarov-type cyclisation1 with palladium chloride acting as a Lewis acid. While we do not have any evidence at this stage to support the Lewis acidic behaviour of palladium chloride,2 however, a Nazarov-type mechanistic scenario is possible. The overall transformation can also be considered as an intramolecular ene-type reaction3 or as an intramolecular Friedel–Crafts-type reaction,4 although our data agrees better with the 5-endo-trig process facilitated by the LUMO umpolung.5 Further experimental and computational investigations are underway to elucidate the full mechanistic details.
Scheme 1. General representation of the palladium-catalysed intramolecular allylic (hetero)arylation strategy reported by our group.
We thank the reader for the thought-provoking comments and also for their interest in our work.
Previously, the methoxy (–OMe) groups in the structure 2l (Table 2) were wrongly placed. The correct structure of 2l is show below.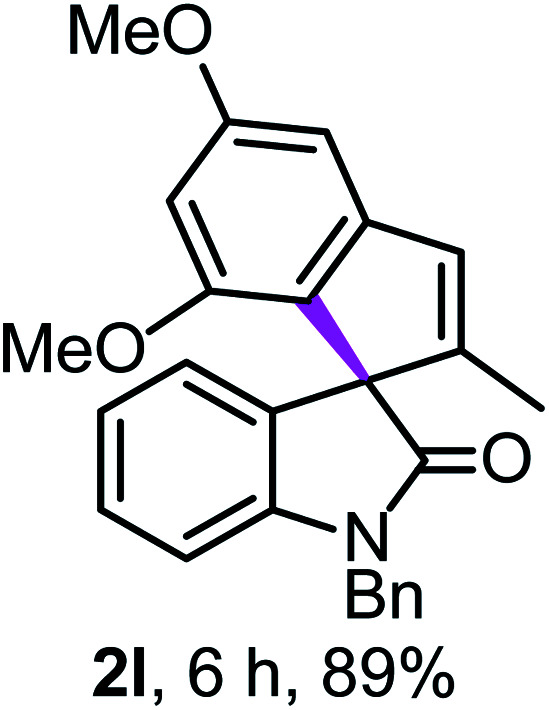
These corrections do not influence any conclusions reported in the main article.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- For some seminal contributions on Nazarov-type cyclisation reactions, see: ; (a) Bee C. Leclerc E. Tius M. A. Org. Lett. 2003;5:4927. doi: 10.1021/ol036017e. [DOI] [PubMed] [Google Scholar]; (b) Frontier A. J. Collison C. Tetrahedron. 2005;61:7577. doi: 10.1016/j.tet.2005.05.019. [DOI] [Google Scholar]; (c) Malona J. A. Colbourne J. M. Frontier A. J. Org. Lett. 2006;8:5661. doi: 10.1021/ol062403v. [DOI] [PubMed] [Google Scholar]; (d) Subramanium S. S. Handa S. Miranda A. J. Slaughter L. M. ACS Catal. 2011;1:1371. doi: 10.1021/cs200449g. [DOI] [Google Scholar]; (e) Spencer W. T. Vaidya T. Frontier A. J. Eur. J. Org. Chem. 2013:3621. doi: 10.1002/ejoc.201300134. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shao J. Hu P. Hong G. Fang M. Li X. Xu X. Synlett. 2014;25:1009. doi: 10.1055/s-0033-1339115. [DOI] [Google Scholar]; (g) Vaidya T. Cheng R. Carlsen P. N. Frontier A. J. Eisenberg R. Org. Lett. 2014;16:800. doi: 10.1021/ol403542k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Kitamura K. Shimada N. Stewart C. Atesin A. C. Atesin T. A. Tius M. A. Angew. Chem., Int. Ed. 2015;54:6288. doi: 10.1002/anie.201500881. [DOI] [PubMed] [Google Scholar]; (i) Martin M. C. Sandridge M. J. Williams C. W. Francis Z. A. France S. Tetrahedron. 2017;73:4093. doi: 10.1016/j.tet.2017.03.041. [DOI] [Google Scholar]
- For some rare examples where palladium(ii) complexes were shown to behave as Lewis acids, see: ; (a) Asao N. Nogami T. Takahashi K. Yamamoto Y. J. Am. Chem. Soc. 2002;124:764. doi: 10.1021/ja017366b. [DOI] [PubMed] [Google Scholar]; (b) Xiao Y. Zhang J. Angew. Chem., Int. Ed. 2008;47:190. doi: 10.1002/anie.200703516. [DOI] [PubMed] [Google Scholar]
- (a) Bankar S. K. Singh B. Tung P. Ramasastry S. S. V. Angew. Chem., Int. Ed. 2018;57:1678. doi: 10.1002/anie.201711797. [DOI] [PubMed] [Google Scholar]; (b) Yadav S. Hazra R. Singh A. Ramasastry S. S. V. Org. Lett. 2019;21:2983. doi: 10.1021/acs.orglett.9b00410. [DOI] [PubMed] [Google Scholar]
- (a) Yadav J. S. Mishra A. K. Das S. Tetrahedron. 2014;70:7560. doi: 10.1016/j.tet.2014.08.001. [DOI] [Google Scholar]; (b) Zhao Z.-L. Xu Q. L. Gu Q. Wu X.-Y. Yu S.-L. Org. Biomol. Chem. 2015;13:3086. doi: 10.1039/C4OB02574A. [DOI] [PubMed] [Google Scholar]; (c) Nemato T. Hamada Y. Synlett. 2016;27:2301. doi: 10.1055/s-0035-1561470. [DOI] [Google Scholar]
- According to the pioneer in this field, Prof. Igor Alabugin, the LUMO umpolung would apply to olefins as long as the π-complex formation is feasible. For details, see: ; (a) Alabugin I. V. Gilmore K. Chem. Commun. 2013;49:11246. doi: 10.1039/C3CC43872D. [DOI] [PubMed] [Google Scholar]; (b) dos Passos Gomes G. Alabugin I. V. J. Am. Chem. Soc. 2017;139:3406. doi: 10.1021/jacs.6b11054. [DOI] [PubMed] [Google Scholar]; (c) Alabugin I. V. Gonzalez-Rodriguez E. Acc. Chem. Res. 2018;51:1206. doi: 10.1021/acs.accounts.8b00026. [DOI] [PubMed] [Google Scholar]